Multiscale Porous Carbon Materials by In Situ Growth of Metal–Organic Framework in the Micro-Channel of Delignified Wood for High-Performance Water Purification
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the TEMPO-Oxidized Wood
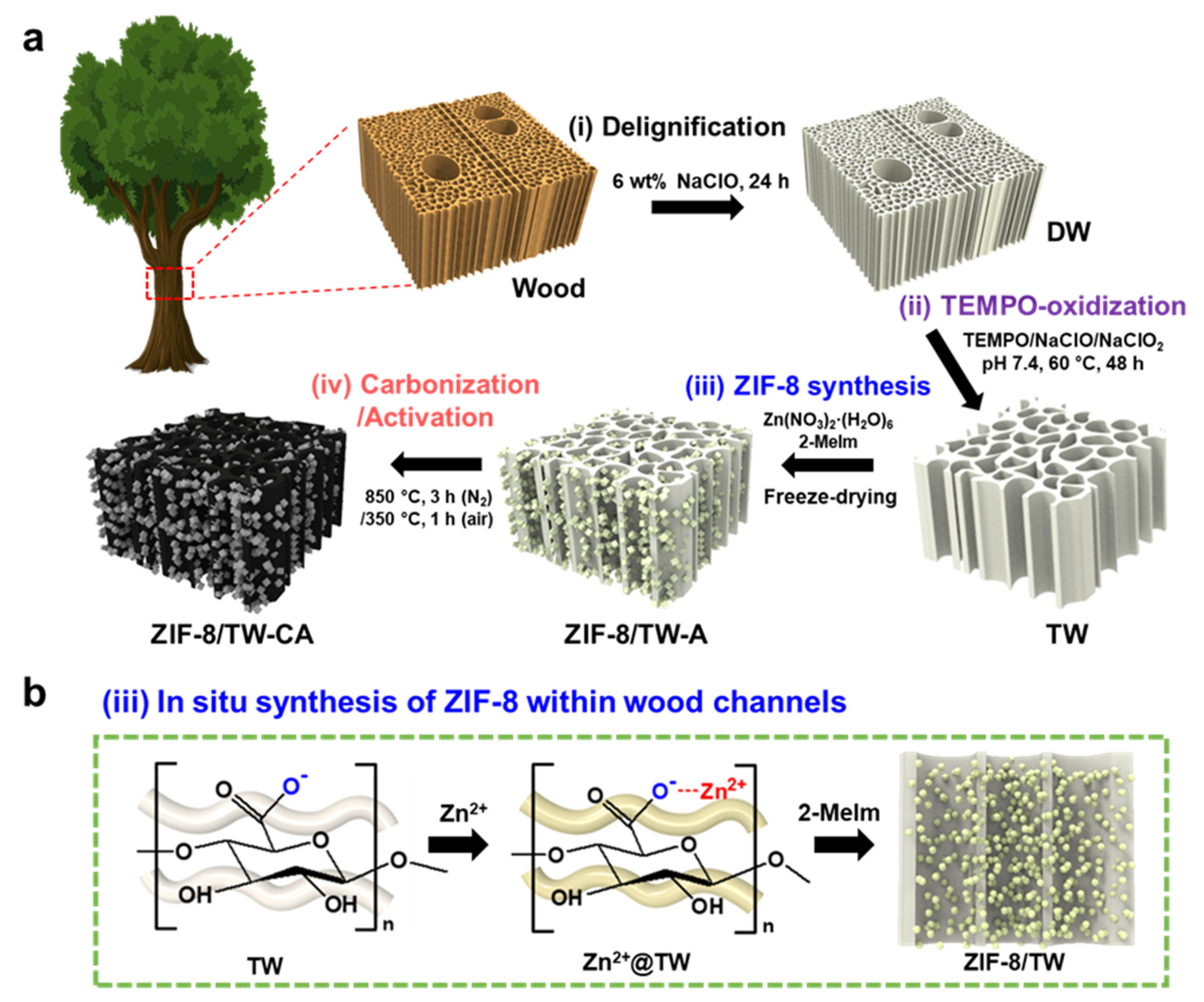
2.3. Preparation of ZIF-8/TW-A
2.4. Preparation of ZIF-8/TW-CA
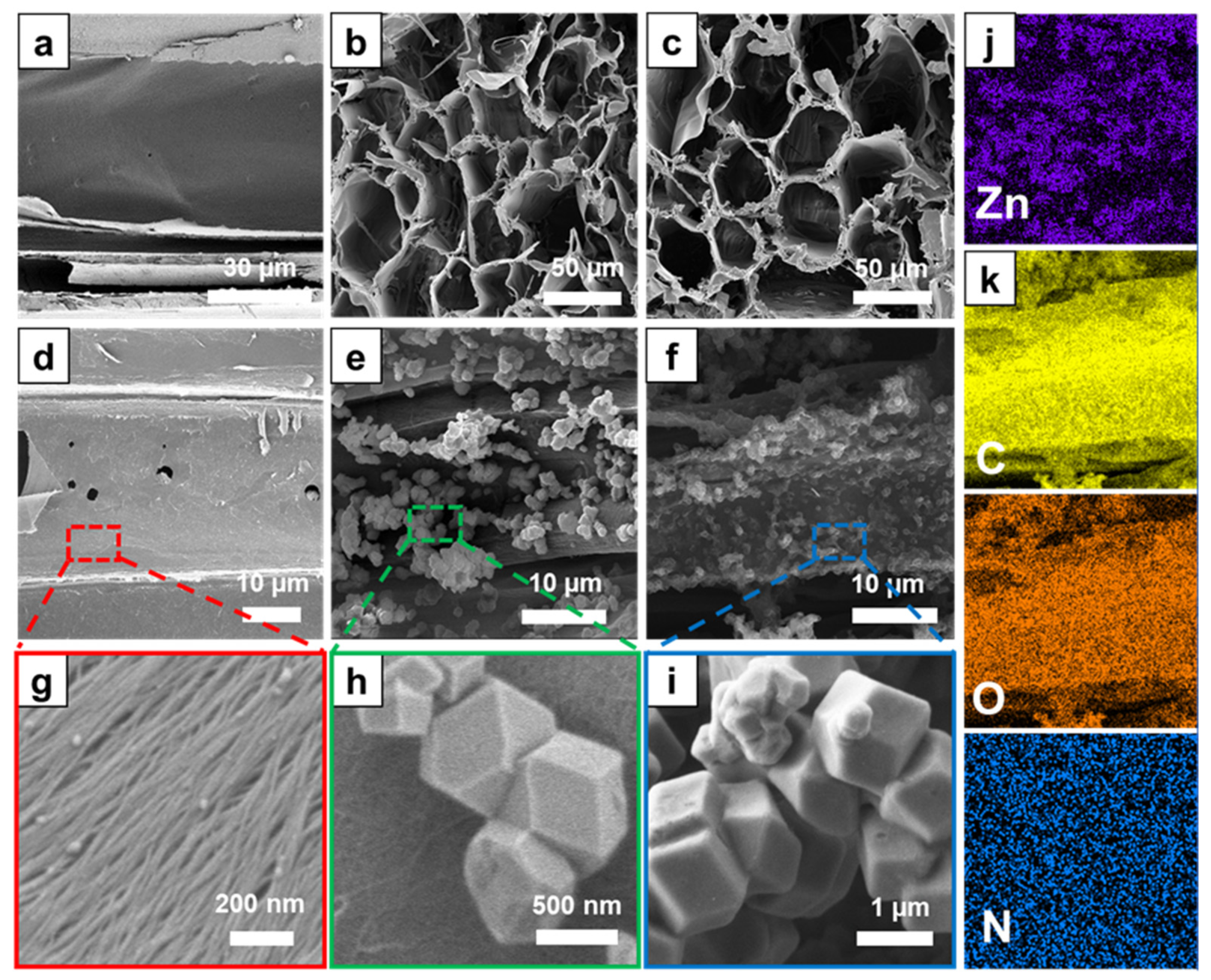
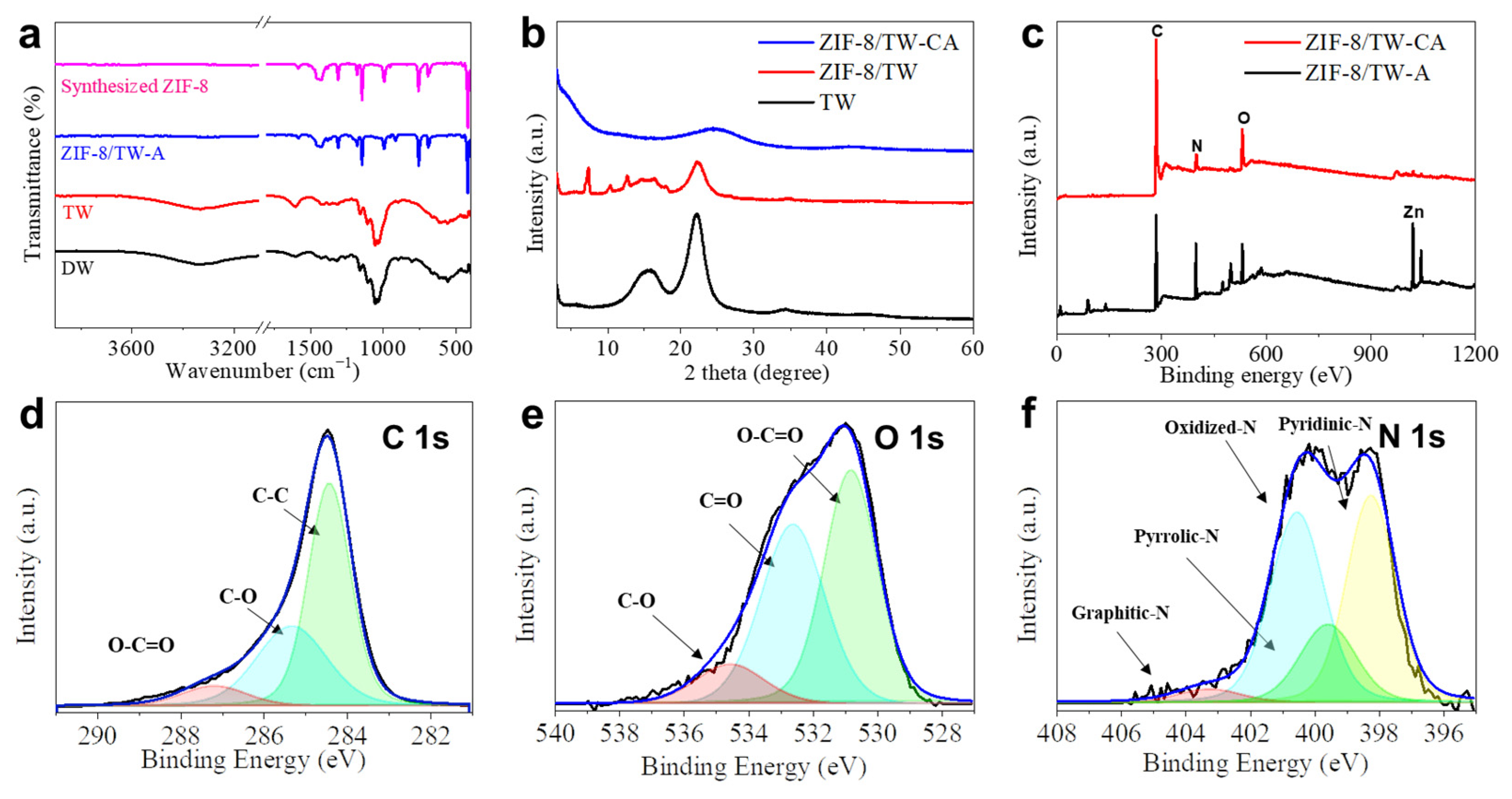
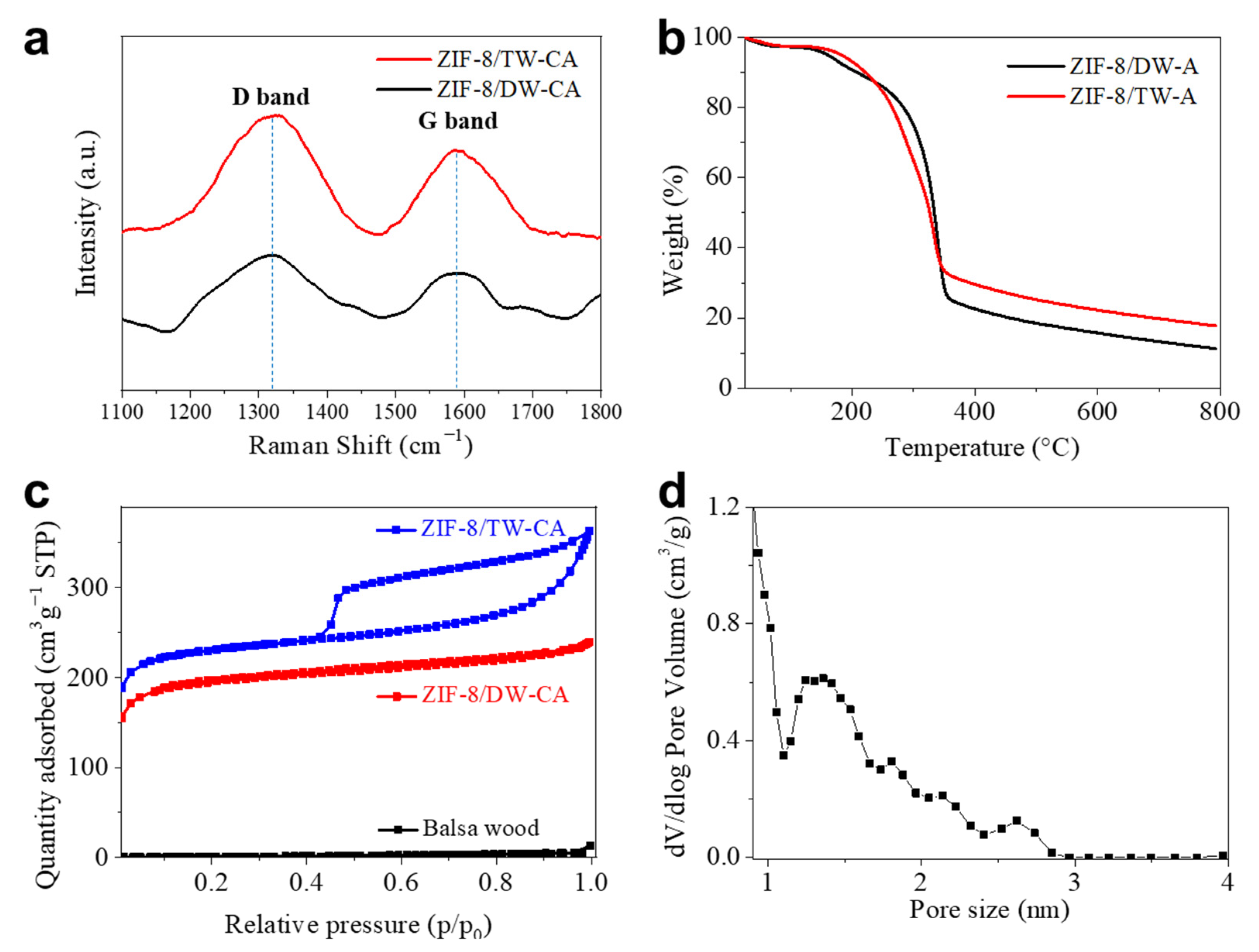
2.5. Characterizations
2.6. Adsorption Studies
Adsorption Models
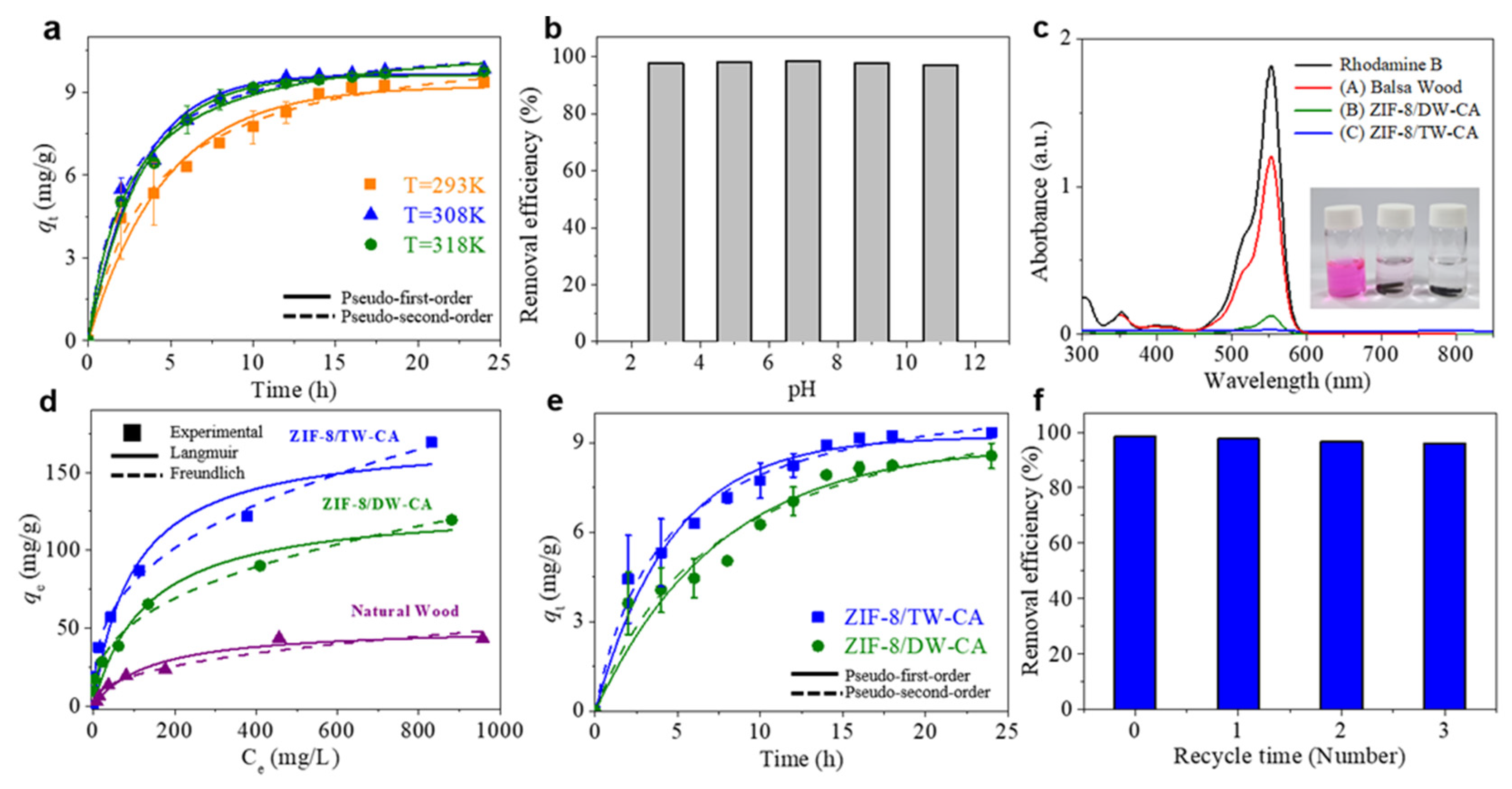
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Cordes, E.E.; Jones, D.O.; Schlacher, T.A.; Amon, D.J.; Bernardino, A.F.; Brooke, S.; Carney, R.; DeLeo, D.M.; Dunlop, K.M.; Escobar-Briones, E.G. Environmental impacts of the deep-water oil and gas industry: A review to guide management strategies. Front. Environ. Sci. 2016, 4, 58. [Google Scholar] [CrossRef]
- Uzma, N.; Khaja Mohinuddin Salar, B.; Kumar, B.S.; Aziz, N.; David, M.A.; Reddy, V.D. Impact of organic solvents and environmental pollutants on the physiological function in petrol filling workers. Int. J. Environ. Res. Public Health 2008, 5, 139–146. [Google Scholar] [CrossRef]
- Velho, S.R.; Brum, L.F.; Petter, C.O.; dos Santos, J.H.Z.; Šimunić, Š.; Kappa, W.H. Development of structured natural dyes for use into plastics. Dyes Pigments 2017, 136, 248–254. [Google Scholar] [CrossRef]
- Benkhaya, S.; M’rabet, S.; El Harfi, A. A review on classifications, recent synthesis and applications of textile dyes. Inorg. Chem. Commun. 2020, 115, 107891. [Google Scholar] [CrossRef]
