Interface Engineering of CoFe-LDH Modified Ti: α-Fe2O3 Photoanode for Enhanced Photoelectrochemical Water Oxidation
Abstract
:1. Introduction
2. Experimental Details
2.1. Synthesis of Photoanodes
2.2. Characterization
2.3. Photoelectrochemical Measurements
3. Results and Discussion
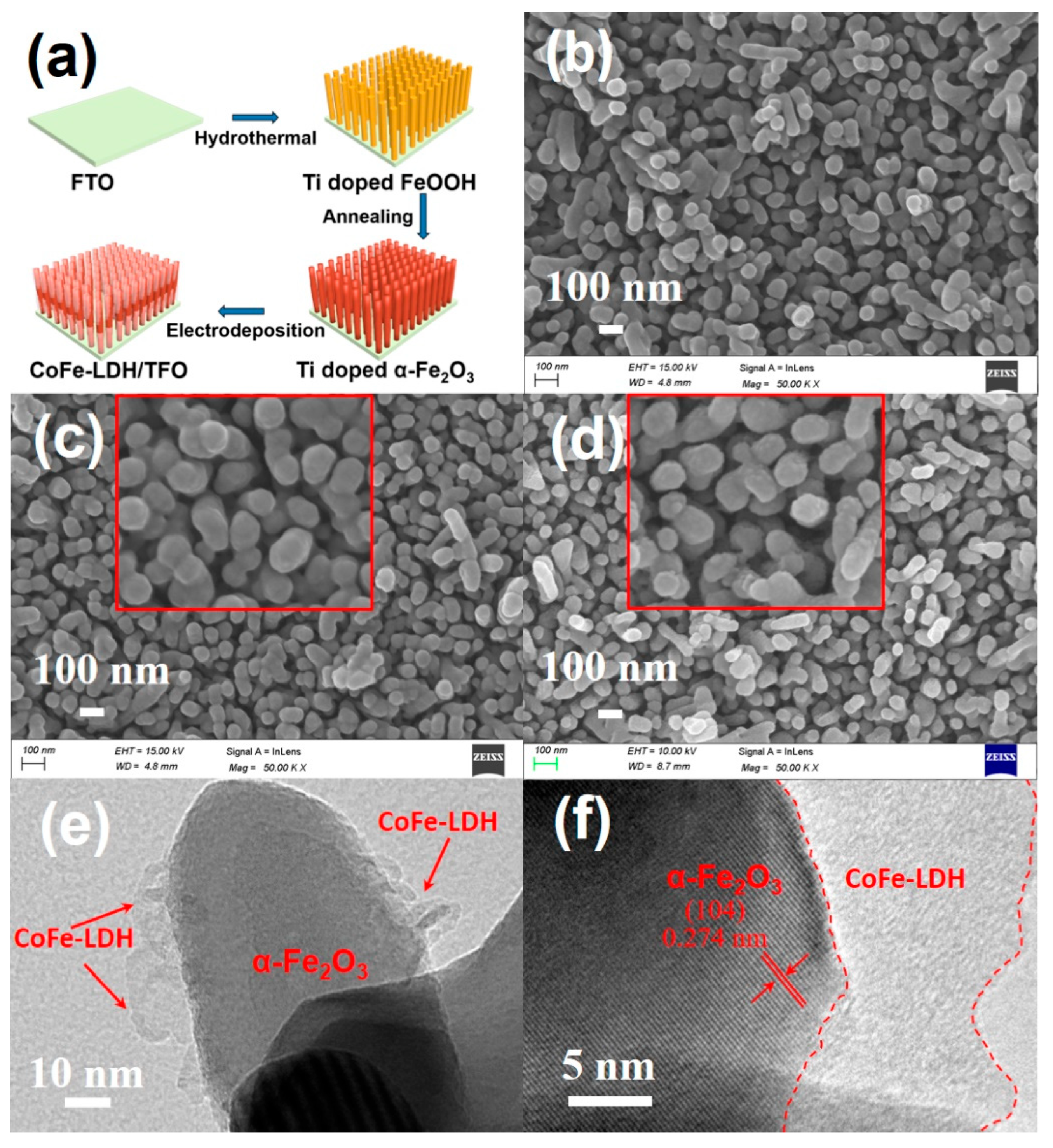
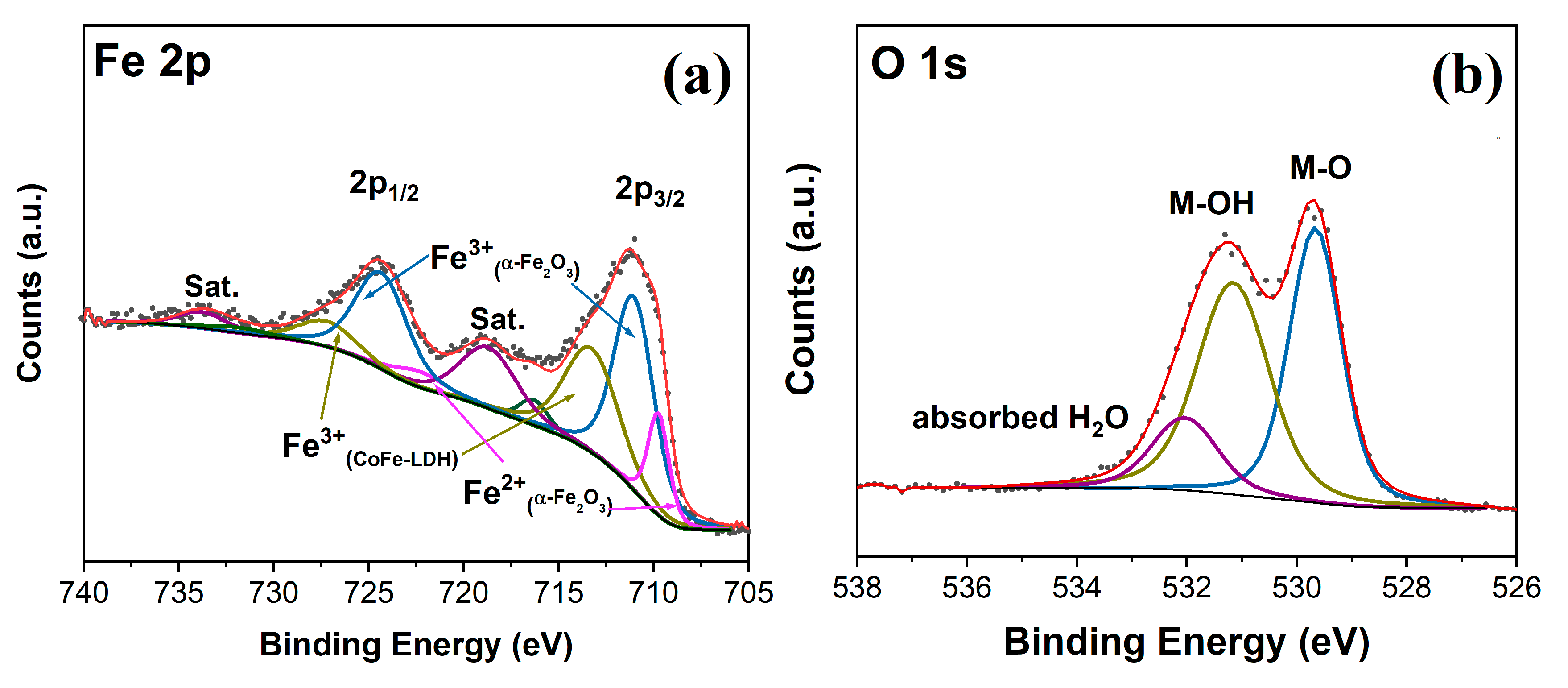
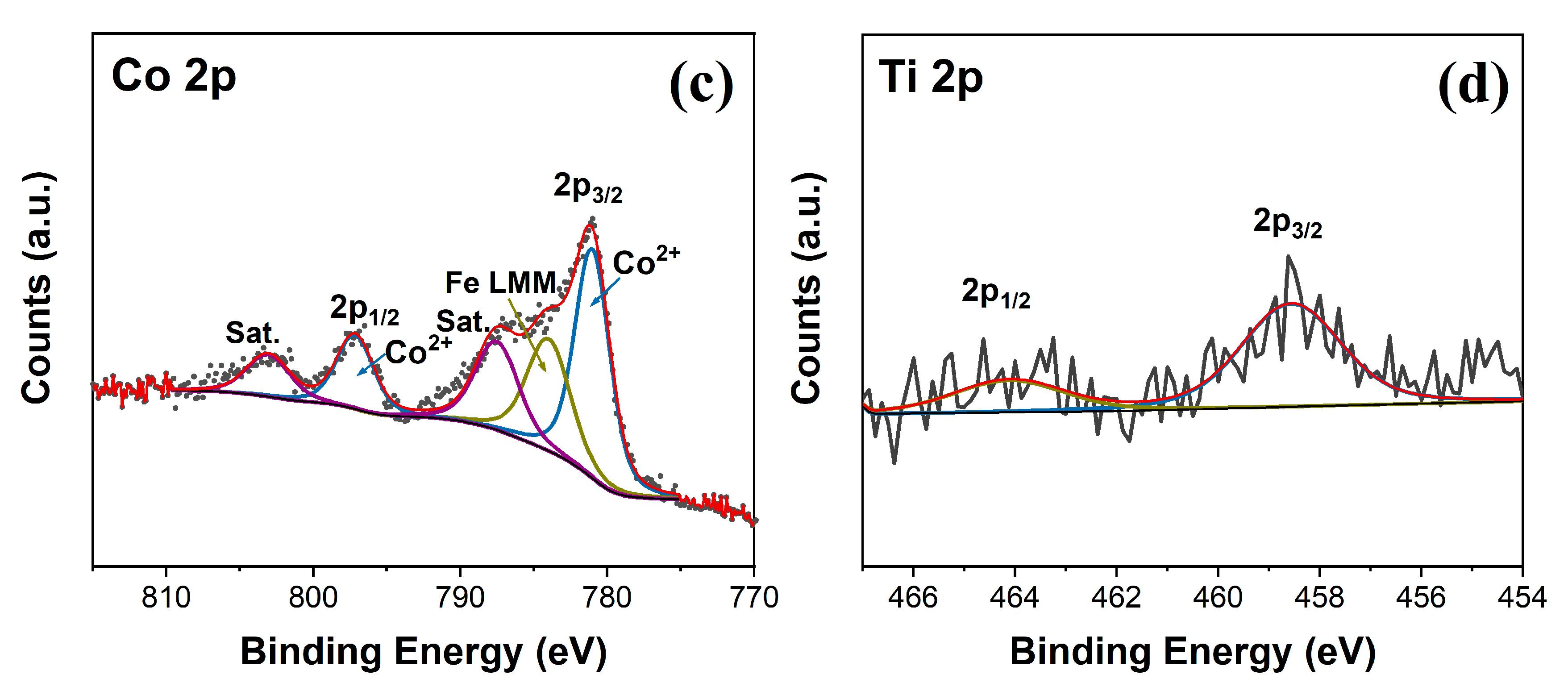
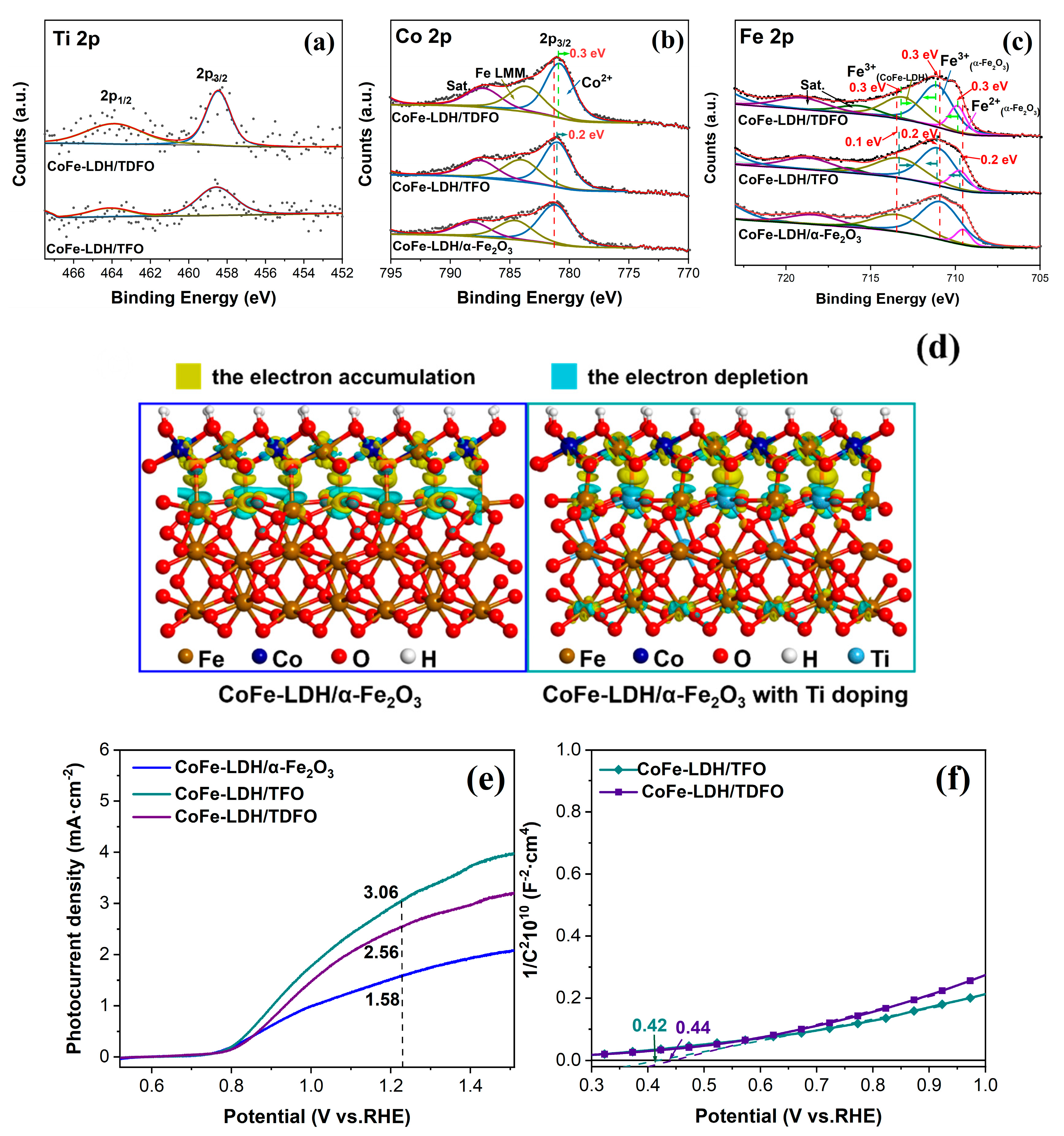
3.1. C and Characterization of the As-Synthesized Photoanode Materials
3.2. The PEC Performance of Water Oxidation for Photoanodes
3.3. The Effect of Ti Dopants and CoFe-LDH Deposition on the Charge Transfer Process of the Photoanodes
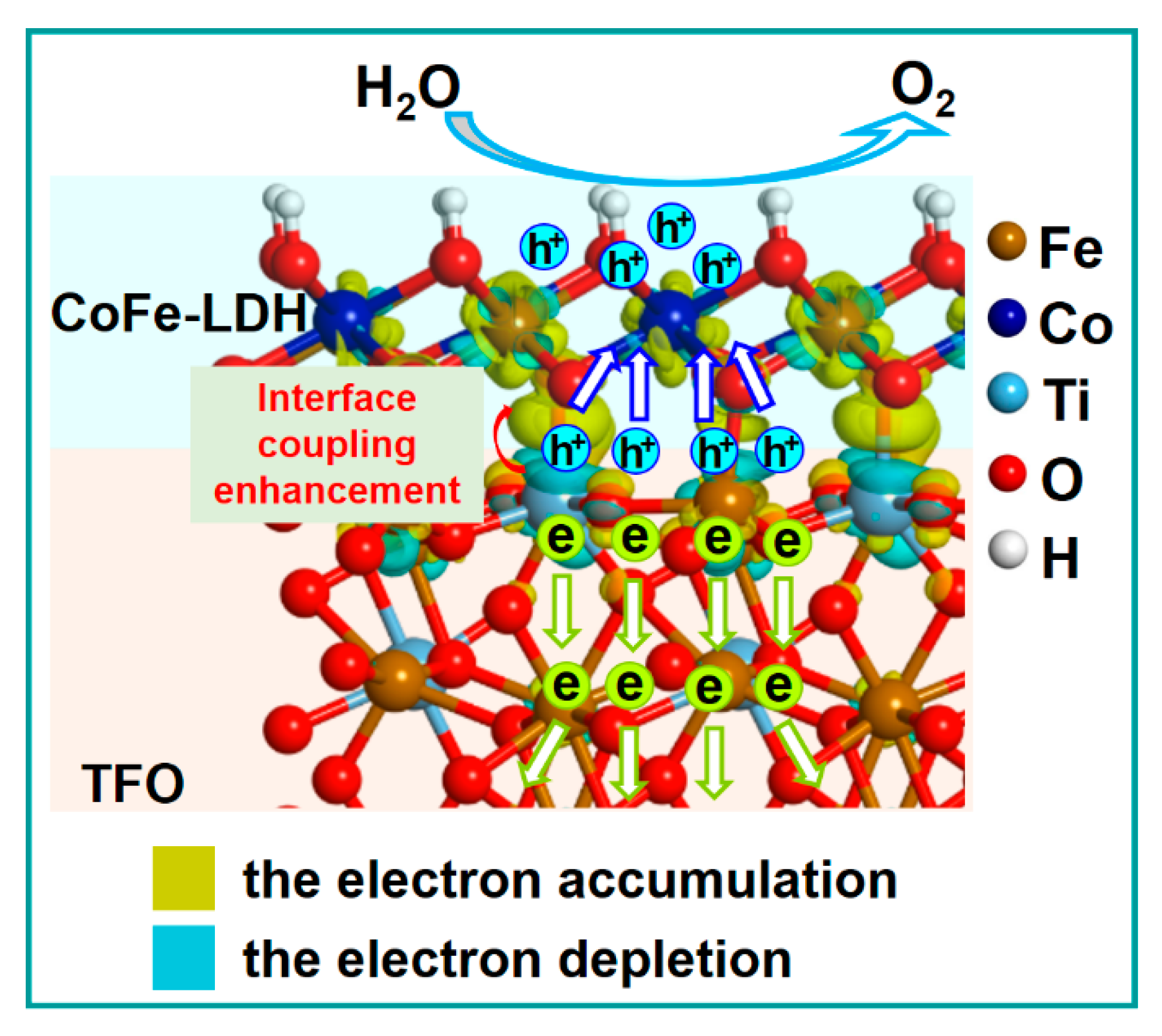
3.4. The Promoting Mechanism of CoFe-LDH/TFO Photoanodes for Water Oxidation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ta, X.M.C.; Daiyan, R.; Nguyen, T.K.A.; Amal, R.; Thanh, T.P.; Tricoli, A. Alternatives to Water Photooxidation for Photoelectrochemical Solar Energy Conversion and Green H2 Production. Adv. Energy Mater. 2022, 12, 202201358. [Google Scholar] [CrossRef]
- Gondolini, A.; Sangiorgi, N. A Sangiorgi and A Sanson. Photoelectrochemical Hydrogen Production by Screen-Printed Copper Oxide Electrodes. Energies 2021, 14, 2942. [Google Scholar] [CrossRef]
- Yi, S.S.; Wang, Z.Y.; Li, H.M.; Zafar, Z.; Zhang, Z.T.; Zhang, L.Y.; Chen, D.L.; Liu, Z.Y.; Yue, X.Z. Coupling effects of indium oxide layer on hematite enabling efficient photoelectrochemical water splitting. Appl. Catal. B Environ. 2021, 283, 119649. [Google Scholar] [CrossRef]
- Yang, W.; Prabhakar, R.R.; Tan, J.; Tilley, S.D.; Moon, J. Strategies for enhancing the photocurrent, photovoltage, and stability of photoelectrodes for photoelectrochemical water splitting. Chem. Soc. Rev. 2019, 48, 4979–5015. [Google Scholar] [CrossRef]
