A Review of the Current State of Magnetic Force Microscopy to Unravel the Magnetic Properties of Nanomaterials Applied in Biological Systems and Future Directions for Quantum Technologies
Abstract
1. Introduction
- Superconducting quantum interference device (SQUID) magnetometry is a highly sensitive technique to measure ultralow magnetic signals up to 5 × 10−14 Tesla (T) with a noise threshold of nearly 3 fT · Hz−1/2 [7]. The signal-to-noise ratio can be improved when the SQUID is damped by low susceptometer resonances [8]. For this reason, SQUID is considered the most sensitive type of quantitative magnetometry with 1 · 10−8 electromagnetic units (emu; 1 emu = 10−3 Am2). SQUID consists of placing a sample in a magnetic field and measuring the magnetic moment of the sample as a function of the applied field strength [9]. Moreover, controlling the applied current by the integration of a heating resistor on the same sample chip makes tunable the SQUID sensor device [10]. This technology has been applied to measure the magnetic properties of biological systems such as magnetosomes from magnetotactic bacteria [11], mesenchymal stem cells for tissue engineering applications [12], or the characterization of iron oxide nanoparticles in biological samples [13], and for the magnetic separation of microplastic bodies from water resources [14], respectively.
- Vibrating sample magnetometry (VSM) consists of the sample mounting on a thin rod under B while simultaneous vibration of the sample at a specific frequency occurs [15]. Multiple magnetic properties including magnetic moment, susceptibility, and coercivity can be measured by varying the strength of B, the sample orientation with respect to B, and its temperature [16]. Nowadays, customized VSM setups can achieve signal sensitivities ranging from 1 · 10−5 to 1 · 10−6 emu [17]. The employment of VSM has revealed the metagenomic analysis of magnetotactic bacteria [18], the detection of ferromagnetic materials in insect tissues responsible for their orientation toward external magnetic fields under both light and dark conditions [19], and the characterization of magnetic nanoparticles in the use of DNA isolation [20] or for hyperthermia therapies [21].
- Magneto-optic Kerr effect (MOKE) is based on measuring the rotation of the reflected light polarization, which is proportional to the magnetic moment of the sample under an external magnetic field [22]. MOKE technology can be used to address many magnetic sample properties like the magnetization process, the magnetic domain structure, and the magnetic anisotropy [23]. The sensitivity of MOKE is slightly higher than VSM, being settled at 1 · 10−7 emu [24]. Recently, a twofold increase in the intensity signal was reported by polarizing the beam splitter-based MOKE setup [25] and the reach of femtosecond-scale time resolution by coupling a free electron laser [26], respectively. The assemblies of magnetosomes from magnetotactic bacteria [27], and the manipulation and trap of magnetic yeast cells on lab-on-chip devices [28] are some of the few examples where MOKE is employed in biological samples. MOKE is conventionally used in the study of multiferroic materials [29].
- Magnetic resonance techniques like nuclear magnetic resonance (NMR) [30], electron paramagnetic resonance (EPR) [31], or ferromagnetic resonance (FMR) [32] apply a magnetic field to the sample and measure the subsequent response of the atomic or electronic spins according to this field, respectively. While NMR is mainly focused on the determination of molecular structure and dynamics independent of the nature of the sample [33], EPR requires magnetic domains embedded inside the sample structure. For this reason, NMR cannot be considered a bulk technique to measure the magnetic properties of biosystems. On the other hand, EPR is capable of detecting 1012 spins per mT linewidth [34]. Alternatively, FMR detects the magnetic moments of non-paramagnetic materials by applying a second microwave pulse, being widely used for ferromagnetic particles [35] or magnetosomes [36]. EPR has satisfactorily ascertained the magnetic sensitivity of cryptochromes in birds [37], the catalytic mechanisms of molecular radicals existing in nature [38], the conformational dynamics of membrane proteins [39] or metalloenzymes [40], and the electron spin relaxation of porphyrins [41], which regulates oxygen transport in the blood and muscles.
- Mössbauer spectroscopy is a versatile technique to study the interaction of certain isotopes with their surroundings [42]. This technology enables the hyperfine interactions between the nuclei and electrons to be measured with an accuracy of 14–15 magnitude orders [43]. Mössbauer spectroscopy was used to unravel the magnetic properties of nanometer-size particles [44], magnetosomes [45], and cryptochromes [46]. Mössbauer spectroscopy is a particularly powerful instrument when it is exploited in combination with synchrotron facilities [47].
- Alternating current (AC) susceptibility refers to the extent to which the material can become magnetized in response to an alternating magnetic field [48]. AC susceptibility measurements are commonly carried out through a magnetic susceptibility meter or a vibrating sample magnetometer. The signal analysis for the moment measurements is processed by a high-speed digital voltmeter leading to sensitivity yields of nearly 3.5 × 10−6 emu [49]. AC susceptibility measurements allow for the detection of directional changes in magnetic fields of small insects [50] and magnetotactic bacteria [51], the nucleus positioning of carcinogenic cells [52], iron detection in ferritins or hemoglobins [53] and human serum albumin [54], or the elasticity of globular proteins labeled with gold nanoparticles [55].
2. Biological Systems Affected by Magnetism
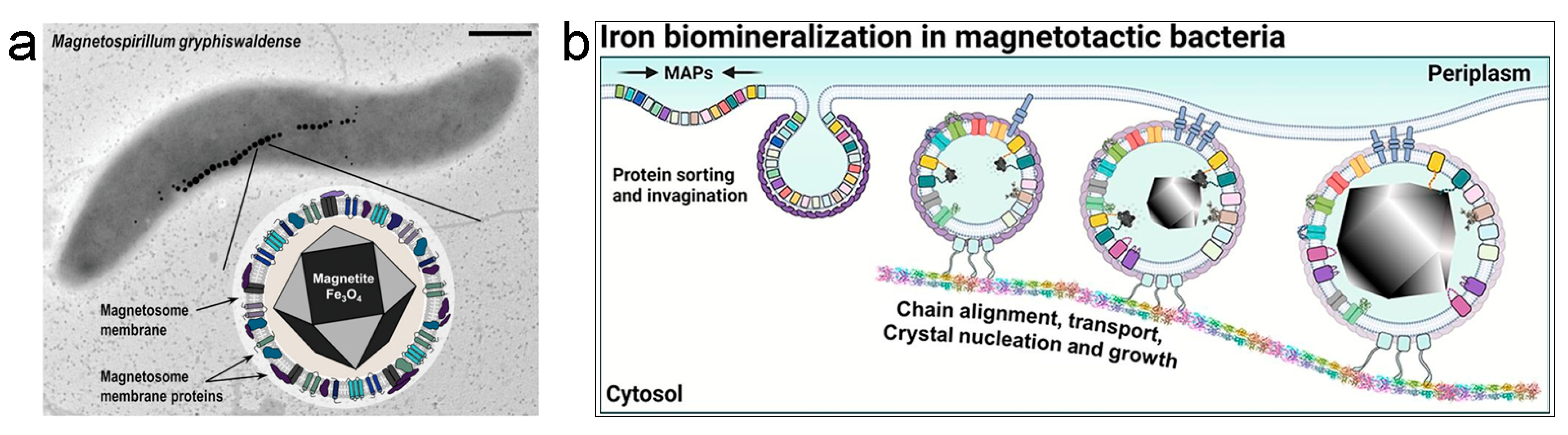
2.1. Magnetosomes from Magnetotactic Bacteria
2.2. Synthetic Magnetic Nanoparticles Used for Biological Applications
2.3. Enzymatic Reactions Involving DNA and Neurodegenerative Diseases
2.4. Cryptochromes
2.4.1. Magnetic Fields and Cryptochromes
2.4.2. Radical Pair Mechanism
2.4.3. Light-Independent Magnetosensing in Cryptochromes
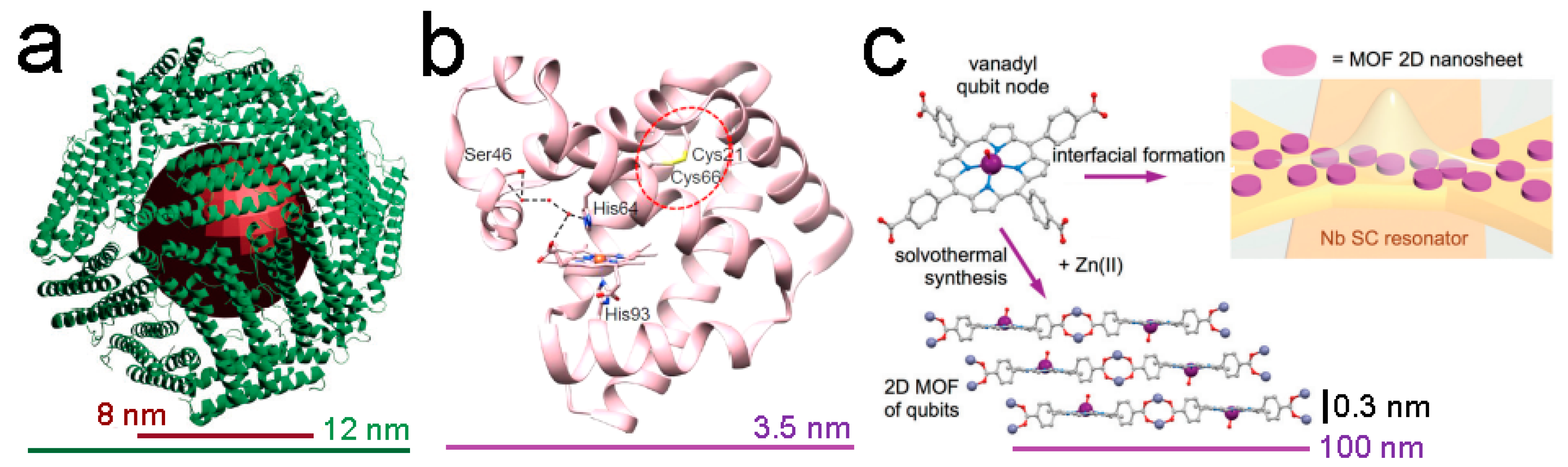
2.5. Biomolecules with Prospective Applications in Quantum Technologies
3. Working Principles of MFM
4. MFM Operational Modes
4.1. Lift Mode
4.1.1. Amplitude Modulation (AM)
4.1.2. Frequency Modulation (FM)
4.2. Constant Height Mode
4.3. Electrostatic and Tip Artifacts
4.4. Magnetic Resonance Force Microscopy (MRFM)
4.5. Nitrogen-Vacancy (NV) Microscopy
5. Magnetic Force Measurements with Commercially Available MFM Tips
6. Development of Ultra-Sharp MFM Tips
6.1. Advanced Coating Approaches
6.2. Nanomachining by Focused Ion Beam Milling

6.3. Carbon Nanotubes, Carbon Nanofibers, and Electrodeposited Wires
6.4. Focused Electron Beam-Induced Deposition
- (1)
- (2)
- (3)
- The magnetic volume can be deposited precisely at the tip region; either on a FIB-milled or FEBID-grown plateau [329], onto an existing tip [330] (Figure 9i), or directly on tipless cantilevers [315]. While the first approach requires an additional process step, the second is a straightforward single-step process. Fabricating on flat/tipless cantilevers requires a more sophisticated FEBID-tip design [315] (Figure 9g), but simplifies the production of more advanced cantilever layouts.
- (4)
- For perpendicular alignment of the cantilever axis to the substrate plane, the technical pre-tilt in AFMs (typically about 10°) can be easily compensated [330].
- (5)
- FEBID is typically performed at room temperature [331], thus avoiding thermal stress for the cantilever.
- (6)
- Flexibility in material properties: The first attempts used the FEBID pillars as a scaffold for sputtering with magnetic materials [332,333]. The development of high-quality magnetic precursor materials for FEBID [325,334] has made this second process step unnecessary, now allowing for true direct-write, single-step fabrication of all magnetic tips [335]. Consequently, FEBID-MFM tips have no risk of delamination, while revealing 10 nm apexes. Different precursor materials have been used for FEBID-MFM probes, listed in Table 2.
- (7)
- Tip dimensions and material quality can be adjusted by the deposition conditions, such as primary electron energies and beam currents, which enable a controlled tuning of magnetic properties [327,336]. This way, FEBID-MFM tips can be adapted to the requirements of the sample and environmental conditions. For example, Jaafar et al. demonstrated exceptional MFM performance under liquid conditions [327] using Fe-based nanorods, which is highly relevant for biological samples. In addition, a range of various post-processing procedures (annealing [337], electron beam irradiation [315]) opens the door to a wide variety of MFM probes with different properties. Looking to the future, the potential of FEBID has not yet been fully exploited, considering the unrivaled possibilities of 3D nanoprinting [338] for the fabrication of advanced probe designs [315,331] (Figure 9g).
| Technique-Tip Type | First Author | Material/Precursor | Tip Radius | Ref. |
|---|---|---|---|---|
| CNT-filled | Wolny | FeC | n.a. ~25 nm | [320] |
| CNT-filled | Wolny | FeC | 25 nm | [321] |
| FEBID-Pillar | Utke | Co2(CO)8 | 25 nm | [339] |
| FEBID-Pillar | Gavagnin | Fe(CO)5 | n.a. (<20 nm) | [330] |
| Electrodeposition | Yang | Ni, Co | 20 nm | [314] |
| FIB milling | Campanella | NdFeB | 20 nm | [309] |
| CNT-coated | Kuramochi | CoFe | 20 nm | [312] |
| CNT-capped | Arie | Ni3C | 17 nm | [318] |
| CNT-coated | Deng | Ti/Co/Ti | 15 nm | [319] |
| FIB milling | Gao | CoPt | 15 nm | [306] |
| CNT-filled | Tanaka | Co3C | 15 nm | [322] |
| CNT-coated | Choi | Co90Fe10 | 15 nm | [317] |
| FEBID-Pillar | Escalante-Quiceno | Fe2(CO)9 | 15 nm | [340] |
| FIB milling | Phillips | Co | 12 nm | [305] |
| CNF-capped | Cui | NiC | 10 nm | [313] |
| FEBID-Pillar | Belova | Co2(CO)8 | 10 nm | [329] |
| FEBID-Cone | Winkler, Brugger-Hatzl | HCo3Fe(CO)12 | 9 nm | [315] |
| FEBID-Pillar | Pablo-Navarro | Fe2(CO)9 | 8 nm | [316] |
| FEBID-Pillar | Jaafar | Fe2(CO)9 | 7 nm | [327] |
7. Discussion and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, C.; Todo, T. The cryptochromes. Genome Biol. 2005, 6, 220. [Google Scholar] [CrossRef] [PubMed]
- Faivre, D.; Schüler, D. Magnetotactic Bacteria and Magnetosomes. Chem. Rev. 2008, 108, 4875–4898. [Google Scholar] [CrossRef] [PubMed]
- Tenuzzo, B.; Chionna, A.; Panzarini, E.; Lanubile, R.; Tarantino, P.; Di Jeso, B.; Dwikat, M.; Dini, L. Biological effects of 6 mT static magnetic fields: A comparative study in different cell types. Bioelectromagnetics 2006, 27, 560–577. [Google Scholar] [CrossRef]
- Liboff, A.R.; Williams, T., Jr.; Strong, D.M.; Wistar, R., Jr. Time-varying magnetic fields: Effect on DNA synthesis. Science 1984, 223, 818–820. [Google Scholar] [CrossRef] [PubMed]
- Ritz, T.; Yoshii, T.; Helfrich-Foerster, C.; Ahmad, M. Crytochrome: A photoreceptor with the properties of a magnetoreceptor? Commun. Integr. Biol. 2010, 3, 24–27. [Google Scholar] [CrossRef]
- Valdés, D.P.; Torres, T.E.; Moreno Maldonado, A.C.; Urretavizcaya, G.; Nadal, M.S.; Vasquez Mansilla, M.; Zysler, R.D.; Goya, G.F.; De Biasi, E.; Lima, E., Jr. Thermographical Method to Assess the Performance of Magnetic Nanoparticles in Hyperthermia Experiments through Spatiotemperal Temperature Profiles. Phys. Rev. Appl. 2023, 19, 014042. [Google Scholar] [CrossRef]
- Drung, D.; Abmann, C.; Beyer, J.; Kirste, A.; Peters, M.; Ruede, F.; Schurig, T. Highly Sensitive and Easy-to-Use SQUID Sensors. IEEE Trans. Appl. Supercond. 2007, 17, 699–704. [Google Scholar] [CrossRef]
- Davis, S.I.; Kirtley, J.R.; Moler, K.A. Influence of Resonances on the Noise Performance of SQUID Susceptometers. Sensors 2019, 20, 204. [Google Scholar] [CrossRef]
- Reith, P.; Wang, X.R.; Hilgenkamp, H. Analysing magnetism using scanning SQUID microscopy. Rev. Sci. Instrum. 2017, 88, 123706. [Google Scholar] [CrossRef]
- Vettoliere, A.; Granata, C. Highly Sensitive Tunable Magnetometer Based on Superconducting Quantum Interference Device. Sensors 2023, 23, 3558. [Google Scholar] [CrossRef]
- Blondeau, M.; Guyodo, Y.; Guyot, F.; Gatel, C.; Menguy, N.; Chebbi, I.; Haye, B.; Durand-Dubief, M.; Alphandery, E.; Brayner, R.; et al. Magnetic-field induced rotation of magnetosome chains in silicified magnetotactic bacteria. Sci. Rep. 2018, 8, 7699. [Google Scholar] [CrossRef] [PubMed]
