Ni-Based SBA-15 Catalysts Modified with CeMnOx for CO2 Valorization via Dry Reforming of Methane: Effect of Composition on Modulating Activity and H2/CO Ratio
Abstract
:1. Introduction
2. Experiment
2.1. Preparation of Support and Catalysts
2.2. Characterization of Materials
2.3. Catalytic Test
3. Results and Discussion
3.1. Characterization of Calcined Supports and Catalysts
3.1.1. X-ray Fluorescence Analysis (XRF)
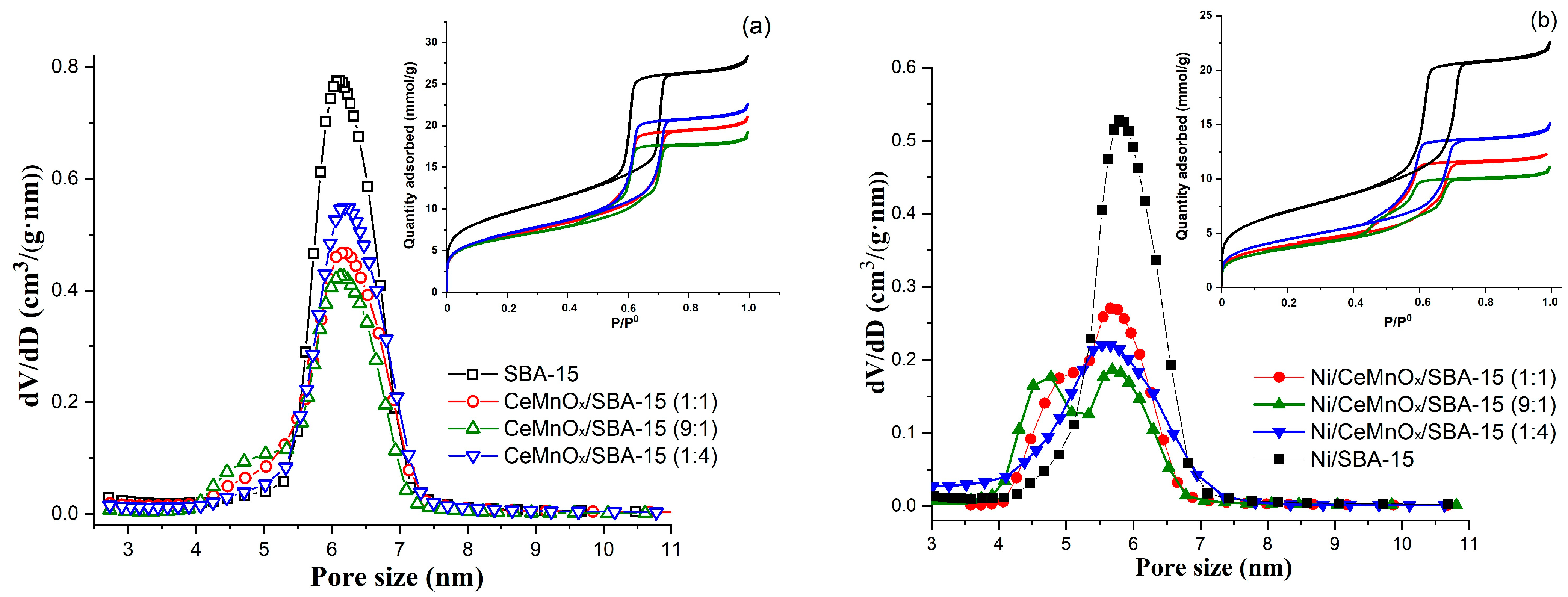
3.1.2. BET Surface Area and Pore Structure
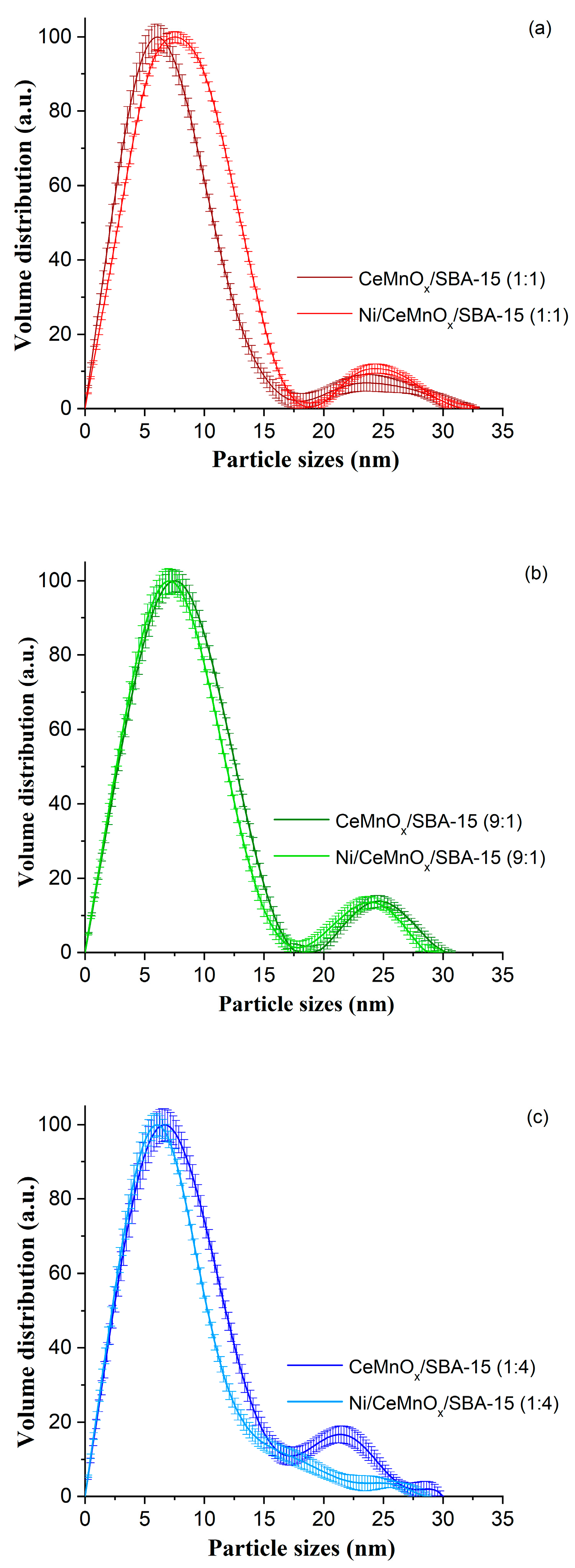
3.1.3. Small-Angle X-ray Scattering (SAXS) and Wide-Angle X-ray Diffraction (XRD)
3.1.4. UV-Vis Diffuse Reflectance Spectroscopy (UV-Vis DRS) and Raman Spectroscopy
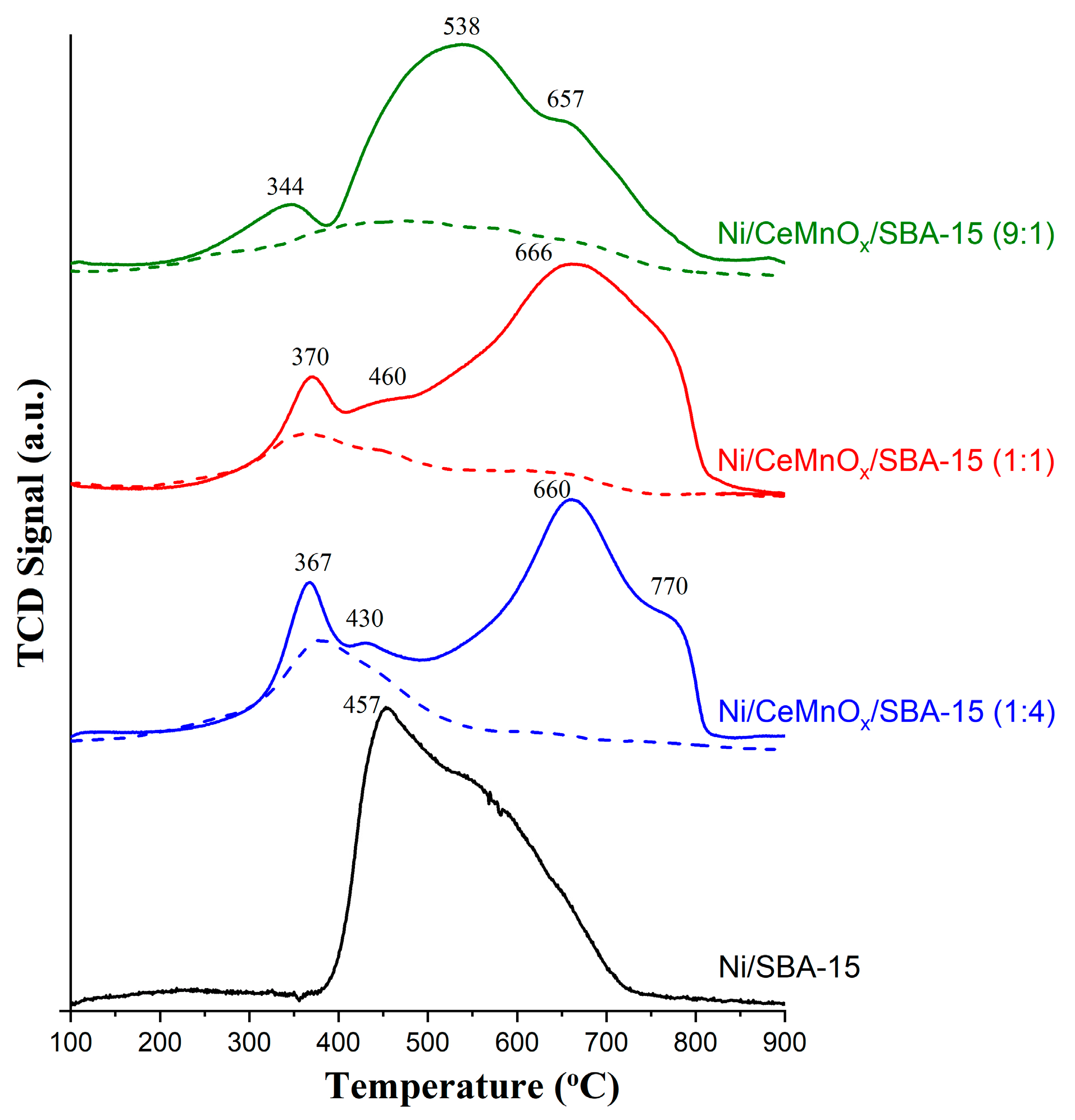
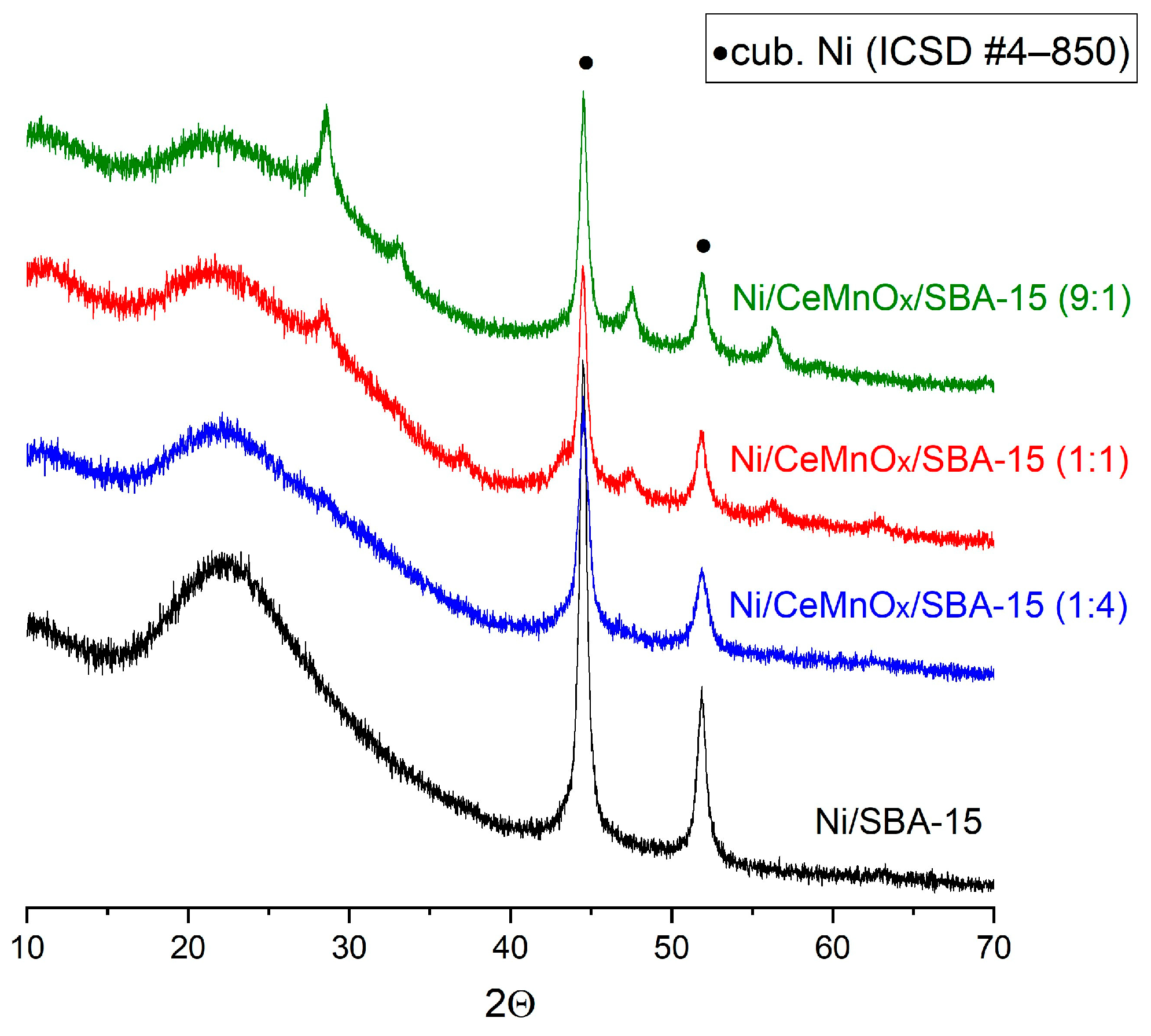
3.2. H2-TPR Profiles and Structural Characterization of Reduced Catalysts
3.3. Catalyst Performance
3.3.1. Gradient Catalytic Tests
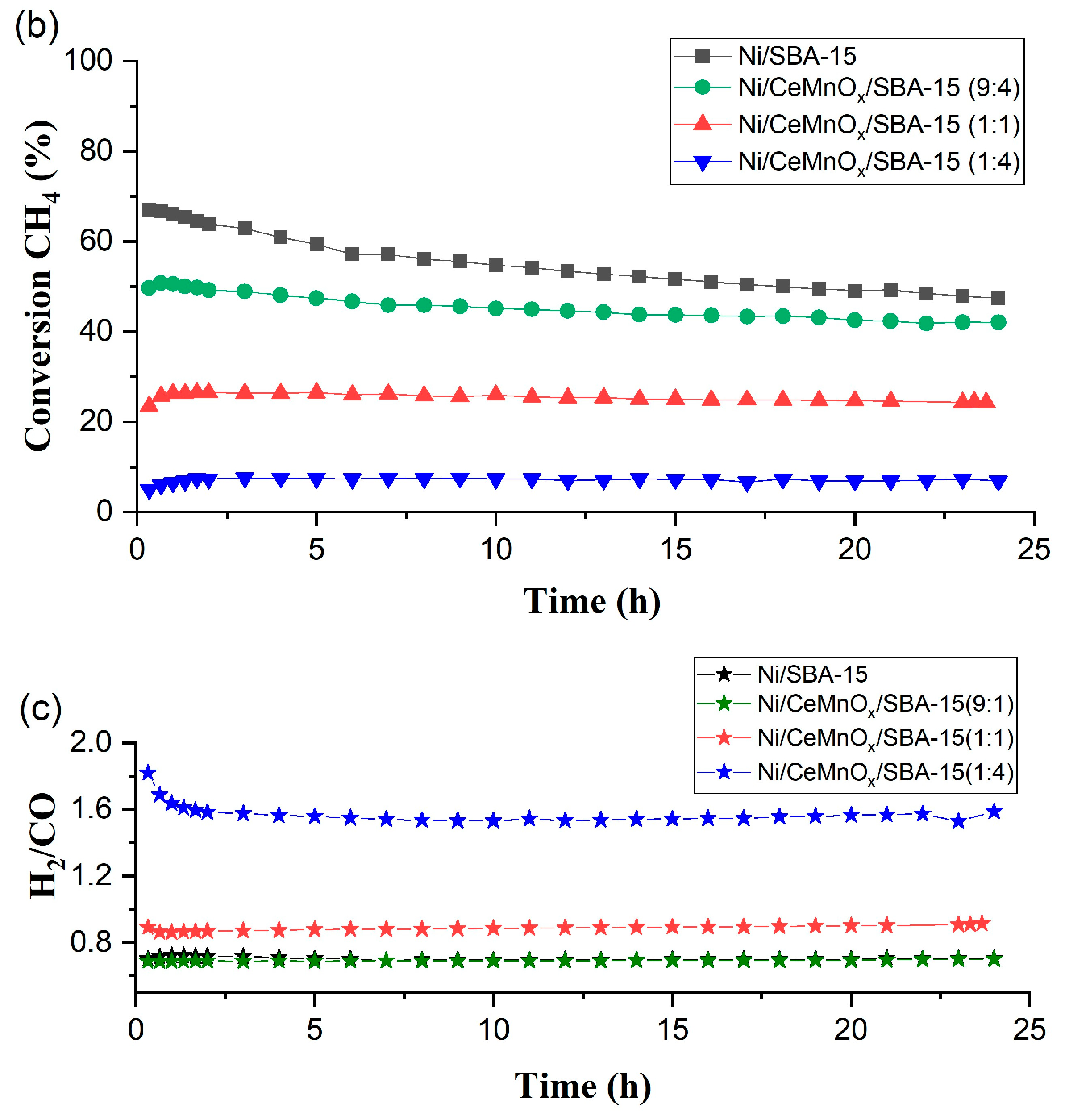
3.3.2. Long-Run Tests
3.4. Characterization of Spent Catalyst
3.4.1. TGA Study after Catalytic Tests
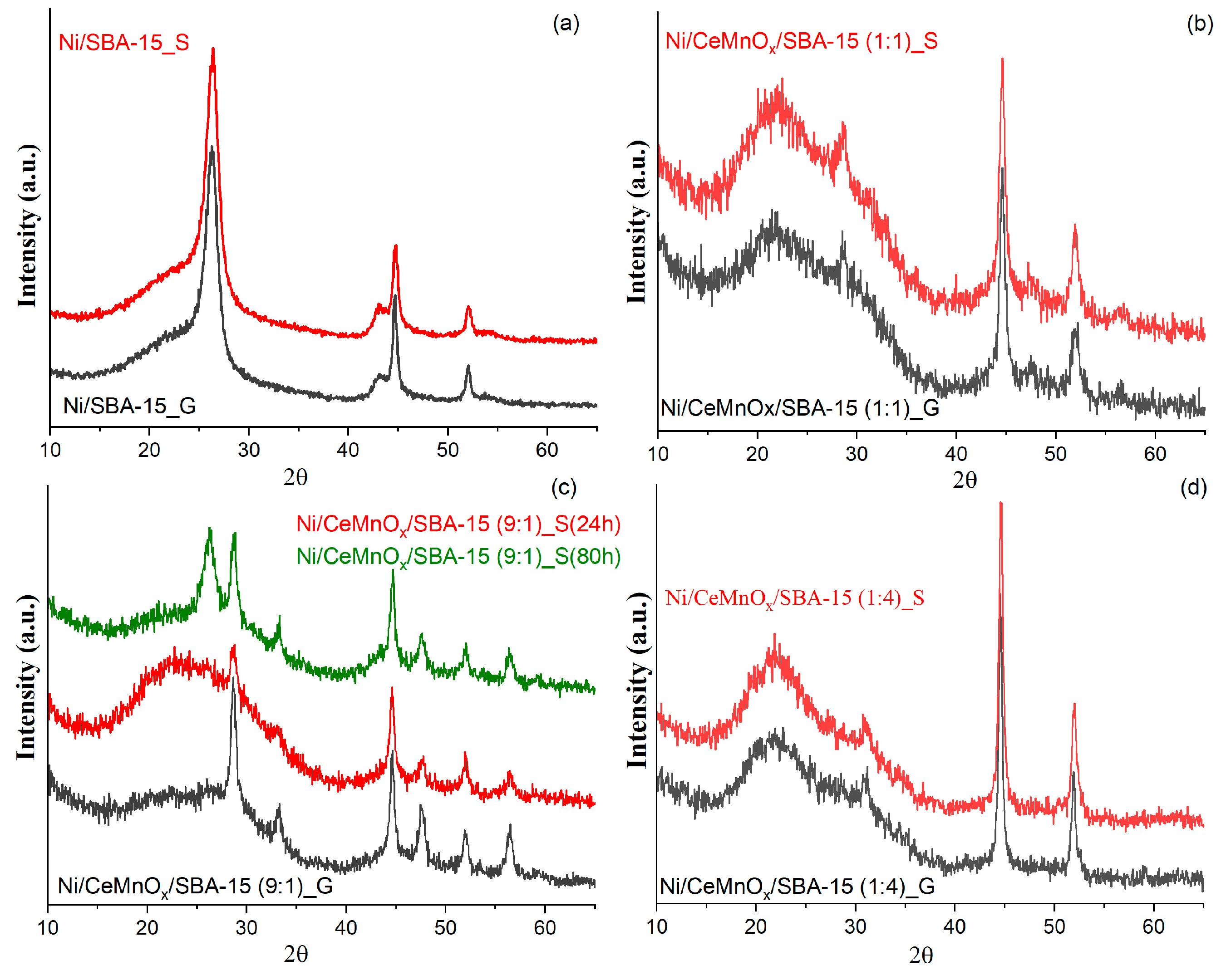
3.4.2. XRD Study after Catalytic Tests
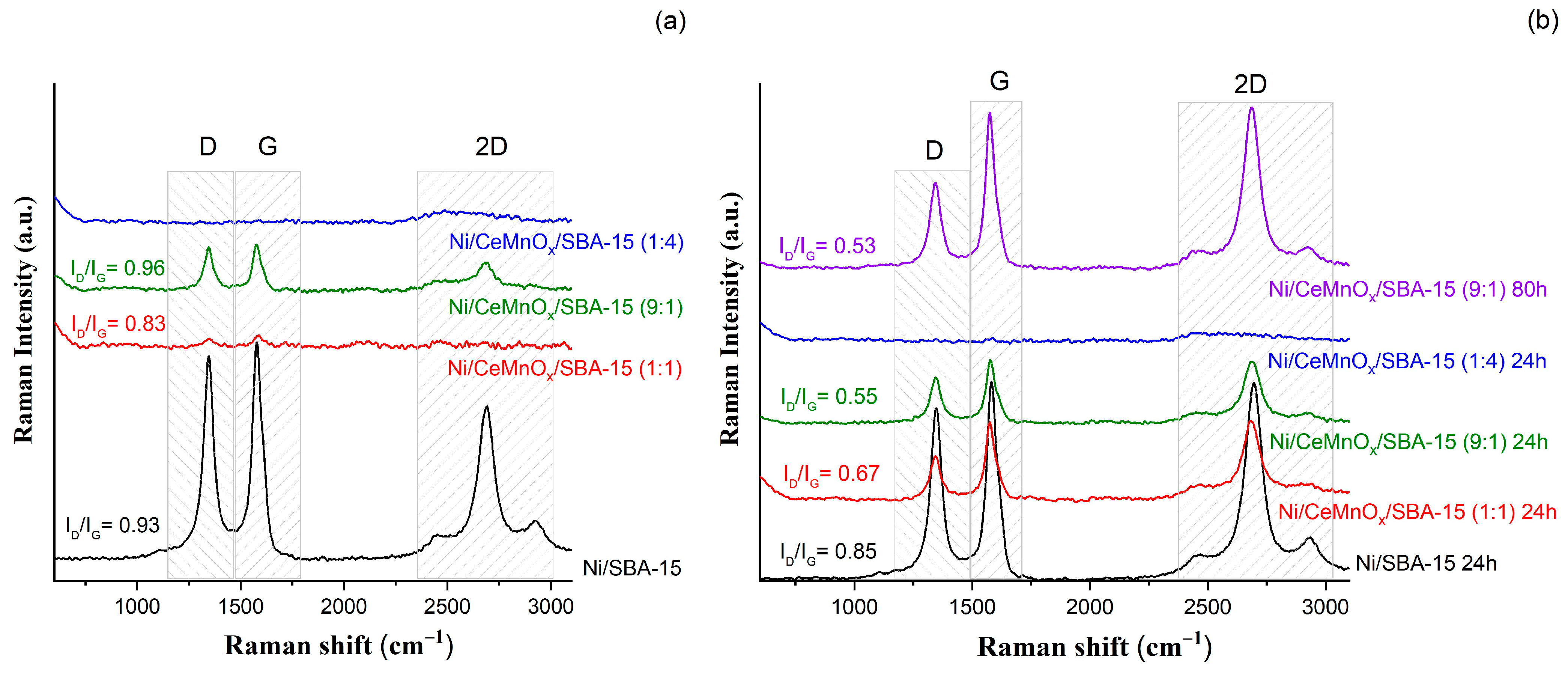
3.4.3. Raman Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aresta, M.; Dibenedetto, A. The Carbon Dioxide Revolution Challenges and Perspectives for a Global Society, 1st ed.; Springer Nature: Switzerland, 2021; p. 277. [Google Scholar]
- Salaev, M.A.; Liotta, L.F.; Vodyankina, O.V. Lanthanoid-containing Ni-based catalysts for dry reforming of methane: A review. Int. J. Hydrogen Energy 2022, 47, 4489–4535. [Google Scholar] [CrossRef]
- Goula, M.A.; Charisiou, N.D.; Siakavelas, G.; Tzounis, L.; Tsiaoussis, I.; Panagiotopoulou, P.; Goula, G.; Yentekakis, I.V. Syngas production via the biogas dry reforming reaction over Ni supported on zirconia modified with CeO2 or La2O3 catalysts. Int. J. Hydrogen Energy 2017, 42, 13724–13740. [Google Scholar] [CrossRef]
- Lavoie, J.M. Review on dry reforming of methane, a potentially more environmentally-friendly approach to the increasing natural gas exploitation. Front. Chem. 2014, 2, 81. [Google Scholar] [CrossRef]
