Engineering Self-Assembled Nanomedicines Composed of Clinically Approved Medicines for Enhanced Tumor Nanotherapy
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
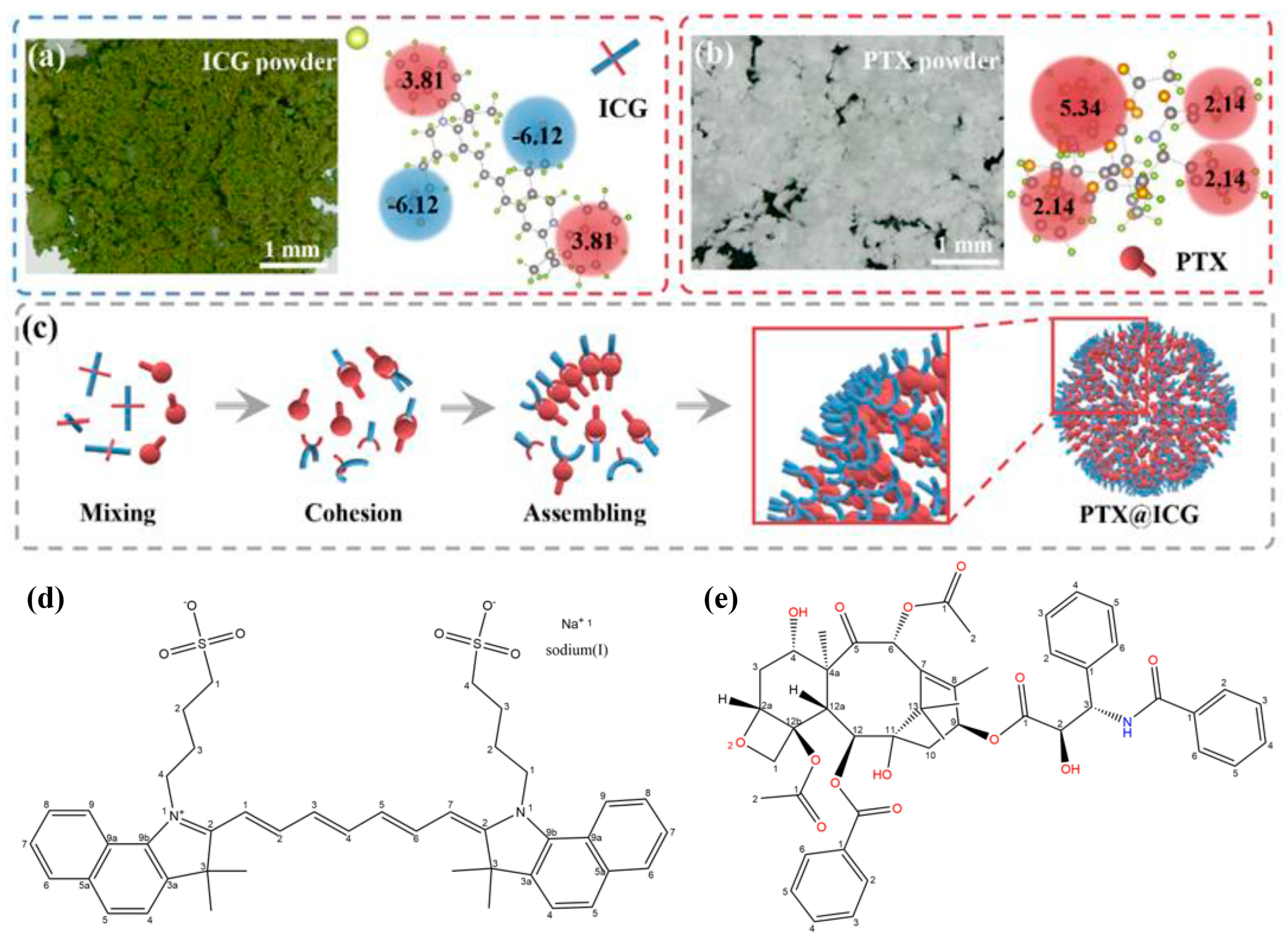
3.1. Preparation and Characterization of PTX@ICG
3.2. The Photothermal Properties and the NIR-Activated Disassembly of PTX@ICG
3.3. In Vitro Chemo-Photothermal Therapy of PTX@ICG
3.4. The Bio-Distribution of PTX@ICG
3.5. In Vivo Chemo-Photothermal Therapy of PTX@ICG
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Stoffel, E.M.; Murphy, C.C. Epidemiology and Mechanisms of the Increasing Incidence of Colon and Rectal Cancers in Young Adults. Gastroenterology 2020, 158, 341–353. [Google Scholar] [CrossRef]
- Saatci, O.; Kaymak, A.; Raza, U.; Ersan, P.G.; Akbulut, O.; Banister, C.E.; Sikirzhytski, V.; Tokat, U.M.; Aykut, G.; Ansari, S.A.; et al. Targeting lysyl oxidase (LOX) overcomes chemotherapy resistance in triple negative breast cancer. Nat. Commun. 2020, 11, 2416. [Google Scholar] [CrossRef]
- Lawrence, M.S.; Stojanov, P.; Polak, P.; Kryukov, G.V.; Cibulskis, K.; Sivachenko, A.; Carter, S.L.; Stewart, C.; Mermel, C.H.; Roberts, S.A.; et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature 2013, 499, 214–218. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Santoro, E.; Carlini, M.; Carboni, F.; Feroce, A. Colorectal carcinoma: Laparoscopic versus traditional open surgery. A clinical trial. Hepato Gastroenterol. 1999, 46, 900–904. [Google Scholar]
- Pretzsch, E.; Bsch, F.; Renz, B.; Werner, J.; Angele, M.; Chaudry, I.H. Operative Trauma and Blood Loss—Impact on Tumor Growth and Recurrence. Shock 2021, 55, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Liu, H.; Zheng, J. SynLethDB: Synthetic lethality database toward discovery of selective and sensitive anticancer drug targets. Nucleic Acids Res. 2016, 44, 1011–1017. [Google Scholar] [CrossRef]
- Palmer, A.C.; Plana, D.; Gao, H.; Korn, J.M.; Yang, G.; Green, J.; Zhang, X.; Velazquez, R.; Mclaughlin, M.E.; Ruddy, D.A. A proof of concept for biomarker-guided targeted therapy against ovarian cancer based on patient-derived tumor xenografts. Cancer Res. 2020, 80, 4278–4287. [Google Scholar] [CrossRef]
- Bakx, R.; Emous, M.; Legemate, D.A.; Zoetmulder, F.A.N.; van Tienhoven, G.; Bemelman, W.A.; van Lanschot, J.J.B. Harm and benefits of short-term pre-operative radiotherapy in patients with resectable rectal carcinomas. Ejso 2006, 32, 520–526. [Google Scholar] [CrossRef]
- Xue, Y.; Bai, H.; Peng, B.; Fang, B.; Baell, J.; Li, L.; Huang, W.; Voelcker, N.H. Stimulus-cleavable chemistry in the field of controlled drug delivery. Chem. Soc. Rev. 2021, 50, 4872–4931. [Google Scholar] [CrossRef]