- Kerans, F.F.A.; Lungaro, L.; Azfer, A.; Salter, D.M. The Potential of Intrinsically Magnetic Mesenchymal Stem Cells for Tissue Engineering. Int. J. Mol. Sci. 2018, 19, 3159. [Google Scholar] [CrossRef] [PubMed]
- Škrátek, M.; Dvurečenskij, A.; Kluknavský, M.; Barta, A.; Bališ, P.; Mičurová, A.; Cigáň, A.; Eckstein-Andicsová, A.; Maňka, J.; Bernátová, I. Sensitive SQUID Bio-Magnetometry for Determination and Differentiation of Biogenic Iron and Iron Oxide Nanoparticles in the Biological Samples. Nanomaterials 2020, 10, 1993. [Google Scholar] [CrossRef] [PubMed]
- Martin, L.M.A.; Sheng, J.; Zimba, P.V.; Zhu, L.; Fadare, O.O.; Haley, C.; Wang, M.; Phillips, T.D.; Conkle, J.; Xu, W. Testing an Iron Oxide Nanoparticle-Based Method for Magnetic Separation of Nanoplastics and Microplastics from Water. Nanomaterials 2022, 12, 2348. [Google Scholar] [CrossRef]
- Foner, S. Versatile and Sensitive Vibrating-Sample Magnetometer. Rev. Sci. Instrum. 1959, 30, 548–557. [Google Scholar] [CrossRef]
- Lopez-Dominguez, V.; Quesada, A.; Guzmán-Mínguiez, J.C.; Moreno, L.; Lere, M.; Spottorno, J.; Giacomone, F.; Fernández, J.F.; Hernado, A.; García, M.A. A simple vibrating simple magnetometer for macroscopic samples. Rev. Sci. Instrum. 2018, 89, 034707. [Google Scholar] [CrossRef]
- Benito, L.; Arnaudas, J.I.; del Moral, A. High-sensitivity vector magnetometer for measuring magnetic torque at low temperatures. Rev. Sci. Instrum. 2006, 77, 025101. [Google Scholar] [CrossRef]
- Chen, S.; Yu, M.; Zhang, W.; He, K.; Pan, H.; Cui, K.; Zhao, Y.; Zhang, X.-H.; Xiao, T.; Zhang, W.; et al. Metagenomic and Microscopic Analysis of Magnetotactic Bacteria in Tangyin Hydrothermal Field of Okinawa Through. Front. Microbiol. 2022, 13, 887136. [Google Scholar] [CrossRef]
- Gao, Y.; Wen, P.; Cardé, R.T.; Xu, H.; Huang, Q. In addition to crytochrome 2, magnetic particles with olfactory co-receptor are important for magnetic orientation in termites. Commun. Biol. 2021, 4, 1121. [Google Scholar] [CrossRef]
- Gerzsenyi, T.B.; Ilosvai, Á.M.; Szilágyi, G.; Szőri, M.; Váradi, C.; Viskolcz, B.; Vanyorek, L.; Szőri-Dorogházi, E. A Simplified and Efficient Method for Production of Manganese Ferrite Magnetic Nanoparticles and Their Applications in DNA Isolation. Int. J. Mol. Sci. 2023, 24, 2156. [Google Scholar] [CrossRef]
- Vassallo, M.; Martella, D.; Barrera, G.; Celegato, F.; Coïson, M.; Ferrero, R.; Olivetti, E.S.; Troia, A.; Sözeri, H.; Parmeggiani, C.; et al. Improvement of Hyperthermia Properties of Iron Oxide Nanoparticles by Surface Coating. ACS Omega 2023, 8, 2143–2154. [Google Scholar] [CrossRef] [PubMed]
- Oppeneer, P.M. Magneto-Optical Kerr Spectra. In Handbook of Magnetic Materials; Buschow, H.H.J., Ed.; North-Holland: Amsterdam, The Netherlands, 2001; pp. 229–422. [Google Scholar]
- Soldatov, I.V.; Zehner, J.; Leistner, K.; Kang, T.; Karnaushenko, D.; Schäfer, R. Advanced, Kerr-microscopy-based MOKE magnetometry for the anisotropy characterization of magnetic films. J. Magn. Magn. Mater. 2021, 529, 167889. [Google Scholar] [CrossRef]
- Teixeira, J.M.; Lusche, R.; Ventura, J.; Fermento, R.; Carpinteiro, F.; Araujo, J.P.; Sousa, J.B.; Cardoso, S.; Freitas, P.P. Versatile, high sensitivity, and automatized angular dependent vectorial Kerr magnetometer for the analysis of nanostructured materials. Rev. Sci. Instrum. 2011, 82, 043902. [Google Scholar] [CrossRef] [PubMed]
- Légaré, K.; Chardonnet, V.; Bermúdez Macias, I.; Hennes, M.; Delaunay, R.; Lassonde, P.; Légaré, F.; Lambert, G.; Jal, E.; Vodungbo, B. Analytic description and optimization of magneto-optical Kerr setups with photoelastic modulation. Rev. Sci. Instrum. 2022, 93, 073001. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Matsuda, I. Measurement of the Resonant Magneto-Optical Kerr Effect Using a Free Electron Laser. Appl. Sci. 2017, 7, 662. [Google Scholar] [CrossRef]
- Huízar-Féliz, A.M.; Muñoz, D.; Orue, I.; Magén, C.; Ibarra, A.; Barandiarán, J.M.; Muela, A.; Fdez-Gubieda, M.L. Assemblies of magnetite nanoparticles extracted from magnetotactic bacteria: A magnetic study. Appl. Phys. Lett. 2016, 108, 063109. [Google Scholar] [CrossRef]
- Donolato, M.; Torti, A.; Kostesha, N.; Deryabina, M.; Sogne, E.; Vavassori, P.; Hansen, M.F.; Bertacco, R. Magnetic domain wall conduits for single cell applications. Lab Chip 2011, 11, 2976–2983. [Google Scholar] [CrossRef]
- Begué, A.; Ciria, M. Strain-Mediated Giant Magnetoelectric Coupling in a Crystalline Multiferroic Heterostructure. ACS Appl. Mater. Interfaces 2021, 13, 6778–6784. [Google Scholar] [CrossRef]
- Lehmann, T.E. Nuclear Magnetic Resonance Spectroscopy. Magnetochemistry 2018, 4, 20. [Google Scholar] [CrossRef]
- Roessler, M.M.; Salvadori, E. Principles and applications of EPR spectroscopy in the chemical sciences. Chem. Soc. Rev. 2018, 47, 2534–2553. [Google Scholar] [CrossRef]
- Von Bardeleben, H.J.; Cantin, J.L.; Gendron, F. Ferromagnetic Resonance Spectroscopy: Basics and Applications. In Electron Paramagnetic Resonance Spectroscopy; Springer: Cham, Switzerland, 2020; Volume 1, pp. 351–383. [Google Scholar] [CrossRef]
- Markwick, P.R.; Malliavin, T.; Nilges, M. Structural biology by NMR: Structure, dynamics, and interactions. PLoS Comput. Biol. 2008, 4, e1000168. [Google Scholar] [CrossRef] [PubMed]
- Möser, J.; Lips, K.; Tseytlin, M.; Eaton, G.R.; Eaton, S.S.; Schnegg, A. Using rapid-scan EPR to improve the detection limit of quantitative EPR by more than one order of magnitude. J. Magn. Reson. 2017, 281, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Benguettat-El Mokhtari, I.; Schmool, D.S. Ferromagnetic Resonance in Magnetic Oxide Nanoparticles: A Short Review of Theory and Experiment. Magnetochemistry 2023, 9, 191. [Google Scholar] [CrossRef]
- Blattmann, T.M.; Lesniak, B.; García-Rubio, I.; Charilaou, M.; Wessels, M.; Eglinton, T.I.; Gehring, A.U. Ferromagnetic resonance of magnetite biominerals traces redox changes. Earth Planet Sci. Lett. 2020, 545, 116400. [Google Scholar] [CrossRef]
- Xu, J.; Jarocha, L.E.; Zollitsch, T.; Konowalczyk, M.; Henbest, K.B.; Richert, S.; Golesworthy, M.J.; Schmidt, J.; Déjean, V.; Sowood, D.J.C.; et al. Magnetic sensitivity of cryptochrome 4 from a migratory songbird. Nature 2021, 594, 535–540. [Google Scholar] [CrossRef] [PubMed]
- Bonke, S.A.; Risse, T.; Schnegg, A.; Brückner, A. In situ electron paramagnetic resonance spectroscopy for catalysis. Nat. Rev. Methods Primers 2021, 1, 33. [Google Scholar] [CrossRef]
- Sahu, I.D.; Lorigan, G.A. Electron Paramagnetic Resonance as a Tool for Studying Membrane Proteins. Biomolecules 2020, 10, 763. [Google Scholar] [CrossRef]
- Barwinska-Sendra, A.; Garcia, Y.M.; Sendra, K.M.; Baslé, A.; Mackenzie, E.S.; Tarrant, E.; Card, P.; Tabares, L.C.; Bicep, C.; Un, S.; et al. An evolutionary path to altered cofactor specificity in a metalloenzyme. Nat. Commun. 2020, 11, 2738. [Google Scholar] [CrossRef]
- Gimeno, I.; Urtizberea, A.; Román-Roche, J.; Zueco, D.; Camón, A.; Alonso, P.J.; Roubeau, O.; Luis, F. Broad-band spectroscopy of a vanadyl porphyrin: A model electronuclear spin qudit. Chem. Sci. 2021, 12, 5621–5630. [Google Scholar] [CrossRef]
- Kuzmann, E.; Homonnay, Z.; Klecsár, Z.; Szalay, R. 57Fe Mössbauer Spectroscopy as a Tool for Study of Spin States and Magnetic Interactions in Inorganic Chemistry. Molecules 2021, 26, 1062. [Google Scholar] [CrossRef]
- Bharut-Ram, K.; Mølholdt, T.E.; Langouche, G.; Geburt, S.; Ronning, C.; Doyle, T.B.; Gunnlaugsson, H.P.; Johnston, K.; Mantovan, R.; Masenda, H.; et al. Sensitivity of 57Fe emission Mössbauer spectroscopy to Ar and C induced defects in ZnO. Hyperfine Interact. 2016, 237, 81. [Google Scholar] [CrossRef]
- Lin, Q.; Xu, J.; Yang, F.; Lin, J.; Yang, H.; He, Y. Magnetic and Mössbauer Spectroscopy Studies of Zinc-Substituted Cobalt Ferrites Prepared by the Sol-Gel Method. Materials 2018, 11, 1799. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Hitchcock, A.P.; Bazylinski, D.A.; Denes, P.; Joseph, J.; Lins, U.; Marchesini, S.; Shiu, H.-W.; Tyliszczak, T.; Shapiro, D.A. Measuring spectroscopy and magnetism of extracted and intracellular magnetosomes using soft X-ray ptychography. Proc. Nat. Acad. Sci. USA 2016, 113, E8219–E8227. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.O.; Graf, D.; Lamparter, T.; Schünermann, V. Characterization of the photolyase-like iron sulfur protein. PhrB from Agrabacterium tumefaciens by Mössbauer spectroscopy. Hyperfine Interact. 2014, 226, 445–449. [Google Scholar] [CrossRef]
- Cini, A.; Poggini, L.; Chumakov, A.I.; Rüffer, R.; Spina, G.; Wattiaux, A.; Duttine, M.; Gonidec, M.; Fittipaldi, M.; Rosa, P.; et al. Synchrotron-based Mössbauer spectroscopy characterization of sublimated spin crossover molecules. Phys. Chem. Chem. Phys. 2020, 22, 6626–6637. [Google Scholar] [CrossRef]
- Nikolo, M. Superconductivity: A guide to alternating current susceptibility measurements and alternating current susceptometer design. Am. J. Phys. 1995, 63, 57–65. [Google Scholar] [CrossRef]
- Postulka, L.; Eibisch, P.; Holzmann, A.; Wolf, B.; Lang, M. Phase-sensitive dynamic susceptibility setup for measurements in pulsed magnetic fields. Rev. Sci. Instrum. 2019, 90, 033901. [Google Scholar] [CrossRef]
- Bazalova, O.; Kvicalova, M.; Valkova, T.; Slaby, P.; Bartos, P.; Netusil, R.; Tomanova, K.; Braeunig, P.; Lee, H.-J.; Sauman, I.; et al. Crytochrome 2 mediated directional magnetoreception in cockroaches. Proc. Natl. Acad. Sci. USA 2016, 113, 1660–1665. [Google Scholar] [CrossRef]
- Gandia, D.; Gandarias, L.; Rodrigo, I.; Robles-García, J.; Das, R.; Garaio, E.; García, J.E.; Phan, M.-H.; Srikanth, H.; Orue, I.; et al. Unlocking the Potential of Magnetotactic Bacteria as Magnetic Hyperthermia Agents. Small 2019, 15, e1902626. [Google Scholar] [CrossRef]
- Tao, Q.; Zhang, L.; Han, X.; Chen, H.; Ji, X.; Zhang, X. Magnetic Susceptibility Difference-Induced Nucleus Positioning in Gradient Ultrahigh Magnetic Field. Biophys. J. 2020, 118, 578–585. [Google Scholar] [CrossRef]
- Fernández-Afonso, Y.; Asín, L.; Beola, L.; Moros, M.; de la Fuente, J.M.; Fratila, R.M.; Grazú, V.; Gutiérrez, L. Iron Speciation in Animal Tissues Using AC Magnetic Susceptibility Measurements: Quantification of Magnetic Nanoparticles, Ferritin, and Other Iron-Containing Species. ACS Appl. Bio. Mater. 2022, 5, 1879–1889. [Google Scholar] [CrossRef] [PubMed]
- Escalante, J.; Nishimura, B.; Tuttobene, M.R.; Subils, T.; Mezcord, V.; Actis, L.A.; Tolmasky, M.E.; Bonomo, R.A.; Ramirez, M.S. The Iron Content of Human Serum Albumin Modulates the Susceptibility of Acinetobacter baumannii to Cefiderocol. Biomedicines 2023, 11, 639. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zocchi, G. Elasticity of globular proteins measured from the ac susceptibility. Phys. Rev. Lett. 2010, 105, 238104. [Google Scholar] [CrossRef] [PubMed]
- Pauling, L. Nature of forces between large molecules of biological interest. Nature 1948, 161, 707–709. [Google Scholar] [CrossRef]
- Franken, L.E.; Grünewald, K.; Boekema, E.J.; Stuart, M.C.A. A Technical Introduction to Transmission Electron Microscopy for Soft-Matter: Imaging, Possibilities, Choices, and Technical Developments. Small 2020, 16, e1906198. [Google Scholar] [CrossRef]
- Phatak, C.; Petford-Long, A.K.; De Graef, M. Recent advances in Lorentz microscopy. Curr. Opin. Solid State Mater. Sci. 2016, 20, 107–114. [Google Scholar] [CrossRef]
- Shibata, N.; Findlay, S.D.; Kohno, Y.; Sawada, H.; Kondo, Y.; Ikuhara, Y. Differential phase-contrast microscopy at atomic resolution. Nature Phys. 2012, 8, 611–615. [Google Scholar] [CrossRef]
- Danev, R.; Baumeister, W. Expanding the boundaries of cryo-EM with phase plates. Curr. Opin. Struct. Biol. 2017, 46, 87–94. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Dahlbom, D.; Barros, K.; Batista, C.D. CP2 skyrmions and skyrmion crystals in realistic quantum magnets. Nat. Commun. 2023, 14, 3626. [Google Scholar] [CrossRef]
- Irfan, M.; Martin, S.; Obeidi, M.A.; Miller, S.; Kuster, F.; Brabazon, D.; Naydenova, I. A magnetic Nanoparticle Doped Photopolymer for Holographic Recording. Polymers 2022, 14, 1858. [Google Scholar] [CrossRef]
