Food Additive Solvents Increase the Dispersion, Solubility, and Cytotoxicity of ZnO Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
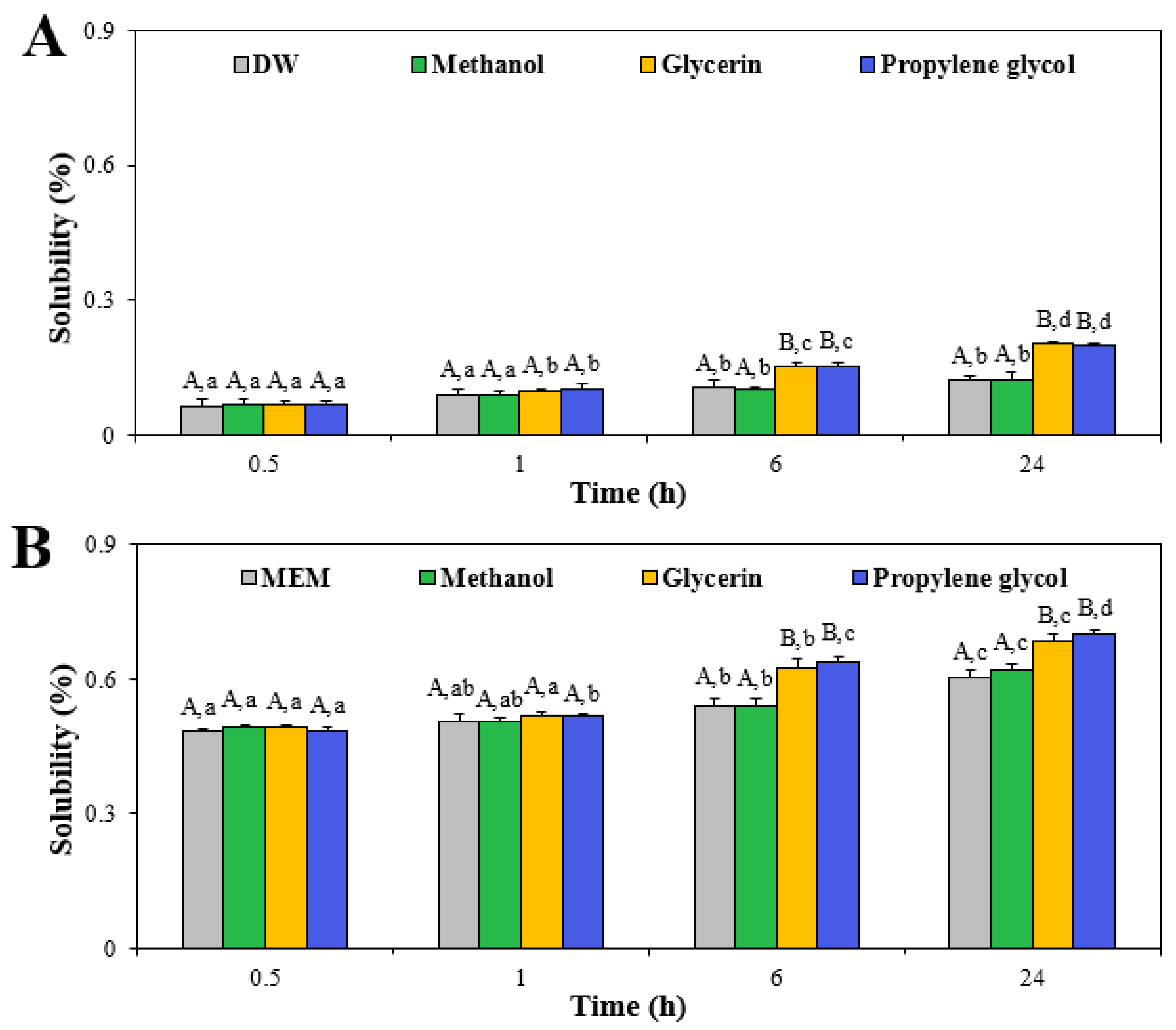
2.3. Solubility
2.4. Digestion and ICP-AES Analysis
2.5. Cell Culture