- Liu, P.; Liu, X.; Cheng, Y.; Zhong, S.; Zhou, W. Core–Shell Nanosystems for Self-Activated Drug–Gene Combinations against Triple-Negative Breast Cancer. ACS Appl. Mater. Interfaces 2020, 12, 53654–53664. [Google Scholar] [CrossRef] [PubMed]
- Ou, K.; Xu, X.; Guan, S.; Zhang, R.; Wu, J. Nanodrug Carrier Based on Poly(Ursolic Acid) with Self-Anticancer Activity against Colorectal Cancer. Adv. Funct. Mater. 2020, 30, 1907857. [Google Scholar] [CrossRef]
- He, W.; Xing, X.; Wang, X.; Wu, D.; Mitragotri, S. Nanocarrier mediated Cytosolic Delivery of Biopharmaceuticals. Adv. Funct. Mater. 2020, 30, 1910566. [Google Scholar] [CrossRef]
- Motta, S.; Siani, P.; Levy, A.; Di Valentin, C. Exploring the drug loading mechanism of photoactive inorganic nanocarriers through molecular dynamics simulations. Nanoscale 2021, 13, 13000–13013. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Zhong, L.; Wang, M.; Li, H.; Qu, Y.; Liu, Q.; Han, R.; Yuan, L.; Shi, K.; Peng, J. Perfluorocarbon-loaded and redox-activatable photosensitizing agent with oxygen supply for enhancement of fluorescence/photoacoustic imaging guided tumor photodynamic therapy. Adv. Funct. Mater. 2019, 29, 1806199. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Guo, X.; Zhao, J.; Zhou, S. A Biomimetic Polymer Magnetic Nanocarrier Polarizing Tumor-Associated Macrophages for Potentiating Immunotherapy. Small 2020, 16, e2003543. [Google Scholar] [CrossRef]
- Zheng, M.B.; Yue, C.X.; Ma, Y.F.; Gong, P.; Zhao, P.F.; Zheng, C.F.; Sheng, Z.H.; Zhang, P.F.; Wang, Z.H.; Cai, L.T. Single-Step Assembly of DOX/ICG Loaded Lipid-Polymer Nanoparticles for Highly Effective Chemo-photothermal Combination Therapy. Acs Nano 2013, 7, 2056–2067. [Google Scholar] [CrossRef]
- Guo, T.; Lin, W.; Chen, W.; Huang, Y.; Zhu, L.; Pan, X. Photodynamic therapy in combination with sorafenib for enhanced immunotherapy of lung cancer. J. Biomed. Nanotechnol. 2020, 16, 1219–1228. [Google Scholar] [CrossRef]
- Ji, J.F.; Ma, F.; Zhang, H.B.; Liu, F.Y.; He, J.; Li, W.L.; Xie, T.T.; Zhong, D.N.; Zhang, T.T.; Tian, M.; et al. Light-Activatable Assembled Nanoparticles to Improve Tumor Penetration and Eradicate Metastasis in Triple Negative Breast Cancer. Adv. Funct. Mater. 2018, 28, 1801738. [Google Scholar] [CrossRef]
- Yang, L.; Tseng, Y.T.; Suo, G.; Chen, L.; Yu, J.; Chiu, W.J.; Huang, C.C.; Lin, C.H. Photothermal Therapeutic Response of Cancer Cells to Aptamer–Gold Nanoparticle-Hybridized Graphene Oxide under NIR Illumination. ACS Appl. Mater. Interfaces 2015, 7, 5097–5106. [Google Scholar] [CrossRef]
