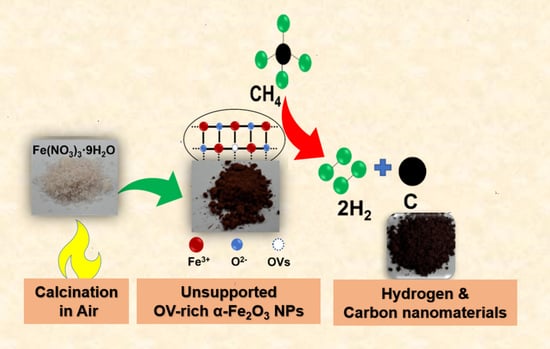
Scalable Synthesis of Oxygen Vacancy-Rich Unsupported Iron Oxide for Efficient Thermocatalytic Conversion of Methane to Hydrogen and Carbon Nanomaterials
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of Unsupported OV-Rich α-Fe2O3 NPs
2.2. Characterization Techniques
2.3. Thermocatalytic Activity Experiments
3. Results and Discussion
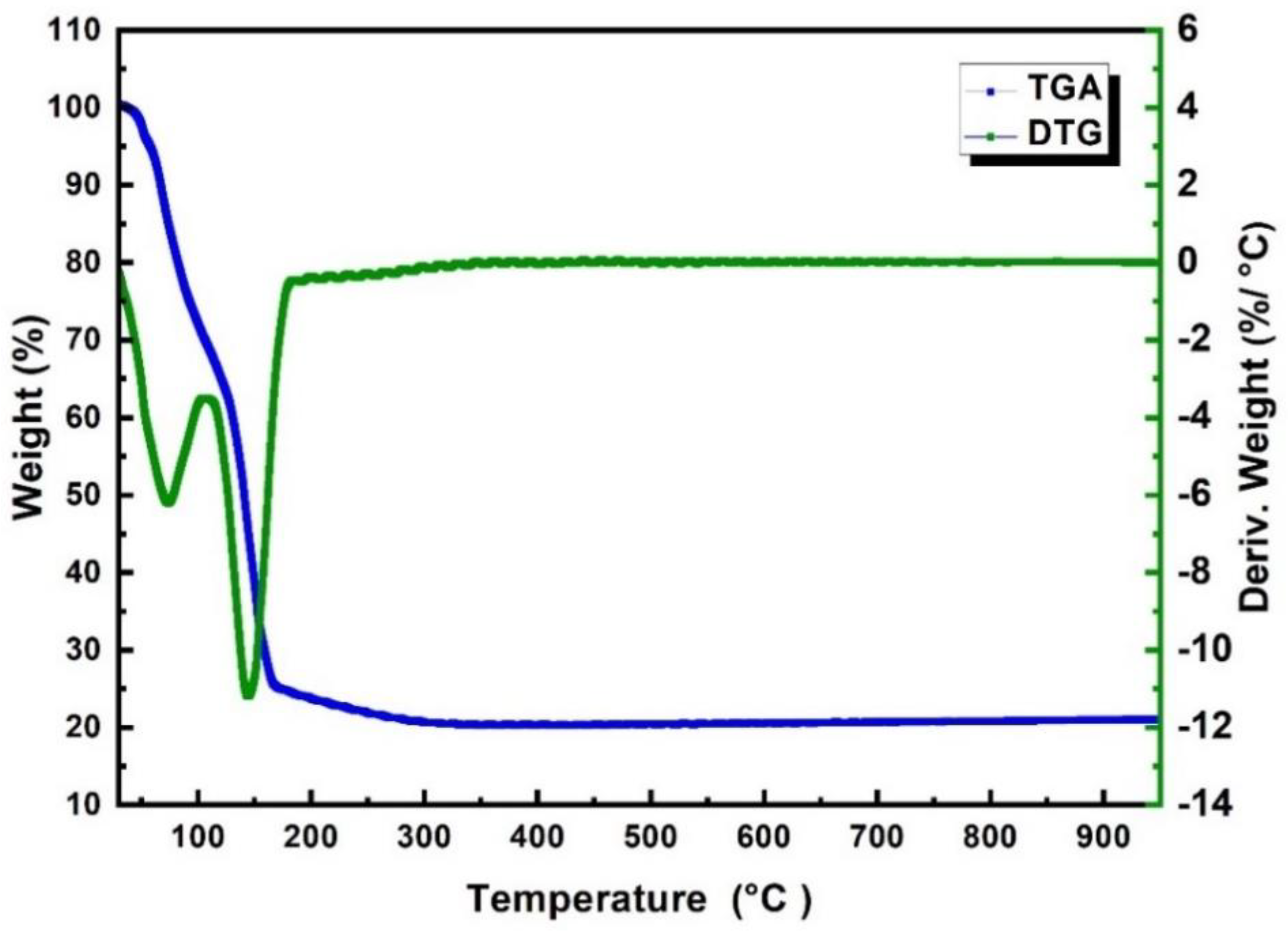
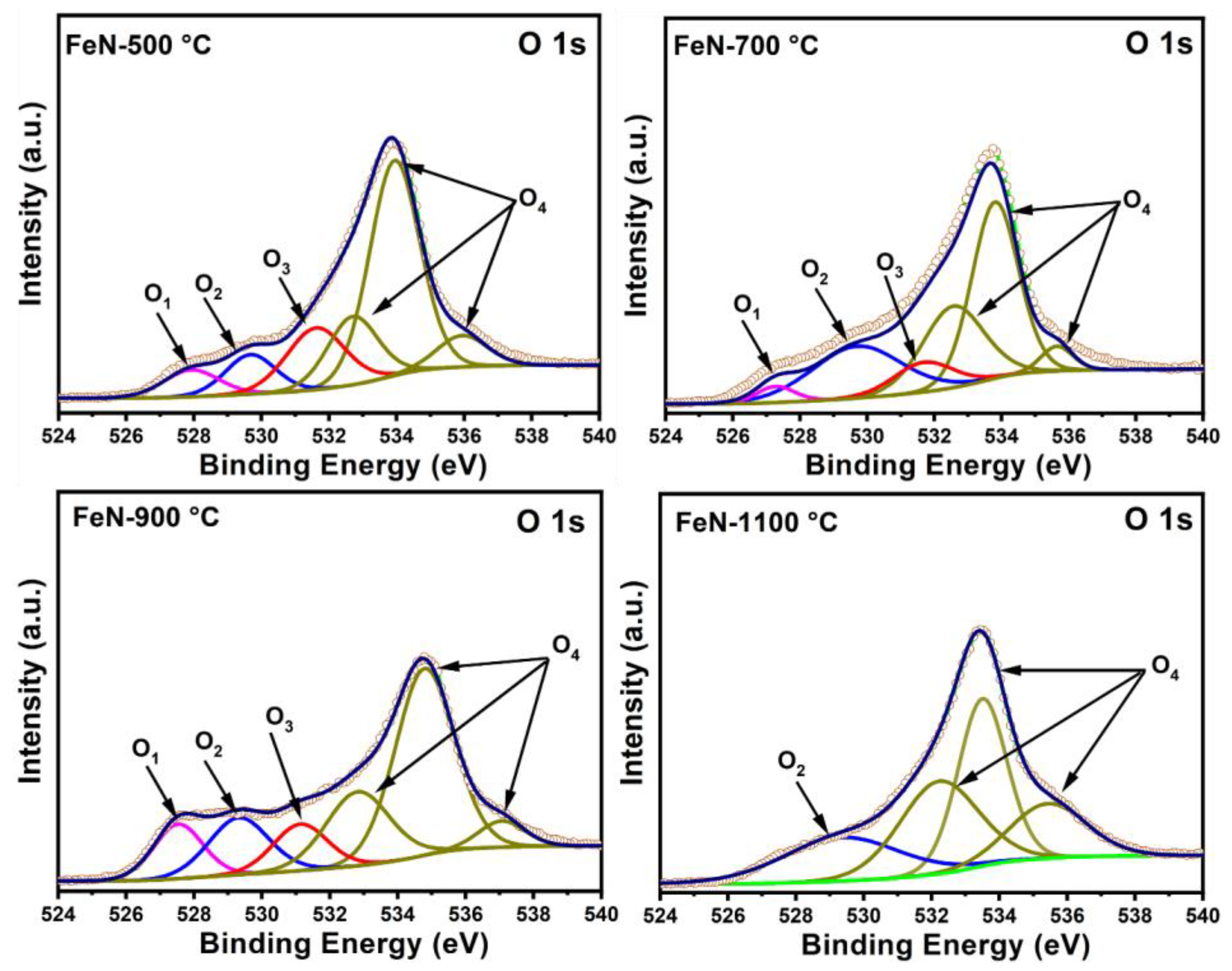
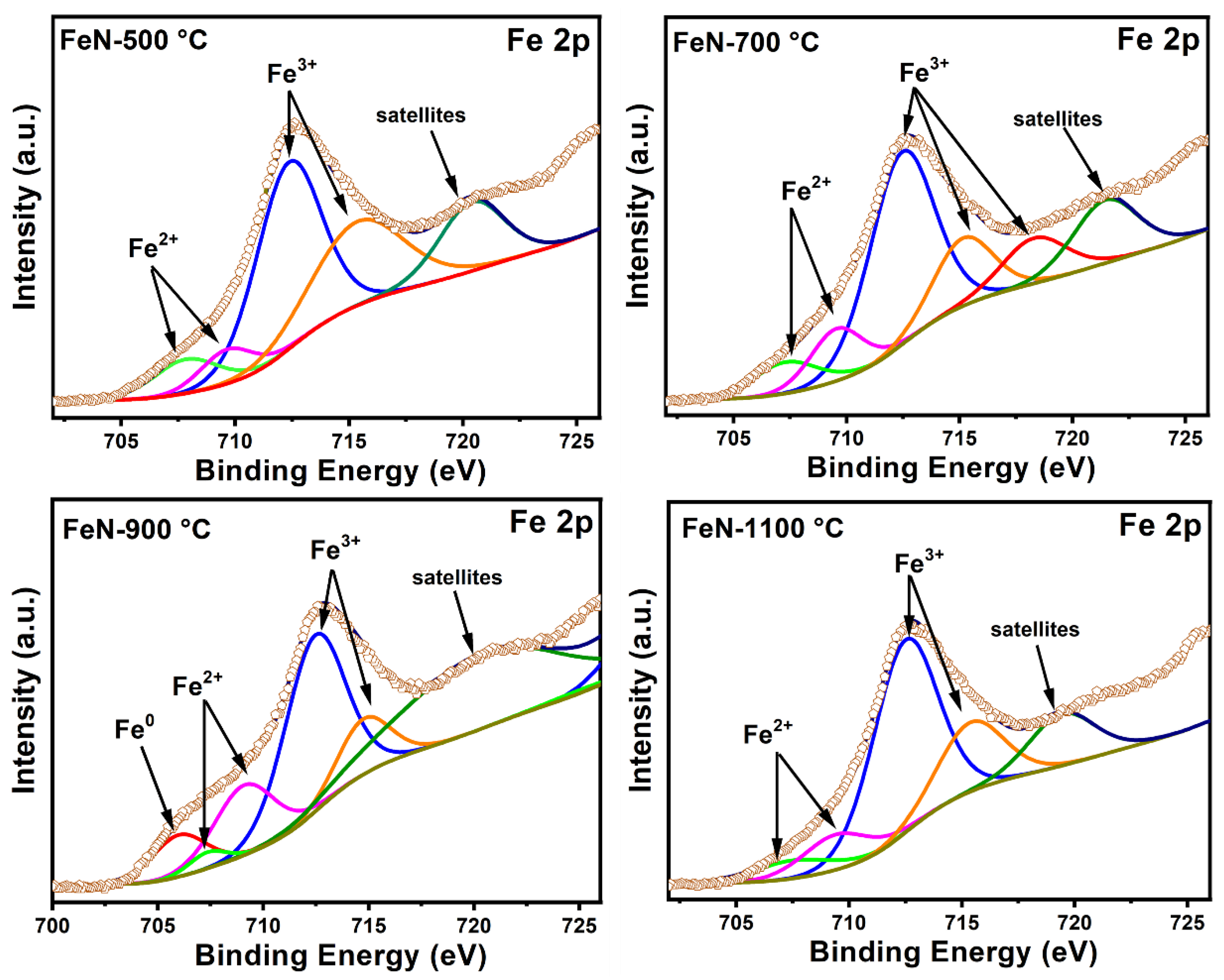
3.1. Characterization of Fresh Samples
3.2. Thermocatalytic Activity of the Obtained OV-Rich α-Fe2O3 NPs
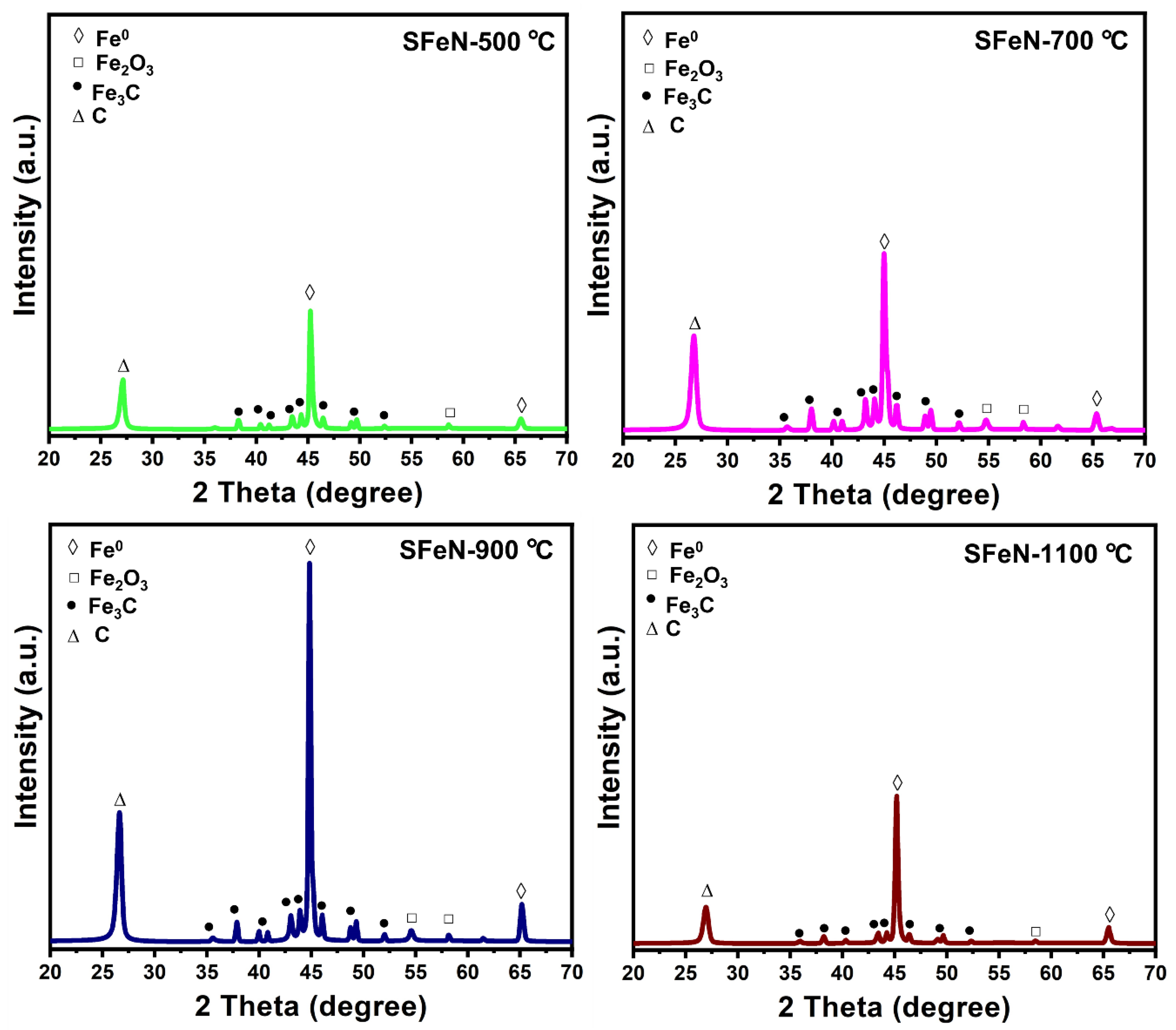
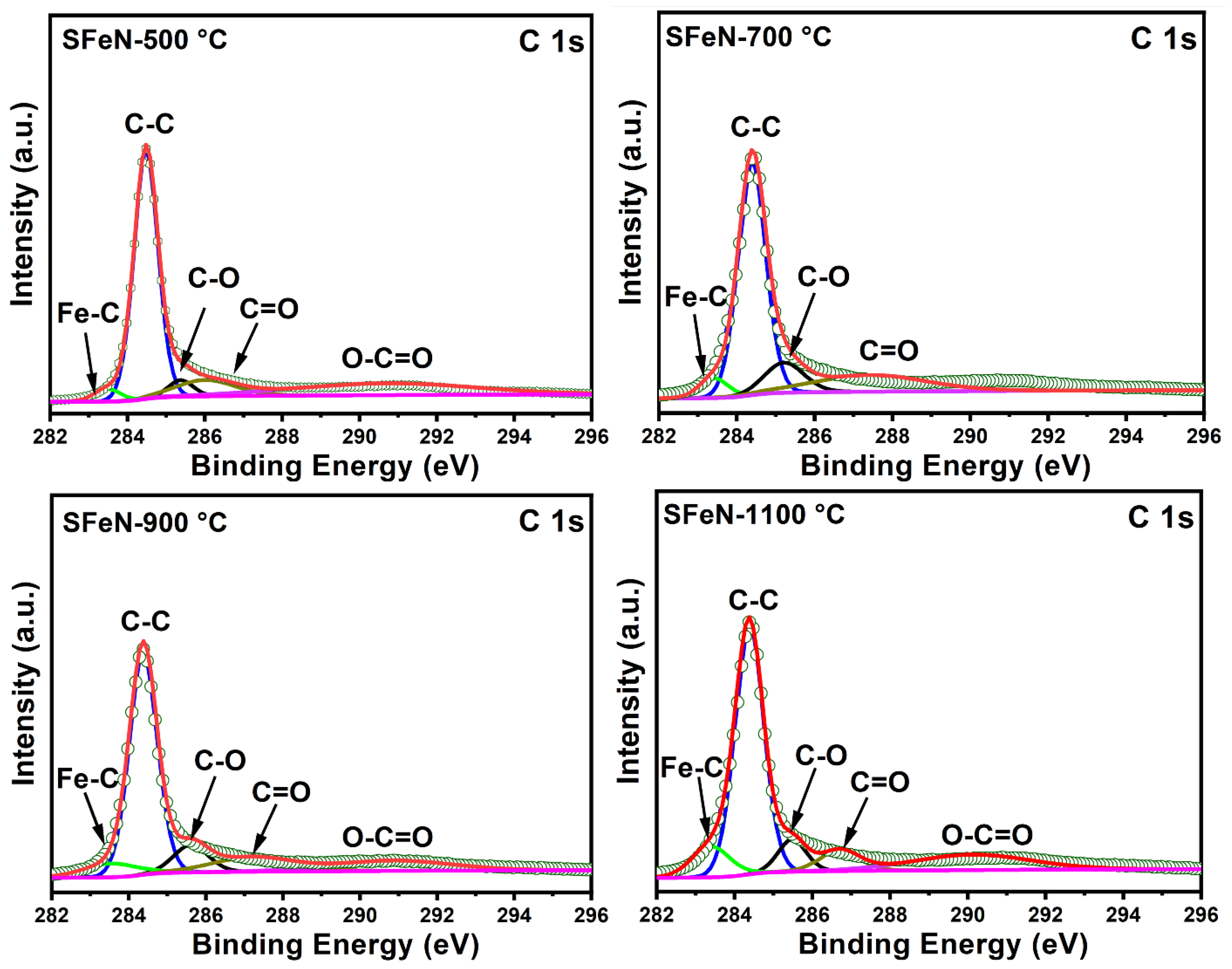
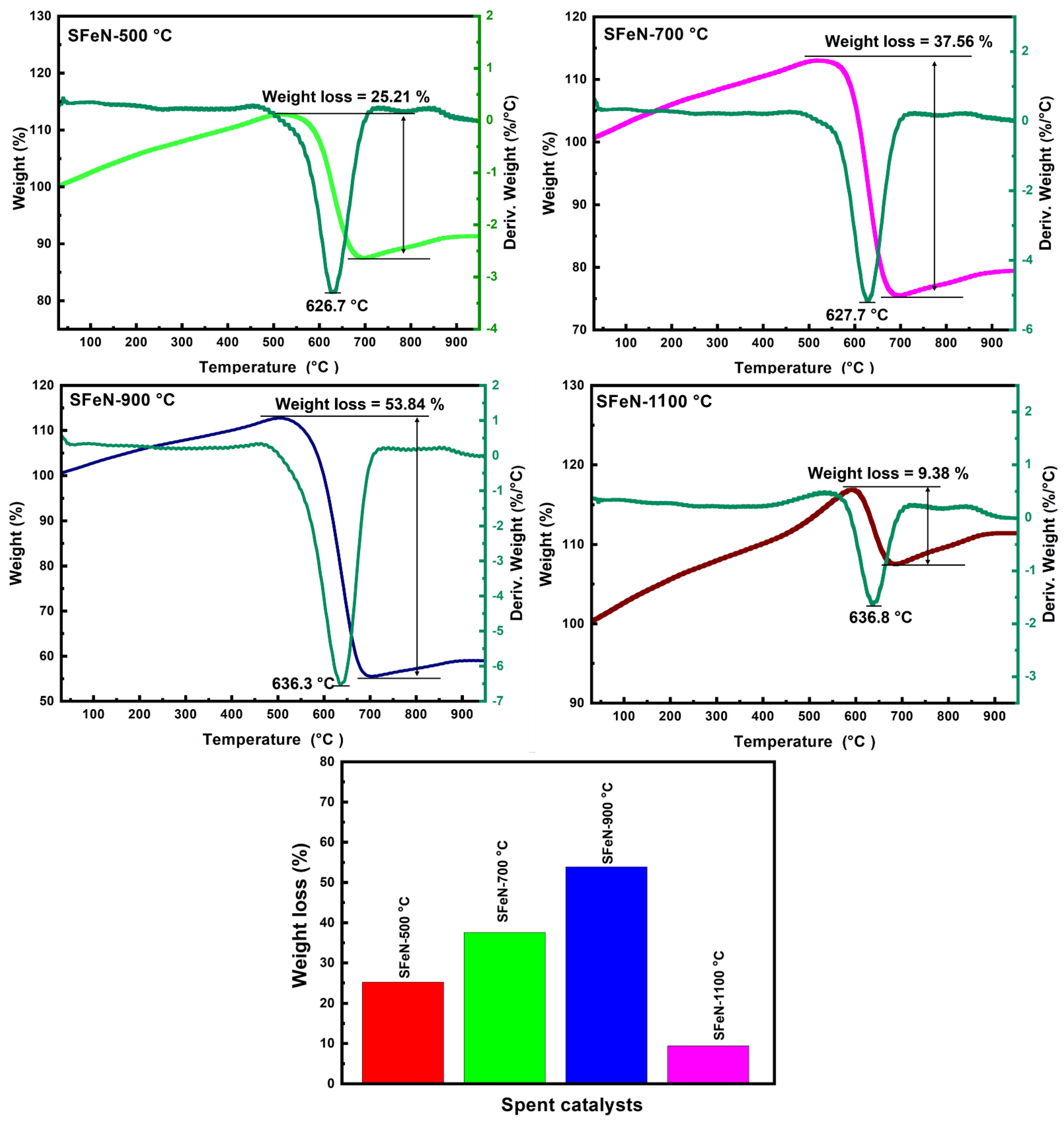
3.3. Characterization of Spent Catalysts after the TCMD
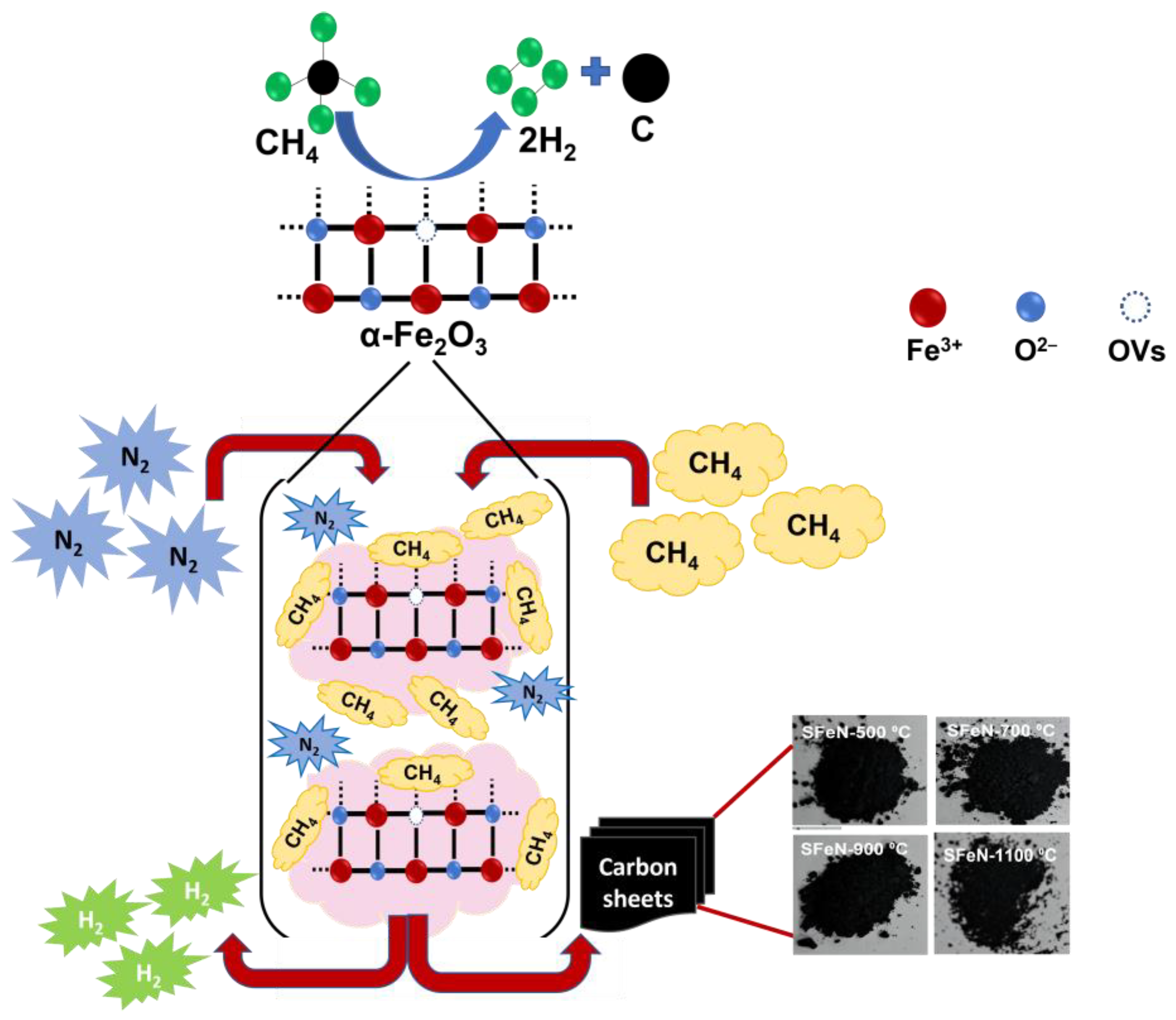
3.4. Proposed Reaction Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Alharthi, A.I. Pd Supported on ZSM-5 with different ratios of Si/Al as catalysts for direct catalytic decomposition of methane. J. Taibah Univ. Sci. 2021, 15, 567–573. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z.; Takriff, M.S. Methane decomposition into COx free hydrogen and multiwalled carbon nanotubes over ceria, zirconia and lanthana supported nickel catalysts prepared via a facile solid state citrate fusion method. Energy Convers. Manag. 2016, 126, 302–315. [Google Scholar] [CrossRef]
- Wang, G.; Jin, Y.; Liu, G.; Li, Y. Production of Hydrogen and Nanocarbon from Catalytic Decomposition of Methane over a Ni–Fe/Al2O3 Catalyst. Energy Fuels 2013, 27, 4448–4456. [Google Scholar] [CrossRef]
- Alharthi, A.I.; Abdel, E.; Hargreaves, J.S.J.; Alotaibi, M.A.; Din, I.U.; Al-Shalwi, M.N. Influence of Zn and Ni dopants on the physicochemical and activity patterns of CoFe2O4 derived catalysts for hydrogen production by catalytic cracking of methane. J. Alloys Compd. 2023, 938, 168437. [Google Scholar] [CrossRef]