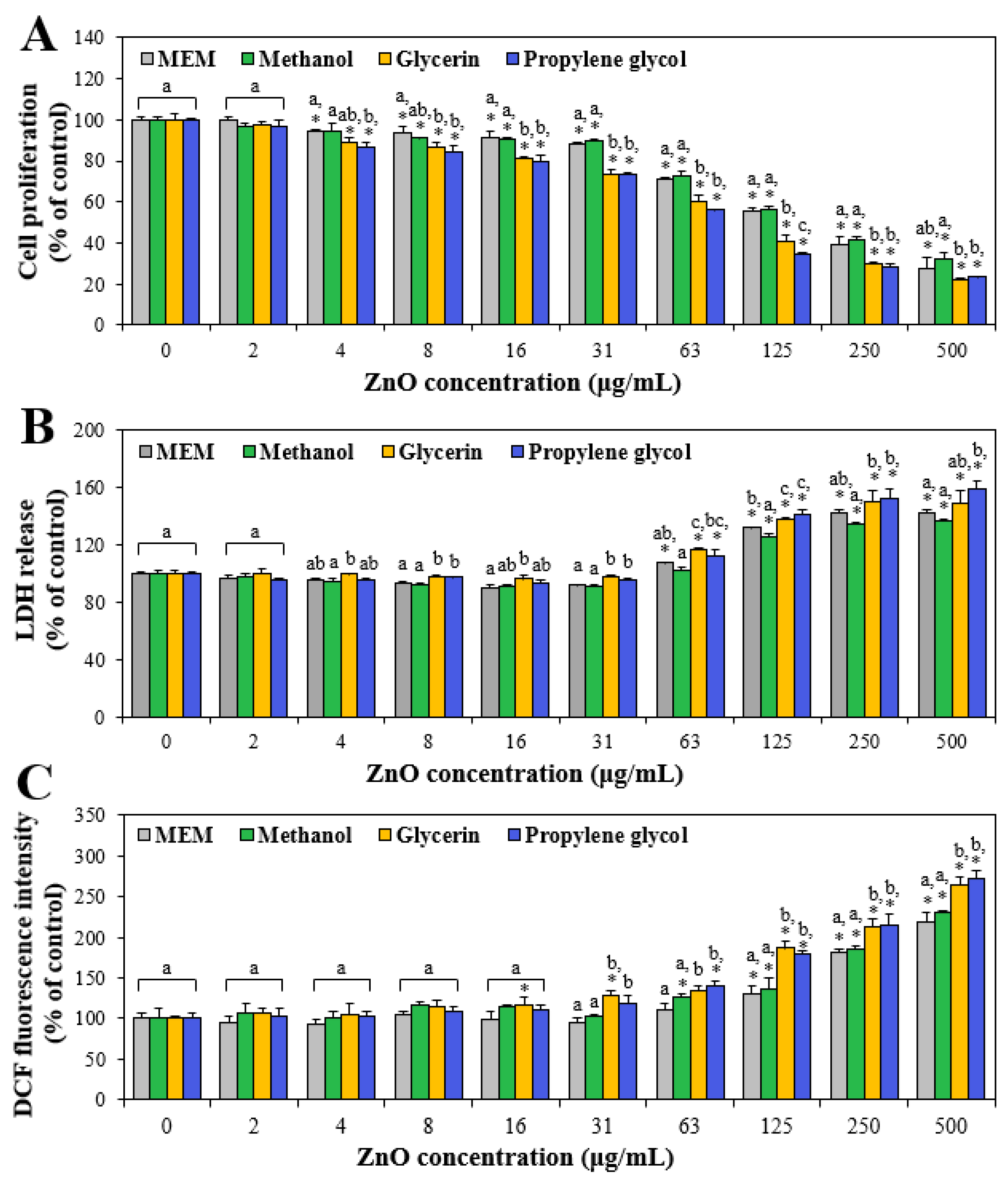
2.6. Cell Proliferation
2.7. Lactate Dehydrogenase (LDH) Leakage
2.8. Reactive Oxygen Species (ROS) Generation
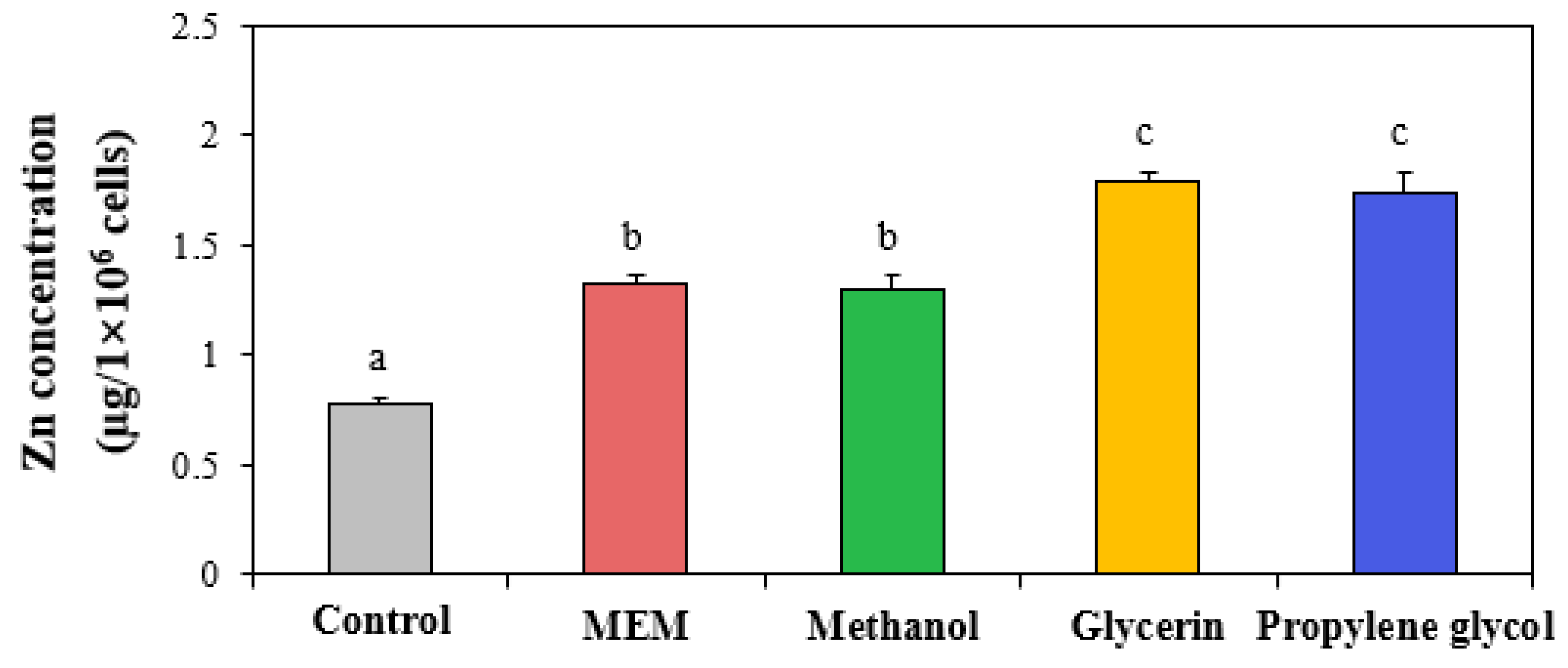
2.9. Cellular Uptake
2.10. Intestinal Transport
2.11. Membrane Permeability
