Synthesis and Characterization of Environmentally Friendly Chitosan–Arabic Gum Nanoparticles for Encapsulation of Oregano Essential Oil in Pickering Emulsion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Methods
2.2.1. Synthesis of Nanoparticles, Preparation, and Complex Coacervation
2.2.2. Size and Zeta Potential
2.2.3. Interfacial Tension and Contact Angles
2.2.4. Biodegradability Test
2.2.5. Encapsulation
2.2.6. Formation of the Capsules: Size and Morphology
2.2.7. OEO Encapsulation Efficiency
3. Results and Discussion
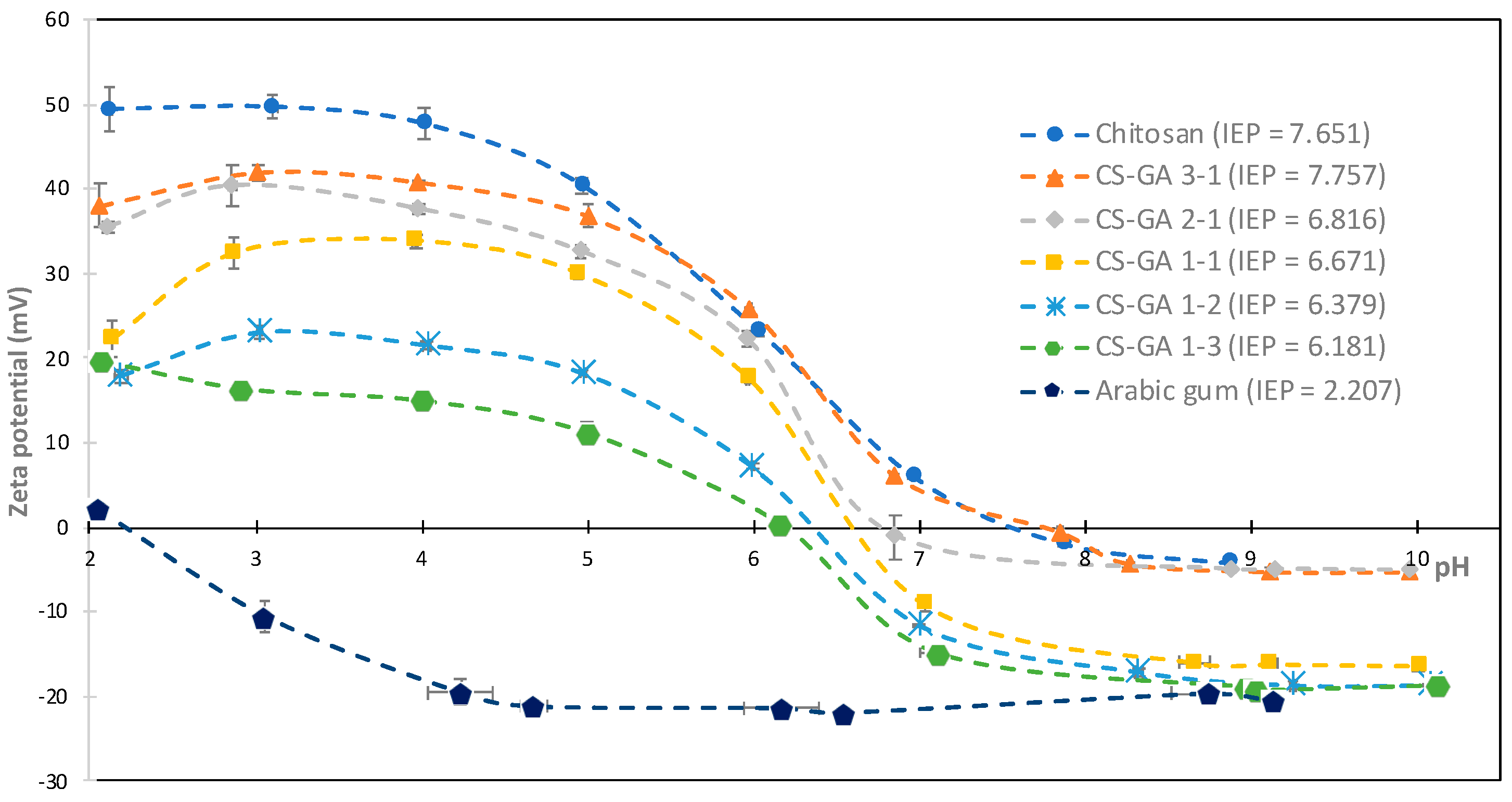
3.1. Formation of CS–GA Nanoparticles and Zeta Potential
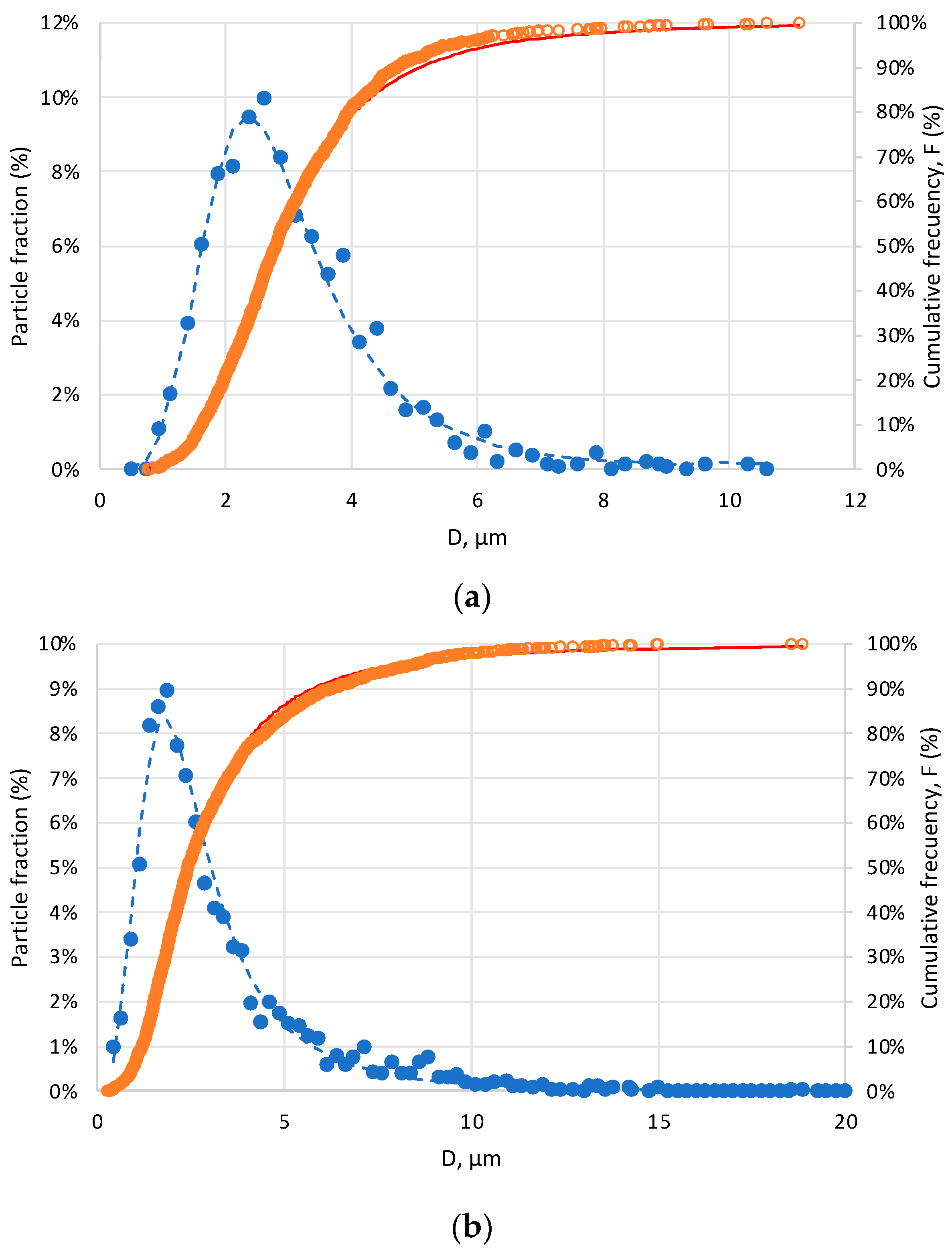
3.2. Particle Size
3.3. Contact Angle
3.4. Interfacial Tension
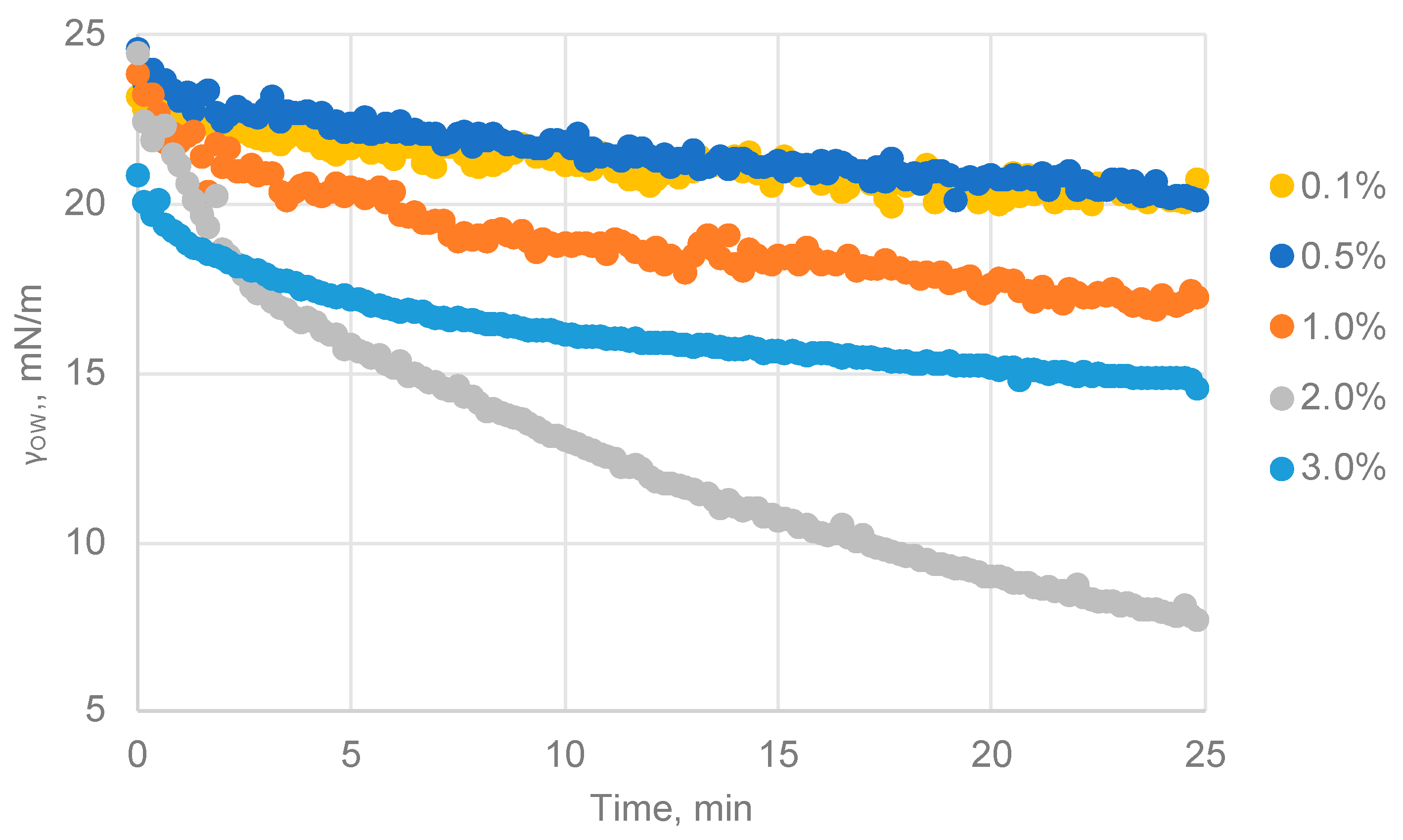
3.4.1. Influence of Nanoparticle Concentrations
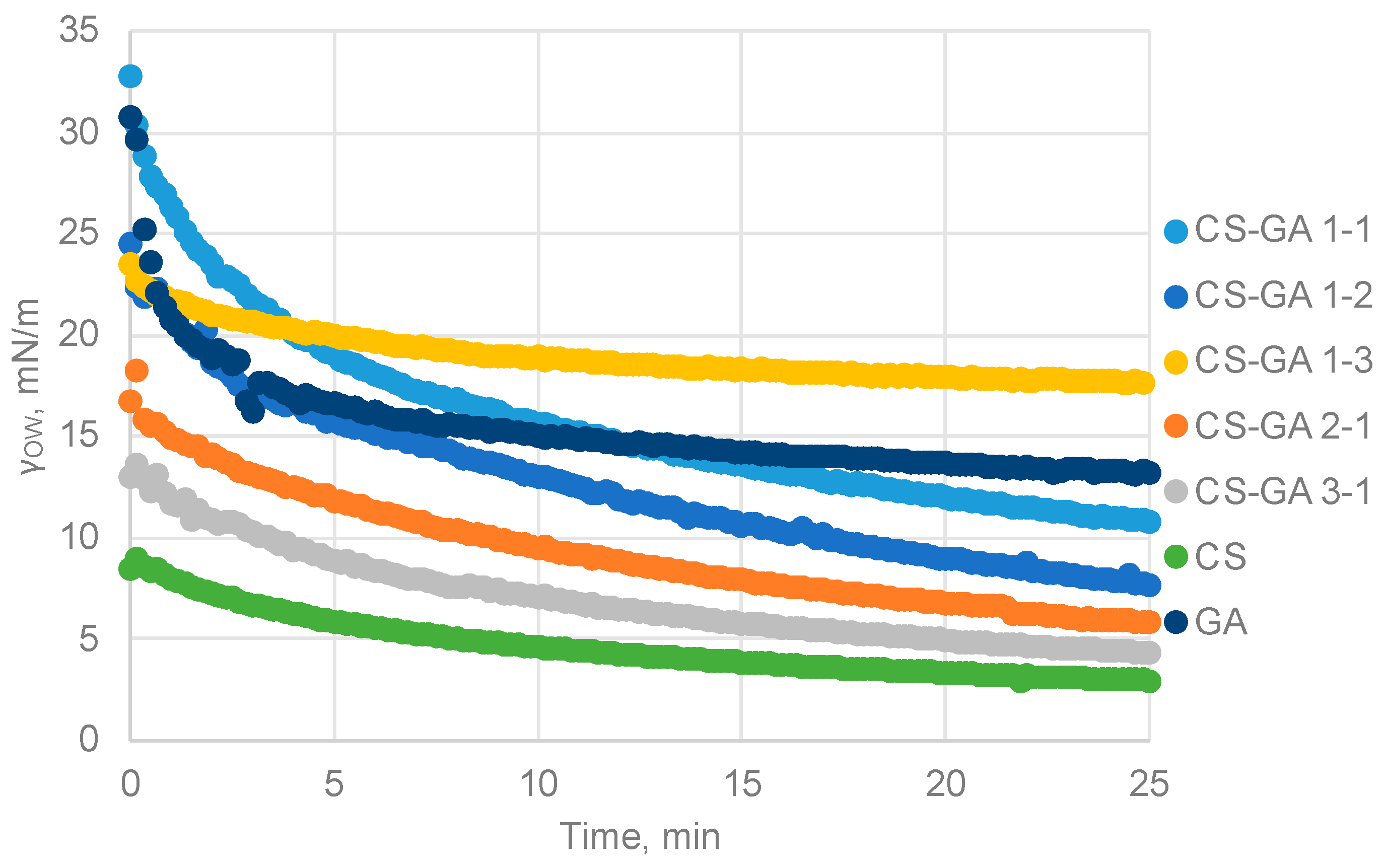
3.4.2. Influence of the Polymer Ratio
3.5. Biodegradability Test
3.6. Encapsulation
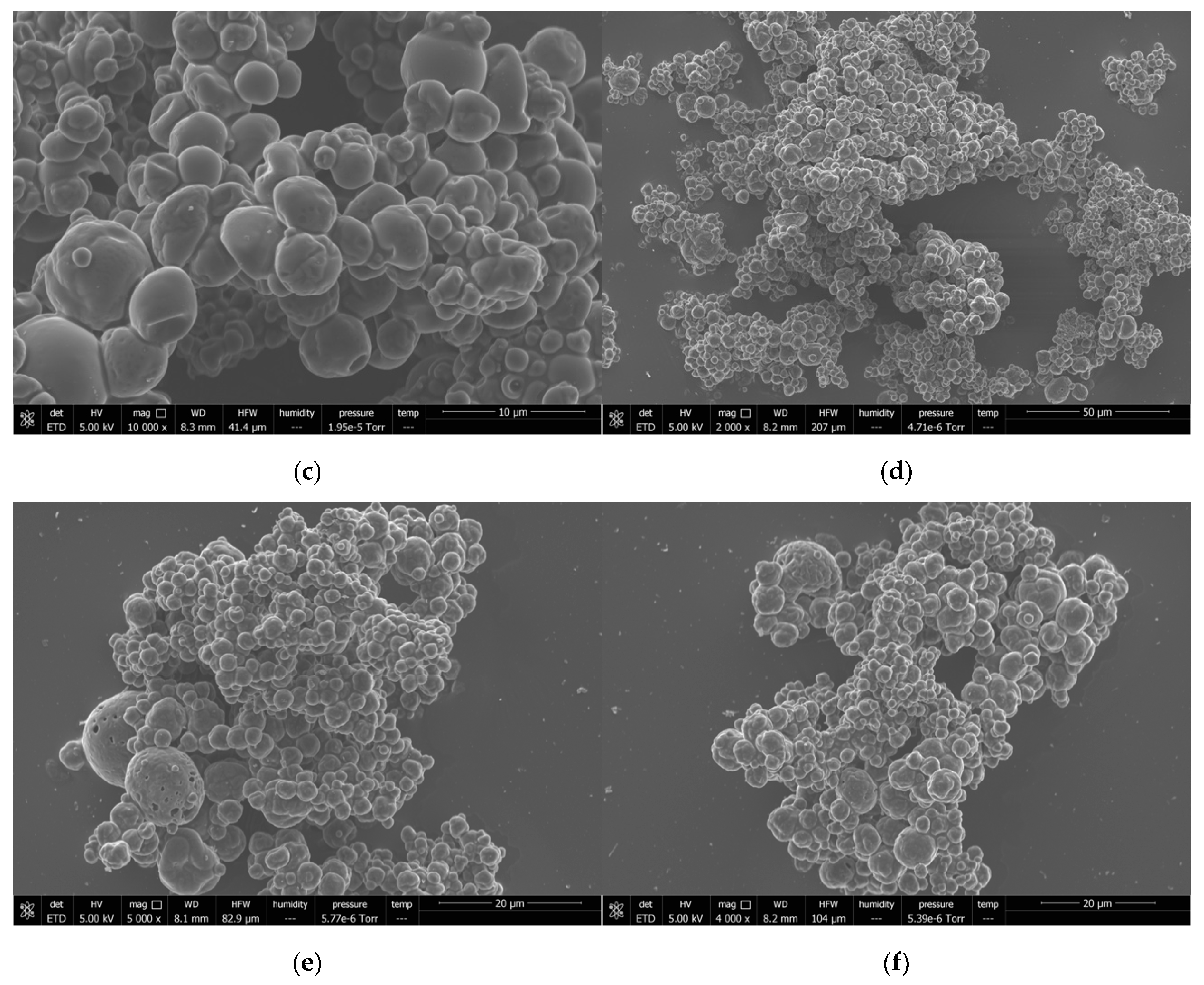
3.6.1. Characterization of Formed Capsules
3.6.2. OEO Encapsulation Efficiency
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shi, Y.; Li, C.; Zhang, L.; Huang, T.; Ma, D.; Tu, Z.C.; Wang, H.; Xie, H.; Zhang, N.H.; Ouyang, B.L. Characterization and Emulsifying Properties of Octenyl Succinate Anhydride Modified Acacia Seyal Gum (Gum Arabic). Food Hydrocoll. 2017, 65, 10–16. [Google Scholar] [CrossRef]
- Vasile, F.E.; Martinez, M.J.; Pizones Ruiz-Henestrosa, V.M.; Judis, M.A.; Mazzobre, M.F. Physicochemical, Interfacial and Emulsifying Properties of a Non-Conventional Exudate Gum (Prosopis Alba) in Comparison with Gum Arabic. Food Hydrocoll. 2016, 56, 245–253. [Google Scholar] [CrossRef]
- Golkar, A.; Taghavi, S.M.; Dehnavi, F.A. The Emulsifying Properties of Persian Gum (Amygdalus Scoparia Spach) as Compared with Gum Arabic. Int. J. Food Prop. 2018, 21, 416–436. [Google Scholar] [CrossRef]