- Pérez-Ibarbia, L.; Majdanski, T.; Schubert, S.; Windhab, N.; Schubert, U.S. Safety and regulatory review of dyes commonly used as excipients in pharmaceutical and nutraceutical applications. Eur. J. Pharm. Sci. 2016, 93, 264–273. [Google Scholar] [CrossRef]
- Wainwright, M. Dyes in the development of drugs and pharmaceuticals. Dyes Pigments 2008, 76, 582–589. [Google Scholar] [CrossRef]
- Levitan, H. Food, drug, and cosmetic dyes: Biological effects related to lipid solubility. Proc. Natl. Acad. Sci. USA 1977, 74, 2914–2918. [Google Scholar] [CrossRef]
- Dey, S.; Nagababu, B.H. Applications of food color and bio-preservatives in the food and its effect on the human health. Food Chem. Adv. 2022, 1, 100019. [Google Scholar] [CrossRef]
- Orts, F.; Del Río, A.; Molina, J.; Bonastre, J.; Cases, F. Electrochemical treatment of real textile wastewater: Trichromy Pro-cion HEXL®. J. Electroanal. Chem. 2018, 808, 387–394. [Google Scholar] [CrossRef]
- Emam, M. Thermal stability of some textile dyes. J. Therm. Anal. Calorim. 2001, 66, 583–591. [Google Scholar] [CrossRef]
- Tkaczyk, A.; Mitrowska, K.; Posyniak, A. Synthetic organic dyes as contaminants of the aquatic environment and their implications for ecosystems: A review. Sci. Total Environ. 2021, 717, 137222. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, J.; Yan, Y.; Yang, L.; Xing, G.; Li, H.; Wu, P.; Wang, M.; Zheng, H. Application of coagulation/flocculation in oily wastewater treatment: A review. Sci. Total Environ. 2021, 765, 142795. [Google Scholar] [CrossRef] [PubMed]
- Ramos, S.; Homem, V.; Alves, A.; Santos, L. A review of organic UV-filters in wastewater treatment plants. Environ. Int. 2016, 86, 24–44. [Google Scholar] [CrossRef] [PubMed]
