Healable Anti-Corrosive and Wear-Resistant Silicone-Oil-Impregnated Porous Oxide Layer of Aluminum Alloy by Plasma Electrolytic Oxidation
Abstract
:1. Introduction
2. Materials and Methods
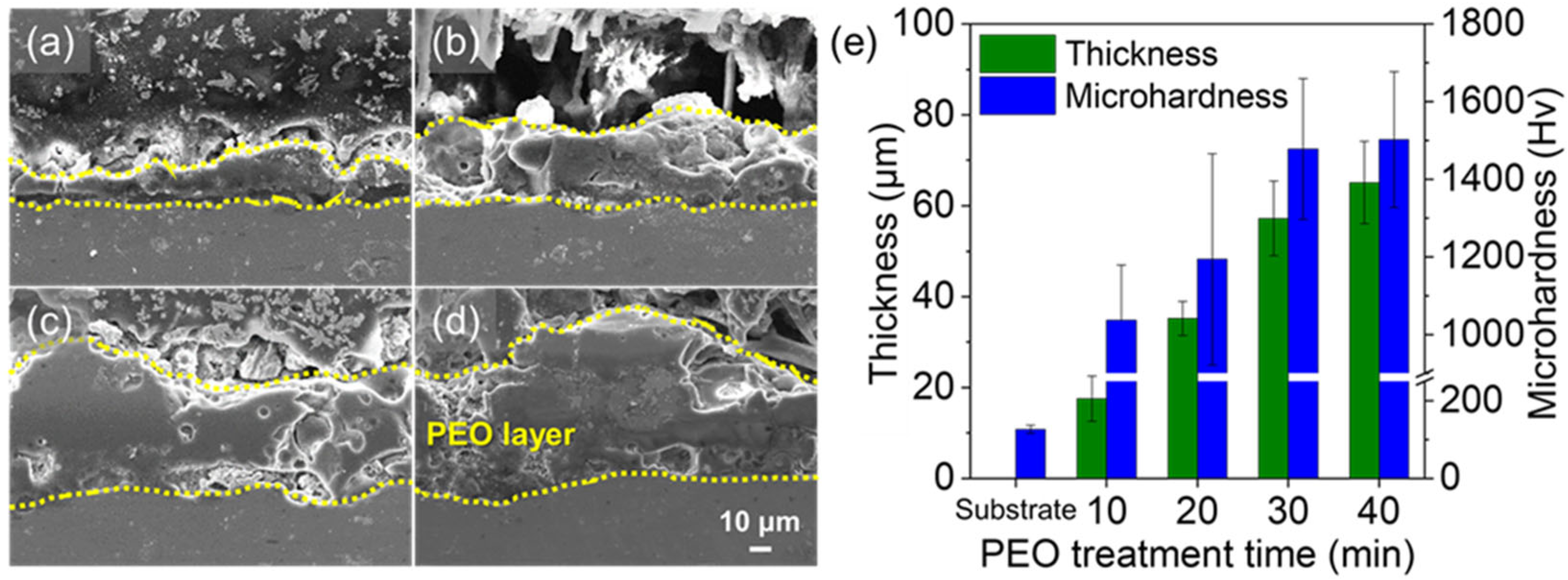
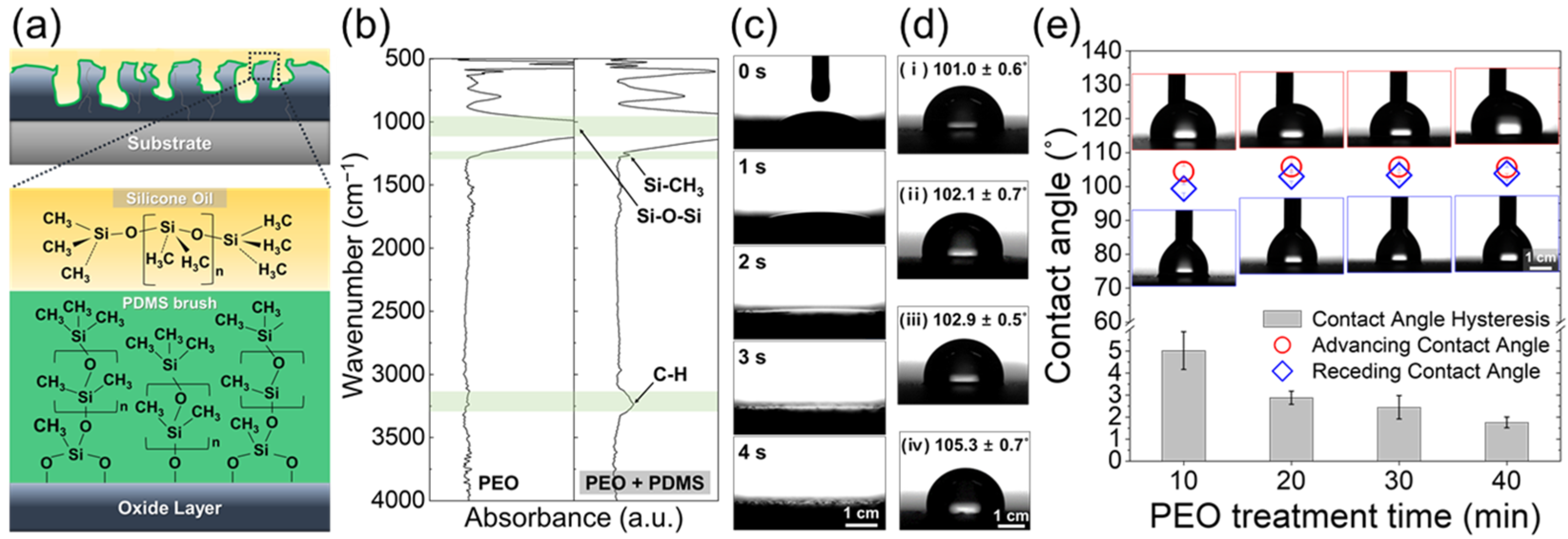
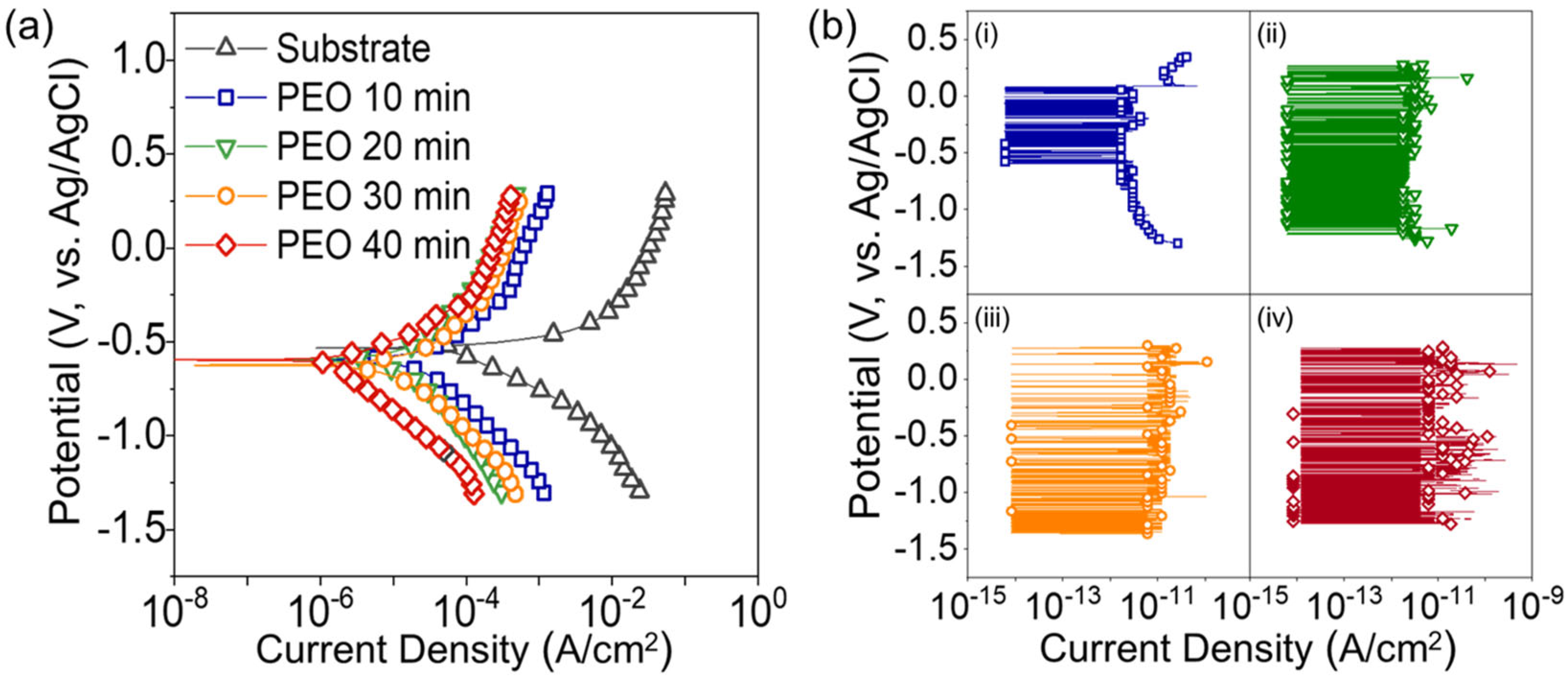
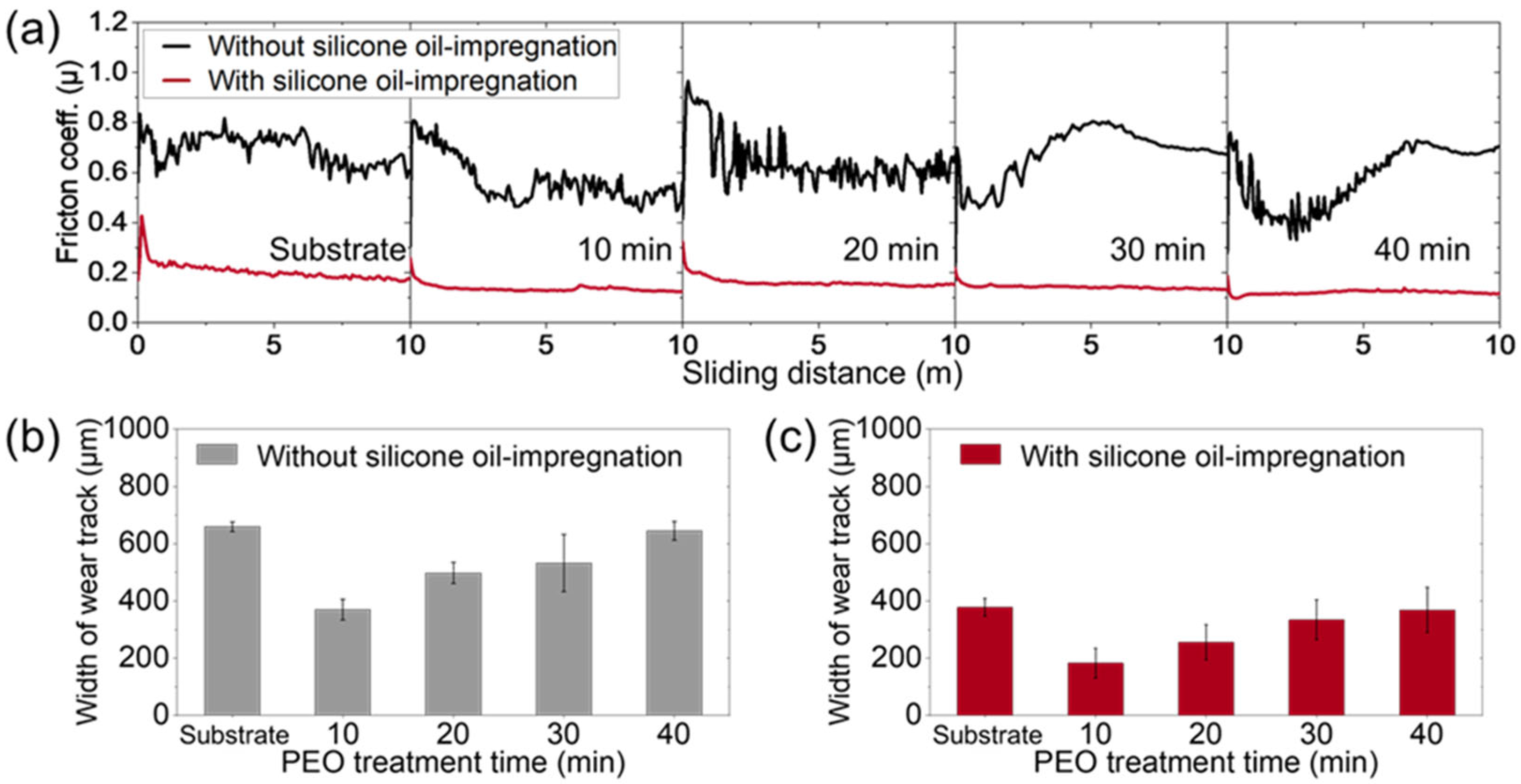
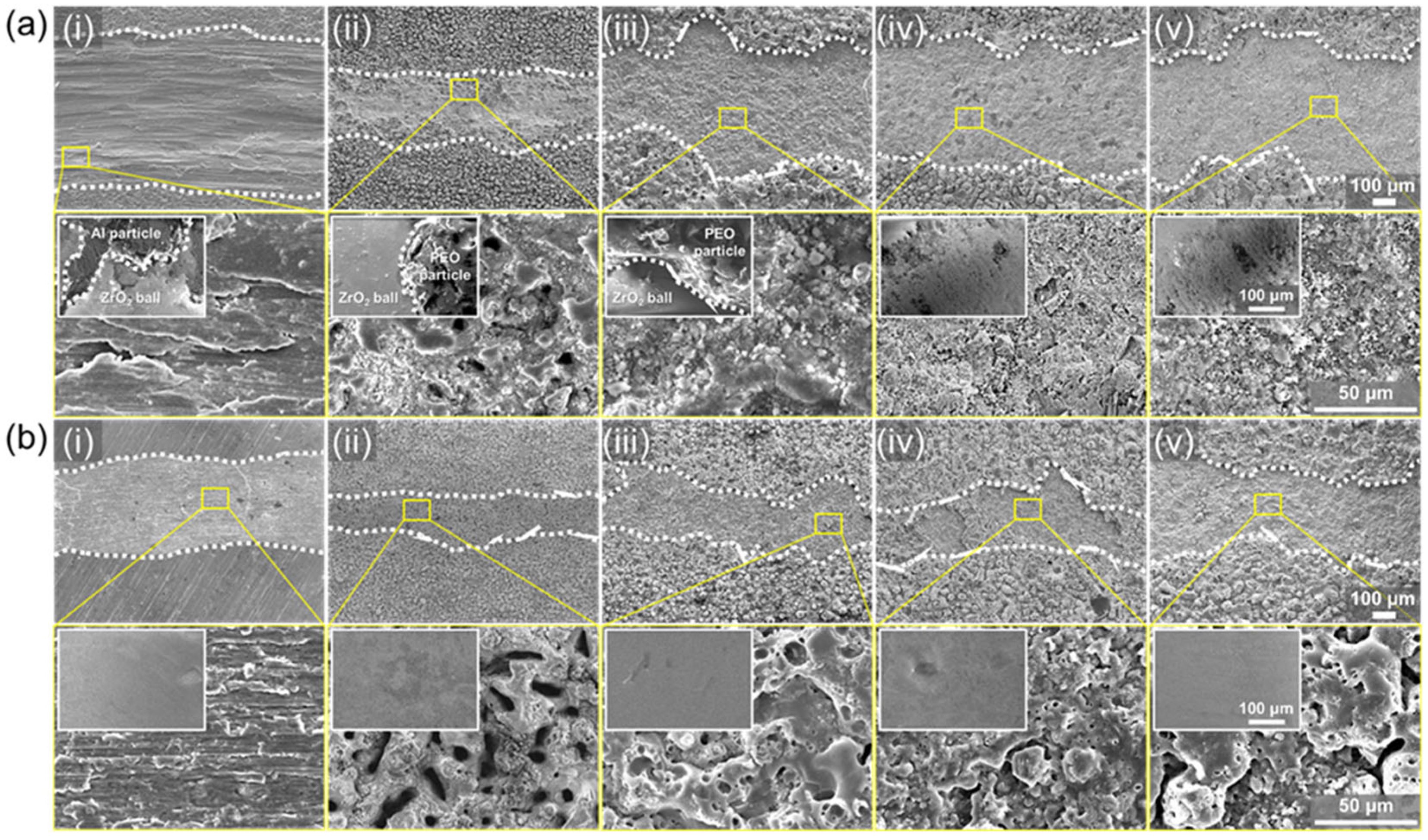
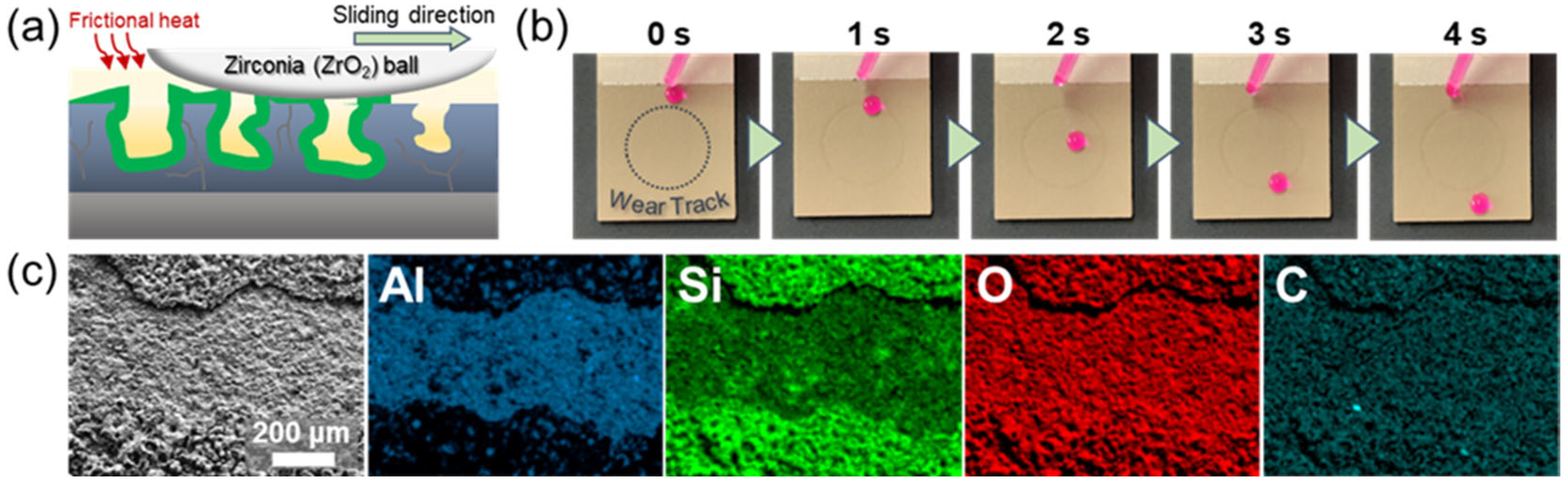
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Clyne, T.W.; Troughton, S.C. A review of recent work on discharge characteristics during plasma electrolytic oxidation of various metals. Int. Mat. Rev. 2019, 64, 127–162. [Google Scholar] [CrossRef]
- Yerokhin, A.; Nie, X.; Leyland, A.; Matthews, A.; Dowey, S. Plasma electrolysis for surface engineering. Surf. Coat. Technol. 1999, 122, 73–93. [Google Scholar] [CrossRef]
- Kaseem, M.; Fatimah, S.; Nashrah, N.; Ko, Y.G. Recent progress in surface modification of metals coated by plasma electrolytic oxidation: Principle, structure, and performance. Prog. Mat. Sci. 2021, 117, 100735. [Google Scholar] [CrossRef]
- Khan, R.; Yerokhin, A.; Li, X.; Dong, H.; Matthews, A. Surface characterisation of DC plasma electrolytic oxidation treated 6082 aluminium alloy: Effect of current density and electrolyte concentration. Surf. Coat. Technol. 2010, 205, 1679–1688. [Google Scholar] [CrossRef]
- Dzhurinskiy, D.V.; Dautov, S.S.; Shornikov, P.G.; Akhatov, I.S. Surface Modification of Aluminum 6061-O Alloy by Plasma Electrolytic Oxidation to Improve Corrosion Resistance Properties. Coatings 2021, 11, 4. [Google Scholar] [CrossRef]
- Wheeler, J.; Curran, J.; Shrestha, S. Microstructure and multi-scale mechanical behavior of hard anodized and plasma electrolytic oxidation (PEO) coatings on aluminum alloy 5052. Surf. Coat. Technol. 2012, 207, 480–488. [Google Scholar] [CrossRef]
