Recent Advances in Bimetallic Nanoporous Gold Electrodes for Electrochemical Sensing
Abstract
:1. Introduction
2. Fabrication
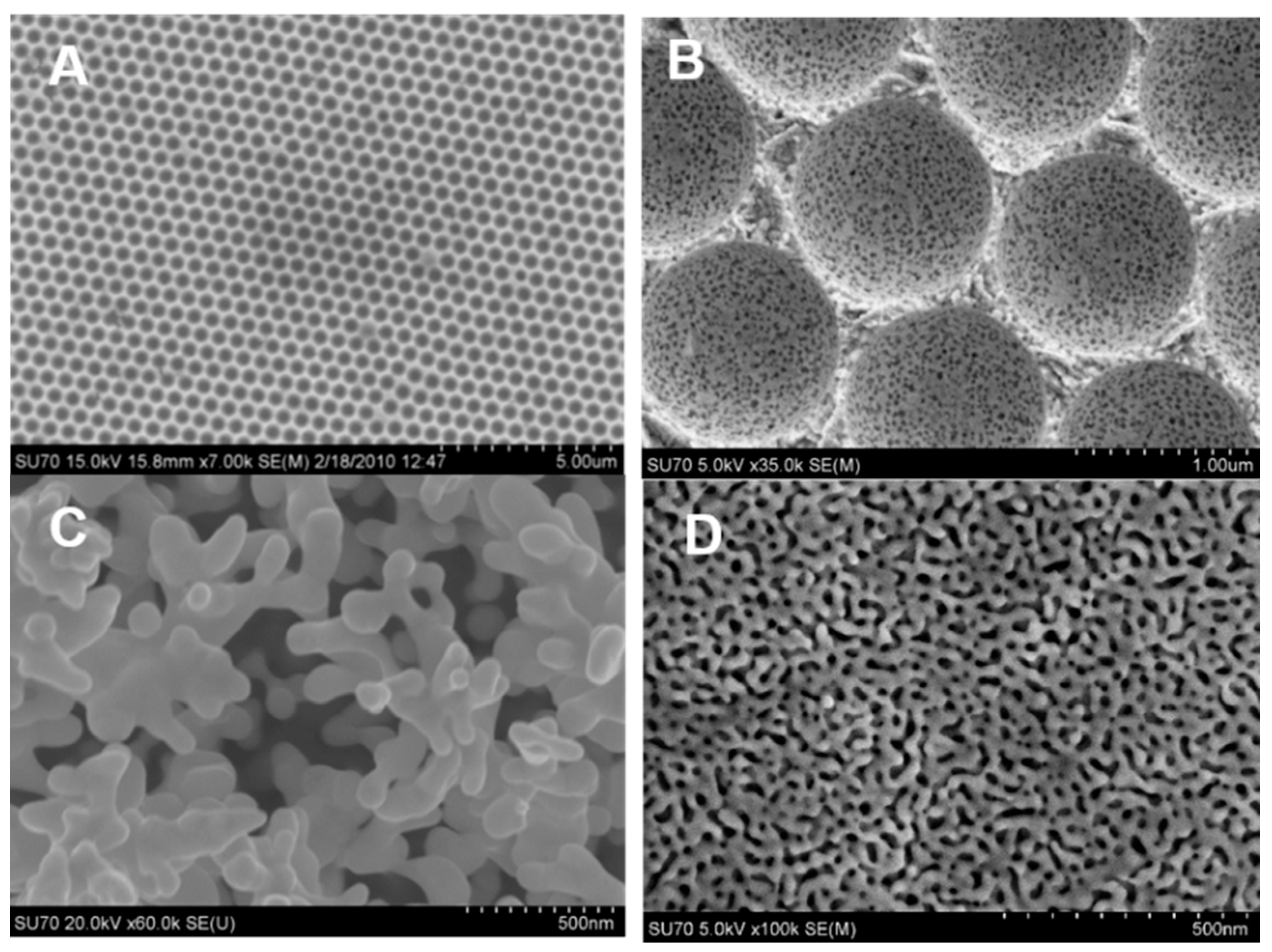
2.1. Templating
2.2. Chemical Dealloying of a Pre-Formed Alloy
2.3. Electrochemical Dealloying of a Pre-Formed Alloy
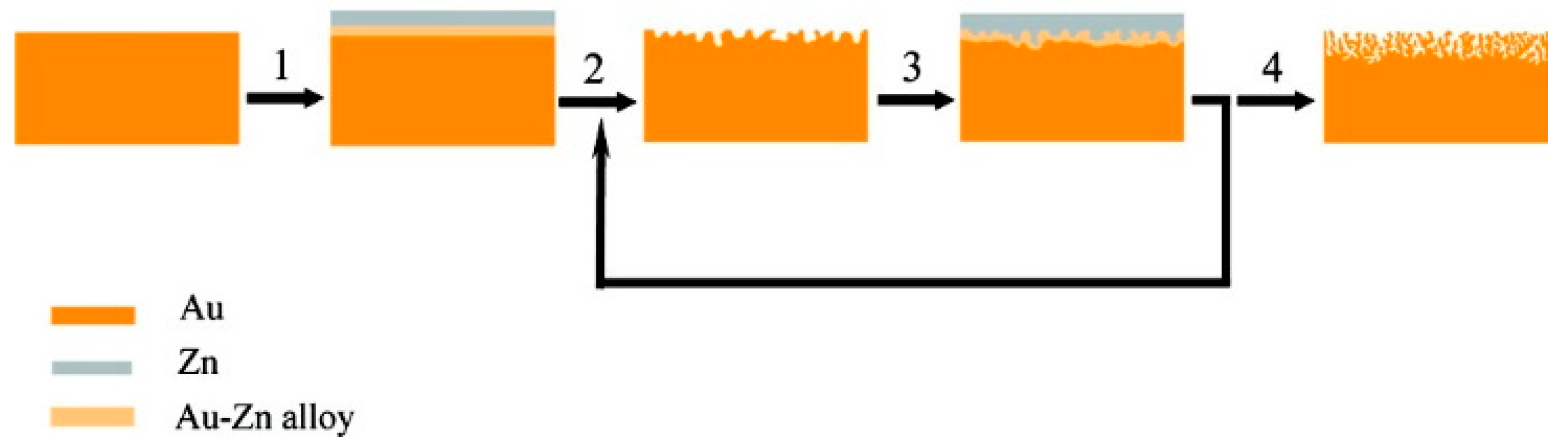
2.4. Electrochemical Formation of the Alloy Followed by Electrochemical Dealloying
2.5. Anodization and Surface Roughening
3. Bimetallic NPG Electrode Fabrication
3.1. Ternary Alloys
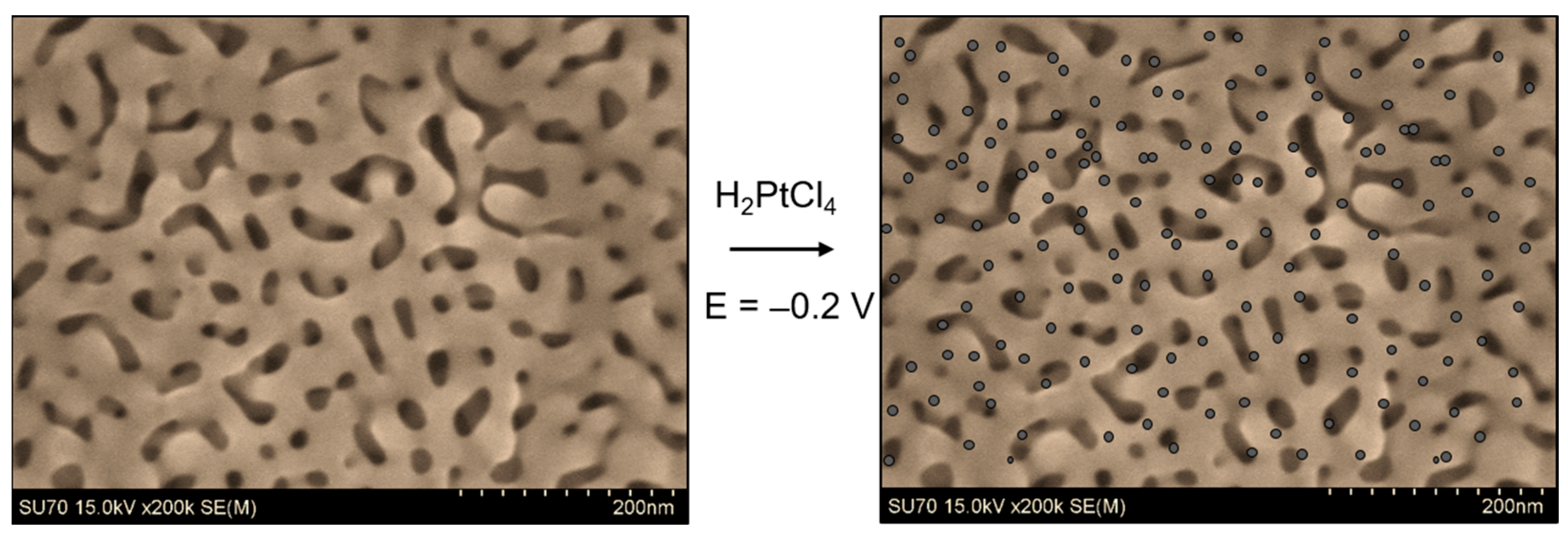
3.2. Immersion Followed by Reduction (Chemical, Electrochemical)
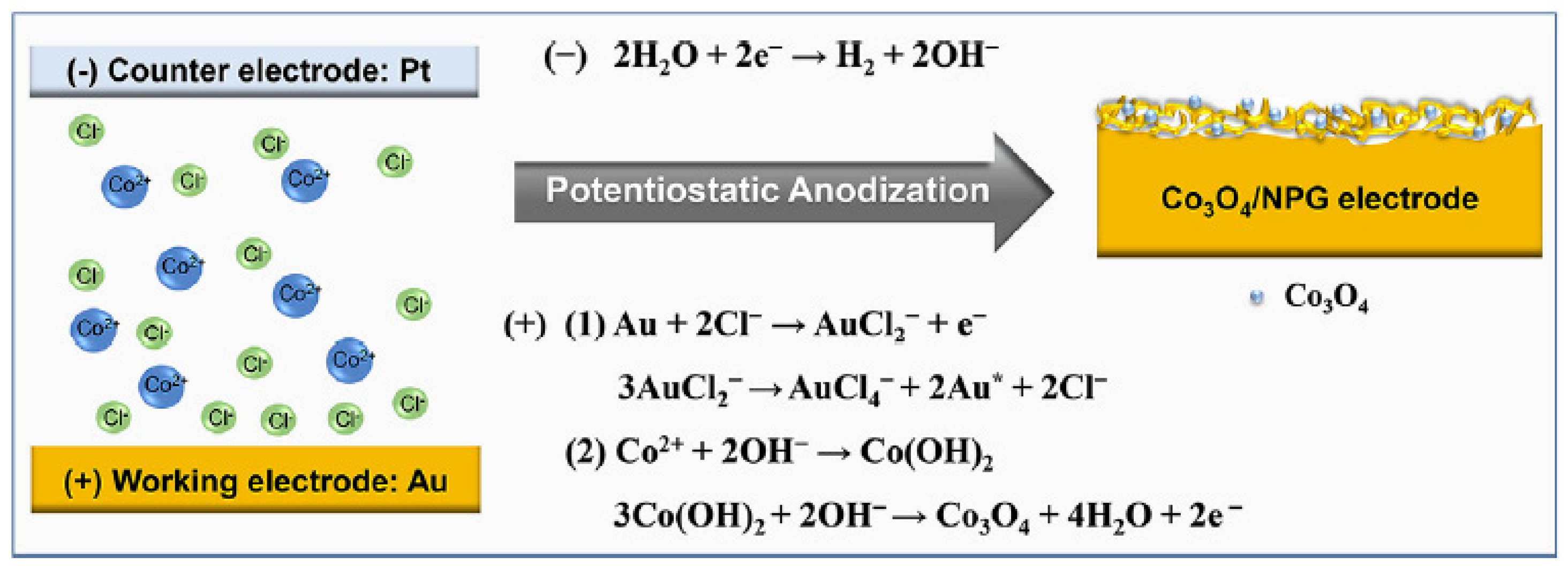
3.3. Electrodeposition–Annealing
3.4. UPD–Surface-Limited Redox Replacement
4. Characterization
4.1. Scanning Electron Microscopy (SEM)
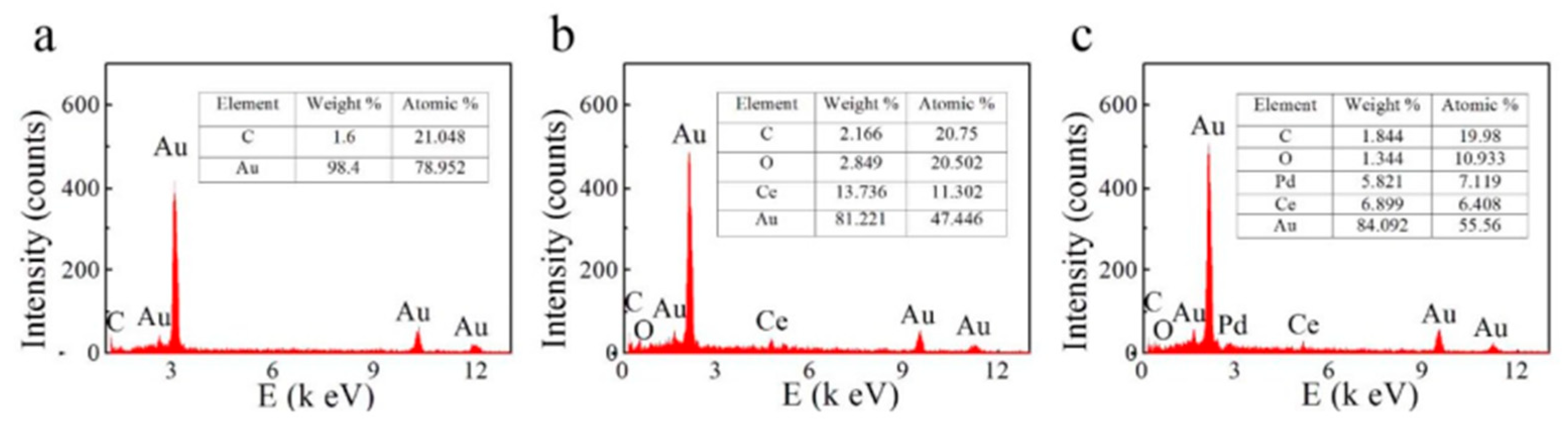
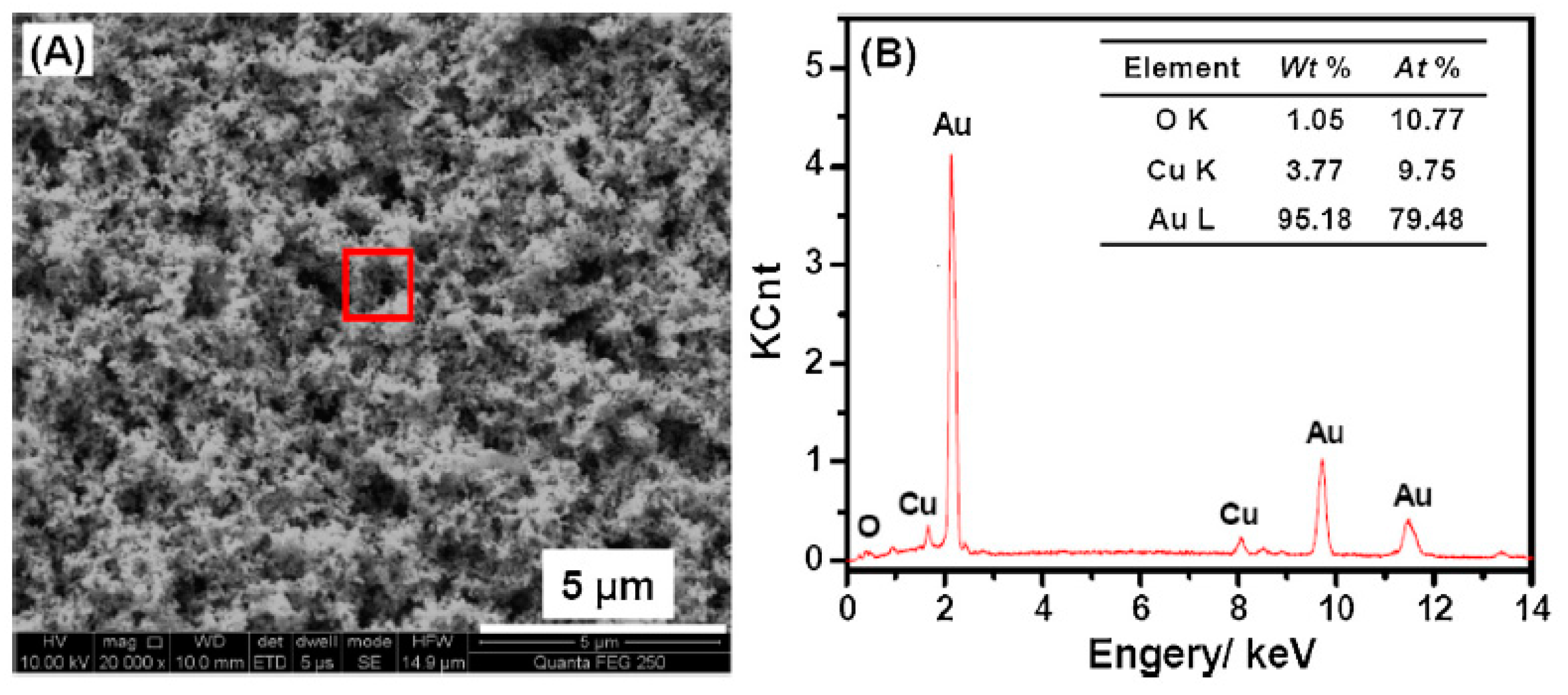
4.2. SEM-Energy Dispersive X-ray Spectroscopy (EDX)
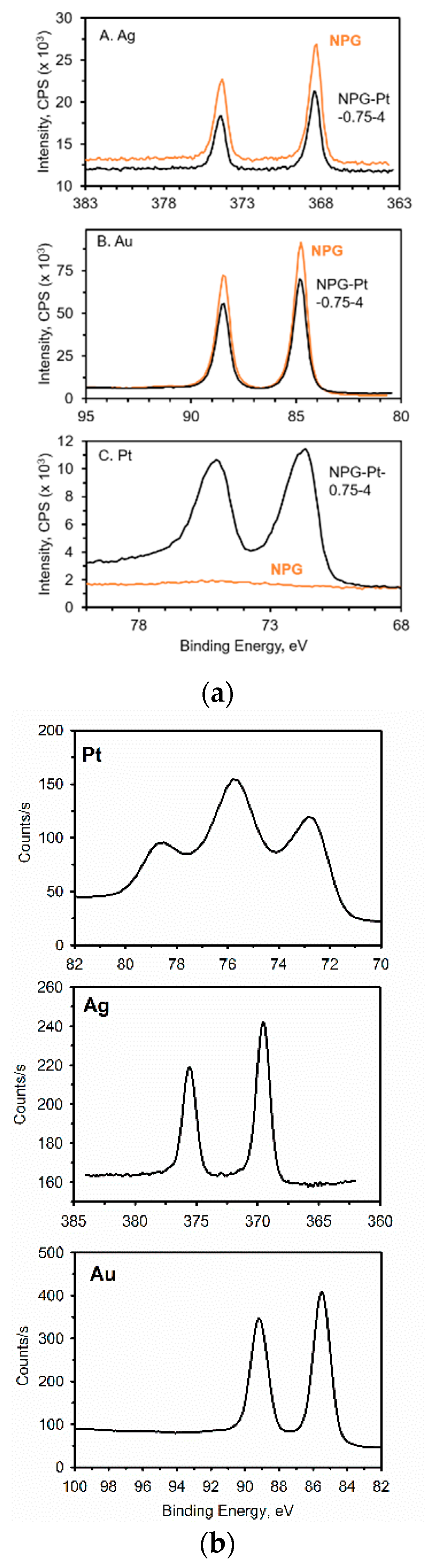
4.3. X-ray Photoelectron Spectroscopy (XPS)
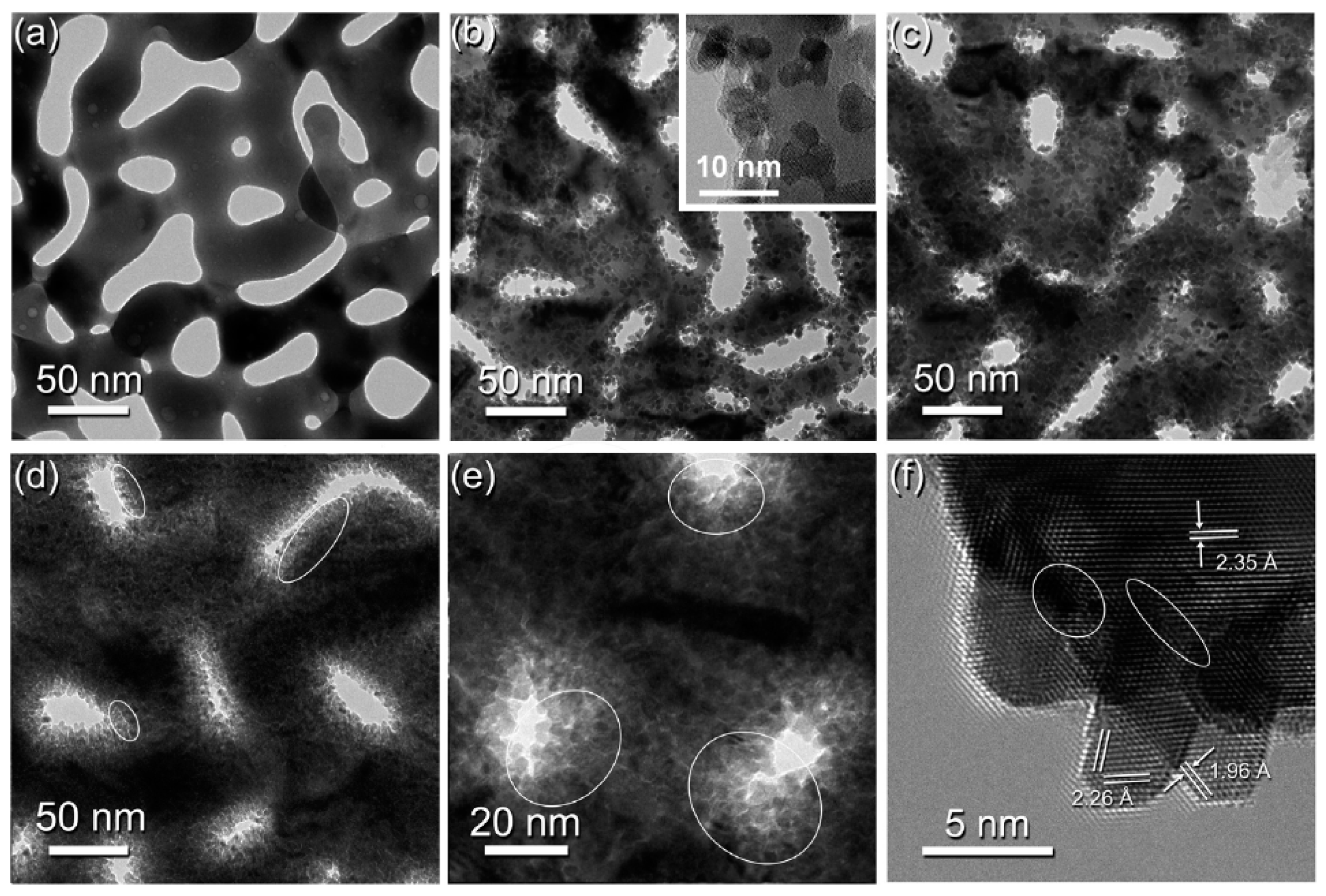
4.4. Transmission Electron Microscopy (TEM)
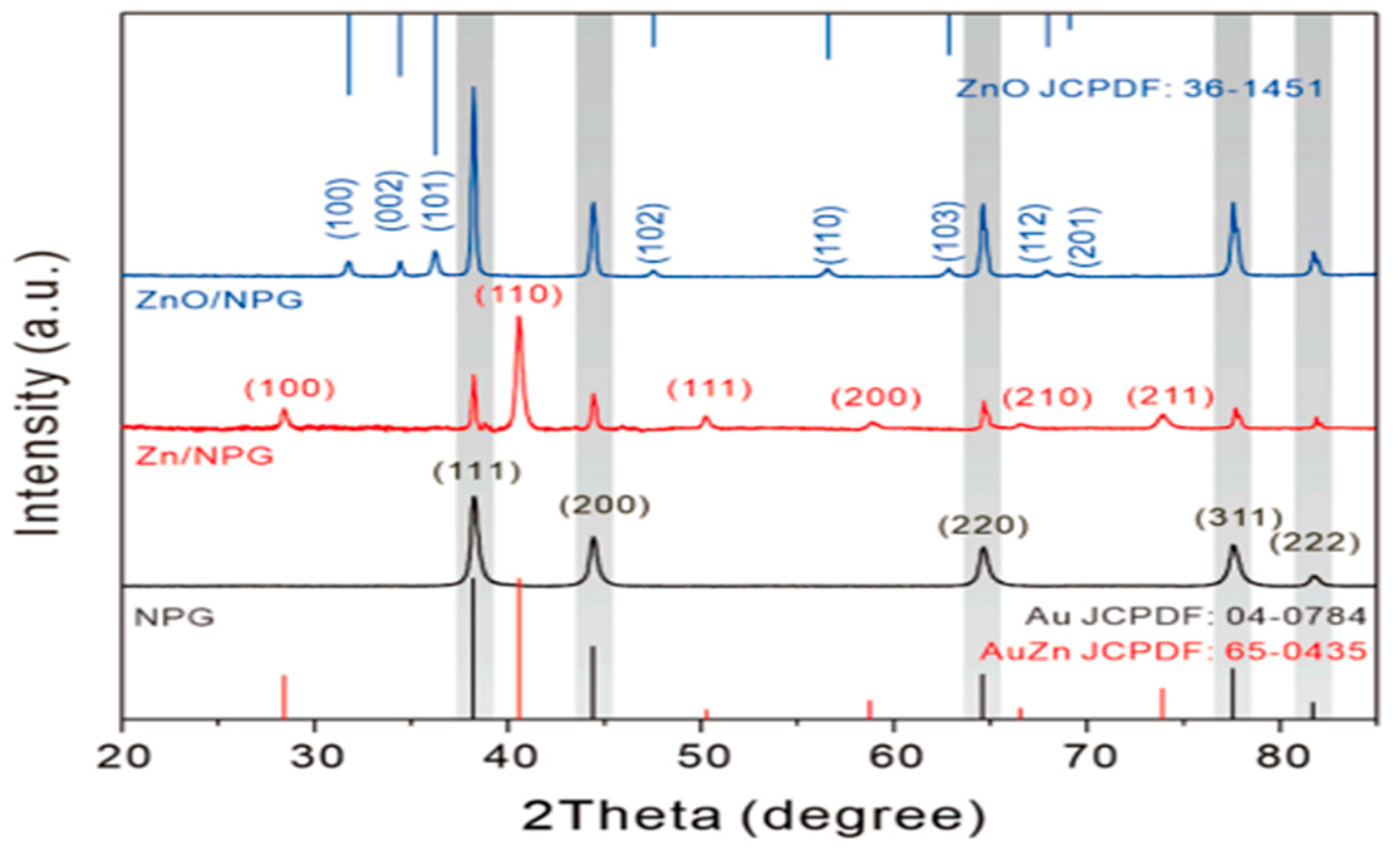
4.5. XRD
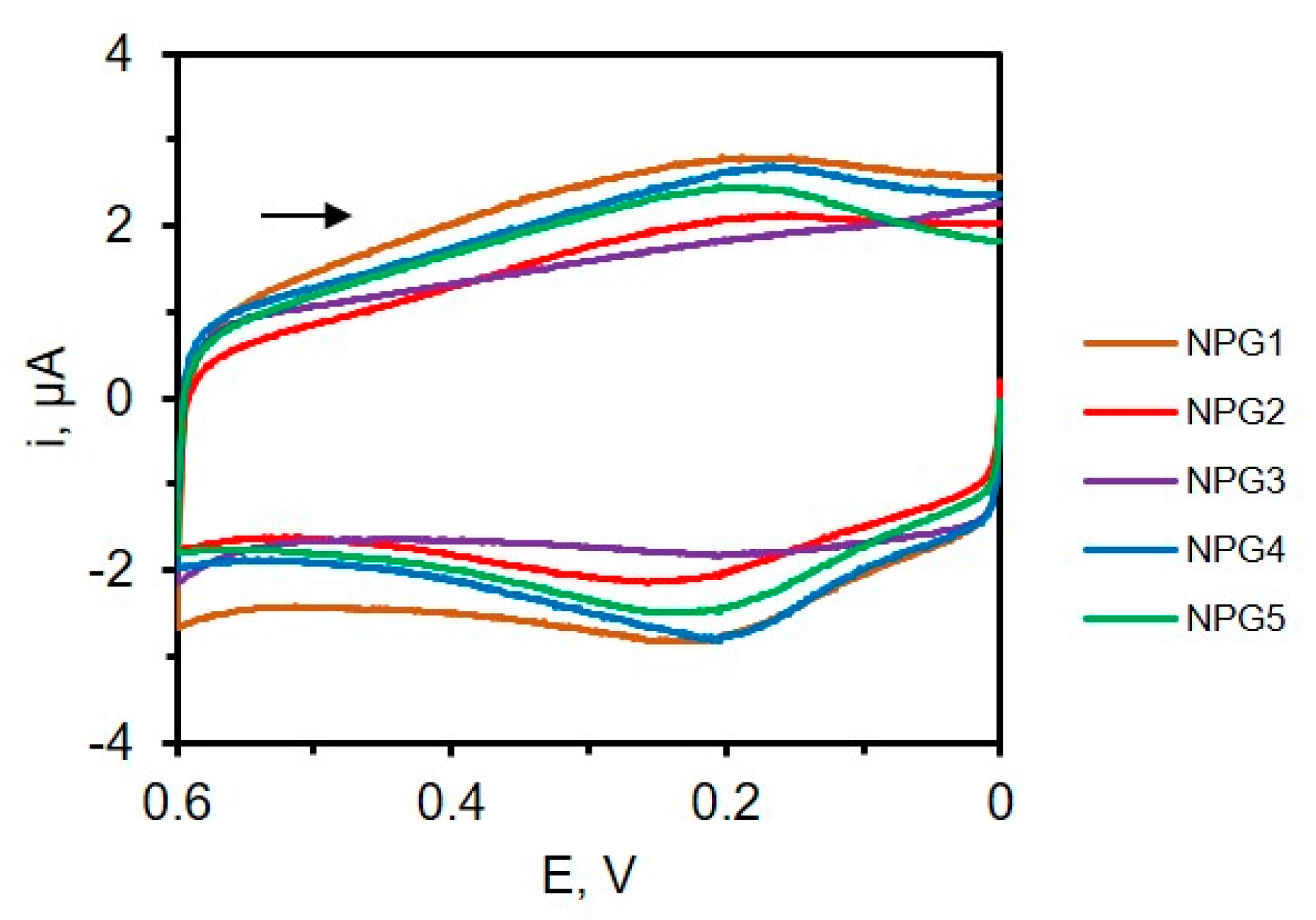
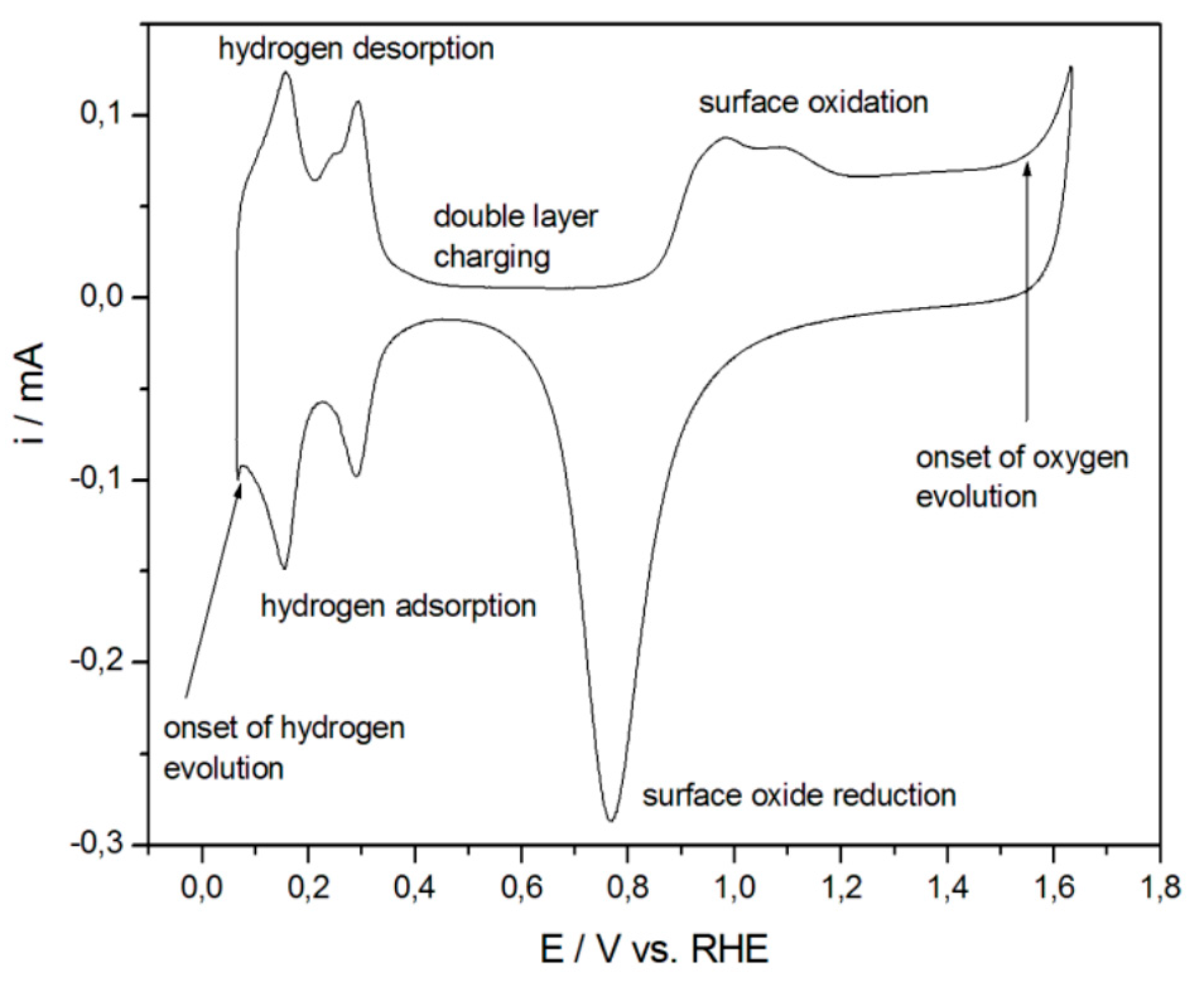
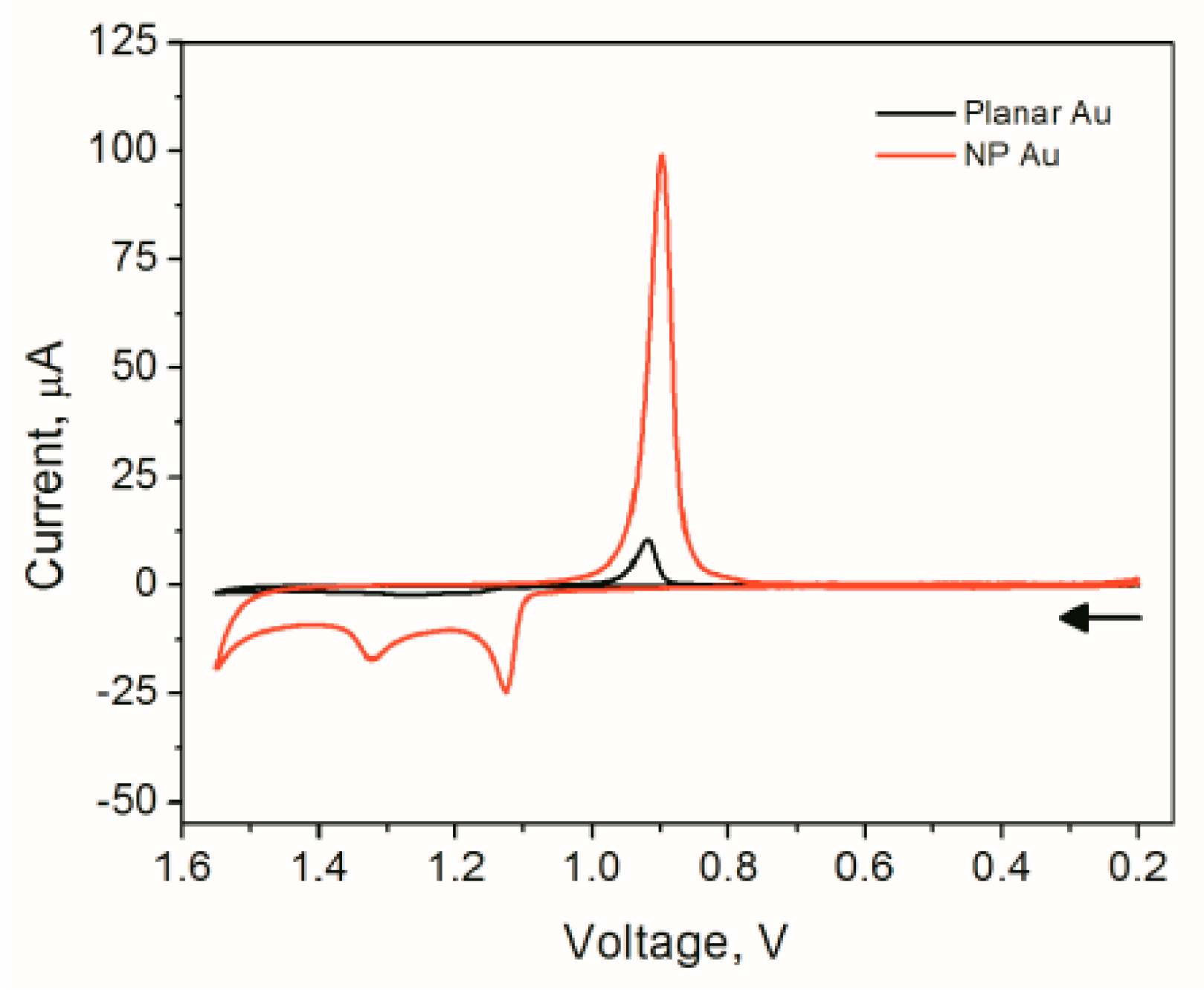
4.6. Surface Area Measurements
5. Electrochemical Applications in Chemical Sensing Using Bimetallic NPG Electrodes
5.1. Hydrogen Peroxide (H2O2) Sensing
5.2. Glucose Sensing
5.3. Sensing Applications of Bimetallic Decorated NPG for Molecules or Ions Other Than H2O2 and Glucose
| Electrode | Method | Analyte | Conditions | Linear Range | LOD, nM | Sensitivity | Interference Study | Ref. |
|---|---|---|---|---|---|---|---|---|
| NPG-Ti-Chitosan | DPV | acetaminophen | Buffer, pH 7 | 60–700 μM | 10 | Yes | [188] | |
| ZnO-NPG | SWASV | As(III) | 0.1 M PBS, pH 5.0 | 1.0–260 ppb | 0.30 ppb | 1.366 µA ppb−1cm−2 | Yes | [150] |
| NPG/ITO | DPASV | As(III) | 0.1 M HCl | 0.1–50 µg/L | 0.054 µg/L | 9.837 μA μg L−1 | Yes | [189] |
| FeOOH-NPG | SWV | Hg(II) | 0.1 M PBS; pH 5.0 | 0.02–2.2 µM | 7.81 | 123.5 μA μM−1 cm−2 | Yes | [89] |
| np-Au NPs/ITO | DPASV | Hg(II) | 0.1 M HCl | 0.1–10 µg/L | 0.15 | Yes | [190] | |
| Pt-NPG | CA | Hydrazine | 0.2 M PBS, pH 7.0 | 5 μM to 6.105 mM | 1030 | 3449.68 μA mM−1 cm−2 | Yes | [185] |
| Pd@CeO2-NPG/CFP | CA | 4-aminophenol | 0.1 M PBS; pH 7.0 | 0.005–0.03; 0.03–9 µM | 4 | 75.4 and 56.5 µA µM−1 | Yes | [131] |
| MoO2/Cu-NPG | DPV | methimazole | 0.1 M PBS; pH 7.0 | 0.01–30 µM | 35 | 4.3 μA μM−1 | Yes | [91] |
| RuPt-NPG | DPV | methionine | 0.1 M PBS; pH 7.0 | 0.006–0.105 and 3–102 μM | 2 | 0.063 μA μM−1 | Yes | [139] |
| RuPd-NPG | CA | Captopril | 0.1 M PBS; pH 7.0 | 0.0025–0.475 and 2.5–32.5 µM | 1.25 | 0.022 mA μM−1 | Yes | [92] |
| PPY-CuO-NPG | DPV, CV | Piroxicam and tramadole | 0.1 M PBS; pH 7.0 | 0.05–30.0 & 50.0–300.0 µM | 10 | 0.428 μA μM−1 | Yes | [95] |
| Pd-NPG | DPV | Dopamine | PBS | 1–220 μM | 1000 | 1.19 μA μΜ−1 | Yes | [125] |
6. Summary and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Taguchi, A.; Schüth, F. Ordered Mesoporous Materials in Catalysis. Microporous Mesoporous Mater. 2005, 77, 1–45. [Google Scholar] [CrossRef]
- Corma, A. From Microporous to Mesoporous Molecular Sieve Materials and Their Use in Catalysis. Chem. Rev. 1997, 97, 2373–2420. [Google Scholar] [CrossRef]
- Ying, J.Y.; Mehnert, C.P.; Wong, M.S. Synthesis and Applications of Supramolecular-Templated Mesoporous Materials. Angew. Chem. Int. Ed. 1999, 38, 56–77. [Google Scholar] [CrossRef]
- Yamauchi, Y.; Kuroda, K. Rational Design of Mesoporous Metals and Related Nanomaterials by a Soft-Template Approach. Chem. Asian J. 2008, 3, 664–676. [Google Scholar] [CrossRef] [PubMed]
- Grosso, D.; Cagnol, F.; Soler-Illia, G.J.D.A.A.; Crepaldi, E.L.; Amenitsch, H.; Brunet-Bruneau, A.; Bourgeois, A.; Sanchez, C. Fundamentals of Mesostructuring through Evaporation-Induced Self-Assembly. Adv. Funct. Mater. 2004, 14, 309–322. [Google Scholar] [CrossRef]
- Bryce, C.T.; Stephen, A.S.; Luther, E.P. Nanoporous Metal Foams. Angew. Chem. Int. Ed. 2010, 49, 4544–4565. [Google Scholar] [CrossRef]
- Ying, J.; Lenaerts, S.; Symes, M.D.; Yang, X.Y. Hierarchical Design in Nanoporous Metals. Adv. Sci. 2022, 9, 2106117. [Google Scholar] [CrossRef]
- Rebbecchi, T.A.; Chen, Y. Template-Based Fabrication of Nanoporous Metals. J. Mater. Res. 2018, 33, 2–15. [Google Scholar] [CrossRef]
- Díaz, U.; Corma, A. Ordered Covalent Organic Frameworks, COFs and PAFs. From Preparation to Application. Coord. Chem. Rev. 2016, 311, 85–124. [Google Scholar] [CrossRef]
- Geng, K.; He, T.; Liu, R.; Dalapati, S.; Tan, K.T.; Li, Z.; Tao, S.; Gong, Y.; Jiang, Q.; Jiang, D. Covalent Organic Frameworks: Design, Synthesis, and Functions. Chem. Rev. 2020, 120, 8814–8933. [Google Scholar] [CrossRef]
- Sang, Q.; Hao, S.; Han, J.; Ding, Y. Dealloyed Nanoporous Materials for Electrochemical Energy Conversion and Storage. EnergyChem 2022, 4, 100069. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.M. Nanoporous Metals: Fabrication Strategies and Advanced Electrochemical Applications in Catalysis, Sensing and Energy Systems. Chem. Soc. Rev. 2012, 41, 7016–7031. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, H.C.; Chung, T.D. Electrochemical Analysis Based on Nanoporous Structures. Analyst 2012, 137, 3891. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Ju, H. Bioanalysis Based on Nanoporous Materials. TrAC Trends Anal. Chem. 2012, 39, 149–162. [Google Scholar] [CrossRef]
- Şeker, E.; Shih, W.C.; Stine, K.J. Nanoporous Metals by Alloy Corrosion: Bioanalytical and Biomedical Applications. MRS Bull. 2018, 43, 49–56. [Google Scholar] [CrossRef]
- Gonçalves, J.M.; Kumar, A.; da Silva, M.I.; Toma, H.E.; Martins, P.R.; Araki, K.; Bertotti, M.; Angnes, L. Nanoporous Gold-Based Materials for Electrochemical Energy Storage and Conversion. Energy Technol. 2021, 9, 2000927. [Google Scholar] [CrossRef]
- Seker, E.; Reed, M.L.; Begley, M.R. Nanoporous Gold: Fabrication, Characterization, and Applications. Materials 2009, 2, 2188–2215. [Google Scholar] [CrossRef]