- Alharthi, A.I.; Abdel-Fattah, E.; Alotaibi, M.A.; Al-Shalwi, M.N. Facile modification of cobalt ferrite by SiO2 and H-ZSM-5 support for hydrogen and filamentous carbon production from methane decomposition. Int. J. Energy Res. 2022, 46, 17497–17510. [Google Scholar] [CrossRef]
- Tsyganok, A.I.; Tsunoda, T.; Hamakawa, S.; Suzuki, K.; Takehira, K.; Hayakawa, T. Dry reforming of methane over catalysts derived from nickel-containing Mg–Al layered double hydroxides. J. Catal. 2003, 213, 191–203. [Google Scholar] [CrossRef]
- Izquierdo, U.; Barrio, V.L.; Cambra, J.F.; Requies, J.; Güemez, M.B.; Arias, P.L.; Kolb, G.; Zapf, R.; Gutiérrez, A.M.; Arraibi, J.R. Hydrogen production from methane and natural gas steam reforming in conventional and microreactor reaction systems. Int. J. Hydrog. Energy 2012, 37, 7026–7033. [Google Scholar] [CrossRef]
- Abbas, H.F.; Daud, W.M.A.W. Hydrogen production by methane decomposition: A review. Int. J. Hydrog. Energy 2010, 35, 1160–1190. [Google Scholar] [CrossRef]
- Li, Y.; Li, D.; Wang, G. Methane decomposition to COx-free hydrogen and nano-carbon material on group 8–10 base metal catalysts: A review. Catal. Today 2011, 162, 1–48. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z.; Takriff, M.S. Methane decomposition over Pd promoted Ni/MgAl2O4 catalysts for the production of COx free hydrogen and multiwalled carbon nanotubes. Appl. Surf. Sci. 2015, 356, 1320–1326. [Google Scholar] [CrossRef]
- Awadallah, A.E.; Aboul-Enein, A.A.; Aboul-Gheit, A.K. Effect of progressive Co loading on commercial Co–Mo/Al2O3 catalyst for natural gas decomposition to COx-free hydrogen production and carbon nanotubes. Energy Convers. Manag. 2014, 77, 143–151. [Google Scholar] [CrossRef]