- Bai, L.; Huan, S.; Li, Z.; McClements, D.J. Comparison of Emulsifying Properties of Food-Grade Polysaccharides in Oil-in-Water Emulsions: Gum Arabic, Beet Pectin, and Corn Fiber Gum. Food Hydrocoll. 2017, 66, 144–153. [Google Scholar] [CrossRef]
- Yanat, M.; Schroën, K. Preparation Methods and Applications of Chitosan Nanoparticles; with an Outlook toward Reinforcement of Biodegradable Packaging. React. Funct. Polym. 2021, 161, 104849. [Google Scholar] [CrossRef]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar. Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Tavares, L.; Barros, H.L.B.; Vaghetti, J.C.P.; Noreña, C.P.Z. Microencapsulation of Garlic Extract by Complex Coacervation Using Whey Protein Isolate/Chitosan and Gum Arabic/Chitosan as Wall Materials: Influence of Anionic Biopolymers on the Physicochemical and Structural Properties of Microparticles. Food Bioprocess Technol. 2019, 12, 2093–2106. [Google Scholar] [CrossRef]
- Ding, S.; Wang, Y.; Li, J.; Chen, S. Progress and Prospects in Chitosan Derivatives: Modification Strategies and Medical Applications. J. Mater. Sci. Technol. 2021, 89, 209–224. [Google Scholar] [CrossRef]
- Sharkawy, A.; Barreiro, M.F.; Rodrigues, A.E. Preparation of Chitosan/Gum Arabic Nanoparticles and Their Use as Novel Stabilizers in Oil/Water Pickering Emulsions. Carbohydr. Polym. 2019, 224, 115190. [Google Scholar] [CrossRef]
- Sharkawy, A.; Barreiro, F.; Rodrigues, A. Pickering Emulsions Stabilized with Chitosan/Gum Arabic Particles: Effect of Chitosan Degree of Deacetylation on the Physicochemical Properties and Cannabidiol (CBD) Topical Delivery. J. Mol. Liq. 2022, 355, 118993. [Google Scholar] [CrossRef]