- Collinson, M.M. Nanoporous Gold Electrodes and Their Applications in Analytical Chemistry. ISRN Anal. Chem. 2013, 2013, 1–21. [Google Scholar] [CrossRef]
- Wittstock, A.; Wichmann, A.; Bäumer, M. Nanoporous Gold as a Platform for a Building Block Catalyst. ACS Catal. 2012, 2, 2199–2215. [Google Scholar] [CrossRef]
- Wittstock, A.; Biener, J.; Erlebacher, J.; Bäumer, M. Nanoporous Gold: From an Ancient Technology to a High-Tech Material; The Royal Society of Chemistry: London, UK, 2012. [Google Scholar]
- Xiao, S.; Wang, S.; Wang, X.; Xu, P. Nanoporous Gold: A Review and Potentials in Biotechnological and Biomedical Applications. Nano Sel. 2021, 2, 1437–1458. [Google Scholar] [CrossRef]
- Ruffino, F.; Grimaldi, M.G. Nanoporous Gold-Based Sensing. Coatings 2020, 10, 899. [Google Scholar] [CrossRef]
- Xiao, X.; Si, P.; Magner, E. An Overview of Dealloyed Nanoporous Gold in Bioelectrochemistry. Bioelectrochemistry 2016, 109, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Fujita, T.; Chen, M.W. Biofunctionalized Nanoporous Gold for Electrochemical Biosensors. Electrochim. Acta 2012, 67, 1–5. [Google Scholar] [CrossRef]
- Qiu, H.; Xu, C.; Huang, X.; Ding, Y.; Qu, Y.; Gao, P. Immobilization of Laccase on Nanoporous Gold: Comparative Studies on the Immobilization Strategies and the Particle Size Effects. J. Phys. Chem. C 2009, 113, 2521–2525. [Google Scholar] [CrossRef]
- Ding, Y.; Erlebacher, J. Nanoporous Metals with Controlled Multimodal Pore Size Distribution. J. Am. Chem. Soc. 2003, 125, 7772–7773. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q. Bicontinuous Nanoporous Metals with Self-Organized Functionalities. Chem. Mater. 2022, 34, 10237–10248. [Google Scholar] [CrossRef]
- Fujita, T.; Guan, P.; McKenna, K.; Lang, X.; Hirata, A.; Zhang, L.; Tokunaga, T.; Arai, S.; Yamamoto, Y.; Tanaka, N.; et al. Atomic Origins of the High Catalytic Activity of Nanoporous Gold. Nat. Mater. 2012, 11, 775–780. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, M. Nanoporous Metals for Catalytic and Optical Applications. MRS Bull. 2009, 34, 569–576. [Google Scholar] [CrossRef]
- Ge, X.; Wang, R.; Liu, P.; Ding, Y. Platinum-Decorated Nanoporous Gold Leaf for Methanol Electrooxidation. Chem. Mater. 2007, 19, 5827–5829. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, P.; Ma, H.; Ding, Y. Nanostructured Porous Gold for Methanol Electro-Oxidation. J. Phys. Chem. C 2007, 111, 10382–10388. [Google Scholar] [CrossRef]
- Qiu, H.; Xu, C.; Huang, X.; Ding, Y.; Qu, Y.; Gao, P. Adsorption of Lacease on the Surface of Nanoporous Gold and the Direct Electron Transfer between Them. J. Phys. Chem. C 2008, 112, 14781–14785. [Google Scholar] [CrossRef]
- Stine, K.J. Enzyme Immobilization on Nanoporous Gold: A Review. Biochem. Insights 2017, 10, 117862641774860. [Google Scholar] [CrossRef] [PubMed]
- Patel, J.; Radhakrishnan, L.; Zhao, B.; Uppalapati, B.; Daniels, R.C.; Ward, K.R.; Collinson, M.M. Electrochemical Properties of Nanostructured Porous Gold Electrodes in Biofouling Solutions. Anal. Chem. 2013, 85, 11610–11618. [Google Scholar] [CrossRef]
- Daggumati, P.; Matharu, Z.; Wang, L.; Seker, E. Biofouling-Resilient Nanoporous Gold Electrodes for DNA Sensing. Anal. Chem. 2015, 87, 8618–8622. [Google Scholar] [CrossRef]
- Stephanie, R.; Kim, M.W.; Kim, S.H.; Kim, J.K.; Park, C.Y.; Park, T.J. Recent Advances of Bimetallic Nanomaterials and Its Nanocomposites for Biosensing Applications. TrAC Trends Anal. Chem. 2021, 135, 116159. [Google Scholar] [CrossRef]
- Lu, L. Nanoporous Noble Metal-Based Alloys: A Review on Synthesis and Applications to Electrocatalysis and Electrochemical Sensing. Microchim. Acta 2019, 186, 664. [Google Scholar] [CrossRef]
- Rajeev, R.; Datta, R.; Varghese, A.; Sudhakar, Y.N.N.; George, L. Recent Advances in Bimetallic Based Nanostructures: Synthesis and Electrochemical Sensing Applications. Microchem. J. 2021, 163, 105910. [Google Scholar] [CrossRef]
- Rick, J.; Tsai, M.C.; Hwang, B.J. Biosensors Incorporating Bimetallic Nanoparticles. Nanomaterials 2015, 6, 5. [Google Scholar] [CrossRef]
- Arora, N.; Thangavelu, K.; Karanikolos, G.N. Bimetallic Nanoparticles for Antimicrobial Applications. Front. Chem. 2020, 8, 412. [Google Scholar] [CrossRef]
- Kannan, P.; Maduraiveeran, G. Bimetallic Nanomaterials-Based Electrochemical Biosensor Platforms for Clinical Applications. Micromachines 2022, 13, 76. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y. Bimetallic Nanocrystals: Liquid-Phase Synthesis and Catalytic Applications. Adv. Mater. 2011, 23, 1044–1060. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, K.D.; Ruditskiy, A.; Peng, H.C.; Qin, D.; Xia, Y. Bimetallic Nanocrystals: Syntheses, Properties, and Applications. Chem. Rev. 2016, 116, 10414–10472. [Google Scholar] [CrossRef] [PubMed]
- Mandal, R.; Baranwal, A.; Srivastava, A.; Chandra, P. Evolving Trends in Bio/Chemical Sensor Fabrication Incorporating Bimetallic Nanoparticles. Biosens. Bioelectron. 2018, 117, 546–561. [Google Scholar] [CrossRef] [PubMed]
- Loza, K.; Heggen, M.; Epple, M. Synthesis, Structure, Properties, and Applications of Bimetallic Nanoparticles of Noble Metals. Adv. Funct. Mater. 2020, 30, 1909260. [Google Scholar] [CrossRef]
- Porter, N.S.; Wu, H.; Quan, Z.; Fang, J. Shape-Control and Electrocatalytic Activity-Enhancement of Pt-Based Bimetallic Nanocrystals. Acc. Chem. Res. 2013, 46, 1867–1877. [Google Scholar] [CrossRef]
- Jiang, J.; Zhou, X.L.; Lv, H.G.; Yu, H.Q.; Yu, Y. Bimetallic-Based Electrocatalysts for Oxygen Evolution Reaction. Adv. Funct. Mater. 2023, 33, 2212160. [Google Scholar] [CrossRef]
- Chavan, V.A.; Bhagat, D.S.; Gangawane, A.K.; Khawashi, H.P.; Thorat, B.R. Bimetallic Nanomaterials-Based Electroanalytical Methods for Detection of Pesticide Residues. Biointerface Res. Appl. Chem. 2023, 13, 468. [Google Scholar] [CrossRef]
- Cui, C.; Hu, X.; Wen, L. Recent Progress on Nanostructured Bimetallic Electrocatalysts for Water Splitting and Electroreduction of Carbon Dioxide. J. Semicond. 2020, 41, 091705. [Google Scholar] [CrossRef]