- Kohno, Y.; Seki, T.; Findlay, S.D.; Ikuhara, Y.; Shibata, N. Real-space visualization of intrinsic magnetic fields of an antiferromagnet. Nature 2022, 602, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Carlino, E. In-Line Holography in Transmission Electron Microscopy for the Atomic Resolution Imaging of Single Particle of Radiation-Sensitive Matter. Materials 2020, 13, 1413. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lazic, I.; Huang, X.; Wirix, M.; Wang, L.; Deng, Y.; Niu, T.; Wu, D.; Yu, L.; Sun, F. Imaging biological samples by integrated differential phase contrast (iDPC) STEM technique. J. Struct. Biol. 2022, 214, 107837. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, C.J.; Chemla, Y.R.; Liu, S.; Wang, M.D. Optical tweezers in single-molecule biophysics. Nat. Rev. Methods Primers 2021, 1, 25. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, R.; Rybenkov, V.V. A Guide to Magnetic Tweezers and Their Applications. Front. Phys. 2016, 4, 48. [Google Scholar] [CrossRef]
- Moghram, W.I.; Kruger, A.; Sander, E.A.; Selby, J.C. Magnetic tweezers with magnetic flux density feedback control. Rev. Sci. Instrum. 2021, 92, 034101. [Google Scholar] [CrossRef]
- Binnig, G.; Quate, C.F.; Gerber, C. Atomic Force Microscope. Phys. Rev. Lett. 1986, 56, 930. [Google Scholar] [CrossRef]
- Yang, Q.; Ma, Q.; Herum, K.M.; Wang, C.; Patel, N.; Lee, J.; Wang, S.; Yen, T.M.; Wang, J.; Tang, H.; et al. Array atomc force microscopy for real-time multiparametric analysis. Proc. Natl. Acad. Sci. USA 2019, 116, 5872–5877. [Google Scholar] [CrossRef]
- Vega, S.; Neira, J.L.; Marcuello, C.; Lostao, A.; Abian, O.; Velazquez-Campoy, A. NS3 protease from hepatitis C virus: Biophysical studies on an intrinsically disordered protein domain. Int. J. Mol. Sci. 2013, 14, 13282–13306. [Google Scholar] [CrossRef]
- Iturri, J.; Toca-Herrera, J.L. Characterization of Cell Scaffolds by Atomic Force Microscopy. Polymers 2017, 9, 383. [Google Scholar] [CrossRef]
- Marcuello, C.; Frempong, G.A.; Balsera, M.; Medina, M.; Lostao, A. Atomic Force Microscopy to Elicit Conformational Transitions of Ferredoxin-Dependent Flavin Thioredoxin Reductases. Antioxidants 2021, 10, 1437. [Google Scholar] [CrossRef] [PubMed]
- Sebastián, M.; Lira-Navarrete, E.; Serrano, A.; Marcuello, C.; Velázquez-Campoy, A.; Lostao, A.; Hurtado-Guerrero, R.; Medina, M.; Martínez-Júlvez, M. The FAD synthetase from the human pathogen Streptococcus pneumoniae: A bifunctional enzyme exhibiting activity-dependent redox requirements. Sci. Rep. 2017, 7, 7609. [Google Scholar] [CrossRef]
- Sergunova, V.; Leesment, S.; Kozlov, A.; Inozemtsev, V.; Platitsina, P.; Lyapunova, S.; Onufrievich, A.; Polyakov, V.; Shertyukova, E. Investigation of Red Blood Cells by Atomic Force Microscopy. Sensors 2022, 22, 2055. [Google Scholar] [CrossRef] [PubMed]
- Dinarelli, S.; Longo, G.; Germanova-Taneva, S.; Todinova, S.; Krumova, S.; Girasole, M. Surprising Structural and Functional Properties of Favism Erythrocytes Are Linked to Special Metabolic Regulation: A Cell Aging Study. Int. J. Mol. Sci. 2022, 24, 637. [Google Scholar] [CrossRef] [PubMed]
- Gerbin, E.; Frapart, Y.-M.; Marcuello, C.; Cottyn, B.; Foulon, L.; Pernes, M.; Crônier, D.; Molinari, M.; Chabbert, B.; Ducrot, P.-H.; et al. Dual Antioxidant Properties and Organic Radical Stabilization in Cellulose Nanocomposite Films Functionalized by In Situ Polymerization of Coniferyl Alcohol. Biomacromolecules 2020, 21, 3163–3172. [Google Scholar] [CrossRef] [PubMed]
- Berzin, F.; Lemkhanter, L.; Marcuello, C.; Chabbert, B.; Aguié-Béghin, V.; Molinari, M.; Castellani, R.; Vergnes, B. Influence of the polarity of the matrix on the breakage mechanisms of Lignocellulosic fibers during twin-screw extrusion. Polym. Compos. 2020, 41, 1106–1117. [Google Scholar] [CrossRef]
- Pele, K.G.; Amaveda, H.; Mora, M.; Marcuello, C.; Lostao, A.; Alamán-Díez, P.; Pérez-Huertas, S.; Pérez, M.A.; García-Aznar, J.M.; García-Gareta, E. Hydrocolloids of Egg White and Gelatin as a Platform for Hydrogel-Based Tissue Engineering. Gels 2023, 9, 605. [Google Scholar] [CrossRef]
- Lostao, A.; Lim, K.; Pallarés, M.C.; Ptak, A.; Marcuello, C. Recent advances in sensing the inter-biomolecular interactions at the nanoscale—A comprehensive review of AFM-based force spectroscopy. Int. J. Biol. Macromol. 2023, 238, 124089. [Google Scholar] [CrossRef]
- Marcuello, C.; de Miguel, R.; Martínez-Júlvez, M.; Gómez-Moreno, C.; Lostao, A. Mechanostability of the Single-Electron-Transfer Complexes of Anabaena Ferredoxin-NADP(+) Reductase. Chemphyschem 2015, 16, 3161–3169. [Google Scholar] [CrossRef]
- Pérez-Domínguez, S.; Caballero-Mancebo, S.; Marcuello, C.; Martínez-Júlvez, M.; Medina, M.; Lostao, A. Nanomechanical Study of Enzyme: Coenzyme Complexes: Bipartite Sites in Plastidic Ferredoxin-NADP+ Reductase for the Interaction with NADP. Antioxidants 2022, 11, 537. [Google Scholar] [CrossRef]
- Marcuello, C.; de Miguel, R.; Lostao, A. Molecular Recognition of Proteins through Quantitative Force Maps at Single Molecule Level. Biomolecules 2022, 12, 594. [Google Scholar] [CrossRef] [PubMed]
- Lipke, P.N.; Rauceo, J.M.; Viljoen, A. Cell-Cell Mating Interactions: Overview and Potential of Single-Cell Force Spectroscopy. Int. J. Mol. Sci. 2022, 23, 1110. [Google Scholar] [CrossRef]
- Ding, Y.; Apostolidou, D.; Marszalek, P. Mechanical Stability of a Small, Highly-Luminescent Engineered Protein NanoLuc. Int. J. Mol. Sci. 2020, 22, 55. [Google Scholar] [CrossRef] [PubMed]
- Marcuello, C.; Chabbert, B.; Berzin, F.; Bercu, N.B.; Molinari, M.; Aguié-Béghin, V. Influence of Surface Chemistry of Fiber and Lignocellulosic Materials on Adhesion Properties with Polybutylene Succinate at Nanoscale. Materials 2023, 16, 2440. [Google Scholar] [CrossRef]
- Magazzù, A.; Marcuello, C. Investigation of Soft Matter Nanomechanics by Atomic Force Microscopy and Optical Tweezers: A Comprehensive Review. Nanomaterials 2023, 13, 963. [Google Scholar] [CrossRef] [PubMed]
- Zambito, M.; Viti, F.; Bosio, A.G.; Ceccherini, I.; Florio, T.; Vassalli, M. The Impact of Experimental Conditions on Cell Mechanics as Measured with Nanoindentation. Nanomaterials 2023, 13, 1190. [Google Scholar] [CrossRef]
- Kontomaris, S.V.; Stylianou, A.; Malamou, A. Atomic Force Microscopy Nanoindentation Method on Collagen Fibrils. Materials 2022, 15, 2477. [Google Scholar] [CrossRef]
- Marcuello, C.; Foulon, L.; Chabbert, B.; Aguié-Béghin, V.; Molinari, M. Atomic force microscopy reveals how relative humidity impacts the Young’s modulus of lignocellulosic polymers and their adhesion with cellulose nanocrystals at the nanoscale. Int. J. Biol. Macromol. 2020, 147, 1064–1075. [Google Scholar] [CrossRef]
- Carapeto, A.P.; Vitorino, M.V.; Santos, J.D.; Ramalho, S.S.; Robalo, T.; Rodrigues, M.S.; Farinha, C.M. Mechanical Properties of Human Bronchial Epithelial Cells Expressing Wt- and Mutant CFTR. Int. J. Mol. Sci. 2020, 21, 2916. [Google Scholar] [CrossRef]
- Brás, M.M.; Cruz, T.B.; Maia, A.F.; Oliveira, M.J.; Sousa, S.R.; Granja, P.L.; Radmacher, M. Mechanical Properties of Colorectal Cancer Cells Determined by Dynamic Atomic Force Microscopy: A Novel Biomaker. Cancers 2022, 14, 5053. [Google Scholar] [CrossRef]
- Kubiak, A.; Zieliński, T.; Pabijan, J.; Lekka, M. Nanomechanics in Monitoring the Effectiveness of Drugs Targeting the Cancer Cell Cytoskeleton. Int. J. Mol. Sci. 2020, 21, 8786. [Google Scholar] [CrossRef] [PubMed]
- Dazzi, A.; Prazeres, R.; Glotin, F.; Ortega, J.M. Analysis of nano-chemical mapping performed by an AFM-based (“AFMIR”) acousto-optic technique. Ultramicroscopy 2007, 107, 1194–1200. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, F.S.; Habchi, J.; Chia, S.; Horne, R.I.; Vendruscolo, M.; Knowles, T.P.J. Infrared nanospectroscopy reveals the molecular fingerprint of an aggregation inhibitor with single Aβ42 oligomers. Nat. Commun. 2021, 12, 688. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Khanat, D.; Kalionis, B.; Chrzanowski, W. High-fidelity probing of the structure and heterogeneity of extracellular vesicles by resonance-enhanced atomic force microscopy infrared spectroscopy. Nat. Protoc. 2019, 14, 576–593. [Google Scholar] [CrossRef] [PubMed]
- Saboo, S.; Bapat, P.; Moseson, D.E.; Kestur, U.S.; Taylor, L.S. Exploring the Role of Surfactants in Enhancing Drug Release from Amorphous Solid Dispersions at Higher Drug Loadings. Pharmaceutics 2021, 13, 735. [Google Scholar] [CrossRef]
- Martin, Y.; Wickramasinghe, H.K. Magnetic imaging by “force microscopy” with 1000 Å resolution. Appl. Phys. Lett. 1987, 50, 1455–1457. [Google Scholar] [CrossRef]
- Wang, C.H.; Yang, Z. Submicrometer resolution far field high sensitivity Kerr microscopy for in-plane magnetization detection. Rev. Sci. Instrum. 2009, 80, 073107. [Google Scholar] [CrossRef]
- Buchner, M.; Höfler, K.; Henne, B.; Ney, V.; Ney, A. Tutorial: Basic principles, limits of detection, and pitfalls of highly sensitive SQUID magnetometry for nanomagnetism and spintronics. J. Appl. Phys. 2018, 124, 161101. [Google Scholar] [CrossRef]
- Kalendra, V.; Turčak, J.; Banys, J.; Morton, J.J.L.; Šimenas, M. X- and Q-band EPR with cryogenic amplifiers independent of sample temperature. J. Magn. Reson. 2023, 346, 107356. [Google Scholar] [CrossRef]
- Ares, P.; Jaafar, M.; Gil, A.; Gómez-Herrero, J.; Asenjo, A. Magnetic Force Microscopy in Liquids. Small 2015, 11, 4731–4736. [Google Scholar] [CrossRef]
- Kim, G.; Yun, J.; Lee, Y.; Kim, J. Construction of a vector-field cryogenic magnetic force microscope. Rev. Sci. Instrum. 2022, 93, 063701. [Google Scholar] [CrossRef] [PubMed]
- Suehira, N.; Tomiyoshi, Y.; Sugawara, Y.; Morita, S. Low-temperature noncontact atomic-force microscope with quick simple and cantilever Exchange mechanism. Rev. Sci. Instrum. 2001, 72, 2971–2976. [Google Scholar] [CrossRef]
- Lorusso, G.; Jenkins, M.; González-Monje, P.; Arauzo, A.; Sesé, J.; Ruiz-Molina, D.; Roubeau, O.; Evangelisti, M. Surface-confined molecular coolers for cryogenics. Adv. Mater. 2013, 25, 2984–2988. [Google Scholar] [CrossRef] [PubMed]
- Amor, M.; Mathon, F.P.; Monteil, C.L.; Busigny, V.; Léfevre, C.T. Iron-biomineralizing organelle in magnetotactic bacteria: Function, synthesis and preservation in ancient rock samples. Environ. Microbiol. 2020, 22, 3611–3622. [Google Scholar] [CrossRef] [PubMed]
- Baaziz, W.; Ghica, C.; Cypriano, J.; Abreu, F.; Anselme, K.; Ersen, O.; Farina, M.; Werckmann, J. New Phenotype and Mineralization of Biogenic Iron Oxide in Magnetotactic Bacteria. Nanomaterials 2021, 11, 3189. [Google Scholar] [CrossRef] [PubMed]
- Müller, F.D.; Schüler, D.; Pfeiffer, D. A Compass to Boost Navigation: Cell Biology of Bacterial Magnetotaxis. J. Bacteriol. 2020, 202, e00398-20. [Google Scholar] [CrossRef]
- Raschdorf, O.; Forstner, Y.; Kolinko, I.; Uebe, R.; Plitzko, J.M.; Schüler, D. Genetic and Ultrastructural Analysis Reveals the Key Players and Initial Steps of Bacterial Magnetosome Membrane Biogenesis. PLoS Genet. 2016, 12, e1006101. [Google Scholar] [CrossRef]
- Ozyamak, E.; Kollman, J.; Agard, D.A.; Komeili, A. The bacterial actin MamK: In vitro assembly behavior and filament architecture. J. Biol. Chem. 2013, 288, 4265–4277. [Google Scholar] [CrossRef]
- Toro-Nahuelpan, M.; Giacomelli, G.; Raschdorf, O.; Borg, S.; Plitzko, J.M.; Bramkamp, M.; Schüler, D.; Müller, F.-D. MamY is a membrane-bound protein that aligns magnetosomes and the motility axis of helical magnetotactic bacteria. Nat. Microbiol. 2019, 4, 1978–1989. [Google Scholar] [CrossRef]
- Uebe, R.; Junge, K.; Henn, V.; Poxleitner, G.; Katzmann, E.; Plitzko, J.M.; Zarivach, R.; Kasama, T.; Wanner, G.; Pósfai, M.; et al. The cation diffusion facilitator proteins MamB and MamM of Magnetospirillum gryphiswaldense have distinct and complex functions, and are involved in magnetite biomineralization and magnetosome membrane assembly. Mol. Microbiol. 2011, 82, 818–835. [Google Scholar] [CrossRef]
- Peigneux, A.; Jabalera, Y.; Fernández Vivas, M.A.; Casares, S.; Azuaga, A.I.; Jimenez-Lopez, C. Tuning properties of biomimetic magnetic nanoparticles by combining magnetosome associated proteins. Sci. Rep. 2019, 9, 8804. [Google Scholar] [CrossRef] [PubMed]
- Moisescu, C.; Ardelean, I.I.; Benning, L.G. The effect and role of environmental conditions on magnetosome synthesis. Front. Microbiol. 2014, 5, 49. [Google Scholar] [CrossRef] [PubMed]
- Gandia, D.; Gandarias, L.; Marcano, L.; Orue, I.; Gil-Cartón, D.; Alonso, J.; García-Arribas, A.; Muela, A.; Fdez-Gubieda, M.L. Elucidating the role of shape anisotropy in faceted magnetic nanoparticles using biogenic magnetosomes as a model. Nanoscale 2020, 12, 16081–16090. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, J.; Bertinetti, L.; Widdrat, M.; Hirt, A.M.; Faivre, D. Formation of magnetite nanoparticles at low temperature: From superparamagnetic to stable single domains particles. PLoS ONE 2013, 8, e57070. [Google Scholar] [CrossRef] [PubMed]
