Abstract
In this study, Pt nanoparticles-loaded nitrogen-doped mesoporous carbon nanotube (Pt/NMCT) was successfully synthesized through a polydopamine-mediated “one-pot” co-deposition strategy. The Pt source was introduced during the co-deposition of polydopamine and silica on the surface of SiO2 nanowire (SiO2 NW), and Pt atoms were fixed in the skeleton by the chelation of polydopamine. Thus, in the subsequent calcination process in nitrogen atmosphere, the growth and agglomeration of Pt nanoparticles were effectively restricted, achieving the in situ loading of uniformly dispersed, ultra-small (~2 nm) Pt nanoparticles. The method is mild, convenient, and does not require additional surfactants, reducing agents, or stabilizers. At the same time, the use of the dual silica templates (SiO2 NW and the co-deposited silica nanoclusters) brought about a hierarchical pore structure with a high specific surface area (620 m2 g−1) and a large pore volume (1.46 cm3 g−1). The loading process of Pt was studied by analyzing the electron microscope and X-ray photoelectron spectroscopy of the intermediate products. The catalytic performance of Pt/NMCT was investigated in the reduction of 4-nitrophenol. The Pt/NMCT with a hierarchical pore structure had an apparent reaction rate constant of 0.184 min−1, significantly higher than that of the sample, without the removal of the silica templates to generate the hierarchical porosity (0.017 min−1). This work provides an outstanding contribution to the design of supported noble metal catalysts and also highlights the importance of the hierarchical pore structure for catalytic activity.
1. Introduction
Pt catalysts have important applications in many kinds of reactions, especially reduction reactions, because of their high activity, selectivity, and stability [1,2,3]. The catalytic efficiency of Pt nanoparticles supported on a substrate can be significantly enhanced due to their small size, homogeneous dispersion, expansive active surface area, and resistance to sintering both during preparation and catalytic reactions. Moreover, the interaction between Pt species and supportive materials is advantageous for regulating the electronic configuration of Pt [4,5,6,7]. In the past few decades, because carbon-based carriers usually have a high surface area, large pore volume, chemical stability, and thermal conductivity, carbon-supported metal catalysts have gained a lot of attention [8,9,10,11]. However, the microporous structure of commonly used carbon supports restricts the transport and diffusion of reaction molecules to some extent; at the same time, the anchorable site used to supply the active phase is insufficient, which leads to the uneven dispersion of metal nanoparticles on the carbon surface and may cause further aggregation and leaching. Therefore, it is urgent to develop porous carbon materials with open pores and more supporting sites to prepare metal/carbon catalysts to expand their application prospects.
Researchers have developed porous carbon materials doped with heteroatoms (B, N, S, etc.) [12,13,14,15]. The doping of heteroatoms in carbon materials is able to alter the surface properties, thereby broadening their potential application range. Among the various dopants, nitrogen has been widely studied. Previous work shows that nitrogen is conducive to improving the catalytic performance of heterogeneous catalytic reactions of metal/nitrogen-doped carbon catalysts [16,17,18]. The doped nitrogen can change the acid-base substance on the surface of the carrier, increase the electron interaction between the metal and the carrier, and accelerate the electron transfer in the catalytic system [19].
In order to realize the loading of platinum nanoparticles, the commonly used method is post-loading, that is, depositing metal precursors on pre-synthesized nitrogen-doped carbon materials through traditional methods such as impregnation and deposition precipitation [20]. This usually requires the addition of reducing agents like sodium borohydride and ethylene glycol [21,22]. And, for smaller and highly dispersed metal nanoparticles, it is often necessary to add certain stabilizers, such as citric acid and polyvinylpyrrolidone [23,24]. In recent years, a more convenient, “one-pot” co-deposition method has been developed for the in situ deposition of metal nanoparticles on the functionalized surface with amino groups, polydopamine (PDA), and others, avoiding the use of stabilizers [25,26,27,28].
Recently, we reported a method for the preparation of nitrogen-doped mesoporous carbon nanotubes (NMCTs) through the co-deposition of PDA and silica on a sacrificial silica nanowire (SiO2 NW) [29]. Herein, we extended this “dual-silica template” strategy to the synthesis of ultra-small Pt nanoparticles-loaded N-doped mesoporous carbon nanotubes (Pt/NMCT) by combing the PDA-mediated one-pot co-deposition approach. The chloroplatinic acid (H2PtCl6, platinum source) introduced in the “co-deposition” step was fixed by the chelation of the amino group of PDA. During the subsequent carbonization process, the overgrowth of the Pt nanoparticles in the PDA skeleton was restricted, thus achieving the in situ loading of the ultra-small and uniformly dispersed Pt nanoparticles without the need for additional protective agents or reducing agents. The prepared Pt/NMCT had a high specific surface area, a large porosity, a hierarchical pore structure, and abundant nitrogen-doping. The loaded Pt nanoparticles had a uniform particle size (ca. 2 nm), and the content of Pt was 9.5 wt%. The chemical structure and morphological changes in Pt in the process of material synthesis were studied. Also, the catalytic performance was evaluated through the reduction reaction of 4-nitrophenol (4-NP) to 4-aminophenol (4-AP).
2. Materials and Methods
2.1. Materials
Dopamine hydrochloride (DA·HCl, 98%), tetraethoxysilane (TEOS, A.R.), and chloroplatinic acid (H2PtCl6·6H2O, ≥37.50% Pt basis) were provided from Sigma-Aldrich. Hydrofluoric acid (40 wt%), ammonium hydroxide solution (28 wt%), ethanol (EtOH, A.R.), and sodium borohydride (A.R.) were purchased from Sinopharm Chemical Reagent. 4-nitrophenol (4-NP, A.R.) was purchased from Aladdin. The water used in the experiment was ultrapure water (18.2 MΩ cm−1).
2.2. Synthesis of Pt/NMCT
The monodispersed SiO2 NW was prepared according to the literature [30]. A total of 50 mg of SiO2 NW was fully dispersed in a mixture of 30 mL of EtOH, 4 mL of water, and 2.5 mL of concentrated ammonia. A total of 0.9 mL of a 100 mg mL−1 DA aqueous solution was added while stirring. Forty-five minutes later, 50 μL of 0.1 g mL−1 H2PtCl6 solution and 100 μL of TEOS were added, and the solution was then stirred for 4 h. After centrifugation and washing three times, the sediment was dried at 70 °C in an oven. The obtained powder (SiO2 NW@Pt/PDA-SiO2) was then calcined at 300 °C and 800 °C for 2 h under nitrogen atmosphere. The calcined samples (SiO2 NW@Pt/C-SiO2) were dispersed into a 5.0 M HF solution to remove SiO2. The obtained product was named Pt/NMCT. For comparison, Pt nanoparticles-loaded mesoporous carbon sphere (Pt/NMCS) was also synthesized by the same experimental method as Pt/NMCT, except that no SiO2 NW template was added.
2.3. Catalytic Reduction Reaction of 4-NP
A total of 3 mL of 0.1 mM 4-NP aqueous solution (light yellow color) was taken, and 200 μL of newly prepared 0.1 M NaBH4 solution was added, and the solution turned a bright yellow color. After that, 20 μL of catalyst dispersion (48 μg mL−1 Pt) was quickly added, and the change in the absorption peak at 400 nm was monitored at one-minute intervals using a UV-Vis spectrophotometer.
2.4. Characterization
The morphology of the samples was observed using a scanning electron microscope (SEM, Ultra 55, Carl Zeiss, Jena, Germany) at 5 kV, a Hitachi HT7800 transmission electron microscope (TEM, Tokyo, Japan) operating at 100 kV, and a FEI Tecnai G2 F20 S-Twin TEM (Hillsboro, OR, USA) operating at 200 kV. The element distribution and content were obtained by an energy-dispersive X-ray spectrometer (EDS, FEI, Hillsboro, OR, USA) and an inductively coupled plasma-optical emission spectrometer (ICP-OES, PerkinElmer Optima 8000, Waltham, MA, USA). The phase information was evaluated by a Bruker AXS D2 PHASER diffractometer (Karlsruhe, Germany) with a tube voltage of 5 kV and a current of 30 mA (Cu-K, λ = 1.54056 Å). N2 sorption analysis was performed on a Quadrasorb apparatus (Quantachrome Instruments, Boynton Beach, FL, USA). The specific surface area was calculated by the Brunauer–Emmett–Teller (BET) method. Pore volumes were determined at p/p0 = 0.989. Pore size distributions were acquired using the quenched solid density functional theory (DFT) model with the desorption branch. UV-vis spectra were obtained with a Shimadzu UV-2600 spectrophotometer (Kyoto, Japan). A Kratos Axis Ultra DLD spectrometer (Manchester, UK) was engaged to obtain the X-ray photoelectron spectroscopy (XPS) spectra. The binding energies in the XPS spectra were adjusted for specimen charging with reference to C 1s at 284.6 eV.