- Zaleska-Medynska, A.; Marchelek, M.; Diak, M.; Grabowska, E. Noble Metal-Based Bimetallic Nanoparticles: The Effect of the Structure on the Optical, Catalytic and Photocatalytic Properties. Adv. Colloid Interface Sci. 2016, 229, 80–107. [Google Scholar] [CrossRef]
- Dindar, C.K.; Erkmen, C.; Uslu, B. Electroanalytical Methods Based on Bimetallic Nanomaterials for Determination of Pesticides: Past, Present, and Future. Trends Environ. Anal. Chem. 2021, 32, e00145. [Google Scholar] [CrossRef]
- Ferrando, R.; Jellinek, J.; Johnston, R.L. Nanoalloys: From Theory to Applications of Alloy Clusters and Nanoparticles. Chem. Rev. 2008, 108, 846–910. [Google Scholar] [CrossRef] [PubMed]
- Noyhouzer, T.; Valdinger, I.; Mandler, D. Enhanced Potentiometry by Metallic Nanoparticles. Anal. Chem. 2013, 85, 8347–8353. [Google Scholar] [CrossRef]
- Islam, M.S.; Collinson, M.M. Improved Sensitivity and Selectivity for the Redox Potentiometric Measurement of Biological Redox Molecules Using Nafion-Coated Platinum Decorated Nanoporous Gold Electrodes. J. Electrochem. Soc. 2022, 169, 057503. [Google Scholar] [CrossRef]
- Vukovic, I.; ten Brinke, G.; Loos, K. Block Copolymer Template-Directed Synthesis of Well-Ordered Metallic Nanostructures. Polymer 2013, 54, 2591–2605. [Google Scholar] [CrossRef]
- Raman, N.K.; Anderson, M.T.; Brinker, C.J. Template-Based Approaches to the Preparation of Amorphous, Nanoporous Silicas. Chem. Mater. 1996, 8, 1682–1701. [Google Scholar] [CrossRef]
- Bartlett, P.N.; Birkin, P.R.; Ghanem, M.A. Electrochemical Deposition of Macroporous Platinum, Palladium and Cobalt Films Using Polystyrene Latex Sphere Templates. Chem. Commun. 2000, 1671–1672. [Google Scholar] [CrossRef]
- Bartlett, P.N.; Baumberg, J.J.; Birkin, P.R.; Ghanem, M.A.; Netti, M.C. Highly Ordered Macroporous Gold and Platinum Films Formed by Electrochemical Deposition through Templates Assembled from Submicron Diameter Monodisperse Polystyrene Spheres. Chem. Mater. 2002, 14, 2199–2208. [Google Scholar] [CrossRef]
- Zhao, B.; Collinson, M.M. Hierarchical Porous Gold Electrodes: Preparation, Characterization, and Electrochemical Behavior. J. Electroanal. Chem. 2012, 684, 53–59. [Google Scholar] [CrossRef]
- Yang, X.Y.; Chen, L.H.; Li, Y.; Rooke, J.C.; Sanchez, C.; Su, B.L. Hierarchically Porous Materials: Synthesis Strategies and Structure Design. Chem. Soc. Rev. 2017, 46, 481–558. [Google Scholar] [CrossRef]
- Pedireddy, S.; Lee, H.K.; Tjiu, W.W.; Phang, I.Y.; Tan, H.R.; Chua, S.Q.; Troadec, C.; Ling, X.Y. One-Step Synthesis of Zero-Dimensional Hollow Nanoporous Gold Nanoparticles with Enhanced Methanol Electrooxidation Performance. Nat. Commun. 2014, 5, 4947. [Google Scholar] [CrossRef]
- Zhao, B.; Collinson, M.M. Well-Defined Hierarchical Templates for Multimodal Porous Material Fabrication. Chem. Mater. 2010, 22, 4312–4319. [Google Scholar] [CrossRef]
- Hsueh, H.Y.; Chen, H.Y.; Hung, Y.C.; Ling, Y.C.; Gwo, S.; Ho, R.M. Well-Defined Multibranched Gold with Surface Plasmon Resonance in near-Infrared Region from Seeding Growth Approach Using Gyroid Block Copolymer Template. Adv. Mater. 2013, 25, 1780–1786. [Google Scholar] [CrossRef] [PubMed]
- Farghaly, A.A.; Collinson, M.M. Electroassisted Codeposition of Sol-Gel Derived Silica Nanocomposite Directs the Fabrication of Coral-like Nanostructured Porous Gold. Langmuir 2014, 30, 5276–5286. [Google Scholar] [CrossRef]
- Erlebacher, J.; Aziz, M.J.; Karma, A.; Dimitrov, N.; Sieradzki, K. Evolution of Nanoporosity in Dealloying. Nature 2001, 410, 450–453. [Google Scholar] [CrossRef] [PubMed]
- McCue, I.; Benn, E.; Gaskey, B.; Erlebacher, J. Dealloying and Dealloyed Materials. Annu. Rev. Mater. Res. 2016, 46, 263–286. [Google Scholar] [CrossRef]
- Xu, Y.; Ke, X.; Yu, C.; Liu, S.; Zhao, J.; Cui, G.; Higgins, D.; Chen, Z.; Li, Q.; Wu, G. A Strategy for Fabricating Nanoporous Gold Films through Chemical Dealloying of Electrochemically Deposited Au-Sn Alloys. Nanotechnology 2014, 25, 445602. [Google Scholar] [CrossRef]
- Chen, S.; Chu, Y.; Zheng, J.; Li, Z. Study on the Two Dealloying Modes in the Electrooxidation of Au-Sn Alloys by in Situ Raman Spectroscopy. Electrochim. Acta 2009, 54, 1102–1108. [Google Scholar] [CrossRef]
- Jia, F.; Yu, C.; Ai, Z.; Zhang, L. Fabrication of Nanoporous Gold Film Electrodes with Ultrahigh Surface Area and Electrochemical Activity. Chem. Mater. 2007, 19, 3648–3653. [Google Scholar] [CrossRef]
- Huang, J.F.; Sun, I.W. Fabrication and Surface Functionalization of Nanoporous Gold by Electrochemical Alloying/Dealloying of Au-Zn in an Ionic Liquid, and the Self-Assembly of L-Cysteine Monolayers. Adv. Funct. Mater. 2005, 15, 989–994. [Google Scholar] [CrossRef]
- Xue, Y.; Scaglione, F.; Paschalidou, E.M.; Rizzi, P.; Battezzati, L. Excellent Surface Enhanced Raman Scattering Obtained with Nanoporous Gold Fabricated by Chemical De-Alloying. Chem. Phys. Lett. 2016, 665, 6–9. [Google Scholar] [CrossRef]
- Yu, J.; Ding, Y.; Xu, C.; Inoue, A.; Sakurai, T.; Chen, M. Nanoporous Metals by Dealloying Multicomponent Metallic Glasses. Chem. Mater. 2008, 20, 4548–4550. [Google Scholar] [CrossRef]
- Cheng, C.; Lührs, L. Robust Metallic Actuators Based on Nanoporous Gold Rapidly Dealloyed from Gold–Nickel Precursors. Adv. Funct. Mater. 2021, 31, 2107241. [Google Scholar] [CrossRef]
- Zhong, Y.; Markmann, J.; Jin, H.J.; Ivanisenko, Y.; Kurmanaeva, L.; Weissmüller, J. Crack Mitigation during Dealloying of Au25Cu75. Adv. Eng. Mater. 2014, 16, 389–398. [Google Scholar] [CrossRef]
- Raj, D.; Scaglione, F.; Fiore, G.; Rizzi, P. Cost-Effective Nanoporous Gold Obtained by Dealloying Metastable Precursor, Au33Fe67, Reveals Excellent Methanol Electro-Oxidation Performance. Coatings 2022, 12, 831. [Google Scholar] [CrossRef]
- Zhonghua, Z.; Yan, W.; Zhen, Q.; Jikui, L.; Xiufang, B. Nanoporous Gold Ribbons with Bimodal Channel Size Distributions by Chemical Dealloying of Al-Au Alloys. J. Phys. Chem. C 2009, 113, 1308–1314. [Google Scholar] [CrossRef]
