Highly Efficient Fluorescent Detection of Vitamin B12 Based on the Inner Filter Effect of Dithiol-Functionalized Silver Nanoparticles
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis and Characterization of AgNPs and PDT-AgNPs
2.3. Fluorescent Detection of VB12
3. Results and Discussion
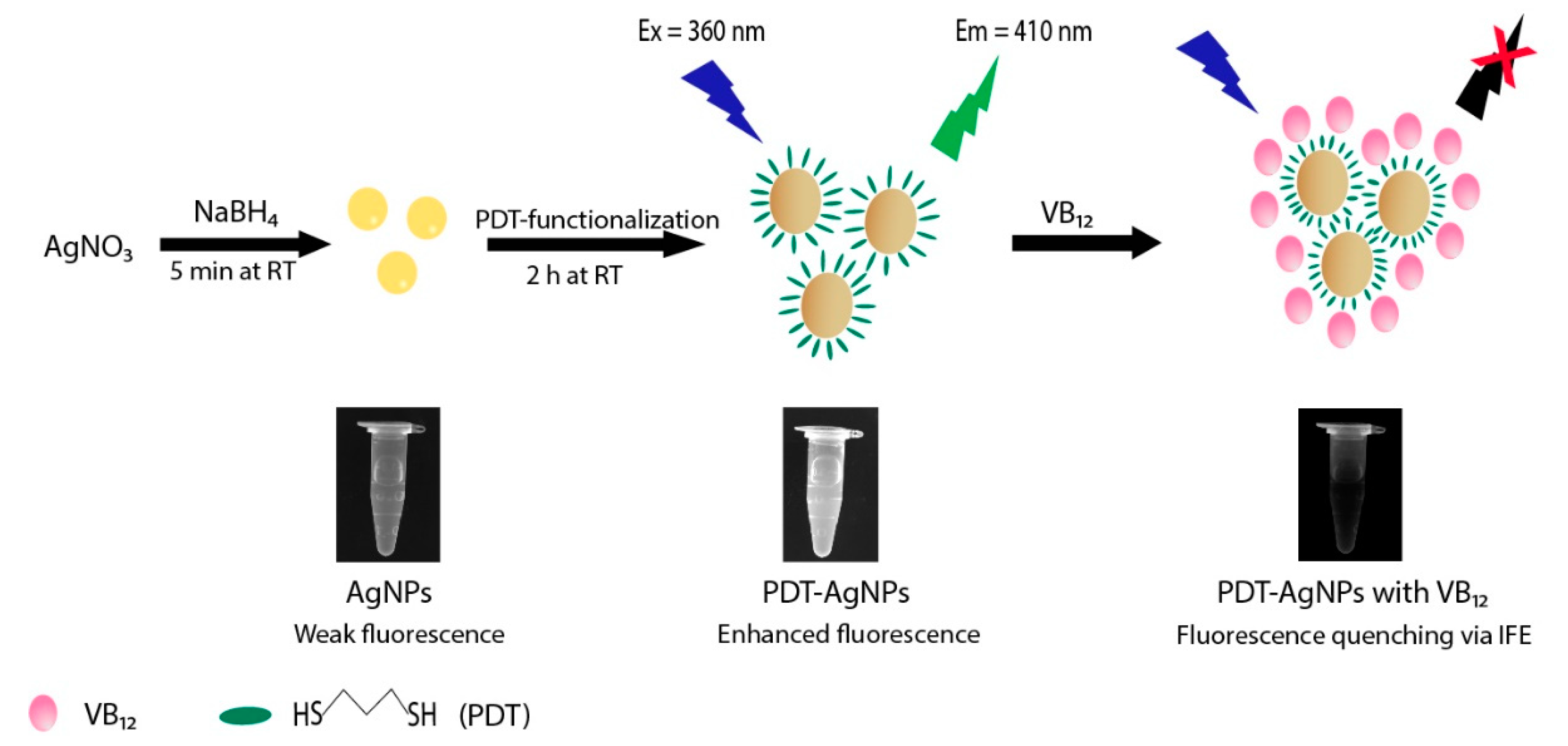
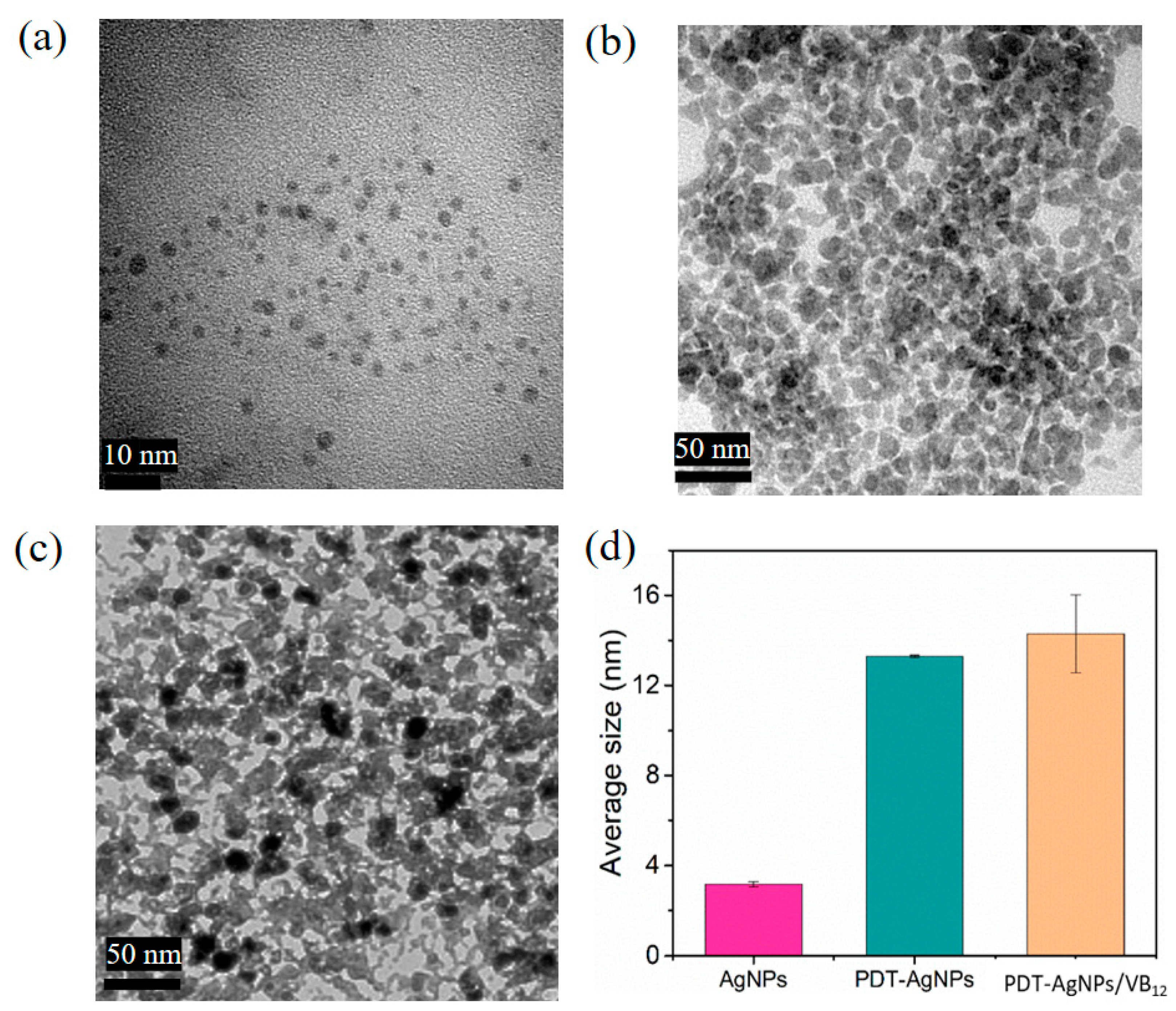
3.1. Synthesis and Characterization of PDT-AgNPs
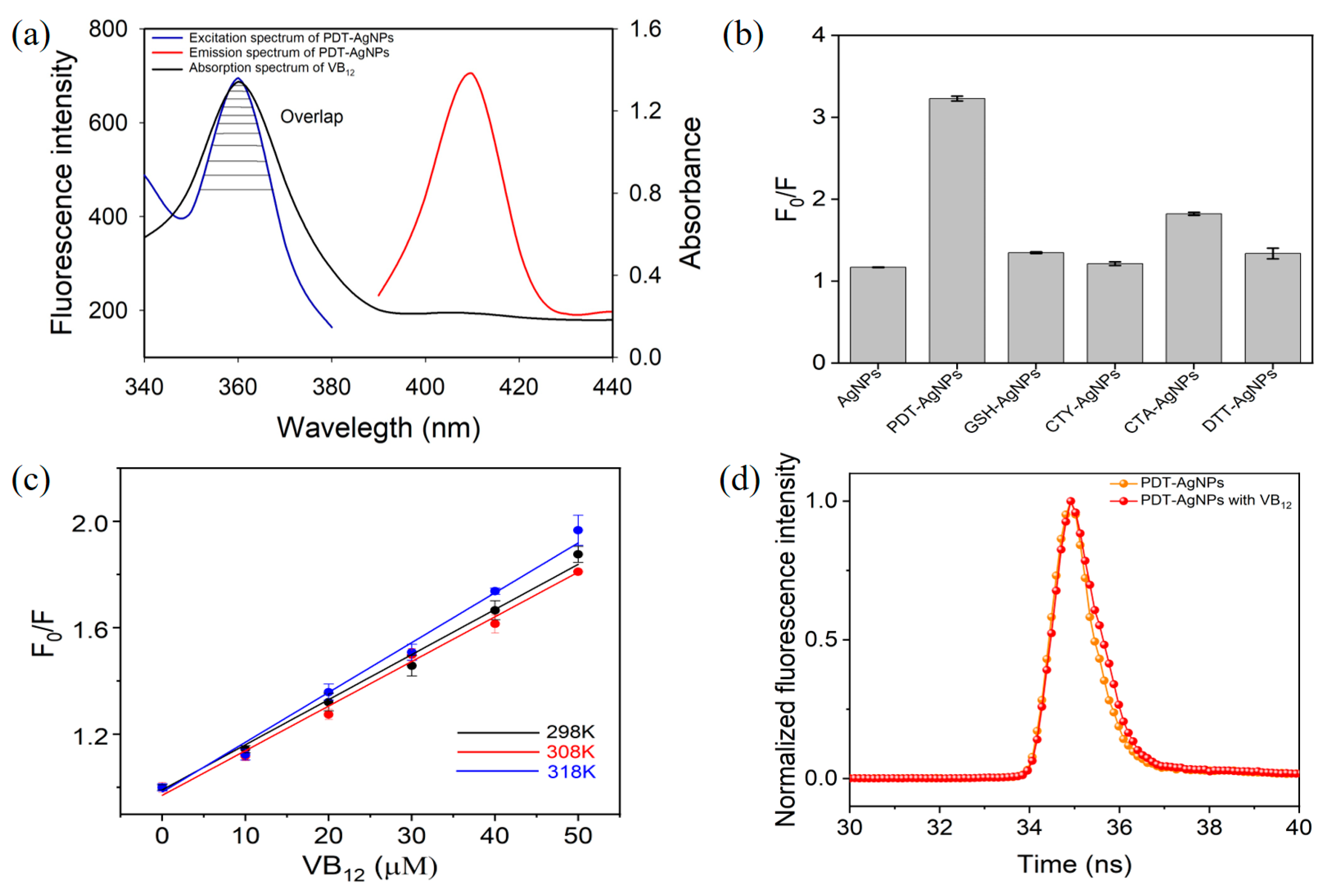
3.2. IFE-Based Fluorescence Quenching of PDT-AgNPs in the Presence of VB12
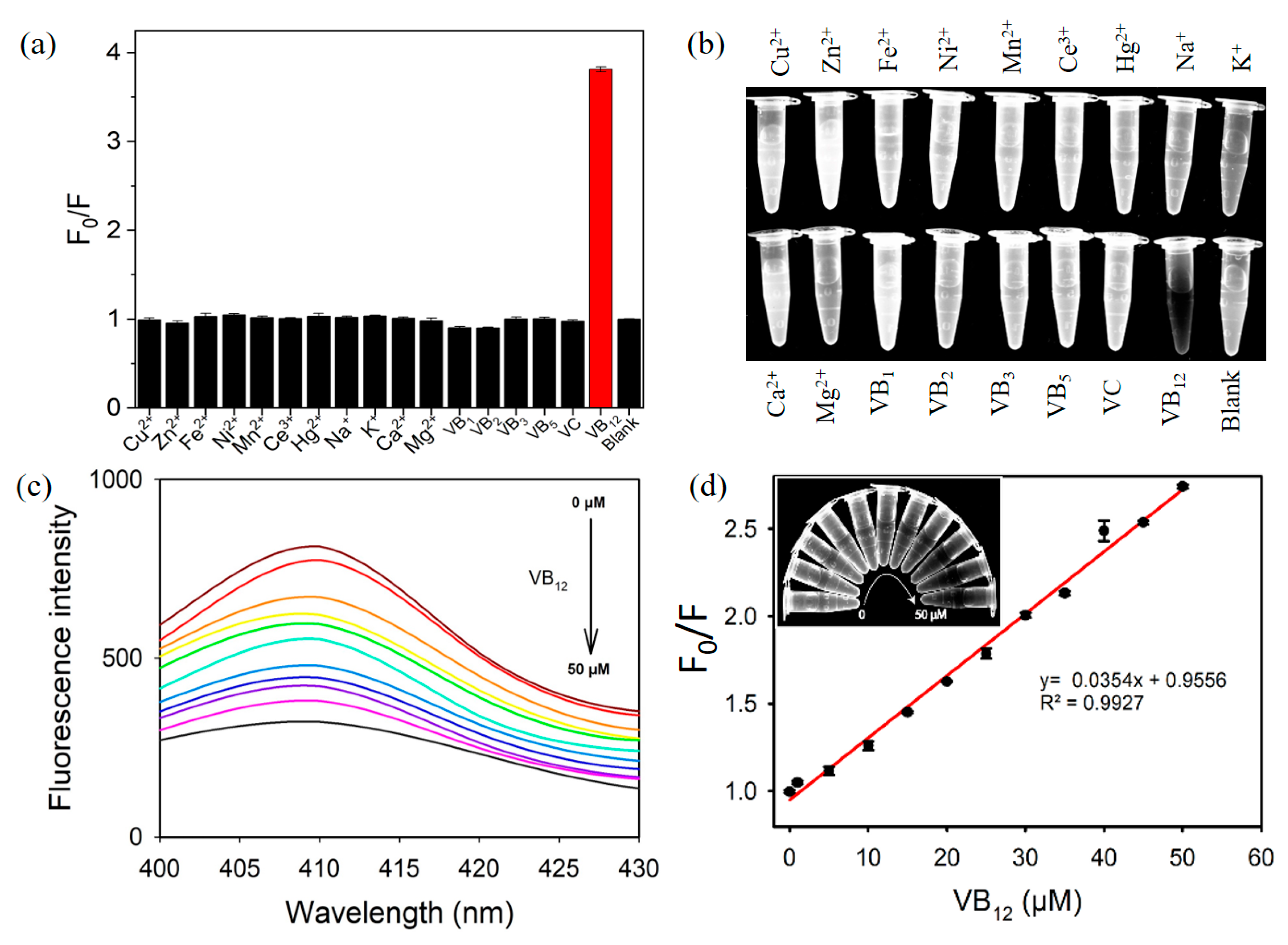
3.3. Fluorescent Detection of VB12 Using PDT-AgNPs-Mediated Detection System
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Eschenmoser, A. Vitamin B12: Experiments concerning the origin of its molecular structure. Angew. Chem.-Int. Edit 1988, 27, 5–39. [Google Scholar] [CrossRef]
- Kumar, N. Neurologic aspects of cobalamin (B12) deficiency. Handb. Clin. Neurol. 2014, 120, 915–926. [Google Scholar] [PubMed]
- Stabler, S.P. Vitamin B12 deficiency. N. Engl. J. Med. 2013, 368, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Hu, Y.; Li, Y.; Zeng, Q.; Jiang, X.; Cheng, Z.J.M.A. Boron doped carbon dots as a multifunctional fluorescent probe for sorbate and vitamin B12. Microchim. Acta 2019, 186, 84. [Google Scholar] [CrossRef]
- Vachhani, M.; Desai, J.; Parmar, V. Development and validation of HPLC method for simultaneous estimation of Methylcobalamin and Duloxetine Hydrochloride in Capsules. Charusat J. 2017, 1, 26–30. [Google Scholar]
