Thermally Stable Ceramic-Salt Electrolytes for Li Metal Batteries Produced from Cold Sintering Using DMF/Water Mixture Solvents
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
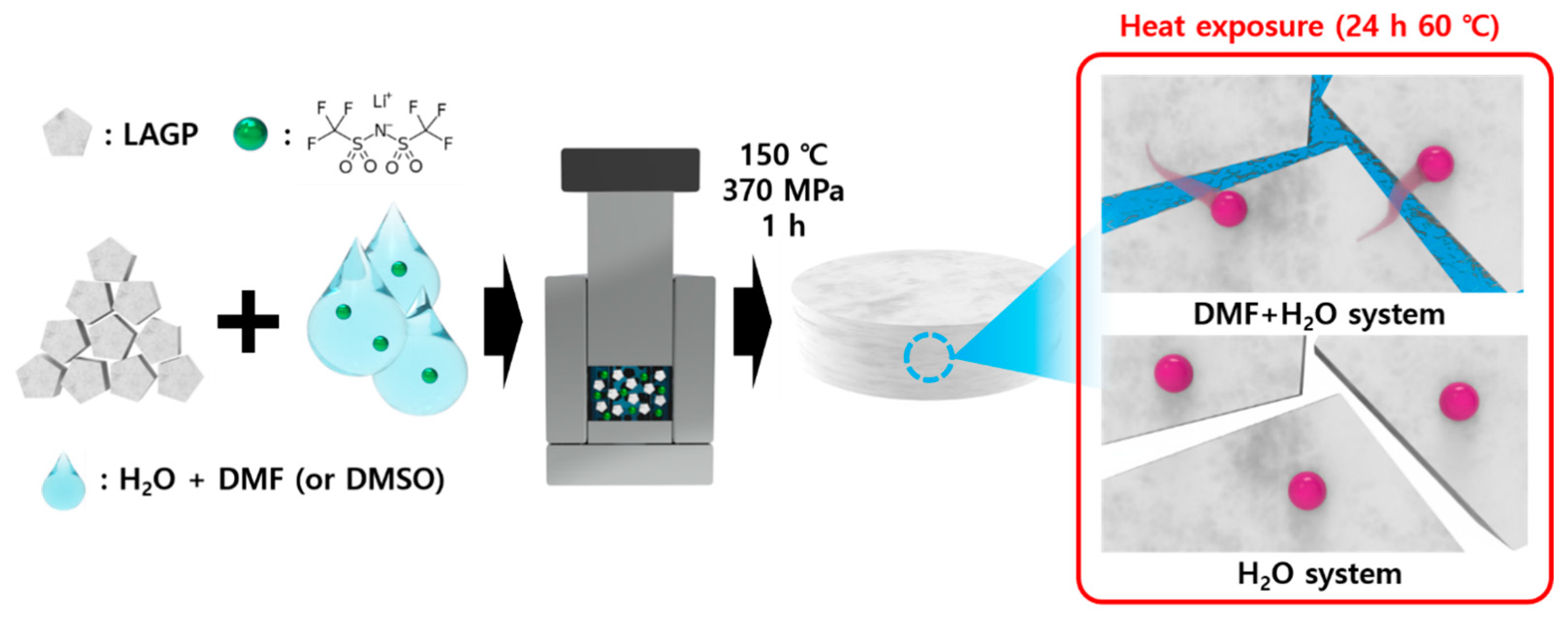
2.2. Preparation of Electrolytes Using CSP
2.3. Characterization
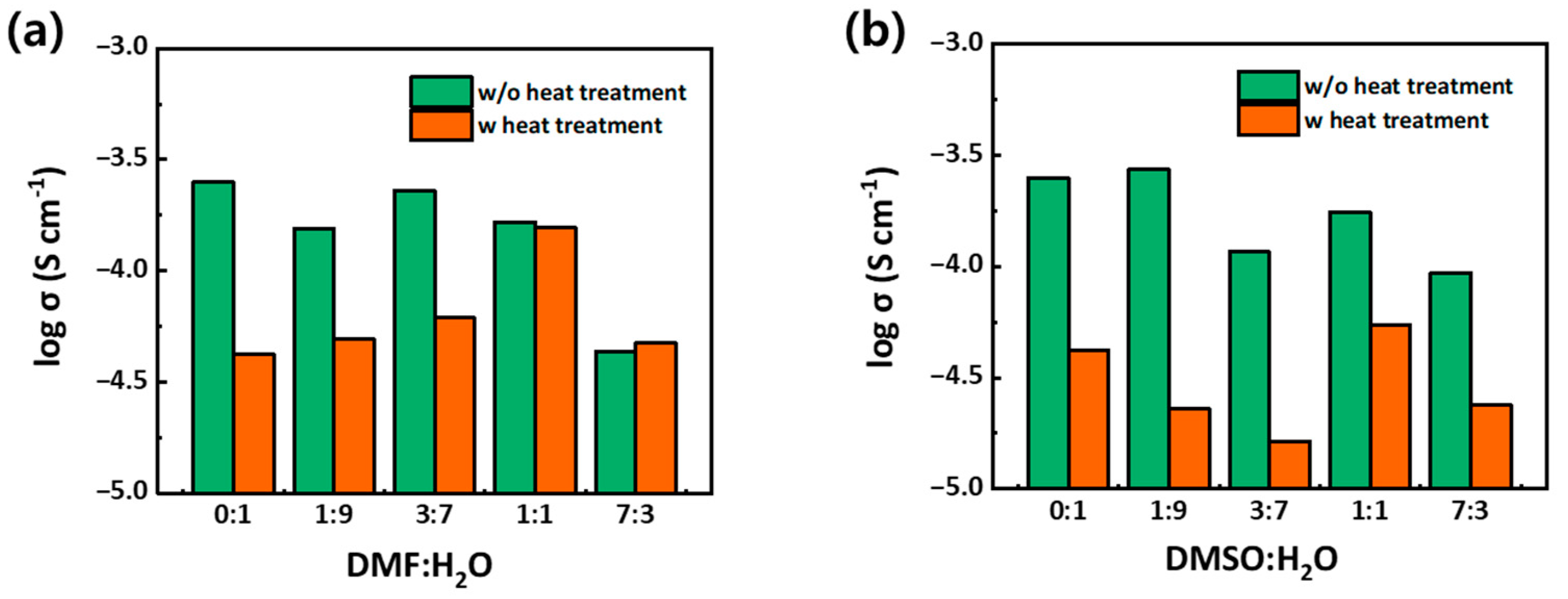
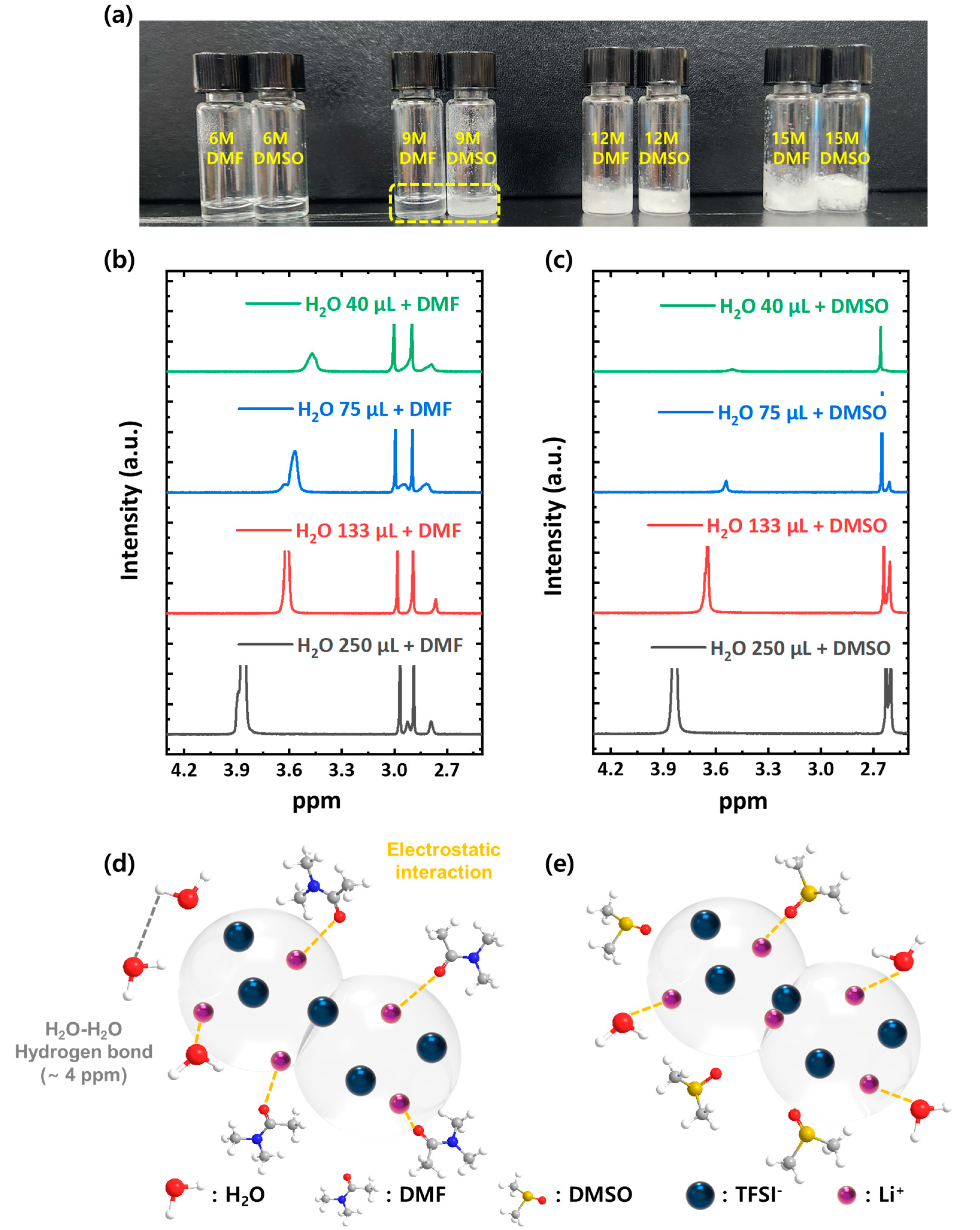
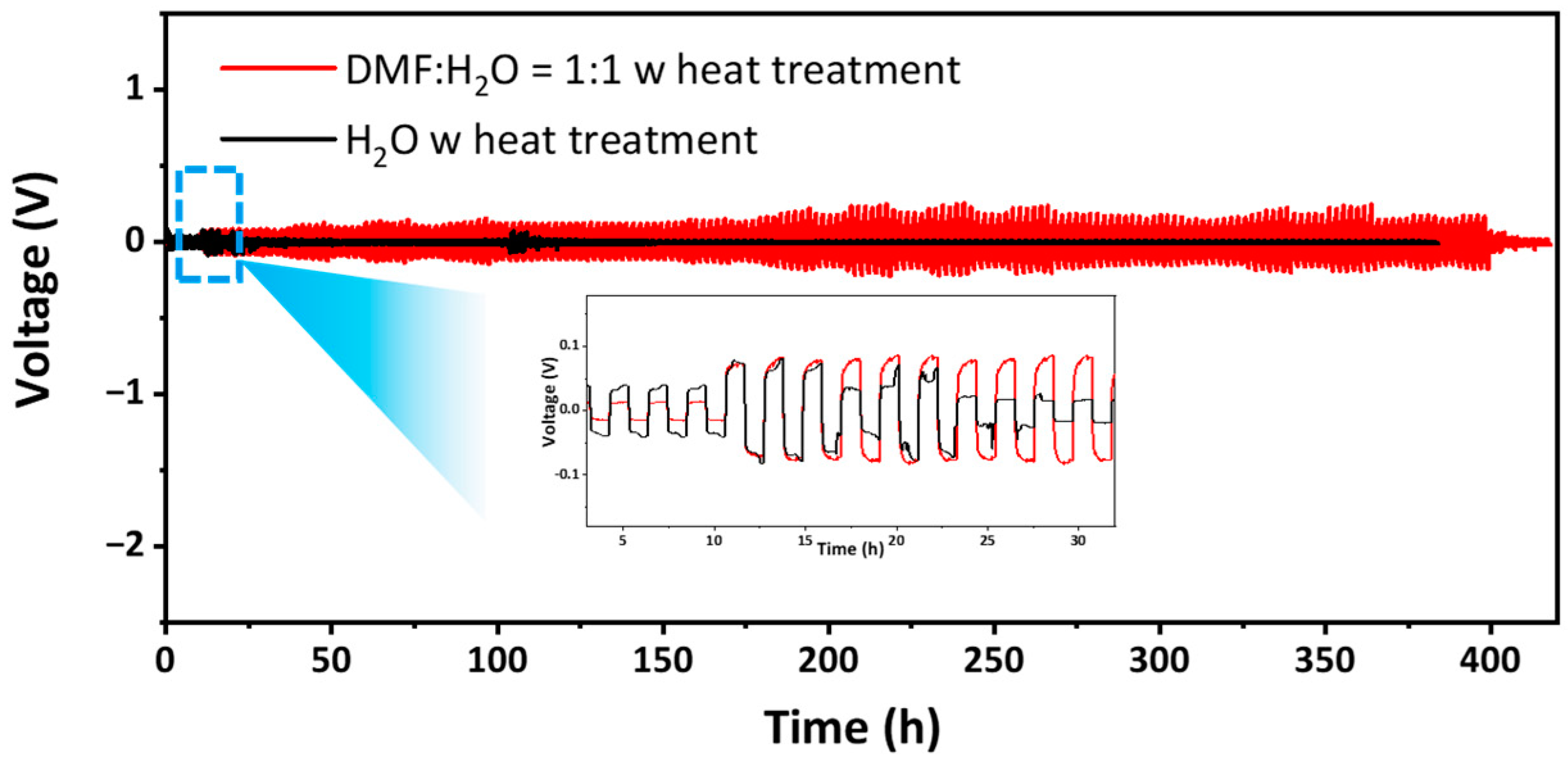
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Kim, T.; Song, W.; Son, D.-Y.; Ono, L.K.; Qi, Y. Lithium-ion batteries: Outlook on present, future, and hybridized technologies. J. Mater. Chem. A 2019, 7, 2942–2964. [Google Scholar] [CrossRef]
- Jie, Y.; Ren, X.; Cao, R.; Cai, W.; Jiao, S. Advanced liquid electrolytes for rechargeable li metal batteries. Adv. Funct. Mater. 2020, 30, 1910777. [Google Scholar] [CrossRef]
- Xu, L.; Li, J.; Deng, W.; Shuai, H.; Li, S.; Xu, Z.; Li, J.; Hou, H.; Peng, H.; Zou, G.; et al. Garnet solid electrolyte for advanced All-Solid-State li batteries. Adv. Energy Mater. 2020, 11, 2000648. [Google Scholar] [CrossRef]
- Chen, X.-R.; Zhao, B.-C.; Yan, C.; Zhang, Q. Review on li deposition in working batteries: From nucleation to early growth. Adv. Mater. 2021, 33, 2004128. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Toward safe lithium metal anode in rechargeable batteries: A review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef]
- Yang, C.; Fu, K.; Zhang, Y.; Hitz, E.; Hu, L. Protected lithium-metal anodes in batteries: From liquid to solid. Adv. Mater. 2017, 29, 1701169. [Google Scholar] [CrossRef]
- Fan, L.; Wei, S.; Li, S.; Li, Q.; Lu, Y. Recent progress of the solid-state electrolytes for high-energy metal-based batteries. Adv. Energy Mater. 2018, 8, 1702657. [Google Scholar] [CrossRef]