- Ding, Y.; Kim, Y.-J.; Erlebacher, J. Nanoporous Gold Leaf: “Ancient Technology”. Adv. Mater. 2004, 16, 1897–1900. [Google Scholar] [CrossRef]
- Bozzini, B.; De Gaudenzi, G.P.; Mele, C. A SERS Investigation of the Electrodeposition of Ag-Au Alloys from Free-Cyanide Solutions. J. Electroanal. Chem. 2004, 563, 133–143. [Google Scholar] [CrossRef]
- Kim, M.; Ha, W.J.; Anh, J.W.; Kim, H.S.; Park, S.W.; Lee, D. Fabrication of Nanoporous Gold Thin Films on Silicon Substrate by Multilayer Deposition of Au and Ag. J. Alloys Compd. 2009, 484, 28–32. [Google Scholar] [CrossRef]
- Islam, M.S.; Branigan, A.J.; Ullah, B.; Freeman, C.J.; Collinson, M.M. The Measurement of Mixed Potentials Using Platinum Decorated Nanoporous Gold Electrodes. J. Electrochem. Soc. 2022, 169, 016503. [Google Scholar] [CrossRef]
- Erlebacher, J. An Atomistic Description of Dealloying Porosity Evolution, the Critical Potential, and Rate-Limiting Behavior. J. Electrochem. Soc. 2004, 151, C614–C626. [Google Scholar] [CrossRef]
- Silva Olaya, A.R.; Kühling, F.; Mahr, C.; Zandersons, B.; Rosenauer, A.; Weissmüller, J.; Wittstock, G. Promoting Effect of the Residual Silver on the Electrocatalytic Oxidation of Methanol and Its Intermediates on Nanoporous Gold. ACS Catal. 2022, 12, 4415–4429. [Google Scholar] [CrossRef]
- Wittstock, A.; Neumann, B.; Schaefer, A.; Dumbuya, K.; Kübel, C.; Biener, M.M.; Zielasek, V.; Steinrüek, H.P.; Gottfried, J.M.; Biener, J.; et al. Nanoporous Au: An Unsupported Pure Gold Catalyst? J. Phys. Chem. C 2009, 113, 5593–5600. [Google Scholar] [CrossRef]
- Silva Olaya, A.R.; Zandersons, B.; Wittstock, G. Effect of the Residual Silver and Adsorbed Lead Anions towards the Electrocatalytic Methanol Oxidation on Nanoporous Gold in Alkaline Media. Electrochim. Acta 2021, 383, 138348. [Google Scholar] [CrossRef]
- Kwon, H.; Barad, H.N.; Silva Olaya, A.R.; Alarcón-Correa, M.; Hahn, K.; Richter, G.; Wittstock, G.; Fischer, P. Dry Synthesis of Pure and Ultrathin Nanoporous Metallic Films. ACS Appl. Mater. Interfaces 2023, 15, 5620–5627. [Google Scholar] [CrossRef] [PubMed]
- Sieradzki, K.; Dimitrov, N.; Movrin, D.; McCall, C.; Vasiljevic, N.; Erlebacher, J. The Dealloying Critical Potential. J. Electrochem. Soc. 2002, 149, B370. [Google Scholar] [CrossRef]
- Xiao, C.; Liu, Y.L.; Xu, J.Q.; Lv, S.W.; Guo, S.; Huang, W.H. Real-Time Monitoring of H2O2 Release from Single Cells Using Nanoporous Gold Microelectrodes Decorated with Platinum Nanoparticles. Analyst 2015, 140, 3753–3758. [Google Scholar] [CrossRef]
- Li, W.; Qi, H.; Wang, B.; Wang, Q.; Wei, S.; Zhang, X.; Wang, Y.; Zhang, L.; Cui, X. Ultrathin NiCo2O4 Nanowalls Supported on a 3D Nanoporous Gold Coated Needle for Non-Enzymatic Amperometric Sensing of Glucose. Microchim. Acta 2018, 185, 124. [Google Scholar] [CrossRef]
- Liu, Z.; Puumala, E.; Chen, A. Sensitive Electrochemical Detection of Hg(II) via a FeOOH Modified Nanoporous Gold Microelectrode. Sens. Actuators B Chem. 2019, 287, 517–525. [Google Scholar] [CrossRef]
- Ikegami, M.; Hirano, Y.; Mie, Y.; Komatsu, Y. Adsorptive Stripping Voltammetry for the Determination of Dissolved Oxygen Using a Mesoporous Pt Microelectrode. J. Electrochem. Soc. 2019, 166, B542–B546. [Google Scholar] [CrossRef]
- Tavakkoli, N.; Soltani, N.; Sadeghi, M.; Salavati, H. Electrochemical Determination of Methimazole Using Nanoporous Gold Film Electrode Modified with MoO2 Thin Film. Microchem. J. 2019, 150, 104153. [Google Scholar] [CrossRef]
- Tavakkoli, N.; Soltani, N.; Khorshidi, E. Fabrication of Ru-Pd Bimetallic Monolayer on Nanoporous Gold Film Electrode with Excellent Electrocatalytic Performance towards Captopril Oxidation. Electrochim. Acta 2015, 164, 1–11. [Google Scholar] [CrossRef]
- Tavakkoli, N.; Nasrollahi, S. Non-Enzymatic Glucose Sensor Based on Palladium Coated Nanoporous Gold Film Electrode. Aust. J. Chem. 2013, 66, 1097–1104. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, W.; Chen, X.; Li, Z. Facile Fabrication of Nanoporous Gold Film Electrodes. Electrochem. Commun. 2008, 10, 810–813. [Google Scholar] [CrossRef]
- Sadeghi, M.; Shabani-Nooshabadi, M.; Ansarinejad, H. A Nanoporous Gold Film Sensor Modified with Polypyrrole/CuO Nanocomposite for Electrochemical Determination of Piroxicam and Tramadole. Environ. Res. 2023, 216, 114633. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.; Liu, A.; Chen, X.; Wang, K.; Lian, Z.; Liu, Q.; Chen, Y.; Du, M.; Lin, X. Electrochemical Biosensor Based on Nanoporous Gold Electrode for Detection of PML/RARα Fusion Gene. Biosens. Bioelectron. 2011, 26, 3812–3817. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, B.; Damiri, S. Fabrication of a Nanostructure Thin Film on the Gold Electrode Using Continuous Pulsed-Potential Technique and Its Application for the Electrocatalytic Determination of Metronidazole. Electrochim. Acta 2010, 55, 1801–1808. [Google Scholar] [CrossRef]
- Lee, E.; Sung, M.; Wang, Y.; Kim, J. Atomic Layer Electrodeposition of Pt on Nanoporous Au and Its Application in PH Sensing. Electroanalysis 2018, 30, 2028–2034. [Google Scholar] [CrossRef]
- Qiu, H.; Huang, X. Effects of Pt Decoration on the Electrocatalytic Activity of Nanoporous Gold Electrode toward Glucose and Its Potential Application for Constructing a Nonenzymatic Glucose Sensor. J. Electroanal. Chem. 2010, 643, 39–45. [Google Scholar] [CrossRef]
- Xia, Y.; Huang, W.; Zheng, J.; Niu, Z.; Li, Z. Nonenzymatic Amperometric Response of Glucose on a Nanoporous Gold Film Electrode Fabricated by a Rapid and Simple Electrochemical Method. Biosens. Bioelectron. 2011, 26, 3555–3561. [Google Scholar] [CrossRef]
- Huang, W.; Wang, M.; Zheng, J.; Li, Z. Facile Fabrication of Multifunctional Three-Dimensional Hierarchical Porous Gold Films via Surface Rebuilding. J. Phys. Chem. C 2009, 113, 1800–1805. [Google Scholar] [CrossRef]
- Snyder, J.; Asanithi, P.; Dalton, A.B.; Erlebacher, J. Stabilized Nanoporous Metals by Dealloying Ternary Alloy Precursors. Adv. Mater. 2008, 20, 4883–4886. [Google Scholar] [CrossRef]