- Lai, W.; Li, D.; Wang, Q.; Ma, Y.; Tian, J.; Fang, Q. Bacterial Magnetosomes Release Iron Ions and Induce Regulation of Iron Homeostasis in Endothelial Cells. Nanomaterials 2022, 12, 3995. [Google Scholar] [CrossRef]
- Schmidt, C.K.; Medina-Sánchez, M.; Edmondson, R.J.; Schmidt, O.G. Engineering microrobots for targeted cancer therapies from a medical perspective. Nat. Commun. 2020, 11, 5618. [Google Scholar] [CrossRef]
- Xie, M.; Zhang, W.; Fan, C.; Wu, C.; Feng, Q.; Wu, J.; Li, Y.; Gao, R.; Li, Z.; Wang, Q.; et al. Bioinspired Soft Microrobots with Precise Magneto-Collective Control for Microvascular Thrombolysis. Adv. Mater. 2020, 32, e2000366. [Google Scholar] [CrossRef]
- Liu, P.; Zheng, Y.; Zhang, R.; Bai, J.; Zhu, K.; Benzerara, K.; Menguy, N.; Roberts, A.P.; Pan, Y. Key gene networks that control magnetosome biomineralization in magnetotactic bacteria. Natl. Sci. Rev. 2022, 10, nwac238. [Google Scholar] [CrossRef]
- Nan, X.; Lai, W.; Li, D.; Tian, J.; Hu, Z.; Fang, Q. Biocompatibility of Bacterial Magnetosomes as MRI Contrast Agent: A Long-Term In Vivo Follow-Up Study. Nanomaterials 2021, 11, 1235. [Google Scholar] [CrossRef]
- Usov, N.A.; Gubanova, E.M. Application of Magnetosomes in Magnetic Hyperthermia. Nanomaterials 2020, 10, 1320. [Google Scholar] [CrossRef]
- Mickoleit, F.; Jörke, C.; Richter, R.; Rosenfeldt, S.; Market, S.; Rehberg, I.; Schenk, A.S.; Bäumchen, O.; Schüler, D.; Clement, J.H. Long-Term Stability, Biocompatibility, and Magnetization of Suspensions of Isolated Bacterial Magnetosomes. Small 2023, 19, e2206244. [Google Scholar] [CrossRef] [PubMed]
- Ben-Shimon, S.; Stein, D.; Zarivach, R. Current view of iron biomineralization in magnetotactic bacteria. J. Struct. Biol. X 2021, 5, 100052. [Google Scholar] [CrossRef] [PubMed]
- Vodyashkin, A.A.; Kezimana, P.; Prokonov, F.Y.; Vasilenko, I.A.; Stanishevskiy, Y.M. Current Methods for Synthesis and Potential Applications of Cobalt Nanoparticles: A Review. Crystals 2022, 12, 272. [Google Scholar] [CrossRef]
- Mubraiz, N.; Bano, A.; Mahmood, T.; Khan, N. Microbial and Plant Assisted Synthesis of Cobalt Oxide Nanoparticles and Their Antimicrobial Activities. Agronomy 2021, 11, 1607. [Google Scholar] [CrossRef]
- Fatima, A.; Ahmad, M.W.; Saidi, A.K.A.A.; Choudhury, A.; Chang, Y.; Lee, G.H. Recent Advances in Gadolinium Based Contrast Agents for Bioimaging Applications. Nanomaterials 2021, 11, 2449. [Google Scholar] [CrossRef]
- Cruje, C.; Dunmore-Buyze, P.J.; Grolman, E.; Holdsworth, D.W.; Gillies, E.R.; Drangova, M. PEG-modified gadolinium nanoparticles as contrast agents for in vivo micro-CT. Sci. Rep. 2021, 11, 16603. [Google Scholar] [CrossRef]
- Saeidi, H.; Mozaffari, M.; Ilbey, S.; Dutz, S.; Zahn, D.; Azimi, G.; Bock, M. Effect of Europium Substitution on the Structural, Magnetic and Relaxivity Properties of Mn-Zn Ferrite Nanoparticles: A Dual-Mode MRI Contrast-Agent Candidate. Nanomaterials 2023, 13, 331. [Google Scholar] [CrossRef]
- Hulsure, N.R.; Inamdar, A.K.; Bandgar, S.S.; Mulik, R.N.; Shelke, S.B. Effect of samarium substitutium on Mn0.5Zn0.5SmxFe2−xO4 ferrite nanoparticles. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Tayal, A.; Chen, Y.; Song, C.; Hiroi, S.; Kumara, L.S.R.; Palina, N.; Seo, O.; Mukoyoshi, M.; Kobayashi, H.; Kitagawa, H.; et al. Local Geometry and Electronic Properties of Nickel Nanoparticles Prepared via Thermal Decomposition of Ni-MOF-74. Inorg. Chem. 2018, 57, 10072–10080. [Google Scholar] [CrossRef]
- Rehman, Z.U.; Nawaz, M.; Ullah, H.; Uddin, I.; Shad, S.; Eldin, E.; Alshgari, R.A.; Bahajjaj, A.A.A.; Arifeen, W.U.; Javed, M.S. Synthesis and Characterization of Ni Nanoparticles via the Microemulsion Technique and Its Applications for Energy Storage Devices. Materials 2022, 16, 325. [Google Scholar] [CrossRef]
- Ahmad, F.; Salem-Bekhit, M.M.; Khan, F.; Alshehri, S.; Khan, A.; Ghoneim, M.M.; Wu, H.-F.; Taha, E.I.; Elbagory, I. Unique Properties of Surface-Functionalized Nanoparticles for Bio-Application: Functionalization Mechanisms and Importance in Application. Nanomaterials 2022, 12, 1333. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, L.; de la Cueva, L.; Moros, M.; Mazarío, E.; de Bernardo, S.; de la Fuente, J.M.; Morales, M.P.; Salas, G. Aggregation effects on the magnetic properties of iron oxide colloids. Nanotechnology 2019, 30, 112001. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; He, Q.; Jiang, C. Magnetic iron oxide nanoparticles: Synthesis and surface functionalization strategies. Nanoscale Res. Lett. 2008, 3, 397–415. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, A.; Alpízar, A.; Beola, L.; Rubio, C.; Gavilán, H.; Marciello, M.; Rodríguez-Ramiro, I.; Ciordia, S.; Morris, C.J.; Del Puerto Morales, M. Understanding the Influence of a Bifunctional Polyethylene Glycol Derivative in Protein Corona Formation around Iron Oxide Nanoparticles. Materials 2019, 12, 2218. [Google Scholar] [CrossRef]
- Wang, Q.; Ma, X.; Liao, H.; Liang, Z.; Li, F.; Tian, J.; Ling, D. Artificially Engineered Cubic Iron Oxide Nanoparticle as a High-Performance Magnetic Particle Imaging Tracer for Stem Cell Tracking. ACS Nano 2020, 14, 2053–2062. [Google Scholar] [CrossRef]
- Theodosiou, M.; Sakellis, E.; Boukos, N.; Kusigerski, V.; Kalska-Szostko, B.; Efthimiadou, E. Iron oxide nanoflowers encapsulated in thermosensitive fluorescent liposomes for hyperthermia treatment of lung adenocarcinoma. Sci. Rep. 2022, 12, 8697. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhu, X.; Wu, D.; Chen, Q.; Huang, D.; Sun, C.; Xin, J.; Ni, K.; Gao, J. Anisotropic Shaped Iron Oxide Nanostructures: Controlled Synthesis and Proton Relaxation Shortening Effects. Chem. Mater. 2015, 27, 3505–3515. [Google Scholar] [CrossRef]
- Mohapatra, J.; Mitra, A.; Tyagi, H.; Bahadur, D.; Aslam, M. Iron oxide nanorods as high-performance magnetic resonance imaging contrast agents. Nanoscale 2015, 7, 9174–9184. [Google Scholar] [CrossRef]
- Park, J.; An, K.; Hwang, Y.; Park, J.-G.; Noh, H.-J.; Kim, J.-Y.; Park, J.-H.; Hwang, N.-M.; Hyeon, T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef]
- Zavaleta, C.; Ho, D.; Chung, E.J. Theranostic Nanoparticles for Tracking and Monitoring Disease State. SLAS Technol. 2018, 23, 281–293. [Google Scholar] [CrossRef]
- Zheng, P.; Paria, D.; Wang, H.; Li, M.; Barman, I. Optical properties of symmetry-breaking tetrahedral nanoparticles. Nanoscale 2020, 12, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Demortière, A.; Panissod, P.; Pichon, B.P.; Pourroy, G.; Guillon, D.; Donnio, B.; Bégin-Colin, C. Size-dependent properties of magnetic iron oxidenanocrystals. Nanoscale 2011, 3, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, F.H.; Zélis, P.M.; Arciniegas, M.L.; Pasquevich, G.A.; van Raap, M.B.F. Dipolar interaction and demagnetizing effects in magnetic nanoparticle dispersions: Introducing the mean-field interacting superparamagnet model. Phys. Rev. B 2017, 95, 134421. [Google Scholar] [CrossRef]
- Zhong, Z.; He, J.; Li, G.; Xia, L. Recent Advances in Magnetic Nanoparticles-Assisted Microfluidic Bioanalysis. Chemosensors 2023, 11, 173. [Google Scholar] [CrossRef]
- Liu, Z.; Liu, J.; Cui, X.; Wang, X.; Zhang, L.; Tang, P. Recent advances on Magnetic Sensitive Hydrogels in Tissue Engineering. Front Chem. 2020, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Bell, S.B.; Dutta, A. DNA replication in eukaryotic cells. Annu. Rev. Biochem. 2002, 71, 333–374. [Google Scholar] [CrossRef]
- Ubersax, J.A.; Ferrell, J.E., Jr. Mechanisms of specificity in protein phosphorylation. Nat. Rev. Mol. Cell Biol. 2007, 8, 530–541. [Google Scholar] [CrossRef]
- Li, H.; Stillman, B. The origin recognition complex: A biochemical and structural view. Subcell Biochem. 2012, 62, 37–58. [Google Scholar] [CrossRef]
- Carvajal-Maldonado, D.; Beckham, L.D.; Wood, R.D.; Doublié, S. When DNA Polymerases Multitask: Functions Beyond Nucleotidyl Transfer. Front. Mol. Biosci. 2022, 8, 815845. [Google Scholar] [CrossRef]
- Patil, M.; Pabla, N.; Dong, Z. Checkpoint kinase 1 in DNA damage response and cell cycle regulation. Cell. Mol. Life Sci. 2013, 70, 4009–4021. [Google Scholar] [CrossRef]
- Wilson, D.F. Oxidative phosphorylation: Regulation and role in cellular and tissue metabolism. J. Physiol. 2017, 595, 7023–7038. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Pérez, D.; Santos-Argumedo, L.; Rodríguez-Alba, J.C.; López-Herrera, G. Role of Protein Kinase A Activation in the Immune System with an Emphasis on Lipopolysaccharide-Responsive and Beige-like Anchor Protein in B Cells. Int. J. Mol. Sci. 2023, 24, 3098. [Google Scholar] [CrossRef] [PubMed]
- Vorotnikov, A.V.; Krymsky, M.A.; Shirinsky, V.P. Signal transduction and protein phosphorylation in smooth muscle contraction. Biochemistry 2002, 67, 1309–1328. [Google Scholar] [CrossRef]
- Stovbun, S.V.; Zlenko, D.V.; Bukhvostov, A.A.; Vedenkin, A.S.; Skoblin, A.A.; Kuznetsov, D.A.; Buchachenko, A.L. Magnetic field and nuclear spin influence on the DNA synthesis rate. Sci. Rep. 2023, 13, 465. [Google Scholar] [CrossRef]
- Buchachenko, A.L.; Kuznetsov, D.A. Magnetic field affects enzymatic ATP synthesis. J. Am. Chem. Soc. 2008, 130, 12868–12869. [Google Scholar] [CrossRef]
- Xue, X.; Ali, Y.F.; Hong, Z.; Luo, W.; Nie, J.; Li, B.; Jiao, Y.; Liu, N.-A. Geomagnetic Shielding Enhances Radiation Resistance by Promoting DNA Repair Process in Human Bronchial Epithelial Cells. Int. J. Mol. Sci. 2020, 21, 9304. [Google Scholar] [CrossRef]
- Luukkonen, J.; Hakulinen, P.; Mäki-Paakkanen, J.; Juutilainen, J.; Naarala, J. Enhancement of chemically induced reactive oxygen species production and DNA damage in human SH-SY5Y neuroblastoma cells by 872 MHz radiofrequency radiation. Mutat. Res. 2009, 662, 54–58. [Google Scholar] [CrossRef]
- De Iuliis, G.N.; Newey, R.J.; King, B.V.; Aitken, R.J. Mobile phone radiation induces reactive oxygen species production and DNA damage in human spermatozoa in vitro. PLoS ONE 2009, 4, e6446. [Google Scholar] [CrossRef]
- Manna, D.; Ghosh, R. Effect of radiofrequency radiation in cultured mammalian cells: A review. Electromagn. Biol. Med. 2016, 35, 265–301. [Google Scholar] [CrossRef]
- Pallarés, M.C.; Marcuello, C.; Botello-Morte, L.; González, A.; Fillat, M.F.; Lostao, A. Sequential binding of FurA from Anabaena sp. PCC 7120 to iron boxes: Exploring regulation at the nanoscale. Biochim. Biophys. Acta 2014, 1844, 623–631. [Google Scholar] [CrossRef]
- Carey, J. Affinity, Specifity, and Cooperativity of DNA Binding by Bacterial Gene Regulatory Proteins. Int. J. Mol. Sci. 2022, 23, 562. [Google Scholar] [CrossRef] [PubMed]
- Novo, N.; Romero-Tamayo, S.; Marcuello, C.; Boneta, S.; Blasco-Machin, I.; Velázquez-Campoy, A.; Villanueva, R.; Moreno-Loshuertos, R.; Lostao, A.; Medina, M.; et al. Beyond a platform protein for the degradosome assembly: The Apoptosis-Inducing Factor as an efficient nuclease involved in chromatinolysis. PNAS Nexus 2023, 2, pgac312. [Google Scholar] [CrossRef] [PubMed]
- Wei, T.; Cheng, Q.; Min, Y.-L.; Olson, E.N.; Siegwart, D.J. Systematic nanoparticle delivery of CRISPR-Cas9 ribonucleoproteins for effective tissue specific genome editing. Nat. Commun. 2020, 11, 3232. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Li, C.; Gao, C. Applications of CRISPR-Cas in agricultural and plant biotechnology. Nat. Rev. Mol. Cell. Biol. 2020, 21, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Ding, C.; Wu, Y.; Chen, X.; Chen, Y.; Wu, Z.; Lin, Z.; Kang, D.; Fang, W.; Chen, F. Global, regional, and national burden and attributable risk factors of neurological disorders: The Global Burden of Disease study 1990–2019. Front. Public Health 2022, 10, 952161. [Google Scholar] [CrossRef]
- Makky, A.; Bousset, L.; Madiona, K.; Melki, R. Atomic Force Microscopy Imaging and Nanomechanical Properties of Six Tau Isoform Assemblies. Biophys. J. 2020, 119, 2497–2507. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Komatsu, H.; Ma, J.; Axelsen, P.H.; Fakhraai, Z. Identifying Polymorphs of Amyloid-β (1-40) Fibrils Using High-Resolution Atomic Force Microscopy. J. Phys. Chem. B 2019, 123, 10376–10383. [Google Scholar] [CrossRef]
- Adegbuyiro, A.; Sedighi, F.; Jain, P.; Pinti, M.V.; Siriwardhana, C.; Hollander, J.M.; Legleiter, J. Mitochondrial membranes modify mutant hintingtin aggregation. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183663. [Google Scholar] [CrossRef]
