MOF-Derived Nitrogen-Doped Porous Carbon Polyhedrons/Carbon Nanotubes Nanocomposite for High-Performance Lithium–Sulfur Batteries
Abstract
:1. Introduction
2. Experimental
2.1. Materials
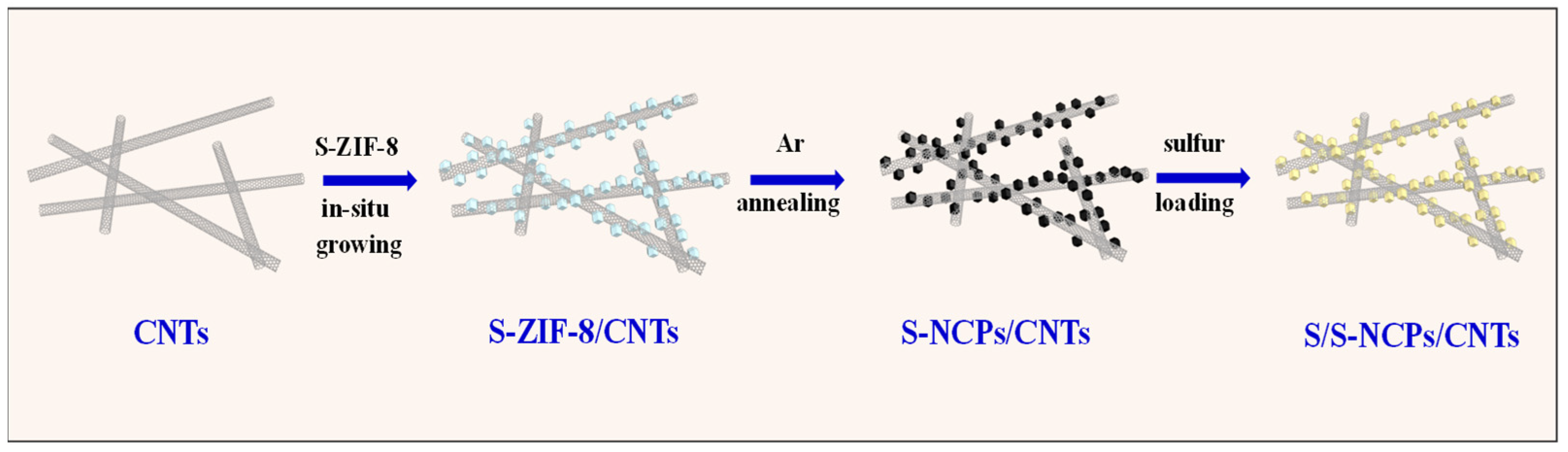
2.2. Synthesis of S-NCP/CNT Nanocomposite
2.3. Fabrication of S/S-NCP/CNT Nanocomposite
2.4. Materials Characterization
2.5. Electrochemical Measurements
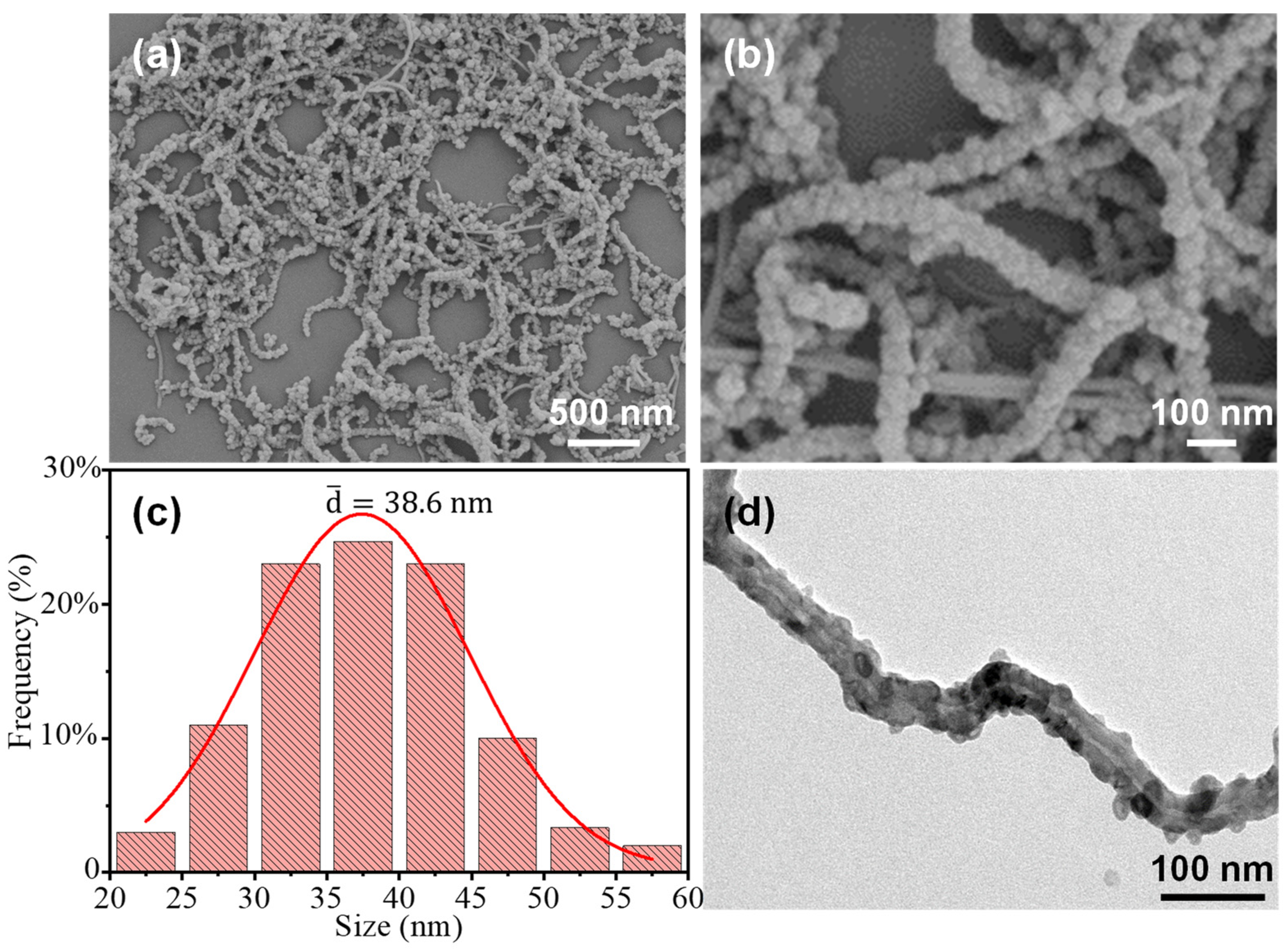
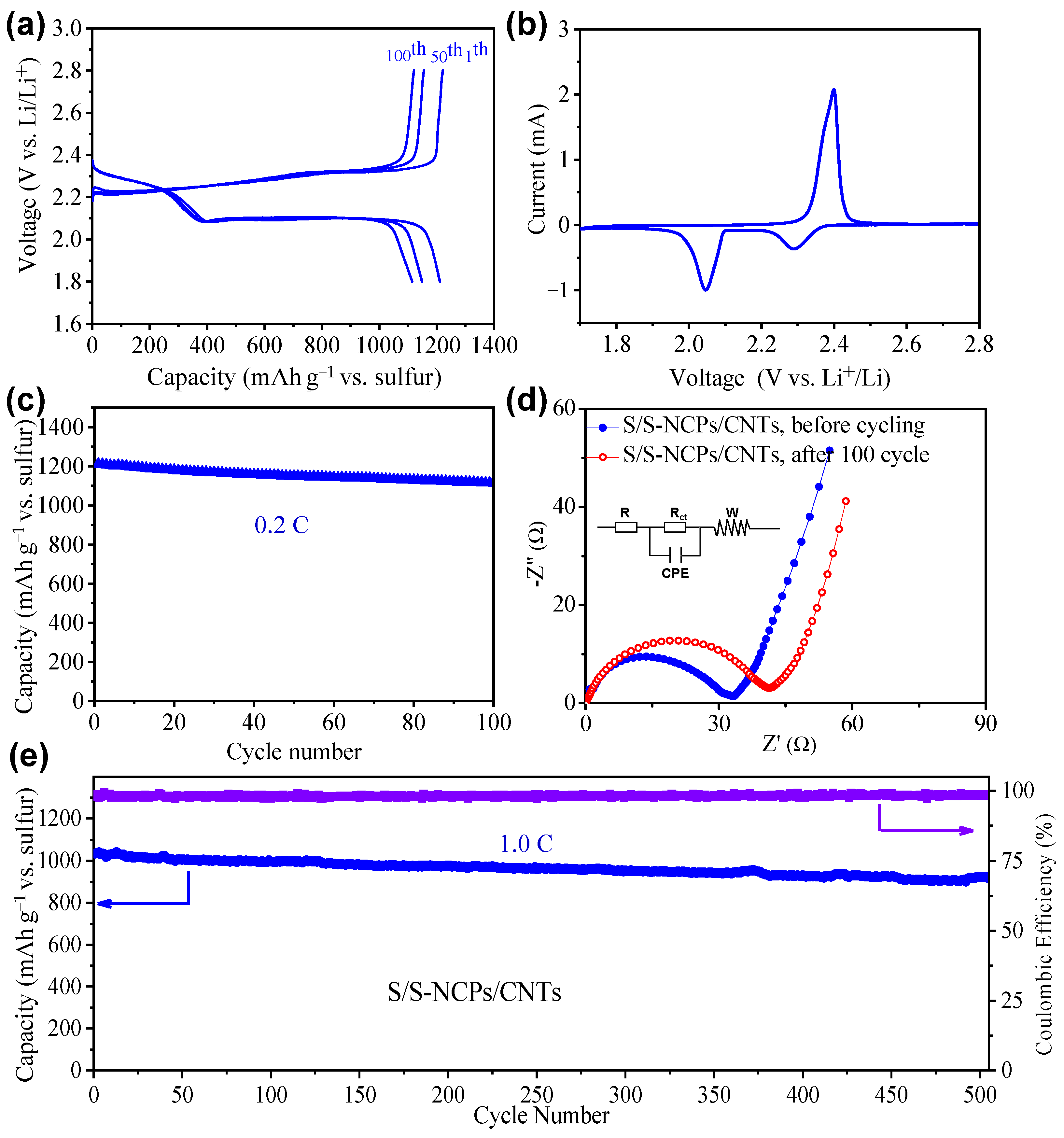
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, D.; Jiang, S.; Yu, S.; Ren, J.; Zhang, Z.; Liu, S.; Liu, X.; Wang, Z.; Wu, Y.; Zhang, Y. Lychee seed-derived microporous carbon for high-performance sodium-sulfur batteries. Carbon 2023, 201, 864–870. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, D.; Xu, Y.; Liu, S.; Xu, X.; Zhou, J.; Gao, F.; Tang, H.; Wang, Z.; Wu, Y.; et al. A Review on Electrode Materials of Fast-Charging Lithium-Ion Batteries. Chem. Rec. 2022, 22, e202200127. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Wang, Z.; Xu, P.; Wang, C.; Gao, F.; Zhao, D.; Liu, S.; Yang, H.; Wang, D.; Niu, C.; et al. Porous Co2VO4 Nanodisk as a High-Energy and Fast-Charging Anode for Lithium-Ion Batteries. Nano-Micro Lett. 2022, 14, 5. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, H.; Qi, Z.; Liu, X.; Ren, Y. The relationship between the adsorption configuration and fermi energy levels in the conversion process of Li2S6/Li2S8 on the ZrO2 surface. Mater. Today Chem. 2023, 29, 101467. [Google Scholar] [CrossRef]
- Gao, F.; Yue, X.; Xu, X.; Xu, P.; Zhang, F.; Fan, H.; Wang, Z.; Wu, Y.; Liu, X.; Zhang, Y. A N/Co co-doped three-dimensional porous carbon as cathode host for advanced lithium–selenium batteries. Rare Metals 2023, 42, 2670–2678. [Google Scholar] [CrossRef]
- Ye, H.; Li, Y. Towards practical lean-electrolyte Li-S batteries: Highly solvating electrolytes or sparingly solvating electrolytes? Nano Res. Energy 2022, 1, e9120012. [Google Scholar] [CrossRef]