- Tikekar, M.D.; Choudhury, S.; Tu, Z.; Archer, L.A. Design principles for electrolytes and interfaces for stable lithium-metal batteries. Nat. Energy 2016, 1, 16114. [Google Scholar] [CrossRef]
- Deng, X.; Zou, K.; Momen, R.; Cai, P.; Chen, J.; Hou, H.; Zou, G.; Ji, X. High content anion (S/Se/P) doping assisted by defect engineering with fast charge transfer kinetics for high-performance sodium ion capacitors. Sci. Bull. 2021, 66, 1858–1868. [Google Scholar] [CrossRef]
- Liu, C.; Wang, B.; Xu, L.; Zou, K.; Deng, W.; Hou, H.; Zou, G.; Ji, X. Novel nonstoichiometric niobium oxide anode material with rich oxygen vacancies for advanced lithium-ion capacitors. ACS Appl. Mater. Interfaces 2023, 15, 5387–5398. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Fu, X.; Liao, S.; Zou, G.; Yang, H. Interface engineering enables high-performance Sb anode for sodium storage. Nanomaterials 2023, 13, 254. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.-J.; Liu, C.; Li, J.-Y.; Hu, Z.-L.; Zhou, R.-Y.; Guo, C.-C.; Liu, X.-R.; Yang, F.-F.; Zhu, Y.-R. 3D nitrogen and boron dual-doped carbon quantum dots/reduced graphene oxide aerogel for advanced aqueous and flexible quasi-solid-state zinc-ion hybrid capacitors. Rare Met. 2023, 42, 2307–2323. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, J.-G.; Xu, W. Advancing lithium metal batteries. Joule 2018, 2, 833–845. [Google Scholar] [CrossRef]
- Kim, J.; Kwon, K.; Kim, K.; Han, S.; Kim, P.J.; Choi, J. Size effect of a piezoelectric material as a separator coating layer for suppressing dendritic li growth in li metal batteries. Nanomaterials 2022, 13, 90. [Google Scholar] [CrossRef]
- Ding, F.; Xu, W.; Graff, G.L.; Zhang, J.; Sushko, M.L.; Chen, X.; Shao, Y.; Engelhard, M.H.; Nie, Z.; Xiao, J.; et al. Dendrite-free lithium deposition via self-healing electrostatic shield mechanism. J. Am. Chem. Soc. 2013, 135, 4450–4456. [Google Scholar] [CrossRef]