- Liu, M.; Weissmüller, J. Phase Decomposition in Nanoporous Au-Pt. Acta Mater. 2022, 241, 118419. [Google Scholar] [CrossRef]
- Jin, H.J.; Wang, X.L.; Parida, S.; Wang, K.; Seo, M.; Weissmüller, J. Nanoporous Au-Pt Alloys as Large Strain Electrochemical Actuators. Nano Lett. 2010, 10, 187–194. [Google Scholar] [CrossRef]
- Vega, A.A.; Newman, R.C. Nanoporous Metals Fabricated through Electrochemical Dealloying of Ag-Au-Pt with Systematic Variation of Au:Pt Ratio. J. Electrochem. Soc. 2014, 161, C1–C10. [Google Scholar] [CrossRef]
- Gao, P.; Zhu, Z.; Ye, X.; Wu, Y.; Jin, H.; Volinsky, A.A.; Qiao, L.; Su, Y. Defects Evolution in Nanoporous Au(Pt) during Dealloying. Scr. Mater. 2016, 113, 68–70. [Google Scholar] [CrossRef]
- Xu, C.; Wang, R.; Chen, M.; Zhang, Y.; Ding, Y. Dealloying to Nanoporous Au/Pt Alloys and Their Structure Sensitive Electrocatalytic Properties. Phys. Chem. Chem. Phys. 2010, 12, 239–246. [Google Scholar] [CrossRef]
- Xie, Y.; Dimitrov, N. Ultralow Pt Loading Nanoporous Au-Cu-Pt Thin Film as Highly Active and Durable Catalyst for Formic Acid Oxidation. Appl. Catal. B 2020, 263, 118366. [Google Scholar] [CrossRef]
- Li, D.; Meng, F.; Wang, H.; Jiang, X.; Zhu, Y. Nanoporous AuPt Alloy with Low Pt Content: A Remarkable Electrocatalyst with Enhanced Activity towards Formic Acid Electro-Oxidation. Electrochim. Acta 2016, 190, 852–861. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, C.; Wang, X.; Ji, H.; Zhao, C.; Wang, Y.; Zhang, Z. Fabrication of Bi-Modal Nanoporous Bimetallic Pt–Au Alloy with Excellent Electrocatalytic Performance towards Formic Acid Oxidation. Green Chem. 2011, 13, 1914–1922. [Google Scholar] [CrossRef]
- Chiu, H.Y.; Liu, Y.C.; Hsieh, Y.T.; Sun, I.W. Some Aspects on the One-Pot Fabrication of Nanoporous Pd-Au Surface Films by Electrochemical Alloying/Dealloying of (Pd-Au)-Zn from a Chlorozincate Ionic Liquid. ACS Omega 2017, 2, 4911–4919. [Google Scholar] [CrossRef]
- Wang, X.; Sun, J.; Zhang, C.; Kou, T.; Zhang, Z. On the Microstructure, Chemical Composition, and Porosity Evolution of Nanoporous Alloy through Successive Dealloying of Ternary Al-Pd-Au Precursor. J. Phys. Chem. C 2012, 116, 13271–13280. [Google Scholar] [CrossRef]
- Xiao, S.; Xiao, F.; Hu, Y.; Yuan, S.; Wang, S.; Qian, L.; Liu, Y. Hierarchical Nanoporous Gold-Platinum with Heterogeneous Interfaces for Methanol Electrooxidation. Sci. Rep. 2014, 4, 4370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ma, H.; Zhang, D.; Liu, P.; Tian, F.; Ding, Y. Electrocatalytic Activity of Bimetallic Platinum-Gold Catalysts Fabricated Based on Nanoporous Gold. Phys. Chem. Chem. Phys. 2008, 10, 3250–3255. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Wang, R.; Cui, S.; Tian, F.; Xu, L.; Ding, Y. Structure Dependent Electrooxidation of Small Organic Molecules on Pt-Decorated Nanoporous Gold Membrane Catalysts. Electrochem. Commun. 2008, 10, 1494–1497. [Google Scholar] [CrossRef]
- Xue, Y.; Scaglione, F.; Rizzi, P.; Battezzati, L.; Denis, P.; Fecht, H.J. Electrodeposited Platinum on De-Alloyed Nanoporous Gold with Enhanced Electro-Catalytic Performance. Appl. Surf. Sci. 2019, 476, 412–417. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, B. Recent Advances in Porous Pt-Based Nanostructures: Synthesis and Electrochemical Applications. Chem. Soc. Rev. 2014, 43, 2439–2450. [Google Scholar] [CrossRef]
- Ding, Y.; Chen, M.; Erlebacher, J. Metallic Mesoporous Nanocomposites for Electrocatalysis. J. Am. Chem. Soc. 2004, 126, 6876–6877. [Google Scholar] [CrossRef]
- Zhong, Y.; Liu, M.M.; Chen, Y.; Yang, Y.J.; Wu, L.N.; Bai, F.; Lei, Y.; Gao, F.; Liu, A.L. A High-Performance Amperometric Sensor Based on a Monodisperse Pt–Au Bimetallic Nanoporous Electrode for Determination of Hydrogen Peroxide Released from Living Cells. Microchim. Acta 2020, 187, 3250–3255. [Google Scholar] [CrossRef]
- Freeman, C.J.; Ullah, B.; Shafiul Islam, M.D.; Collinson, M.M. Potentiometric Biosensing of Ascorbic Acid, Uric Acid, and Cysteine in Microliter Volumes Using Miniaturized Nanoporous Gold Electrodes. Biosensors 2021, 11, 10. [Google Scholar] [CrossRef]
- Jiang, J.Z.; Ren, L.Q.; Huang, Y.P.; Li, X.D.; Wu, S.H.; Sun, J.J. 3D Nanoporous Gold-Supported Pt Nanoparticles as Highly Accelerating Catalytic Au-Pt Micromotors. Adv. Mater. Interfaces 2018, 5, 1701689. [Google Scholar] [CrossRef]
- Xiao, X.; Wang, M.; Li, H.; Pan, Y.; Si, P. Non-Enzymatic Glucose Sensors Based on Controllable Nanoporous Gold/Copper Oxide Nanohybrids. Talanta 2014, 125, 366–371. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.M.; Wang, P.S.; Zhou, C.H.; Xia, Y.; Huang, W.; Li, Z. An Ultrasensitive Non-Enzymatic Amperometric Glucose Sensor Based on a Cu-Coated Nanoporous Gold Film Involving Co-Mediating. Sens. Actuators B Chem. 2014, 203, 388–395. [Google Scholar] [CrossRef]
- Hernández-Saravia, L.P.; Martinez, T.; Llanos, J.; Bertotti, M. A Cu-NPG/SPE Sensor for Non-Enzymatic and Non-Invasive Electrochemical Glucose Detection. Microchem. J. 2021, 160, 105629. [Google Scholar] [CrossRef]
- Yi, X.; Wu, Y.; Tan, G.; Yu, P.; Zhou, L.; Zhou, Z.; Chen, J.; Wang, Z.; Pang, J.; Ning, C. Palladium Nanoparticles Entrapped in a Self-Supporting Nanoporous Gold Wire as Sensitive Dopamine Biosensor. Sci. Rep. 2017, 7, 7941. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.M.; Zhou, C.H.; Xia, Y.; Huang, W.; Li, Z. Ultrasensitive Nonenzymatic Sensing of Glucose on Ni(OH)2-Coated Nanoporous Gold Film with Two Pairs of Electron Mediators. Electrochim. Acta 2014, 142, 351–358. [Google Scholar] [CrossRef]
- Zhou, C.; Tang, X.; Xia, Y.; Li, Z. Electrochemical Fabrication of Cobalt Oxides/Nanoporous Gold Composite Electrode and Its Nonenzymatic Glucose Sensing Performance. Electroanalysis 2016, 28, 2149–2157. [Google Scholar] [CrossRef]
- Pei, Y.; Hu, M.; Tang, X.; Huang, W.; Li, Z.; Chen, S.; Xia, Y. Ultrafast One-Pot Anodic Preparation of Co3O4/Nanoporous Gold Composite Electrode as an Efficient Nonenzymatic Amperometric Sensor for Glucose and Hydrogen Peroxide. Anal. Chim. Acta 2019, 1059, 49–58. [Google Scholar] [CrossRef]