- Alharthi, A.I. Nickel-iron catalyst for decomposition of methane to hydrogen and filamentous carbon: Effect of calcination and reaction temperatures. Alex. Eng. J. 2023, 67, 129–141. [Google Scholar] [CrossRef]
- Moghaddam, E.A.; Ahlgren, S.; Nordberg, Å. Assessment of Novel Routes of Biomethane Utilization in a Life Cycle Perspective. Front. Bioeng. Biotechnol. 2016, 4, 89. [Google Scholar] [CrossRef]
- Koornneef, J.; van Breevoort, P.; Noothout, P.; Hendriks, C.; Luning, u.; Camps, A. Global Potential for Biomethane Production with Carbon Capture, Transport and Storage up to 2050. Energy Procedia 2013, 37, 6043–6052. [Google Scholar] [CrossRef]
- Zhou, L.; Enakonda, L.R.; Harb, M.; Saih, Y.; Aguilar-Tapia, A.; Ould-Chikh, S.; Hazemann, J.; Li, J.; Wei, N.; Gary, D.; et al. Fe catalysts for methane decomposition to produce hydrogen and carbon nano materials. Appl. Catal. B: Environ. 2017, 208, 44–59. [Google Scholar] [CrossRef]
- Enakonda, L.R.; Zhou, L.; Saih, Y.; Ould-Chikh, S.; Lopatin, S.; Gary, D.; Del-Gallo, P.; Basset, J.-M. Methane-induced Activation Mechanism of Fused Ferric Oxide–Alumina Catalysts during Methane Decomposition. ChemSusChem 2016, 9, 1911–1915. [Google Scholar] [CrossRef]
- Torres, D.; de Llobet, S.; Pinilla, J.L.; Lázaro, M.J.; Suelves, I.; Moliner, R. Hydrogen production by catalytic decomposition of methane using a Fe-based catalyst in a fluidized bed reactor. J. Nat. Gas Chem. 2012, 21, 367–373. [Google Scholar] [CrossRef]
- Adánez, J.; de Diego, L.F.; García-Labiano, F.; Gayán, P.; Abad, A.; Palacios, J.M. Selection of Oxygen Carriers for Chemical-Looping Combustion. Energy Fuels 2004, 18, 371–377. [Google Scholar] [CrossRef]
- Mattisson, T.; Lyngfelt, A.; Cho, P. The use of iron oxide as an oxygen carrier in chemical-looping combustion of methane with inherent separation of CO2. Fuel 2001, 80, 1953–1962. [Google Scholar] [CrossRef]