- Famiyeh, L.; Huang, X. Plasma electrolytic oxidation coatings on aluminum alloys: Microstructures, properties, and applications. Mod. Concepts Mater. Sci. 2019, 2, 000526. [Google Scholar] [CrossRef]
- Lu, X.; Blawert, C.; Zheludkevich, M.L.; Kainer, K.U. Insights into plasma electrolytic oxidation treatment with particle addition. Corros. Sci. 2015, 101, 201–207. [Google Scholar] [CrossRef]
- Yürektürk, Y.; Muhaffel, F.; Baydoğan, M. Characterization of micro arc oxidized 6082 aluminum alloy in an electrolyte containing carbon nanotubes. Surf. Coat. Technol. 2015, 269, 83–90. [Google Scholar] [CrossRef]
- Tran, Q.; Chin, T.; Kuo, Y.; Jin, C.; Trung, T.; Van Tuan, C.; Dang, D.Q. Diamond powder incorporated oxide layers formed on 6061 Al alloy by plasma electrolytic oxidation. J. Alloys Compd. 2018, 751, 289–298. [Google Scholar] [CrossRef]
- Sun, M.; Matthews, A.; Yerokhin, A. Plasma electrolytic oxidation coatings on cp-Mg with cerium nitrate and benzotriazole immersion post-treatments. Surf. Coat. Technol. 2018, 344, 330–341. [Google Scholar] [CrossRef]
- Pak, S.; Jiang, Z.; Yao, Z.; Ju, J.; Ju, K.; Pak, U. Fabrication of environmentally friendly anti-corrosive composite coatings on AZ31B Mg alloy by plasma electrolytic oxidation and phytic acid/3-aminopropyltrimethoxysilane post treatment. Surf. Coat. Technol. 2017, 325, 579–587. [Google Scholar] [CrossRef]
- Ao, N.; Liu, D.; Zhang, X.; Liu, C. Enhanced fatigue performance of modified plasma electrolytic oxidation coated Ti-6Al-4V alloy: Effect of residual stress and gradient nanostructure. Appl. Surf. Sci. 2019, 489, 595–607. [Google Scholar] [CrossRef]
- Yin, B.; Peng, Z.; Liang, J.; Jin, K.; Zhu, S.; Yang, J.; Qiao, Z. Tribological behavior and mechanism of self-lubricating wear-resistant composite coatings fabricated by one-step plasma electrolytic oxidation. Tribol. Int. 2016, 97, 97–107. [Google Scholar] [CrossRef]