- Fernández-Serrano, M.; Routh, A.F.; Rios, F.; Caparros-Salvador, F.; Salih-Ortega, M.A. Calcium Alginate as a Novel Sealing Agent for Colloidosomes. Langmuir 2020, 36, 8398–8406. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Andrews, H.; Báez-González, J.G.; Cruz-Sosa, F.; Vernon-Carter, E.J. Gum Arabic−Chitosan Complex Coacervation. Biomacromolecules 2007, 8, 1313–1318. [Google Scholar] [CrossRef] [PubMed]
- Butstraen, C.; Salaün, F. Preparation of Microcapsules by Complex Coacervation of Gum Arabic and Chitosan. Carbohydr. Polym. 2014, 99, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Abreu, F.O.M.; Da Silva, N.A.; De Sousa Sipauba, M.; Pires, T.F.M.; Bomfim, T.A.; De Castro Monteiro, O.A.; De Camargo Forte, M.M. Chitosan and Gum Arabic Nanoparticles for Heavy Metal Adsorption. Polimeros 2018, 28, 231–238. [Google Scholar] [CrossRef]
- Slyusarenko, N.V.; Vasilyeva, N.Y.; Kazachenko, A.S.; Gerasimova, M.A.; Romanchenko, A.S.; Slyusareva, E.A. Synthesis and Properties of Interpolymer Complexes Based on Chitosan and Sulfated Arabinogalactan. Polym. Sci. Ser. B 2020, 62, 272–278. [Google Scholar] [CrossRef]
- Avadi, M.R.; Sadeghi, A.M.M.; Mohamadpour Dounighi, N.; Dinarvand, R.; Atyabi, F.; Rafiee-Tehrani, M. Ex Vivo Evaluation of Insulin Nanoparticles Using Chitosan and Arabic Gum. ISRN Pharm. 2011, 2011, 860109. [Google Scholar] [CrossRef]
- Ibekwe, C.A.; Oyatogun, G.M.; Esan, T.A.; Oluwasegun, K.M. Synthesis and Characterization of Chitosan/Gum Arabic Nanoparticles for Bone Regeneration. Am. J. Mater. Sci. Eng. 2017, 5, 28–36. [Google Scholar] [CrossRef]
- Cota-Arriola, O.; Onofre Cortez-Rocha, M.; Burgos-Hernández, A.; Marina Ezquerra-Brauer, J.; Plascencia-Jatomea, M. Controlled Release Matrices and Micro/Nanoparticles of Chitosan with Antimicrobial Potential: Development of New Strategies for Microbial Control in Agriculture. J. Sci. Food Agric. 2013, 93, 1525–1536. [Google Scholar] [CrossRef]