2.12. Statistical Analysis
3. Results and Discussion
3.1. Characterization
3.2. Dissolution Property
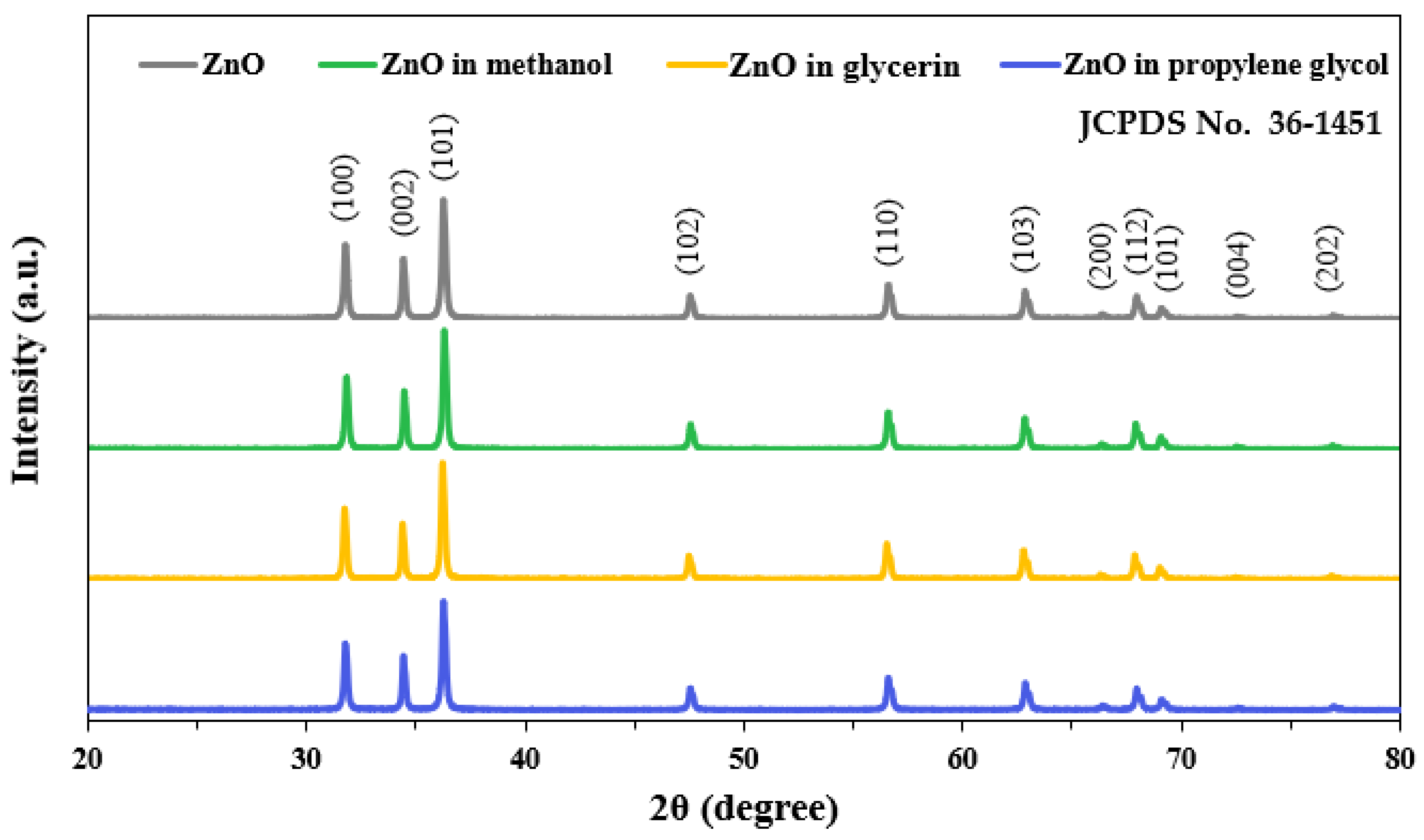
3.3. Crystal Structure
3.4. Cytotoxicity
3.5. Cellular Uptake
3.6. Intestinal Transport
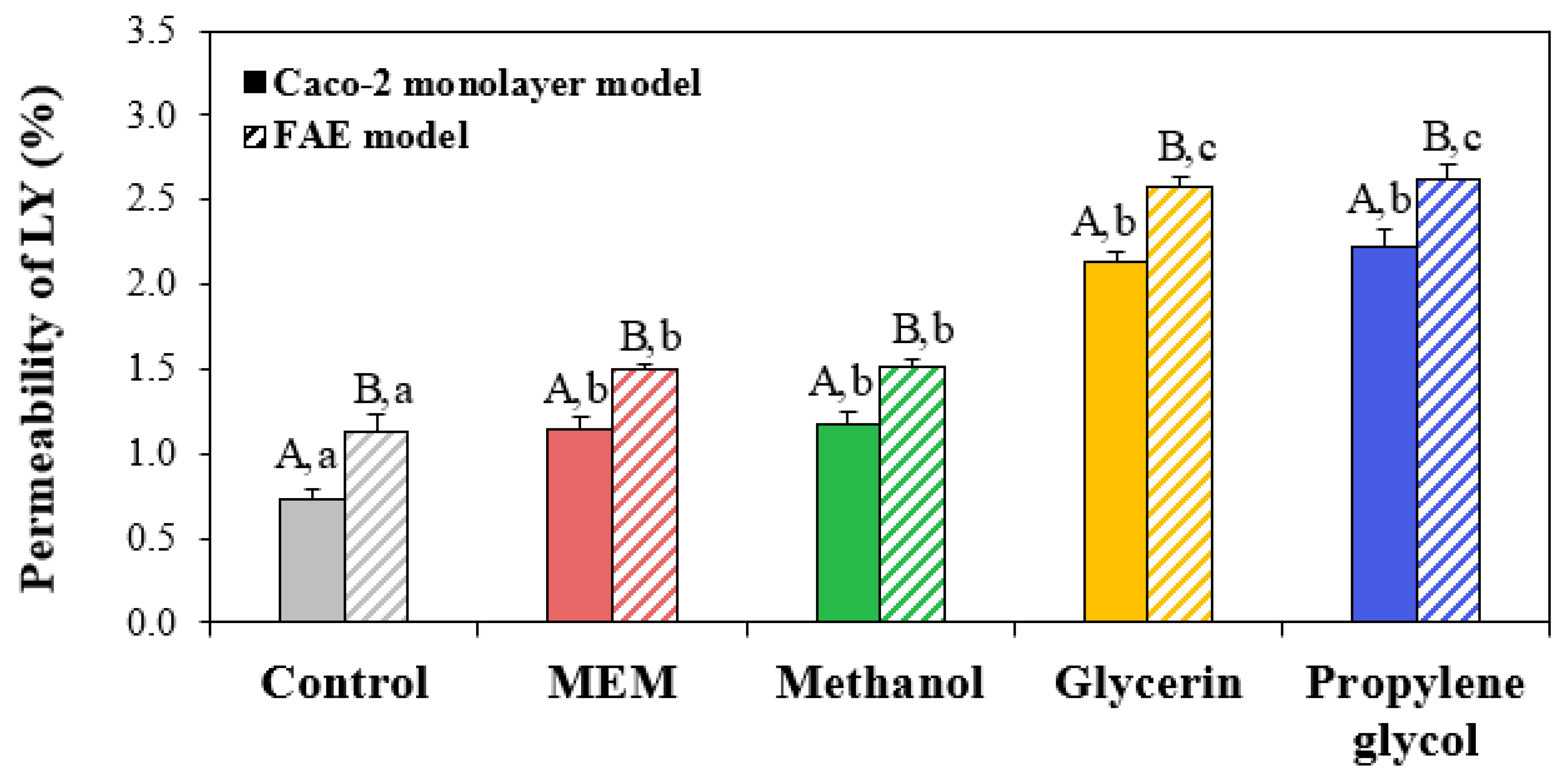
3.7. Membrane Permeability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- National Institutes of Health (NIH). Zinc: Fact Sheet for Health Professionals. Available online: https://ods.od.nih.gov/factsheets/Zinc-HealthProfessional (accessed on 10 August 2023).