- Van Phuong, N.; Fazal, B.R.; Moon, S. Cerium-and phosphate-based sealing treatments of PEO coated AZ31 Mg alloy. Surf. Coat. Technol. 2017, 309, 86–95. [Google Scholar] [CrossRef]
- Li, Z.; Jing, X.; Yuan, Y.; Zhang, M. Composite coatings on a Mg–Li alloy prepared by combined plasma electrolytic oxidation and sol–gel techniques. Corros. Sci. 2012, 63, 358–366. [Google Scholar] [CrossRef]
- Pezzato, L.; Rigon, M.; Martucci, A.; Brunelli, K.; Dabalà, M. Plasma Electrolytic Oxidation (PEO) as pre-treatment for sol-gel coating on aluminum and magnesium alloys. Surf. Coat. Technol. 2019, 366, 114–123. [Google Scholar] [CrossRef]
- Guo, J.; Wang, L.; Wang, S.; Liang, J.; Xue, Q.; Yan, F. Preparation and performance of a novel multifunctional plasma electrolytic oxidation composite coating formed on magnesium alloy. J. Mat. Sci. 2009, 44, 1998–2006. [Google Scholar] [CrossRef]
- Lee, J.; Shin, S.; Jiang, Y.; Jeong, C.; Stone, H.A.; Choi, C.H. Oil-impregnated nanoporous oxide layer for corrosion protection with self-healing. Adv. Funct. Mat. 2017, 27, 1606040. [Google Scholar] [CrossRef]
- Huang, X.; Chrisman, J.D.; Zacharia, N.S. Omniphobic slippery coatings based on lubricant-infused porous polyelectrolyte multilayers. ACS Macro Lett. 2013, 2, 826–829. [Google Scholar] [CrossRef]
- Tuo, Y.; Zhang, H.; Chen, W.; Liu, X. Corrosion protection application of slippery liquid-infused porous surface based on aluminum foil. Appl. Surf. Sci. 2017, 423, 365–374. [Google Scholar] [CrossRef]
- Lee, S.J.; Kim, H.N.; Choi, W.; Yoon, G.Y.; Seo, E. A nature-inspired lubricant-infused surface for sustainable drag reduction. Soft Matter. 2019, 15, 8459–8467. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, B.J.; Van Buren, T.; Fu, M.K.; Smits, A.J. Turbulent drag reduction over air-and liquid-impregnated surfaces. Phys. Fluids 2016, 28, 015103. [Google Scholar] [CrossRef]
