Scanning Tunneling Microscopy Study of Lipoic Acid, Mannose, and cRGD@AuNPs Conjugates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. ALA@AuNPs
2.3. MAN@AuNPs
2.4. cRGD@AuNPs
2.5. Characterization
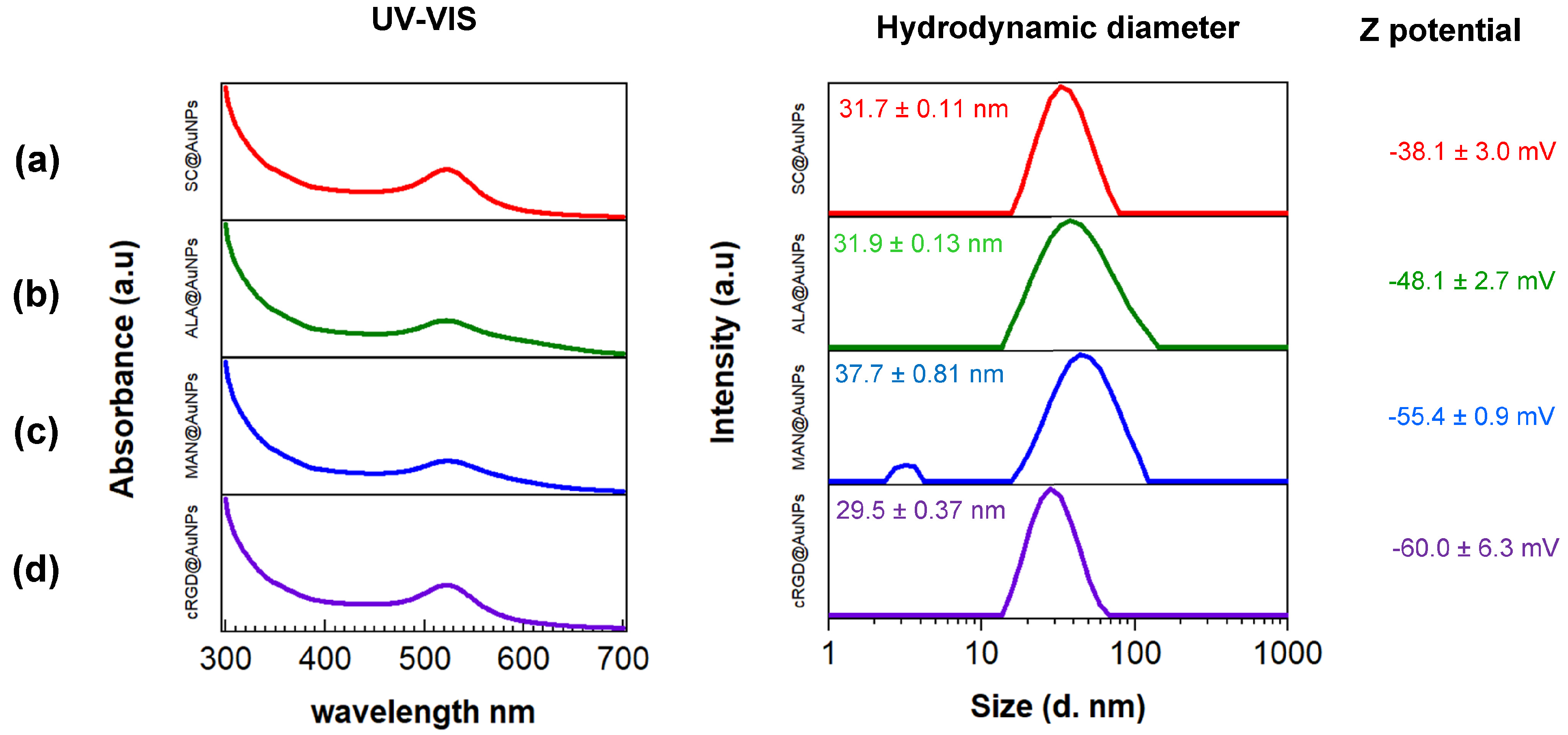
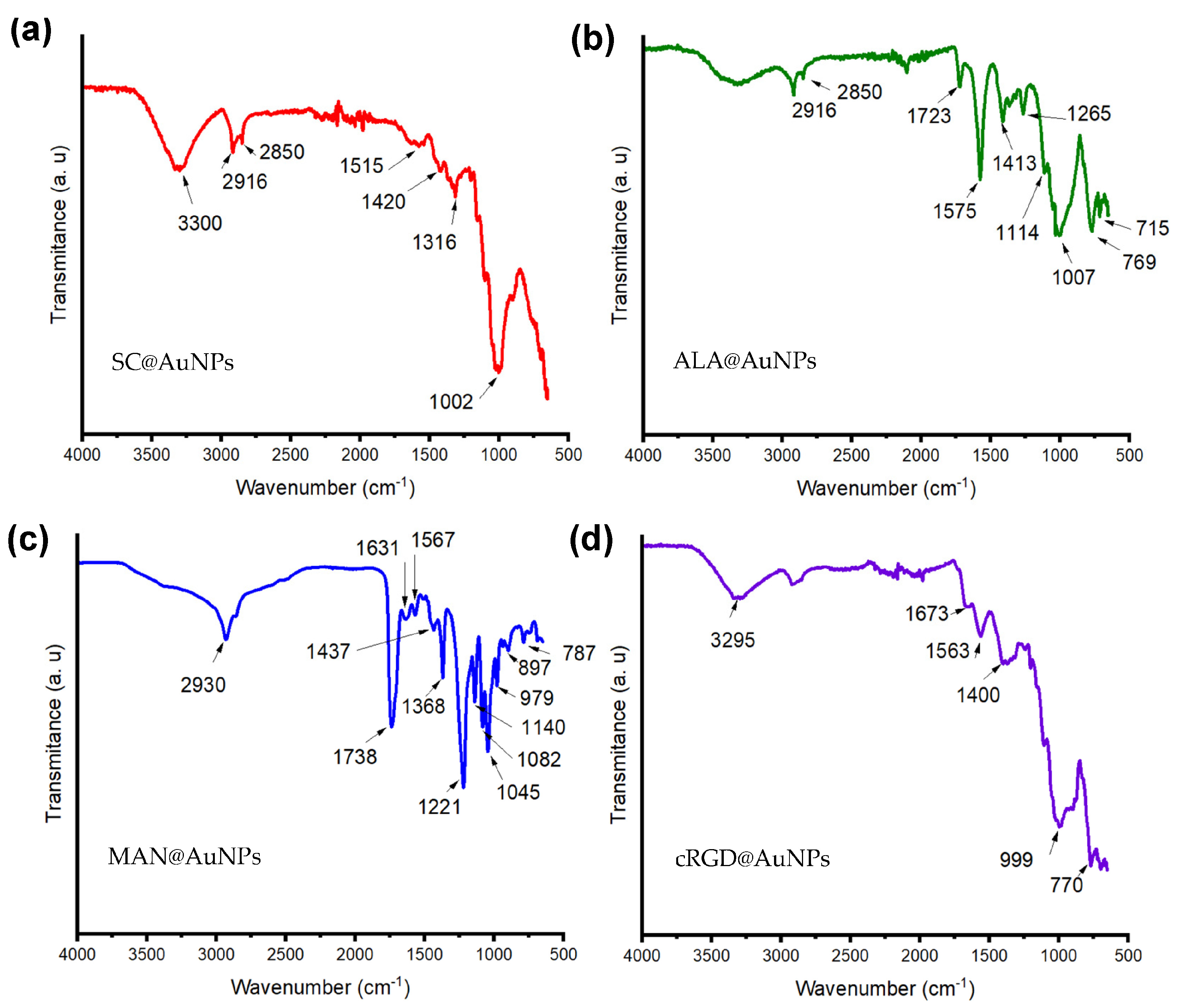
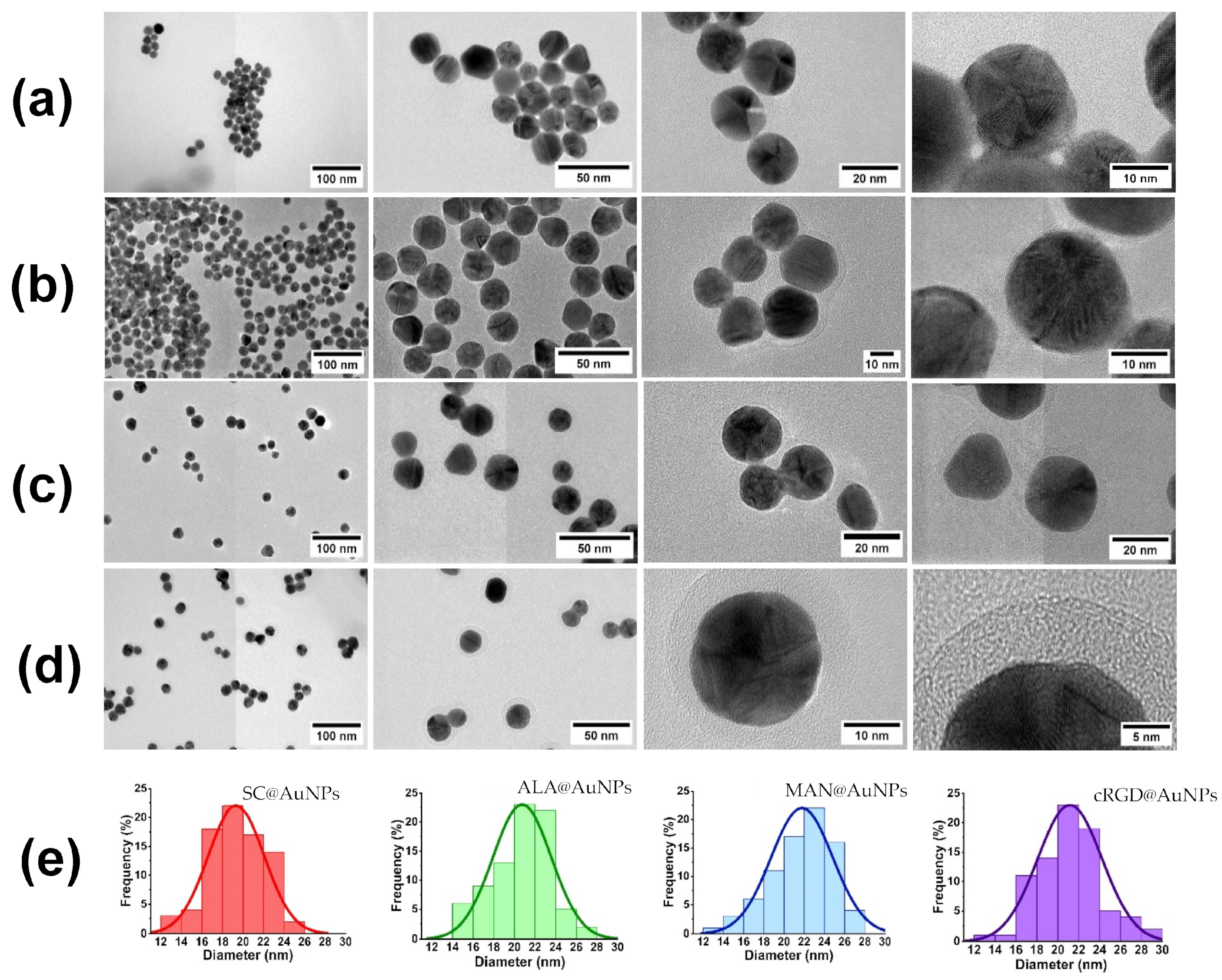
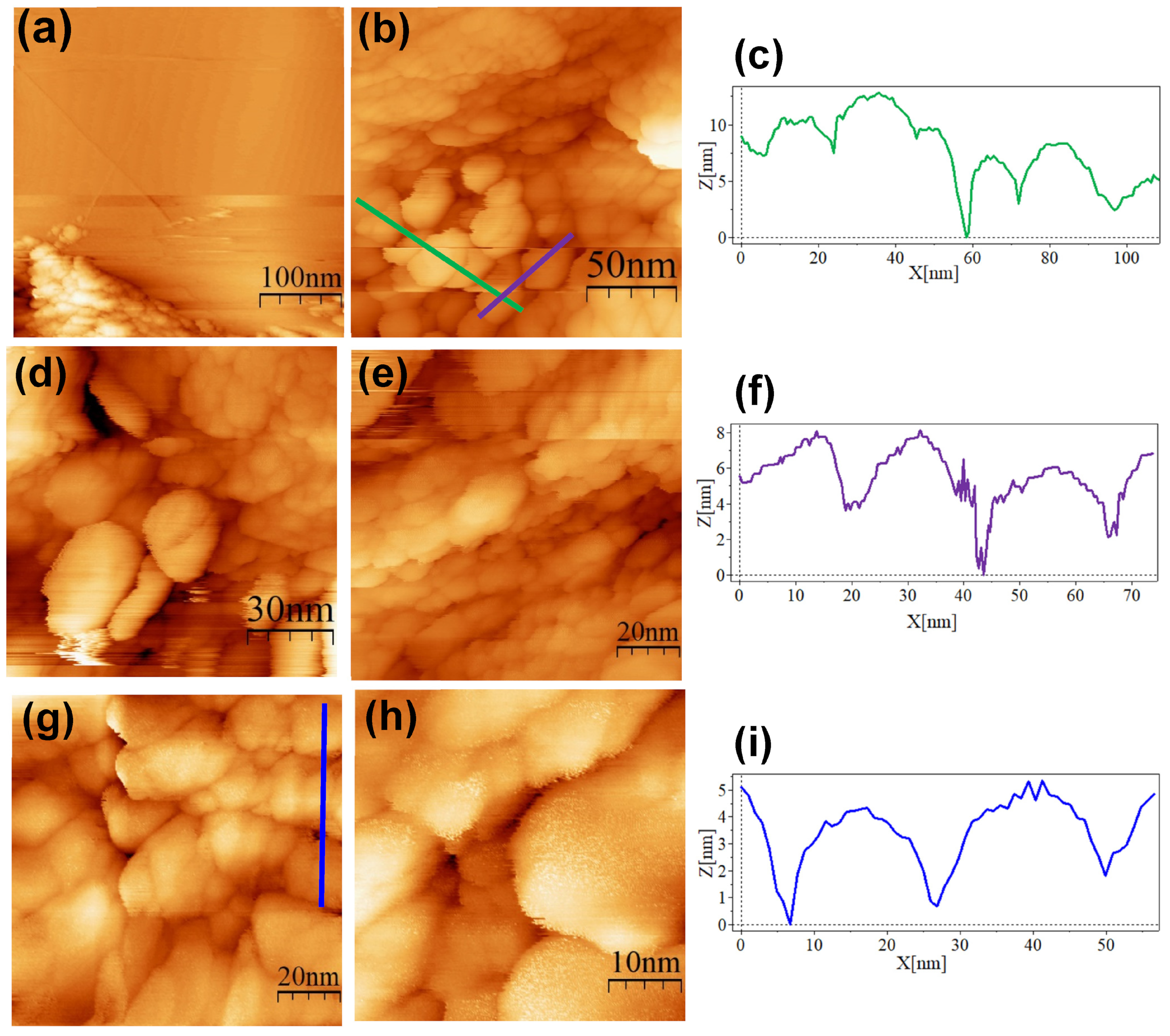
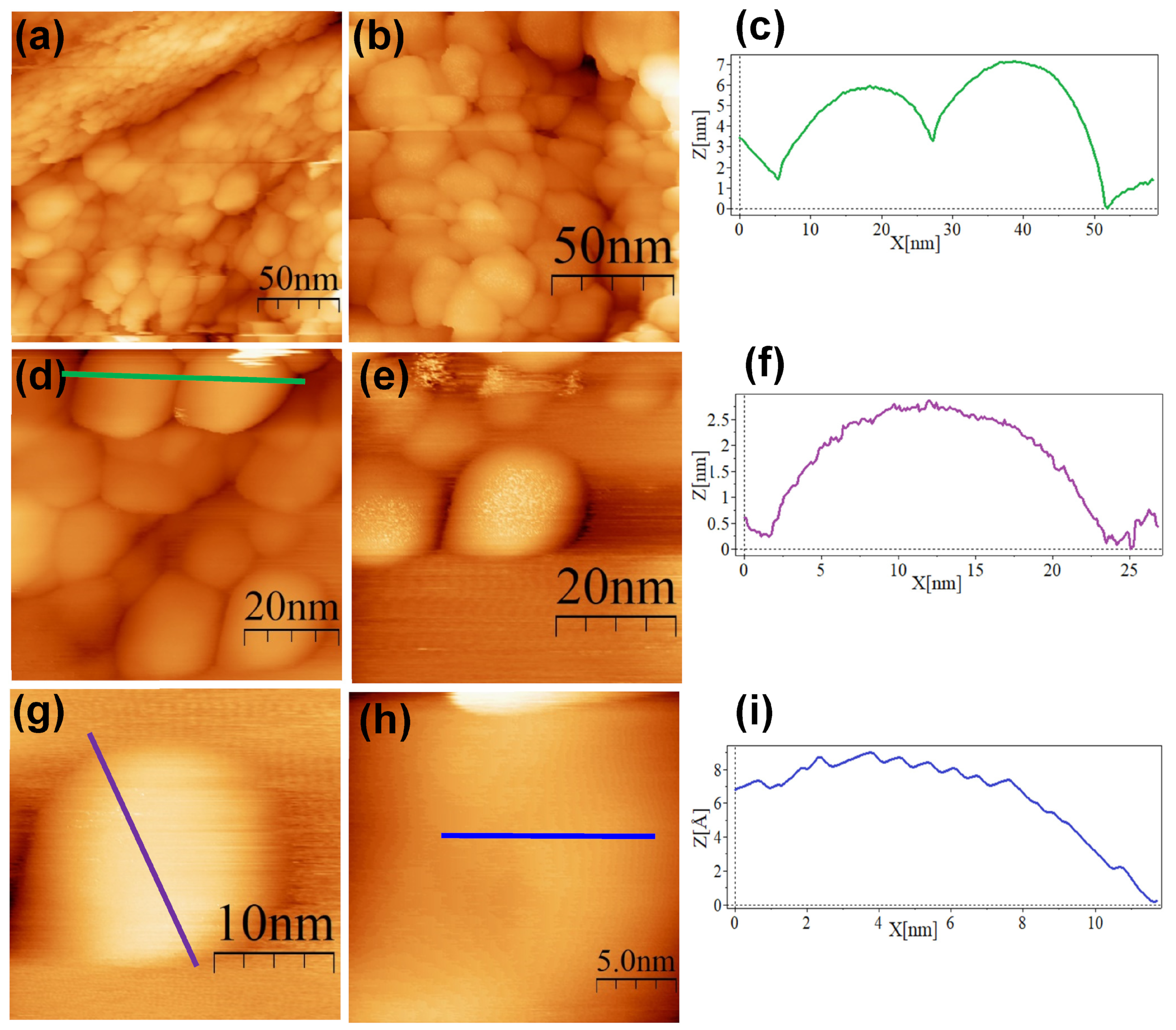
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Anik, M.I.; Mahmud, N.; Al Masud, A.; Hasan, M. Gold Nanoparticles (GNPs) in Biomedical and Clinical Applications: A Review. Nano Sel. 2022, 3, 792–828. [Google Scholar] [CrossRef]
- Amina, S.J.; Guo, B. A Review on the Synthesis and Functionalization of Gold Nanoparticles as a Drug Delivery Vehicle. Int. J. Nanomed. 2020, 15, 9823–9857. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Wang, W.; Wang, Z.; Zhao, M. Gold Nanoparticles Enhance Antibody Effect through Direct Cancer Cell Cytotoxicity by Differential Regulation of Phagocytosis. Nat. Commun. 2021, 12, 6371. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, H.; Zeng, D.; San, L.; Mi, X. DNA Nanotechnology Mediated Gold Nanoparticle Conjugates and Their Applications in Biomedicine. Chin. J. Chem. 2016, 34, 299–307. [Google Scholar] [CrossRef]