- Liu, Q.; Cui, Z.-M.; Ma, Z.; Bian, S.-W.; Song, W.-G.; Wan, L.-J. Morphology control of Fe2O3 nanocrystals and their application in catalysis. Nanotechnology 2007, 18, 385605. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, D.; Yang, P. Morphology control of α-Fe2O3 towards super electrochemistry performance. RSC Adv. 2019, 9, 21947–21955. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, N.; Wang, C.; Zhao, F.; Yu, Y. Construction of Fe2O3@CuO Heterojunction Nanotubes for Enhanced Oxygen Evolution Reaction. ACS Appl. Energy Mater. 2020, 3, 666–674. [Google Scholar] [CrossRef]
- Lei, R.; Wei, J.; He, W.; Ao, X.; Song, J.; Wang, C.; Zhang, Q.; Xie, H.; Li, J.; Ni, J. Construction of an α-Fe2O3/Bi2O3 p–n heterojunction with exceptional visible-light photocatalytic performance for dye removal. New J. Chem. 2023. advance article. [Google Scholar] [CrossRef]
- Palimi, M.J.; Rostami, M.; Mahdavian, M.; Ramezanzadeh, B. Surface modification of Fe2O3 nanoparticles with 3-aminopropyltrimethoxysilane (APTMS): An attempt to investigate surface treatment on surface chemistry and mechanical properties of polyurethane/Fe2O3 nanocomposites. Appl. Surf. Sci. 2014, 320, 60–72. [Google Scholar] [CrossRef]
- Liu, F.; Shan, W.; Lian, Z.; Liu, J.; He, H. The smart surface modification of Fe2O3 by WOx for significantly promoting the selective catalytic reduction of NOx with NH3. Appl. Catal. B: Environ. 2018, 230, 165–176. [Google Scholar] [CrossRef]
- Xiong, T.; Zhang, Y.; Lee, W.S.V.; Xue, J. Defect Engineering in Manganese-Based Oxides for Aqueous Rechargeable Zinc-Ion Batteries: A Review. Adv. Energy Mater. 2020, 10, 2001769. [Google Scholar] [CrossRef]