- Stefanidou, M.; Maravelias, C.; Dona, A.; Spiliopoulou, C. Zinc: A multipurpose trace element. Arch. Toxicol. 2006, 80, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.I.; Sarmento-Ribeiro, A.B.; Goncalves, A.C. Zinc: From Biological Functions to Therapeutic Potential. Int. J. Mol. Sci. 2023, 24, 4822. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization (WHO). Environmental Health Criteria 221: Zinc; World Health Organization: Geneva, Switzerland, 2001; p. 360. [Google Scholar]
- U.S. Food and Drug Administration (FDA). Food Additive Status List. Available online: https://www.fda.gov/food/foodadditives-petitions/food-additive-status-list (accessed on 10 August 2023).
- Fosmire, G.J. Zinc toxicity. Am. J. Clin. Nutr. 1990, 51, 225–227. [Google Scholar] [CrossRef]
- Nriagu, J. Zinc toxicity in humans. In Encyclopedia of Environmental Health; Nriagu, J.O., Ed.; Elsevier: Hoboken, NJ, USA, 2011; pp. 801–807. [Google Scholar]
- Maret, W.; Sandstead, H.H. Zinc requirements and the risks and benefits of zinc supplementation. J. Trace Elem. Med. Biol. 2006, 20, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Saper, R.B.; Rash, R. Zinc: An essential micronutrient. Am. Fam. Physician 2009, 79, 768. [Google Scholar] [PubMed]
- Ge, W.; Zhao, Y.; Lai, F.-N.; Liu, J.-C.; Sun, Y.-C.; Wang, J.-J.; Cheng, S.-F.; Zhang, X.-F.; Sun, L.-L.; Li, L.; et al. Cutaneous applied nano-ZnO reduce the ability of hair follicle stem cells to differentiate. Nanotoxicology 2017, 11, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, D.; Narcy, A.; Durosoy, S.; Bordes, C.; Chevalier, Y. Dissolution kinetics of zinc oxide and its relationship with physicochemical characteristics. Powder Technol. 2021, 378, 746–759. [Google Scholar] [CrossRef]
- Bian, S.W.; Mudunkotuwa, I.A.; Rupasinghe, T.; Grassian, V.H. Aggregation and dissolution of 4 nm ZnO nanoparticles in aqueous environments: Influence of pH, ionic strength, size, and adsorption of humic acid. Langmuir 2011, 27, 6059–6068. [Google Scholar] [CrossRef]
- Yu, J.; Kim, H.-J.; Go, M.-R.; Bae, S.-H.; Choi, S.-J. ZnO Interactions with Biomatrices: Effect of Particle Size on ZnO-Protein Corona. Nanomaterials 2017, 7, 377. [Google Scholar] [CrossRef]
- Jeon, Y.R.; Yu, J.; Choi, S.J. Fate Determination of ZnO in Commercial Foods and Human Intestinal Cells. Int. J. Mol. Sci. 2020, 21, 433. [Google Scholar] [CrossRef]
- Duan, L.; Zhang, L.; Yan, F.; Liu, Z.; Bao, H.; Liu, T. Solubility of ZnO Nanoparticles in Food Media: An Analysis Using a Novel Semiclosed Dynamic System. J. Agric. Food Chem. 2021, 69, 11065–11073. [Google Scholar] [CrossRef]
- Youn, S.-M.; Choi, S.-J. Food Additive Zinc Oxide Nanoparticles: Dissolution, Interaction, Fate, Cytotoxicity, and Oral Toxicity. Int. J. Mol. Sci. 2022, 23, 6074. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhang, Y.; Mao, Z.; Yu, D.; Gao, C. Toxicity of ZnO nanoparticles to macrophages due to cell uptake and intracellular release of zinc ions. J. Nanosci. Nanotechnol. 2014, 14, 5688–5896. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Zhang, J.; Guo, J.; Zhang, J.; Ding, F.; Li, L.; Sun, Z. Role of the dissolved zinc ion and reactive oxygen species in cytotoxicity of ZnO nanoparticles. Toxicol. Lett. 2010, 199, 389–397. [Google Scholar] [CrossRef]