- Sena, M.; Hicks, A. Life cycle assessment review of struvite precipitation in wastewater treatment. Resour. Conserv. Recycl. 2018, 139, 194–204. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A state-of-the-art review on wastewater treatment techniques: The effectiveness of adsorption method. Environ. Sci. Pollut. Res. 2021, 28, 9050–9066. [Google Scholar] [CrossRef]
- Huang, X.; Guida, S.; Jefferson, B.; Soares, A. Economic evaluation of ion-exchange processes for nutrient removal and recovery from municipal wastewater. NPJ Clean Water 2020, 3, 7. [Google Scholar] [CrossRef]
- Ma, D.; Yi, H.; Lai, C.; Liu, X.; Huo, X.; An, Z.; Li, L.; Fu, Y.; Li, B.; Zhang, M. Critical review of advanced oxidation processes in organic wastewater treatment. Chemosphere 2021, 275, 130104. [Google Scholar] [CrossRef]
- Lu, T.; Cao, W.; Liang, H.; Deng, Y.; Zhang, Y.; Zhu, M.; Ma, W.; Xiong, R.; Huang, C. Blow-Spun nanofibrous membrane for simultaneous treatment of emulsified oil/water mixtures, dyes, and bacteria. Langmuir 2022, 38, 15729–15739. [Google Scholar] [CrossRef]
- Banerjee, P.; Dinda, P.; Kar, M.; Uchman, M.; Mandal, T.K. Ionic liquid cross-linked high-absorbent polymer hydrogels: Kinetics of swelling and dye adsorption. Langmuir 2023, 39, 9757–9772. [Google Scholar] [CrossRef]
- Ugwu, E.; Othmani, A.; Nnaji, C. A review on zeolites as cost-effective adsorbents for removal of heavy metals from aqueous environment. Int. J. Environ. Sci. Technol. 2022, 19, 8061–8084. [Google Scholar] [CrossRef]