3. Results and Discussion
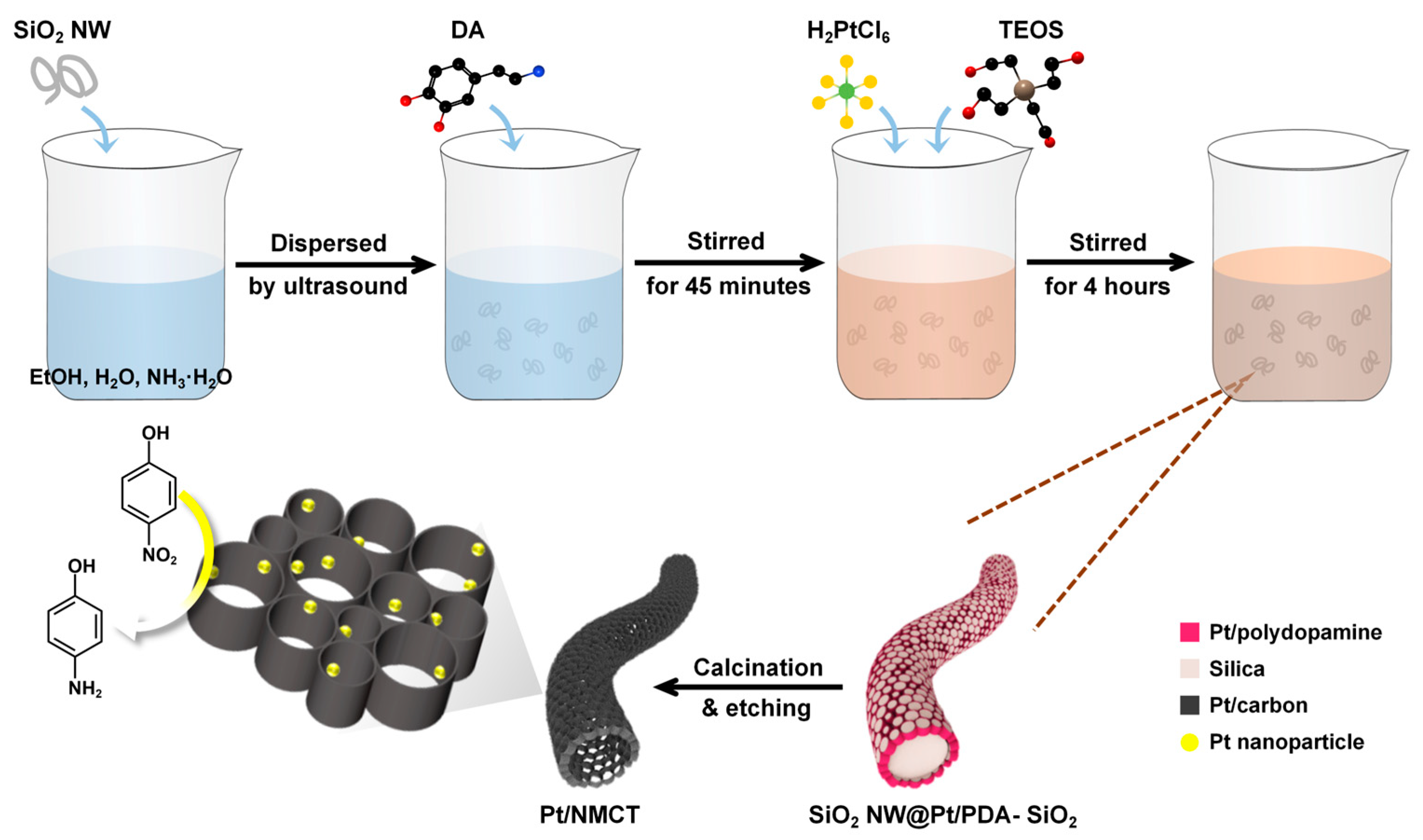
As shown in Figure 1, Pt/NMCT is prepared by a polydopamine-mediated “one-pot” co-deposition method, followed by carbonization and removal of the silica template. To start with, the DA in the reaction solution was self-polymerized and sequestered the Pt species from the solution. The SiO2 NW used in synthesis is shown in Figure 2a. The formed PDA oligomers and SiO2 nanoparticles (from the hydrolysis of added TEOS) were co-deposited on the surface of SiO2 NW, producing a core-shell structured SiO2 NW with a co-deposited SiO2 and Pt-loaded PDA shell. During the following carbonization procedure, the PDA-loaded Pt was simultaneously reduced by utilizing the reducibility of PDA. After removal of the co-deposited SiO2 nanoparticles and the SiO2 NW, hierarchically porous Pt/NMCT was obtained.
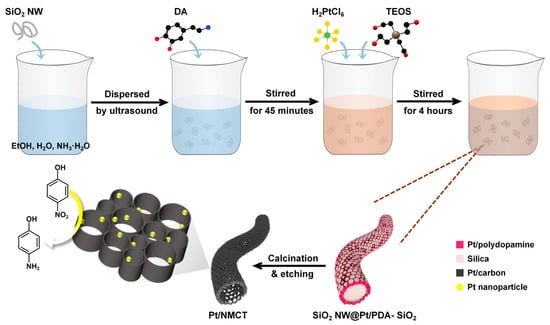
Figure 1.
Schematic illustration for the synthesis of the Pt nanoparticles loaded-N-doped mesoporous carbon nanotube (Pt/NMCT).
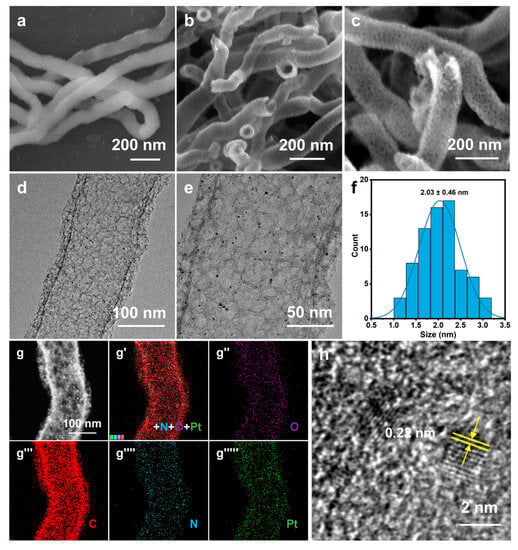
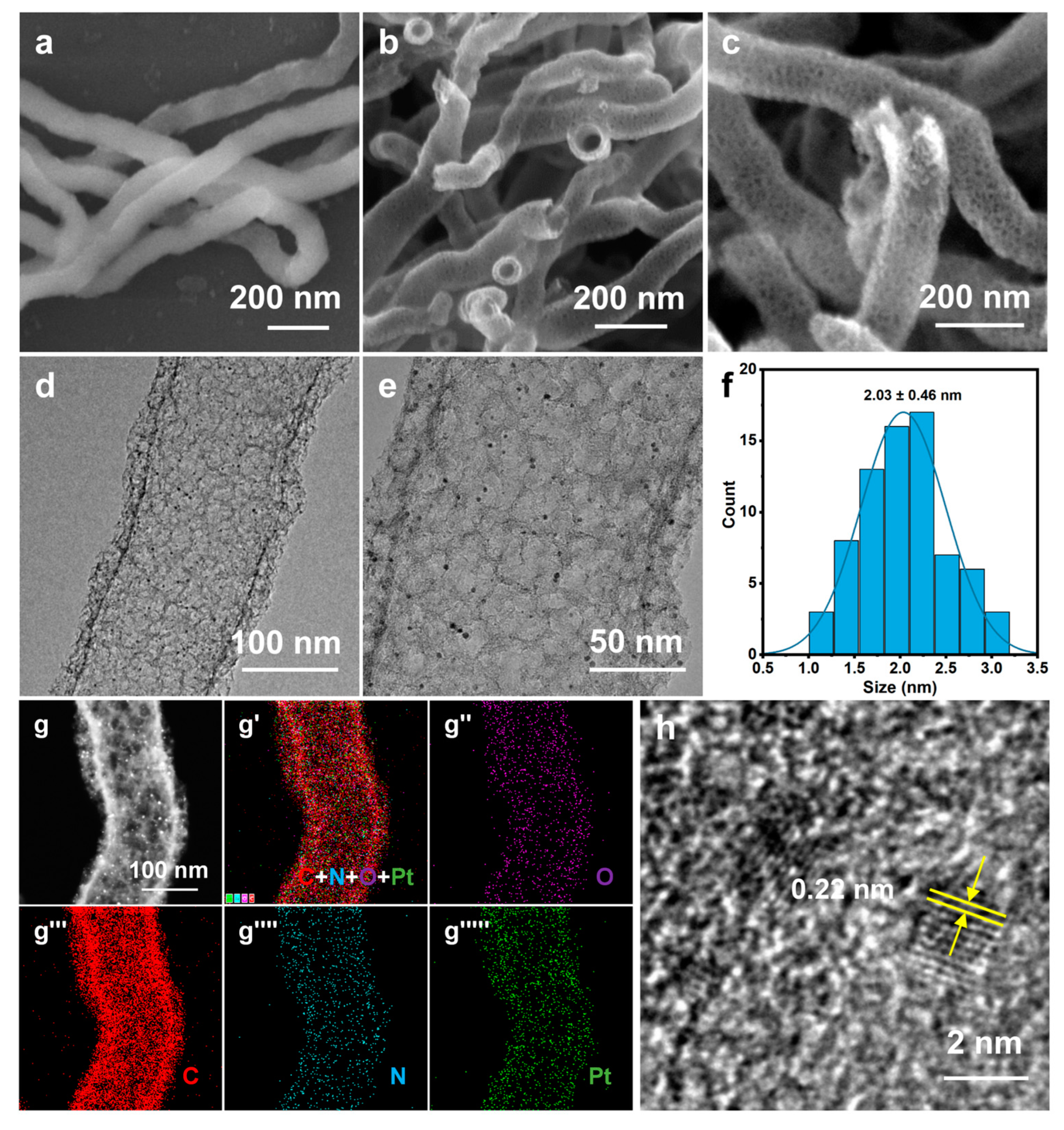
Figure 2.