- Estudiante-Mariquez, O.J.; Rodríguez-Galván, A.; Ramírez-Hernández, D.; Contreras-Torres, F.F.; Medina, L.A. Technetium-Radiolabeled Mannose-Functionalized Gold Nanoparticles as Nanoprobes for Sentinel Lymph Node Detection. Molecules 2020, 25, 1982. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, P.; Han, G.; De, M.; Kim, C.K.; Rotello, V.M. Gold Nanoparticles in Delivery Applications. Adv. Drug Deliv. Rev. 2008, 60, 1307–1315. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Choi, S.-R.; Heo, J.H. Simultaneous Stabilization and Functionalization of Gold Nanoparticles via Biomolecule Conjugation: Progress and Perspectives. ACS Appl. Mater. Interfaces 2021, 13, 42311–42328. [Google Scholar] [CrossRef] [PubMed]
- Iijima, M.; Somiya, M.; Yoshimoto, N.; Niimi, T.; Kuroda, S. Nano-Visualization of Oriented-Immobilized IgGs on Immunosensors by High-Speed Atomic Force Microscopy. Sci. Rep. 2012, 2, 790. [Google Scholar] [CrossRef]
- Adak, A.K.; Lin, H.-J.; Lin, C.-C. Multivalent Glycosylated Nanoparticles for Studying Carbohydrate–Protein Interactions. Org. Biomol. Chem. 2014, 12, 5563–5573. [Google Scholar] [CrossRef]