- Qiang, Z.; Li, R.; Yang, Z.; Guo, M.; Cheng, F.; Zhang, M. Zeolite X adsorbent with high stability synthesized from bauxite tailings for cyclic adsorption of CO2. Energy Fuels 2019, 33, 6641–6649. [Google Scholar] [CrossRef]
- Awasthi, A.; Jadhao, P.; Kumari, K. Clay nano-adsorbent: Structures, applications and mechanism for water treatment. SN Appl. Sci. 2019, 1, 1–21. [Google Scholar] [CrossRef]
- Wang, Z.K.; Li, T.T.; Peng, H.K.; Ren, H.T.; Lin, J.H.; Lou, C.W. Natural-clay-reinforced hydrogel adsorbent: Rapid ad-sorption of heavy-metal ions and dyes from textile wastewater. Water Env. Res. 2022, 94, e10698. [Google Scholar] [CrossRef]
- Raj, S.I.; Jaiswal, A.; Uddin, I. Tunable porous silica nanoparticles as a universal dye adsorbent. RSC Adv. 2019, 9, 11212–11219. [Google Scholar] [CrossRef]
- Huang, C.H.; Chang, K.P.; Ou, H.D.; Chiang, Y.C.; Wang, C.F. Adsorption of cationic dyes onto mesoporous silica Microporous. Mesoporous Mater. 2011, 141, 102–109. [Google Scholar] [CrossRef]
- Gan, G.; Li, X.; Fan, S.; Wang, L.; Qin, M.; Yin, Z.; Chen, G. Carbon aerogels for environmental clean-up. Eur. J. Inorg. Chem. 2019, 27, 3126–3141. [Google Scholar] [CrossRef]
- Geng, S.; Wei, J.; Jonasson, S.; Hedlund, J.; Oksman, K. Multifunctional carbon aerogels with hierarchical anisotropic structure derived from lignin and cellulose nanofibers for CO2 capture and energy storage. ACS Appl. Mater. Interfaces 2020, 12, 7432–7441. [Google Scholar] [CrossRef]
- Moreno-Castilla, C.; Maldonado-Hódar, F. Carbon aerogels for catalysis applications: An overview. Carbon 2005, 43, 455–465. [Google Scholar] [CrossRef]