- Zhang, H.; Li, P.; Zhou, H.; Xu, J.; Jiang, Q.; Hadden, J.H.L.; Wang, Y.; Wang, M.; Chen, S.; Xie, F.; et al. Unravelling the synergy of oxygen vacancies and gold nanostars in hematite for the electrochemical and photoelectrochemical oxygen evolution reaction. Nano Energy 2022, 94, 106968. [Google Scholar] [CrossRef]
- Yoon, K.Y.; Park, J.; Lee, H.; Seo, J.H.; Kwak, M.; Lee, J.H.; Jang, J. Unveiling the Role of the Ti Dopant and Viable Si Doping of Hematite for Practically Efficient Solar Water Splitting. ACS Catal. 2022, 12, 5112–5122. [Google Scholar] [CrossRef]
- Ahart, C.S.; Rosso, K.M.; Blumberger, J. Electron and Hole Mobilities in Bulk Hematite from Spin-Constrained Density Functional Theory. J. Am. Chem. Soc. 2022, 144, 4623–4632. [Google Scholar] [CrossRef]
- Yang, G.L.; Li, Y.X.; Pang, H.; Chang, K.; Ye, J.H. Ultrathin Cobalt–Manganese Nanosheets: An Efficient Platform for Enhanced Photoelectrochemical Water Oxidation with Electron-Donating Effect. Adv. Funct. Mater. 2019, 29, 1904622. [Google Scholar] [CrossRef]
- Bedin, K.C.; Muche, D.N.F.; Melo, M.A.; Freitas, A.L.M.; Goncalves, R.V.; Souza, F.L. Role of Cocatalysts on Hematite Photoanodes in Photoelectrocatalytic Water Splitting: Challenges and Future Perspectives. ChemCatchem 2020, 12, 3156–3169. [Google Scholar] [CrossRef]
- Wang, P.P.; Fu, P.; Ma, J.P.; Gao, Y.Y.; Li, Z.; Wang, H.; Fan, F.T.; Shi, J.Y.; Li, C. Ultrathin Cobalt Oxide Interlayer Facilitated Hole Storage for Sustained Water Oxidation over Composited Tantalum Nitride Photoanodes. ACS Catal. 2021, 11, 12736–12744. [Google Scholar] [CrossRef]
- Ning, X.M.; Du, P.Y.; Han, Z.G.; Chen, J.; Lu, X.Q. Insight into the Transition-Metal Hydroxide Cover Layer for Enhancing Photoelectrochemical Water Oxidation. Angew. Chem. Int. Ed. 2021, 60, 3504–3509. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, L.; Zhang, J.; Zhang, W.; Yao, W.; Jiang, G. NiFe-layered Double Hydroxide/Vertical Bi2WO6 Nanoplate Arrays with Oriented {001} Facets Supported on ITO Glass: Improved Photoelectrocatalytic Activity and Mechanism Insight. ChemCatchem 2021, 13, 3414–3420. [Google Scholar] [CrossRef]