- Feng, J.; Wang, L.; Chen, Y.; Wang, P.; Zhang, H.; He, X. PEO based polymer-ceramic hybrid solid electrolytes: A review. Nano Converg. 2021, 8, 2. [Google Scholar] [CrossRef]
- Yu, X.; Jiang, Z.; Yuan, R.; Song, H. A review of the relationship between gel polymer electrolytes and solid electrolyte interfaces in lithium metal batteries. Nanomaterials 2023, 13, 1789. [Google Scholar] [CrossRef]
- Rangasamy, E.; Wolfenstine, J.; Sakamoto, J. The role of al and li concentration on the formation of cubic garnet solid electrolyte of nominal composition Li7La3Zr2O12. Solid. State Ion. 2012, 206, 28–32. [Google Scholar] [CrossRef]
- Xu, A.; Wang, R.; Yao, M.; Cao, J.; Li, M.; Yang, C.; Liu, F.; Ma, J. Electrochemical properties of an Sn-doped LATP ceramic electrolyte and its derived sandwich-structured composite solid electrolyte. Nanomaterials 2022, 12, 2082. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 16103. [Google Scholar] [CrossRef]
- Qian, J.; Henderson, W.A.; Xu, W.; Bhattacharya, P.; Engelhard, M.; Borodin, O.; Zhang, J.-G. High rate and stable cycling of lithium metal anode. Nat. Commun. 2015, 6, 6362. [Google Scholar] [CrossRef]
- Ramasubramanian, B.; Reddy, M.V.; Zaghib, K.; Armand, M.; Ramakrishna, S. Growth mechanism of micro/nano metal dendrites and cumulative strategies for countering its impacts in metal ion batteries: A review. Nanomaterials 2021, 11, 2476. [Google Scholar] [CrossRef]
- Seo, J.-H.; Nakaya, H.; Takeuchi, Y.; Fan, Z.; Hikosaka, H.; Rajagopalan, R.; Gomez, E.D.; Iwasaki, M.; Randall, C.V. Broad temperature dependence, high conductivity, and structure-property relations of cold sintering of LLZO-based composite electrolytes. J. Eur. Ceram. 2020, 40, 6241–6248. [Google Scholar] [CrossRef]