- Zhao, X.; Tang, D.; Wu, Y.; Chen, S.; Wang, C. An artificial cell system for biocompatible gene delivery in cancer therapy. Nanoscale 2020, 12, 10189–10195. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, R.; Wu, C.; Feng, W.; Zhong, Q.; Chen, X.; Wang, T.; Mao, C. Diffusion-mediated carving of interior topologies of all-natural protein nanoparticles to tailor sustained drug release for effective breast cancer therapy. Biomaterials 2023, 295, 122027. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Meng, Y.; Xu, X.; Tong, T.; He, C.; Wang, L.; Wang, K.; Zhao, M.; You, X.; Zhang, W. A ferroptosis-inducing and leukemic cell-targeting drug nanocarrier formed by redox-responsive cysteine polymer for acute myeloid leukemia therapy. ACS Nano 2023, 17, 3334–3345. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Zheng, M.; Yue, C.; Luo, Z.; Gong, P.; Gao, G.; Sheng, Z.; Zheng, C.; Cai, L. Improving drug accumulation and photothermal efficacy in tumor depending on size of ICG loaded lipid-polymer nanoparticles. Biomaterials 2014, 35, 6037–6046. [Google Scholar] [CrossRef]
- Cheng, Y.; Cheng, H.; Jiang, C.; Qiu, X.; Wang, K.; Huan, W.; Yuan, A.; Wu, J.; Hu, Y. Perfluorocarbon nanoparticles enhance reactive oxygen levels and tumour growth inhibition in photodynamic therapy. Nat. Commun. 2015, 6, 8785. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Meng, J.; Huang, L.; Wu, F.; Yi, X.; Su, G.; Li, Y.; Hou, Z.; Fan, Z. Platinum-Coordinated Engineered Nanoreactors with O2 Self-Amplificationand On-Demand Cascade Chemo-Drug Synthesis for Self-Reinforcing Hypoxic Oncotherapy. ACS Appl. Mater. Interfaces 2023, 15, 17495–17506. [Google Scholar] [CrossRef] [PubMed]
- Emerich, D.F.; Thanos, C.G. Targeted nanoparticle-based drug delivery and diagnosis. J. Drug Target. 2007, 15, 163–183. [Google Scholar] [CrossRef]
- Denkova, A.G.; de Kruijff, R.M.; Serra-Crespo, P. Nanocarrier-Mediated Photochemotherapy and Photoradiotherapy. Adv. Healthc. Mater. 2018, 7, e1701211. [Google Scholar] [CrossRef]
- Dai, Y.; Ding, Y.; Li, L. Nanozymes for regulation of reactive oxygen species and disease therapy. Chin. Chem. Lett. 2021, 32, 2715–2728. [Google Scholar] [CrossRef]
- Hosseini, S.H.; Alipour, S.; Zohreh, N. Delivery of doxorubicin using double-layered core–shell nanocarrier based on magnetic Fe3O4 core and salep shells. Langmuir 2018, 34, 13735–13744. [Google Scholar] [CrossRef]
- Du, B.; Liu, J.; Ding, G.; Han, X.; Li, D.; Wang, E.; Wang, J. Positively charged graphene/Fe3O4/polyethylenimine with enhanced drug loading and cellular uptake for magnetic resonance imaging and magnet-responsive cancer therapy. Nano Res. 2017, 10, 2280–2295. [Google Scholar] [CrossRef]
