A Portable Device for I–V and Arrhenius Plots to Characterize Chemoresistive Gas Sensors: Test on SnO2-Based Sensors
Abstract
:1. Introduction
2. Materials and Methods
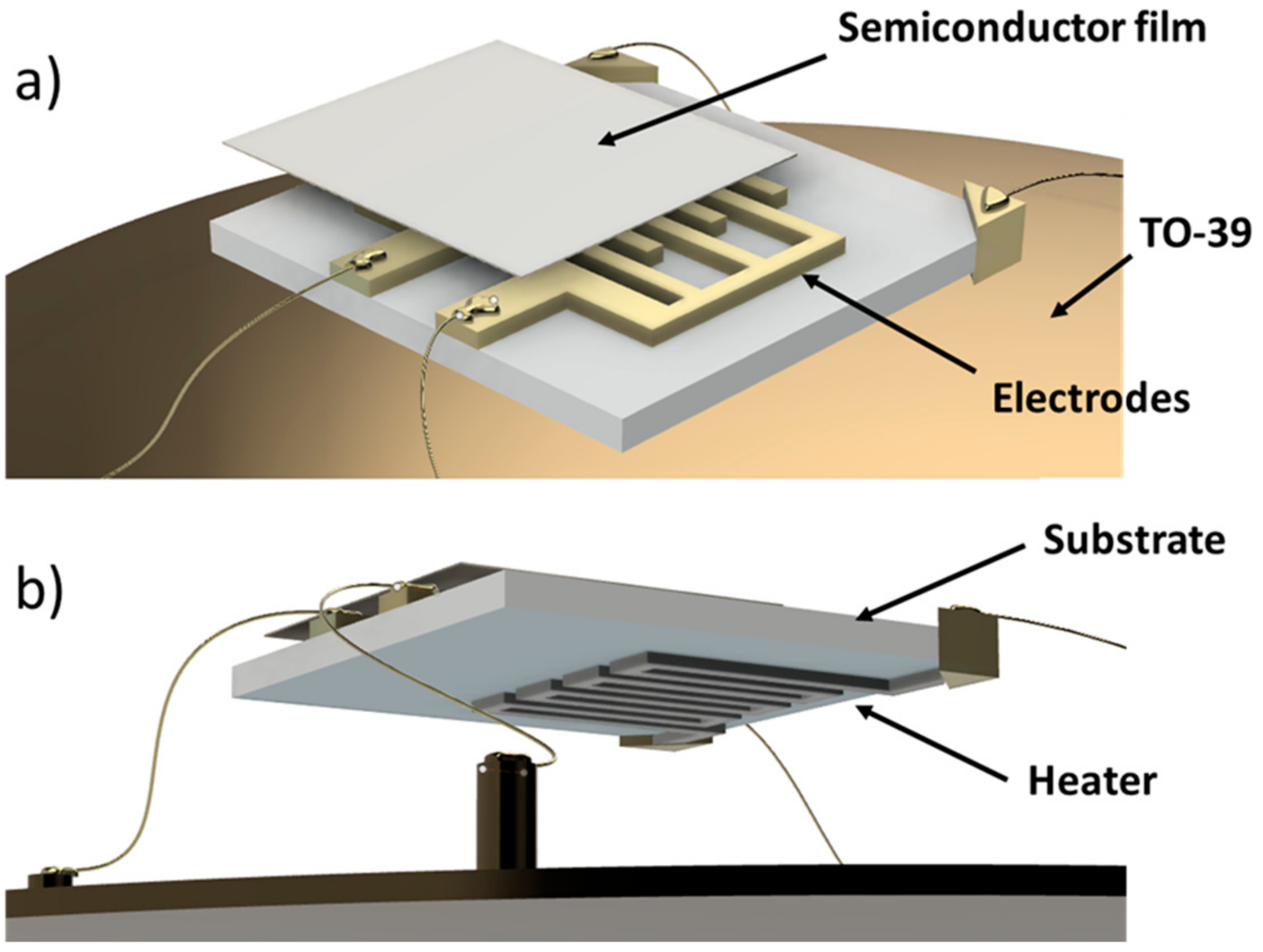
2.1. Chemoresistive Sensors Fabrication
- the substrate: an alumina-made insulating layer hosting two interdigitated comb-shaped gold electrodes, connecting the sensor to the readout circuit;
- the sensing material (or active material): a porous thick film (thickness ~20 µm) of interconnected MOX nanoparticles;
- the heater: a platinum meander aimed to heat the sensor to the proper WT by controlling the current flowing through it.
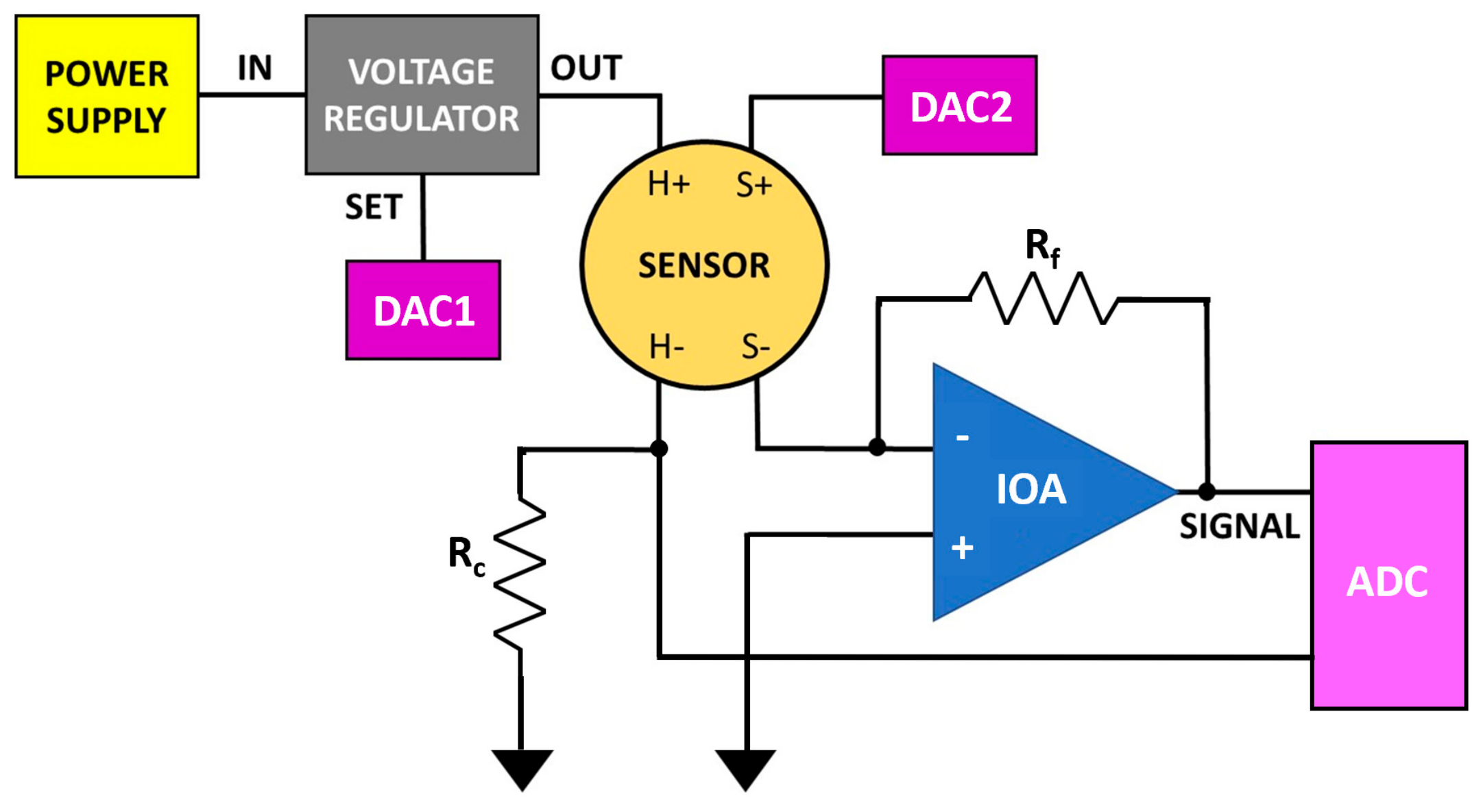
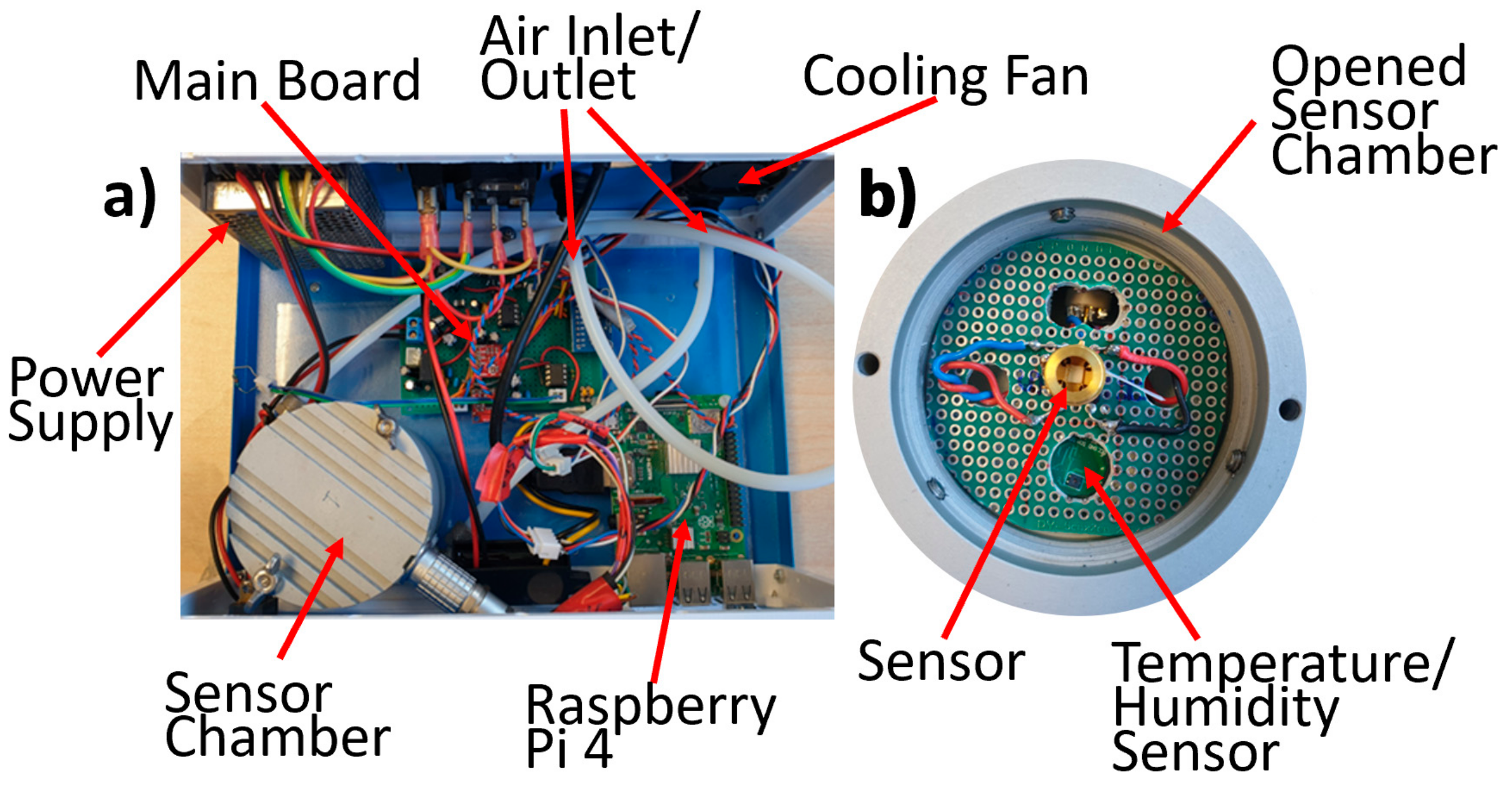
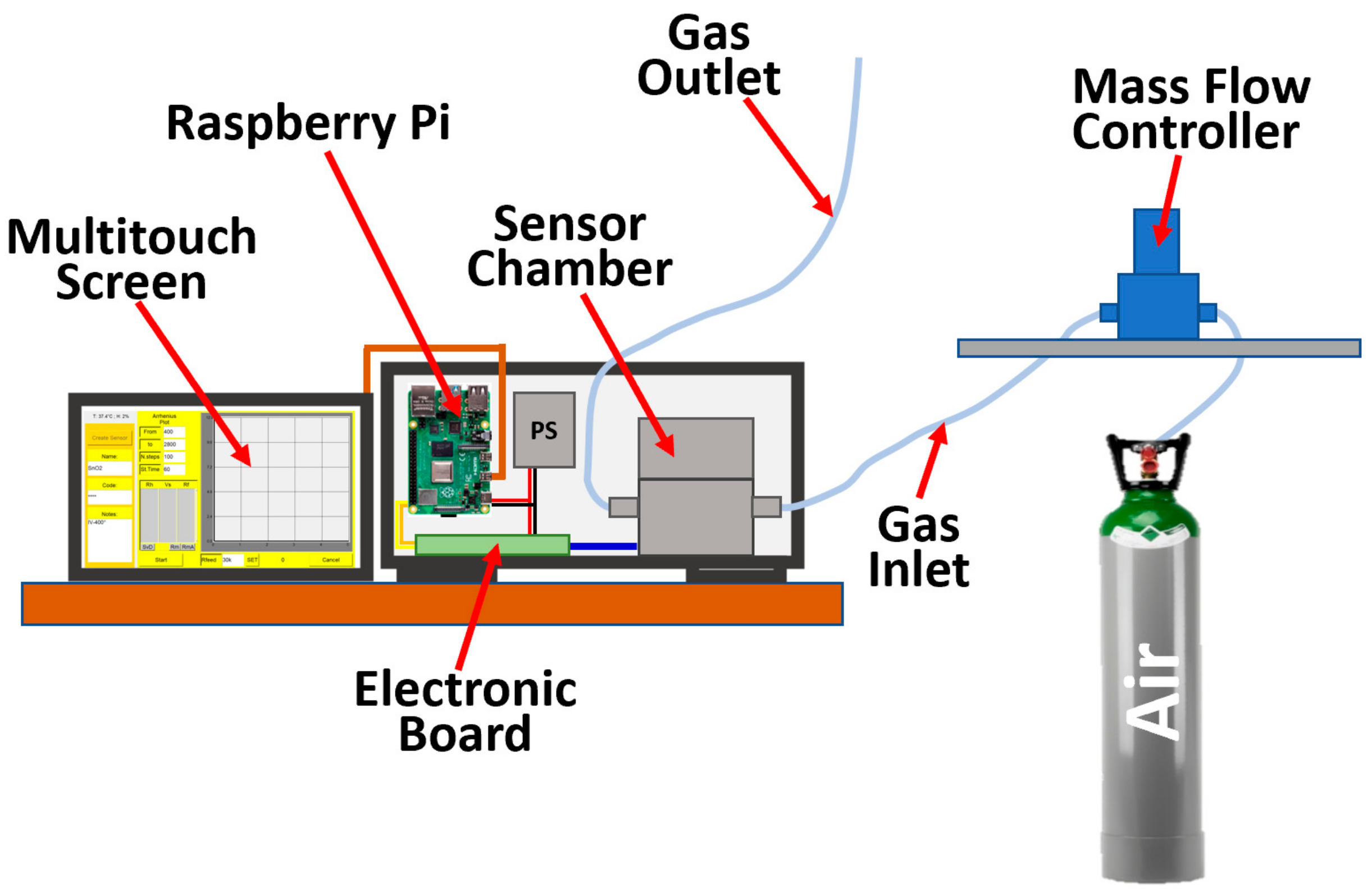
2.2. Single Sensor Device and Set-Up
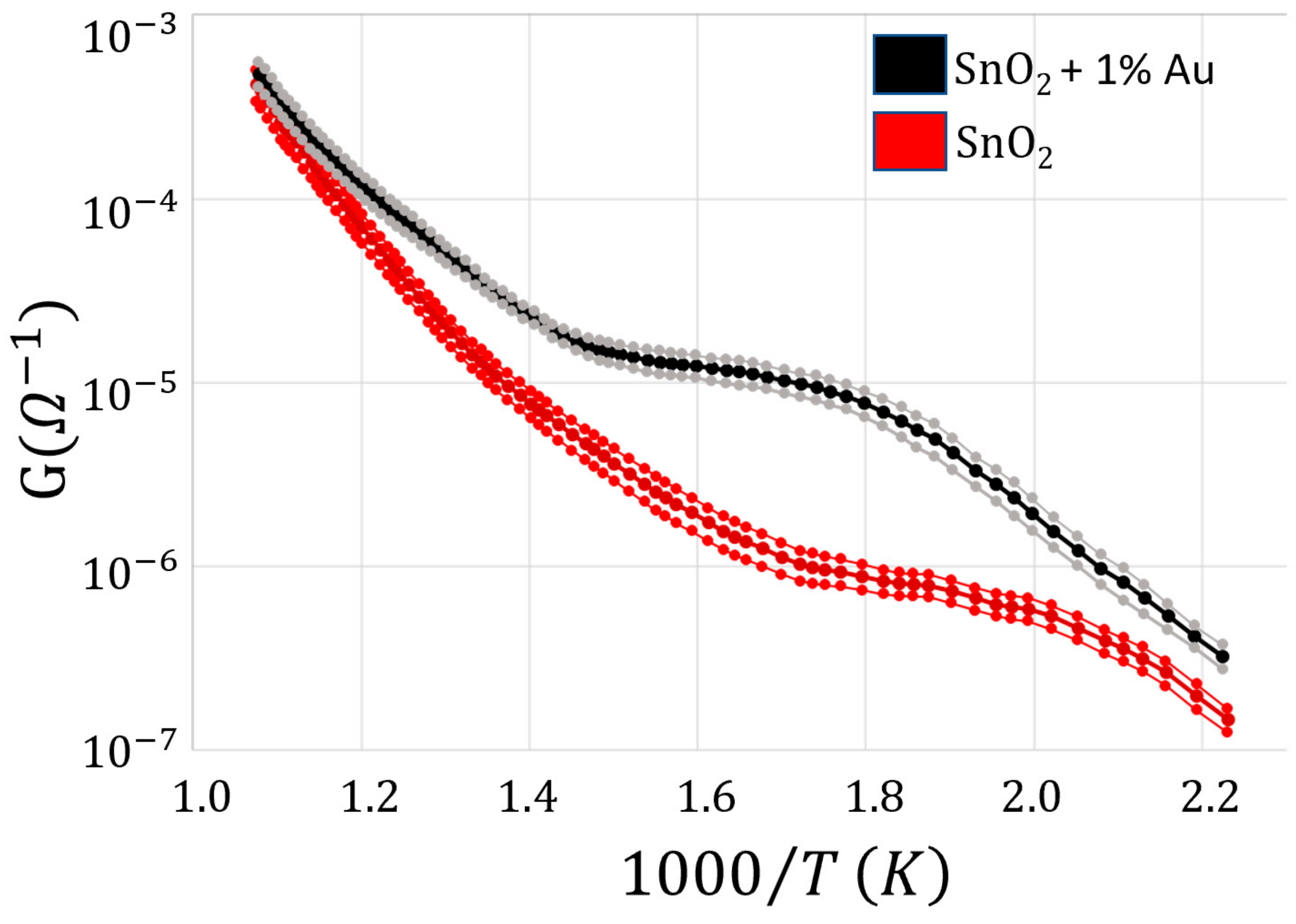
2.3. WT Determination
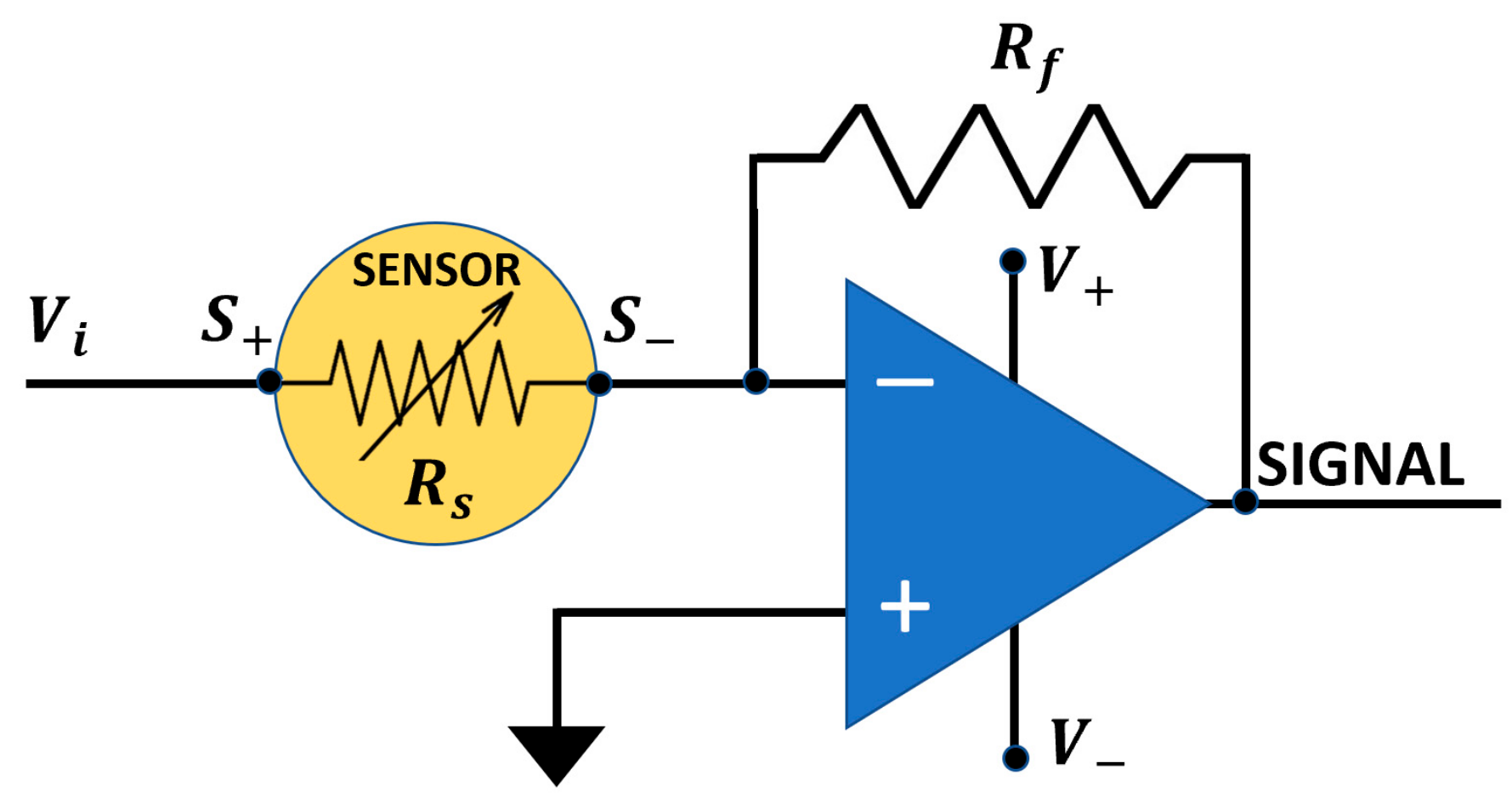
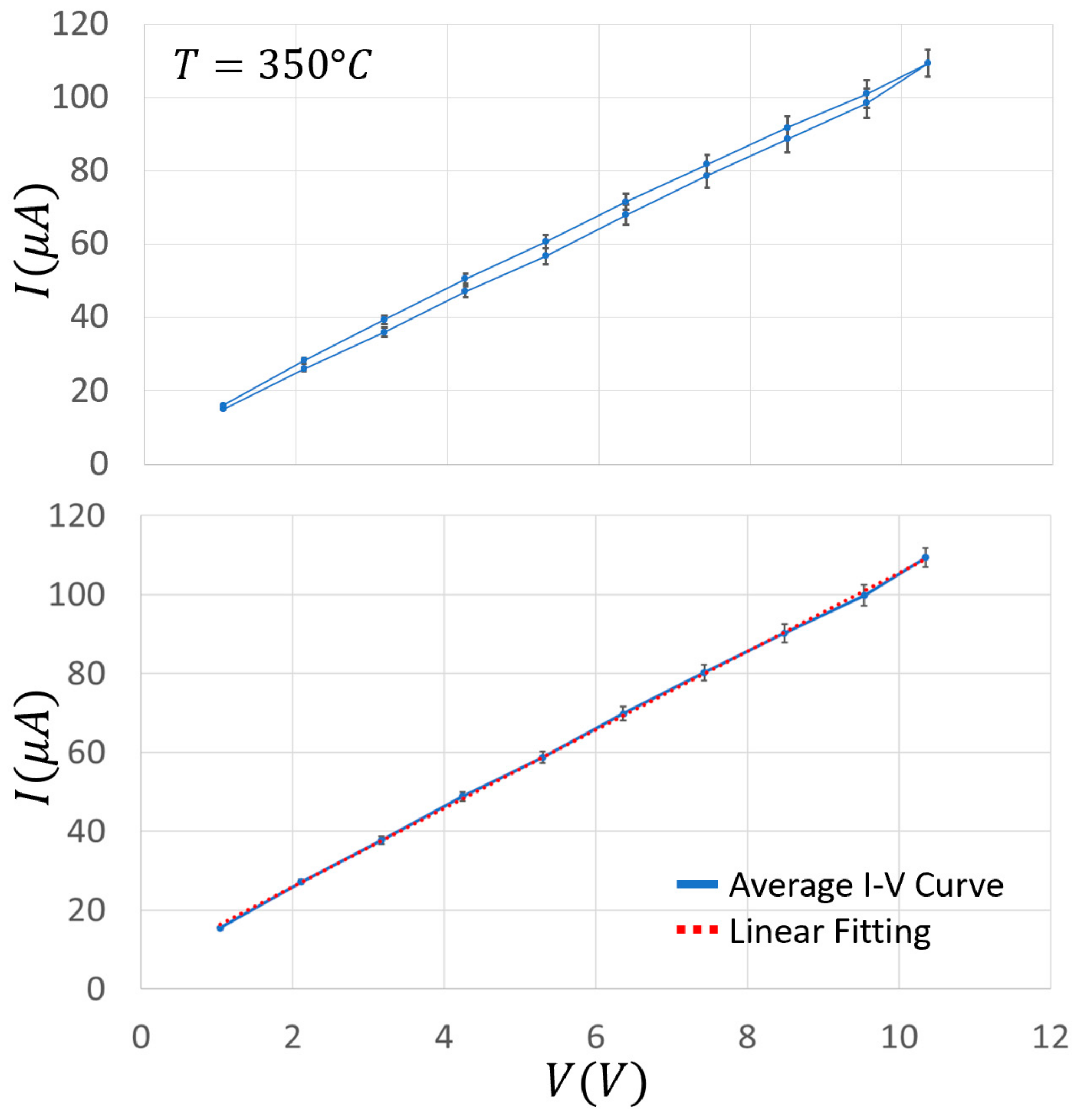
2.4. I–V Characteristic Curves Determination
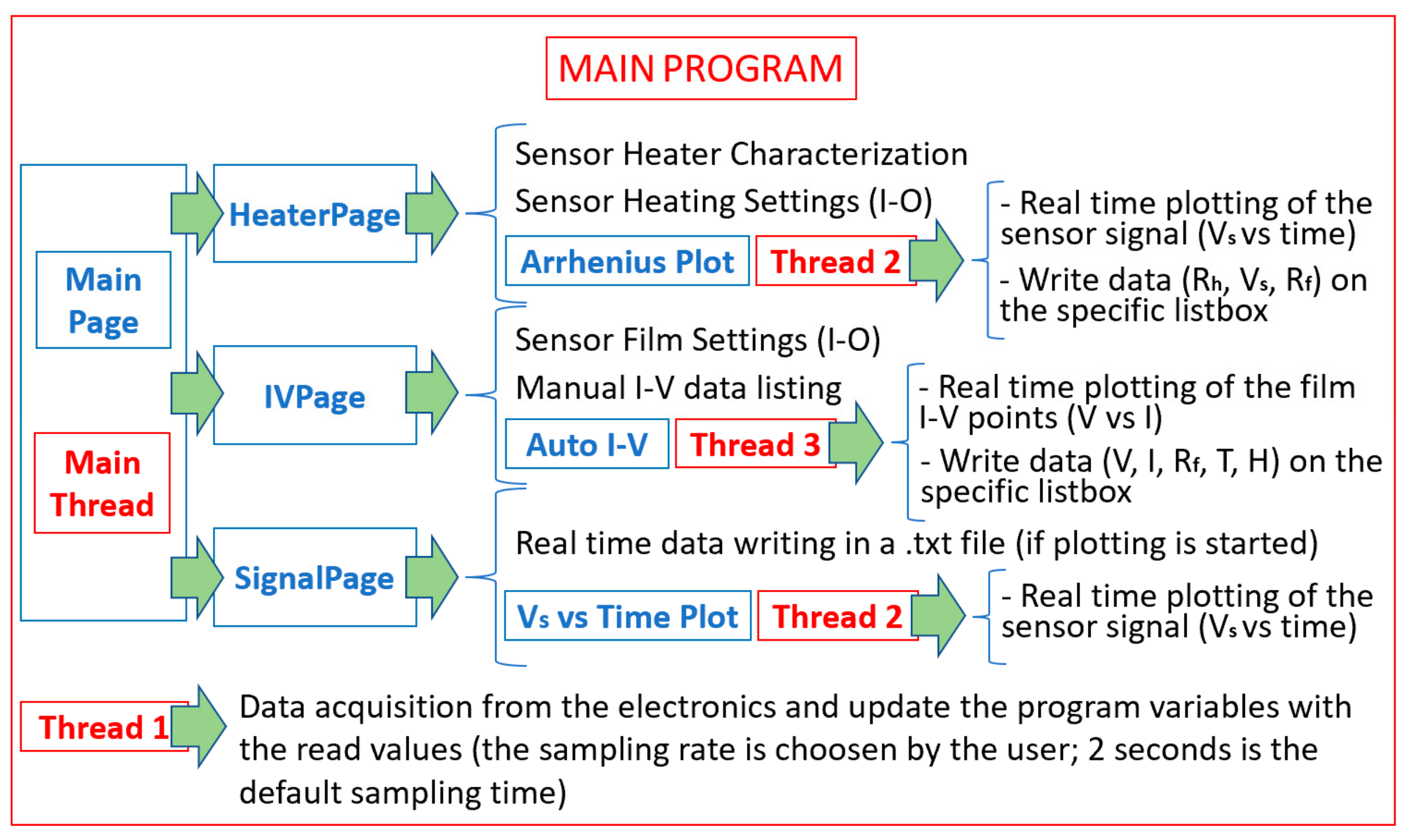
2.5. The Managing Software
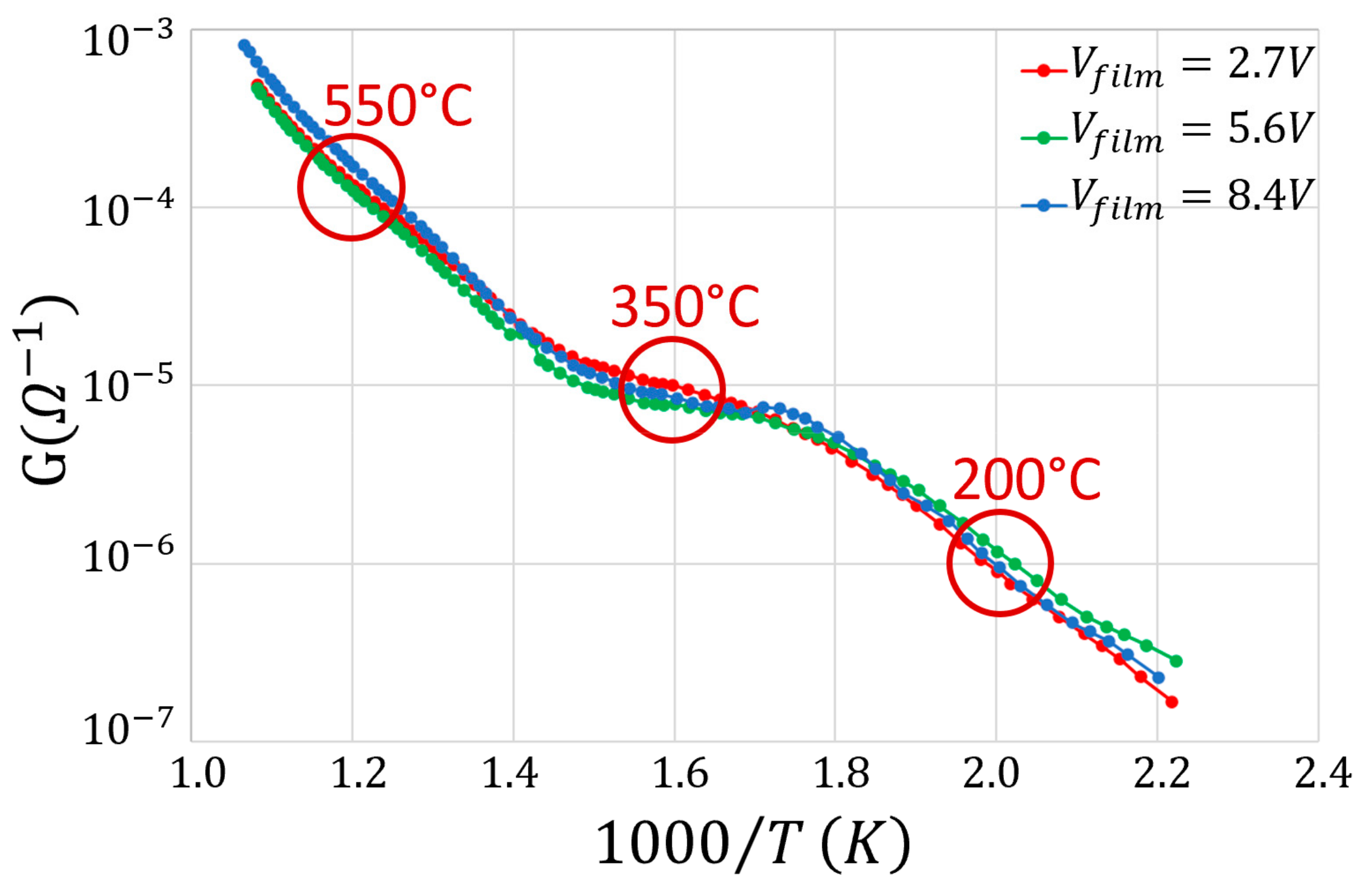
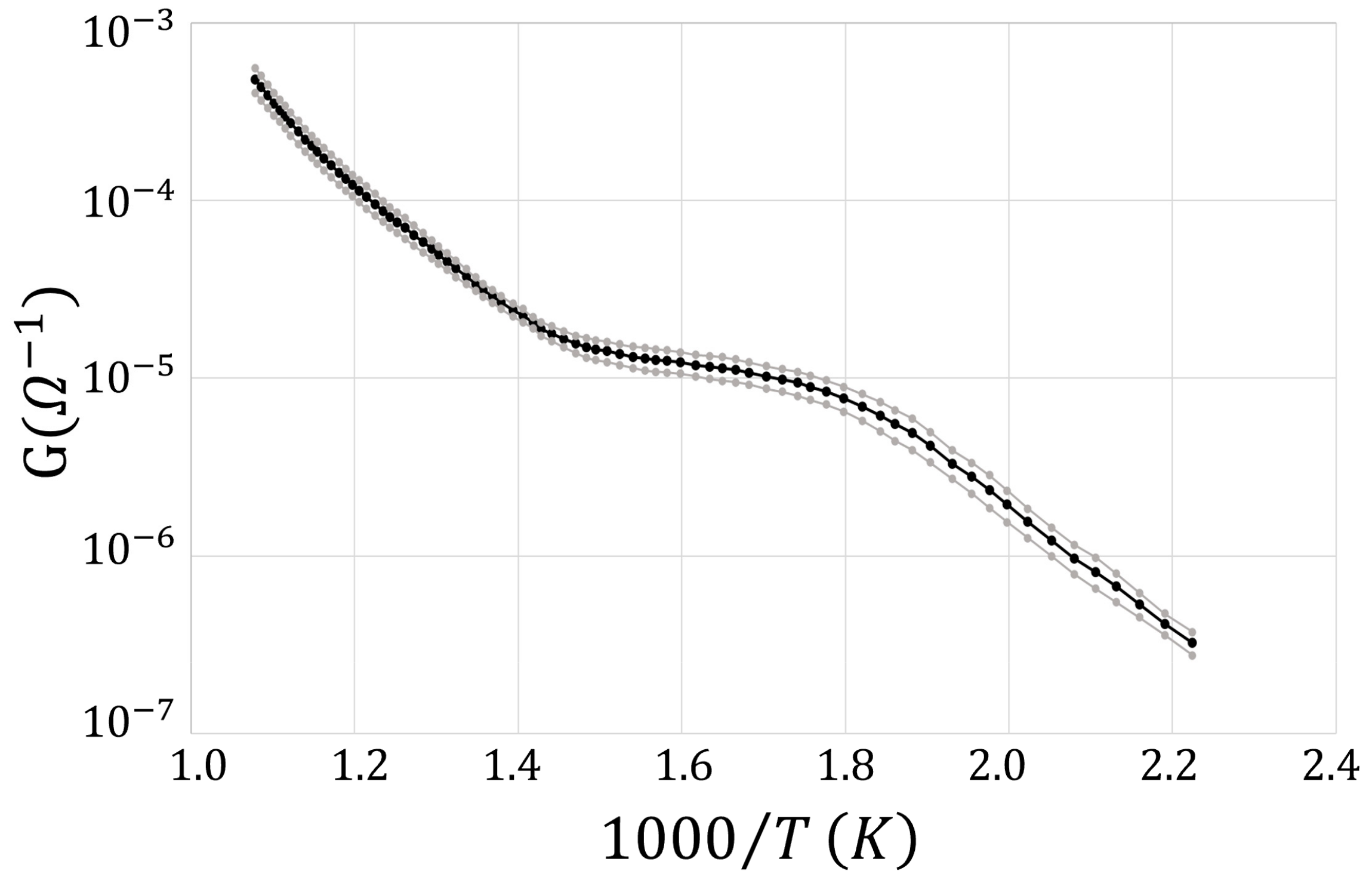
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spagnoli, E.; Gaiardo, A.; Fabbri, B.; Valt, M.; Krik, S.; Ardit, M.; Cruciani, G.; Della Ciana, M.; Vanzetti, L.; Vola, G.; et al. Design of a Metal-Oxide Solid Solution for Sub-Ppm H2 Detection. ACS Sens. 2022, 7, 573–583. [Google Scholar] [CrossRef] [PubMed]