- Hernández-Téllez, C.N.; Plascencia-Jatomea, M.; Cortez-Rocha, M.O. Chitosan-Based Bionanocomposites: Development and Perspectives in Food and Agricultural Applications. In Chitosan in the Preservation of Agricultural Commodities, 1st ed.; Bautista-Baños, S., Romanazzi, G., Jiménez-Aparicio, A., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 315–338. [Google Scholar] [CrossRef]
- Raj, V.; Kim, Y.; Kim, Y.G.; Lee, J.H.; Lee, J. Chitosan-Gum Arabic Embedded Alizarin Nanocarriers Inhibit Biofilm Formation of Multispecies Microorganisms. Carbohydr. Polym. 2022, 284, 118959. [Google Scholar] [CrossRef]
- Tan, C.; Xie, J.; Zhang, X.; Cai, J.; Xia, S. Polysaccharide-Based Nanoparticles by Chitosan and Gum Arabic Polyelectrolyte Complexation as Carriers for Curcumin. Food Hidrocoll. 2016, 57, 236–245. [Google Scholar] [CrossRef]
- Rajabi, H.; Jafari, S.M.; Rajabzadeh, G.; Sarfarazi, M.; Sedaghati, S. Chitosan-Gum Arabic Complex Nanocarriers for Encapsulation of Saffron Bioactive Components. Colloids Surf. A Physicochem. Eng. 2019, 578, 123644. [Google Scholar] [CrossRef]
- De Oliveira, J.L.; Campos, E.V.R.; Pereira, A.E.S.; Nunes, L.E.S.; Da Silva, C.C.L.; Pasquoto, T.; Lima, R.; Smaniotto, G.; Polanczyk, R.A.; Fraceto, L.F. Geraniol Encapsulated in Chitosan/Gum Arabic Nanoparticles: A Promising System for Pest Management in Sustainable Agriculture. J. Agric. Food Chem. 2018, 66, 5325–5334. [Google Scholar] [CrossRef] [PubMed]
- Hasani, M.; Hasani, S. Nano-Encapsulation of Thyme Essential Oil in Chitosan-Arabic Gum System: Evaluation of Its Antioxidant and Antimicrobial Properties. Trends Phytochem. Res. 2018, 2, 75–82. [Google Scholar]
- Leyva-López, N.; Gutiérrez-Grijalva, E.P.; Vazquez-Olivo, G.; Heredia, J.B. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules 2017, 22, 989. [Google Scholar] [CrossRef] [PubMed]