- Condello, M.; Berardis, B.D.; Ammendolia, M.G.; Barone, F.; Condello, G.; Degan, P.; Meschini, S. ZnO nanoparticle tracking from uptake to genotoxic damage in human colon carcinoma cells. Toxicology 2016, 35, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Prach, M.; Stone, V.; Proudfoot, L. Zinc oxide nanoparticles and monocytes: Impact of size, charge and solubility on activation status. Toxicol. Appl. Pharmacol. 2013, 266, 19–26. [Google Scholar] [CrossRef]
- Kang, T.; Guan, R.; Chen, X.; Song, Y.; Jiang, H.; Zhao, J. In vitro toxicity of different-sized ZnO nanoparticles in Caco-2 cells. Nanoscale Res. Lett. 2013, 8, 496. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Choi, S.-J. Particle Size and Biological Fate of ZnO Do Not Cause Acute Toxicity, but Affect Toxicokinetics and Gene Expression Profiles in the Rat Livers after Oral Administration. Int. J. Mol. Sci. 2021, 22, 1698. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Feng, W.Y.; Wang, T.C.; Jia, G.; Wang, M.; Shi, J.W.; Zhang, F.; Zhao, Y.L.; Chai, Z.F. Acute toxicity of nano- and micro-scale zinc powder in healthy adult mice. Toxicol. Lett. 2006, 161, 115–123. [Google Scholar] [CrossRef]
- Go, M.-R.; Yu, J.; Bae, S.-H.; Kim, H.-J.; Choi, S.-J. Effects of Interactions between ZnO Nanoparticles and Saccharides on Biological Responses. Int. J. Mol. Sci. 2018, 19, 486. [Google Scholar] [CrossRef]
- Bae, S.-H.; Yu, J.; Lee, T.G.; Choi, S.-J. Protein Food Matrix—ZnO Nanoparticle Interactions Affect Protein Conformation, but May not Be Biological Responses. Int. J. Mol. Sci. 2018, 19, 3926. [Google Scholar] [CrossRef] [PubMed]
- Anders, C.B.; Chess, J.J.; Wingett, D.G.; Punnoose, A. Serum Proteins Enhance Dispersion Stability and Influence the Cytotoxicity and Dosimetry of ZnO Nanoparticles in Suspension and Adherent Cancer Cell Models. Nanoscale Res. Lett. 2015, 10, 448. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.-B.; Yu, J.; Choi, S.-J. Interaction between ZnO Nanoparticles and Albumin and Its Effect on Cytotoxicity, Cellular Uptake, Intestinal Transport, Toxicokinetics, and Acute Oral Toxicity. Nanomaterials 2021, 11, 2922. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-B.; Yoo, N.-K.; Choi, S.-J. Interactions between ZnO Nanoparticles and Polyphenols Affect Biological Responses. Nanomaterials 2022, 12, 3337. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food and Drug Administration (FDA). CFR-Code of Federal Regulations Title 21 CFR 173.250. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=173.250 (accessed on 10 August 2023).
- Joint FAO/WHO Expert Committee on Food Additives (JECFA). Combined Compendium of Food Additive Specifications, Monograph 1. Available online: https://www.fao.org/food/food-safety-quality/scientific-advice/jecfa/jecfa-additives/detail/en/c/133/ (accessed on 10 August 2023).
- Government of Canada. Chemical Substance—Methyl Alcohol. Available online: https://webprod.hc-sc.gc.ca/nhpid-bdipsn/ingredReq.do?id=3463 (accessed on 10 August 2023).
- Ministry of Food and Drug Safety (MFDS). Food Code. Available online: https://various.foodsafetykorea.go.kr/fsd/#/ext/Document/FC (accessed on 10 August 2023).
- EFSA Panel on Food Additives and Nutrient Sources Added to Food (ANS). Re-evaluation of glycerol (E 422) as a food additive. EFSA J. 2017, 15, e04720. [Google Scholar]
- U.S. Food and Drug Administration (FDA). CFR-Code of Federal Regulations Title 21 CFR 182.1320. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=182.1320 (accessed on 10 August 2023).