- Qin, Q.; Liu, T.; Zhang, J.; Wei, R.; You, S.; Xu, Y. Facile synthesis of oxygen vacancies enriched α-Fe2O3 for peroxymonosulfate activation: A non-radical process for sulfamethoxazole degradation. J. Hazard. Mater. 2021, 419, 126447. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Qin, L.; Guo, M.; Fan, J.A.; Xu, D.; Fan, L.-S. Methane adsorption and dissociation on iron oxide oxygen carriers: The role of oxygen vacancies. Phys. Chem. Chem. Phys. 2016, 18, 16423–16435. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, L.; Wen, T.; Liu, H.; Zhang, J.; Liu, Z.; Zhu, C.; Long, C. Mesoporous poorly crystalline α-Fe2O3 with abundant oxygen vacancies and acid sites for ozone decomposition. Sci. Total Environ. 2022, 804, 150161. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, W.M. Thermal behaviour of pure and binary Fe(NO3)3·9H2O and (NH4)6Mo7O24·4H2O systems. Mater. Sci. Eng. A 2007, 445–446, 113–121. [Google Scholar] [CrossRef]
- Geng, S.; Han, Z.; Hu, Y.; Cui, Y.; Yue, J.; Yu, J.; Xu, G. Methane Decomposition Kinetics over Fe2O3 Catalyst in Micro Fluidized Bed Reaction Analyzer. Ind. Eng. Chem. Res. 2018, 57, 8413–8423. [Google Scholar] [CrossRef]
- Sangeetha, A.; Seeli, S.J.; Bhuvana, K.P.; Kader, M.A.; Nayak, S.K. Correlation between calcination temperature and optical parameter of zinc oxide (ZnO) nanoparticles. J. Sol. Gel. Sci. Technol. 2019, 91, 261–272. [Google Scholar] [CrossRef]
- Park, C.; Jung, J.; Lee, C.W.; Cho, J. Synthesis of Mesoporous α-Fe2O3 Nanoparticles by Non-ionic Soft Template and Their Applications to Heavy Oil Upgrading. Sci. Rep. 2016, 6, 39136. [Google Scholar] [CrossRef]
- Hassan, S.M.; Ahmed, A.I.; Mannaa, M.A. Preparation and characterization of SnO2 doped TiO2 nanoparticles: Effect of phase changes on the photocatalytic and catalytic activity. J. Sci. Adv. Mater. Devices 2019, 4, 400–412. [Google Scholar] [CrossRef]