- Liese, S.; Netz, R.R. Quantitative Prediction of Multivalent Ligand–Receptor Binding Affinities for Influenza, Cholera, and Anthrax Inhibition. ACS Nano 2018, 12, 4140–4147. [Google Scholar] [CrossRef]
- Tjandra, K.C.; Thordarson, P. Multivalency in Drug Delivery—When Is It Too Much of a Good Thing? Bioconjug. Chem. 2019, 30, 503–514. [Google Scholar] [CrossRef] [PubMed]
- Hinterwirth, H.; Kappel, S.; Waitz, T.; Prohaska, T.; Lindner, W.; Lämmerhofer, M. Quantifying Thiol Ligand Density of Self-Assembled Monolayers on Gold Nanoparticles by Inductively Coupled Plasma–Mass Spectrometry. ACS Nano 2013, 7, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Volkert, A.A.; Subramaniam, V.; Ivanov, M.R.; Goodman, A.M.; Haes, A.J. Salt-Mediated Self-Assembly of Thioctic Acid on Gold Nanoparticles. ACS Nano 2011, 5, 4570–4580. [Google Scholar] [CrossRef] [PubMed]
- Grönbeck, H.; Curioni, A.; Andreoni, W. Thiols and Disulfides on the Au(111) Surface: The Headgroup−Gold Interaction. J. Am. Chem. Soc. 2000, 122, 3839–3842. [Google Scholar] [CrossRef]
