A Gram Scale Soft-Template Synthesis of Heteroatom Doped Nanoporous Hollow Carbon Spheres for Oxygen Reduction Reaction
Abstract
:1. Introduction
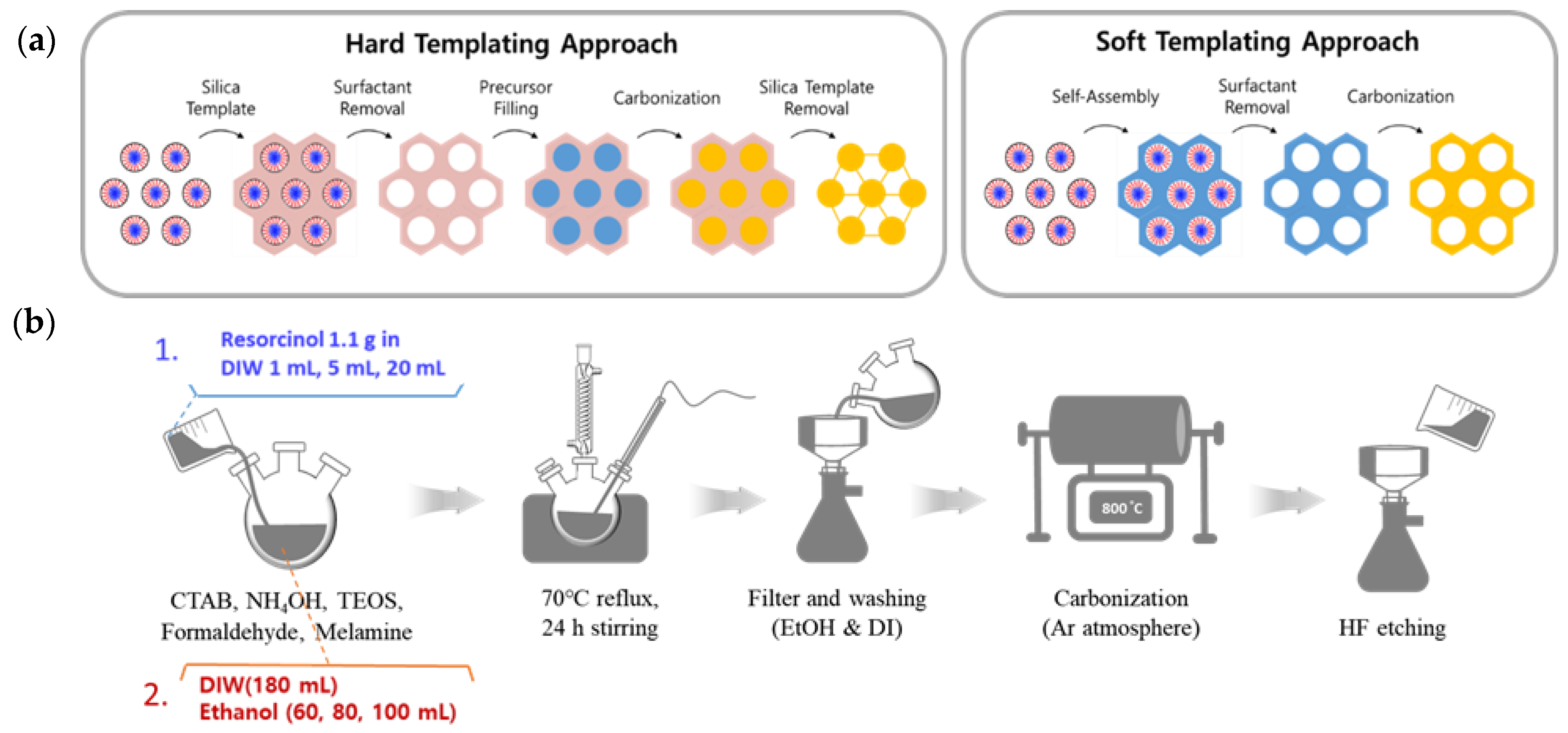
2. Materials and Methods
2.1. Synthesis of Nitrogen-Doped Hallow Nanoporous Carbon Sphere (N-HNCS)
2.2. Synthesis of Cobalt and Nitrogen-Doped Hallow Nanoporous Carbon Sphere (Co-N-HNCS)
2.3. Analysis of Physicochemical Properties
3. Results
3.1. Synthesis of Nitrogen-Doped Hollow Nanoporous Carbon Spheres (N-HNCS)
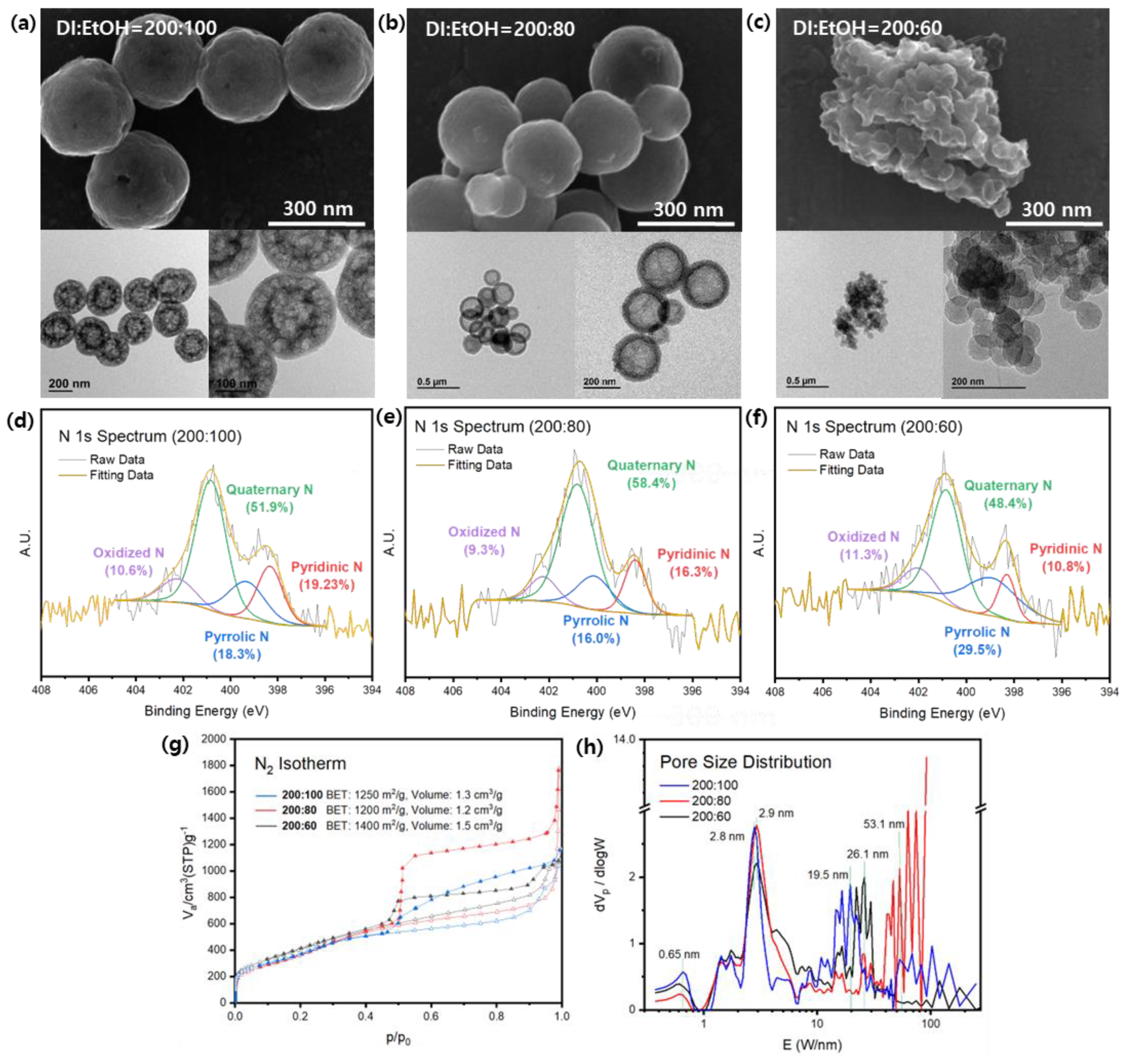
3.1.1. Effect of Resorcinol Solution Concentration
3.1.2. Effect of Resorcinol Solvent Ratio
3.2. Design of Cobalt and Nitrogen-Doped Hollow Nanoporous Carbon Spheres (Co-N-HNCS)
Effect of Additives on Electrochemical Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Davis, M.E. Ordered porous materials for emerging applications. Nature 2002, 417, 813–821. [Google Scholar]
- Liang, C.; Li, Z.; Dai, S. Mesoporous carbon materials: Synthesis and modification. Angew. Chem. Int. Ed. 2008, 47, 3696–3717. [Google Scholar]
- Lan, K.; Wei, Q.; Wang, R.; Xia, Y.; Tan, S.; Wang, Y.; Elzatahry, A.; Feng, P.; Mai, L.; Zhao, D. Two-dimensional mesoporous heterostructure delivering superior pseudocapacitive sodium storage via bottom-up monomicelle assembly. J. Am. Chem. Soc. 2019, 141, 16755–16762. [Google Scholar]
- Liu, C.; Wang, J.; Li, J.; Zeng, M.; Luo, R.; Shen, J.; Sun, X.; Han, W.; Wang, L. Synthesis of N-doped hollow-structured mesoporous carbon nanospheres for high-performance supercapacitors. ACS Appl. Mater. Interfaces 2016, 8, 7194–7204. [Google Scholar]
- Wang, C.; Lai, Q.; Xu, P.; Zheng, D.; Li, X.; Zhang, H. Cage-like porous carbon with superhigh activity and Br2-complex-entrapping capability for bromine-based flow batteries. Adv. Mater. 2017, 29, 1605815. [Google Scholar]
- Zheng, W.; Li, Z.; Han, G.; Zhao, Q.; Lu, G.; Hu, X.; Sun, J.; Wang, R.; Xu, C. Nitrogen-doped activated porous carbon for 4.5 V lithium-ion capacitor with high energy and power density. J. Energy Storage 2022, 47, 103675. [Google Scholar]
- Kim, J.G.; Han, S.; Pak, C. Pore modification and phosphorus doping effect on phosphoric acid-activated Fe-NC for alkaline oxygen reduction reaction. Nanomaterials 2021, 11, 1519. [Google Scholar]