- Zhuo, H.; Hu, Y.; Chen, Z.; Peng, X.; Liu, L.; Luo, Q.; Yi, J.; Liu, C.; Zhong, L. A carbon aerogel with super mechanical and sensing performances for wearable piezoresistive sensors. J. Mater. Chem. A 2019, 7, 8092–8100. [Google Scholar] [CrossRef]
- Luo, Q.; Zheng, H.; Hu, Y.; Zhuo, H.; Chen, Z.; Peng, X.; Zhong, L. Carbon nanotube/chitosan-based elastic carbon aerogel for pressure sensing. Indus. Eng. Chem. Res. 2019, 58, 17768–17775. [Google Scholar] [CrossRef]
- Ma, Y.; Liu, Q.; Li, W.; Zheng, Y.; Shi, Q.; Zhou, Z.; Shao, G.; Yang, W.; Chen, D.; Fang, X. Ultralight and robust carbon nano-fiber aerogels for advanced energy storage. J. Mater. Chem. A 2021, 9, 900–907. [Google Scholar] [CrossRef]
- Fan, W.; Shi, Y.; Gao, W.; Sun, Z.; Liu, T. Graphene–carbon nanotube aerogel with a scroll-interconnected-sheet structure as an advanced framework for a high-performance asymmetric supercapacitor electrode. ACS Appl. Nano Mater. 2018, 1, 4435–4441. [Google Scholar] [CrossRef]
- Lee, Y.J.; Jung, J.C.; Yi, J.; Baeck, S.-H.; Yoon, J.R.; Song, I.K. Preparation of carbon aerogel in ambient conditions for electrical double-layer capacitor. Curr. Appl. Phys. 2010, 10, 682–686. [Google Scholar] [CrossRef]
- Pekala, R.; Farmer, J.; Alviso, C.; Tran, T.; Mayer, S.; Miller, J.; Dunn, B. Carbon aerogels for electrochemical applications. J. Non-Cryst. Solids 1998, 225, 74–80. [Google Scholar] [CrossRef]
- Chen, G.; He, S.; Shi, G.; Ma, Y.; Ruan, C.; Jin, X.; Chen, Q.; Liu, X.; Dai, H.; Chen, X. In-situ immobilization of ZIF-67 on wood aerogel for effective removal of tetracycline from water. Chem. Eng. J. 2021, 423, 130184. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Wang, Z.; Song, L.; Yao, J. In situ growth of ZIF-8 within wood channels for water pollutants removal. Sep. Purif. Technol. 2021, 266, 118527. [Google Scholar] [CrossRef]