- Elmi, I.; Zampolli, S.; Cozzani, E.; Mancarella, F.; Cardinali, G.C. Development of Ultra-Low-Power Consumption MOX Sensors with Ppb-Level VOC Detection Capabilities for Emerging Applications. Sens. Actuators B Chem. 2008, 135, 342–351. [Google Scholar] [CrossRef]
- Gherardi, S.; Zonta, G.; Astolfi, M.; Malagù, C. Humidity Effects on SnO2 and (SnTiNb)O2 Sensors Response to CO and Two-Dimensional Calibration Treatment. Mater. Sci. Eng. B 2021, 265, 115013. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor Metal Oxide Gas Sensors: A Review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Astolfi, M.; Rispoli, G.; Gherardi, S.; Zonta, G.; Malagù, C. Reproducibility and Repeatability Tests on (SnTiNb)O2 Sensors in Detecting Ppm-Concentrations of CO and Up to 40% of Humidity: A Statistical Approach. Sensors 2023, 23, 1983. [Google Scholar] [CrossRef] [PubMed]
- Getino, J.; Arés, L.; Robla, J.I.; Horrillo, C.; Sayago, I.; Fernández, M.J.; Rodrigo, J.; Gutiérrez, J. Environmental Applications of Gas Sensor Arrays: Combustion Atmospheres and Contaminated Soils. Sens. Actuators B Chem. 1999, 59, 249–254. [Google Scholar] [CrossRef]
- Martinelli, G.; Carotta, M.C.; Ferroni, M.; Sadaoka, Y.; Traversa, E. Screen-Printed Perovskite-Type Thick Films as Gas Sensors for Environmental Monitoring. Sens. Actuators B Chem. 1999, 55, 99–110. [Google Scholar] [CrossRef]