- Akatsuka, K.; Atsuya, I. Determination of vitamin B12 as cobalt by electrothermal atomic absorption spectrometry using the solid sampling technique. Z. Anal. Chem. 1989, 335, 200–204. [Google Scholar] [CrossRef]
- Kumar, P.; Shim, Y.-B.J.T. A novel cobalt (II)-selective potentiometric sensor based on p-(4-n-butylphenylazo) calix [4] arene. Talanta 2009, 77, 1057–1062. [Google Scholar] [CrossRef]
- Sato, K.; Muramatsu, K.; Amano, S. Application of vitamin B12-targeting site on Lactobacillus helveticus B-1 to vitamin B12 assay by chemiluminescence method. Anal. Biochem. 2002, 308, 1–4. [Google Scholar] [CrossRef]
- Chen, S.; Yu, Y.-L.; Wang, J.-H. Inner filter effect-based fluorescent sensing systems: A review. Anal. Chim. Acta 2018, 999, 13–26. [Google Scholar] [CrossRef]
- Xiao, N.; Liu, S.G.; Mo, S.; Li, N.; Ju, Y.J.; Ling, Y.; Li, N.B.; Luo, H.Q. Highly selective detection of p-nitrophenol using fluorescence assay based on boron, nitrogen co-doped carbon dots. Talanta 2018, 184, 184–192. [Google Scholar] [CrossRef]
- Zhang, Y.; Fang, X.; Zhao, H.; Li, Z. A highly sensitive and selective detection of Cr (VI) and ascorbic acid based on nitrogen-doped carbon dots. Talanta 2018, 181, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Vaishnavi, E.; Renganathan, R. CdTe quantum dot as a fluorescence probe for vitamin B12 in dosage form. Spectrochim. Acta A 2013, 115, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.H.; Kale, M.B.; Anbhule, P.V.; Patil, S.R.; Kolekar, G.B. A novel FRET probe for selective and sensitive determination of vitamin B12 by functionalized CdS QDs in aqueous media: Applications to pharmaceutical and biomedical analysis. RSC Adv. 2014, 4, 683–692. [Google Scholar] [CrossRef]