- Bang, S.H.; Tsuji, K.; Ndayishimiye, A.; Dursun, S.; Seo, J.-H.; Otieno, S.; Randall, C.A. Toward a size scale-up cold sintering process at reduced uniaxial pressure. J. Am. Ceram. Soc. 2020, 103, 2322–2327. [Google Scholar] [CrossRef]
- Hamao, N.; Yamaguchi, Y.; Hamamoto, K. Densification of a NASICON-type LATP electrolyte sheet by a cold-sintering process. Materials 2021, 14, 4737. [Google Scholar] [CrossRef] [PubMed]
- Grady, Z.; Fan, Z.; Ndayishimiye, A.; Randall, C.A. Design and sintering of all-solid-state composite cathodes with tunable mixed conduction properties via the cold sintering process. ACS Appl. Mater. Interfaces 2021, 13, 48071–48087. [Google Scholar] [CrossRef]
- Sharafi, A.; Kazyak, E.; Davis, A.L.; Yu, S.; Thompson, T.; Siegel, D.J.; Dasgupta, N.P.; Sakamoto, J. Surface chemistry mechanism of ultra-low interfacial resistance in the solid-state electrolyte Li7La3Zr2O12. Chem. Mater. 2017, 29, 7961–7968. [Google Scholar] [CrossRef]
- Liu, C.; Deng, L.; Li, X.; Wu, T.; Zhang, W.; Cui, H.; Yang, H. Metal-organic frameworks for solid-state electrolytes: A mini review. Electrochem. Commun. 2023, 150, 107491. [Google Scholar] [CrossRef]
- Jiang, W.; Dong, L.; Liu, S.; Ai, B.; Zhao, S.; Zhang, W.; Pan, K.; Zhang, L. Improvement of the interface between the lithium anode and a garnet-type solid electrolyte of lithium batteries using an aluminum-nitride layer. Nanomaterials 2022, 12, 2023. [Google Scholar] [CrossRef]
- DeWees, R.; Wang, H. Synthesis and properties of NaSICON-type LATP and LAGP solid electrolytes. ChemSusChem 2019, 12, 3713–3725. [Google Scholar] [CrossRef] [PubMed]
- Berbano, S.S.; Guo, J.; Guo, H.; Lanagan, M.T.; Randall, C.A. Cold sintering process of Li1.5Al0.5Ge1.5(PO4) 3 solid electrolyte. J. Am. Ceram. Soc. 2017, 100, 2123–2135. [Google Scholar] [CrossRef]