- Ong, Q.; Luo, Z.; Stellacci, F. Characterization of Ligand Shell for Mixed-Ligand Coated Gold Nanoparticles. Acc. Chem. Res. 2017, 50, 1911–1919. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Kaappa, S.; Malola, S.; Lu, H.; Guan, D.; Li, Y.; Wang, H.; Xie, Z.; Ma, Z.; Häkkinen, H.; et al. Real-Space Imaging with Pattern Recognition of a Ligand-Protected Ag 374 Nanocluster at Sub-Molecular Resolution. Nat. Commun. 2018, 9, 2948. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Galván, A.; Contreras-Torres, F.F. Scanning Tunneling Microscopy of Biological Structures: An Elusive Goal for Many Years. Nanomaterials 2022, 12, 3013. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Galván, A.; Heredia, A.; Amelines-Sarria, O.; Rivera, M.; Medina, L.A.; Basiuk, V.A. Non-Covalent Attachment of Silver Nanoclusters onto Single-Walled Carbon Nanotubes with Human Serum Albumin as Linking Molecule. Appl. Surf. Sci. 2015, 331, 271–277. [Google Scholar] [CrossRef]
- Rodríguez-Galván, A.; Amelines-Sarria, O.; Rivera, M.; del Pilar Carreón-Castro, M.; Basiuk, V.A. Adsorption and Self-Assembly of Anticancer Antibiotic Doxorubicin on Single-Walled Carbon Nanotubes. Nano 2015, 11, 1650038. [Google Scholar] [CrossRef]