- Kim, P.; Wong, T.; Alvarenga, J.; Kreder, M.J.; Adorno-Martinez, W.E.; Aizenberg, J. Liquid-infused nanostructured surfaces with extreme anti-ice and anti-frost performance. ACS Nano 2012, 6, 6569–6577. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, K.; Tao, C.; Zhao, Y.; Li, X.; Zhu, K.; Yuan, X. Strategies for anti-icing: Low surface energy or liquid-infused? RSC Adv. 2016, 6, 70251–70260. [Google Scholar] [CrossRef]
- Subramanyam, S.B.; Rykaczewski, K.; Varanasi, K.K. Ice adhesion on lubricant-impregnated textured surfaces. Langmuir 2013, 29, 13414–13418. [Google Scholar] [CrossRef]
- MacCallum, N.; Howell, C.; Kim, P.; Sun, D.; Friedlander, R.; Ranisau, J.; Ahanotu, O.; Lin, J.J.; Vena, A.; Hatton, B.; et al. Liquid-infused silicone as a biofouling-free medical material. ACS Biomat. Sci. Eng. 2015, 1, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Villegas, M.; Zhang, Y.; Abu Jarad, N.; Soleymani, L.; Didar, T.F. Liquid-infused surfaces: A review of theory, design, and applications. Acs Nano 2019, 13, 8517–8536. [Google Scholar] [CrossRef] [PubMed]
- Howell, C.; Vu, T.L.; Lin, J.J.; Kolle, S.; Juthani, N.; Watson, E.; Weaver, J.C.; Alvarenga, J.; Aizenberg, J. Self-replenishing vascularized fouling-release surfaces. ACS Appl. Mat. Interfaces 2014, 6, 13299–13307. [Google Scholar] [CrossRef] [PubMed]
- Xiang, T.; Zhang, M.; Sadig, H.R.; Li, Z.; Zhang, M.; Dong, C.; Yang, L.; Chan, W.; Li, C. Slippery liquid-infused porous surface for corrosion protection with self-healing property. Chem. Eng. J. 2018, 345, 147–155. [Google Scholar] [CrossRef]
- Wong, T.; Kang, S.H.; Tang, S.K.; Smythe, E.J.; Hatton, B.D.; Grinthal, A.; Aizenberg, J. Bioinspired self-repairing slippery surfaces with pressure-stable omniphobicity. Nature 2011, 477, 443–447. [Google Scholar] [CrossRef] [PubMed]