- Wetchakun, K.; Samerjai, T.; Tamaekong, N.; Liewhiran, C.; Siriwong, C.; Kruefu, V.; Wisitsoraat, A.; Tuantranont, A.; Phanichphant, S. Semiconducting Metal Oxides as Sensors for Environmentally Hazardous Gases. Sens. Actuators B Chem. 2011, 160, 580–591. [Google Scholar] [CrossRef]
- Deininger, D.J. Metal Oxide Sensors Applied to Industrial and Consumer Applications: Examples and Requirements for Successful Implementation. Proceedings 2019, 14, 31. [Google Scholar] [CrossRef]
- Abbatangelo, M.; Carmona, E.N.; Sberveglieri, V.; Comini, E.; Sberveglieri, G. Overview of Iot Mox Chemical Sensors Arrays for Agri-Food Applications. In Proceedings of the 2019 IEEE International Symposium on Olfaction and Electronic Nose (ISOEN), Fukuoka, Japan, 26–29 May 2019; pp. 1–3. [Google Scholar]
- Bonaccorsi, L.; Donato, A.; Fotia, A.; Frontera, P.; Gnisci, A. Competitive Detection of Volatile Compounds from Food Degradation by a Zinc Oxide Sensor. Appl. Sci. 2022, 12, 2261. [Google Scholar] [CrossRef]
- Falasconi, M.; Concina, I.; Gobbi, E.; Sberveglieri, V.; Pulvirenti, A.; Sberveglieri, G. Electronic Nose for Microbiological Quality Control of Food Products. Int. J. Electrochem. 2012, 2012, e715763. [Google Scholar] [CrossRef]
- Ponzoni, A.; Comini, E.; Concina, I.; Ferroni, M.; Falasconi, M.; Gobbi, E.; Sberveglieri, V.; Sberveglieri, G. Nanostructured Metal Oxide Gas Sensors, a Survey of Applications Carried out at SENSOR Lab, Brescia (Italy) in the Security and Food Quality Fields. Sensors 2012, 12, 17023–17045. [Google Scholar] [CrossRef] [PubMed]