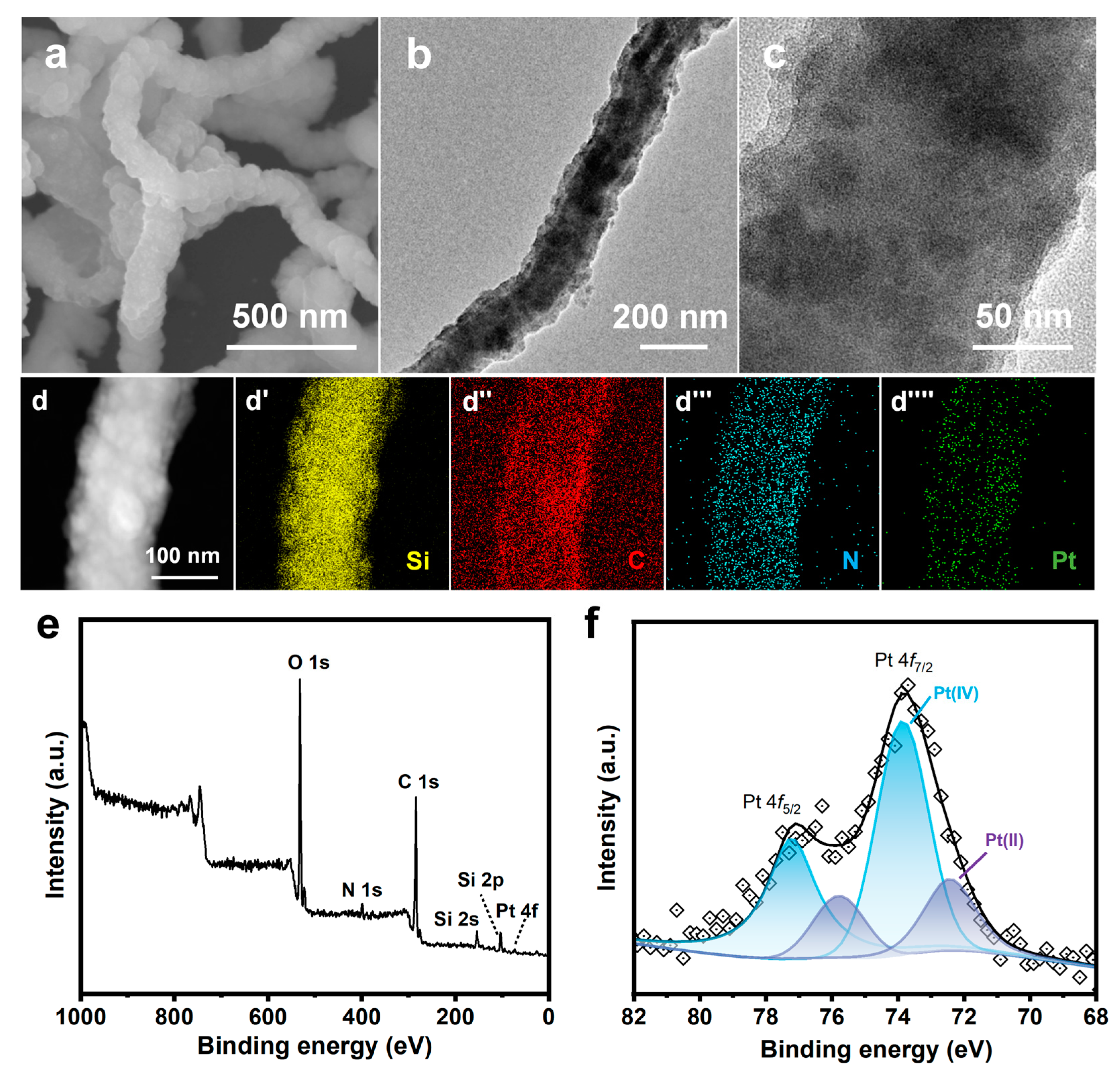
(a) SEM image of SiO2 NW; (b,c) SEM images, (d,e) TEM images, (f) Pt particle size distribution, (g) HAADF-STEM image, (g′–g′′′′′) EDS elemental mappings, and (h) high-resolution TEM image of Pt/NMCT.
The electron microscopy (SEM/TEM) was used to characterize the morphology of Pt/NMCT. According to the SEM images (Figure 2b,c), Pt/NMCT has a hollow tubular structure and a mesoporous tube wall. The TEM image (Figure 2d) indicates that the Pt nanoparticles loaded in the NMCT are evenly distributed and uniform in particle size. The statistics of the particle size from the TEM image (Figure 2e) show that the particle size is about 2 nm (Figure 2f). The HAADF-STEM image (Figure 2g) further shows that Pt nanoparticles have a small size, a uniform size distribution, and good dispersion throughout the carrier NMCT. The EDS elemental mappings (Figure 2g′–g′′′′′) demonstrate that the N and Pt elements are uniformly distributed in Pt/NMCT. The content of Pt was measured to be 9.5 wt% by ICP-OES. From Figure 2h, the lattice fringe of Pt nanoparticles can be seen in the HRTEM image with a crystal plane spacing of 0.22 nm, which is consistent with the (111) crystal plane of Pt [31].
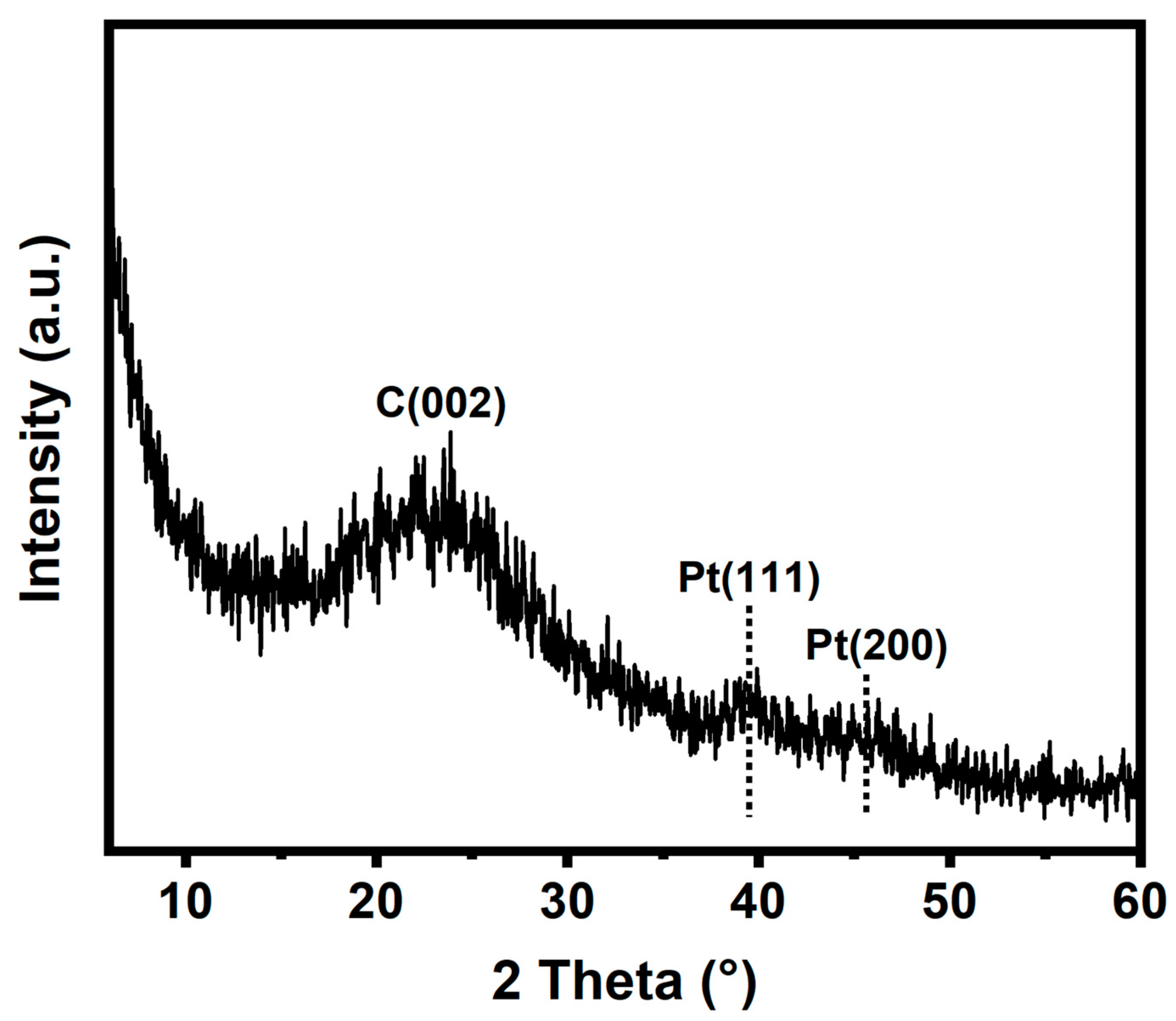
The crystal structure of Pt/NMCT was characterized by XRD. In the XRD pattern of Pt/NMCT (Figure 3), the material exhibits diffraction peaks at around 39.8° and 46.2°, corresponding to the (111) and (200) crystalline planes of Pt, respectively [32]. The weakening and broadening of the XRD diffraction peak also confirm the small particle size of Pt nanoparticles [20]. The wide diffraction peak near 23° is due to partially graphitized carbon [33].