- Rodríguez-Galván, A.; Heredia, A.; Plascencia-Villa, G.; Ramírez, O.T.; Palomares, L.A.; Basiuk, V.A. Scanning Tunneling Microscopy of Rotavirus VP6 Protein Self-Assembled into Nanotubes and Nanospheres. J. Scanning Probe Microsc. 2008, 3, 25–31. [Google Scholar] [CrossRef]
- Dzwonek, M.; Załubiniak, D.; Piątek, P.; Cichowicz, G.; Męczynska-Wielgosz, S.; Stępkowski, T.; Kruszewski, M.; Więckowska, A.; Bilewicz, R. Towards Potent but Less Toxic Nanopharmaceuticals—Lipoic Acid Bioconjugates of Ultrasmall Gold Nanoparticles with an Anticancer Drug and Addressing Unit. RSC Adv. 2018, 8, 14947–14957. [Google Scholar] [CrossRef] [PubMed]
- Abad, J.M.; Mertens, S.F.L.; Pita, M.; Fernández, V.M.; Schiffrin, D.J. Functionalization of Thioctic Acid-Capped Gold Nanoparticles for Specific Immobilization of Histidine-Tagged Proteins. J. Am. Chem. Soc. 2005, 127, 5689–5694. [Google Scholar] [CrossRef] [PubMed]
- Gajbhiye, K.R.; Gajbhiye, V.; Siddiqui, I.A.; Gajbhiye, J.M. cRGD Functionalised Nanocarriers for Targeted Delivery of Bioactives. J. Drug Target. 2019, 27, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A Software for Scanning Probe Microscopy and a Tool for Nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.; Yin, J.-Y.; Nie, S.-P.; Xie, M.-Y. Applications of Infrared Spectroscopy in Polysaccharide Structural Analysis: Progress, Challenge and Perspective. Food Chem. X 2021, 12, 100168. [Google Scholar] [CrossRef] [PubMed]