- Wang, C.; Ping, W.; Bai, Q.; Cui, H.; Hensleigh, R.; Wang, R.; Brozena, A.H.; Xu, Z.; Dai, J.; Pei, Y.; et al. A general method to synthesize and sinter bulk ceramics in seconds. Science 2020, 368, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; Lin, Y.; Chamas, M.; Hu, C.; Grasso, S. Cold isostatic sintering to enhance the ionic conductivity of LiFePO4. Ceram. Int. 2021, 47, 9296–9302. [Google Scholar] [CrossRef]
- Maria, J.-P.; Kang, X.; Floyd, R.D.; Dickey, E.C.; Guo, H.; Guo, J.; Baker, A.; Funihashi, S.; Randall, C.A. Cold sintering: Current status and prospects. J. Mater. Res. 2017, 32, 3205–3218. [Google Scholar] [CrossRef]
- Bouville, F.; Studart, A.R. Geologically-inspired strong bulk ceramics made with water at room temperature. Nat. Commun. 2017, 8, 14655. [Google Scholar] [CrossRef] [PubMed]
- Galotta, A.; Sglavo, V.M. The cold sintering process: A review on processing features, densification mechanisms and perspectives. J. Eur. Ceram. 2021, 41, 1–17. [Google Scholar] [CrossRef]
- Grasso, S.; Biesuz, M.; Zoli, L.; Taveri, G.; Duff, A.I.; Ke, D.; Jiang, A.; Reece, M.J. A review of cold sintering processes. Adv. Appl. Ceram. 2020, 119, 115–143. [Google Scholar] [CrossRef]
- Dursun, S.; Tsuji, K.; Bang, S.H.; Ndayishimiye, A.; Randall, C.A. A route towards fabrication of functional ceramic/polymer nanocomposite devices using the cold sintering process. ACS Appl. Electron. Mater. 2020, 2, 1917–1924. [Google Scholar] [CrossRef]
- Ndayishimiye, A.; Sengul, M.Y.; Bang, S.H.; Tsuji, K.; Takashima, K.; Beauvoir, T.H.; Denux, D.; Thibaud, J.-M.; Duin, A.C.; Elissalde, C.; et al. Comparing hydrothermal sintering and cold sintering process: Mechanisms, microstructure, kinetics and chemistry. J. Eur. Ceram. 2020, 40, 1312–1324. [Google Scholar] [CrossRef]