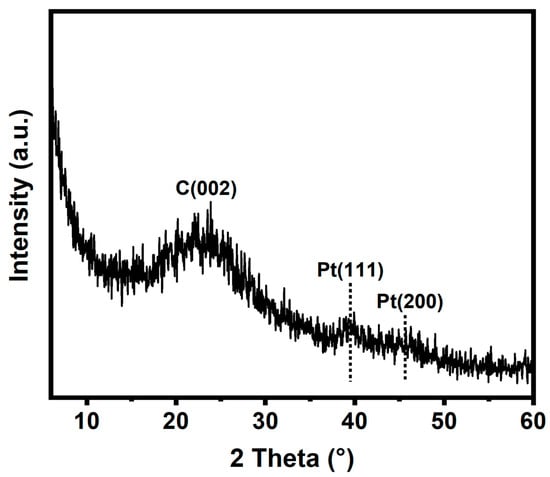
Figure 3.
XRD pattern of Pt/NMCT.
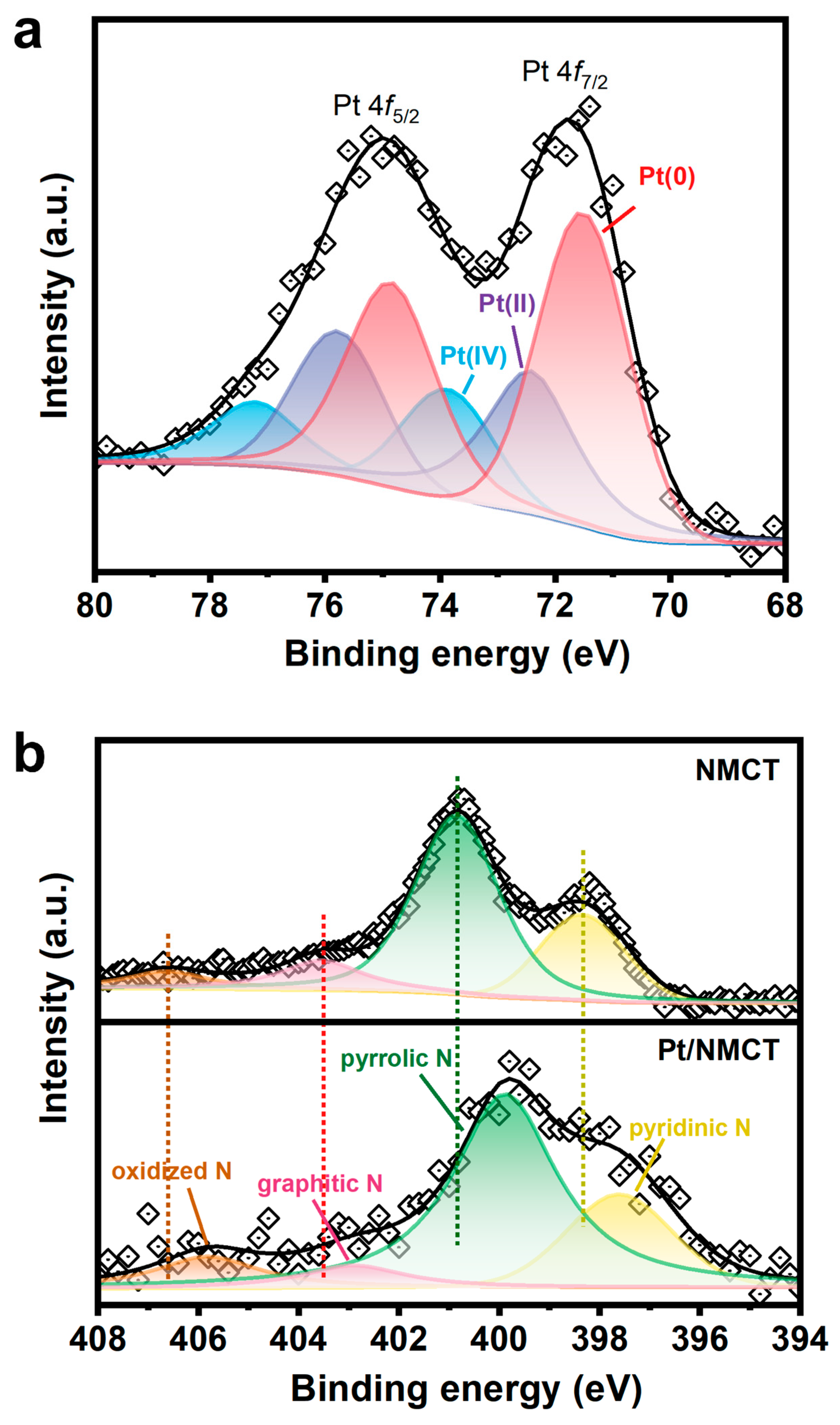
XPS was applied to investigate the chemical states of Pt/NMCT. The Pt 4f XPS spectrum of Pt/NMCT (Figure 4a) was subjected to peak fitting, and three groups of 4f split peaks (4f7/2 and 4f5/2) were obtained. The peaks of 4f7/2 are located at 71.5, 72.4 and 73.9 eV, respectively, corresponding to Pt at 0, +2 and +4 valence states [20,34]. This shows that most Pt is reduced, accounting for more than half of the total Pt content. The N 1s spectrum (Figure 4b) of Pt/NMCT can be divided into four peaks centered at 397.6, 399.9, 402.9 and 405.8 eV, assigned to pyridinic N, pyrrolic N, graphitic N, and oxidized N, respectively [35,36]. The relative contributions of pyridinic N, pyrrolic N, graphitic N, and oxidized N are 21.7, 60.8, 7.3 and 10.2%, respectively, demonstrating the successful doping of N atoms in the carbon skeleton. Compared with the N 1s spectrum of NMCT synthesized in the absence of a Pt source, the peak in the N 1s spectrum moves in the direction of low binding energy, which indicates a strong interaction between Pt nanoparticles and the N atom-containing surface of NMCT [33]. The interaction between the empty orbitals of Pt atoms and the free electron pairs of N atoms (such as pyridinic N) can limit the mobility of Pt nanoparticles and prevent them from aggregation, enhancing their durability and stability [23]. In addition, the sp2-hybridized N atoms that supply electrons to the delocalized π bond in carbon materials (such as pyridinic N and graphitic N) can modify the electronic structure of Pt nanoparticles or enhance the adsorption of 4-NP, thereby improving the intrinsic catalytic activity of the catalyst [37,38].
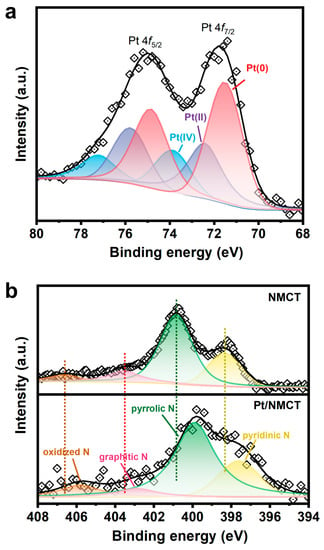
Figure 4.
(a) Pt 4f high-resolution X-ray photoelectron diffraction spectra (XPS) of Pt/NMCT; (b) N 1s XPS of NMCT and Pt/NMCT.
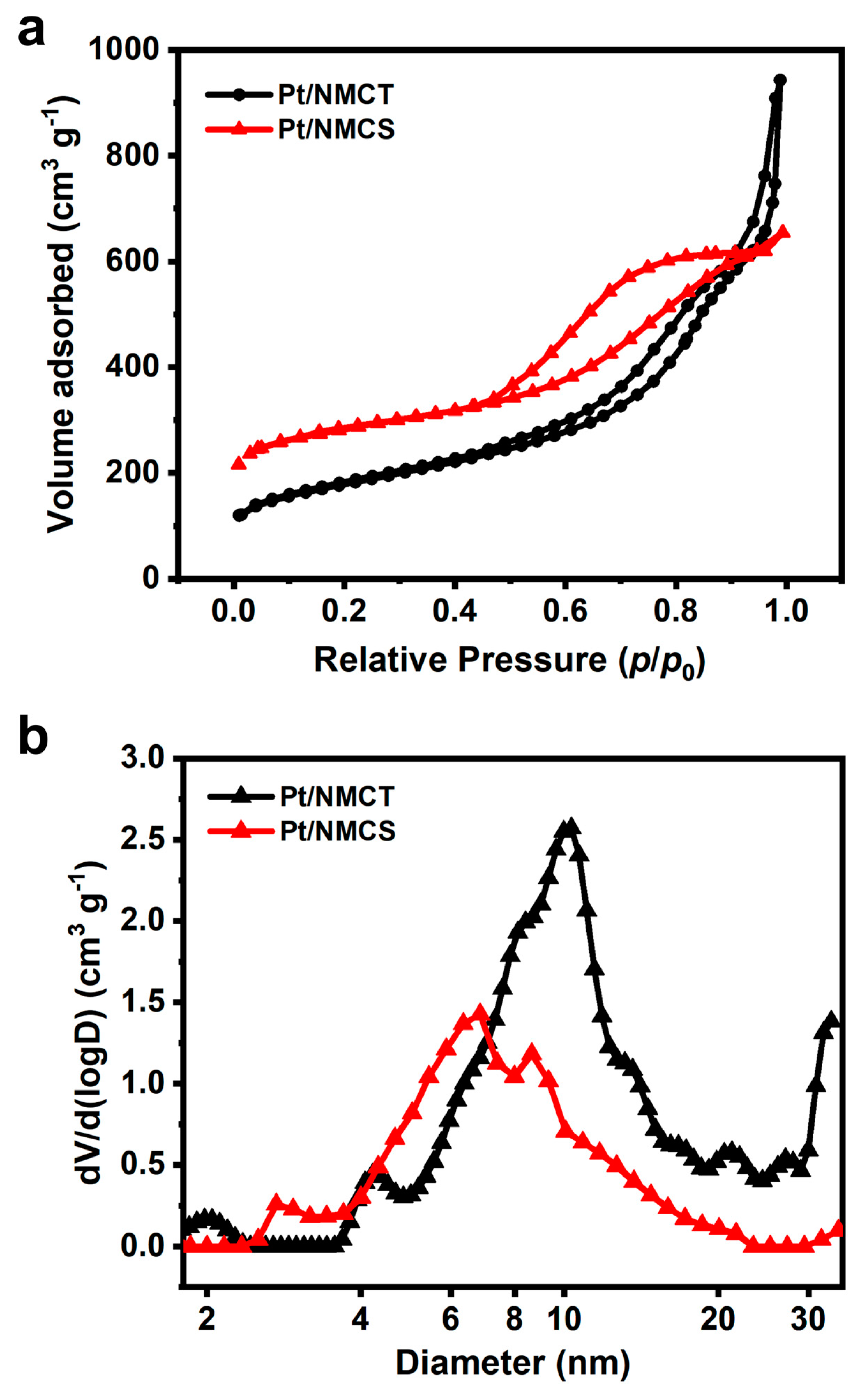
The texture property of the Pt/NMCT was studied through the low-temperature nitrogen gas adsorption experiment, and the isotherms and pore size distribution are shown in Figure 5. The nitrogen adsorption/desorption isotherms can be classified as type IV with an H4 hysteresis loop according to the IUPAC classification. This shows that Pt/NMCT has a hierarchical pore structure with micropores, mesopores, and macropores, and the pore distribution curve (Figure 5b) also verifies this conclusion. From the pore size distribution plot, the mesopores are mainly distributed in the range of 4–20 nm. The mesopores are from co-deposited silica, and the macropores are mainly from the hollow tube structure brought about by the SiO2 NW template. Based on the calculation, the specific surface area of the material is 620 m2 g−1, and the total pore volume is 1.46 cm3 g−1. The high surface area and pore volume of the sample are conducive to the diffusion of molecules, the thorough dispersion of Pt nanoparticles, and the exposure of active sites, which are beneficial for improved catalyst activity.