- Sun, C.; Liu, J.; Li, L.; Cheng, J.; Peng, Y.; Xie, Q. Photoanode synthesis of ammonia based on a light reflex strategy and NiCe-layered double hydroxide and oxygen-vacancy Bi2O3 catalysts. Chem. Eng. J. 2023, 464, 142447. [Google Scholar] [CrossRef]
- Wang, H.; Xia, Y.; Wang, X.; Han, Y.; Jiao, X.; Chen, D. Interfacial Coupling Effect on Electron Transport in Hierarchical TaON/Au/ZnCo-LDH Photoanode with Enhanced Photoelectrochemical Water Oxidation. ACS Appl. Mater. Inter. 2019, 11, 33062–33073. [Google Scholar] [CrossRef]
- Zhong, Y.; Wu, C.; Jia, X.; Sun, S.; Chen, D.; Yao, W.; Ding, H.; Zhang, J.; Ma, T. Coupling of self-healing atomic layer CoAl-LDH onto Mo:BiVO4 photoanode for fast surface charge transfer toward stable and high-performance water splitting. Chem. Eng. J. 2023, 465, 142893. [Google Scholar] [CrossRef]
- Chung, D.Y.; Lopes, P.P.; Martins, P.F.B.D.; He, H.; Kawaguchi, T.; Zapol, P.; You, H.; Tripkovic, D.; Strmcnik, D.; Zhu, Y.; et al. Dynamic stability of active sites in hydr(oxy)oxides for the oxygen evolution reaction. Nat. Energy 2020, 5, 222–230. [Google Scholar] [CrossRef]
- Hung, S.F.; Chan, Y.T.; Chang, C.C.; Tsai, M.K.; Liao, Y.F.; Hiraoka, N.; Hsu, C.S.; Chen, H.M. Identification of Stabilizing High-Valent Active Sites by Operando High-Energy Resolution Fluorescence-Detected X-ray Absorption Spectroscopy for High-Efficiency Water Oxidation. J. Am. Chem. Soc. 2018, 140, 17263–17270. [Google Scholar] [CrossRef]
- Bhowmik, T.; Kundu, M.K.; Barman, S. CoFe Layered Double Hydroxide Supported on Graphitic Carbon Nitrides: An Efficient and Durable Bifunctional Electrocatalyst for Oxygen Evolution and Hydrogen Evolution Reactions. Acs Appl. Energy Mater. 2018, 1, 1200–1209. [Google Scholar] [CrossRef]
- Xu, X.; Jin, S.; Yang, C.; Pan, J.; Du, W.; Hu, J.; Zeng, H.; Zhou, Y. Engineering Interfaces to Steer Hole Dynamics of BiVO4 Photoanodes for Solar Water Oxidation. Sol. RRL 2019, 3, 1900115. [Google Scholar] [CrossRef]