- Gatti, L.; Troiano, F.; Vacchini, V.; Cappitelli, F.; Balloi, A. An In Vitro Evaluation of the Biocidal Effect of Oregano and Cloves’ Volatile Compounds against Microorganisms Colonizing an Oil Painting—A Pioneer Study. Appl. Sci. 2021, 11, 78. [Google Scholar] [CrossRef]
- Rhoades, J.; Gialagkolidou, K.; Gogou, M.; Mavridou, O.; Blatsiotis, N.; Ritzoulis, C.; Likotrafiti, E. Oregano Essential Oil as an Antimicrobial Additive to Detergent for Hand Washing and Food Contact Surface Cleaning. J. Appl. Microbiol. 2013, 115, 987–994. [Google Scholar] [CrossRef]
- Ríos, F.; Lechuga, M.; Lobato-Guarnido, I.; Fernández-Serrano, M. Antagonistic Toxic Effects of Surfactants Mixtures to Bacteria Pseudomonas putida and Marine Microalgae Phaeodactylum tricornutum. Toxics 2023, 11, 344. [Google Scholar] [CrossRef]
- OECD. OECD Guidelines for Testing Chemicals; Test No. 301: Ready Biodegradability; Adopted by the Council on 17 July 1992; OECD: Paris, France, 1992. [Google Scholar] [CrossRef]
- Bayat, H.; Rastgo, M.; Mansouri Zadeh, M.; Vereecken, H. Particle Size Distribution Models, Their Characteristics and Fitting Capability. J. Hydrol. 2015, 529, 872–889. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Zandi, M.; Rezaei, M.; Farahmandghavi, F. Two-Step Method for Encapsulation of Oregano Essential Oil in Chitosan Nanoparticles: Preparation, Characterization and in Vitro Release Study. Carbohydr. Polym. 2013, 95, 50–56. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 7, 603–632. [Google Scholar] [CrossRef]
- Bordi, F.; Chronopoulou, L.; Palocci, C.; Bomboi, F.; Di Martino, A.; Cifani, N.; Pompili, B.; Ascenzioni, F.; Sennato, S. Chitosan-DNA Complexes: Effect of Molecular Parameters on the Efficiency of Delivery. Colloids Surf. A Physicochem. Eng. 2014, 460, 184–190. [Google Scholar] [CrossRef]