- Li, C.; Zhu, L.; Yang, W.; He, X.; Zhao, S.; Zhang, X.; Tang, W.; Wang, J.; Yue, T.; Li, Z. Amino-functionalized Al–MOF for fluorescent detection of tetracyclines in milk. J. Agric. Food Chem. 2019, 67, 1277–1283. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Yang, H.; Ge, S.; Yu, J. Fluorescent carbon dots nanosensor for label-free determination of vitamin B12 based on inner filter effect. Spectrochim. Acta A 2018, 193, 305–309. [Google Scholar] [CrossRef]
- Meng, Y.; Jiao, Y.; Zhang, Y.; Lu, W.; Wang, X.; Shuang, S.; Dong, C. Facile synthesis of orange fluorescence multifunctional carbon dots for label-free detection of vitamin B12 and endogenous/exogenous peroxynitrite. J. Hazard. Mater. 2021, 408, 124422. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.T.; Chen, B.B.; Jiang, L.; Lv, J.; Chang, S.; Wang, Y.; Hafez, M.E. Dual-emitting carbonized polymer dots synthesized at room temperature for ratiometric fluorescence sensing of vitamin B12. ACS Appl. Mater. Inter. 2021, 13, 50228–50235. [Google Scholar] [CrossRef]
- Chen, B.B.; Liu, Z.X.; Deng, W.C.; Zhan, L.; Liu, M.L.; Huang, C.Z. A large-scale synthesis of photoluminescent carbon quantum dots: A self-exothermic reaction driving the formation of the nanocrystalline core at room temperature. Green Chem. 2016, 18, 5127–5132. [Google Scholar] [CrossRef]
- Zhu, J.; Chang, H.; Li, J.-J.; Li, X.; Zhao, J.-W. Dual-mode melamine detection based on gold nanoparticles aggregation-induced fluorescence “turn-on” and “turn-off” of CdTe quantum dots. Sens. Actuator B-Chem. 2017, 239, 906–915. [Google Scholar] [CrossRef]