- Dhandole, L.K.; Anushkkaran, P.; Chae, W.-S.; Chung, H.S.; Lee, H.H.; Choi, S.H.; Cho, M.; Jang, J.S. Efficient charge transfers in hematite photoanode integrated by fluorine and zirconia co-doping for photoelectrochemical water splitting. Chem. Eng. J. 2022, 446, 136957. [Google Scholar] [CrossRef]
- Chen, S.; Huang, D.L.; Xu, P.A.; Xue, W.J.; Lei, L.; Cheng, M.; Wang, R.Z.; Liu, X.G.; Deng, R. Semiconductor-based photocatalysts for photocatalytic and photoelectrochemical water splitting: Will we stop with photocorrosion? J. Mater. Chem. A 2020, 8, 2286–2322. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, Y.; Yao, R.; Li, N.; Wang, M.; Ren, H.; Li, J.; Zhao, C. Realizing high performance solar water oxidation for Ti-doped hematite nanoarrays by synergistic decoration with ultrathin cobalt-iron phosphate nanolayers. Chem. Eng. J. 2019, 355, 49–57. [Google Scholar] [CrossRef]
- Apriandanu, D.O.B.; Nomura, S.; Nakayama, S.; Tateishi, C.; Amano, F. Effect of two-step annealing on photoelectrochemical properties of hydrothermally prepared Ti-doped Fe2O3 films. Catal. Today 2023, 411–412, 113826. [Google Scholar] [CrossRef]
- Bai, J.; Gao, R.-T.; Guo, X.; He, J.; Liu, X.; Zhang, X.; Wang, L. Reduction of charge carrier recombination by Ce gradient doping and surface polarization for solar water splitting. Chem. Eng. J. 2022, 448, 137602. [Google Scholar] [CrossRef]
- Quang, N.D.; Van, P.C.; Majumder, S.; Jeong, J.-R.; Kim, D.; Kim, C. Rational construction of S-doped FeOOH onto Fe2O3 nanorods for enhanced water oxidation. J. Colloid Interf. Sci. 2022, 616, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Jiang, T.; Liu, Z.; Wang, D.; Wang, L.; Xie, T. Highly photoactive Ti-doped α-Fe2O3 nanorod arrays photoanode prepared by a hydrothermal method for photoelectrochemical water splitting. Electrochim. Acta. 2014, 129, 358–363. [Google Scholar] [CrossRef]