- Marceau, H.; Kim, C.; Paolella, A.; Ladouceur, S.; Lagacé, M.; Chaker, M.; Vijh, A.; Guerfi, A.; Julien, C.M.; Mauger, A.; et al. In operando scanning electron microscopy and ultraviolet–visible spectroscopy studies of lithium/sulfur cells using all solid-state polymer electrolyte. J. Power Sources 2016, 319, 247–254. [Google Scholar] [CrossRef]
- Wang, M.; Qin, B.; Wu, S.; Li, Y.; Liu, C.; Zhang, Y.; Zeng, L.; Fan, H. Interface ion-exchange strategy of MXene@FeIn2S4 hetero-structure for super sodium ion half/full batteries. J. Colloid Interface Sci. 2023, 650, 1457–1465. [Google Scholar] [CrossRef]
- Zhou, Y.; Cheng, W.; Bai, Y.; Hou, C.; Li, K.; Huang, Y. Rise of flexible high-temperature electronics. Rare Metals 2023, 42, 1773–1777. [Google Scholar] [CrossRef]
- Zhou, Y.; Qu, Y.; Yin, L.; Cheng, W.; Huang, Y.; Fan, R. Coassembly of elastomeric microfibers and silver nanowires for fabricating ultra-stretchable microtextiles with weakly and tunable negative permittivity. Compos. Sci. Technol. 2022, 223, 109415. [Google Scholar] [CrossRef]
- Li, H.; Shao, F.; Wen, X.; Ding, Y.; Zhou, C.; Zhang, Y.; Wei, H.; Hu, N. Graphene/MXene fibers-enveloped sulfur cathodes for high-performance Li-S batteries. Electrochim. Acta 2021, 371, 137838. [Google Scholar] [CrossRef]
- Yuan, Z.; Xiao, F.; Fang, Y.; Xiong, P.; Sun, X.; Duan, X.; Yang, X.; Fan, H.; Wei, M.; Qian, Q.; et al. Defect engineering on VO2(B) nanoleaves/graphene oxide for the high performance of cathodes of zinc-ion batteries with a wide temperature range. J. Power Sources 2023, 559, 232688. [Google Scholar] [CrossRef]
- Zeng, L.; Fang, Y.; Xu, L.; Zheng, C.; Yang, M.-Q.; He, J.; Xue, H.; Qian, Q.; Wei, M.; Chen, Q. Rational design of few-layer MoSe2 confined within ZnSe-C hollow porous spheres for high-performance lithium-ion and sodium-ion batteries. Nanoscale 2019, 11, 6766–6775. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Ge-Zhang, S.; Zhang, Z.; Tang, H.; Xu, Y.; Gao, F.; Xu, X.; Liu, S.; Zhou, J.; Wang, Z.; et al. Three-Dimensional Honeycomb-Like Carbon as Sulfur Host for Sodium–Sulfur Batteries without the Shuttle Effect. ACS Appl. Mater. Interfaces 2022, 14, 54662–54669. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Cao, G.; Yuan, M.; Zhong, S.; Wang, Y.; Liu, X.; Cao, D.; Peng, W.; Liu, J.; Wang, G.; et al. Core-Shell Engineering of Conductive Fillers toward Enhanced Dielectric Properties: A Universal Polarization Mechanism in Polymer Conductor Composites. Adv. Mater. 2023, 35, 2207829. [Google Scholar] [CrossRef]
- Wu, S.; Xu, F.; Li, Y.; Liu, C.; Zhang, Y.; Fan, H. Synergistically enhanced sodium ion storage from encapsulating highly dispersed cobalt nanodots into N, P, S tri-doped hexapod carbon framework. J. Colloid Interface Sci. 2023, 649, 741–749. [Google Scholar] [CrossRef]
- Du, X.-L.; You, Y.; Yan, Y.; Zhang, D.; Cong, H.-P.; Qin, H.; Zhang, C.; Cao, F.-F.; Jiang, K.-C.; Wang, Y.; et al. Conductive Carbon Network inside a Sulfur-Impregnated Carbon Sponge: A Bioinspired High-Performance Cathode for Li–S Battery. ACS Appl. Mater. Interfaces 2016, 8, 22261–22269. [Google Scholar] [CrossRef]
- Su, Z.; Wang, R.; Huang, J.; Sun, R.; Qin, Z.; Zhang, Y.; Fan, H. Silver vanadate (Ag0.33V2O5) nanorods from Ag intercalated vanadium pentoxide for superior cathode of aqueous zinc-ion batteries. Rare Metals 2022, 41, 2844–2852. [Google Scholar] [CrossRef]
- Gu, X.; Tong, C.-J.; Rehman, S.; Liu, L.-M.; Hou, Y.; Zhang, S. Multifunctional Nitrogen-Doped Loofah Sponge Carbon Blocking Layer for High-Performance Rechargeable Lithium Batteries. ACS Appl. Mater. Interfaces 2016, 8, 15991–16001. [Google Scholar] [CrossRef]
- Zhao, Q.N.; Wang, R.H.; Wen, J.; Hu, X.L.; Li, Z.Y.; Li, M.H.; Pan, F.S.; Xu, C.H. Separator engineering toward practical Li-S batteries: Targeted electrocatalytic sulfur conversion, lithium plating regulation, and thermal tolerance. Nano Energy 2022, 95, 106982. [Google Scholar] [CrossRef]
- Yang, M.H.; Wang, X.W.; Wu, J.F.; Tian, Y.; Huang, X.Y.; Liu, P.; Li, X.Y.; Li, X.R.; Liu, X.Y.; Li, H.X. Dual electrocatalytic heterostructures for efficient immobilization and conversion of polysulfides in Li-S batteries. J. Mater. Chem. A 2021, 9, 18477–18487. [Google Scholar] [CrossRef]