- Qiu, R.; Zhang, Q.; Wang, P.; Jiang, L.; Hou, J.; Guo, W.; Zhang, H. Fabrication of slippery liquid-infused porous surface based on carbon fiber with enhanced corrosion inhibition property. Colloids Surf. A Physicochem. Eng. Asp. 2014, 453, 132–141. [Google Scholar] [CrossRef]
- Tian, X.; Banerjee, S.; Gonzalez-Alfonzo, I.; Cademartiri, L. Suppressing evaporative loss in slippery liquid-infused porous surfaces (SLIPS) with self-suspended perfluorinated nanoparticles. Langmuir 2020, 36, 5106–5111. [Google Scholar] [CrossRef]
- Asawa, K.; Kumar, S.; Huang, Y.; Choi, C. Guiding light via slippery liquid-infused porous surfaces. Appl. Phys. Lett. 2021, 118, 091602. [Google Scholar] [CrossRef]
- Ganne, A.A. On the Issue of the Stability of Water-Repellent Infusion Liquids on Hydrophilic and Hydrophobic Silica Substrates. Colloid J. 2022, 84, 411–415. [Google Scholar] [CrossRef]
- Emelyanenko, K.A.; Feoktistova, L.S.; Lunev, I.V.; Galiullin, A.A.; Malyshkina, I.A.; Krasovskiy, V.G. Stability of Some Silicone Lubricating Interlayers in Liquid-Infused Coatings. Colloid J. 2023, 85, 348–357. [Google Scholar] [CrossRef]
- Agureev, L.; Savushkina, S.; Ashmarin, A.; Borisov, A.; Apelfeld, A.; Anikin, K.; Tkachenko, N.; Gerasimov, M.; Shcherbakov, A.; Ignatenko, V. Study of plasma electrolytic oxidation coatings on aluminum composites. Metals 2018, 8, 459. [Google Scholar] [CrossRef]
- Leger, C.; Lira, H.D.L.; Paterson, R. Preparation and properties of surface modified ceramic membranes. Part II. Gas and liquid permeabilities of 5 nm alumina membranes modified by a monolayer of bound polydimethylsiloxane (PDMS) silicone oil. J. Membr. Sci. 1996, 120, 135–146. [Google Scholar] [CrossRef]
- Wooh, S.; Butt, H.J. A Photocatalytically Active Lubricant-Impregnated Surface. Angew. Chem. 2017, 129, 5047–5051. [Google Scholar] [CrossRef]
- Zhu, X.; Lu, J.; Li, X.; Wang, B.; Song, Y.; Miao, X.; Wang, Z.; Ren, G. Simple way to a slippery lubricant impregnated coating with ultrastability and self-replenishment property. Ind. Eng. Chem. Res. 2019, 58, 8148–8153. [Google Scholar] [CrossRef]