- Kim, Y.H.; Kim, G.H.; Kim, M.S.; Jung, S.D. Iridium Oxide-Electrodeposited Nanoporous Gold Multielectrode Array with Enhanced Stimulus Efficacy. Nano Lett. 2016, 16, 7163–7168. [Google Scholar] [CrossRef]
- Lang, X.Y.; Fu, H.Y.; Hou, C.; Han, G.F.; Yang, P.; Liu, Y.B.; Jiang, Q. Nanoporous Gold Supported Cobalt Oxide Microelectrodes as High-Performance Electrochemical Biosensors. Nat. Commun. 2013, 4, 2169. [Google Scholar] [CrossRef]
- Li, G.; Sun, P.; Wu, F.; Zhao, J.; Han, D.; Cui, G. Significant Enhancement in the Electrochemical Determination of 4-Aminophenol from Nanoporous Gold by Decorating with a Pd@CeO2 Composite Film. New J. Chem. 2020, 44, 3087–3096. [Google Scholar] [CrossRef]
- Guo, H.; Yin, H.; Yan, X.; Shi, S.; Yu, Q.; Cao, Z.; Li, J. Pt-Bi Decorated Nanoporous Gold for High Performance Direct Glucose Fuel Cell. Sci. Rep. 2016, 6, 39162. [Google Scholar] [CrossRef] [PubMed]
- Farghaly, A.A.; Khan, R.K.; Collinson, M.M. Biofouling-Resistant Platinum Bimetallic Alloys. ACS Appl. Mater. Interfaces 2018, 10, 21103–21112. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cao, L.; Huang, W.; Li, Z. Preparation of AuPt Alloy Foam Films and Their Superior Electrocatalytic Activity for the Oxidation of Formic Acid. ACS Appl. Mater. Interfaces 2011, 3, 3552–3558. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, N. Recent Advances in the Growth of Metals, Alloys, and Multilayers by Surface Limited Redox Replacement (SLRR) Based Approaches. Electrochim. Acta 2016, 209, 599–622. [Google Scholar] [CrossRef]
- Herrero, E.; Buller, L.J.; Abruña, H.D. Underpotential Deposition at Single Crystal Surfaces of Au, Pt, Ag and Other Materials. Chem. Rev. 2001, 101, 1897–1930. [Google Scholar] [CrossRef]
- McCurry, D.A.; Kamundi, M.; Fayette, M.; Wafula, F.; Dimitrov, N. All Electrochemical Fabrication of a Platinized Nanoporous Au Thin-Film Catalyst. ACS Appl. Mater. Interfaces 2011, 3, 4459–4468. [Google Scholar] [CrossRef]
- Xia, J.; Ambrozik, S.; Crane, C.C.; Chen, J.; Dimitrov, N. Impact of Structure and Composition on the Dealloying of CuxAu(1-x) Bulk and Nanoscale Alloys. J. Phys. Chem. C 2016, 120, 2299–2308. [Google Scholar] [CrossRef]
- Tavakkoli, N.; Soltani, N.; Khorshidi, E. Preparation of Ru-Pt Bimetallic Monolayer on Nanoporous Gold Film Electrode and Its Application as an Ultrasensitive Sensor for Determination of Methionine. RSC Adv. 2017, 7, 21827–21836. [Google Scholar] [CrossRef]
- Huang, J.F. Facile Preparation of an Ultrathin Nickel Film Coated Nanoporous Gold Electrode with the Unique Catalytic Activity to Oxidation of Glucose. Chem. Commun. 2009, 1270–1272. [Google Scholar] [CrossRef]
- Liu, P.; Ge, X.; Wang, R.; Ma, H.; Ding, Y. Facile Fabrication of Ultrathin Pt Overlayers onto Nanoporous Metal Membranes via Repeated Cu UPD and in Situ Redox Replacement Reaction. Langmuir 2009, 25, 561–567. [Google Scholar] [CrossRef]
- Yoo, S.H.; Park, S. Platinum-Coated, Nanoporous Gold Nanorod Arrays: Synthesis and Characterization. Adv. Mater. 2007, 19, 1612–1615. [Google Scholar] [CrossRef]
- Kiani, A.; Fard, E.N. Fabrication of Palladium Coated Nanoporous Gold Film Electrode via Underpotential Deposition and Spontaneous Metal Replacement: A Low Palladium Loading Electrode with Electrocatalytic Activity. Electrochim. Acta 2009, 54, 7254–7259. [Google Scholar] [CrossRef]
- Xia, J.; Achari, I.; Ambrozik, S.; Dimitrov, N. Synthesis, Characterization, and Testing of Pt-NPG Catalysts Developed by de-Alloying of Electrodeposited CuxAu(1−x) Thin Films. Mater. Res. Bull. 2017, 85, 1–9. [Google Scholar] [CrossRef]
- Fayette, M.; Liu, Y.; Bertrand, D.; Nutariya, J.; Vasiljevic, N.; Dimitrov, N. From Au to Pt via Surface Limited Redox Replacement of Pb UPD in One-Cell Configuration. Langmuir 2011, 27, 5650–5658. [Google Scholar] [CrossRef]
- Cherevko, S.; Kulyk, N.; Chung, C.H. Nanoporous Pt@AuXCu100-x by Hydrogen Evolution Assisted Electrodeposition of AuXCu100-x and Galvanic Replacement of Cu with Pt: Electrocatalytic Properties. Langmuir 2012, 28, 3306–3315. [Google Scholar] [CrossRef] [PubMed]
- Rouya, E.; Cattarin, S.; Reed, M.L.; Kelly, R.G.; Zangari, G. Electrochemical Characterization of the Surface Area of Nanoporous Gold Films. J. Electrochem. Soc. 2012, 159, K97–K102. [Google Scholar] [CrossRef]
- Lee, B.; Yoon, S.; Lee, J.W.; Kim, Y.; Chang, J.; Yun, J.; Ro, J.C.; Lee, J.-S.; Lee, J.H. Statistical Characterization of the Morphologies of Nanoparticles through Machine Learning Based Electron Microscopy Image Analysis. ACS Nano 2020, 14, 17125–17133. [Google Scholar] [CrossRef]
- Chen, A.Y.; Shi, S.S.; Wang, J.W.; Liu, F.; Wang, F.; Wang, Y.; Ruan, H.H.; Xie, X.F. Microstructure and Electrocatalytic Performance of Nanoporous Gold Foils Decorated by TiO2 Coatings. Surf. Coat. Technol. 2016, 286, 113–118. [Google Scholar] [CrossRef]
- Zhuang, Z.; Chen, Y.; Chen, K.; Liu, Z.; Guo, Z.; Huang, X. In-Situ Synthesis of ZnO onto Nanoporous Gold Microelectrode for the Electrochemical Sensing of Arsenic(III) in near-Neutral Conditions. Sens. Actuators B Chem. 2023, 378, 133184. [Google Scholar] [CrossRef]
- Trasatti, S.; Petrii, O.A. Real Surface Area Measurements in Electrochemistry. J. Electroanal. Chem. 1992, 327, 353–376. [Google Scholar] [CrossRef]
- Tan, Y.H.; Davis, J.A.; Fujikawa, K.; Ganesh, N.V.; Demchenko, A.V.; Stine, K.J. Surface Area and Pore Size Characteristics of Nanoporous Gold Subjected to Thermal, Mechanical, or Surface Modification Studied Using Gas Adsorption Isotherms, Cyclic Voltammetry, Thermogravimetric Analysis, and Scanning Electron Microscopy. J. Mater. Chem. 2012, 22, 6733–6745. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Park, J.Y.; Kim, Y.; Ko, J.W. Amperometric Sensing of Hydrogen Peroxide via Highly Roughened Macroporous Gold-/Platinum Nanoparticles Electrode. Curr. Appl. Phys. 2011, 11, 211–216. [Google Scholar] [CrossRef]
- Daggumati, P.; Matharu, Z.; Seker, E. Effect of Nanoporous Gold Thin Film Morphology on Electrochemical DNA Sensing. Anal. Chem. 2015, 87, 8149–8156. [Google Scholar] [CrossRef] [PubMed]