- Alharthi, A.; Blackley, R.A.; Flowers, T.H.; Hargreaves, J.S.J.; Pulford, I.D.; Wigzell, J.; Zhou, W. Iron ochre—A pre-catalyst for the cracking of methane. J. Chem. Technol. Biotechnol. 2014, 89, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Motozuka, S.; Tagaya, M.; Ikoma, T.; Morinaga, M.; Yoshioka, T.; Tanaka, J. Efficient Methane Conversion to Hydrogen by the Force-Activated Oxides on Iron Particle Surfaces. J. Phys. Chem. C 2013, 117, 16104–16118. [Google Scholar] [CrossRef]
- Li, L.; Ma, P.; Hussain, S.; Jia, L.; Lin, D.; Yin, X.; Lin, Y.; Cheng, Z.; Wang, L. FeS2/carbon hybrids on carbon cloth: A highly efficient and stable counter electrode for dye-sensitized solar cells. Sustain. Energy Fuels 2019, 3, 1749–1756. [Google Scholar] [CrossRef]
- Liu, D.; Wu, C.; Yan, M.; Wang, J. Correlating the microstructure, growth mechanism and magnetic properties of FeSiAl soft magnetic composites fabricated via HNO3 oxidation. Acta Mater. 2018, 146, 294–303. [Google Scholar] [CrossRef]
- Sushil, S.; Alabdulrahman, A.M.; Balakrishnan, M.; Batra, V.S.; Blackley, R.A.; Clapp, J.; Hargreaves, J.S.J.; Monaghan, A.; Pulford, I.D.; Rico, J.L.; et al. Carbon deposition and phase transformations in red mud on exposure to methane. J. Hazard. Mater. 2010, 180, 409–418. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z.; Jia, Q.; Takriff, M.S. Catalytic decomposition of methane over rare earth metal (Ce and La) oxides supported iron catalysts. Appl. Surf. Sci. 2019, 467–468, 236–248. [Google Scholar] [CrossRef]
- Awadallah, A.E.; Abdel-Mottaleb, M.S.; Aboul-Enein, A.A.; Yonis, M.M.; Aboul-Gheit, A.K. Catalytic Decomposition of Natural Gas to CO/CO2-Free Hydrogen Production and Carbon Nanomaterials Using MgO-Supported Monometallic Iron Family Catalysts. Chem. Eng. Commun. 2015, 202, 163–174. [Google Scholar] [CrossRef]
- Torres, D.; Pinilla, J.L.; Lázaro, M.J.; Moliner, R.; Suelves, I. Hydrogen and multiwall carbon nanotubes production by catalytic decomposition of methane: Thermogravimetric analysis and scaling-up of Fe–Mo catalysts. Int. J. Hydrog. Energy 2014, 39, 3698–3709. [Google Scholar] [CrossRef]