- Krimm, S.; Bandekar, J. Vibrational Spectroscopy and Conformation of Peptides, Polypeptides, and Proteins. In Advances in Protein Chemistry; Anfinsen, C.B., Edsall, J.T., Richards, F.M., Eds.; Academic Press: Cambridge, MA, USA, 1986; Volume 38, pp. 181–364. [Google Scholar]
- Pensa, E.; Albrecht, T. Controlling the Dynamic Instability of Capped Metal Nanoparticles on Metallic Surfaces. J. Phys. Chem. Lett. 2018, 9, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, W.-M.; Bolan, N.S.; Tsang, D.C.W.; Li, Y.; Qin, M.; Hou, D. Environmental Fate, Toxicity and Risk Management Strategies of Nanoplastics in the Environment: Current Status and Future Perspectives. J. Hazard. Mater. 2021, 401, 123415. [Google Scholar] [CrossRef]
- Xu, M.; Soliman, M.G.; Sun, X.; Pelaz, B.; Feliu, N.; Parak, W.J.; Liu, S. How Entanglement of Different Physicochemical Properties Complicates the Prediction of in Vitro and in Vivo Interactions of Gold Nanoparticles. ACS Nano 2018, 12, 10104–10113. [Google Scholar] [CrossRef]
- Egorova, E.A.; van Rijt, M.M.J.; Sommerdijk, N.; Gooris, G.S.; Bouwstra, J.A.; Boyle, A.L.; Kros, A. One Peptide for Them All: Gold Nanoparticles of Different Sizes Are Stabilized by a Common Peptide Amphiphile. ACS Nano 2020, 14, 5874–5886. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Galván, A.; Reyes, M.; Ávila-Cruz, M.; Rivera, M.; Basiuk, V.A. Scanning Tunneling Microscopy Study of Lipoic Acid, Mannose, and cRGD@AuNPs Conjugates. Nanomaterials 2023, 13, 2596. https://doi.org/10.3390/nano13182596
Rodríguez-Galván A, Reyes M, Ávila-Cruz M, Rivera M, Basiuk VA. Scanning Tunneling Microscopy Study of Lipoic Acid, Mannose, and cRGD@AuNPs Conjugates. Nanomaterials. 2023; 13(18):2596. https://doi.org/10.3390/nano13182596
Chicago/Turabian StyleRodríguez-Galván, Andrés, Mitzi Reyes, Marisol Ávila-Cruz, Margarita Rivera, and Vladimir A. Basiuk. 2023. "Scanning Tunneling Microscopy Study of Lipoic Acid, Mannose, and cRGD@AuNPs Conjugates" Nanomaterials 13, no. 18: 2596. https://doi.org/10.3390/nano13182596
APA StyleRodríguez-Galván, A., Reyes, M., Ávila-Cruz, M., Rivera, M., & Basiuk, V. A. (2023). Scanning Tunneling Microscopy Study of Lipoic Acid, Mannose, and cRGD@AuNPs Conjugates. Nanomaterials, 13(18), 2596. https://doi.org/10.3390/nano13182596