- Manikandan, V.S.; Liu, Z.; Chen, A. Simultaneous Detection of Hydrazine, Sulfite, and Nitrite Based on a Nanoporous Gold Microelectrode. J. Electroanal. Chem. 2018, 819, 524–532. [Google Scholar] [CrossRef]
- Du, Y.; Xu, J.J.; Chen, H.Y. Ultrathin Platinum Film Covered High-Surface-Area Nanoporous Gold for Methanol Electro-Oxidation. Electrochem. Commun. 2009, 11, 1717–1720. [Google Scholar] [CrossRef]
- Do, U.P.; Seland, F.; Johannessen, E.A. The Real Area of Nanoporous Catalytic Surfaces of Gold and Palladium in Aqueous Solutions. J. Electrochem. Soc. 2018, 165, H219. [Google Scholar] [CrossRef]
- Lukaszewski, M.; Soszko, M.; Czerwiński, A. Electrochemical Methods of Real Surface Area Determination of Noble Metal Electrodes—An Overview. Int. J. Electrochem. Sci. 2016, 11, 4442–4469. [Google Scholar] [CrossRef]
- Liu, Y.; Bliznakov, S.; Dimitrov, N. Comprehensive Study of the Application of a Pb Underpotential Deposition-Assisted Method for Surface Area Measurement of Metallic Nanoporous Materials. J. Phys. Chem. C 2009, 113, 12362–12372. [Google Scholar] [CrossRef]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical Biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef]
- Wang, J. Electrochemical Glucose Biosensors. Chem. Rev. 2008, 108, 814–825. [Google Scholar] [CrossRef]
- Chen, R.J.; Choi, H.C.; Bangsaruntip, S.; Yenilmez, E.; Tang, X.; Wang, Q.; Chang, Y.L.; Dai, H. An Investigation of the Mechanisms of Electronic Sensing of Protein Adsorption on Carbon Nanotube Devices. J. Am. Chem. Soc. 2004, 126, 1563–1568. [Google Scholar] [CrossRef] [PubMed]
- Im, H.; Huang, X.J.; Gu, B.; Choi, Y.K. A Dielectric-Modulated Field-Effect Transistor for Biosensing. Nat. Nanotechnol. 2007, 2, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Lu, Y. Using Personal Glucose Meters and Functional DNA Sensors to Quantify a Variety of Analytical Targets. Nat. Chem. 2011, 3, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical Sensors and Biosensors Based on Nanomaterials and Nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef]
- Yang, X.; Qiu, P.; Yang, J.; Fan, Y.; Wang, L.; Jiang, W.; Cheng, X.; Deng, Y.; Luo, W. Mesoporous Materials–Based Electrochemical Biosensors from Enzymatic to Nonenzymatic. Small 2021, 17, 1904022. [Google Scholar] [CrossRef]
- Chen, W.; Cai, S.; Ren, Q.Q.; Wen, W.; Zhao, Y.D. Recent Advances in Electrochemical Sensing for Hydrogen Peroxide: A Review. Analyst 2012, 137, 49–58. [Google Scholar] [CrossRef]
- Thatikayala, D.; Ponnamma, D.; Sadasivuni, K.K.; Cabibihan, J.J.; Al-Ali, A.K.; Malik, R.A.; Min, B. Progress of Advanced Nanomaterials in the Non-Enzymatic Electrochemical Sensing of Glucose and H2O2. Biosensors 2020, 10, 151. [Google Scholar] [CrossRef]
- Sies, H. Hydrogen Peroxide as a Central Redox Signaling Molecule in Physiological Oxidative Stress: Oxidative Eustress. Redox Biol. 2017, 11, 613–619. [Google Scholar] [CrossRef]
- Dhara, K.; Mahapatra, D.R. Recent Advances in Electrochemical Nonenzymatic Hydrogen Peroxide Sensors Based on Nanomaterials: A Review. J. Mater. Sci. 2019, 54, 12319–12357. [Google Scholar] [CrossRef]
- Meier, J.; Hofferber, E.M.; Stapleton, J.A.; Iverson, N.M.; Hofferber, E.; Stapleton, J.A.; Iverson, N.M. Hydrogen Peroxide Sensors for Biomedical Applications. Chemosensors 2019, 7, 64. [Google Scholar] [CrossRef]
- Patel, V.; Kruse, P.; Selvaganapathy, P.R. Solid State Sensors for Hydrogen Peroxide Detection. Biosensors 2020, 11, 9. [Google Scholar] [CrossRef]
- Sukeri, A.; Lima, A.S.; Bertotti, M. Development of Non-Enzymatic and Highly Selective Hydrogen Peroxide Sensor Based on Nanoporous Gold Prepared by a Simple Unusual Electrochemical Approach. Microchem. J. 2017, 133, 149–154. [Google Scholar] [CrossRef]
- Khan, R.K.; Silva, T.A.; Fatibello-Filho, O.; Collinson, M.M.; Farghaly, A.A. Nanoporous Pt(Au) Alloys for the Enhanced, Non-enzymatic Detection of Hydrogen Peroxide under Biofouling Conditions. Electroanalysis 2022, 34, 1893–1901. [Google Scholar] [CrossRef]
- Ke, X.; Li, Z.; Gan, L.; Zhao, J.; Cui, G.; Kellogg, W.; Matera, D.; Higgins, D.; Wu, G. Three-Dimensional Nanoporous Au Films as High-Efficiency Enzyme-Free Electrochemical Sensors. Electrochim. Acta 2015, 170, 337–342. [Google Scholar] [CrossRef]
- Ke, X.; Xu, Y.; Yu, C.; Zhao, J.; Cui, G.; Higgins, D.; Li, Q.; Wu, G. Nanoporous Gold on Three-Dimensional Nickel Foam: An Efficient Hybrid Electrode for Hydrogen Peroxide Electroreduction in Acid Media. J. Power Sources 2014, 269, 461–465. [Google Scholar] [CrossRef]
- Zhang, C.; Huang, B.; Qian, L.; Yuan, S.; Wang, S.; Chen, R. Electrochemical Biosensor Based on Nanoporous Au/CoO Core-Shell Material with Synergistic Catalysis. ChemPhysChem 2016, 17, 98–104. [Google Scholar] [CrossRef]
- Yin, G.; Xing, L.; Ma, X.J.; Wan, J. Non-Enzymatic Hydrogen Peroxide Sensor Based on a Nanoporous Gold Electrode Modified with Platinum Nanoparticles. Chem. Pap. 2014, 68, 435–441. [Google Scholar] [CrossRef]
- Si, P.; Huang, Y.; Wang, T.; Ma, J. Nanomaterials for Electrochemical Non-Enzymatic Glucose Biosensors. RSC Adv. 2013, 3, 3487–3502. [Google Scholar] [CrossRef]
- Chen, C.; Xie, Q.; Yang, D.; Xiao, H.; Fu, Y.; Tan, Y.; Yao, S. Recent Advances in Electrochemical Glucose Biosensors: A Review. RSC Adv. 2013, 3, 4473–4491. [Google Scholar] [CrossRef]
- Niu, X.H.; Shi, L.B.; Zhao, H.L.; Lan, M.B. Advanced Strategies for Improving the Analytical Performance of Pt-Based Nonenzymatic Electrochemical Glucose Sensors: A Minireview. Anal. Methods 2016, 8, 1755–1764. [Google Scholar] [CrossRef]
- Mohamad Nor, N.; Ridhuan, N.S.; Abdul Razak, K. Progress of Enzymatic and Non-Enzymatic Electrochemical Glucose Biosensor Based on Nanomaterial-Modified Electrode. Biosensors 2022, 12, 1136. [Google Scholar] [CrossRef]