- Zhu, Y.K.; Zhao, X.L.; Li, J.Z.; Zhang, H.W.; Chen, S.; Han, W.; Yang, D.J. Surface modification of hematite photoanode by NiFe layered double hydroxide for boosting photoelectrocatalytic water oxidation. J. Alloy. Compd. 2018, 764, 341–346. [Google Scholar] [CrossRef]
- Chang, Y.; Xuan, Y.; Zhang, C.; Hao, H.; Yu, K.; Liu, S. Z-Scheme Pt@CdS/3DOM-SrTiO3 composite with enhanced photocatalytic hydrogen evolution from water splitting. Catal. Today 2019, 327, 315–322. [Google Scholar] [CrossRef]
- Ding, P.; Luo, F.; Wang, P.; Xia, W.; Xu, X.; Hu, J.; Zeng, H. Photo-induced charge kinetic acceleration in ultrathin layered double hydroxide nanosheets boosts the oxygen evolution reaction. J. Mater. Chem. A 2020, 8, 1105–1112. [Google Scholar] [CrossRef]
- Yi, S.S.; Wulan, B.R.; Yan, J.-M.; Jiang, Q. Highly Efficient Photoelectrochemical Water Splitting: Surface Modification of Cobalt-Phosphate-Loaded Co3O4/Fe2O3 p–n Heterojunction Nanorod Arrays. Adv. Funct. Mater. 2019, 29, 1801902. [Google Scholar] [CrossRef]
- Bai, S.; Chu, H.; Xiang, X.; Luo, R.; He, J.; Chen, A. Fabricating of Fe2O3/BiVO4 heterojunction based photoanode modified with NiFe-LDH nanosheets for efficient solar water splitting. Chem. Eng. J. 2018, 350, 148–156. [Google Scholar] [CrossRef]
- Wang, G.; Ling, Y.; Wheeler, D.A.; George, K.E.N.; Horsley, K.; Heske, C.; Zhang, J.Z.; Li, Y. Facile Synthesis of Highly Photoactive alpha-Fe2O3-Based Films for Water Oxidation. Nano Lett. 2011, 11, 3503–3509. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wang, P.; Chai, H.; Li, S.; Jin, J.; Ma, J. Expediting hole transfer via surface states in hematite-based composite photoanodes. Nanoscale 2022, 14, 17044–17052. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Liu, P.; Li, R.; Wang, L.; Zhang, K.; Zhou, P.; Huang, X.; Wang, G. Constructing accelerated charge transfer channels along V-Co-Fe via introduction of V into CoFe-layered double hydroxides for overall water splitting. Appl. Catal. B Environ. 2021, 298, 120587. [Google Scholar] [CrossRef]