- John, A.T.; Murugappan, K.; Nisbet, D.R.; Tricoli, A. An Outlook of Recent Advances in Chemiresistive Sensor-Based Electronic Nose Systems for Food Quality and Environmental Monitoring. Sensors 2021, 21, 2271. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, M.; Rispoli, G.; Benedusi, M.; Zonta, G.; Landini, N.; Valacchi, G.; Malagù, C. Chemoresistive Sensors for Cellular Type Discrimination Based on Their Exhalations. Nanomaterials 2022, 12, 1111. [Google Scholar] [CrossRef] [PubMed]
- Vasiliev, A.A.; Varfolomeev, A.E.; Volkov, I.A.; Simonenko, N.P.; Arsenov, P.V.; Vlasov, I.S.; Ivanov, V.V.; Pislyakov, A.V.; Lagutin, A.S.; Jahatspanian, I.E.; et al. Reducing Humidity Response of Gas Sensors for Medical Applications: Use of Spark Discharge Synthesis of Metal Oxide Nanoparticles. Sensors 2018, 18, 2600. [Google Scholar] [CrossRef] [PubMed]
- Zonta, G.; Anania, G.; Astolfi, M.; Feo, C.; Gaiardo, A.; Gherardi, S.; Giberti, A.; Guidi, V.; Landini, N.; Palmonari, C.; et al. Chemoresistive Sensors for Colorectal Cancer Preventive Screening through Fecal Odor: Double-Blind Approach. Sens. Actuators B Chem. 2019, 301, 127062. [Google Scholar] [CrossRef]
- Astolfi, M.; Rispoli, G.; Anania, G.; Nevoso, V.; Artioli, E.; Landini, N.; Benedusi, M.; Melloni, E.; Secchiero, P.; Tisato, V.; et al. Colorectal Cancer Study with Nanostructured Sensors: Tumor Marker Screening of Patient Biopsies. Nanomaterials 2020, 10, 606. [Google Scholar] [CrossRef] [PubMed]