- Kaushik, J.; Kumar, V.; Garg, A.K.; Dubey, P.; Tripathi, K.M.; Sonkar, S.K. Bio-mass derived functionalized graphene aerogel: A sustainable approach for the removal of multiple organic dyes and their mixtures. New J. Chem. 2021, 45, 9073–9083. [Google Scholar] [CrossRef]
- Wang, M.-L.; Zhang, S.; Zhou, Z.-H.; Zhu, J.-L.; Gao, J.-F.; Dai, K.; Huang, H.-D.; Li, Z.-M. Facile heteroatom doping of bio-mass-derived carbon aerogels with hierarchically porous architecture and hybrid conductive network: Towards high electro-magnetic interference shielding effectiveness and high absorption coefficient. Compos. B Eng. 2021, 224, 109175. [Google Scholar] [CrossRef]
- Wang, C.; Kim, J.; Tang, J.; Na, J.; Kang, Y.M.; Kim, M.; Lim, H.; Bando, Y.; Li, J.; Yamauchi, Y. Large-scale synthesis of MOF-derived superporous carbon aerogels with extraordinary adsorption capacity for organic solvents. Angew. Chem. 2020, 132, 2082–2086. [Google Scholar] [CrossRef]
- Grossman, P.; Wold, M. Compression fracture of wood parallel to the grain. Wood Sci. Technol. 1971, 5, 147–156. [Google Scholar] [CrossRef]
- Kelley, S.S.; Rials, T.G.; Glasser, W.G. Relaxation behaviour of the amorphous components of wood. J. Mater. Sci. 1987, 22, 617–624. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, Y.; Wang, X.; Wu, Y.; Han, M.; You, J.; Jia, C.; Kim, J. Aerogel nanoarchitectonics based on cellulose nanocrystals and nanofibers from eucalyptus pulp: Preparation and comparative study. Cellulose 2022, 29, 817–833. [Google Scholar] [CrossRef]