- Zhao, A.; Zhang, Z.; Zhang, P.; Xiao, S.; Wang, L.; Dong, Y.; Yuan, H.; Li, P.; Sun, Y.; Jiang, X.; et al. 3D Nanoporous Gold Scaffold Supported on Graphene Paper: Freestanding and Flexible Electrode with High Loading of Ultrafine PtCo Alloy Nanoparticles for Electrochemical Glucose Sensing. Anal. Chim. Acta 2016, 938, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lv, S.; Wang, Y.; Zhang, L.; Cui, X. Nanoporous Gold Induced Vertically Standing 2D NiCo Bimetal-Organic Framework Nanosheets for Non-Enzymatic Glucose Biosensing. Sens. Actuators B Chem. 2019, 281, 652–658. [Google Scholar] [CrossRef]
- Pei, Y.; Hu, M.; Xia, Y.; Huang, W.; Li, Z.; Chen, S. Electrochemical Preparation of Pt Nanoparticles Modified Nanoporous Gold Electrode with Highly Rough Surface for Efficient Determination of Hydrazine. Sens. Actuators B Chem. 2020, 304, 127416. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.; Chen, H.; Chen, L.; Chen, W.; Yin, X.; Liu, A.; Lin, X.; Weng, S.; Zheng, Y. A Facile Approach for Fabrication of Three-Dimensional Platinum-Nanoporous Gold Film and Its Application for Sensitive Detection of MicroRNA-126 Combining with Catalytic Hairpin Assembly Reaction. J. Electroanal. Chem. 2021, 886, 115109. [Google Scholar] [CrossRef]
- Fog, A.; Buck, R.P. Electronic Semiconducting Oxides as PH Sensors. Sens. Actuators 1984, 5, 137–146. [Google Scholar] [CrossRef]
- Sadeghi, M.; Shabani-Nooshabadi, M. High Sensitive Titanium/Chitosan-Coated Nanoporous Gold Film Electrode for Electrochemical Determination of Acetaminophen in the Presence of Piroxicam. Prog. Org. Coat. 2021, 151, 106100. [Google Scholar] [CrossRef]
- Chen, C.; Yu, S.; Jiang, S.; Liu, J.; Wang, Z.; Ye, B.-C. A Novel and Sensitive Electrochemical Sensor Based on Nanoporous Gold for Determination of As(III). Microchim. Acta 2020, 187, 395. [Google Scholar] [CrossRef]
- Lin, Y.; Peng, Y.; Di, J. Electrochemical Detection of Hg(II) Ions Based on Nanoporous Gold Nanoparticles Modified Indium Tin Oxide Electrode. Sens. Actuators B Chem. 2015, 220, 1086–1090. [Google Scholar] [CrossRef]







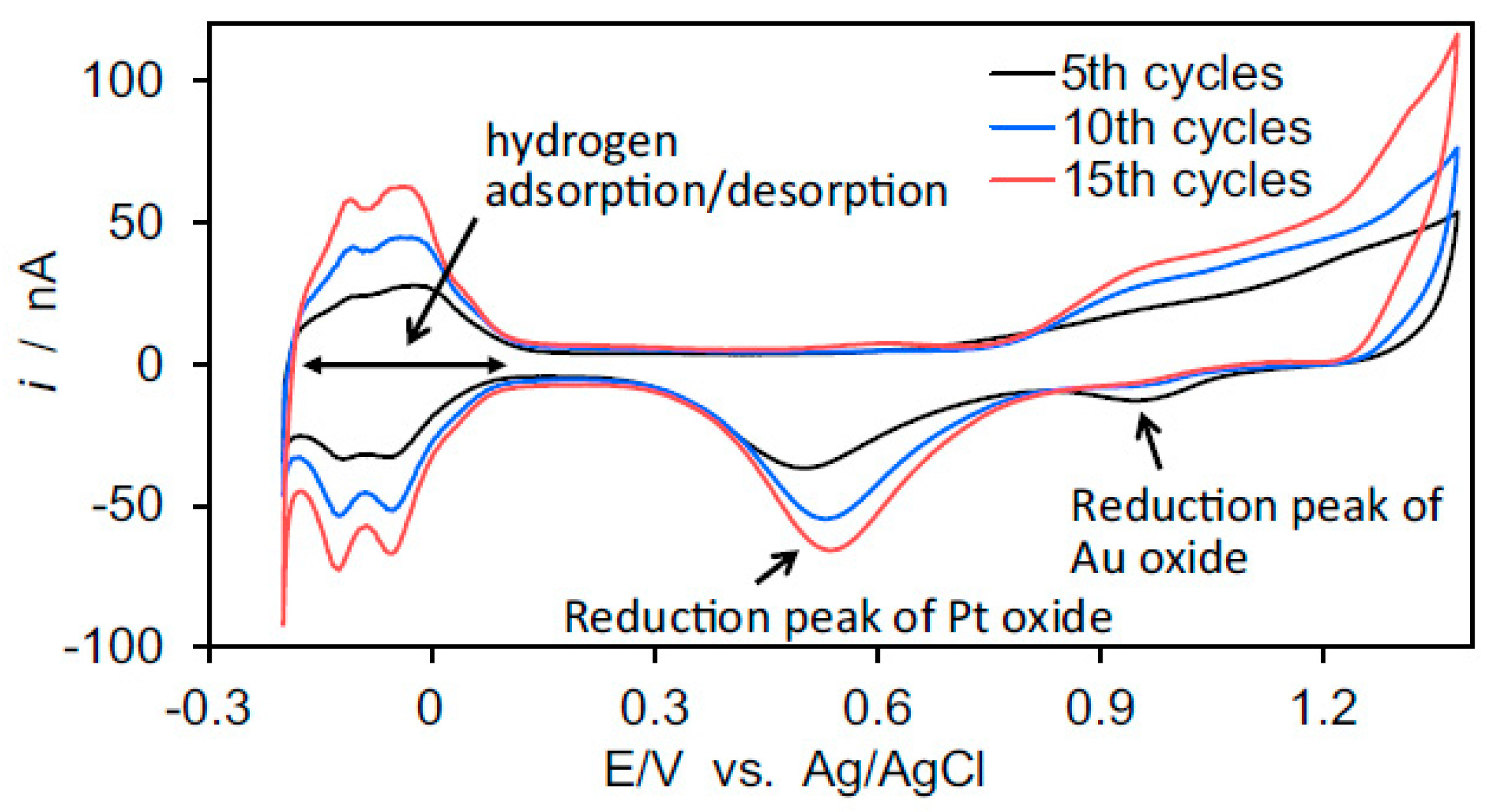
| Method | Requirements | Advantages | Concerns |
|---|---|---|---|
Templating
|
|
|
|
| Chemical dealloying a pre-formed alloy |
|
|
|
| Electrochemical dealloying of a pre-formed alloy |
|
|
|
| Electrochemical alloying–dealloying |
|
|
|
| Anodization–Roughening |
|
|
|
| Method | Requirements | Advantages | Concerns/Limitations |
|---|---|---|---|
| Ternary alloys |
|
|
|
| Immersion followed by reduction (chemical, electrochemical) |
|
|
|
| Electrodeposition–annealing |
|
|
|
| UPD-surface-limited redox replacement |
|
|
|
| Substrate | Roughness Factor | Method | References |
|---|---|---|---|
| Gold slide | 3–5 | Template (-Polystyrene) | [34,59] |
| Gold slide | 20.6 | Template (-Silica) | [64] |
| Steel mold | 88.6 | Template (-Alumina) | [153] |
| Gold slide–gold leaf | ~12–25 | Chemical dealloying | [34,80] |
| Gold slide–sputtered alloy | 2.4–9.3 | Chemical dealloying | [154] |
| Gold–glassy carbon; electrodeposited alloy | 4.2–6.5 | Electrochemical dealloying | [137] |
| Gold microwire | 27.7 | Electrochemically alloying–dealloying | [155] |
| Gold microwire | 86–115 | Electrochemically alloying–dealloying | [87] |
| Gold wire | 55–560 | Electrochemically alloying–dealloying | [69] |
| Gold wire | 18–213 | Electrochemically alloying–dealloying | [70] |
| Gold rod | 280–1020 | Anodization, buffer | [98] |
| Gold CD-R | 17.8 | Anodization | [93] |
| Gold disk | 43.7 | Anodization | [156] |
| Gold disk | 34 | Anodization, square-wave pulse, NaOH | [99] |
| Electrode | Linear Range (mM) | LOD (µM) | Sensitivity, μAcm−2mM−1 | Interference Study | Biofouling Test | Ref. |
|---|---|---|---|---|---|---|
| NP-Pt(Au) | 0.0709 to 1.25 | 39.3 | 148 | Yes | Yes | [174] |
| NPG/PtNPs | 0.001 to 0.005 | 0.0003 | Yes | No | [87] | |
| Pt NPs/NPG | 10−4 to 0.02 | 0.072 | Yes | No | [178] | |
| Au-/nPts | up to ~10 | 50 | 264 | Yes | No | [153] |
| NPG/CoO | 0.1 to 100 | 100 | 62.5 | Yes | No | [177] |
| Co3O4/(NPG) | 0.02 to 19.1 | 6.4 | 1338.7 | Yes | No | [128] |
| NPG@Ni foam | 0.02 to 9.74 | 10 | 2880 | Yes | No | [175] |
| Glucose Sensors | Solution pH | Linear Range, mM | Detection Limit, μM | Sensitivity µAcm−2 mM−1 | Storage Ability | Interference Study | Ref. |
|---|---|---|---|---|---|---|---|
| NPG-Pt (24%) | Neutral | 0.5–10 | 0.6 | 145.7 | 1 month | Yes | [99] |
| Pd-NPGF | Neutral | 1–33 | 5 | yes | [93] | ||
| Ni@NPG | Alkaline | 1–105 µM | 5070.9 | No | [140] | ||
| Ni(OH)2/NPG | Alkaline | 0.002–7 | 0.73 | 3529 | 3 weeks | Yes | [126] |
| CoOx/NPG | Alkaline | 0.002–2 | 0.094 | 2025 | 3 weeks | Yes | [127] |
| NPG/NiCo2O4 | Alkaline | 0.01–21 | 1 | 0.3871 | Yes | [88] | |
| NPG/Co3O4 | Alkaline | 0.005 | 12.5 | Yes | [130] | ||
| Cu/NPG | Alkaline | 0.002–8.11 | 0.59 | 3643 | >3 weeks | Yes | [123] |
| NPG/CuO | Alkaline | Up to 12 | 2.8 | 374 | Yes | [122] | |
| NPG/CoO | Alkaline | Up to 100 | Yes | [177] | |||
| PtCo/NPG/GP | Alkaline | 0.035–30 | 5 | 7.84 | Yes | [183] | |
| Cu-NPG/SPE | Synthetic saliva, pH 7.5 | 10−3–13 | 0.13 | 659.9 | Yes | [124] | |
| NiCo-MOF/NPG | Alkaline | 0.001–8 | 0.29 | 684.4 | Yes | [184] | |
| Co3O4/NPG | Alkaline | 0.002–2.1 | 0.085 | 4470.4 | Yes | [128] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Islam, M.S.; Banik, S.; Collinson, M.M. Recent Advances in Bimetallic Nanoporous Gold Electrodes for Electrochemical Sensing. Nanomaterials 2023, 13, 2515. https://doi.org/10.3390/nano13182515
Islam MS, Banik S, Collinson MM. Recent Advances in Bimetallic Nanoporous Gold Electrodes for Electrochemical Sensing. Nanomaterials. 2023; 13(18):2515. https://doi.org/10.3390/nano13182515
Chicago/Turabian StyleIslam, Md. Shafiul, Subrata Banik, and Maryanne M. Collinson. 2023. "Recent Advances in Bimetallic Nanoporous Gold Electrodes for Electrochemical Sensing" Nanomaterials 13, no. 18: 2515. https://doi.org/10.3390/nano13182515
APA StyleIslam, M. S., Banik, S., & Collinson, M. M. (2023). Recent Advances in Bimetallic Nanoporous Gold Electrodes for Electrochemical Sensing. Nanomaterials, 13(18), 2515. https://doi.org/10.3390/nano13182515