- Jones, O.G.; McClements, D.J. Functional Biopolymer Particles: Design, Fabrication, and Applications. Compr. Rev. Food Sci. Food Saf. 2010, 9, 374–397. [Google Scholar] [CrossRef] [PubMed]
- Beyer, M.; Reichert, J.; Heurich, E.; Jandt, K.D.; Sigusch, B.W. Pectin, Alginate and Gum Arabic Polymers Reduce Citric Acid Erosion Effects on Human Enamel. Dent. Mater. J. 2010, 26, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, R.S.; Tavares, G.M.; Prata, A.S.; Hubinger, M.D. Complexation of Chitosan with Gum Arabic, Sodium Alginate and κ-Carrageenan: Effects of pH, Polymer Ratio and Salt Concentration. Carbohydr. Polym. 2019, 223, 115120. [Google Scholar] [CrossRef] [PubMed]
- Sabet, S.; Rashidinejad, A.; Melton, L.D.; Zujovic, Z.; Akbarinejad, A.; Nieuwoudt, M.; Seal, C.K.; McGillivray, D.J. The Interactions between the Two Negatively Charged Polysaccharides: Gum Arabic and Alginate. Food Hydrocoll. 2021, 112, 106343. [Google Scholar] [CrossRef]
- Weinbrreck, F.; Tromp, R.H.; de Kruif, C.G. Composition and Structure of Whey Protein/Gum Arabic Coacervates. Biomacromolecules 2004, 5, 1437–1445. [Google Scholar] [CrossRef]
- Gulão, E.d.S.; de Souza, C.J.F.; da Silva, F.A.S.; Coimbra, J.S.R.; Garcia-Rojas, E.E. Complex Coacervates Obtained from Lactoferrin and Gum Arabic: Formation and Characterization. Food Res. Int. 2014, 65, 367–374. [Google Scholar] [CrossRef]
- Yeh, H.Y.; Lin, J.C. Surface Characterization and in Vitro Platelet Compatibility Study of Surface Sulfonated Chitosan Membrane with Amino Group Protection–Deprotection Strategy. J. Biomater. Sci. Polym. Ed. 2008, 19, 291–310. [Google Scholar] [CrossRef]