- Chen, L.; Park, S.; Yoo, J.; Hwang, H.; Kim, H.; Lee, J.; Hong, J.; Wooh, S. One-Step Fabrication of Universal Slippery Lubricated Surfaces. Adv. Mater. Interfaces 2020, 7, 2000305. [Google Scholar] [CrossRef]
- Inoue, T.; Koyama, A.; Kowalski, D.; Zhu, C.; Aoki, Y.; Habazaki, H. Fluorine-Free Slippery Liquid-Infused Porous Surfaces Prepared Using Hierarchically Porous Aluminum. Phys. Status Solidi 2020, 217, 1900836. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.; Chung, W. Effect of Ar bubbling during plasma electrolytic oxidation of AZ31B magnesium alloy in silicate electrolyte. Appl. Surf. Sci. 2012, 259, 454–459. [Google Scholar] [CrossRef]
- Song, J.; Nam, K.; Moon, J.; Choi, Y.; Lim, D. Influence of the duty cycle on structural and mechanical properties of oxide layers on Al-1050 by a plasma electrolytic oxidation process. Metals Mat. Int. 2014, 20, 451–458. [Google Scholar] [CrossRef]
- Jadhav, P.; Bongale, A.; Kumar, S. A review of process characteristics of plasma electrolytic oxidation of aluminium alloy. J. Phys. Conf. Ser. 2021, 1854, 012030. [Google Scholar] [CrossRef]
- Peng, Q.; Wang, P.; Huang, W.; Chen, H. The irradiation-induced grafting of nano-silica with methyl silicone oil. Polymer 2020, 192, 122315. [Google Scholar] [CrossRef]
- Shahri, Z.; Allahkaram, S.; Soltani, R.; Jafari, H. Optimization of plasma electrolyte oxidation process parameters for corrosion resistance of Mg alloy. J. Magnes. Alloys 2020, 8, 431–440. [Google Scholar] [CrossRef]
- Javidi, M.; Fadaee, H. Plasma electrolytic oxidation of 2024-T3 aluminum alloy and investigation on microstructure and wear behavior. Appl. Surf. Sci. 2013, 286, 212–219. [Google Scholar] [CrossRef]