- de Lasa, H.; Salaices, E.; Mazumder, J.; Lucky, R. Catalytic Steam Gasification of Biomass: Catalysts, Thermodynamics and Kinetics. Chem. Rev. 2011, 111, 5404–5433. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, T.; Shi, Y.; Zhao, B.; Wang, M.; Liu, Q.; Wang, J.; Long, K.; Duan, Y.; Ning, P. A sintering and carbon-resistant Ni-SBA-15 catalyst prepared by solid-state grinding method for dry reforming of methane. J. CO2 Util. 2017, 17, 10–19. [Google Scholar] [CrossRef]
- Taherian, Z.; Khataee, A.; Han, N.; Orooji, Y. Hydrogen production through methane reforming processes using promoted-Ni/mesoporous silica: A review. J. Ind. Eng. Chem. 2022, 107, 20–30. [Google Scholar] [CrossRef]
- Abdulrasheed, A.A.; Jalil, A.A.; Hamid, M.Y.S.; Siang, T.J.; Fatah, N.A.A.; Izan, S.M.; Hassan, N.S. Dry reforming of methane to hydrogen-rich syngas over robust fibrous KCC-1 stabilized nickel catalyst with high activity and coke resistance. Int. J. Hydrogen Energy 2020, 45, 18549–18561. [Google Scholar] [CrossRef]
- Palanichamy, K.; Umasankar, S.; Ganesh, S.; Sasirekha, N. Highly coke resistant Ni–Co/KCC-1 catalysts for dry reforming of methane. Int. J. Hydrogen Energy 2023, 48, 11727–11745. [Google Scholar] [CrossRef]
- Xu, S.; Slater, T.J.A.; Huang, H.; Zhou, Y.; Jiao, Y.; Parlett, C.M.A.; Guan, S.; Chansai, S.; Xue, S.; Wang, X.; et al. Developing silicalite-1 encapsulated Ni nanoparticles as sintering-/coking-resistant catalysts for dry reforming of methane. J. Chem. Eng. 2022, 446, 137439. [Google Scholar] [CrossRef]
- Bukhari, S.N.; Chin, C.Y.; Setiabudi, H.D.; Vo, D.-V.N. Tailoring the properties and catalytic activities of Ni/SBA-15 via different TEOS/P123 mass ratios for CO2 reforming of CH4. J. Environ. Chem. Eng. 2017, 5, 3122–3128. [Google Scholar] [CrossRef]
- Abdullah, N.; Ainirazali, N.; Chong, C.C.; Razak, H.A.; Setiabudi, H.D.; Jalil, A.A.; Vo, D.-V.N. Influence of impregnation assisted methods of Ni/SBA-15 for production of hydrogen via dry reforming of methane. Int. J. Hydrogen Energy 2019, 45, 18426–18439. [Google Scholar] [CrossRef]