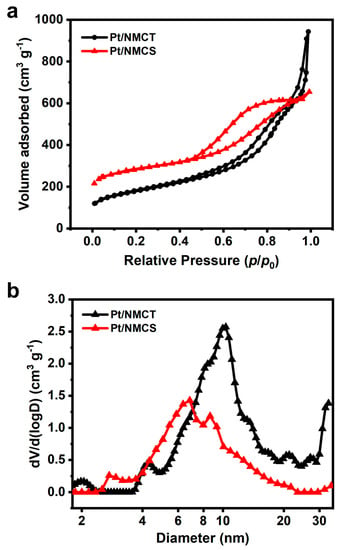
Figure 5.
(a) N2 sorption isotherms and (b) pore size distribution of Pt/NMCT.
In order to monitor the Pt loading and reduction process, we also analyzed the intermediates SiO2 NW@Pt/PDA-SiO2 and SiO2 NW@Pt/C-SiO2 by the SEM, TEM, and XPS techniques. A growth mechanism for Pt nanoparticles was also speculated.
There are no observable metal particles in SEM and TEM images of the intermediate SiO2 NW@Pt/PDA-SiO2 prepared in the co-deposition step (Figure 6a–c). From the EDS elemental mapping (Figure 6d) and the XPS full spectrum (Figure 6e), the loading of the Pt element can be clearly confirmed. In the XPS high-resolution spectrum of Pt 4f (Figure 6f), it can be seen that the main peak position of Pt is at a higher binding energy. After deconvolution of the 4f peaks, they are rearranged into two groups of peaks. The two 4f7/2 located at 72.4 eV and 73.9 eV, corresponding to the +2 and +4 valent, account for 26.2% and 73.7%, respectively. In other words, the Pt element in SiO2 NW@Pt/PDA-SiO2 exists in its ionic state. To demonstrate the interactions between PDA and Pt species during the co-deposition step, a simulated adsorption experiment was performed. In this simulated experiment, the PDA nanoparticles, synthesized under the same conditions except for the addition of SiO2 NW and TEOS, were suspended in a H2PtCl6 solution of an equivalent concentration to that in the co-deposition process. EDS analyses of the sediment revealed that PDA adsorbed a certain amount of Pt, probably through electrostatic and coordination interactions with PDA [39,40,41,42].
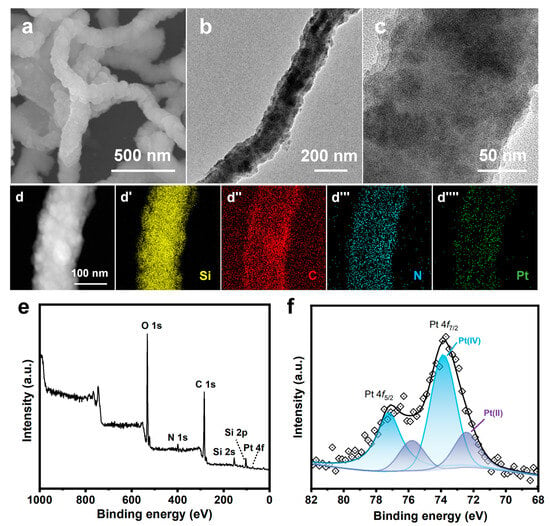
Figure 6.
(a) SEM image, (b,c) TEM images, (d) HAADF-STEM image, (d′–d′′′′) EDS elemental mappings, (e) XPS full spectrum of SiO2 NW@Pt/PDA-SiO2, and (f) Pt 4f XPS high-resolution spectrum of SiO2 NW@Pt/PDA-SiO2.
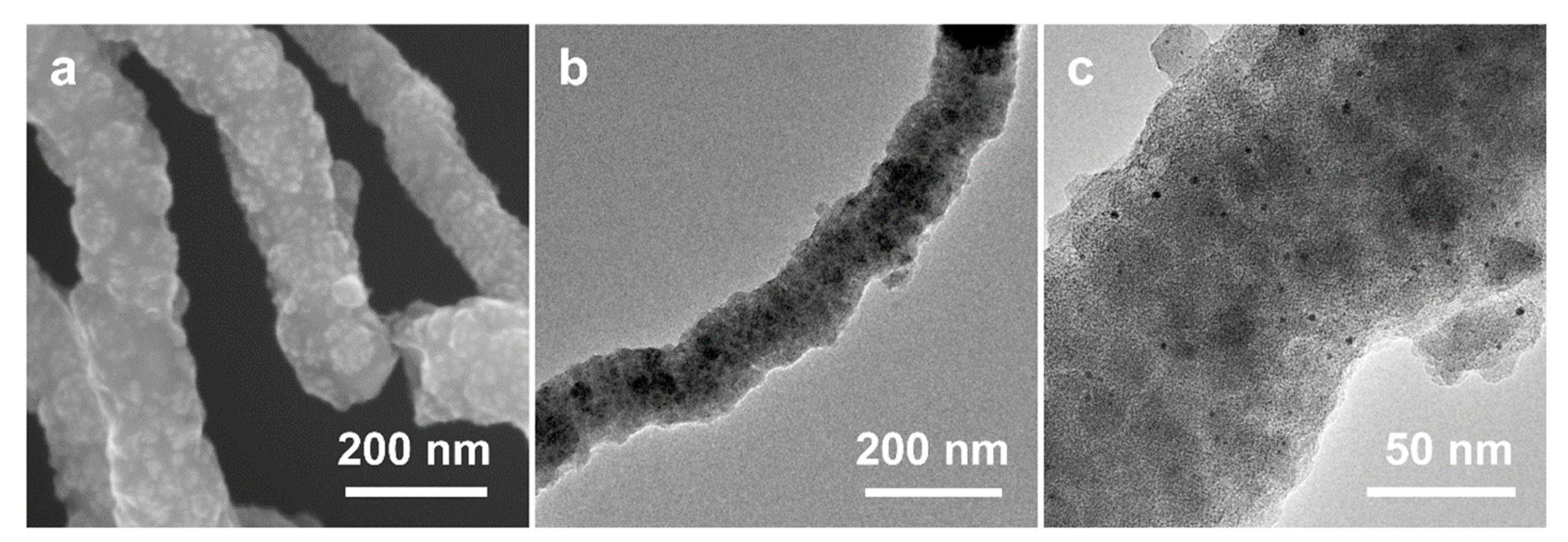
After the high-temperature calcination of SiO2 NW@Pt/PDA-SiO2 in a nitrogen atmosphere, it was transformed into SiO2 NW@Pt/C-SiO2 along with PDA carbonization. The SiO2 NW@Pt/C-SiO2 has a relatively rough surface without an obvious pore structure (Figure 7a,b), which does not seem to be very different from the SiO2 NW@Pt/PDA-SiO2. However, the presence of metal particles can be clearly seen in the TEM image of SiO2 NW@Pt/C-SiO2 (Figure 7c), which is significantly different from SiO2 NW@Pt/PDA-SiO2. After the subsequent HF etching, Pt/NMCT was obtained. Comparing its SEM and TEM images (Figure 2c,e) with those of SiO2 NW@Pt/C-SiO2 (Figure 7a,c), it can be found that the lumpy species in the SEM image of SiO2 NW@Pt/C-SiO2 (the patchy, slightly dark part in the TEM image) has been removed, leaving behind a honeycombed carbon skeleton structure. In addition, by comparing the TEM images of SiO2 NW@Pt/C-SiO2 (Figure 7b) and Pt/NMCT (Figure 2d), we can see that the original SiO2 NW was removed and formed a hollow tubular structure. Based on these results, the function of the dual-silica template agents was verified, namely that the co-deposited silica nanoclusters were used as mesoporous template agents, while the SiO2 NW acted as a sacrificial template for the formation of the tubular structure. In addition, from the TEM images of Pt/NMCT (Figure 2d,e), it can be seen that the Pt nanoparticles formed during the carbonization process are well-retained during the silica template etching process. To further prove the impact of the chelation and spatial restriction of the PDA-SiO2 composite on the growth of Pt nanoparticles, a comparison experiment was conducted by loading a similar content of Pt species onto a pre-prepared NMCT using rotary evaporation before calcination at the same temperature program used in the Pt/NMCT synthesis. The NMCT was synthesized by the same process as Pt/NMCT, except no H2PtCl6 was added. The resulting material exhibited a noticeable sintering of Pt nanoparticles of about 22.6 nm (Figure S1a) and very large Pt particles with a particle size of up to 200 nm (Figure S1b). These results demonstrated that the interaction between Pt and PDA and the confinement of Pt species in the PDA-SiO2 skeleton are important to suppress the overgrowth of the Pt nanoparticles in the calcination process [43,44].