- Ha, K.; Park, Y.K.; Kim, T.; Baek, G.Y.; Jeon, J.B.; Shim, D.-s.; Moon, Y.H.; Lee, W. Effects of Heat Treatment Condition and Counter Materials on the Wear Behavior of Laser Direct Energy Deposited Fe-8Cr-3V-2Mo-2W Alloy. Korean J. Met. Mater. 2020, 58, 680–692. [Google Scholar] [CrossRef]
- Bae, K.; Kim, D.; Lee, W.; Park, Y. Wear Behavior of Conventionally and Directly Aged Maraging 18Ni-300 Steel Produced by Laser Powder Bed Fusion. Materials 2021, 14, 2588. [Google Scholar] [CrossRef] [PubMed]
- Elias, C.; dos Santos, H.; Garbossa, M.; dos Santos, C. Mechanical properties of zirconia Y-TZP core veneered for dentistry applications. J. Ceram. Sci. Technol. 2017, 8, 525–530. [Google Scholar] [CrossRef]
- Lv, T.; Cheng, Z.; Zhang, E.; Kang, H.; Liu, Y.; Jiang, L. Self-Restoration of Superhydrophobicity on Shape Memory Polymer Arrays with Both Crushed Microstructure and Damaged Surface Chemistry. Small 2017, 13, 1503402. [Google Scholar] [CrossRef]
- Joo, J.; Kang, M.; Moon, H.; Wooh, S.; Lee, J. Design and experimental studies of self-healable anti-corrosion coating: Passivation of metal surfaces by silicone oil impregnated porous oxides. Surf. Coat. Technol. 2020, 404, 126595. [Google Scholar] [CrossRef]
- Mohedano, M.; Matykina, E.; Arrabal, R.; Mingo, B.; Pardo, A. PEO of pre-anodized Al–Si alloys: Corrosion properties and influence of sealings. Appl. Surf. Sci. 2015, 346, 57–67. [Google Scholar] [CrossRef]
- Krishna, L.R.; Somaraju, K.; Sundararajan, G. The tribological performance of ultra-hard ceramic composite coatings obtained through microarc oxidation. Surf. Coat. Technol. 2003, 163, 484–490. [Google Scholar] [CrossRef]
- Dehnavi, V.; Luan, B.L.; Liu, X.Y.; Shoesmith, D.W.; Rohani, S. Correlation between plasma electrolytic oxidation treatment stages and coating microstructure on aluminum under unipolar pulsed DC mode. Surf. Coat. Technol. 2015, 269, 91–99. [Google Scholar] [CrossRef]

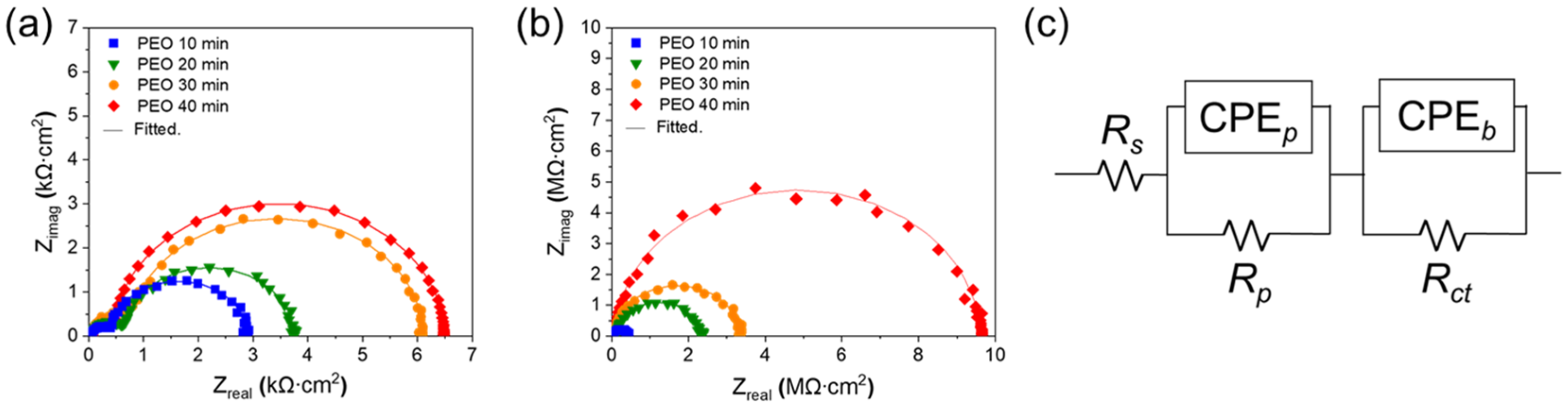
| Sample Name | Silicone Oil-Impregnation | CPEp, nF/cm2 | np | Rp, Ω cm2 | CPEb, nF/cm2 | nb | Rct, Ω cm2 |