- Gai, H.; Xue, S.; Wang, X.; Zhou, J.; Jioang, H.; Huang, M. Sandwich-like hierarchical porous dual-carbon catalyst with more accessible sites for boosting oxygen reduction reaction. Mater. Today Energy 2021, 21, 100809. [Google Scholar]
- Zaman, S.; Wang, M.; Liu, H.; Sun, F.; Yu, Y.; Shui, J.; Chen, M.; Wang, H. Carbon-based catalyst supports for oxygen reduction in proton-exchange membrane fuel cells. Trends Chem. 2022, 4, 886–906. [Google Scholar]
- Liu, X.; Zhou, Y.; Wang, C.-L.; Liu, Y.; Tao, D.-J. Solvent-free self-assembly synthesis of N-doped ordered mesoporous carbons as effective and bifunctional materials for CO2 capture and oxygen reduction reaction. Chem. Eng. J. 2022, 427, 130878. [Google Scholar]
- Xue, C.; Liu, Y.; Zhao, J.; Li, X.; Zhang, J.; Zhang, J. Micro–mesoporous nitrogen-doped hollow carbon nanospheres as anodes for lithium-ion batteries with high-rate capability and outstanding cycling performance. Ceram. Int. 2022, 48, 5434–5441. [Google Scholar]
- Zhong, X.; Li, Y.; Zhang, L.; Tang, J.; Li, X.; Liu, C.; Shao, M.; Lu, Z.; Pan, H.; Xu, B. High-performance sodium-ion batteries based on nitrogen-doped mesoporous carbon spheres with ultrathin nanosheets. ACS Appl. Mater. Interfaces 2019, 11, 2970–2977. [Google Scholar]
- Zaman, S.; Su, Y.-Q.; Dong, C.-L.; Qi, R.; Huang, L.; Qin, Y.; Huang, Y.-C.; Li, F.-M.; You, B.; Guo, W.; et al. Scalable molten salt synthesis of platinum alloys planted in metal–nitrogen–graphene for efficient oxygen reduction. Angew. Chem. Int. Ed. 2022, 61, e202115835. [Google Scholar]
- Du, J.; Liu, L.; Yu, Y.; Qin, Y.; Wu, H.; Chen, A. A confined space pyrolysis strategy for controlling the structure of hollow mesoporous carbon spheres with high supercapacitor performance. Nanoscale 2019, 11, 4453–4462. [Google Scholar]
- Benzigar, M.R.; Talapaneni, S.N.; Joseph, S.; Ramadass, K.; Singh, G.; Scaranto, J.; Ravon, U.; Al-Bahily, K.; Vinu, A. Recent advances in functionalized micro and mesoporous carbon materials: Synthesis and applications. Chem. Soc. Rev. 2018, 47, 2680–2721. [Google Scholar]
- Wang, L.; Yang, R.T. Significantly increased CO2 adsorption performance of nanostructured templated carbon by tuning surface area and nitrogen doping. J. Phys. Chem. C 2012, 116, 1099–1106. [Google Scholar]