- Panigrahi, S.K.; Mishra, A.K. Inner filter effect in fluorescence spectroscopy: As a problem and as a solution. J. Photochem. Photobiol. C-Photochem. Rev. 2019, 41, 100318. [Google Scholar] [CrossRef]
- Chavada, V.D.; Bhatt, N.M.; Sanyal, M.; Shrivastav, P.S. Modulation of inner filter effect of non-conjugated silver nanoparticles on blue emitting ZnS quantum dots for the quantitation of betahistine. Spectrochim. Acta A 2020, 240, 118575. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Chen, C.; Sun, J.; Yang, X. Carbon dots-assisted colorimetric and fluorometric dual-mode protocol for acetylcholinesterase activity and inhibitors screening based on the inner filter effect of silver nanoparticles. Analyst 2016, 141, 3280–3288. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.B.; Liu, H.; Huang, C.Z.; Ling, J.; Wang, J. Rapid and convenient synthesis of stable silver nanoparticles with kiwi juice and its novel application for detecting protease K. New J. Chem. 2015, 39, 1295–1300. [Google Scholar] [CrossRef]
- Shang, L.; Qin, C.; Jin, L.; Wang, L.; Dong, S. Turn-on fluorescent detection of cyanide based on the inner filter effect of silver nanoparticles. Analyst 2009, 134, 1477–1482. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, B.; Shahshahanipour, M.; Ensafi, A.A. In situ production of silver nanoparticles for high sensitive detection of ascorbic acid via inner filter effect. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 71, 663–668. [Google Scholar] [CrossRef] [PubMed]
- Swinehart, D.F. The beer-lambert law. J. Chem. Educ. 1962, 39, 333. [Google Scholar] [CrossRef]