- de Morais, W.A.; Silva, G.T.M.; Nunes, J.S.; Wanderley Neto, A.O.; Pereira, M.R.; Fonseca, J.L.C. Interpolyelectrolyte Complex Formation: From Lyophilic to Lyophobic Colloids. Colloids Surf. A Physicochem. Eng. 2016, 498, 112–120. [Google Scholar] [CrossRef]
- Aveyard, R.; Binks, B.P.; Clint, J.H. Emulsions Stabilised Solely by Colloidal Particles. Adv. Colloid Interface Sci. 2003, 100–102, 503–546. [Google Scholar] [CrossRef]
- Blute, I.; Pugh, R.J.; van de Pas, J.; Callaghan, I. Industrial Manufactured Silica Nanoparticle Sols. 2: Surface Tension, Particle Concentration, Foam Generation and Stability. Colloids Surf. A Physicochem. Eng. 2009, 337, 127–135. [Google Scholar] [CrossRef]
- Dave, P.N.; Gor, A. Natural Polysaccharide-Based Hydrogels and Nanomaterials: Recent Trends and Their Applications. In Handbook of Nanomaterials for Industrial Applications; Hussain, C.M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 36–66. [Google Scholar]
- Prasad, N.; Thombare, N.; Sharma, S.C.; Kumar, S. Gum Arabic—A Versatile Natural Gum: A Review on Production, Processing, Properties and Applications. Ind. Crops Prod. 2022, 187, 115304. [Google Scholar] [CrossRef]
- Ocak, B. Gum Arabic and Collagen Hydrolysate Extracted from Hide Fleshing Wastes as Novel Wall Materials for Microencapsulation of Origanum onites L. Essential Oil through Complex Coacervation. Environ. Sci. Pollut. Res. 2020, 27, 42727–42737. [Google Scholar] [CrossRef] [PubMed]
- Rahman Mazumder, M.A.; Ranganathan, T.V. Encapsulation of Isoflavone with Milk, Maltodextrin and Gum Acacia Improves Its Stability. Curr. Res. Nutr. Food Sci. 2020, 2, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Angelo, L.M.; Franca, D.; Faez, R. Biodegradation and viability of chitosan-based microencapsulated fertilizers. Carbohydr. Polym. 2021, 257, 117635. [Google Scholar] [CrossRef] [PubMed]