- Gibson, L.J. The hierarchical structure and mechanics of plant materials. J. R. Soc. Interface 2012, 9, 2749–2766. [Google Scholar] [CrossRef]
- Xia, L.; Li, X.; Wu, Y.; Rong, M. Wood-derived carbons with hierarchical porous structures and monolithic shapes prepared by biological-template and self-assembly strategies. ACS Sustain. Chem. Eng. 2015, 3, 1724–1731. [Google Scholar] [CrossRef]
- Zhang, W.; Taheri-Ledari, R.; Saeidirad, M.; Qazi, F.S.; Kashtiaray, A.; Ganjali, F.; Tian, Y.; Maleki, A. Regulation of Porosity in MOFs: A Review on Tunable Scaffolds and Related Effects and Advances in Different Applications. J. Environ. Chem. Eng. 2022, 10, 108836. [Google Scholar] [CrossRef]
- Baumann, A.E.; Burns, D.A.; Liu, B.; Thoi, V.S. Metal-organic framework functionalization and design strategies for advanced electrochemical energy storage devices. Commun. Chem. 2019, 2, 86. [Google Scholar] [CrossRef]
- Qian, Q.; Asinger, P.A.; Lee, M.J.; Han, G.; Mizrahi Rodriguez, K.; Lin, S.; Benedetti, F.M.; Wu, A.X.; Chi, W.S.; Smith, Z.P. MOF-based membranes for gas separations. Chem. Rev. 2020, 120, 8161–8266. [Google Scholar] [CrossRef]
- Han, M.; Zhu, W.; Hossain, M.S.A.; You, J.; Kim, J. Recent progress of functional metal–organic framework materials for water treatment using sulfate radicals. Environ. Res. 2022, 211, 112956. [Google Scholar] [CrossRef]
- Shet, S.P.; Priya, S.S.; Sudhakar, K.; Tahir, M. A review on current trends in potential use of metal-organic framework for hydrogen storage. Int. J. Hydro. Energy 2021, 46, 11782–11803. [Google Scholar] [CrossRef]