- Seo, J.-H.; Fan, Z.; Nakaya, H.; Rajagopalan, R.; Gomez, E.D.; Iwasaki, M.; Randall, C.A. Cold sintering, enabling a route to co-sinter an all-solid-state lithium-ion battery. Jpn. J. Appl. Phys. 2021, 60, 037001. [Google Scholar] [CrossRef]
- Chung, H.; Kang, B. Increase in grain boundary ionic conductivity of Li1.5Al0.5Ge1.5(PO4)3 by adding excess lithium. Solid. State Ion. 2014, 263, 125–130. [Google Scholar] [CrossRef]
- Kang, J.; Chung, H.; Doh, C.; Kang, B.; Han, B. Integrated study of first principles calculations and experimental measurements for li-ionic conductivity in al-doped solid-state LiGe2(PO4)3 electrolyte. J. Power Sources 2015, 293, 11–16. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Sun, Q.; Wang, D.; Adair, K.R.; Liang, J.; Zhang, C.; Zhang, L.; Lu, X.; Huang, H.; et al. Insight into the microstructure and ionic conductivity of cold sintered NASICON solid electrolyte for solid-state batteries. ACS Appl. Mater. Interfaces 2019, 11, 27890–27896. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Guo, P.; Shi, Y.; Li, S.; Li, K.; Lu, X.Y.; Zhang, Z.; He, D.; Bian, J.; Lu, X. Solid-state li metal battery enabled by cold sintering at 120 °C. Mater. Today Phys. 2021, 20, 100476. [Google Scholar] [CrossRef]
- Lee, W.; Lyon, C.K.; Seo, J.-H.; Lopez-Hallman, R.; Leng, Y.; Wang, C.-Y.; Hickner, M.A.; Randall, C.A.; Gomez, E.D. Ceramic–Salt composite electrolytes from cold sintering. Adv. Funct. Mater. 2019, 29, 1807872. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Z.; Ji, Z.; Wang, P.; Wang, J.; Ling, W.; Huang, Y. Temperature adaptability issue of aqueous rechargeable batteries. Mater. Today Energy 2021, 19, 100577. [Google Scholar] [CrossRef]
- Yuan, X.; Li, Y.; Zhu, Y.; Deng, W.; Li, C.; Zhou, Z.; Hu, J.; Zhang, M.; Chen, M.; Li, R. Low concentration DMF/H2O hybrid electrolyte: A new opportunity for anode materials in aqueous potassium-ion batteries. ACS Appl. Mater. Interfaces 2021, 13, 38248–38255. [Google Scholar] [CrossRef]