- Ji, X.L.; Nazar, L.F. Advances in Li-S batteries. J. Mater. Chem. 2010, 20, 9821–9826. [Google Scholar] [CrossRef]
- Qiu, S.; Zhang, J.; Liang, X.; Li, Y.; Cui, J.; Chen, M. Tunable MOFs derivatives for stable and fast sulfur electrodes in Li-S batteries. Chem. Eng. J. 2022, 450, 138287. [Google Scholar] [CrossRef]
- Liu, Z.; Balbuena, P.; Mukherjee, P. Revealing Charge Transport Mechanisms in Li2S2 for Li-Sulfur Batteries. J. Phys. Chem. Lett. 2017, 8, 1324–1330. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, L.; Pan, H.; Zhang, J.; Xiang, S.; Cheng, Z.; Zhang, Z. Pore-space-partitioned MOF separator promotes high-sulfur-loading Li–S batteries with intensified rate capability and cycling life. J. Mater. Chem. A 2021, 47, 26413–27104. [Google Scholar] [CrossRef]
- He, D.; Yue, C.; Tang, L.; Wang, B.; Tang, H.; Li, X.; Chen, J.; Gao, M.; Liu, N. NiCoSe4@CNFs derived from MOF compounds enabling robust polysulfide adsorption and catalysis in Li-S batteries. J. Alloys Compd. 2023, 957, 170449. [Google Scholar] [CrossRef]
- Raulo, A.; Gupta, A.; Srivastava, R.; Nandan, B. Cotton cloth templated in situ encapsulation of sulfur into carbon fibers for lithium-sulfur batteries. Chem. Commun. 2021, 57, 544–547. [Google Scholar] [CrossRef]
- Ren, F.; Wu, T.; Zhang, J.; Lu, Z.; Duan, Q.; Pei, L.; Ren, P. Realization of water resistant, durable and self-cleaning on oriented cellulose nanocomposite packaging films. J. Polym. Res. 2022, 30, 3. [Google Scholar] [CrossRef]
- He, Y.; Chang, Z.; Wu, S.; Qiao, Y.; Bai, S.; Jiang, K.; He, P.; Zhou, H. Simultaneously Inhibiting Lithium Dendrites Growth and Polysulfides Shuttle by a Flexible MOF-Based Membrane in Li–S Batteries. Adv. Energy Mater. 2018, 8, 1802130. [Google Scholar] [CrossRef]
- Sonawane, A.; Murthy, Z. Synthesis, characterization, and application of ZIF-8/Ag3PO4, MoS2/Ag3PO4, and h-BN/Ag3PO4 based photocatalytic nanocomposite polyvinylidene fluoride mixed matrix membranes for effective removal of drimaren orange P2R. J. Membr. Sci. 2022, 641, 119939. [Google Scholar] [CrossRef]