- Abdullah, N.; Ainirazali, N.; Chong, C.C.; Razak, H.A.; Setiabudi, H.D.; Chin, S.Y.; Jalil, A.A. Effect of Ni loading on SBA-15 synthesized from palm oil fuel ash waste for hydrogen production via CH4 dry reforming. Int. J. Hydrogen Energy 2019, 45, 18411–18425. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, R.; Setiabudi, H.D.; Nanda, S.; Vo, D.V.N. Advanced synthesis strategies of mesoporous SBA-15 supported catalysts for catalytic reforming applications: A state-of-the-art review. Appl. Catal. A Gen. 2018, 559, 57–74. [Google Scholar] [CrossRef]
- Kang, D.; Lim, H.S.; Lee, J.W. Enhanced catalytic activity of methane dry reforming by the confinement of Ni nanoparticles into mesoporous silica. Int. J. Hydrogen Energy 2017, 42, 11270–11282. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H.; Ye, H.; Hu, R.; Yang, S.; Tang, G.; Li, K.; Yang, Y. Vacuum thermal treated Ni-CeO2/SBA-15 catalyst for CO2 methanation. Nanomaterials 2018, 8, 759. [Google Scholar] [CrossRef]
- Li, D.; Zeng, L.; Li, X.; Wang, X.; Ma, H.; Assabumrungrat, S.; Gong, J. Ceria-promoted Ni/SBA-15 catalysts for ethanol steam reforming with enhanced activity and resistance to deactivation. Appl. Catal. B Environ. 2015, 176–177, 532–541. [Google Scholar] [CrossRef]
- Li, J.F.; Xia, C.; Au, C.T.; Liu, B.S. Y2O3-promoted NiO/SBA-15 catalysts highly active for CO2/CH4 reforming. Int. J. Hydrogen Energy 2014, 39, 10927–10940. [Google Scholar] [CrossRef]
- Sun, C.; Świrk, K.; Wang, Y.; Scheidl, K.S.; Breiby, D.W.; Rønning, M.; Hu, C.; Da Costa, P. Tailoring the yttrium content in Ni-Ce-Y/SBA-15 mesoporous silicas for CO2 methanation. Catal. Today 2021, 382, 104–119. [Google Scholar] [CrossRef]
- Oemar, U.; Kathiraser, Y.; Mo, L.; Hoa, X.K.; Kawi, S. CO2 reforming of methane over highly active La-promoted Ni supported on SBA-15 catalysts: Mechanism and kinetic modelling. Catal. Sci. Technol. 2016, 6, 1173–1186. [Google Scholar] [CrossRef]
- Dai, Y.M.; Lu, C.Y.; Chang, C.J. Catalytic activity of mesoporous Ni/CNT, Ni/SBA-15 and (Cu, Ca, Mg, Mn, Co)–Ni/SBA-15 catalysts for CO2 reforming of CH4. RSC Adv. 2016, 6, 73887–73896. [Google Scholar] [CrossRef]
- Yao, L.; Wang, Y.; Shi, J.; Xu, H.; Shen, W.; Hu, C. The influence of reduction temperature on the performance of ZrOx/Ni-MnOx/SiO2 catalyst for low-temperature CO2 reforming of methane. Catal. Today 2017, 281, 259–267. [Google Scholar] [CrossRef]