- U.S. Food and Drug Administration (FDA). CFR-Code of Federal Regulations Title 21 CFR 184.1666. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=184.1666 (accessed on 11 August 2023).
- Blekas, G.A. Food Additives: Classification, Uses and Regulation. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 731–736. [Google Scholar]
- Fowles, J.R.; Banton, M.I.; Pottenger, L.J. A toxicological review of the propylene glycols. Crit. Rev. Toxicol. 2013, 43, 363–390. [Google Scholar] [CrossRef]
- des Rieux, A.; Ragnarsson, E.G.E.; Gullberg, E.; Préat, V.; Schneider, Y.-J.; Artursson, P. Transport of nanoparticles across an in vitro model of the human intestinal follicle associated epithelium. Eur. J. Pharm. Sci. 2005, 25, 455–465. [Google Scholar] [CrossRef]
- Silva, C.M.; Veiga, F.; Ribeiro, A.J.; Zerrouk, N.; Arnaud, P. Effect of Chitosan-Coated Alginate Microspheres on the Permeability of Caco-2 Cell Monolayers. Drug Dev. Ind. Pharm. 2006, 32, 1079–1088. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 1030, Propylene Glycol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Propylene-Glycol (accessed on 22 August 2023).
- National Center for Biotechnology Information. PubChem Compound Summary for CID 887, Methanol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Methanol (accessed on 22 August 2023).
- Bacchetta, R.; Maran, B.; Marelli, M.; Santo, N.; Tremolada, P. Role of soluble zinc in ZnO nanoparticle cytotoxicity in Daphnia magna: A morphological approach. Environ. Res. 2016, 148, 376–385. [Google Scholar] [CrossRef]
- Mudunkotuwa, I.A.; Rupasinghe, T.; Wu, C.-M.; Grassian, V.H. Dissolution of ZnO Nanoparticles at Circumneutral pH: A Study of Size Effects in the Presence and Absence of Citric Acid. Langmuir 2012, 28, 396–403. [Google Scholar] [CrossRef]
- Cho, W.-S.; Duffin, R.; Howie, S.E.; Scotton, C.J.; Wallace, W.A.; MacNee, W.A.; MacNee, M.; Bradley, M.; Megson, I.L.; Donaldson, K. Progressive severe lung injury by zinc oxide nanoparticles; the role of Zn2+ dissolution inside lysosomes. Part. Fibre Toxicol. 2011, 8, 27. [Google Scholar] [CrossRef] [PubMed]
- Talam, S.; Karumuri, S.R.; Gunnam, N. Synthesis, Characterization, and Spectroscopic Properties of ZnO Nanoparticles. ISRN Nanotechnology 2012, 2012, 372505. [Google Scholar] [CrossRef]
- Gonçalves, N.S.; Carvalho, J.A.; Lima, Z.M.; Sasaki, J.M. Size–strain study of NiO nanoparticles by X-ray powder diffraction line broadening. Mater. Lett. 2012, 72, 36–38. [Google Scholar] [CrossRef]
- Chatterjee, A.K. X-Ray Diffraction; Ramachandran, V.S., Beaudoin, J., Eds.; Building Materials Series: Hoboken, NJ, USA, 2000; pp. 275–332. [Google Scholar]
- Lee, S.H.; Lee, H.R.; Kim, Y.R. Toxic response of zinc oxide nanoparticles in human epidermal keratinocyte HaCaT cells. Toxicol. Environ. Health Sci. 2012, 4, 14–18. [Google Scholar] [CrossRef]
- Chen, P.; Wang, H.; He, M.; Chen, B.; Yang, B.; Hu, B. Size-dependent cytotoxicity study of ZnO nanoparticles in HepG2 cells. Ecotoxicol. Environ. Saf. 2019, 171, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-N.; Zhang, M.; Xia, L.; Zhang, J.; Xing, G. The Toxic Effects and Mechanisms of CuO and ZnO Nanoparticles. Materials 2012, 5, 2850–2871. [Google Scholar] [CrossRef]
- Yu, J.; Baek, M.; Chung, H.E.; Choi, S.J. Effects of physicochemical properties of zinc oxide nanoparticles on cellular uptake. J. Phys. Conf. Ser. 2011, 304, 012007. [Google Scholar] [CrossRef]