- Yao, T.; Zhou, W.; Cao, G.; Peng, W.; Liu, J.; Dong, X.; Chen, X.; Zhang, Y.; Chen, Y.; Yuan, M. Engineering of core@double-shell structured Zn@ZnO@PS particles in poly(vinylidene fluoride) composites towards significantly enhanced dielectric performances. J. Appl. Polym. Sci. 2023, 140, e53772. [Google Scholar] [CrossRef]
- Li, C.; Liu, R.; Xiao, Y.; Cao, F.F.; Zhang, H. Recent progress of separators in lithium-sulfur batteries. Energy Storage Mater. 2021, 40, 439–460. [Google Scholar] [CrossRef]
- Wang, M.Y.; Bai, Z.C.; Yang, T.; Nie, C.H.; Xu, X.; Wang, Y.X.; Yang, J.; Dou, S.X.; Wang, N.N. Advances in High Sulfur Loading Cathodes for Practical Lithium-Sulfur Batteries. Adv. Energy Mater. 2022, 12, 2201585. [Google Scholar] [CrossRef]
- Zou, K.Y.; Zhou, T.F.; Chen, Y.Z.; Xiong, X.Y.; Jing, W.T.; Dai, X.; Shi, M.; Li, N.; Sun, J.J.; Zhang, S.L.; et al. Defect Engineering in a Multiple Confined Geometry for Robust Lithium-Sulfur Batteries. Adv. Energy Mater. 2022, 12, 2103981. [Google Scholar] [CrossRef]
- Yin, Y.X.; Xin, S.; Guo, Y.G.; Wan, L.J. Lithium-Sulfur Batteries: Electrochemistry, Materials, and Prospects. Angew. Chem.-Int. Ed. 2013, 52, 13186–13200. [Google Scholar] [CrossRef]
- Lin, C.; Yang, X.; Xiong, P.; Lin, H.; He, L.; Yao, Q.; Wei, M.; Qian, Q.; Chen, Q.; Zeng, L. High-Rate, Large Capacity, and Long Life Dendrite-Free Zn Metal Anode Enabled by Trifunctional Electrolyte Additive with a Wide Temperature Range. Adv. Sci. 2022, 9, 2201433. [Google Scholar] [CrossRef]
- Yang, X.X.; Li, X.T.; Zhao, C.F.; Fu, Z.H.; Zhang, Q.S.; Hu, C. Promoted Deposition of Three-Dimensional Li2S on Catalytic Co Phthalocyanine Nanorods for Stable High-Loading Lithium-Sulfur Batteries. ACS Appl. Mater. Interfaces 2020, 12, 32752–32763. [Google Scholar] [CrossRef]
- Li, Y.; Peng, X.; Li, X.; Duan, H.; Xie, S.; Dong, L.; Kang, F. Functional Ultrathin Separators Proactively Stabilizing Zinc Anodes for Zinc-Based Energy Storage. Adv. Mater. 2023, 35, 2300019. [Google Scholar] [CrossRef]