- Landini, N.; Anania, G.; Astolfi, M.; Fabbri, B.; Guidi, V.; Rispoli, G.; Valt, M.; Zonta, G.; Malagù, C. Nanostructured Chemoresistive Sensors for Oncological Screening and Tumor Markers Tracking: Single Sensor Approach Applications on Human Blood and Cell Samples. Sensors 2020, 20, 1411. [Google Scholar] [CrossRef]
- Shin, W. Medical Applications of Breath Hydrogen Measurements. Anal. Bioanal. Chem. 2014, 406, 3931–3939. [Google Scholar] [CrossRef]
- Astolfi, M.; Rispoli, G.; Anania, G.; Artioli, E.; Nevoso, V.; Zonta, G.; Malagù, C. Tin, Titanium, Tantalum, Vanadium and Niobium Oxide Based Sensors to Detect Colorectal Cancer Exhalations in Blood Samples. Molecules 2021, 26, 466. [Google Scholar] [CrossRef]
- Nikolic, M.V.; Milovanovic, V.; Vasiljevic, Z.Z.; Stamenkovic, Z. Semiconductor Gas Sensors: Materials, Technology, Design, and Application. Sensors 2020, 20, 6694. [Google Scholar] [CrossRef]
- Haidry, A.A.; Kind, N.; Saruhan, B. Investigating the Influence of Al-Doping and Background Humidity on NO2 Sensing Characteristics of Magnetron-Sputtered SnO2 Sensors. J. Sens. Sens. Syst. 2015, 4, 271–280. [Google Scholar] [CrossRef]
- Rydosz, A.; Brudnik, A.; Staszek, K. Metal Oxide Thin Films Prepared by Magnetron Sputtering Technology for Volatile Organic Compound Detection in the Microwave Frequency Range. Materials 2019, 12, 877. [Google Scholar] [CrossRef] [PubMed]