- Zhang, K.; Dong, T.; Xie, G.; Guan, L.; Guo, B.; Xiang, Q.; Dai, Y.; Tian, L.; Batool, A.; Jan, S.U.; et al. Sacrificial Interlayer for Promoting Charge Transport in Hematite Photoanode. ACS Appl. Mater. Inter. 2017, 9, 42723–42733. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Long, X.; Wei, S.; Wang, T.; Li, F.; Gao, L.; Hu, Y.; Li, S.; Jin, J. Conformally Coupling CoAl-Layered Double Hydroxides on Fluorine-Doped Hematite: Surface and Bulk Co-Modification for Enhanced Photoelectrochemical Water Oxidation. ACS Appl. Mater. Inter. 2019, 11, 29799–29806. [Google Scholar] [CrossRef]
- Laskowski, F.A.L.; Nellist, M.R.; Qiu, J.; Boettcher, S.W. Metal Oxide/(oxy)hydroxide Overlayers as Hole Collectors and Oxygen-Evolution Catalysts on Water-Splitting Photoanodes. J. Am. Chem. Soc. 2019, 141, 1394–1405. [Google Scholar] [CrossRef]
- Liu, W.; Liu, H.; Dang, L.; Zhang, H.; Wu, X.; Yang, B.; Li, Z.; Zhang, X.; Lei, L.; Jin, S. Amorphous Cobalt–Iron Hydroxide Nanosheet Electrocatalyst for Efficient Electrochemical and Photo-Electrochemical Oxygen Evolution. Adv. Funct. Mater. 2017, 27, 1603904. [Google Scholar] [CrossRef]
- Bergamini, L.; Sangiorgi, N. A Gondolini and A Sanson. Hematite heterostructures for photoelectrochemical water splitting: Rational materials design and charge carrier dynamics. Solar Energy 2020, 212, 62–72. [Google Scholar] [CrossRef]
- Yu, Z.; Chen, L.; Luo, L.; Huang, R.; Tang, Z.; Xiao, P.; Zhang, Y. Combining Bulk Charge Transport and Surface Charge Transfer to Design Titanium-Doped Hematite Homojunction Photoanodes. J. Phys. Chem. C 2022, 126, 4296–4305. [Google Scholar] [CrossRef]
- Miao, Y.; Li, Z.; Song, Y.; Fan, K.; Guo, J.; Li, R.; Shao, M. Surface active oxygen engineering of photoanodes to boost photoelectrochemical water and alcohol oxidation coupled with hydrogen production. Appl. Catal. B Environ. 2023, 323, 122147. [Google Scholar] [CrossRef]