- Grabchenko, M.V.; Dorofeeva, N.V.; Lapin, I.N.; Parola, V.L.; Liotta, L.F.; Vodyankina, O.V. Study of Nickel Catalysts Supported on MnOx–CeO2 Mixed Oxides in Dry Reforming of Methane. Kinet. Catal. 2021, 62, 765–777. [Google Scholar] [CrossRef]
- Mikheeva, N.N.; Zaikovskii, V.I.; Larichev, Y.V.; Mamontov, G.V. Toluene abatement on Ag-CeO2/SBA-15 catalysts: Synergistic effect of silver and ceria. Mater. Today Chem. 2021, 21, 100530. [Google Scholar] [CrossRef]
- Larichev, Y.V.; Tuzikov, F.V. Advances in small-angle X-ray scattering for the study of supported catalysts. J. Appl. Cryst. 2013, 46, 752–757. [Google Scholar] [CrossRef]
- Konarev, P.V.; Petoukhov, M.V.; Volkov, V.V.; Svergun, D.I. ATSAS 2.1, a program package for small angle scattering data analysis. J. Appl. Cryst. 2006, 39, 277–286. [Google Scholar] [CrossRef]
- Mikheeva, N.N.; Zaikovskii, V.I.; Mamontov, G.V. Synthesis of ceria nanoparticles in pores of SBA-15: Pore size effect and influence of citric acid addition. Microporous Mesoporous Mater. 2019, 277, 10–16. [Google Scholar] [CrossRef]
- Chen, L.F.; Arellano, U.; Wang, J.A.; Balcazar, L.M.; Sotelo, R.; Solis, S.; Azomosa, M.; Gonzalez, J.; González Vargas, O.A.; Song, Y.; et al. Oxygen defect, electron transfer and photocatalytic activity of Ag/CeO2/SBA-15 hybrid catalysts. Catal. Today 2022, 394–396, 62–80. [Google Scholar] [CrossRef]
- Khan, M.M.; Ansari, S.A.; Lee, J.-H.; Omaish Ansari, M.; Lee, J.; Cho, M.H. Electrochemically active biofilm assisted synthesis of Ag@CeO2 nanocomposites for antimicrobial activity, photocatalysis and photoelectrodes. J. Colloid Interface Sci. 2014, 431, 255–263. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, B.; Jiang, S.; Bai, H.; Zhang, S. Use of CeO2 Nanoparticles to Enhance UV-Shielding of Transparent Regenerated Cellulose Films. Polymers 2019, 11, 458. [Google Scholar] [CrossRef]
- Ho, C.; Yu, J.C.; Kwong, T.; Mak, A.C.; Lai, S. Morphology-Controllable Synthesis of Mesoporous CeO2 Nano- and Microstructures. Chem. Mater. 2005, 17, 4514–4522. [Google Scholar] [CrossRef]
- Ansari, A.A.; Labis, J.P.; Alam, M.; Ramay, S.; Ahmad, N.; Mahmood, A. Synthesis, Structural and Optical Properties of Mn-Doped Ceria Nanoparticles: A Promising Catalytic Material. Acta Metall. Sin. 2016, 29, 265–273. [Google Scholar] [CrossRef]