- Moreira, G.G.; Cantrelle, F.-X.; Quezada, A.; Carvalho, F.S.; Cristóvão, J.S.; Sengupta, U.; Puangmalai, N.; Carapeto, A.P.; Rodrigues, M.S.; Cardoso, I.; et al. Dynamic interactions and Ca2+-binding modúlate the holdase-type chaperone activity of S100B preventing tau aggregation and seeding. Nat. Commun. 2021, 12, 6292. [Google Scholar] [CrossRef]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef]
- Moloney, C.M.; Labuzan, S.A.; Crook, J.E.; Siddiqui, H.; Castanedes-Casey, M.; Lachner, C.; Petersen, R.C.; Duara, R.; Graff-Radford, N.R.; Dickson, D.W.; et al. Phosphorylated tau sites that are elevated in Alzheimer’s disease fluid biomarkers are visualized in early neurofibrillary tangle maturity levels in the post mortem brain. Alzheimers Dement. 2023, 19, 1029–1040. [Google Scholar] [CrossRef]
- Kletetschka, G.; Bazala, R.; Takáč, M.; Svecova, E. Magnetic domains oscillation in the brain with neurodegenerative disease. Sci. Rep. 2021, 11, 714. [Google Scholar] [CrossRef]
- Filippini, T.; Hatch, E.E.; Vincenti, M. Residential exposure to electromagnetic fields and risk of amyotrophic lateral sclerosis: A dose-response meta-analysis. Sci. Rep. 2021, 11, 11939. [Google Scholar] [CrossRef]
- Ahmad, M.; Cahsmore, A.R. HY4 gene of A. thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature 1993, 366, 162–166. [Google Scholar] [CrossRef]
- Chaves, I.; Pokorny, R.; Byrdin, M.; Hoang, N.; Ritz, T.; Brettel, K.; Essen, L.-O.; van der Horst, G.T.J.; Batschauer, A.; Ahmad, M. The Cryptochromes: Blue Light Photoreceptors in Plants and Animals. Annu. Rev. Plant Biol. 2011, 62, 335–364. [Google Scholar] [CrossRef]
- Wang, Q.; Lin, C. Mechanisms of Cryptochrome-Mediated Photoresponses in Plants. Annu. Rev. Plant Biol. 2020, 71, 103–129. [Google Scholar] [CrossRef]
- Fantini, E.; Facella, P. Cryptochromes in the field: How blue light influences crop development. Physiol. Plant 2020, 169, 336–346. [Google Scholar] [CrossRef]
- Sancar, A.; Lindsey-Boltz, L.A.; Unsal-Kaçmaz, K.; Linn, S. Molecular mechanisms of mammalian DNA repair and the DNA damage checkpoints. Annu. Rev. Biochem. 2004, 73, 39–85. [Google Scholar] [CrossRef]
- Kavakli, I.; Ozturk, N. DNA Repair by Photolyases. Adv. Protein Chem. Struct. Biol. 2019, 115, 1–19. [Google Scholar] [CrossRef]
- Kondoh, M.; Terazima, M. Conformational and Intermolecular Interaction Dynamics of Photolyase/Cryptochrome Proteins Monitored by the Time-Resolved Diffusion Technique. Photochem. Photobiol. 2017, 93, 15–25. [Google Scholar] [CrossRef]
- Hao, Y.; Zhang, X.; Liu, Y.; Ma, M.; Huang, X.; Liu, H.; Zhang, P. Cryo-EM structure of the CRY2 and CIB1 fragment complex provides insights into CIB1-mediated photosignaling. Plant. Commun. 2023, 4, 100475. [Google Scholar] [CrossRef]
- Ponnu, J.; Hoecker, U. Signaling Mechanisms by Arabidopsis Cryptochromes. Front. Plant Sci. 2022, 13, 844714. [Google Scholar] [CrossRef]
- Ozturk, N. Phylogenetic and Functional Classification of the Photolyase/Crytochrome Family. Photochem. Photobiol. 2017, 93, 104–111. [Google Scholar] [CrossRef]
- Vechtomova, Y.L.; Telegina, T.A.; Kritsky, M.S. Evolution of Proteins of the DNA Photolyase/Cryptochrome Family. Biochemistry 2020, 85, S131–S153. [Google Scholar] [CrossRef]
- Michael, A.K.; Fribourgh, J.L.; Van Gelder, R.N.; Partch, C.L. Animal Cryptochromes: Divergent Roles in Light Perception, Circadian Timekeeping and Beyond. Photochem. Photobiol. 2017, 93, 128–140. [Google Scholar] [CrossRef]
- Brettel, K.; Byrdin, M. Reaction mechanisms of DNA photolyase. Curr. Opin. Struct. Biol. 2010, 20, 693–710. [Google Scholar] [CrossRef]
- Ahmad, M. Photocycle and signaling mechanism of plant cryptochromes. Curr. Opin. Plant Biol. 2016, 33, 108–115. [Google Scholar] [CrossRef]
- Müller, P.; Ahmad, M. Light-activated cryptochrome reacts with molecular oxygen to form a flavin-superoxide radical pair consistent with magnetoreception. J. Biol. Chem. 2011, 286, 21033–21040. [Google Scholar] [CrossRef]
- Kattnig, D.R. Radical-Pair-Based Magnetoreception Amplified by Radical Scavenging: Resilience to Spin Relaxation. J. Phys. Chem. B 2017, 121, 10215–10227. [Google Scholar] [CrossRef]
- Wiltschko, R.; Wiltschko, W. Magnetoreception in birds. J. R. Soc. Interface 2019, 16, 20190295. [Google Scholar] [CrossRef]
- Wiltschko, R.; Wiltschko, W. Magnetoreception and Bird Navigation. In Oxford Research Encyclopedia of Neuroscience; Oxford: London, UK, 2023. [Google Scholar] [CrossRef]
- Pooam, M.; Arthaut, L.-D.; Burdick, D.; Link, J.; Martino, C.F.; Ahmad, M. Magnetic sensitivity mediated by the Arabidopsis blue-light receptor cryptochrome occurs during flavin reoxidation in the dark. Planta 2019, 249, 319–322. [Google Scholar] [CrossRef]
- Kyriacou, C.P.; Rosato, E. Genetic analysis of cryptochrome in insect magnetosensitivity. Front. Physiol. 2022, 13, 928416. [Google Scholar] [CrossRef]
- Sherrard, R.M.; Morellini, N.; Jourdan, N.; El-Esawi, M.; Arthaut, L.-D.; Niessner, C.; Rouyer, F.; Klarsfeld, A.; Doulazmi, M.; Witczak, J.; et al. Low-intensity electromagnetic fields induce human cryptochrome to modulate intracellular reactive oxygen species. PLoS Biol. 2018, 16, e2006229. [Google Scholar] [CrossRef]
- Lohof, A.M.; Dufor, T.; Sherrard, R.M. Neural Circuit Repair by Low-Intensity rTMS. Cerebellum 2022, 21, 750–754. [Google Scholar] [CrossRef]
- Wiltschko, R.; Nieβner, C.; Wiltschko, W. The Magnetic Compass of Birds: The Role of Cryptochrome. Front. Physiol. 2021, 12, 667000. [Google Scholar] [CrossRef]
- Mitsui, H.; Maeda, T.; Yamaguchi, C.; Tsuji, Y.; Watari, R.; Kubo, Y.; Okano, K.; Okano, T. Overexpression in yeast, photocycle, and in vitro structural change of an avian putative magnetoreceptor cryptochrome4. Biochemistry 2015, 54, 1908–1917. [Google Scholar] [CrossRef]
- Hore, P.J.; Mouritsen, H. The Radical-Pair Mechanism of Magnetoreception. Annu. Rev. Biophys. 2016, 45, 299–344. [Google Scholar] [CrossRef]
- Rodgers, C.T.; Hore, P.J. Chemical magnetoreception in birds: The radical pair mechanism. Proc. Natl. Acad. Sci. USA 2009, 106, 253–360. [Google Scholar] [CrossRef]
- Hammad, M.; Albaqami, M.; Pooam, M.; Kernevez, E.; Witczak, J.; Ritz, T.; Martino, C.; Ahmad, M. Cryptochrome mediated magnetic sensitivity in Arabidopsis occurs independently of light-induced electron transfer to the flavin. Photochem. Photobiol. Sci. 2020, 19, 341–352. [Google Scholar] [CrossRef]
- Pooam, M.; El-Esawi, M.; Aguida, B.; Ahmad, M. Arabidopsis cryptochrome and Quantum Biology: New insights for plant science and crop improvement. J. Plant Biochem. Biotechnol. 2020, 29, 636–651. [Google Scholar] [CrossRef]
- El-Esawi, M.; Arthaut, L.-D.; Jourdan, N.; d’Harlingue, A.; Link, J.; Martino, C.F.; Ahmad, M. Blue-light induced biosynthesis of ROS contributes to the signaling mechanism of Arabidopsis cryptochrome. Sci. Rep. 2017, 7, 13875. [Google Scholar] [CrossRef] [PubMed]
- Pooam, M.; Jourdan, N.; Esawi, M.E.; Sherrard, R.M.; Ahmad, M. HEK293 cell response to static magnetic fields via the radical pair mechanism may explain therapeutic effects of pulsed electromagnetic fields. PLoS ONE 2020, 15, e0243038. [Google Scholar] [CrossRef] [PubMed]
- Bradlaugh, A.A.; Fedele, G.; Munro, A.L.; Hansen, C.N.; Hares, J.M.; Patel, S.; Kyriacou, C.P.; Jones, A.R.; Rosato, E.; Baines, R.A. Essential elements of radical pair magnetosensitivity in Drosophila. Nature 2023, 615, 111–116. [Google Scholar] [CrossRef]
- Ikeya, N.; Woodward, J.R. Cellular autofluorescence is magnetic field sensitive. Proc. Nat. Acad. Sci. USA 2021, 118, e2018043118. [Google Scholar] [CrossRef]
- Usselman, R.J.; Chavarriaga, C.; Castello, P.R.; Procopio, M.; Ritz, T.; Dratz, E.A.; Singel, D.J.; Martino, C.F. The Quantum Biology of Reactive Oxygen Species Partitioning Impacts Cellular Bioenergetics. Sci. Rep. 2016, 6, 38543. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, J.; McKenzie, R.H. Quantum dynamics of electronic excitations in biomolecular chromophores: Role of the protein environment and solvent. J. Phys. Chem. A 2008, 112, 2162–2176. [Google Scholar] [CrossRef]
- Liu, L.; Cui, G.; Fang, W.-H. Excited States and Photochemistry of Chromophores in the Photoactive Proteins Explored by the Combined Quantum Mechanical and Molecular Mechanical Calculations. Adv. Protein Chem. Struct. Biol. 2015, 100, 255–284. [Google Scholar] [CrossRef]
- Rashid, M.H.; Koel, A.; Rang, T.; Nasir, N.; Sabir, N.; Ameen, F.; Rasheed, A. Optical Dynamics of Copper-Doped Cadmium Sulfide (CdS) and Zinc Sulfide (ZnS) Quantum-Dots Core/Shell Nanocrystals. Nanomaterials 2022, 12, 2277. [Google Scholar] [CrossRef]
- Johnson, E.; Cascio, D.; Sawaya, M.R.; Gingery, M.; Schröder, O. Crystal structures of a tetrahedral open pore ferriting form the hyperthermophilic archaeon Archaeoglobus fulgidus. Structure 2005, 13, 637–648. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, X.; Zhao, G. Ferritin Nanocage: A Versatile Nanocarrier Utilized in the Field of Food, Nutrition, and Medicine. Nanomaterials 2020, 10, 1894. [Google Scholar] [CrossRef]
- Brinza, C.; Floria, M.; Popa, I.V.; Burlacu, A. The Prognostic Performance of Ferritin in Patients with Acute Myocardial Infarction: A Systematic Review. Diagnostics 2022, 12, 476. [Google Scholar] [CrossRef]
- Allen, P.D.; St Pierre, T.G.; Chua-anusorn, W.; Ström, V.; Rao, K.V. Low-frequency low-field magnetic susceptibility of ferritin and hemosiderin. Biochim. Biophys. Acta 2020, 1500, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Alamrani, N.A.; Greenway, G.M.; Pamme, N.; Goddard, N.J. Method for Determining Average Iron Content of Ferritin by Measuring its Optical Dispersion. Anal. Chem. 2019, 91, 7366–7372. [Google Scholar] [CrossRef] [PubMed]
- Labra-Muñoz, J.A.; de Reuver, A.; Koeleman, F.; Huber, M.; van der Zant, H.S.J. Ferritin-Based Single-Electron Devices. Biomolecules 2022, 12, 705. [Google Scholar] [CrossRef]
- Grant, E.S.; Hall, L.T.; Hollenberg, L.C.L.; McColl, G.; Simpson, D.A. Nonmonotonic Superparamagnetic Behavior of the Ferritin Iron Core Revealed via Quantum Spin Relaxometry. ACS Nano 2023, 17, 372–381. [Google Scholar] [CrossRef]
- Masserschmidt, A.; Hubber, R.; Wieghardt, K.; Poulos, T. Handbook of Metalloproteins; John Wiley & Sons: New York, NY, USA, 2001; pp. 1136–1147. [Google Scholar]
- Yusuf, A.P.; Abubakar, M.B.; Malami, I.; Ibrahim, K.G.; Abubakar, B.; Bello, M.B.; Qusty, N.; Elazab, S.T.; Imam, M.U.; Alexiou, A.; et al. Zinc Metalloproteins in Epigenetics and Their Crosstalk. Life 2021, 11, 186. [Google Scholar] [CrossRef]
- Chen, A.Y.; Adamek, R.N.; Dick, B.L.; Credille, C.V.; Morrison, C.N.; Cohen, S.M. Targeting Metalloenzymes for Therapeutic Intervention. Chem. Rev. 2019, 119, 1323–1455. [Google Scholar] [CrossRef]
- Jeong, W.J.; Yu, J.; Song, W.J. Proteins as diverse, efficient, and evolvable scaffolds for artificial metalloenzymes. Chem. Commun. 2020, 56, 9586–9599. [Google Scholar] [CrossRef] [PubMed]
- Sidorov, P.; Aksenov, M.; Zalivako, I.; Borisenko, A.; Semerikov, I.; Khabarova, K.; Kolachevsky, N. Coherent effects of pulsed fast gate in 1D- and 2D ion quantum computer architectures. Phys. Lett. A 2022, 450, 128370. [Google Scholar] [CrossRef]
- Motta, M.; Rice, J.E. Emerging quantum computing algorithms for quantum chemistry. WIREs Comput. Mol. Sci. 2022, 12, e1580. [Google Scholar] [CrossRef]
- Gao, E.-F.; Kang, K.L.; Kim, J.H. Immobilization of alkine phosphatase on solid surface through self-assembled monolayer and by active-site protection. J. Nanosci. Nanotechnol. 2014, 14, 4626–4632. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Min, J.; Kim, S.-U.; Choi, J.-W. Multifunctional 4-bit biomemory chip consisting of recombinant azurin variants. Biomaterials 2011, 32, 3815–3821. [Google Scholar] [CrossRef] [PubMed]
- Bostelaar, T.; Vitvitsky, V.; Kumutima, J.; Lewis, B.E.; Yadav, P.K.; Brunold, T.C.; Filipovic, M.; Lehnert, N.; Stemmler, T.L.; Banerjee, R. Hydrogen Sulfide Oxidation by Myoglobin. J. Am. Chem. Soc. 2016, 138, 8476–8488. [Google Scholar] [CrossRef] [PubMed]
- Van Stappen, C.; Deng, Y.; Liu, Y.; Heidari, H.; Wang, J.-X.; Zhou, Y.; Ledray, A.P.; Lu, Y. Designing Artificial Metalloenzymes by Tuning of the Environment beyond the Primary Coordination Sphere. Chem. Rev. 2022, 122, 11974–12045. [Google Scholar] [CrossRef]
- Urtizberea, A.; Natividad, E.; Alonso, P.J.; Pérez-Martínez, L.; Andrés, M.A.; Gascón, I.; Gimeno, I.; Luis, F.; Roubeau, O. Vanadyl spin qubit 2D arrays and their integration on superconducting resonators. Mater. Horiz. 2020, 7, 885–897. [Google Scholar] [CrossRef]