- Kim, K.; Lee, T.; Kwon, Y.; Seo, Y.; Song, J.; Park, J.K.; Lee, H.; Park, J.Y.; Ihee, H.; Cho, S.J.; et al. Lanthanum-catalysed synthesis of microporous 3D graphene-like carbons in a zeolite template. Nature 2016, 535, 131–135. [Google Scholar]
- Kim, K.; Kwon, Y.; Lee, T.; Cho, S.J.; Ryoo, R. Facile large-scale synthesis of three-dimensional graphene-like ordered microporous carbon via ethylene carbonization in CaX zeolite template. Carbon 2017, 118, 517–523. [Google Scholar]
- Guo, D.; Fu, Y.; Bu, F.; Liang, H.; Duan, L.; Zhao, Z.; Wang, C.; El-Toni, A.M.; Li, W.; Zhao, D. Monodisperse ultrahigh nitrogen-containing mesoporous carbon nanospheres from melamine-formaldehyde resin. Small Methods 2021, 5, 2001137. [Google Scholar]
- Zhu, X.; Chong, J.; Hu, T.; Wang, X.; Tian, Y. Enhanced stability and metallic modification of polymeric and carbonaceous nanospheres through precursor engineering via a one-pot aqueous strategy assisted by iron ions. J. Mater. Chem. A 2017, 5, 8297–8306. [Google Scholar]
- Kim, J.G.; Cho, J.; Han, S.; Lee, H.; Yuk, E.; Bae, B.; Jang, S.S.; Pak, C. Boosting activity toward oxygen reduction reaction of a mesoporous FeCuNC catalyst via heteroatom doping-induced electronic state modulation. J. Mater. Chem. A 2022, 10, 5361–5372. [Google Scholar]
- Peng, L.; Hung, C.T.; Wang, S.W.; Zhang, X.M.; Zhu, X.H.; Zhao, Z.W.; Wang, C.Y.; Tang, Y.; Li, W.; Zhao, D.Y. Versatile nanoemulsion assembly approach to synthesize functional mesoporous carbon nanospheres with tunable pore sizes and architectures. J. Am. Chem. Soc. 2019, 141, 7073–7080. [Google Scholar]
- Li, M.; Han, K.H.; Teng, Z.C.; Li, J.X.; Wang, M.M.; Li, X. Comparison of porous carbons derived from sodium alginate and calcium alginate and their electrochemical properties. RSC Adv. 2020, 10, 2209–2215. [Google Scholar]
- Peng, L.; Peng, H.R.; Hung, C.T.; Guo, D.Y.; Duan, L.L.; Ma, B.; Liu, L.L.; Li, W.; Zhao, D.Y. Programmable synthesis of radially gradient-structured mesoporous carbon nanospheres with tunable core-shell architectures. Chem 2021, 7, 1020–1032. [Google Scholar]