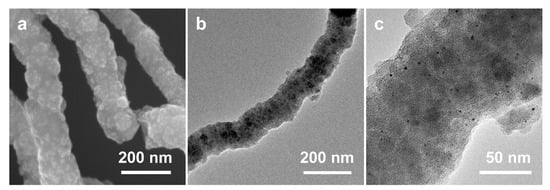
Figure 7.
(a) SEM image and (b,c) TEM images of SiO2 NW@Pt/C-SiO2.
Based on the above results, we speculated that the Pt nanoparticles undergo the following growth mechanism. With the addition of H2PtCl6 in the co-deposition step, the Pt is chelated by the amino group of dopamine and then evenly distributed in the composite shell. In the subsequent calcination process, the Pt is reduced in situ in the carbonization process of PDA, and the growth in reduced Pt nanoparticles was spatially restricted. After removal of the silica template, Pt/NMCT with enriched meso/macroporosity and uniformly distributed Pt nanoparticles is obtained.
To demonstrate the advantage of hollow structure in Pt/NMCT, a control sample of Pt nanoparticle-loaded nitrogen-doped carbon nanospheres (Pt/NMCS) was prepared without adding the SiO2 NWs as a template. As shown in Figure S2, the particles of Pt/NMCSs are highly agglomerated, and their mesopores are loaded with abundant Pt nanoparticles. The N2 sorption isotherms and pore size distribution curves (Figure 5) show that Pt/NMCS has less macroporosity and smaller mesopores than Pt/NMCT. Additionally, Pt/NMCS has a smaller pore volume (1.01 cm3 g−1) but a larger BET specific surface area (1032.6 m2 g−1) than Pt/NMCT due to the smaller mesopores and more abundant micropores that exist in Pt/NMCS.
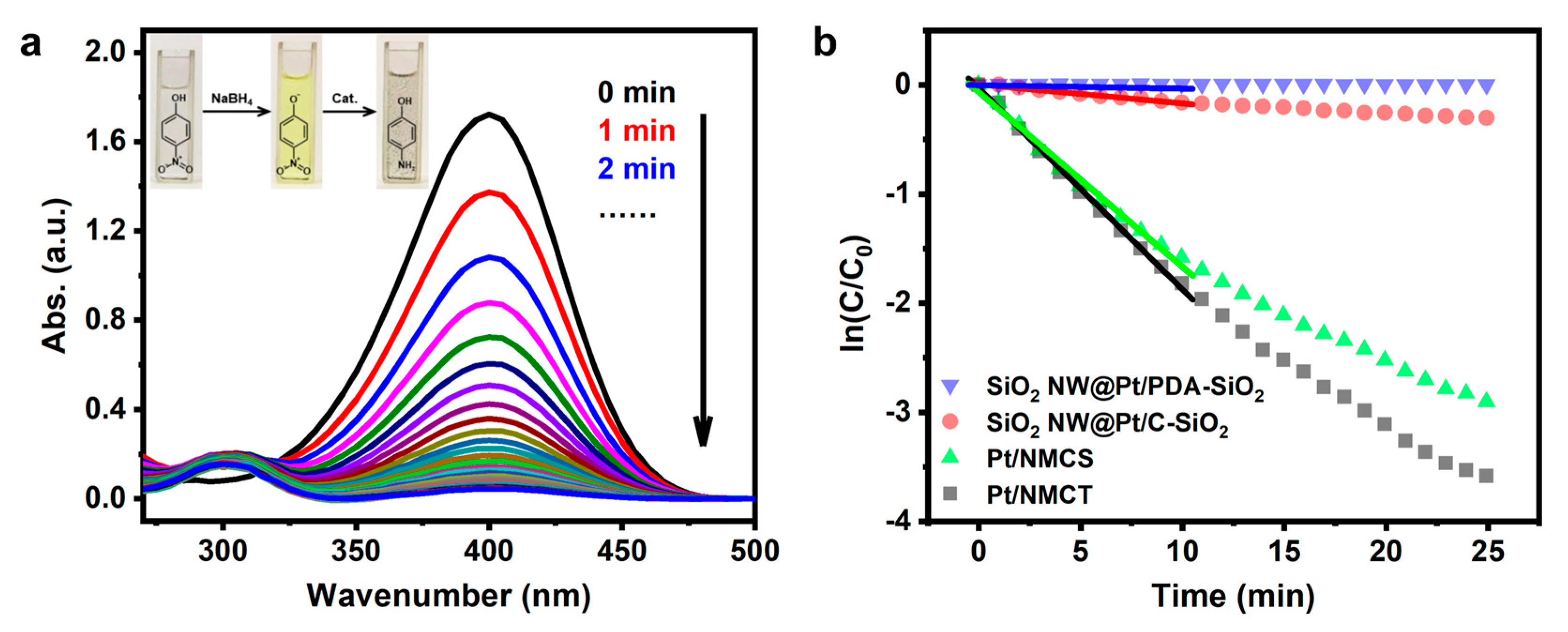
As a proof of concept, we evaluated the catalytic performance of Pt/NMCT in the reduction reaction of 4-NP. The 4-NP solution, after adding freshly prepared NaBH4 solution, showed a bright yellow color, due to the emergence of 4-nitrophenol ions induced by NaBH4 in an alkaline environment (Figure 8a-inset) [45]. When Pt/NMCT was added to the solution containing 4-NP and NaBH4, the yellow color gradually diminished until it became colorless. The reaction’s progress could also be quantitatively monitored by UV-vis spectroscopy. With the extension of reaction time, the peak intensity of 4-NP at 400 nm gradually decreases and finally disappears (Figure 8a). Meanwhile, a new absorption peak of 4-AP appears near 300 nm, indicating that the weakening of the peak is due to the reduction of 4-NP to 4-AP rather than the adsorption of 4-NP on the surface of the material [31,46].
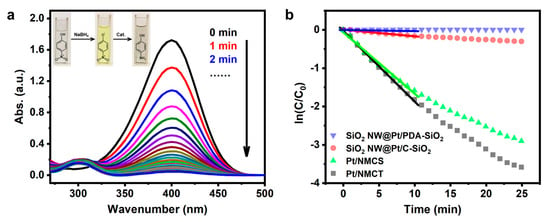
Figure 8.
(a) UV-vis absorption spectra at different reaction times for the reduction of 4-nitrophenol using Pt/NMCT as the catalyst; (b) The curves of ln(ct/c0) versus time t using Pt/NMCT, Pt/NMCS, SiO2 NW@Pt/C-SiO2, and SiO2 NW@Pt/PDA-SiO2 as catalysts. Reaction conditions: [4-NP] = 0.093 mM, [NaBH4] = 6.2 mM, T = 25 °C, [Pt] = 0.30 mg L−1.
In addition, the kinetics of the 4-NP reduction reaction were further studied. As the concentration of NaBH4 is much higher than that of the reactant (4-NP) in this study, the concentration of NaBH4 can be considered to be constant under reaction conditions, and the catalytic reaction follows pseudo first-order reaction kinetics [47]. The reaction rate of pseudo-first-order kinetics can be determined according to Equation (1) [25]:
where k is the apparent rate constant in min−1 unit, and c0 and ct are the initial concentration and concentration of 4-NP at time t, respectively. As shown in Figure 8b (black square), ln(ct/c0) has a good linear correlation with the reaction time t, indicating quasi-first-order kinetics. The apparent rate constant k calculated from the slope of the fitting line is 0.184 min−1, which is similar to that of some promising Pt nanoparticles-supported catalysts in the literature [25,48,49].
ln (ct/c0) = −kt
In order to further illustrate the structural advantages of the materials prepared by the dual-silica template method and the necessary calcination to obtain active Pt species, we also investigated the performance of the control catalysts in the 4-NP reduction reaction, i.e., Pt/NMCS, the silica-retained sample SiO2 NW@Pt/C-SiO2, and the uncalcined sample SiO2 NW@Pt/PDA-SiO2. The curve between ln(ct/c0) and t obtained from UV-vis spectra is shown in Figure 8b. By calculating the slopes of the fitting curves, the apparent rate constants of SiO2 NW@Pt/PDA-SiO2, SiO2 NW@Pt/C-SiO2 and Pt/NMCS are 0.003, 0.017 and 0.160 min−1, respectively. The catalytic performance of Pt/NMCS is slightly worse than that of Pt/NMCT (0.184 min−1). Although Pt/NMCS has a higher surface area, which is normally beneficial to improving the catalyst activity, the longer diffusion pathway and the lack of macropores in Pt/NMCS may restrict the diffusion of guest molecules, thereby causing the inferior activity of Pt/NMCS [50]. Also, SiO2 NW@Pt/C-SiO2 shows significantly lower catalytic activity than Pt/NMCT due to the less porous structure of SiO2 NW@Pt/C-SiO2 without removal of the silica template. The worst activity was found in SiO2 NW@Pt/PDA-SiO2, which suggests that oxidized Pt is less active than the reduced one in this reaction.