- Shujah, T.; Ikram, M.; Butt, A.R.; Shahzad, M.K.; Rashid, K.; Zafar, Q.; Ali, S. H2S Gas Sensor Based on WO3 Nanostructures Synthesized via Aerosol Assisted Chemical Vapor Deposition Technique. Nanosci. Nanotechnol. Lett. 2019, 11, 1247–1256. [Google Scholar] [CrossRef]
- Bârsan, N.; Weimar, U. Understanding the Fundamental Principles of Metal Oxide Based Gas Sensors; the Example of CO Sensing with SnO2 Sensors in the Presence of Humidity. J. Phys. Condens. Matter 2003, 15, R813. [Google Scholar] [CrossRef]
- Navas, D.; Fuentes, S.; Castro-Alvarez, A.; Chavez-Angel, E. Review on Sol-Gel Synthesis of Perovskite and Oxide Nanomaterials. Gels 2021, 7, 275. [Google Scholar] [CrossRef] [PubMed]
- Parashar, M.; Shukla, V.; Singh, R. Metal Oxides Nanoparticles via Sol–Gel Method: A Review on Synthesis, Characterization and Applications. J. Mater. Sci. Mater. Electron. 2020, 31, 3729–3749. [Google Scholar] [CrossRef]
- Zhou, C.; Yao, J.; Zhan, L.; Yuan, C.; Kudryavtsev, A.; Saifutdinov, A.; Wang, Y.; Yu, Z.; Zhou, Z. Using Collisional Electron Spectroscopy to Detect Gas Impurities in an Open Environment: CH4-Containing Mixtures. Molecules 2022, 27, 6066. [Google Scholar] [CrossRef]
- Zhou, C.; Yao, J.; Saifutdinov, A.I.; Kudryavtsev, A.A.; Yuan, C.; Ma, G.; Dou, Z.; Cao, J.; Ma, M.; Zhou, Z. Determination of Organic Impurities by Plasma Electron Spectroscopy in Nonlocal Plasma at Intermediate and High Pressures. Plasma Sources Sci. Technol. 2022, 31, 107001. [Google Scholar] [CrossRef]