- Rothemund, P. A New Porphyrin Synthesis. The Synthesis of Porphin. J. Am. Chem. Soc. 1936, 58, 625–627. [Google Scholar] [CrossRef]
- Koczorowski, T.; Cerbin-Koczorowska-Cerbin, M.; Rębiś, T. Azaporphyrins Embedded on Carbon-Based Nanomaterials for Potential Use in Electrochemical Sensing-A Review. Nanomaterials 2021, 11, 2861. [Google Scholar] [CrossRef]
- Chen, Y.; Li, A.; Huang, Z.-H.; Wang, L.-N.; Kang, F. Porphyrin-Based Nanostructures for Photocatalytic Applications. Nanomaterials 2016, 6, 51. [Google Scholar] [CrossRef]
- Pyrzynska, K.; Kilian, K.; Pęgier, M. Porphyrins as Chelating Agents for Molecular Imaging in Nuclear Medicine. Molecules 2022, 27, 3311. [Google Scholar] [CrossRef]
- Vodyashkin, A.A.; Sergorodceva, A.V.; Kezimana, P.; Stanishevskiy, Y.M. Metal-Organic Framework (MOF)—A Universal Material for Biomedicine. Int. J. Mol. Sci. 2023, 24, 7819. [Google Scholar] [CrossRef]
- Urtizberea, A.; Natividad, E.; Alonso, P.J.; Andrés, M.A.; Gascón, I.; Goldmann, M.; Roubeau, O. A Porhyrin Spin Qubit and Its 2D Framework Nanosheets. Adv. Funct. Mat. 2018, 28, 1801695. [Google Scholar] [CrossRef]
- Fataftah, M.S.; Krzyaniak, M.D.; Vlaisavljevich, B.; Wasielewski, M.R.; Zadrozny, J.M.; Freedman, D.E. Metal-ligand covalency enables room temperature molecular qubit candidates. Chem. Sci. 2019, 10, 6707–6714. [Google Scholar] [CrossRef]
- Aguilà, D.; Roubeau, O.; Aromí, G. Designed polynuclear lanthanide complexes for quantum information processing. Dalton Trans. 2021, 50, 12045–12057. [Google Scholar] [CrossRef]
- Sáenz, J.J.; García, N.; Grütter, P.; Meyer, E.; Heinzelmann, H.; Wiesendanger, R.; Rosenthaler, L.; Hidber, H.R.; Güntherodt, H.-J. Observation of magnetic forces by the atomic force microscope. J. Appl. Phys. 1987, 62, 4293–4295. [Google Scholar] [CrossRef]
- Xu, K.; Liu, Y. Studies of probe tip materials by atomic force microscopy: A review. Beilstein J. Nanotechnol. 2022, 13, 1256–1267. [Google Scholar] [CrossRef]
- Hooke, R. De Potentia Restitutiva, or of Spring. Explaining the Power of Springing Bodies; John Martyn: London, UK, 1678. [Google Scholar]
- Hutter, J.L.; Bechhoefer, J. Calibration of atomic-force microscope tips. Rev. Sci. Instrum. 1993, 64, 1868–1873. [Google Scholar] [CrossRef]
- Sader, J.E.; Larson, I.; Mulvaney, P.; White, L.R. Method for the calibration of atomic force microscope cantilevers. Rev. Sci. Instrum. 1995, 66, 3789–3798. [Google Scholar] [CrossRef]
- Schillers, H.; Rianna, C.; Schäpe, J.; Luque, T.; Doschke, H.; Wälte, M.; Uriarte, J.J.; Campillo, N.; Michanetzis, G.P.A.; Bobrowska, J.; et al. Standardized Nanomechanical Atomic Force Microscopy Procedure (SNAP) for Measuring Soft and Biological Samples. Sci. Rep. 2017, 7, 5117. [Google Scholar] [CrossRef]
- Butt, H.-J.; Cappella, B.; Kappl, M. Force measurements with the atomic force microscope: Technique, interpretation and applications. Surf. Sci. Rep. 2005, 59, 1–152. [Google Scholar] [CrossRef]
- Jalili, N.; Laxminarayana, K. A review of atomic force microscopy imaging systems: Application to molecular metrology and biological sciences. Mechatronics 2004, 14, 907–945. [Google Scholar] [CrossRef]
- Giessibl, F.J. Advances in atomic force microscopy. Rev. Mod. Phys. 2003, 75, 949. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, W.; Wang, W.; Chen, Y. Accurate determination of MFM tip’s magnetic parameters on nanoparticles by decoupling the influence of electrostatic force. Nanotechnology 2022, 33, 475703. [Google Scholar] [CrossRef]
- Lohau, J.; Kirsch, S.; Carl, A.; Dumpich, G.; Wassermann, E.F. Quantitative determination of effective dipole and monopole moments of magnetic force microscopy tips. J. Appl. Phys. 1999, 86, 3410–3417. [Google Scholar] [CrossRef]
- Gisbert, V.G.; Amo, C.A.; Jaafar, M.; Asenjo, A.; Garcia, R. Quantitative mapping of magnetic properties at the nanoscale with bimodal AFM. Nanoscale 2021, 13, 2026–2033. [Google Scholar] [CrossRef]
- Schwenk, J.; Zhao, X.; Bacani, M.; Marioni, M.A.; Romer, S.; Hug, H.J. Bimodal magnetic force microscopy with capacitive tip-sample distance control. Appl. Phys. Lett. 2015, 107, 132407. [Google Scholar] [CrossRef]
- Zhao, X.; Schwenk, J.; Mandru, A.O.; Penedo, M.; Bacani, M.; Marioni, M.A.; Hug, H.J. Magnetic force microscopy with frequency-modulated capacitive tip-sample distance control. New J. Phys. 2018, 20, 013018. [Google Scholar] [CrossRef]
- Jaafar, M.; Iglesias-Freire, O.; Serrano-Ramón, L.; Ibarra, M.R.; de Teresa, J.M.; Asenjo, A. Distinguishing magnetic and electrostatic interactions by a Kelvin probe force microscopy-magnetic force microscopy combination. Beilstein J. Nanotechnol. 2011, 2, 552–560. [Google Scholar] [CrossRef]
- Angeloni, L.; Passeri, D.; Corsetti, S.; Peddis, D.; Mantovani, D.; Rossi, M. Single nanoparticles magnetization curves by controlled tip magnetization magnetic force microscopy. Nanoscale 2017, 9, 18000–18011. [Google Scholar] [CrossRef]
- Angeloni, L.; Passeri, D.; Reggente, M.; Mantovani, D.; Rossi, M. Removal of electrostatic artifact in magnetic force microscopy by controlled magnetization of the tip: Application to superparamagnetic nanoparticles. Sci. Rep. 2016, 6, 26293. [Google Scholar] [CrossRef]
- Poggio, M.; Degen, C.L. Force-detected nuclear magnetic resonance: Recent advances and future challenges. Nanotechnology 2010, 21, 342001. [Google Scholar] [CrossRef]
- Kuehn, S.; Hickman, S.A.; Marohn, J.A. Advances in mechanical detection of magnetic resonance. J. Chem. Phys. 2008, 128, 052208. [Google Scholar] [CrossRef]
- Longenecker, J.G.; Mamin, H.J.; Senko, A.W.; Chen, L.; Rettner, C.T.; Rugar, D.; Marohn, J.A. High-gradient nanomagnets on cantilevers for sensitive detection of nuclear magnetic resonance. ACS Nano 2012, 6, 9637–9645. [Google Scholar] [CrossRef]
- Schnoz, S.; Däpp, A.; Hunkeler, A.; Meier, B.H. Detection of liquids by magnetic resonance force microscopy in the gradient-on-cantilever geometry. J. Magn. Reson. 2019, 298, 85–90. [Google Scholar] [CrossRef]
- Boucher, M.C.; Isaac, C.E.; Sun, P.; Borbat, P.P.; Marohn, J.A. A Non-Perturbative, Low-Noise Surface Coating for Sensitive Force-Gradient Detection of Electron Spin Resonance in Thin Films. ACS Nano 2023, 17, 1153–1165. [Google Scholar] [CrossRef]
- Pelekhov, D.V.; Selcu, C.; Banerjee, P.; Fong, K.C.; Hammel, P.C.; Bhaskaran, H.; Schwab, K. Light-free magnetic resonance force microscopy for studies of electron spin polarized systems. J. Magn. Magn. Mater. 2005, 286, 324–328. [Google Scholar] [CrossRef]
- Peddibhotla, P.; Xue, F.; Hauge, H.I.T.; Assali, S.; Bakkers, E.P.A.M.; Poggio, M. Harnessing nuclear spin polarization fluctuations in a semiconductor nanowire. Nat. Phys. 2013, 9, 631–635. [Google Scholar] [CrossRef]
- Schirhagl, R.; Chang, K.; Loretz, M.; Degen, C.L. Nitrogen-vacancy centers in diamond: Nanoscale sensors for physics and biology. Annu. Rev. Phys. Chem. 2014, 65, 83–105. [Google Scholar] [CrossRef]
- Babinec, T.M.; Hausmann, B.J.M.; Khan, M.; Zhang, Y.; Maze, J.R.; Hemmer, P.R.; Loncar, M. A diamond nanowire single-photon source. Nat. Nanotechnol. 2010, 5, 195–199. [Google Scholar] [CrossRef]
- Childress, L.; Hanson, R. Diamond NV centers for quantum computing and quantum networks. MRS Bull. 2013, 38, 134–138. [Google Scholar] [CrossRef]
- Haque, A.; Sumaiya, S. An Overview on the Formation and Processing of Nitrogen-Vacancy Photonic Centers in Diamond by Ion Implantation. J. Manuf. Mater. Process. 2017, 1, 6. [Google Scholar] [CrossRef]
- Stanwix, P.L.; Pham, L.M.; Maze, J.R.; Le Sage, D.; Yeung, T.K.; Cappellaro, P.; Hemmer, P.R.; Yacoby, A.; Lukin, M.D.; Walsworth, R.L. Coherence of nitrogen-vacancy electronic spin ensembles in diamond. Phys. Rev. B 2010, 82, 201201. [Google Scholar] [CrossRef]
- Segawa, T.F.; Igarashi, R. Nanoscale quantum sensing with Nitrogen-Vacancy centers in nanodiamonds—A magnetic resonance perspective. Prog. Nucl. Magn. Reson. Spectrosc. 2023, 134–135, 20–38. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, V.R.; Alemán, B.J.; Christle, D.J.; Cleland, A.N.; Awschalom, D.D. Electron spin resonance of nitrogen-vacancy centers in optically trapped nanodiamonds. Proc. Natl. Acad. Sci. USA 2012, 109, 13493–13497. [Google Scholar] [CrossRef] [PubMed]
- Zeeman, P. XXXII. On the influence of magnetism on the nature of the light emitted by a substance. Lond. Edimb. Dublin Philos. Magn. J. Sci. 1897, 43, 226–239. [Google Scholar] [CrossRef]
- Barry, J.F.; Schloss, J.M.; Bauch, E.; Turner, M.J.; Hart, C.A.; Pham, L.M.; Walsworth, R.L. Sensitivity optimization for NV-diamond magnetometry. Rev. Mod. Phys. 2020, 92, 015004. [Google Scholar] [CrossRef]
- Zhang, M.; Li, B.-Y.; Liu, J. Monitoring Dark-State Dynamics of a Single Nitrogen-Vacancy Center in Nanodiamond by Auto-Correlation Spectroscopy: Photonionization and Recharging. Nanomaterials 2021, 11, 979. [Google Scholar] [CrossRef]
- Chen, S.; Li, W.; Zheng, X.; Yu, P.; Wang, P.; Sun, Z.; Xu, Y.; Jiao, D.; Ye, X.; Cai, M.; et al. Immunomagnetic microscopy of tumor tissues using quantum sensors in diamond. Proc. Natl. Acad. Sci. USA 2022, 9, e2118876119. [Google Scholar] [CrossRef]
- Janitz, E.; Herb, K.; Völker, L.A.; Huxter, W.S.; Degen, C.L.; Abendroth, J.M. Diamond surface engineering for molecular sensing with nitrogen-vacancy centers. J. Mater. Chem. C 2022, 10, 13533–13569. [Google Scholar] [CrossRef]
- Katsumasa, N.; Tobari, K.; Ohtake, M.; Futamoto, M. Effect of Magnetic Film Thickness on the Spatial Resolution of Magnetic Force Microscope Tips. J. Phys. Conf. Ser. 2011, 303, 012014. [Google Scholar] [CrossRef]
- Rheem, Y.; Saito, H.; Ishio, S. Fabrication of FePt/FeCo/FePt exchange-spring trilayer with very thin FeCo Layer for high-resolution MFM tips. IEEE Trans. Magn. 2005, 41, 3793–3795. [Google Scholar] [CrossRef]
- Ishihara, S.; Ohtake, M.; Futamoto, M. Magnetic force microscopy tips coated with FePd, FePt, and CoPt alloy films. Thin Solid Film. 2013, 546, 205–210. [Google Scholar] [CrossRef]
- Venturi, F.; Gazzadi, G.C.; Tavabi, A.H.; Rota, A.; Dunin-Borkowski, R.E.; Frabboni, S. Magnetic characterization of cobalt nanowires and square nanorings fabricated by focused electron beam induced deposition. Beilstein J. Nanotechnol. 2018, 9, 1040–1049. [Google Scholar] [CrossRef] [PubMed]
- Marcuello, C.; Chambel, L.; Rodrigues, M.S.; Ferreira, L.P.; Cruz, M.M. Magnetotactic Bacteria: Magnetism Beyond Magnetosomes. IEEE Trans. NanoBiosci. 2018, 17, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Albrecht, M.; Janke, V.; Sievers, S.; Siegner, U.; Schüler, D.; Heyen, U. Scanning force microscopy study of biogenic nanoparticles for medical applications. J. Magn. Magn. Mater. 2005, 290–291, 269–271. [Google Scholar] [CrossRef]
- Elfick, A.; Rischitor, G.; Mouras, R.; Azfer, A.; Lungaro, L.; Uhlarz, M.; Herrmannsdörfer, T.; Lococq, J.; Gamal, W.; Bagnaninchi, P.; et al. Biosynthesis of magnetic nanoparticles by human mesenchymal stem cells following transfection with the magnetotactic bacterial gene mms6. Sci. Rep. 2017, 7, 39755. [Google Scholar] [CrossRef] [PubMed]
- Dufour, S.C.; Laurich, J.R.; Batstone, R.T.; McCuaig, B.; Elliott, A.; Poduska, K.M. Magnetosome-containing bacteria living as symbionts of bivalves. ISME J. 2014, 8, 2453–2462. [Google Scholar] [CrossRef]
- Sangiao, S.; Magén, C.; Mofakhami, D.; de Loubens, G.; de Teresa, J.M. Magnetic properties of optimized cobalt nanospheres grown by focused electron beam induced deposition (FEBID) on cantilever tips. Beilstein J. Nanotechnol. 2017, 8, 2106–2115. [Google Scholar] [CrossRef]
- Sievers, S.; Braun, K.-F.; Eberbeck, D.; Gustafsson, S.; Olsson, E.; Schumacher, H.W.; Siegner, U. Quantitative measurement of the magnetic moment of individual magnetic nanoparticles by magnetic force microscopy. Small 2012, 8, 2675–2679. [Google Scholar] [CrossRef]
- Li, X.; Lu, W.; Song, Y.; Wang, Y.; Chen, A.; Yan, B.; Yoshimura, S.; Saito, H. Quantitatively probing the magnetic behavior of individual nanoparticles by an AC field-modulated magnetic force microscopy. Sci. Rep. 2016, 6, 22467. [Google Scholar] [CrossRef]
- Li, X.; Zhu, X.; Pan, D.; Xue, Y.; Jia, Q.; Liu, F.; Li, Z. Magnetic domains characterization of crystalline Fe3O4 under DC and AC magnetic field. Microscopy 2019, 68, 310–315. [Google Scholar] [CrossRef]