- Silva, J.A.; Santos, J.B.O.; Torres, D.; Pinilla, J.L.; Suelves, I. Natural Fe-based catalysts for the production of hydrogen and carbon nanomaterials via methane decomposition. Int. J. Hydrog. Energy 2021, 46, 35137–35148. [Google Scholar] [CrossRef]
- Pudukudy, M.; Kadier, A.; Yaakob, Z.; Takriff, M.S. Non-oxidative thermocatalytic decomposition of methane into COx free hydrogen and nanocarbon over unsupported porous NiO and Fe2O3 catalysts. Int. J. Hydrog. Energy 2016, 41, 18509–18521. [Google Scholar] [CrossRef]
- Yan, P.; Zhang, K.; Peng, Y. Study of Fe2O3-Al2O3 catalyst reduction parameters and conditions for catalytic methane decomposition. Chem. Eng. Sci. 2022, 250, 117410. [Google Scholar] [CrossRef]
- Muradov, N.Z. CO2-Free Production of Hydrogen by Catalytic Pyrolysis of Hydrocarbon Fuel. Energy Fuels 1998, 12, 41–48. [Google Scholar] [CrossRef]
- Alharthi, A.I.; Alotaibi, M.A.; Din, I.U.; Abdel-Fattah, E.; Bakht, M.A.; Al-Fatesh, A.S.; Alanazi, A.A. Mg and Cu incorporated CoFe2O4 catalyst: Characterization and methane cracking performance for hydrogen and nano-carbon production. Ceram. Int. 2021, 47, 27201–27209. [Google Scholar] [CrossRef]
- Tian, Z.; Wang, C.; Yue, J.; Zhang, X.; Ma, L. Effect of a potassium promoter on the Fischer–Tropsch synthesis of light olefins over iron carbide catalysts encapsulated in graphene-like carbon. Catal. Sci. Technol. 2019, 9, 2728–2741. [Google Scholar] [CrossRef]


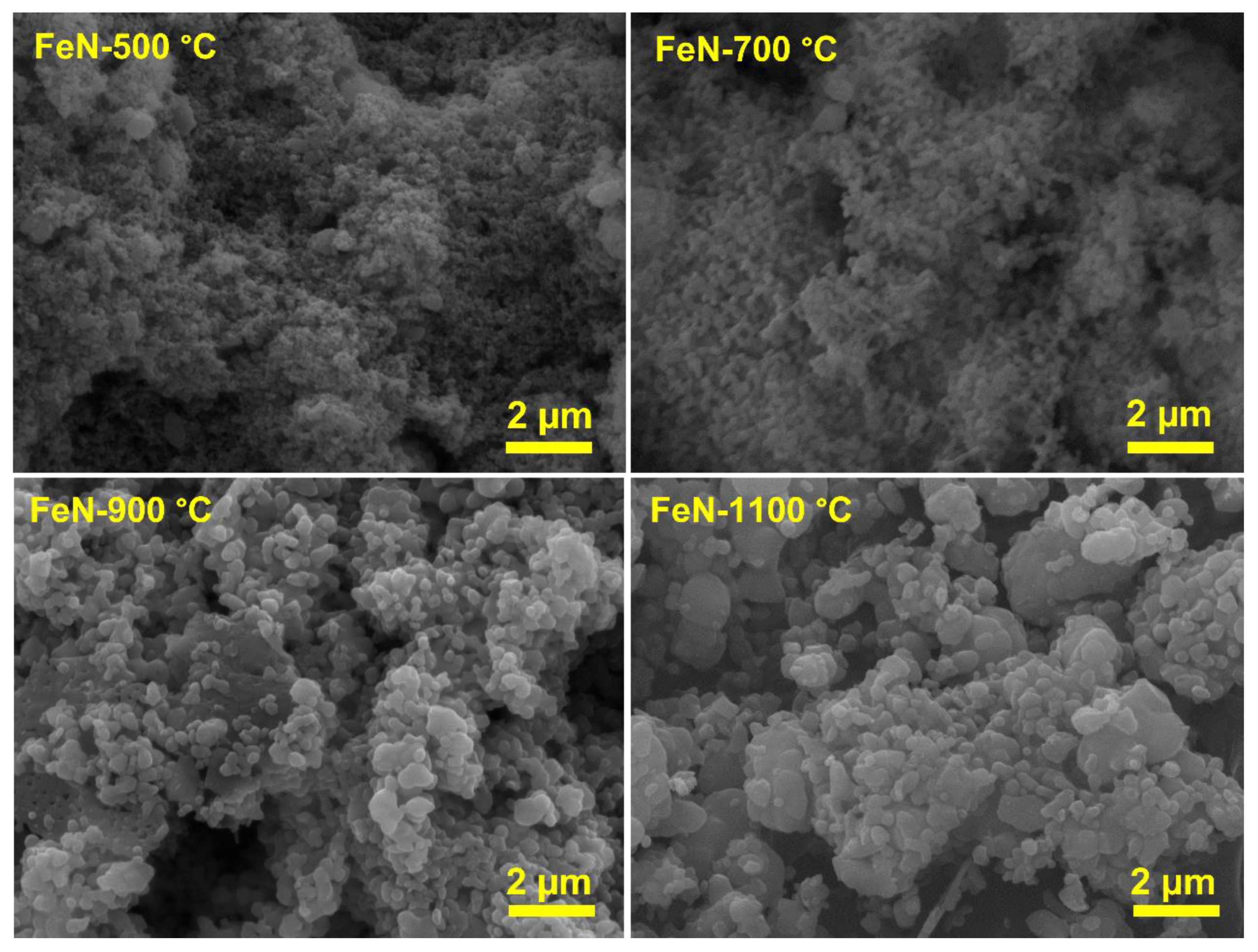

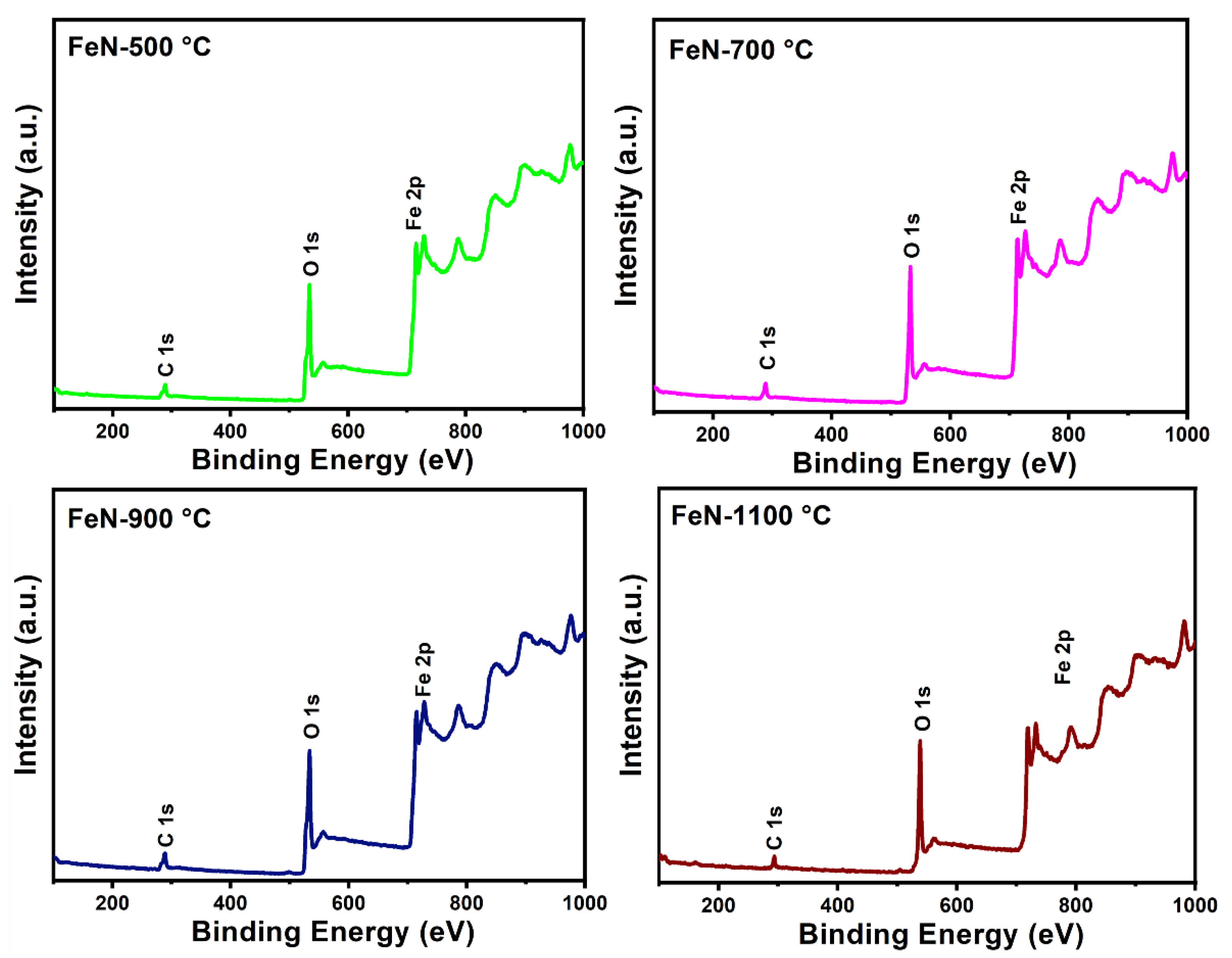


| Catalyst | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Radius (nm) |
|---|---|---|---|
| FeN-500 °C | 16.7 | 0.34 | 19.1 |
| FeN-700 °C | 7.8 | 0.23 | 17.0 |
| FeN-900 °C | 5.5 | 0.04 | 15.3 |
| FeN-1100 °C | 3.9 | 0.04 | 21.6 |