- Peng, L.; Peng, H.R.; Liu, Y.; Wang, X.; Hung, C.T.; Zhao, Z.W.; Chen, G.; Li, W.; Mai, L.Q.; Zhao, D.Y. Spiral self-assembly of lamellar micelles into multi-shelled hollow nanospheres with unique chiral architecture. Sci. Adv. 2021, 7, eabi7403. [Google Scholar]
- Guan, B.Y.; Zhang, S.L.; Lou, X.W. Realization of walnut-shaped particles with macro-/mesoporous open channels through pore architecture manipulation and their use in electrocatalytic oxygen reduction. Angew. Chem. Int. Ed. 2018, 130, 6284–6288. [Google Scholar]
- Zhao, Y.J.; Lyu, H.L.; Liu, Y.J.; Liu, W.J.; Tian, Y.; Wang, X.F. Rational design and synthesis of multimorphology mesoporous carbon@silica nanoparticles with tailored structure. Carbon 2021, 183, 912–928. [Google Scholar]
- Trivedi, M.; Peng, F.; Xia, X.H.; Sepulveda-Medina, P.I.; Vogt, B.D. Control of pore size in ordered mesoporous carbon-silica by Hansen solubility parameters of swelling agent. Langmuir 2019, 35, 14049–14059. [Google Scholar]
- Zhao, Z.W.; Duan, L.L.; Zhao, Y.J.; Wang, L.P.; Zhang, J.Y.; Bu, F.X.; Sun, Z.H.; Zhang, T.S.; Liu, M.L.; Chen, H.X.; et al. Constructing unique mesoporous carbon superstructures via monomicelle interface confined assembly. J. Am. Chem. Soc. 2022, 144, 11767–11777. [Google Scholar]
- Kim, H.S.; Lee, C.H.; Jang, J.H.; Kang, M.S.; Jin, H.; Lee, K.S.; Lee, S.U.; Yoo, S.J.; Yoo, W.C. Single-atom oxygen reduction reaction electrocatalysts of Fe, Si, and N co-doped carbon with 3D interconnected mesoporosity. J. Mater. Chem. A 2021, 9, 4297–4309. [Google Scholar]
- Pan, Y.; Li, H.D.; Xiong, J.; Yu, Y.D.; Du, H.Y.; Li, S.X.; Wu, Z.C.; Li, S.P.; Lai, J.P.; Wang, L. Protecting the state of Cu clusters and nanoconfinement engineering over hollow mesoporous carbon spheres for electrocatalytical C-C coupling. Appl. Catal. B Environ. 2022, 306, 121111. [Google Scholar]
- Zhou, Y.; Yu, Y.N.; Ma, D.S.; Foucher, A.C.; Xiong, L.; Zhang, J.H.; Stach, E.A.; Yue, Q.; Kang, Y.J. Atomic Fe dispersed hierarchical mesoporous Fe-N-C nanostructures for an efficient oxygen reduction reaction. ACS Catal. 2021, 11, 74–81. [Google Scholar]
- Su, Y.H.; Zhu, Y.H.; Jiang, H.L.; Shen, J.H.; Yang, X.L.; Zou, W.J.; Chen, J.D.; Li, C.Z. Cobalt nanoparticles embedded in N-doped carbon as an efficient bifunctional electrocatalyst for oxygen reduction and evolution reactions. Nanoscale 2014, 6, 15080–15089. [Google Scholar]