- Huang, M.; Tong, C. Silicon nanoparticles/gold nanoparticles composite as a fluorescence probe for sensitive and selective detection of Co2+ and vitamin B12 based on the selective aggregation and inner filter effect. Spectrochim. Acta A 2022, 268, 120706. [Google Scholar] [CrossRef]
- Xue, M.; Mao, W.; Chen, J.; Zheng, F.; Chen, W.; Shen, W.; Tang, S. Application of Au or Ag nanomaterials for colorimetric detection of glucose. Analyst 2021, 146, 6726–6740. [Google Scholar] [CrossRef]
- Ravindran, A.; Chandran, P.; Khan, S. Biofunctionalized silver nanoparticles: Advances and prospects. Colloids Surf. B Biointerfaces 2013, 105, 342–352. [Google Scholar] [CrossRef]
- Li, S.; He, J.; Xu, Q.-H. Aggregation of metal-nanoparticle-induced fluorescence enhancement and its application in sensing. ACS Omega 2019, 5, 41–48. [Google Scholar] [CrossRef]
- Zhai, X.; Zhang, P.; Liu, C.; Bai, T.; Li, W.; Dai, L.; Liu, W. Highly luminescent carbon nanodots by microwave-assisted pyrolysis. Chem. Commun. 2012, 48, 7955–7957. [Google Scholar] [CrossRef] [PubMed]
- Kravets, V.; Almemar, Z.; Jiang, K.; Culhane, K.; Machado, R.; Hagen, G.; Kotko, A.; Dmytruk, I.; Spendier, K.; Pinchuk, A. Imaging of biological cells using luminescent silver nanoparticles. Nanoscale Res. Lett. 2016, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Coates, J. Interpretation of infrared spectra, a practical approach. In Encyclopedia of Analytical Chemistry; Meyers, R.A., Ed.; John Wiley & Sons, Ltd: Chichester, UK, 2000; Volume 15, pp. 10815–10837. ISBN 978-0-4719-7670-7. [Google Scholar]