The above results suggest that the Pt nanoparticles are mainly distributed in the co-deposited shell rather than on the outer surface of the material. After etching the silica, the Pt nanoparticles embedded in the shell are exposed, and the catalytic activity of Pt nanoparticles is greatly improved. The above results clearly demonstrate that Pt/NMCT has a high catalytic efficiency in the 4-NP catalytic reduction reaction, and its hierarchical pore structure should be the main reason for the increased activity.
4. Conclusions
In summary, this work successfully synthesized Pt/NMCT through the polydopamine-mediated “one-pot” co-deposition process, followed by calcination and etching processes. The loaded Pt nanoparticles had a high loading capacity (9.5 wt%), a small particle size (~2 nm), and a uniform distribution on the carrier. The use of the dual-silica template brought about a hierarchical pore structure with a high specific surface area (620 m2 g−1) and a large pore volume (1.46 cm3 g−1). After high-temperature calcination in a nitrogen atmosphere, most of the Pt was reduced and formed ultra-small nanoparticles. In the 4-NP reduction reaction, Pt/NMCT had an apparent rate constant of 0.184 min−1, which was significantly superior to SiO2 NW@Pt/C-SiO2 and Pt/NMCS. This work makes an excellent contribution to the design of supported noble metal catalysts and also highlights the importance of hierarchical pore structures for catalytic activity.
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/nano13192633/s1: Figure S1. TEM images of the Pt loaded NMCT synthesized by rotary evaporation and calcination; Figure S2. TEM image of Pt/NMCS.
Author Contributions
Data curation, Q.Z. and M.W.; funding acquisition, Y.W. and Y.T.; Investigation, Q.Z., M.W. and Y.F.; methodology, Y.W., C.D. and H.-H.S.; writing—original draft, Q.Z.; writing—review and editing, Y.W., Y.T. and H.-H.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the National Key R&D Program of China (2018YFA0209402) and the National Natural Science Foundation of China (22175132, 22088101).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Meemken, F.; Baiker, A. Recent progress in heterogeneous asymmetric hydrogenation of C=O and C=C bonds on supported noble metal catalysts. Chem. Rev. 2017, 117, 11522–11569. [Google Scholar] [CrossRef]
- Mergler, Y.J.; Van Aalst, A.; Van Delft, J.; Nieuwenhuys, B.E. CO oxidation over promoted Pt catalysts. Appl. Catal. B 1996, 10, 245–261. [Google Scholar] [CrossRef]
- Shan, Y.; Hu, H.M.; Fan, X.Q.; Zhao, Z. Recent progress in catalytic dehydrogenation of propane over Pt-based catalysts. Phys. Chem. Chem. Phys. 2023, 25, 18609–18622. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.B.; Du, Z.; Fan, H.S.; Wang, R.M. Nanostructure optimization of platinum-based nanomaterials for catalytic applications. Nanomaterials 2018, 8, 949. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.Y.; Foucher, A.C.; Montini, T.; Stach, E.A.; Fornasiero, P.; Gorte, R.J. Epitaxial and strong support interactions between Pt and LaFeO3 films stabilize Pt dispersion. J. Am. Chem. Soc. 2020, 142, 10373–10382. [Google Scholar] [CrossRef] [PubMed]
- Jayabal, S.; Saranya, G.; Geng, D.S.; Lin, L.Y.; Meng, X.B. Insight into the correlation of Pt-support interactions with electrocatalytic activity and durability in fuel cells. J. Mater. Chem. A 2020, 8, 9420–9446. [Google Scholar] [CrossRef]
- Esteve-Adell, I.; Bakker, N.; Primo, A.; Hensen, E.; Garcia, H. Oriented Pt nanoparticles supported on few-layers graphene as highly active catalyst for aqueous-phase reforming of ethylene glycol. ACS Appl. Mater. Interfaces 2016, 8, 33690–33696. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.X.; Ying, J.; Liu, H.W.; Yang, X.Y. Pt-C interactions in carbon-supported Pt-based electrocatalysts. Front. Chem. Sci. Eng. 2023. [Google Scholar] [CrossRef]
- Fiorio, J.L.; Garcia, M.A.S.; Gothe, M.L.; Galvan, D.; Troise, P.C.; Conte, C.A.; Vidinha, P.; Camargo, P.H.C.; Rossi, L.M. Recent advances in the use of nitrogen-doped carbon materials for the design of noble metal catalysts. Coord. Chem. Rev. 2023, 481, 215053. [Google Scholar] [CrossRef]
- Hu, X.Y.; Yang, B.Z.; Ke, S.R.; Liu, Y.A.; Fang, M.H.; Huang, Z.H.; Min, X. Review and perspectives of carbon-supported platinum-based catalysts for proton exchange membrane fuel cells. Energy Fuels 2023, 37, 11532–11566. [Google Scholar] [CrossRef]
- Devrim, Y.; Albostan, A. Graphene-supported platinum catalyst-based membrane electrode assembly for PEM fuel cell. J. Electron. Mater. 2016, 45, 3900–3907. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Z.; Gong, Y.T.; Han, C.L.; Li, H.R.; Wang, Y. One-step production of sulfur and nitrogen co-doped graphitic carbon for oxygen reduction: Activation effect of oxidized sulfur and nitrogen. ChemCatChem 2014, 6, 1204–1209. [Google Scholar] [CrossRef]
- You, C.H.; Liao, S.J.; Li, H.L.; Hou, S.Y.; Peng, H.L.; Zeng, X.Y.; Liu, F.F.; Zheng, R.P.; Fu, Z.Y.; Li, Y.W. Uniform nitrogen and sulfur co-doped carbon nanospheres as catalysts for the oxygen reduction reaction. Carbon 2014, 69, 294–301. [Google Scholar] [CrossRef]
- Choi, C.H.; Chung, M.W.; Kwon, H.C.; Park, S.H.; Woo, S.I. B, N- and P, N-doped graphene as highly active catalysts for oxygen reduction reactions in acidic media. J. Mater. Chem. A 2013, 1, 3694–3699. [Google Scholar] [CrossRef]
- Park, J.E.; Jang, Y.J.; Kim, Y.J.; Song, M.S.; Yoon, S.; Kim, D.H.; Kim, S.J. Sulfur-doped graphene as a potential alternative metal-free electrocatalyst and Pt-catalyst supporting material for oxygen reduction reaction. Phys. Chem. Chem. Phys. 2014, 16, 103–109. [Google Scholar] [CrossRef]
- Li, M.M.; Xu, F.; Li, H.R.; Wang, Y. Nitrogen-doped porous carbon materials: Promising catalysts or catalyst supports for heterogeneous hydrogenation and oxidation. Catal. Sci. Technol. 2016, 6, 3670–3693. [Google Scholar] [CrossRef]
- He, L.; Weniger, F.; Neumann, H.; Beller, M. Synthesis, characterization, and application of metal nanoparticles supported on nitrogen-doped carbon: Catalysis beyond electrochemistry. Angew. Chem. Int. Ed. 2016, 55, 12582–12594. [Google Scholar] [CrossRef]
- Mayoral, E.P.; Ojer, M.G.; Ventura, M.; Matos, I. New insights into N-doped porous carbons as both heterogeneous catalysts and catalyst supports: Opportunities for the catalytic synthesis of valuable compounds. Nanomaterials 2023, 13, 2013. [Google Scholar] [CrossRef]
- Cao, Y.L.; Mao, S.J.; Li, M.M.; Chen, Y.Q.; Wang, Y. Metal/porous carbon composites for heterogeneous catalysis: Old catalysts with improved performance promoted by N-doping. ACS Catal. 2017, 7, 8090–8112. [Google Scholar] [CrossRef]
- Liu, Y.X.; Yang, X.J.; Liu, H.Y.; Ye, Y.H.; Wei, Z.J. Nitrogen-doped mesoporous carbon supported Pt nanoparticles as a highly efficient catalyst for decarboxylation of saturated and unsaturated fatty acids to alkanes. Appl. Catal. B 2017, 218, 679–689. [Google Scholar] [CrossRef]
- Afkhami-Ardekani, M.; Naimi-Jamal, M.R.; Doaee, S.; Rostamnia, S. Solvent-free mechanochemical preparation of metal-organic framework ZIF-67 impregnated by Pt nanoparticles for water purification. Catalysts 2022, 13, 9. [Google Scholar] [CrossRef]
- Xia, L.; Li, D.; Long, J.; Huang, F.; Yang, L.; Guo, Y.; Jia, Z.; Xiao, J.; Liu, H. N-doped graphene confined Pt nanoparticles for efficient semi-hydrogenation of phenylacetylene. Carbon 2019, 145, 47–52. [Google Scholar] [CrossRef]