- Fuhrmann, M.; Musyanovych, A.; Thoelen, R.; von Bomhard, S.; Möbius, H. Magnetic Imaging of Encapsulated Superparamagnetic Nanoparticles by Data Fusion of Magnetic Force Microscopy and Atomic Force Microscopy Signals for Correction of Topographic Crosstalk. Nanomaterials 2020, 10, 2486. [Google Scholar] [CrossRef] [PubMed]
- Alavarse, A.C.; Silva, J.B.; Ulrich, H.; Petri, D.F.S. Poly(vinyl alcohol)/sodium alginate/magnetite composites: Magnetic force microscopy for tracking magnetic domains. Soft Matter 2023, 19, 2612–2622. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.J.; Shah, S.V.; Wei, P.; Oberdick, S.D.; Karn, N.M.; McTigue, D.M.; Agarwal, G. Effects of fixatives on histomagnetic evaluation of iron in rodent spleen. J. Magn. Magn. Mater. 2021, 521, 167531. [Google Scholar] [CrossRef] [PubMed]
- Walsh, K.J.; Shiflett, O.; Shah, S.; Renner, T.; Soulas, N.; Scharre, D.; McTigue, D.; Agarwal, G. Artifacts in magnetic force microscopy of histological sections. J. Magn. Magn. Mater. 2022, 564, 170116. [Google Scholar] [CrossRef]
- Tsuji, S.; Yoshinari, Y.; Kawai, E.; Nakajima, K.; Park, H.S.; Shindo, D. Magnetic resonance force microscopy combined with surface topography. J. Magn. Reson. 2007, 188, 380–386. [Google Scholar] [CrossRef]
- Tsuji, S.; Masumizu, T.; Yoshinari, Y. Magnetic resonance imaging of isolated single liposome by magnetic resonance force microscopy. J. Magn. Reson. 2004, 167, 211–220. [Google Scholar] [CrossRef]
- Hemmer, P.; Gomes, C. Single proteins under a diamond spotlight. Science 2015, 347, 1072–1073. [Google Scholar] [CrossRef]
- Nocera, T.M.; Zeng, Y.; Agarwal, G. Distinguishing ferritin from apoferritin using magnetic force microscopy. Nanotechnology 2014, 25, 461001. [Google Scholar] [CrossRef]
- Blissett, A.R.; Ollander, B.; Penn, B.; McTigue, D.M.; Agarwal, G. Magnetic mapping of iron in rodent spleen. Nanomedicine 2017, 13, 977–986. [Google Scholar] [CrossRef]
- Blisset, A.R.; Deng, B.; Walsh, K.J.; Ollander, B.; Sifford, J.; Sauerberck, A.D.; McComb, D.W.; McTigue, D.M.; Agarwal, G. Sub-cellular In-situ Characterization of Ferritin(iron) in a Rodent Model of Spinal Cord Injury. Sci. Rep. 2018, 8, 3567. [Google Scholar] [CrossRef]
- Chuang, C.; Matsunaga, M.; Wang, T.-H.; Roy, P.; Ravindranath, R.; Ananthula, M.; Aoki, N. Investigation of plant leaf-derived graphene quantum dot clusters via magnetic force microscopy. Nanotechnology 2021, 32, 245704. [Google Scholar] [CrossRef] [PubMed]
- Hui, L.S.; Munir, M.; Vuong, A.; Hilke, M.; Wong, V.; Fanchini, G.; Scharber, M.C.; Sariciftci, N.S.; Turak, A. Universal Transfer Printing of Micelle-Templated Nanoparticles Using Plasma-Functionalized Graphene. ACS Appl. Mater. Interfaces 2020, 12, 46530–46538. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Mironov, V.L.; Gribkov, B.A.; Fraerman, A.A.; Gusev, S.A.; Vdovichev, S.N. Magnetic state control of ferromagnetic nanodots by magnetic force microscopy probe. J. Appl. Phys. 2006, 100, 104304. [Google Scholar] [CrossRef]
- Futamoto, M.; Hagami, T.; Ishihara, S.; Soneta, K.; Ohtake, M. Improvement of Magnetic Force Microscope Resolution and Application to High-Density Recording Media. IEEE Trans. Magn. 2013, 49, 2748–2754. [Google Scholar] [CrossRef]
- Lisunova, Y.; Heidler, J.; Levkivskyi, I.; Gaponenko, I.; Weber, A.; Caillier, C.; Heyderman, L.J.; Kläui, M.; Paruch, P. Optimal ferromagnetically-coated carbon nanotube tips for ultra-high resolution magnetic force microscopy. Nanotechnology 2013, 24, 105705. [Google Scholar] [CrossRef]
- Available online: https://www.nanosensors.com/supersharpsilicon-high-quality-factor-magnetic-force-microscopy-reflex-coating-afm-tip-SSS-QMFMR (accessed on 9 August 2023).
- Ishihara, S.; Ohtake, M.; Futamoto, M. Magnetic Force Microscope Tip with High Resolution and High Switching Field Prepared by Coating Si Tip with L11 Ordered CoPt-Alloy Film. J. Magn. Soc. Jpn. 2013, 37, 255–258. [Google Scholar] [CrossRef]
- Panchal, V.; Corte-León, H.; Gribkov, B.; Rodriguez, L.A.; Snoeck, E.; Manzin, A.; Simonetto, E.; Vock, S.; Neu, V.; Kazakova, O. Calibration of multi-layered probes with low/high magnetic moments. Sci. Rep. 2017, 7, 7224. [Google Scholar] [CrossRef]
- Precner, M.; Fedor, J.; Šoltýs, J.; Cambel, V. Dual-tip magnetic force microscopy with suppressed influence on magnetically soft samples. Nanotechnology 2015, 26, 055304. [Google Scholar] [CrossRef]
- Phillips, G.N.; Siekman, M.; Abelmann, L.; Lodder, J.C. High resolution magnetic force microscopy using focused ion beam modified tips. Appl. Phys. Lett. 2002, 81, 865–867. [Google Scholar] [CrossRef]
- Gao, L.; Yue, L.P.; Yokota, T.; Skomski, R.; Liou, S.H.; Takahoshi, H.; Saito, H.; Ishio, S. Focused ion beam milled CoPt magnetic force microscopy tips for high resolution domain images. IEEE Trans. Magn. 2004, 40, 2194–2196. [Google Scholar] [CrossRef]
- Koblischka, M.R.; Hartmann, U. Recent advances in magnetic force microscopy. Ultramicroscopy 2003, 97, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Campanella, H.; Jaafar, M.; Llobet, J.; Esteve, J.; Vázquez, M.; Asenjo, A.; del Real, R.P.; Plaza, J.A. Nanomagnets with high shape anisotropy and strong crystalline anisotropy: Perspectives on magnetic force microscopy. Nanotechnology 2011, 22, 505301. [Google Scholar] [CrossRef] [PubMed]
- Neu, V.; Vock, S.; Sturm, T.; Schultz, L. Epitaxial hard magnetic SmCo5 MFM tips—A new approach to advanced magnetic force microscopy imaging. Nanoscale 2018, 10, 16881–16886. [Google Scholar] [CrossRef] [PubMed]
- Rettner, C.T.; Anders, S.; Thomson, T.; Albrecht, M.; Ikeda, Y.; Best, M.E.; Terris, B.D. Magnetic characterization and recording properties of patterned Co/sub 70/Cr/sub 18/Pt/sub 12/ perpendicular media. IEEE Trans. Magn. 2002, 38, 1725–1730. [Google Scholar] [CrossRef]
- Höflich, K.; Hobler, G.; Allen, F.I.; Wirtz, T.; Rius, G.; Krasheninnikov, A.V.; Schmidt, M.; Utke, I.; Klingner, N.; Osenberg, M.; et al. Roadmap for focused ion beam technologies. arXiv 2023, arXiv:2305.19631. [Google Scholar] [CrossRef]
- Kuramochi, H.; Uzumaki, T.; Yasutake, M.; Tanaka, A.; Akinaga, H.; Yokoyama, H. A magnetic force microscope using CoFe-coated carbon nanotube probes. Nanotechnology 2005, 16, 24. [Google Scholar] [CrossRef]
- Cui, H.; Kalinin, S.v.; Yang, X.; Lowndes, D.H. Growth of Carbon Nnaofibers on Tipless Cantilevers for High Resolution Topography and Magnetic Force Imaging. Nano Lett. 2004, 4, 2151–2157. [Google Scholar] [CrossRef]
- Yang, G.; Tang, J.; Kato, S.; Zhang, Q.; Qin, L.C.; Woodson, M.; Liu, J.; Kim, J.W.; Lillehei, P.; Park, C.; et al. Magnetic nanowire based high resolution magnetic force microscope probes. Appl. Phys. Lett. 2005, 87, 123507. [Google Scholar] [CrossRef]
- Winkler, R.; Brugger-Hatzl, M.; Seewald, L.M.; Kuhness, D.; Barth, S.; Mairhofer, T.; Kothleitner, G.; Plank, H. Additive Manufacturing of Co3Fe Nano-Probes for Magnetic Force Microscopy. Nanomaterials 2023, 13, 1217. [Google Scholar] [CrossRef]
- Pablo-Navarro, J.; Sangiao, S.; Magén, C.; de Teresa, J.M. Magnetic Functionalized of Scanning Probes by Focused Electron Beam Induced Deposition Technology. Magnetochemistry 2021, 7, 140. [Google Scholar] [CrossRef]
- Choi, S.-J.; Kim, K.-H.; Cho, Y.-J.; Lee, H.-S.; Cho, S.-H.; Kwon, S.-J.; Moon, J.-H.; Lee, K.-J. Demonstration of ultra-high-resolution MFM images using Co90Fe10-coated CNT probes. J. Magn. Magn. Mater. 2010, 322, 332–336. [Google Scholar] [CrossRef]
- Arie, T.; Nishijima, H.; Akita, S.; Nakayama, Y. Carbon-nanotube probe equipped magnetic force microscope. J. Vac. Sci. Technol. B 2000, 18, 104–106. [Google Scholar] [CrossRef]
- Deng, Z.; Yenilmez, E.; Leu, J.; Hoffman, J.E.; Straver, E.W.J.; Dai, H.; Moler, K.A. Metal-coated carbon nanotube tips for magnetic force microscopy. Appl. Phys. Lett. 2004, 85, 6263–6265. [Google Scholar] [CrossRef]
- Wolny, F.; Weissker, U.; Mühl, T.; Leonhardt, A.; Menzel, S.; Winkler, A.; Büchner, B. Iron-filled carbon nanotubes as probes for magnetic force microscopy. J. Appl. Phys. 2008, 104, 064908. [Google Scholar] [CrossRef]
- Wolny, F.; Mühl, T.; Weissker, U.; Lipert, K.; Schumann, J.; Leonhardt, A.; Büchner, B. Iron filled carbon nanotubes as novel monopole-like sensors for quantitative magnetic force microscopy. Nanotechnology 2010, 21, 435501. [Google Scholar] [CrossRef]
- Tanaka, K.; Yoshimura, M.; Ueda, K. High-Resolution Magnetic Force Microscopy Using Carbon Nanotube Probes Fabricated Directly by Microwave Plasma-Enhanced Chemical Vapor Deposition. J. Nanomater. 2009, 2009, 147204. [Google Scholar] [CrossRef]
- Chen, I.-C.; Chen, L.-H.; Gapin, A.; Jin, S.; Yuan, L.; Liou, S.-H. Iron-platinum-coated carbon nanocone probes on tipless cantilevers for high resolution magnetic force imaging. Nanotechnology 2008, 19, 075501. [Google Scholar] [CrossRef]
- Utke, I.; Hoffmann, P.; Melngailis, J. Gas-assisted focused electron beam and ion beam processing and fabrication. J. Vac. Sci. Technol. B 2008, 26, 1197–1276. [Google Scholar] [CrossRef]
- De Teresa, J.M.; Fernández-Pacheco, A.; Córdoba, R.; Serrano-Ramón, L.; Sangiao, S.; Ibarra, M.R. Review of magnetic nanostructures grown by focused electron beam induced deposition (FEBID). J. Phys. D Appl. Phys. 2016, 49, 243003. [Google Scholar] [CrossRef]
- Pablo-Navarro, J.; Sangiao, S.; Magén, C.; de Teresa, J.M. Nanofabrication—Focused electron beam induced deposition. In Nanofabrication: Nanolithography Techniques and Their Applications; IOP Publishing: Bristol, UK, 2020; pp. 4-1–4-39. [Google Scholar] [CrossRef]
- Jaafar, M.; Pablo-Navarro, J.; Berganza, E.; Ares, P.; Magén, P.; Masseboeuf, A.; Gatel, C.; Snoeck, E.; Gómez-Herrero, J.; de Teresa, J.M.; et al. Customized MFM probes based on magnetic nanorods. Nanoscale 2020, 12, 10090–10097. [Google Scholar] [CrossRef]
- Winkler, A.; Mühl, T.; Menzel, S.; Kozhuharova-Koseva, R.; Hampel, S.; Leonhardt, A.; Büchner, B. Magnetic force microscopy sensors using iron-filled carbon nanotubes. J. Appl. Phys. 2006, 99, 104905. [Google Scholar] [CrossRef]
- Belova, L.M.; Hellwig, O.; Dobisz, E.; Dahlberg, E.D. Rapid preparation of electron beam induced deposition Co magnetic force microscopy tips with 10 nm spatial resolution. Rev. Sci. Instrum. 2012, 83, 093711. [Google Scholar] [CrossRef] [PubMed]
- Gavagnin, M.; Wanzenboeck, H.D.; Wachter, S.; Shawrav, M.M.; Persson, A.; Gunnarsson, K.; Svedlindh, P.; Stöger-Pollach, M.; Bertagnolli, E. Free-standing magnetic nanopillars for 3D nanomagnet logic. ACS Appl. Mater. Interfaces 2014, 6, 20254–20260. [Google Scholar] [CrossRef] [PubMed]
- Plank, H.; Winkler, R.; Schwalb, C.H.; Hütner, J.; Fowlkes, J.D.; Rack, P.D.; Utke, I.; Huth, M. Focused Electron Beam-Based 3D Nanoprinting for Scanning Probe Microscopy: A Review. Micromachines 2019, 11, 48. [Google Scholar] [CrossRef] [PubMed]
- Skidmore, G.D.; Dahlberg, E.D. Improved spatial resolution in magnetic force microscopy. Appl. Phys. Lett. 1997, 71, 3293–3295. [Google Scholar] [CrossRef]
- Rührig, M.; Porthun, S.; Lodder, J.C.; McVitie, S.; Heyderman, L.J.; Johnston, A.B.; Chapman, J.N. Electron beam fabrication and characterization of high-resolution magnetic force microscopy tips. J. Appl. Phys. 1996, 79, 2913–2919. [Google Scholar] [CrossRef]
- Barth, S.; Huth, M.; Jungwirth, F. Precursors for direct-write nanofabrication with electrons. J. Mater. Chem. C 2020, 8, 15884–15919. [Google Scholar] [CrossRef]
- Utke, I.; Cicoira, F.; Jaenchen, G.; Hoffmann, P.; Scandella, L.; Dwir, B.; Kapon, E.; Laub, D.; Buffat, P.; Xanthopoulos, N.; et al. Focused Electron Beam Induced Deposition of High Resolution Magnetic Scanning Probe Tips. MRS Online Proc. Lib. 2001, 706, 9241. [Google Scholar] [CrossRef]
- Magén, C.; Pablo-Navarro, J.; De Teresa, J.M. Focused-Electron-Beam Engineering of 3D Magnetic Nanowires. Nanomaterials 2021, 11, 402. [Google Scholar] [CrossRef]
- Pablo-Navarro, J.; Magén, C.; de Teresa, J.M. Purified and Crystalline Three-Dimensional Electron-Beam-Induced Deposits: The Successful Case of Cobalt for High-Performance Magnetic Nanowires. ACS Appl. Nano Mater. 2018, 1, 38–46. [Google Scholar] [CrossRef]
- Winkler, R.; Fowlkes, J.D.; Rack, P.D.; Plank, H. 3D nanoprinting via focused electron beams. J. Appl. Phys. 2019, 125, 210901. [Google Scholar] [CrossRef]
- Utke, I.; Hoffmann, P.; Berger, R.; Scandella, L. High-resolution magnetic Co supertips grown by a focused electron beam. Appl. Phys. Lett. 2002, 80, 4792–4794. [Google Scholar] [CrossRef]
- Escalante-Quiceno, A.; Novotný, O.; Neuman, J.; Magén, C.; De Teresa, J.M. Long-Term Performance of Magnetic Force Microscopy Tips Grown by Focused Electron Beam Induced Deposition. Sensors 2023, 23, 2879. [Google Scholar] [CrossRef]