- Zamiri, R.; Ahangar, H.A.; Zakaria, A.; Zamiri, G.; Shabani, M.; Singh, B.; Ferreira, J. The structural and optical constants of Ag 2 S semiconductor nanostructure in the Far-Infrared. Chem. Cent. J. 2015, 9, 1–6. [Google Scholar] [CrossRef]
- Gharagozlou, M.; Naghibi, S. Sensitization of ZnO nanoparticle by vitamin B12: Investigation of microstructure, FTIR and optical properties. Mater. Res. Bull. 2016, 84, 71–78. [Google Scholar] [CrossRef]
- Karimi, Z.; Mahjoub, A. Efficient epoxidation over cyanocobalamine containing SBA-15 organic–inorganic nanohybrids. Appl. Surf. Sci. 2010, 256, 4473–4479. [Google Scholar] [CrossRef]
- Gharagozlou, M.; Naghibi, S. Preparation of vitamin B12–TiO2 nanohybrid studied by TEM, FTIR and optical analysis techniques. Mater. Sci. Semicond. Process 2015, 35, 166–173. [Google Scholar] [CrossRef]
- Singh, H.; Du, J.; Singh, P.; Yi, T.H. Ecofriendly synthesis of silver and gold nanoparticles by Euphrasia officinalis leaf extract and its biomedical applications. Artif. Cell. Nanomed. Biotechnol. 2018, 46, 1163–1170. [Google Scholar] [CrossRef]
- Nain, A.; Tseng, Y.T.; Wei, S.C.; Periasamy, A.P.; Huang, C.C.; Tseng, F.G.; Chang, H.T. Capping 1, 3-propanedithiol to boost the antibacterial activity of protein-templated copper nanoclusters. J. Hazard. Mater. 2020, 389, 121821. [Google Scholar] [CrossRef]
- Li, H.; Cui, Z.; Han, C. Glutathione-stabilized silver nanoparticles as colorimetric sensor for Ni2+ ion. Sens. Actuat. B Chem. 2009, 143, 87–92. [Google Scholar] [CrossRef]
- Oćwieja, M.; Barbasz, A.; Walas, S.; Roman, M.; Paluszkiewicz, C. Physicochemical properties and cytotoxicity of cysteine-functionalized silver nanoparticles. Colloids Surf. B Biointerfaces 2017, 160, 429–437. [Google Scholar] [CrossRef]