- Zhang, H.W.; Noonan, O.; Huang, X.D.; Yang, Y.N.; Xu, C.; Zhou, L.; Yu, C.Z. Surfactant-free assembly of mesoporous carbon hollow spheres with large tunable pore sizes. ACS Nano 2016, 10, 4579–4586. [Google Scholar]
- Qu, L.L.; Hu, H.C.; Yu, J.Q.; Yu, X.Y.; Liu, J.; Xu, Y.; Zhang, Q. High-yield synthesis of Janus dendritic mesoporous silica@ resorcinol–formaldehyde nanoparticles: A competing growth mechanism. Langmuir 2017, 33, 5269–5274. [Google Scholar]
- Chen, Z.Y.; Wu, Z.M.; Jiang, L.L.; Li, T.T.; Gao, Y.J. An electrochemical sensor for sunset yellow detection based on hollow mesoporous carbon spheres. J. Food Compo. Anal. 2023, 122, 105480. [Google Scholar]
- Zhang, Q.; Deng, C.; Huang, Z.M.; Zhang, Q.C.; Chai, X.C.; Yi, D.L.; Fang, Y.Y.; Wu, M.Y.; Wang, X.D.; Tang, Y.; et al. Dual-silica template-mediated synthesis of nitrogen-doped mesoporous carbon nanotubes for supercapacitor applications. Small 2022, 19, 2205725. [Google Scholar]
- Fan, J.W.; Chen, Y.Y.; Chen, X.Q.; Wu, Z.X.; Teng, W.; Zhang, W.X. Atomically dispersed iron enables high-efficiency electrocatalytic conversion of nitrate to dinitrogen on a N-coordinated mesoporous carbon architecture. Appl. Catal. B Environ. 2023, 320, 121983. [Google Scholar]
- Cheng, Q.; Yang, L.; Zou, L.; Zou, Z.; Chen, C.; Hu, Z.; Yang, H. Single Cobalt Atom and N Codoped Carbon Nanofibers as Highly Durable Electrocatalyst for Oxygen Reduction Reaction. ACS Catal. 2017, 7, 6864–6871. [Google Scholar]
- Fuertes, A.B.; Valle-Vigon, P.; Sevilla, M. One-step synthesis of silica@resorcinol-formaldehyde spheres and their application for the fabrication of polymer and carbon capsules. Chem. Commun. 2012, 48, 6124–6126. [Google Scholar]
- Du, J.; Zong, S.; Zhang, Y.; Hou, S. Preparation of N/O co-doped porous carbon by a one-step activation method for supercapacitor electrode materials. J. Colloid. Inter. Surf. 2020, 565, 245–253. [Google Scholar]
- Ren, C.C.; Li, H.B.; Li, R.; Xu, S.L.; Wei, D.H.; Kang, W.J.; Wang, L.; Jia, L.P.; Yang, B.C.; Liu, J.F.A. Electrocatalytic study of a 1,10-phenanthroline-cobalt(ii) metal complex catalyst supported on reduced graphene oxide towards oxygen reduction reaction. RSC Adv. 2016, 6, 33302–33307. [Google Scholar]