- Saranya, J.; Ranjith, K.S.; Saravanan, P.; Mangalaraj, D.; Kumar, R.T.R. Cobalt-doped cerium oxide nanoparticles: Enhanced photocatalytic activity under UV and visible light irradiation. Mater. Sci. Semicond. Process. 2014, 26, 218–224. [Google Scholar] [CrossRef]
- Cestaro, R.; Schweizer, P.; Philippe, L.; Maeder, X.; Serra, A. Phase and microstructure control of electrodeposited Manganese Oxide with enhanced optical properties. Appl. Surf. Sci. 2022, 580, 152289. [Google Scholar] [CrossRef]
- Mavuso, M.A.; Makgwane, P.R.; Ray, S.S. Heterostructured CeO2–M (M = Co, Cu, Mn, Fe, Ni) Oxide Nanocatalysts for the Visible-Light Photooxidation of Pinene to Aroma Oxygenates. ACS Omega 2020, 5, 9775–9788. [Google Scholar] [CrossRef]
- Lisboa, J.D.S.; Santos, D.C.T.M.; Passos, F.B.; Noronha, F.B. Influence of the addition of promoters to steam reforming catalysts. Catal. Today 2005, 101, 15–21. [Google Scholar] [CrossRef]
- Lisboa, J.S.; Terra, L.E.; Silva, P.R.J.; Saitovitch, H.; Passos, F.B. Investigation of Ni/Ce–ZrO2 catalysts in the autothermal reforming of methane. Fuel Process. Technol. 2011, 92, 2075–2082. [Google Scholar] [CrossRef]
- Han, Y.-F.; Chen, F.; Zhong, Z.; Ramesh, K.; Chen, L.; Widjaja, E. Controlled Synthesis, Characterization, and Catalytic Properties of Mn2O3 and Mn3O4 Nanoparticles Supported on Mesoporous Silica SBA-15. J. Phys. Chem. B 2006, 110, 24450–24456. [Google Scholar] [CrossRef]
- Loridant, S. Raman spectroscopy as a powerful tool to characterize ceria-based catalysts. Catal. Today 2021, 373, 98–111. [Google Scholar] [CrossRef]
- Grabchenko, M.V.; Mamontov, G.V.; Zaikovskii, V.I.; La Parola, V.; Liotta, L.F.; Vodyankina, O.V. Design of Ag-CeO2/SiO2 catalyst for oxidative dehydrogenation of ethanol: Control of Ag–CeO2 interfacial interaction. Catal. Today 2019, 333, 2–9. [Google Scholar] [CrossRef]
- Guo, D.; Lu, Y.; Ruan, Y.; Zhao, Y.; Zhao, Y.; Wang, S.; Ma, X. Effects of extrinsic defects originating from the interfacial reaction of CeO2-x-nickel silicate on catalytic performance in methane dry reforming. Appl. Catal. B Environ. 2020, 277, 119278. [Google Scholar] [CrossRef]