- Qin, Y.; Guo, Q.; Wu, S.; Huang, C.; Zhang, Z.; Zhang, L.; Zhang, L.; Zhu, D. LHRH/TAT dual peptides-conjugated polymeric vesicles for PTT enhanced chemotherapy to overcome hepatocellular carcinoma. Chin. Chem. Lett. 2020, 31, 3121–3126. [Google Scholar] [CrossRef]
- Hu, D.; Chen, L.; Qu, Y.; Peng, J.; Chu, B.; Shi, K.; Hao, Y.; Zhong, L.; Wang, M.; Qian, Z. Oxygen-generating hybrid polymeric nanoparticles with encapsulated doxorubicin and chlorin e6 for trimodal imaging-guided combined chemo-photodynamic therapy. Theranostics 2018, 8, 1558. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Liu, X.; Fang, Z.; Xu, Q.; Zhang, Q. Synthesis of multifunctional Fe3O4@PLGA-PEG nano-niosomes as a targeting carrier for treatment of cervical cancer. Mater. Sci. Eng. C-Mater. Biol. Appl. 2019, 94, 291–302. [Google Scholar] [CrossRef]
- Wang, J.; Fang, J.; Fang, P.; Li, X.; Wu, S.; Zhang, W.; Li, S. Preparation of hollow core/shell Fe3O4@graphene oxide composites as magnetic targeting drug nanocarriers. J. Biomater. Sci. Polym. Ed. 2017, 28, 337–349. [Google Scholar] [CrossRef]
- Asian, P.A.; Shakibi, S.; Maniati, M.S.; Khorasani, S.N.; Khalili, S. Targeted delivery, drug release strategies, and toxicity study of polymeric drug nanocarriers. Polym. Adv. Technol. 2020, 32, 931–944. [Google Scholar] [CrossRef]
- Ji, X.Y.; Wang, C.Y.; Tang, M.M.; Guo, D.X.; Peng, F.; Zhong, Y.L.; Song, B.; Su, Y.Y.; He, Y. Biocompatible protamine sulfate@silicon nanoparticle-based gene nanocarriers featuring strong and stable fluorescence. Nanoscale 2018, 10, 14455–14463. [Google Scholar] [CrossRef]
- Lin, J.; Li, C.; Guo, Y.; Zou, J.; Wu, P.; Liao, Y.; Zhang, B.; Le, J.; Zhao, R.; Shao, J.-W. Carrier-free nanodrugs for in vivo NIR bioimaging and chemo-photothermal synergistic therapy. J. Mater. Chem. B 2019, 7, 6914–6923. [Google Scholar] [CrossRef]
- Coha, M.; Farinelli, G.; Tiraferri, A.; Minella, M.; Vione, D. Advanced oxidation processes in the removal of organic substances from produced water: Potential, configurations, and research needs. Chem. Eng. J. 2021, 414, 128668. [Google Scholar] [CrossRef]
- Zhang, G.; Li, K.; He, S.; Wang, L.; Xu, B. Electron Donor-Acceptor Effect-Induced Organic/Inorganic Nanohybrids with Low Energy Gap for Highly Efficient Photothermal Therapy. ACS Appl. Mater. Interfaces 2021, 13, 17920–17930. [Google Scholar] [CrossRef]
- Ding, K.; Zheng, C.; Sun, L.; Liu, X.; Yin, Y.; Wang, L. NIR light-induced tumor phototherapy using ICG delivery system based on platelet-membrane-camouflaged hollow bismuth selenide nanoparticles. Chin. Chem. Lett. 2020, 31, 1168–1172. [Google Scholar] [CrossRef]