- Zhang, H.R.; Huang, K.B.; Chen, Z.F.; Liu, Y.C.; Liu, Y.N.; Meng, T.; Qin, Q.P.; Zou, B.Q.; Liang, H. Cobalt(II) 8-hydroxyquinoline complexes: Structure, cytotoxicity and action mechanism. Medchemcomm 2016, 7, 806–812. [Google Scholar]
- Zaman, S.; Tian, X.; Xia, B.Y. Bridging oxygen reduction performance gaps in half and full cells: Challenges and perspectives. Mater. Chem. Front. 2023. [CrossRef]
- Han, Y.; Wang, Y.; Chen, W.; Xu, R.; Zheng, L.; Zhang, J.; Luo, J.; Shen, R.; Zhu, Y.; Cheong, W.; et al. Hollow N-Doped Carbon Spheres with Isolated Cobalt Single Atomic Sites: Superior Electrocatalysts for Oxygen Reduction. J. Am. Chem. Soc. 2017, 139, 48–17269. [Google Scholar]
- You, B.; Jiang, N.; Sheng, M.; Drisdell, W.S.; Yano, J.; Sun, Y. Bimetal–Organic Framework Self-Adjusted Synthesis of Support-Free Nonprecious Electrocatalysts for Efficient Oxygen Reduction. ACS Catal. 2015, 5, 7068–7076. [Google Scholar]
- Cheng, Q.; Han, S.; Mao, K.; Chen, C.; Yang, L.; Zou, Z.; Gu, M.; Hu, Z.; Yang, H. Co nanoparticle embedded in atomically-dispersed Co-N-C nanofibers for oxygen reduction with high activity and remarkable durability. Nano Energy. 2018, 52, 485–493. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, J.; Kim, J.G.; Han, S.; Cho, Y.; Pak, C. A Gram Scale Soft-Template Synthesis of Heteroatom Doped Nanoporous Hollow Carbon Spheres for Oxygen Reduction Reaction. Nanomaterials 2023, 13, 2555. https://doi.org/10.3390/nano13182555
Kang J, Kim JG, Han S, Cho Y, Pak C. A Gram Scale Soft-Template Synthesis of Heteroatom Doped Nanoporous Hollow Carbon Spheres for Oxygen Reduction Reaction. Nanomaterials. 2023; 13(18):2555. https://doi.org/10.3390/nano13182555
Chicago/Turabian StyleKang, Jisue, Jong Gyeong Kim, Sunghoon Han, Youngin Cho, and Chanho Pak. 2023. "A Gram Scale Soft-Template Synthesis of Heteroatom Doped Nanoporous Hollow Carbon Spheres for Oxygen Reduction Reaction" Nanomaterials 13, no. 18: 2555. https://doi.org/10.3390/nano13182555
APA StyleKang, J., Kim, J. G., Han, S., Cho, Y., & Pak, C. (2023). A Gram Scale Soft-Template Synthesis of Heteroatom Doped Nanoporous Hollow Carbon Spheres for Oxygen Reduction Reaction. Nanomaterials, 13(18), 2555. https://doi.org/10.3390/nano13182555