- Taratayko, A.; Larichev, Y.; Zaikovskii, V.; Mikheeva, N.; Mamontov, G. Ag–CeO2/SBA-15 composite prepared from Pluronic P123@SBA-15 hybrid as catalyst for room-temperature reduction of 4-nitrophenol. Catal. Today 2021, 375, 576–584. [Google Scholar] [CrossRef]
- Li, H.; Ren, J.; Qin, X.; Qin, Z.; Lin, J.; Li, Z. Ni/SBA-15 catalysts for CO methanation: Effects of V, Ce, and Zr promoters. RSC Adv. 2015, 5, 96504–96517. [Google Scholar] [CrossRef]
- Pudukudy, M.; Yaakob, Z.; Akmal, Z.S. Direct decomposition of methane over Pd promoted Ni/SBA-15 catalysts. Appl. Surf. Sci. 2015, 353, 127–136. [Google Scholar] [CrossRef]
- Yang, J.; Gong, D.; Lu, X.; Han, C.; Liu, H.; Wanget, L. Ni-CeO2/SBA-15 Catalyst Prepared by Glycine-Assisted Impregnation Method for Low-Temperature Dry Reforming of Methane. Crystals 2022, 12, 713. [Google Scholar] [CrossRef]
- Dorofeeva, N.V.; Kharlamova, T.S.; La Parola, V.; Liotta, L.F.; Vodyankina, O.V. Dry Reforming of Methane on Ni-Containing La2O3 and La2O3–Mn2O3 Catalysts: Effect of the Preparation Method. Dokl. Phys. Chem. 2022, 505, 95–107. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Dalai, A.K. Development of stable bimetallic catalysts for carbon dioxide reforming of methane. J. Catal. 2007, 249, 300–310. [Google Scholar] [CrossRef]
- Touahra, F.; Sehailia, M.; Halliche, D.; Bachari, K.; Saadi, A.; Cherifi, O. (MnO/Mn3O4)-NiAl nanoparticles as smart carbon resistant catalysts for the production of syngas by means of CO2 reforming of methane: Advocating the role of concurrent carbothermic redox looping in the elimination of coke. Int. J. Hydrogen Energy 2016, 41, 21140–21156. [Google Scholar] [CrossRef]
- Yao, L.; Zhu, J.; Peng, X.; Tong, D.; Hu, C. Comparative study on the promotion effect of Mn and Zr on the stability of Ni/SiO2 catalyst for CO2 reforming of methane. Int. J. Hydrogen Energy 2013, 38, 7268–7279. [Google Scholar] [CrossRef]
- Seok, S.H.; Han, S.H.; Lee, J.S. The role of MnO in Ni/MnO-Al2O3 catalysts for carbon dioxide reforming of methane. Appl. Catal. A Gen. 2001, 215, 31–38. [Google Scholar] [CrossRef]
- Omoregbe, O.; Danh, H.T.; Nguyen-Huy, C.; Setiabudi, H.D.; Abidin, S.Z.; Truong, Q.D.; Vo, D.V.N. Syngas production from methane dry reforming over Ni/SBA-15 catalyst: Effect of operating parameters. Int. J. Hydrogen Energy 2017, 42, 11283–11294. [Google Scholar] [CrossRef]