- Preedia Babu, E.; Subastri, A.; Suyavaran, A.; Premkumar, K.; Sujatha, V.; Aristatile, B.; Alshammaari, G.M.; Dharuman, V.; Thirunavukkarasu, C.E. Size Dependent Uptake and Hemolytic Effect of Zinc Oxide Nanoparticles on Erythrocytes and Biomedical Potential of ZnO-Ferulic acid Conjugates. Sci. Rep. 2017, 7, 4203. [Google Scholar] [CrossRef]
- Zhu, M.; Nie, G.; Meng, H.; Xia, T.; Nel, A.; Zhao, Y. Physicochemical properties determine nanomaterial cellular uptake, transport, and fate. Acc. Chem. Res. 2012, 46, 622–631. [Google Scholar] [CrossRef]
- Firoozabadi, T.P.; Shankayi, Z.; Izadi, A.; Firoozabadi, S.M.P. Can Lucifer Yellow Indicate Correct Permeability of Biological Cell Membrane under An Electric and Magnetic Field? Cell J. 2015, 16, 560–563. [Google Scholar]
- Lin, Y.C.; Phua, S.C.; Lin, B.; Inoue, T. Visualizing molecular diffusion through passive permeability barriers in cells: Conventional and novel approaches. Curr. Opin. Chem. Biol. 2013, 17, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Santbergen, M.J.C.; van der Zande, M.; Gerssen, A.; Bouwmeester, H.; Nielen, M.W.F. Dynamic in vitro intestinal barrier model coupled to chip-based liquid chromatography mass spectrometry for oral bioavailability studies. Anal. Bioanal. Chem. 2020, 412, 1111–1122. [Google Scholar] [CrossRef] [PubMed]

| Dispersant | Hydrodynamic Diameters (nm) | Zeta Potential (mV) | ||||
|---|---|---|---|---|---|---|
| Dispersion for 30 Min | Dispersion for 24 h | Dispersion for 30 Min | ||||
| Without Dilution | Dilution in DW or MEM | Without Dilution | Dilution in DW or MEM | Without Dilution | Dilution in DW or MEM | |
| DDW | 285.0 ± 5.2 a | 261.0 ± 0.5 b | 25.7 ± 1.4 a | |||
| Methanol in DDW | 284.6 ± 7.4 a | 282.6 ± 11.9 a | 260.9 ± 0.4 b | 261.1 ± 0.4 b | 24.9 ± 0.6 a | 25.0 ± 0.4 a |
| Glycerin in DDW | 246.7 ± 13.8 b | 246.4 ± 5.8 b | 224.2 ± 1.0 c | 223.2 ± 0.8 c | 25.6 ± 0.3 a | 25.4 ± 0.4 a |
| Propylene glycol in DDW | 247.3 ± 14.4 b | 247.3 ± 7.3 b | 224.3 ± 0.6 c | 223.9 ± 0.8 c | 25.5 ± 0.3 a | 25.3 ± 0.5 a |
| MEM | 246.2 ± 3.0 a | 230.4 ± 1.2 bc | 8.5 ± 0.3 a | |||
| Methanol in MEM | 247.4 ± 1.2 a | 248.0 ± 1.0 a | 229.5 ± 2.5 bc | 230.2 ± 1.9 c | −8.5 ± 0.4 a | −8.4 ± 0.2 a |
| Glycerin in MEM | 234.4 ± 2.1 bc | 233.1 ± 0.4 bc | 213.7 ± 2.0 d | 210.6 ± 1.9 d | −8.6 ± 0.5 a | −8.5 ± 0.2 a |
| Propylene glycol in MEM | 235.0 ± 2.0 bc | 236.1 ± 3.5 b | 213.4 ± 2.3 d | 213.0 ± 2.1 d | −8.5 ± 0.4 a | −8.5 ± 0.2 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-I.; Kwon, R.-Y.; Choi, S.-J. Food Additive Solvents Increase the Dispersion, Solubility, and Cytotoxicity of ZnO Nanoparticles. Nanomaterials 2023, 13, 2573. https://doi.org/10.3390/nano13182573
Lee H-I, Kwon R-Y, Choi S-J. Food Additive Solvents Increase the Dispersion, Solubility, and Cytotoxicity of ZnO Nanoparticles. Nanomaterials. 2023; 13(18):2573. https://doi.org/10.3390/nano13182573
Chicago/Turabian StyleLee, Hye-In, Ri-Ye Kwon, and Soo-Jin Choi. 2023. "Food Additive Solvents Increase the Dispersion, Solubility, and Cytotoxicity of ZnO Nanoparticles" Nanomaterials 13, no. 18: 2573. https://doi.org/10.3390/nano13182573
APA StyleLee, H.-I., Kwon, R.-Y., & Choi, S.-J. (2023). Food Additive Solvents Increase the Dispersion, Solubility, and Cytotoxicity of ZnO Nanoparticles. Nanomaterials, 13(18), 2573. https://doi.org/10.3390/nano13182573