- Liu, G.; Hou, F.; Wang, Y.; Wang, X.; Fang, B. Engineering Ir and Ni3N heterogeneous interfaces for promoted overall water splitting. Appl. Surf. Sci. 2023, 637, 157896. [Google Scholar] [CrossRef]
- Fang, B.; Wei, Y.; Suzuki, K.; Kumagai, M. Surface modification of carbonaceous materials for EDLCs application. Electrochim. Acta 2005, 50, 3616–3621. [Google Scholar] [CrossRef]
- Xu, J.; An, S.H.; Song, X.Y.; Cao, Y.J.; Wang, N.; Qiu, X.; Zhang, Y.; Chen, J.W.; Duan, X.L.; Huang, J.H.; et al. Towards High Performance Li-S Batteries via Sulfonate-Rich COF-Modified Separator. Adv. Mater. 2021, 33, 2105178. [Google Scholar] [CrossRef]
- Fang, R.P.; Zhao, S.Y.; Sun, Z.H.; Wang, W.; Cheng, H.M.; Li, F. More Reliable Lithium-Sulfur Batteries: Status, Solutions and Prospects. Adv. Mater. 2017, 29, 1606823. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Yang, Y.; Yu, S.; Zhang, Y.; Hou, J.; Yu, N.; Fang, B. MOF-Derived Nitrogen-Doped Porous Carbon Polyhedrons/Carbon Nanotubes Nanocomposite for High-Performance Lithium–Sulfur Batteries. Nanomaterials 2023, 13, 2416. https://doi.org/10.3390/nano13172416
Chen J, Yang Y, Yu S, Zhang Y, Hou J, Yu N, Fang B. MOF-Derived Nitrogen-Doped Porous Carbon Polyhedrons/Carbon Nanotubes Nanocomposite for High-Performance Lithium–Sulfur Batteries. Nanomaterials. 2023; 13(17):2416. https://doi.org/10.3390/nano13172416
Chicago/Turabian StyleChen, Jun, Yuanjiang Yang, Sheng Yu, Yi Zhang, Jiwei Hou, Nengfei Yu, and Baizeng Fang. 2023. "MOF-Derived Nitrogen-Doped Porous Carbon Polyhedrons/Carbon Nanotubes Nanocomposite for High-Performance Lithium–Sulfur Batteries" Nanomaterials 13, no. 17: 2416. https://doi.org/10.3390/nano13172416
APA StyleChen, J., Yang, Y., Yu, S., Zhang, Y., Hou, J., Yu, N., & Fang, B. (2023). MOF-Derived Nitrogen-Doped Porous Carbon Polyhedrons/Carbon Nanotubes Nanocomposite for High-Performance Lithium–Sulfur Batteries. Nanomaterials, 13(17), 2416. https://doi.org/10.3390/nano13172416