- Szybowicz, M.; Nowicka, A.B.; Dychalska, A. Chapter 1—Characterization of Carbon Nanomaterials by Raman Spectroscopy. In Characterization of Nanomaterials; Woodhead Publishing: Cambridge, UK, 2018; pp. 1–36. [Google Scholar] [CrossRef]




| Samples | Content, wt.% | Ce/Mn, mol.% | ||
|---|---|---|---|---|
| MnO2 | CeO2 | NiO | ||
| Ni/SBA-15 | - | - | 10.3 | |
| Ni/CeMnOx/SBA-15 (1:1) | 4.7 | 8.6 | 10.6 | 0.93 |
| Ni/CeMnOx/SBA-15 (9:1) | 1.1 | 16.8 | 11.6 | 9.3 |
| Ni/CeMnOx/SBA-15 (1:4) | 7.7 | 3.6 | 12.5 | 0.24 |
| Sample | SBET, m2/g | Vpore, cm3/g | Pore Size, nm | d(100) 1, nm | a 2, nm | D(NiO) 3, nm | D(Ni) 3, nm |
|---|---|---|---|---|---|---|---|
| SBA-15 | 763 | 0.98 | 6.1 | 9.28 | 10.72 | - | - |
| CeMnOx/SBA-15 (1:1) | 547 | 0.75 | 6.2 | 9.07 | 10.47 | - | - |
| CeMnOx/SBA-15 (9:1) | 525 | 0.66 | 6.1 | 9.11 | 10.52 | - | - |
| CeMnOx/SBA-15 (1:4) | 565 | 0.80 | 6.1 | 9.13 | 10.54 | - | |
| Ni/SBA-15 | 494 | 0.70 | 5.8 | 8.85 | 10.22 | 18 | 19 |
| Ni/CeMnOx/SBA-15 (1:1) | 318 | 0.47 | 5.7/5.0 | 8.87 | 10.24 | 10 | 17 |
| Ni/CeMnOx/SBA-15 (9:1) | 294 | 0.41 | 5.7/4.8 | 8.76 | 10.12 | 8 | 16 |
| Ni/CeMnOx/SBA-15 (1:4) | 363 | 0.57 | 5.6 | 8.86 | 10.23 | 9 | 13 |
| Sample | Tmax (°C) | Reactions | Experimental H2 Consumptions (mmol/g) * | Theoretical H2 Consumptions (mmol/g) ** |
|---|---|---|---|---|
| CeMnOx/SBA-15 (9:1) | 240–750 | Ce4+ → Ce3+ | 0.47 | ― |
| CeMnOx/SBA-15 (1:1) | 290–525 | Mn4+/Mn3+ → Mn2+ | 0.38 | ― |
| 240–750 | Ce4+ → Ce3+ | |||
| CeMnOx/SBA-15 (1:4) | 280–570 | Ce4+ → Ce3+ | 0.46 | ― |
| Ni/SBA-15 | 390–730 | Ni2+ → Ni0 | 1.41 | 1.77 |
| Ni/CeMnOx/SBA-15 (9:1) | 230–380 | Ni2+ → Ni0 | 1.54 | 1.55 |
| Ni/CeMnOx/SBA-15 (1:1) | 290–410 | Ni2+ → Ni0 | 1.79 | 1.51 |
| Mn4+/Mn3+ → Mn2+ | ||||
| 410–480 | Ni2+ → Ni0 | |||
| Mn3+ → Mn2+ | ||||
| 480–817 | Ni2+ → Ni0 | |||
| Ni/CeMnOx/SBA-15 (1:4) | 300–380 | Ni2+ → Ni0 | 1.61 | 1.67 |
| Mn4+/Mn3+ → Mn2+ | ||||
| 380–500 | Ni2+ → Ni0 | |||
| Mn3+ → Mn2+ | ||||
| 500–800 | Ni2+ → Ni0 |
| Catalytic Performance | Catalysts | ||||
|---|---|---|---|---|---|