- Liu, Z.Y.; Zhang, C.L.; Luo, L.; Chang, Z.; Sun, X.M. One-pot synthesis and catalyst support application of mesoporous N-doped carbonaceous materials. J. Mater. Chem. 2012, 22, 12149–12154. [Google Scholar] [CrossRef]
- Zhang, M.H.; Wang, T.D.; Zhang, M.W.; Wang, Q.F.; Wang, L.; Zhang, X.W.; Li, G.Z. Tunable selective hydrogenation of cinnamaldehyde by capped Pt/Pd nanoparticles supported on carbon nanotubes. ChemistrySelect 2022, 7, 202200316. [Google Scholar] [CrossRef]
- Bian, S.W.; Liu, S.; Chang, L. Synthesis of magnetically recyclable Fe3O4@polydopamine-Pt composites and their application in hydrogenation reactions. J. Mater. Sci. 2016, 51, 3643–3649. [Google Scholar] [CrossRef]
- Uson, L.; Hueso, J.L.; Sebastian, V.; Arenal, R.; Florea, I.; Irusta, S.; Arruebo, M.; Santamaria, J. In-situ preparation of ultra-small Pt nanoparticles within rod-shaped mesoporous silica particles: 3-D tomography and catalytic oxidation of n-hexane. Catal. Commun. 2017, 100, 93–97. [Google Scholar] [CrossRef]
- Yao, C.X.; Xue, J.; Xu, L.J.; Su, Y.; Bu, J.D.; Priestley, R.D.; Hou, S.F. Facile synthesis of polydopamine-functionalized hollow graphene composite microspheres and their application in methanol oxidation reaction. Appl. Surf. Sci. 2021, 541, 148329. [Google Scholar] [CrossRef]
- Huang, H.L.; He, Z.Y.; Lin, X.M.; Ruan, W.S.; Liu, Y.J.; Yang, Z.H. Ultradispersed platinum nanoclusters on polydopamine-functionalized carbon nanotubes as an excellent catalyst for methanol oxidation reaction. Appl. Catal. A 2015, 490, 65–70. [Google Scholar] [CrossRef]
- Zhang, Q.; Deng, C.; Huang, Z.M.; Zhang, Q.C.; Chai, X.C.; Yi, D.L.; Fang, Y.Y.; Wu, M.Y.; Wang, X.D.; Tang, Y.; et al. Dual-silica template-mediated synthesis of nitrogen-doped mesoporous carbon nanotubes for supercapacitor applications. Small 2022, 19, 2205725. [Google Scholar] [CrossRef]
- Yi, D.L.; Xu, C.L.; Tang, R.D.; Zhang, X.H.; Caruso, F.; Wang, Y.J. Synthesis of discrete alkyl-silica hybrid nanowires and their assembly into nanostructured superhydrophobic membranes. Angew. Chem. Int. Ed. 2016, 55, 8375–8380. [Google Scholar] [CrossRef]
- Yan, Q.; Wang, X.Y.; Feng, J.J.; Mei, L.P.; Wang, A.J. Simple fabrication of bimetallic platinum-rhodium alloyed nano-multipods: A highly effective and recyclable catalyst for reduction of 4-nitrophenol and rhodamine B. J. Colloid Interface Sci. 2021, 582, 701–710. [Google Scholar] [CrossRef]
- Li, J.; Liu, L.; Ai, Y.; Hu, Z.; Xie, L.; Bao, H.; Wu, J.; Tian, H.; Guo, R.; Ren, S.; et al. Facile and large-scale fabrication of sub-3 nm PtNi nanoparticles supported on porous carbon sheet: A bifunctional material for the hydrogen evolution reaction and hydrogenation. Chem. Eur. J. 2019, 25, 7191–7200. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Wang, S.; Wang, X.; Yan, Y.; Wang, K.; Gao, M.; Xu, Q. Facile preparation of highly dispersed Pt nanoparticles supported on heteroatom-containing porous carbon nanospheres and their catalytic properties for the reduction of 4-nitrophenol. J. Porous Mater. 2017, 25, 1081–1089. [Google Scholar] [CrossRef]
- Jiang, X.F.; Wang, X.B.; Shen, L.M.; Wu, Q.; Wang, Y.N.; Ma, Y.W.; Wang, X.Z.; Hu, Z. High-performance Pt catalysts supported on hierarchical nitrogen-doped carbon nanocages for methanol electrooxidation. Chin. J. Catal. 2016, 37, 1149–1155. [Google Scholar] [CrossRef]
- Bian, S.W.; Liu, S.; Guo, M.X.; Xu, L.L.; Chang, L. Pd nanoparticles partially embedded in the inner wall of nitrogen-doped carbon hollow spheres as nanoreactors for catalytic reduction of 4-nitrophenol. RSC Adv. 2015, 5, 11913–11916. [Google Scholar] [CrossRef]
- Ren, Y.P.; Chen, F.; Pan, K.M.; Zhao, Y.; Ma, L.L.; Wei, S.Z. Studies on kinetics, isotherms, thermodynamics and adsorption mechanism of methylene blue by N and S co-doped porous carbon spheres. Nanomaterials 2021, 11, 1819. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.F.; Zhang, X.M.; Jing, L.Y.; Yang, H.Q. N-doped ordered mesoporous carbon as a multifunctional support of ultrafine Pt nanoparticles for hydrogenation of nitroarenes. Chin. J. Catal. 2017, 38, 1252–1260. [Google Scholar] [CrossRef]
- Liu, J.K.; Li, L.Y.; Li, J.H.; Lin, W.G.; Wang, H.P.; Zhao, H.; Chen, X.; Zhang, J.K.; Yang, W.S. Lattice-strained Pt nanoparticles anchored on petroleum vacuum residue derived N-doped porous carbon as highly active and durable cathode catalysts for PEMFCs. Int. J. Hydrog. Energy 2023, 48, 25720–25729. [Google Scholar] [CrossRef]
- Liu, X.C.; Wang, G.C.; Liang, R.P.; Shi, L.; Qiu, J.D. Environment-friendly facile synthesis of Pt nanoparticles supported on polydopamine modified carbon materials. J. Mater. Chem. A 2013, 1, 3945–3953. [Google Scholar] [CrossRef]
- Pinithchaisakula, A.; Themsirimongkon, S.; Promsawan, N.; Weankeaw, P.; Ounnunkad, K.; Saipanya, S. An investigation of a polydopamine-graphene oxide composite as a support for an anode fuel cell catalyst. Electrocatalysis 2017, 8, 36–45. [Google Scholar] [CrossRef]
- Hu, X.C.; Lu, Y.L.; Shi, X.K.; Yao, T.M.; Dong, C.Y.; Shi, S. Integrating in situ formation of nanozymes with mesoporous polydopamine for combined chemo, photothermal and hypoxia-overcoming photodynamic therapy. Chem. Commun. 2019, 55, 14785–14788. [Google Scholar] [CrossRef] [PubMed]
- Ye, W.C.; Hu, H.Y.; Zhang, H.; Zhou, F.; Liu, W.M. Multi-walled carbon nanotube supported Pd and Pt nanoparticles with high solution affinity for effective electrocatalysis. Appl. Surf. Sci. 2010, 256, 6723–6728. [Google Scholar] [CrossRef]
- Ye, W.C.; Chen, Y.; Zhou, Y.X.; Fu, J.J.; Wu, W.C.; Gao, D.Q.; Zhou, F.; Wang, C.M.; Xue, D.S. Enhancing the catalytic activity of flowerike Pt nanocrystals using polydopamine functionalized graphene supports for methanol electrooxidation. Electrochim. Acta 2014, 142, 18–24. [Google Scholar] [CrossRef]
- Yan, L.J.; Bo, X.J.; Zhu, D.X.; Guo, L.P. Well-dispersed Pt nanoparticles on polydopamine-coated ordered mesoporous carbons and their electrocatalytic application. Talanta 2014, 120, 304–311. [Google Scholar] [CrossRef]
- Gao, D.; Li, S.; Wang, X.; Xi, L.; Lange, K.M.; Ma, X.; Lv, Y.; Yang, S.; Zhao, K.; Loussala, H.M.; et al. Ultrafine PtRu nanoparticles confined in hierarchically porous carbon derived from micro-mesoporous zeolite for enhanced nitroarenes reduction performance. J. Catal. 2019, 370, 385–403. [Google Scholar] [CrossRef]
- Zhao, Q.; Bu, D.C.; Li, Z.H.; Zhang, X.L.; Di, L.B. Cold plasma preparation of Pd/graphene catalyst for reduction of p-nitrophenol. Nanomaterials 2021, 11, 1341. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, X.; Qiu, L.; Liu, C.; Wang, S.; Yan, F. Synthesis of carbon–PtAu nanoparticle hybrids originating from triethoxysilane-derivatized ionic liquids for methanol electrooxidation and the catalytic reduction of 4-nitrophenol. J. Mater. Chem. A 2013, 1, 9257–9263. [Google Scholar] [CrossRef]
- Wang, Z.M.; Xu, C.L.; Gao, G.Q.; Li, X. Facile synthesis of well-dispersed Pd-graphene nanohybrids and their catalytic properties in 4-nitrophenol reduction. RSC Adv. 2014, 4, 13644–13651. [Google Scholar] [CrossRef]
- Ye, W.C.; Yu, J.; Zhou, Y.X.; Gao, D.Q.; Wang, D.A.; Wang, C.M.; Xue, D.S. Green synthesis of Pt-Au dendrimer-like nanoparticles supported on polydopamine-functionalized graphene and their high performance toward 4-nitrophenol reduction. Appl. Catal. B 2016, 181, 371–378. [Google Scholar] [CrossRef]
- Pan, Z.D.; Yu, S.; Wang, L.F.; Li, C.Y.; Meng, F.; Wang, N.; Zhou, S.X.; Xiong, Y.; Wang, Z.L.; Wu, Y.T.; et al. Recent advances in porous carbon materials as electrodes for supercapacitors. Nanomaterials 2023, 13, 1744. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).