- Guo, Q.; Dong, Y.; Zhang, Y.; Fu, H.; Duan, Y. Sequential Release of Pooled siRNAs and Paclitaxel by Aptamer-Functionalized Shell-Core Nanoparticles to Overcome Paclitaxel Resistance of Prostate Cancer. ACS Appl. Mater. Interfaces 2021, 13, 13990–14003. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.L.; Zuo, S.Y.; Li, L.X.; Liu, T.; Dong, F.D.; Wang, X.; Zhang, X.B.; He, Z.G.; Zhai, Y.L.; Sun, B.J.; et al. The length of disulfide bond-containing linkages impacts the oral absorption and antitumor activity of paclitaxel prodrug-loaded nanoemulsions. Nanoscale 2021, 13, 10536–10543. [Google Scholar] [CrossRef] [PubMed]
- Schott, H. Hydrophilic-lipophilic balance, solubility parameter, and oil-water partition coefficient as universal parameters of nonionic surfactants. J. Pharm. Sci. 2010, 84, 1215–1222. [Google Scholar] [CrossRef]
- Grillo, I.; Morfin, I.; Prévost, S. Structural characterization of Pluronic micelles swollen with perfume molecules. Langmuir 2018, 34, 13395–13408. [Google Scholar] [CrossRef]
- Miyake, Y.; Owari, T.; Ishiga, F.; Teramoto, M. Enzymatic reaction in water-in-oil microemulsions. Part 2. Rate of hydrolysis of a hydrophobic substrate, 2-naphthyl acetate. J. Chem. Soc. Faraday Trans. 1994, 90, 979–986. [Google Scholar] [CrossRef]
- Han, L.; Wu, J.-L.; Yang, L.-X. Effect of combination of rapamycin and cisplatin on human cervical carcinoma Hela cells. Contemp. Oncol. 2012, 16, 512–515. [Google Scholar] [CrossRef]
- Roper, D.K.; Ahn, W.; Hoepfner, M. Microscale heat transfer transduced by surface plasmon resonant gold nanoparticles. J. Phys. Chem. C 2007, 111, 3636–3641. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Q.; Yu, L.; Chen, Y. Engineering Self-Assembled Nanomedicines Composed of Clinically Approved Medicines for Enhanced Tumor Nanotherapy. Nanomaterials 2023, 13, 2499. https://doi.org/10.3390/nano13182499
Jiang Q, Yu L, Chen Y. Engineering Self-Assembled Nanomedicines Composed of Clinically Approved Medicines for Enhanced Tumor Nanotherapy. Nanomaterials. 2023; 13(18):2499. https://doi.org/10.3390/nano13182499
Chicago/Turabian StyleJiang, Quzi, Luodan Yu, and Yu Chen. 2023. "Engineering Self-Assembled Nanomedicines Composed of Clinically Approved Medicines for Enhanced Tumor Nanotherapy" Nanomaterials 13, no. 18: 2499. https://doi.org/10.3390/nano13182499
APA StyleJiang, Q., Yu, L., & Chen, Y. (2023). Engineering Self-Assembled Nanomedicines Composed of Clinically Approved Medicines for Enhanced Tumor Nanotherapy. Nanomaterials, 13(18), 2499. https://doi.org/10.3390/nano13182499