| Ni/SBA-15 | Ni/CeMnOx/SBA-15 (9:1) | Ni/CeMnOx/SBA-15 (1:1) | Ni/CeMnOx/SBA-15 (1:4) | ||
| Conversion (X, %) and H2/CO at 650 °C in gradient temperature test | X(CH4) | 32 | 40 | 29 | 8 |
| X(CO2) | 55 | 55 | 52 | 17 | |
| H2/CO | 0.70 | 0.72 | 0.73 | 1.2 | |
| Conversion (X, %) and H2/CO at 650 °C in stability test | X(CH4) 1 h | 66 | 51 | 26 | 7 |
| X(CO2) 1 h | 80 | 69 | 50 | 27 | |
| H2/CO 1 h | 0.72 | 0.69 | 0.86 | 1.64 | |
| X(CH4) 24 h | 47 | 42 | 24 | 7 | |
| X(CO2) 24 h | 68 | 60 | 50 | 21 | |
| H2/CO 24 h | 0.71 | 0.70 | 0.91 | 1.59 | |
| C weight loss, % | 42.2 | 2.7 | - | - | |
| Ni0 particle size (nm) (after g/s tests) | 19/17 | 15/12 | 16/10 | 18/21 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabchenko, M.V.; Dorofeeva, N.V.; Svetlichnyi, V.A.; Larichev, Y.V.; La Parola, V.; Liotta, L.F.; Kulinich, S.A.; Vodyankina, O.V. Ni-Based SBA-15 Catalysts Modified with CeMnOx for CO2 Valorization via Dry Reforming of Methane: Effect of Composition on Modulating Activity and H2/CO Ratio. Nanomaterials 2023, 13, 2641. https://doi.org/10.3390/nano13192641
Grabchenko MV, Dorofeeva NV, Svetlichnyi VA, Larichev YV, La Parola V, Liotta LF, Kulinich SA, Vodyankina OV. Ni-Based SBA-15 Catalysts Modified with CeMnOx for CO2 Valorization via Dry Reforming of Methane: Effect of Composition on Modulating Activity and H2/CO Ratio. Nanomaterials. 2023; 13(19):2641. https://doi.org/10.3390/nano13192641
Chicago/Turabian StyleGrabchenko, Maria V., Natalia V. Dorofeeva, Valery A. Svetlichnyi, Yurii V. Larichev, Valeria La Parola, Leonarda Francesca Liotta, Sergei A. Kulinich, and Olga V. Vodyankina. 2023. "Ni-Based SBA-15 Catalysts Modified with CeMnOx for CO2 Valorization via Dry Reforming of Methane: Effect of Composition on Modulating Activity and H2/CO Ratio" Nanomaterials 13, no. 19: 2641. https://doi.org/10.3390/nano13192641
APA StyleGrabchenko, M. V., Dorofeeva, N. V., Svetlichnyi, V. A., Larichev, Y. V., La Parola, V., Liotta, L. F., Kulinich, S. A., & Vodyankina, O. V. (2023). Ni-Based SBA-15 Catalysts Modified with CeMnOx for CO2 Valorization via Dry Reforming of Methane: Effect of Composition on Modulating Activity and H2/CO Ratio. Nanomaterials, 13(19), 2641. https://doi.org/10.3390/nano13192641