- Bhattacharjee, Y.; Chakraborty, A. Label-free cysteamine-capped silver nanoparticle-based colorimetric assay for Hg (II) detection in water with subnanomolar exactitude. ACS Sustain. Chem. Eng. 2014, 2, 2149–2154. [Google Scholar] [CrossRef]
- Ye, Y.; Guo, Y.; Yue, Y.; Huang, H.; Zhao, L.; Gao, Y.; Zhang, Y. Colorimetric sensing of copper ions based on the anti-aggregation of unmodified silver nanoparticles in the presence of 1,4-dithiothreitol. Anal. Methods 2015, 7, 566–572. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Weber, G.J.B. Quenching of fluorescence by oxygen. Probe for structural fluctuations in macromolecules. Biochemistry 1973, 12, 4161–4170. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Banoo, R.; Rahman, G.M.S.; Khan, M.O.F. A convenient colorimetric assay method for determination of vitamin B, content in Pharmaceutical. J. Med. Sci. 2003, 3, 163–168. [Google Scholar] [CrossRef]
- Sun, J.; Zhu, X.; Wu, M. Hydroxypropyl-β-cyclodextrin enhanced determination for the vitamin B 12 by fluorescence quenching method. J. Fluoresc. 2007, 17, 265–270. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, Z.; Luo, J.; Zhu, Z.; Zhang, X.; Liu, R.; Wu, Z.-C. A yellow-emitting nitrogen-doped carbon dots for sensing of vitamin B12 and their cell-imaging. Dyes Pigment. 2020, 176, 108227. [Google Scholar] [CrossRef]
| Method | Material | Linear Range (μM) | LOD (μM) | Reference |
|---|---|---|---|---|
| HPLC | 10.5–28 | 0.93 | [5] | |
| Atomic spectroscopy | 2.6–66.6 | 0.9 | [6] | |
| Electrochemistry | Mn-DNA-CPE | 3.667–236 | 1.21 | [7] |