| Used Catalysts | Preparation Method | WHSV [L/(gcat∙h)] | TCMD Condition | Initial CH4 Conversion | Reaction Time (Min) | Final CH4 Conversion | Ref. |
|---|---|---|---|---|---|---|---|
| Fe–Al, 40% Fe | Fusion | 7.5 | CH4, 750 °C | 66% | 120 | 19% | [16] |
| Fe–Ce, 27% Fe | Co-precipitation | 4.5 | CH4, 800 °C | 35% | 360 | 49% | [40] |
| Fe–La, 27% Fe | Co-precipitation | 4.5 | CH4, 800 °C | 24% | 360 | 33% | [40] |
| Fe-Mg, 50% Fe | Chemical impregnation | 1.5 | CH4, 700 °C | 5% | 600 | 27% | [41] |
| Fe-Mo-Al, 62% Fe | Fusion | 1.5 | CH4, 750 °C | 75% | 180 | 70% | [42] |
| Tierga ore, 52.6% Fe | None | 2 | CH4, 800 °C | 30% | 180 | 32% | [43] |
| Ilmenite ore, 33.3% Fe | None | 2 | CH4, 800 °C | 8% | 180 | 10% | [43] |
| Unsupported α-Fe2O3 | Calcination | 2.4 | CH4, 800 °C | 35.2 | 480 | 22.5% | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alharthi, A.I.; Qahtan, T.F.; Shaddad, M.N.; Alotaibi, M.A.; Alotibi, S.; Alansi, A.M. Scalable Synthesis of Oxygen Vacancy-Rich Unsupported Iron Oxide for Efficient Thermocatalytic Conversion of Methane to Hydrogen and Carbon Nanomaterials. Nanomaterials 2023, 13, 2461. https://doi.org/10.3390/nano13172461
Alharthi AI, Qahtan TF, Shaddad MN, Alotaibi MA, Alotibi S, Alansi AM. Scalable Synthesis of Oxygen Vacancy-Rich Unsupported Iron Oxide for Efficient Thermocatalytic Conversion of Methane to Hydrogen and Carbon Nanomaterials. Nanomaterials. 2023; 13(17):2461. https://doi.org/10.3390/nano13172461
Chicago/Turabian StyleAlharthi, Abdulrahman I., Talal F. Qahtan, Maged N. Shaddad, Mshari A. Alotaibi, Satam Alotibi, and Amani M. Alansi. 2023. "Scalable Synthesis of Oxygen Vacancy-Rich Unsupported Iron Oxide for Efficient Thermocatalytic Conversion of Methane to Hydrogen and Carbon Nanomaterials" Nanomaterials 13, no. 17: 2461. https://doi.org/10.3390/nano13172461