- Bu, Q.; Zhao, Q.; Sun, G.; Liu, Q.; Xie, T. Boosting the photogenerated hole separation and injection of Ti-Fe2O3 by co-modifying carbon quantum dots and NiFe layered double hydroxide. J. Alloy Compd. 2022, 908, 164643. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Zhang, Y.; Ding, Y.; Bi, Y. Ultrathin FeOOH Nanolayers with Abundant Oxygen Vacancies on BiVO4 Photoanodes for Efficient Water Oxidation. Angew. Chem. Int. Ed. 2018, 57, 2248–2252. [Google Scholar] [CrossRef]
- Wang, C.; Sun, S.; Zhang, H.; Zhang, J.; Li, C.; Chen, W.; Li, S. Regulating the Charge Migration in CuInSe2/N-Doped Carbon Nanorod Arrays via Interfacial Engineering for Boosting Photoelectrochemical Water Splitting. Adv. Sci. 2023, 4, 2300034. [Google Scholar] [CrossRef]
- Li, T.; Feng, C.; Yap, B.K.; Zhu, X.; Xiong, B.; He, Z.; Wong, W.Y. Accelerating charge transfer via nonconjugated polyelectrolyte interlayers toward efficient versatile photoredox catalysis. Commun. Chem. 2021, 4, 150. [Google Scholar] [CrossRef]
- Hu, L.; Zeng, X.; Wei, X.; Wang, H.; Wu, Y.; Gu, W.; Shi, L.; Zhu, C. Interface engineering for enhancing electrocatalytic oxygen evolution of NiFe LDH/NiTe heterostructures. Appl. Catal. B Environ. 2020, 273, 119014. [Google Scholar] [CrossRef]
- Zhao, S.; Jin, R.; Abroshan, H.; Zeng, C.; Zhang, H.; House, S.D.; Gottlieb, E.; Kim, H.J.; Yang, J.C.; Jin, R. Gold Nanoclusters Promote Electrocatalytic Water Oxidation at the Nanocluster/CoSe2 Interface. J. Am. Chem. Soc. 2017, 139, 1077–1080. [Google Scholar] [CrossRef]
- Tang, T.; Jiang, W.J.; Niu, S.; Liu, N.; Luo, H.; Chen, Y.Y.; Jin, S.F.; Gao, F.; Wan, L.J.; Hu, J.S. Electronic and Morphological Dual Modulation of Cobalt Carbonate Hydroxides by Mn Doping toward Highly Efficient and Stable Bifunctional Electrocatalysts for Overall Water Splitting. J. Am. Chem. Soc. 2017, 139, 8320–8328. [Google Scholar] [CrossRef]