- Kogan, V.T.; Lebedev, D.S.; Pavlov, A.K.; Chichagov, Y.V.; Antonov, A.S. A Portable Mass Spectrometer for Direct Monitoring of Gases and Volatile Compounds in Air and Water Samples. Instrum. Exp. Tech. 2011, 54, 390–396. [Google Scholar] [CrossRef]
- Hou, C.; Li, J.; Huo, D.; Luo, X.; Dong, J.; Yang, M.; Shi, X. A Portable Embedded Toxic Gas Detection Device Based on a Cross-Responsive Sensor Array. Sens. Actuators B Chem. 2012, 161, 244–250. [Google Scholar] [CrossRef]
- Astolfi, M.; Rispoli, G.; Anania, G.; Zonta, G.; Malagù, C. Chemoresistive Nanosensors Employed to Detect Blood Tumor Markers in Patients Affected by Colorectal Cancer in a One-Year Follow Up. Cancers 2023, 15, 1797. [Google Scholar] [CrossRef] [PubMed]
- Schipani, F.; Aldao, C.M.; Ponce, M.A. Schottky Barriers Measurements through Arrhenius Plots in Gas Sensors Based on Semiconductor Films. AIP Adv. 2012, 2, 032138. [Google Scholar] [CrossRef]
- Yamada, Y.; Seno, Y.; Masuoka, Y.; Nakamura, T.; Yamashita, K. NO2 Sensing Characteristics of Nb Doped TiO2 Thin Films and Their Electronic Properties. Sens. Actuators B Chem. 2000, 66, 164–166. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef]
- Gaiardo, A.; Krik, S.; Valt, M.; Fabbri, B.; Tonezzer, M.; Feng, Z.; Guidi, V.; Bellutti, P. Development of a Sensor Array Based on Pt, Pd, Ag and Au Nanocluster Decorated SnO2 for Precision Agriculture. Electrochem. Soc. Meet. Abstr. 2021, MA2021-01, 1550. [Google Scholar] [CrossRef]