- Mariappan, C.R.; Yada, C.; Rosciano, F.; Roling, B. Correlation between micro-structural properties and ionic conductivity of Li1.5Al0.5Ge1.5(PO4)3 ceramics. J. Power Sources 2011, 196, 6456–6464. [Google Scholar] [CrossRef]
- Guo, J.; Guo, H.; Baker, A.L.; Lanagan, M.T.; Kupp, E.R.; Messing, G.L.; Randall, C.A. Cold sintering: A paradigm shift for processing and integration of ceramics. Angew. Chem. Int. Ed. 2016, 128, 11457–11461. [Google Scholar] [CrossRef]
- Zhang, L.; Yu, G. Hybrid electrolyte engineering enables safe and Wide-Temperature redox flow batteries. Angew. Chem. Int. Ed. 2021, 133, 15028–15035. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Floyd, R.; Lowum, S.; Maria, J.-P.; Beauvoir, T.H.; Seo, J.-H.; Randall, C.A. Cold sintering: Progress, challenges, and future opportunities. Annu. Rev. Mater. Res. 2019, 49, 275–295. [Google Scholar] [CrossRef]
- Alía, J.M.; Edwards, H.G. Ion solvation and ion association in lithium trifluoromethanesulfonate solutions in three aprotic solvents. an FT-raman spectroscopic study. Vib. Spectrosc. 2000, 24, 185–200. [Google Scholar] [CrossRef]
- Fang, L.; Sun, W.; Hou, W.; Mao, Y.; Wang, Z.; Sun, K. Quasi-solid-state polymer electrolyte based on highly concentrated LiTFSI complexing DMF for ambient-temperature rechargeable lithium batteries. Ind. Eng. Chem. Res. 2022, 61, 7971–7981. [Google Scholar] [CrossRef]
- Tang, C.; Li, M.; Du, J.; Wang, Y.; Zhang, Y.; Wang, G.; Shi, X.; Li, Y.; Liu, J.; Lian, C.; et al. Supramolecular-induced 2.40 V 130 °C working-temperature-range supercapacitor aqueous electrolyte of lithium bis(trifluoromethanesulfonyl) imide in dimethyl sulfoxide-water. J. Colloid. Interface Sci. 2022, 608, 1162–1172. [Google Scholar] [CrossRef]