|---|---|---|---|---|---|---|---|
| PEO 10 min | X | 359.00 | 0.98 | 2481 | 2393 | 0.97 | 278.3 |
| PEO 20 min | X | 103.00 | 0.97 | 3427 | 928.2 | 0.99 | 351.3 |
| PEO 30 min | X | 45.44 | 0.99 | 3600 | 780.5 | 0.98 | 400.9 |
| PEO 40 min | X | 35.96 | 0.98 | 6010 | 562.0 | 0.99 | 600.3 |
| PEO 10 min | O | 1.44 | 0.97 | 417,300 | 9.54 | 0.98 | 72,890 |
| PEO 20 min | O | 0.88 | 0.97 | 2,186,000 | 4.49 | 0.99 | 90,600 |
| PEO 30 min | O | 0.53 | 0.98 | 3,265,000 | 2.71 | 0.97 | 115,600 |
| PEO 40 min | O | 0.24 | 0.96 | 9,536,000 | 0.95 | 0.98 | 282,000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, Y.; Bae, K.; Lee, S.; Kim, H.; Shin, D.; Kim, D.; Choi, E.; Moon, H.-S.; Lee, J. Healable Anti-Corrosive and Wear-Resistant Silicone-Oil-Impregnated Porous Oxide Layer of Aluminum Alloy by Plasma Electrolytic Oxidation. Nanomaterials 2023, 13, 2582. https://doi.org/10.3390/nano13182582
Shin Y, Bae K, Lee S, Kim H, Shin D, Kim D, Choi E, Moon H-S, Lee J. Healable Anti-Corrosive and Wear-Resistant Silicone-Oil-Impregnated Porous Oxide Layer of Aluminum Alloy by Plasma Electrolytic Oxidation. Nanomaterials. 2023; 13(18):2582. https://doi.org/10.3390/nano13182582
Chicago/Turabian StyleShin, Yeji, Kichang Bae, Sumin Lee, Hweeyong Kim, Dongmin Shin, Donghyun Kim, Eunyoung Choi, Hyoung-Seok Moon, and Junghoon Lee. 2023. "Healable Anti-Corrosive and Wear-Resistant Silicone-Oil-Impregnated Porous Oxide Layer of Aluminum Alloy by Plasma Electrolytic Oxidation" Nanomaterials 13, no. 18: 2582. https://doi.org/10.3390/nano13182582
APA StyleShin, Y., Bae, K., Lee, S., Kim, H., Shin, D., Kim, D., Choi, E., Moon, H.-S., & Lee, J. (2023). Healable Anti-Corrosive and Wear-Resistant Silicone-Oil-Impregnated Porous Oxide Layer of Aluminum Alloy by Plasma Electrolytic Oxidation. Nanomaterials, 13(18), 2582. https://doi.org/10.3390/nano13182582