- Tang, R.; Zhou, S.; Zhang, Z.; Zheng, R.; Huang, J. Engineering Nanostructure-Interface of Photoanode Materials Toward Photoelectrochemical Water Oxidation. Adv. Mater. 2021, 3, 2005389. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of Ab-Initio Total Energy Calculations for Metals and Semiconductors Using a Plane-Wave Basis Set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B Condens. Matter Mater. Phys. 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B Condens. Matter Mater. Phys. 1999, 59, 1758. [Google Scholar] [CrossRef]
- Ravishankar, S.; Bisquert, J.; Kirchartz, T. Interpretation of Mott–Schottky plots of photoanodes for water splitting. Chem. Sci. 2022, 13, 4828–4837. [Google Scholar] [CrossRef]

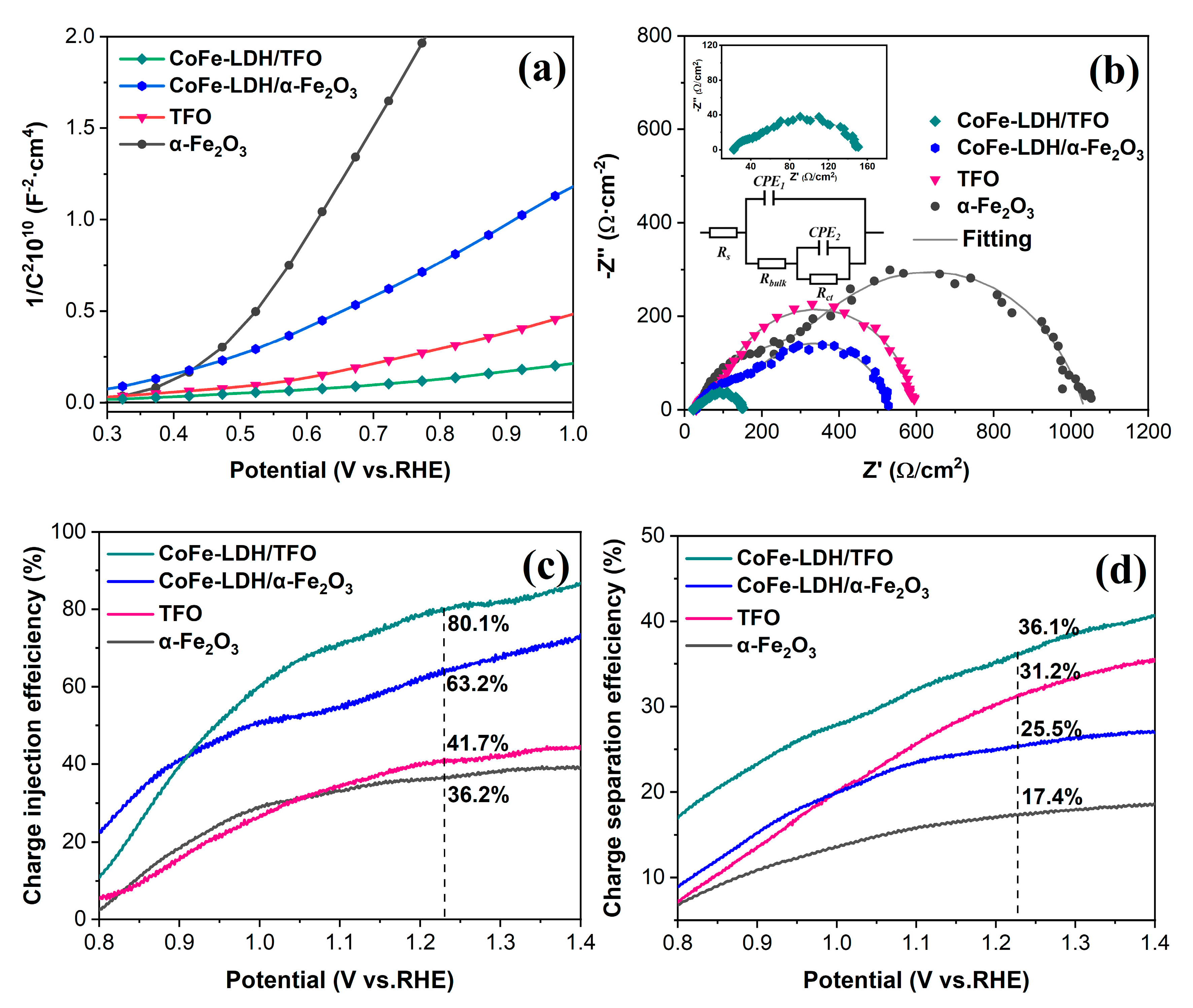
| Photoanodes | Rs (Ω) | Rbulk (Ω) | Rct (Ω) |
|---|---|---|---|
| α-Fe2O3 | 30.5 | 391.9 | 613.3 |
| TFO | 29.8 | 93.7 | 475.6 |
| CoFe-LDH/α-Fe2O3 | 27.5 | 228.8 | 283.7 |
| CoFe-LDH/TFO | 22.5 | 32.4 | 96.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.; Han, M.; Ding, Y.; Wei, H.; Zhang, D.; Luo, H.; Li, X.; Yan, X. Interface Engineering of CoFe-LDH Modified Ti: α-Fe2O3 Photoanode for Enhanced Photoelectrochemical Water Oxidation. Nanomaterials 2023, 13, 2579. https://doi.org/10.3390/nano13182579
Chang Y, Han M, Ding Y, Wei H, Zhang D, Luo H, Li X, Yan X. Interface Engineering of CoFe-LDH Modified Ti: α-Fe2O3 Photoanode for Enhanced Photoelectrochemical Water Oxidation. Nanomaterials. 2023; 13(18):2579. https://doi.org/10.3390/nano13182579
Chicago/Turabian StyleChang, Yue, Minmin Han, Yehui Ding, Huiyun Wei, Dawei Zhang, Hong Luo, Xiaogang Li, and Xiongbo Yan. 2023. "Interface Engineering of CoFe-LDH Modified Ti: α-Fe2O3 Photoanode for Enhanced Photoelectrochemical Water Oxidation" Nanomaterials 13, no. 18: 2579. https://doi.org/10.3390/nano13182579
APA StyleChang, Y., Han, M., Ding, Y., Wei, H., Zhang, D., Luo, H., Li, X., & Yan, X. (2023). Interface Engineering of CoFe-LDH Modified Ti: α-Fe2O3 Photoanode for Enhanced Photoelectrochemical Water Oxidation. Nanomaterials, 13(18), 2579. https://doi.org/10.3390/nano13182579