- Kang, E.; Park, H.R.; Yoon, J.; Yu, H.-Y.; Chang, S.-K.; Kim, B.; Choi, K.; Ahn, S. A simple method to determine the water content in organic solvents using the 1 H NMR chemical shifts differences between water and solvent. Microchem. J. 2018, 138, 395–400. [Google Scholar] [CrossRef]
- Wang, C.; Sun, Q.; Liu, Y.; Zhao, Y.; Li, X.; Lin, X.; Banis, M.N.; Li, M.; Li, W.; Adair, K.R.; et al. Boosting the performance of lithium batteries with solid-liquid hybrid electrolytes: Interfacial properties and effects of liquid electrolytes. Nano Energy 2018, 48, 35–43. [Google Scholar] [CrossRef]
- Chung, H.; Kang, B. Mechanical and thermal failure induced by contact between a Li1.5Al0.5Ge1.5(PO4)3 solid electrolyte and li metal in an all solid-state li cell. Chem. Mater. 2017, 29, 8611–8619. [Google Scholar] [CrossRef]
- Wolfenstine, J.; Allen, J.L.; Sakamoto, J.; Siegel, D.J.; Choe, H. Mechanical behavior of li-ion-conducting crystalline oxide-based solid electrolytes: A brief review. Ionics 2018, 24, 1271–1276. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Gim, Y.; Lee, W. Thermally Stable Ceramic-Salt Electrolytes for Li Metal Batteries Produced from Cold Sintering Using DMF/Water Mixture Solvents. Nanomaterials 2023, 13, 2436. https://doi.org/10.3390/nano13172436
Kim S, Gim Y, Lee W. Thermally Stable Ceramic-Salt Electrolytes for Li Metal Batteries Produced from Cold Sintering Using DMF/Water Mixture Solvents. Nanomaterials. 2023; 13(17):2436. https://doi.org/10.3390/nano13172436
Chicago/Turabian StyleKim, Sunwoo, Yejin Gim, and Wonho Lee. 2023. "Thermally Stable Ceramic-Salt Electrolytes for Li Metal Batteries Produced from Cold Sintering Using DMF/Water Mixture Solvents" Nanomaterials 13, no. 17: 2436. https://doi.org/10.3390/nano13172436