- Bayramoglu, E.E. Unique Biocide for the Leather Industry; Essential Oil of Oregano. J. Am. Leather Chem. Assoc. 2007, 102, 347–352. [Google Scholar]
- ELtai, N.O.; Salisbury, V.; Greenman, J. Efficacy of Oregano Oil as a Biocide Agent against Pathogens In Vitro, Using Lux Reporter Gene Technology. Afr. J. Microbiol. Res. 2015, 9, 2172–2182. [Google Scholar] [CrossRef]




| Polymer Ratio | Size, nm | Std. Dev., nm |
|---|---|---|
| CS | 123.7 | ±4.0 |
| 3–1 | 269.5 | ±5.6 |
| 2–1 | 256.2 | ±2.7 |
| 1–1 | 237.1 | ±3.8 |
| 1–2 | 257.4 | ±1.7 |
| 1–3 | 134.6 | ±4.5 |
| GA | 6.3 | ±0.6 |
| Polymer Ratio | γOW-MIN, mN/m | Std. Dev., mN/m |
|---|---|---|
| CS | 2.89 | ±0.14 |
| 3–1 | 4.39 | ±0.34 |
| 2–1 | 5.88 | ±0.29 |
| 1–1 | 10.82 | ±0.28 |
| 1–2 | 7.72 | ±0.17 |
| 1–3 | 17.68 | ±0.09 |
| GA | 13.13 | ±0.52 |
| %v OEO | N | M | Dg, µm |
|---|---|---|---|
| 0.5% | 3.51 | 1.41 | 2.45 |
| 10% | 2.47 | 1.45 | 2.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lobato-Guarnido, I.; Luzón, G.; Ríos, F.; Fernández-Serrano, M. Synthesis and Characterization of Environmentally Friendly Chitosan–Arabic Gum Nanoparticles for Encapsulation of Oregano Essential Oil in Pickering Emulsion. Nanomaterials 2023, 13, 2651. https://doi.org/10.3390/nano13192651
Lobato-Guarnido I, Luzón G, Ríos F, Fernández-Serrano M. Synthesis and Characterization of Environmentally Friendly Chitosan–Arabic Gum Nanoparticles for Encapsulation of Oregano Essential Oil in Pickering Emulsion. Nanomaterials. 2023; 13(19):2651. https://doi.org/10.3390/nano13192651
Chicago/Turabian StyleLobato-Guarnido, Ismael, Germán Luzón, Francisco Ríos, and Mercedes Fernández-Serrano. 2023. "Synthesis and Characterization of Environmentally Friendly Chitosan–Arabic Gum Nanoparticles for Encapsulation of Oregano Essential Oil in Pickering Emulsion" Nanomaterials 13, no. 19: 2651. https://doi.org/10.3390/nano13192651