- Peterka, D.; Enders, A.; Haas, G.; Kern, K. Combined Kerr microscope and magnetic force microscope for variable temperature ultrahigh vacuum investigations. Rev. Sci. Instrum. 2003, 74, 2744–2748. [Google Scholar] [CrossRef]
- Brocks, O.; Stasiak, A.; Biedinger, J.; Wortmann, M.; Blachowicz, T.; Kaschuba, R.; Ehrmann, A. MOKE and MFM on magnetically coated nanofiber mats: Transferring well-known methods to uncommon samples. Appl. Res. 2023, 2, e202200113. [Google Scholar] [CrossRef]
- Radlinger, T.; Winkler, R.; Knoll, P.; Zweck, J.; Plank, H.; Hofer, F.; Kothleitner, G. A study on the correlation between micro and magnetic domain structure of Cu52Ni34Fe14 spinodal alloys. J. Alloys Compd. 2022, 922, 166214. [Google Scholar] [CrossRef]
- Pang, Z.; Wu, F.; Shi, Y.; Hou, Y.; Li, Q.; Lu, Q. The Design and Test of an STM-MFM Microscope Combo for Use in a ϕ 52 mm 18/20 T Superconducting Magnet. IEEE Trans. Appl. Supercond. 2010, 20, 668–671. [Google Scholar] [CrossRef]
- Binnig, G.; Rohrer, H. Scanning tunneling microscopy. Surf. Sci. 1983, 126, 236–244. [Google Scholar] [CrossRef]
- Lv, S.-L.; Zeng, C.; Yu, Z.; Zheng, J.-F.; Wang, Y.-H.; Shao, Y.; Zhou, X.-S. Recent Advances in Single-Molecule Sensors Based on STM Break Junction Measurements. Biosensors 2022, 12, 565. [Google Scholar] [CrossRef]
- Rana, S.; Johnson, K.N.; Gurdumov, K.; Mazur, U.; Hipps, K.W. Scanning Tunneling Microscopy Reveals Surface Diffusion of Single Double-Decker Phthalocyanine Molecules at the Solution/Solid Interface. J. Phys. Chem. C 2022, 126, 4140–4149. [Google Scholar] [CrossRef]
- Schneiderbauer, M.; Wastl, D.; Giessibl, F.J. qPlus magnetic force microscopy in frequency-modulation mode with millihertz resolution. Beilstein J. Nanotechnol. 2012, 3, 174–178. [Google Scholar] [CrossRef]
- Giessibl, F.J. The qPlus sensor, a powerful core for the atomic force microscope. Rev. Sci. Instrum. 2019, 90, 011101. [Google Scholar] [CrossRef] [PubMed]
- Sadat, M.E.; Bud´ko, S.L.; Ewing, R.C.; Xu, H.; Pauletti, G.M.; Mast, D.B.; Shi, D. Effect of Dipole Interactions on Blocking Temperature and Relaxation Dynamics of Superparamagnetic Iron-Oxide (Fe3O4) Nanoparticle Systems. Materials 2023, 16, 496. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Chung, N.-K.; Allen, S.; Tendler, S.J.B.; Park, J.W. Ferritin-based new magnetic force microscopic probe detecting 10 nm sized magnetic nanoparticles. ACS Nano 2012, 6, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Winkler, R.; Schmidt, F.-P.; Haselmann, U.; Fowlkes, J.D.; Lewis, B.B.; Kothleitner, G.; Rack, P.D.; Plank, R. Direct-Write 3D Nanoprinting of Plasmonic Structures. ACS Appl. Mater. Interfaces 2017, 9, 8233–8240. [Google Scholar] [CrossRef] [PubMed]
- Winkler, R.; Lewis, B.B.; Fowlkes, J.D.; Rack, P.D.; Plank, H. High-Fidelity 3D-Nanoprinting via Focused Electron Beams: Growth Fundamentals. ACS Appl. Nano Mater. 2018, 1, 1014–1027. [Google Scholar] [CrossRef]
- Fernández-Pacheco, A.; Skoric, L.; De Teresa, J.M.; Pablo-Navarro, J.; Huth, M.; Dobrovolskiy, O.V. Writing 3D Nanomagnets Using Focused Electron Beams. Materials 2020, 13, 3774. [Google Scholar] [CrossRef]
- Fowlkes, J.D.; Winkler, R.; Rack, P.D.; Plank, H. 3D Nanoprinting Replication Enhancement Using a Simulation-Informed Analytical Model for Electron Beam Exposure Dose Compensation. ACS Omega 2023, 8, 3148–3175. [Google Scholar] [CrossRef]
- Nanda, G.; van Veldhoven, E.; Maas, D.; Sadeghian, H.; Alkemade, P.F.A. Helium ion beam induced growth of hammerhead AFM probes. J. Vac. Sci. Technol. B 2015, 33, 06F503. [Google Scholar] [CrossRef]
- Keller, L.; Al Mamoori, M.K.I.; Pieper, J.; Gspan, C.; Stockem, I.; Schröder, C.; Barth, S.; Winkler, R.; Plank, H.; Pohlit, M.; et al. Direct-write of free-form building blocks for artificial magnetic 3D lattices. Sci. Rep. 2018, 8, 6160. [Google Scholar] [CrossRef]
- Marcuello, C. Current and future perspective of atomic force microscopy to elicit the intrinsic properties of soft matter at the single molecule level. AIMS Bioeng. 2022, 9, 293–306. [Google Scholar] [CrossRef]
- Marcuello, C. Present and future opportunities in the use of atomic force microscopy to address the phyisco-chemical properties of aquatic ecosystems at the nanoscale level. Int. Aquat. Res. 2022, 14, 231–240. [Google Scholar] [CrossRef]
- Spoilă, A.; Ilie, C.-I.; Motelica, L.; Ficai, D.; Semenescu, A.; Oprea, O.-C.; Ficai, A. Smart Magnetic Drug Delivery Systems for the Treatment of Cancer. Nanomaterials 2023, 13, 876. [Google Scholar] [CrossRef]
- Yuan, M.; Harnett, M.C.; Yan, T.-H.; Georgas, E.; Qin, Y.-X.; Zhou, H.-C.; Wang, Y. Progress, Opportunities, and Challenges of Magneto-Plasmonic Nanoparticles under Remote Magnetic and Light Stimulation for Brain-Tissue and Cellular Regeneration. Nanomaterials 2022, 12, 2242. [Google Scholar] [CrossRef] [PubMed]
- Simonescu, C.M.; Culita, D.C.; Tatarus, A.; Mocanu, T.; Marinescu, G.; Mitran, R.A.; Atkinson, I.; Kuncser, A.; Stanica, N. Novel Magnetic Nanocomposites Based on Carboxyl-Functionalized SBA-15 Silica for Effective Dye Adsorption from Aqueous Solutions. Nanomaterials 2022, 12, 2247. [Google Scholar] [CrossRef]
- Marcuello, C.; Foulon, L.; Chabbert, B.; Molinari, M.; Aguié-Béghin, V. Langmuir-Blodgett Procedure to Precisely Control the Coverage of Functionalized AFM Cantilevers for SMFS Measurements: Application with Cellulose Nanocrystals. Langmuir 2018, 34, 9376–9386. [Google Scholar] [CrossRef]
- Gimeno, I.; Kersten, W.; Pallarés, M.C.; Hermosilla, P.; Martínez-Pérez, M.J.; Jenkins, M.D.; Angerer, A.; Sánchez-Azqueta, C.; Zueco, D.; Majer, J.; et al. Enhanced Molecular Spin-Photon Coupling at Superconducting Nanoconstrictions. ACS Nano 2020, 14, 8707–8715. [Google Scholar] [CrossRef]
- Demidov, V.E.; Urazhdin, S.; Zholud, A.; Sadovnikov, A.V.; Demokritov, S.O. Nanoconstriction-based spin-Hall nano-oscillator. Appl. Phys. Lett. 2014, 105, 172410. [Google Scholar] [CrossRef]
- Liu, G.; Petrosko, S.H.; Zheng, Z.; Mirkin, C.A. Evolution of Dip-Pen Nanolithography (DPN): From Molecular Patterning to Materials Discovery. Chem. Rev. 2020, 120, 6009–6047. [Google Scholar] [CrossRef]
- Martínez-Pérez, M.J.; Bellido, E.; de Miguel, R.; Sesé, J.; Lostao, A.; Gómez-Moreno, C.; Drung, D.; Schurig, T.; Ruiz-Molina, D.; Luis, F. Alternating current magnetic susceptibility of a molecular magnet submonolayer directly patterned onto a micro superconducting quantum interference device. Appl. Phys. Lett. 2011, 99, 032504. [Google Scholar] [CrossRef]
- Baljozović, M.; Liu, X.; Popova, O.; Girovsky, J.; Nowakowski, J.; Rossmann, H.; Nijs, T.; Moradi, M.; Mousavi, S.F.; Plumb, N.C.; et al. Self-Assembly and Magnetic Order of Bi-Molecular 2D Spin Lattices of M(II,III) Phthalocyanines on Au(111). Magnetochemistry 2021, 7, 119. [Google Scholar] [CrossRef]
- Zhang, Y.; Berman, G.P.; Kais, S. The radical pair mechanism and the avian chemical compass: Quantum coherence and entanglement. Int. J. Quantum Chem. 2015, 115, 1327–1341. [Google Scholar] [CrossRef]
- Harvey, S.M.; Wasielewski, M.R. Photogenerated Spin-Correlated Radical Paris: From Photosynthetic Energy Transduction to Quantum Information Science. J. Am. Chem. Soc. 2021, 143, 15508–15529. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.D.; Chowdhury, F.T.; Peasgood, I.; Dawkins, N.; Kattnig, D.R. Driven Radical Motion Enhances Cryptochrome Magnetoreception: Toward Live Quantum Sensing. J. Phys. Chem. Lett. 2022, 13, 10500–10506. [Google Scholar] [CrossRef]
- Freitag, N.H.; Reiche, C.F.; Neu, V.; Devi, P.; Burkhardt, U.; Felser, C.; Wolf, D.; Lubk, A.; Büchner, B.; Mühl, T. Simultaneous magnetic field and field gradient mapping of hexagonal MnNiGa by quantitative magnetic force microscopy. Commun. Phys. 2023, 6, 11. [Google Scholar] [CrossRef]
- Banuazizi, S.A.H.; Houshang, A.; Awad, A.A.; Mohammadi, J.; Åkerman, J.; Belova, L.M. Magnetic force microscopy of an operational spin nano-oscillator. Microsyst. Nanoeng. 2022, 8, 65. [Google Scholar] [CrossRef]
- Talapatra, A.; Gajera, U.; Prasad, P.S.; Chelvane, J.A.; Mohanty, J.R. Understanding the Magnetic Microstructure through Experiments and Machine Learning Algorithms. ACS Appl. Mater. Interfaces 2022, 14, 50318–50330. [Google Scholar] [CrossRef]
- Zheng, X.-X.; Sun, W.-F. Magnetic Force Probe Characterizations of Nanoscale Ferromagnetic Domains: Finite-Element Magnetostatic Simulations. Nanomaterials 2022, 12, 2212. [Google Scholar] [CrossRef]
- Vock, S.; Wolny, F.; Mühl, T.; Kaltofen, R.; Schultz, L.; Büchner, B.; Hassel, C.; Lindner, J.; Neu, V. Monopolelike probes for quantitative magnetic force microscopy: Calibration and application. Appl. Phys. Lett. 2010, 97, 252505. [Google Scholar] [CrossRef]
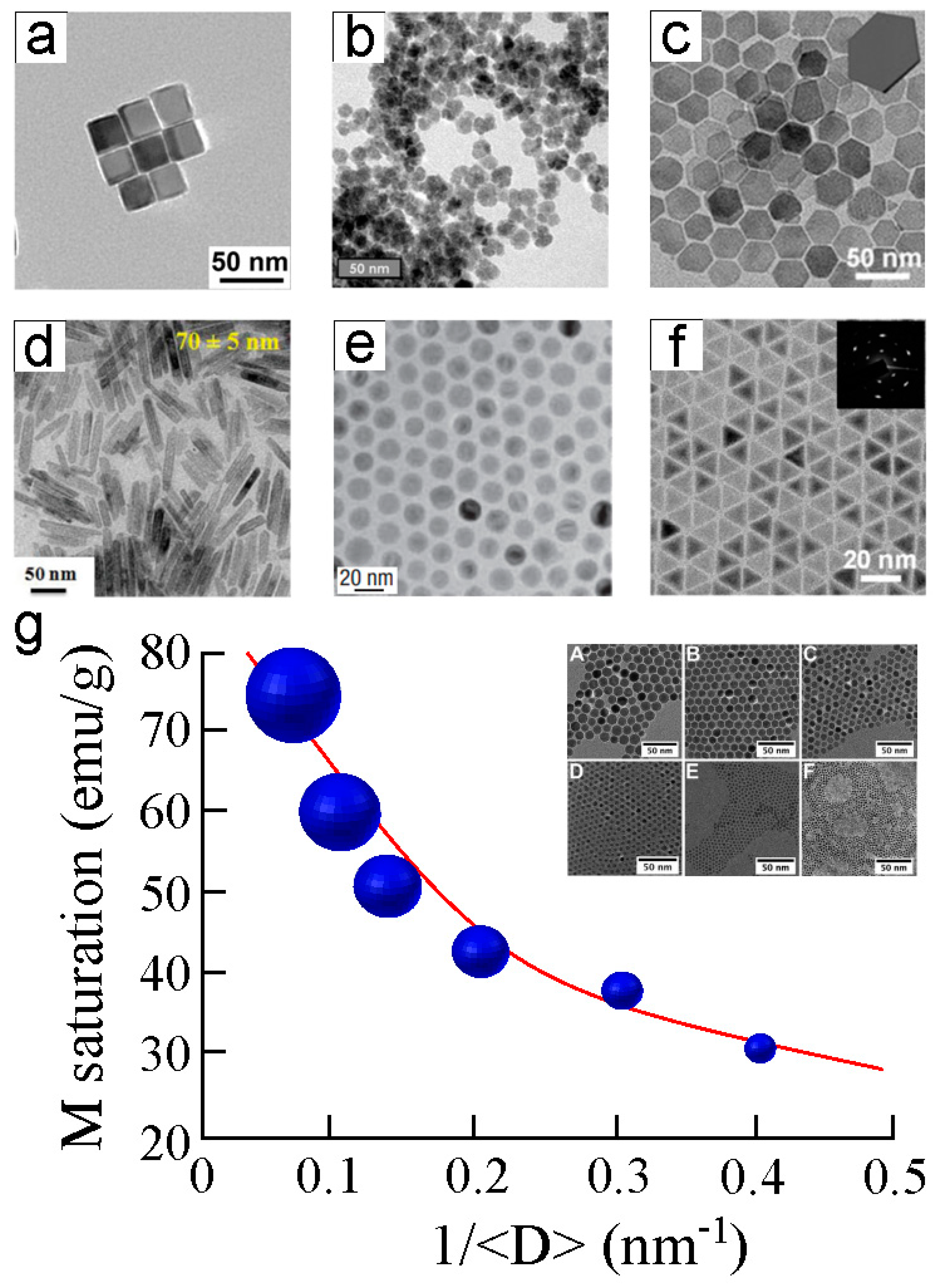

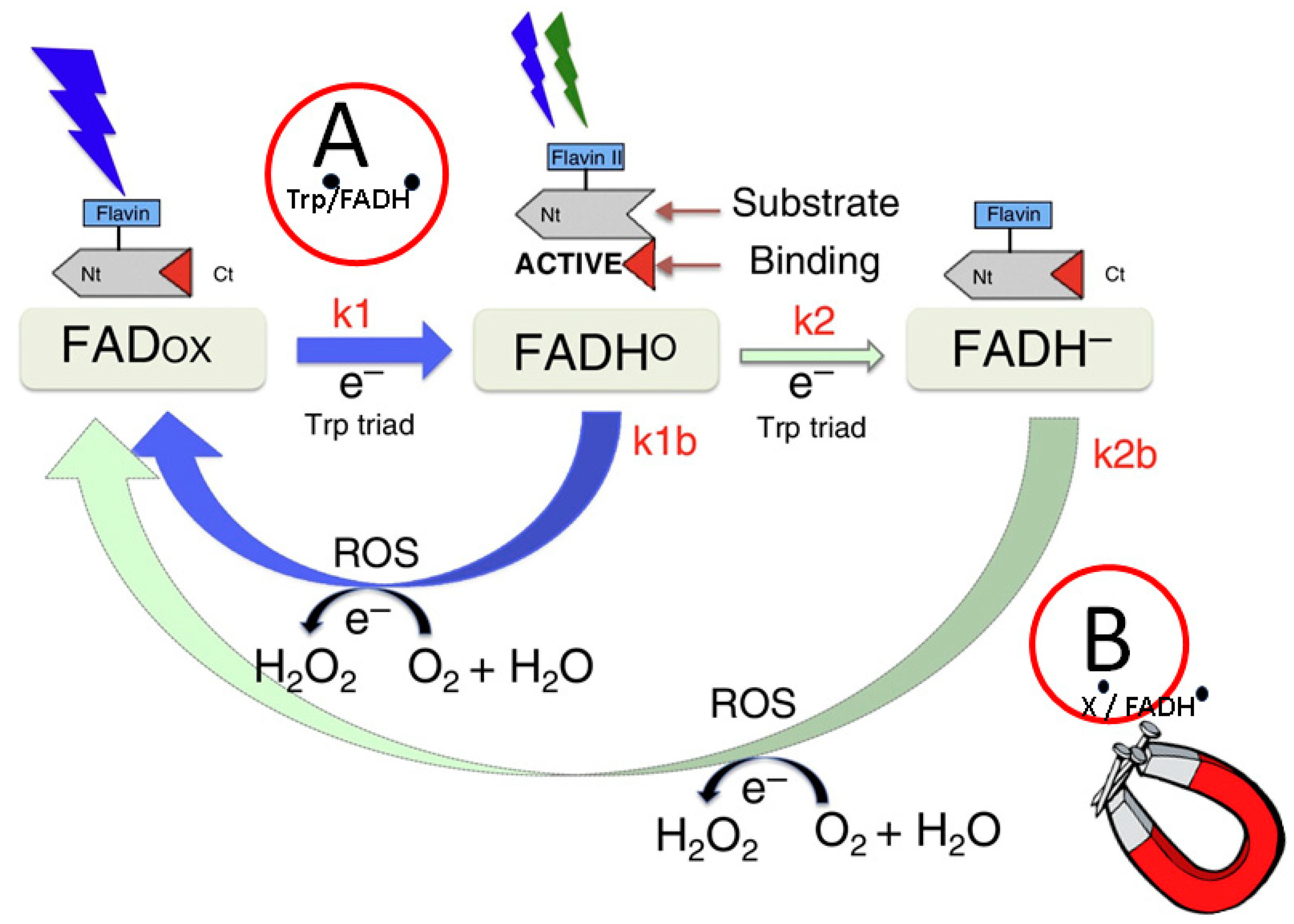
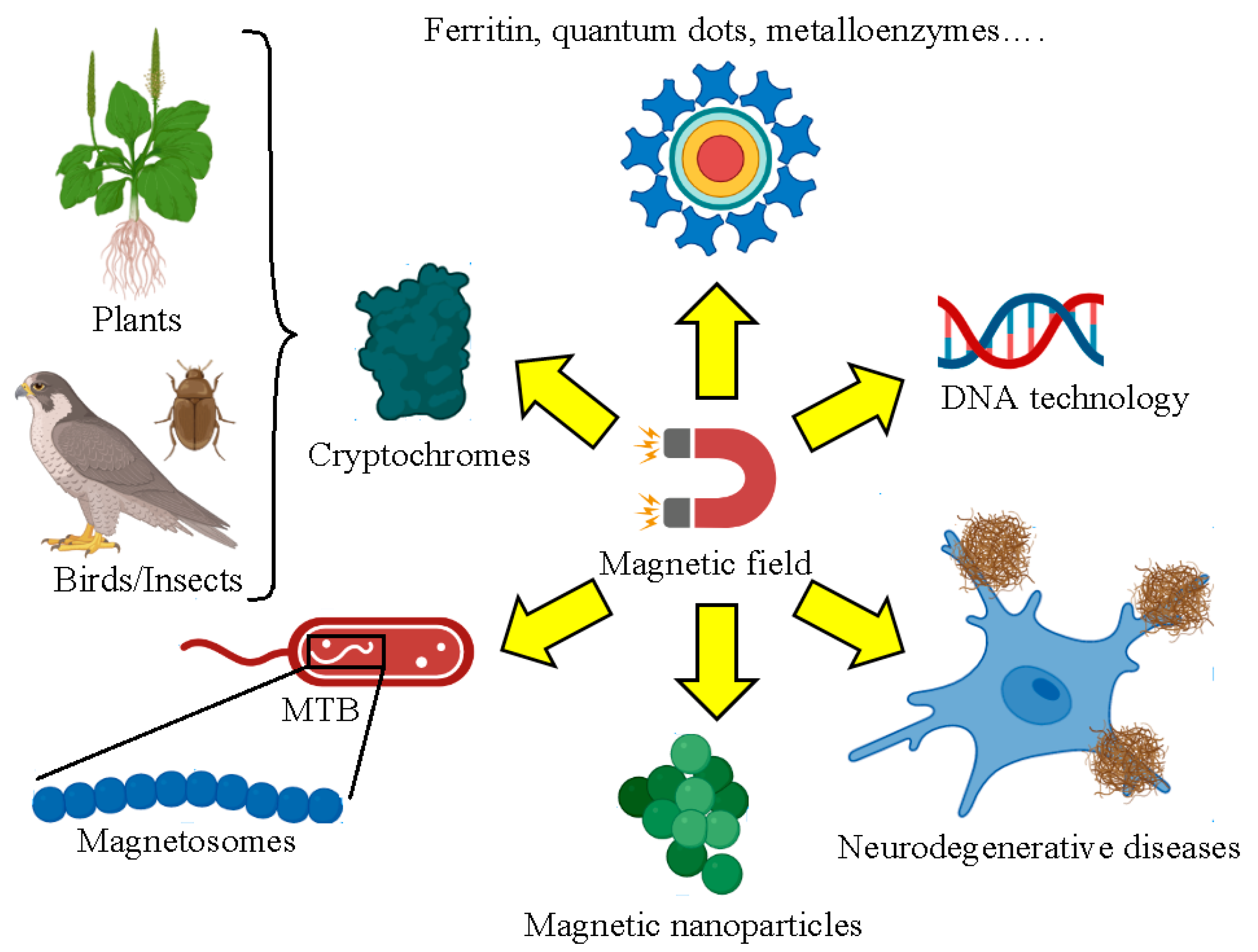
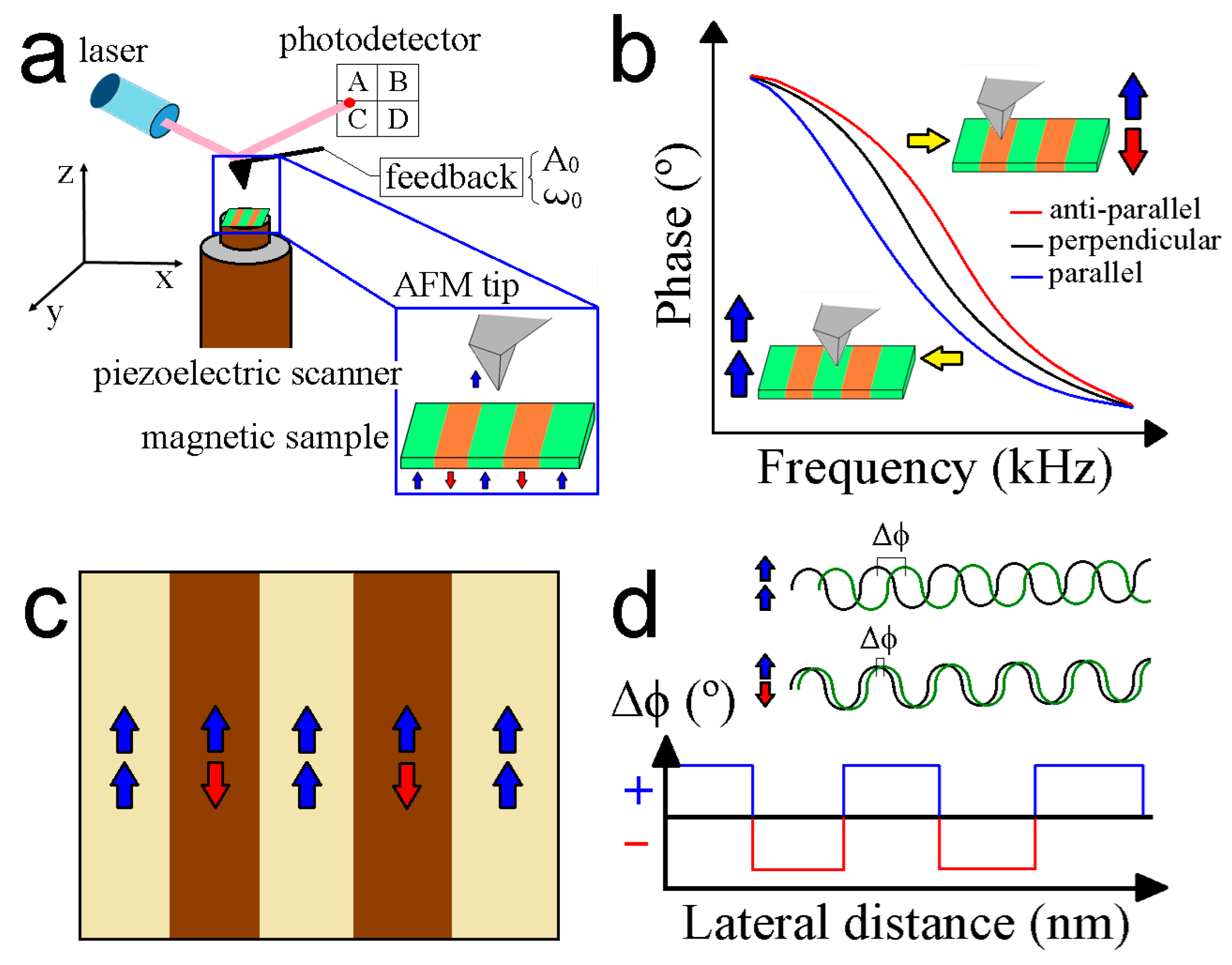
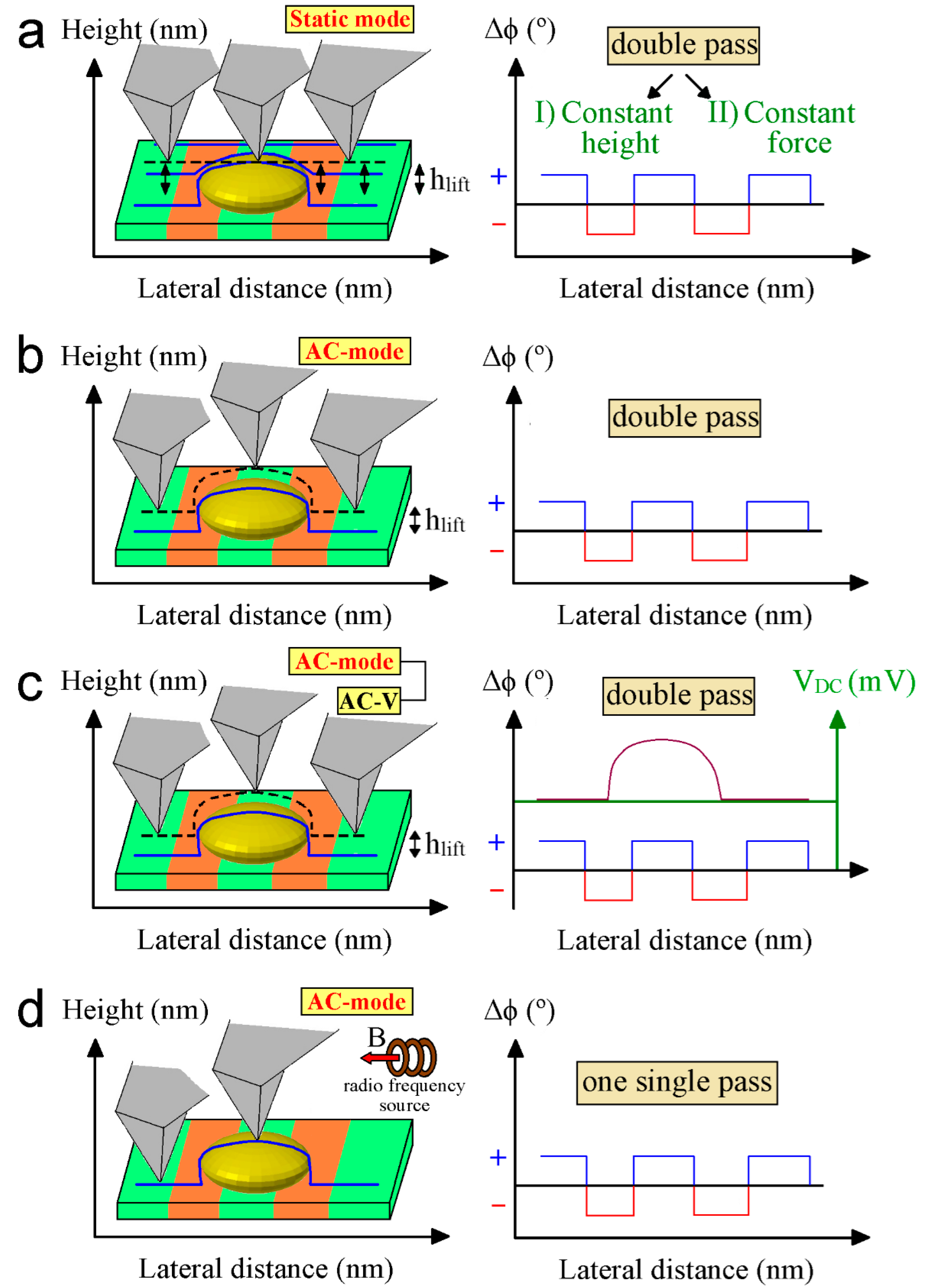
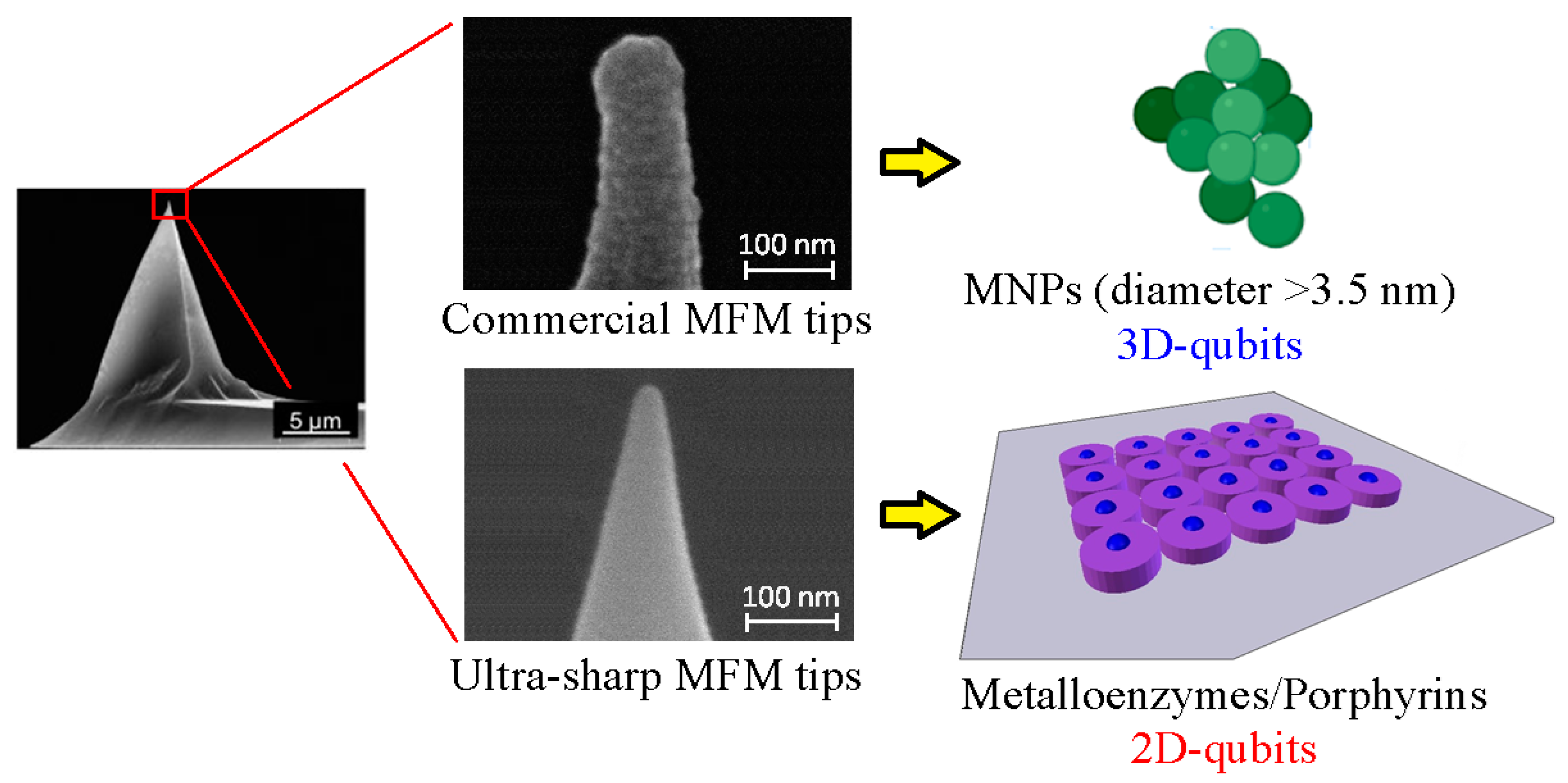
| Biological Sample | MFM Mode | Lift Height | Sample Height | Ref. |
|---|---|---|---|---|
| Magnetosomes from M. spirillum magnetotacticum | Lift mode | 60–300 nm | ~20 nm (glob.) | [278] |
| Magnetosomes from M. spirillum magnetotacticum | Lift mode | 60–300 nm | ~1.5 × 24 × 2000 nm (rod) | [278] |
| Magnetosomes from M. spirillum gryphiswaldense | Const. height | - | 21.0 ± 2.5 nm (glob.) | [279] |
| Magnetosomes transfected to mesenchymal cells | Lift mode | 20 nm | ~12 nm (glob.) | [280] |
| Magnetosomes in bivalve Thasyra cf. gouldi | Lift mode | 35–150 | 72.9 ± 28.9 (glob.) | [281] |
| Cobalt nanospheres | MRFM | - | ~500 nm (glob.) | [282] |
| Cobalt nanowires | Lift mode | 30 nm | ~25 × 85 × 2750 nm (rod) | [277] |
| Cobalt nanorings | Lift mode | 30 nm | 1 × 0.1 µm (L., W.) (sq.) | [277] |
| Magnetite (Fe3O4) nanoparticles | Lift mode | 50 nm | 18.7 ± 3.0 (glob.) | [283] |
| Magnetite (Fe3O4) nanoparticles | Lift mode | 10 nm | ~4.8 nm (glob.) | [284] |
| Magnetite (Fe3O4) nanoparticles | Lift mode | 10 nm | ~20 nm (glob.) | [285] |
| Iron oxide MNPs in polymer matrix | KPFM | 50 nm | ~8 to 12 nm | [286] |
| Gadolinium nanoparticles | Const. height | 150 nm | ~12 nm | [105] |
| Fe3O4 in hydrogels | Lift mode | 50 nm | 34.0 ± 1.0 nm (glob.) | [287] |
| Iron in rodent spleen | Lif mode | 30–100 nm | 3.8 ± 0.2 nm (glob.) | [288] |
| Iron deposits in brain histological sections | Lift mode | 30 nm | ~5 to 8 nm (glob.) | [289] |
| Diphenylpicrylhydrazil (DPPH) radicals | MRFM | - | ~5 to 8 µm (glob.) | [290] |
| Liposome membrane labeled with DPPH | MRFM | - | ~5 to 15 µm (glob.) | [291] |
| Mitotic arrest deficient 2 (MAD2) protein | MRFM | - | ~4.5 nm (glob.) | [292] |
| Ferritin | Lift Mode | 10–50 nm | ~12 nm (glob.) | [293] |
| Ferritin | Lift mode | 30–50 nm | ~12 nm (glob.) | [294] |
| Ferritin iron core | Lift Mode | 30 nm | ~5 nm (glob.) | [295] |
| Graphene quantum dots | Lift mode | 50 nm | ~6.5 nm (glob.) | [296] |
| Graphene functionalized with Fe2O3 particles | Const. height | - | 5–10 nm | [297] |
| Co-FeCo dots | Cont. height | - | ~25 nm (glob.) | [298] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Winkler, R.; Ciria, M.; Ahmad, M.; Plank, H.; Marcuello, C. A Review of the Current State of Magnetic Force Microscopy to Unravel the Magnetic Properties of Nanomaterials Applied in Biological Systems and Future Directions for Quantum Technologies. Nanomaterials 2023, 13, 2585. https://doi.org/10.3390/nano13182585
Winkler R, Ciria M, Ahmad M, Plank H, Marcuello C. A Review of the Current State of Magnetic Force Microscopy to Unravel the Magnetic Properties of Nanomaterials Applied in Biological Systems and Future Directions for Quantum Technologies. Nanomaterials. 2023; 13(18):2585. https://doi.org/10.3390/nano13182585
Chicago/Turabian StyleWinkler, Robert, Miguel Ciria, Margaret Ahmad, Harald Plank, and Carlos Marcuello. 2023. "A Review of the Current State of Magnetic Force Microscopy to Unravel the Magnetic Properties of Nanomaterials Applied in Biological Systems and Future Directions for Quantum Technologies" Nanomaterials 13, no. 18: 2585. https://doi.org/10.3390/nano13182585
APA StyleWinkler, R., Ciria, M., Ahmad, M., Plank, H., & Marcuello, C. (2023). A Review of the Current State of Magnetic Force Microscopy to Unravel the Magnetic Properties of Nanomaterials Applied in Biological Systems and Future Directions for Quantum Technologies. Nanomaterials, 13(18), 2585. https://doi.org/10.3390/nano13182585