| Colorimetric assay | Nitroso-R-salt | 0–12.25 | Not given | [45] |
| Fluorescent assay | Hydroxypropyl-β-cyclodextrin | 0–21 | 0.18 | [46] |
| Fluorescent assay (FRET) | CdTe quantum dots | 0.7–9.8 | 0.1 | [12] |
| Fluorescent assay (FRET) | CdS quantum dots | 3.7–74 | 5.1 | [13] |
| Fluorescent assay (IFE) | Nitrogen-doped carbon dots | 0–200 | 2.045 | [47] |
| Fluorescent assay (IFE) | Carbon dots | 1–65 | 0.62 | [16] |
| Fluorescent assay (IFE) | Carbon quantum dots | 0.75–100 | 0.2 | [18] |
| Fluorescent assay (IFE) | Carbon dots | 0–60 | 0.1 | [15] |
| Fluorescent assay (IFE) | PDT-AgNPs | 1–50 | 0.5 | This work |
| Compound | Spiked Level (µM) | Measured (µM) a | Recovery (%) b | CV(%) c |
|---|---|---|---|---|
| VB12 tablet | 0 | 3.56 | 96.50 | 3.37 |
| 10 | 13.52 | 98.77 | 3.70 | |
| 20 | 24.24 | 102.33 | 2.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chau, P.B.K.; Vu, T.H.; Kim, M.I. Highly Efficient Fluorescent Detection of Vitamin B12 Based on the Inner Filter Effect of Dithiol-Functionalized Silver Nanoparticles. Nanomaterials 2023, 13, 2444. https://doi.org/10.3390/nano13172444
Chau PBK, Vu TH, Kim MI. Highly Efficient Fluorescent Detection of Vitamin B12 Based on the Inner Filter Effect of Dithiol-Functionalized Silver Nanoparticles. Nanomaterials. 2023; 13(17):2444. https://doi.org/10.3390/nano13172444
Chicago/Turabian StyleChau, Phan Ba Khanh, Trung Hieu Vu, and Moon Il Kim. 2023. "Highly Efficient Fluorescent Detection of Vitamin B12 Based on the Inner Filter Effect of Dithiol-Functionalized Silver Nanoparticles" Nanomaterials 13, no. 17: 2444. https://doi.org/10.3390/nano13172444