- Merdrignac-Conanec, O.; Moseley, P.T. Gas Sensing Properties of the Mixed Molybdenum Tungsten Oxide, W0.9Mo0.1O3. J. Mater. Chem. 2002, 12, 1779–1781. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Astolfi, M.; Zonta, G.; Gherardi, S.; Malagù, C.; Vincenzi, D.; Rispoli, G. A Portable Device for I–V and Arrhenius Plots to Characterize Chemoresistive Gas Sensors: Test on SnO2-Based Sensors. Nanomaterials 2023, 13, 2549. https://doi.org/10.3390/nano13182549
Astolfi M, Zonta G, Gherardi S, Malagù C, Vincenzi D, Rispoli G. A Portable Device for I–V and Arrhenius Plots to Characterize Chemoresistive Gas Sensors: Test on SnO2-Based Sensors. Nanomaterials. 2023; 13(18):2549. https://doi.org/10.3390/nano13182549
Chicago/Turabian StyleAstolfi, Michele, Giulia Zonta, Sandro Gherardi, Cesare Malagù, Donato Vincenzi, and Giorgio Rispoli. 2023. "A Portable Device for I–V and Arrhenius Plots to Characterize Chemoresistive Gas Sensors: Test on SnO2-Based Sensors" Nanomaterials 13, no. 18: 2549. https://doi.org/10.3390/nano13182549
APA StyleAstolfi, M., Zonta, G., Gherardi, S., Malagù, C., Vincenzi, D., & Rispoli, G. (2023). A Portable Device for I–V and Arrhenius Plots to Characterize Chemoresistive Gas Sensors: Test on SnO2-Based Sensors. Nanomaterials, 13(18), 2549. https://doi.org/10.3390/nano13182549