- Zhu, W.; Han, M.; Kim, D.; Zhang, Y.; Kwon, G.; You, J.; Jia, C.; Kim, J. Facile preparation of nanocellulose/Zn-MOF-based catalytic filter for water purification by oxidation process. Environ. Res. 2022, 205, 112417. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Kim, J.; Tang, J.; Kim, M.; Lim, H.; Malgras, V.; You, J.; Xu, Q.; Li, J.; Yamauchi, Y. New strategies for novel MOF-derived carbon materials based on nanoarchitectures. Chem 2020, 6, 19–40. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Kim, J.H.; Park, H.S. Hierarchically open-porous nitrogen-incorporated carbon polyhedrons derived from metal-organic frameworks for improved CDI performance. Chem. Eng. J. 2020, 382, 122996. [Google Scholar] [CrossRef]
- Kim, M.; Xu, X.; Xin, R.; Earnshaw, J.; Ashok, A.; Kim, J.; Park, T.; Nanjundan, A.K.; El-Said, W.A.; Yi, J.W. KOH-activated hollow ZIF-8 derived porous carbon: Nanoarchitectured control for upgraded capacitive deionization and supercapacitor. ACS Appl. Mater. Interfaces 2021, 13, 52034–52043. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Xin, R.; Earnshaw, J.; Tang, J.; Hill, J.P.; Ashok, A.; Nanjundan, A.K.; Kim, J.; Young, C.; Sugahara, Y. MOF-derived nanoporous carbons with diverse tunable nanoarchitectures. Nat. Protoc. 2022, 17, 2990–3027. [Google Scholar] [CrossRef]
- Kajal, N.; Singh, V.; Gupta, R.; Gautam, S. Metal organic frameworks for electrochemical sensor applications: A review. Environ. Res. 2022, 204, 112320. [Google Scholar] [CrossRef]
- Lu, T.; Liang, H.; Cao, W.; Deng, Y.; Qu, Q.; Ma, W.; Xiong, R.; Huang, C. Blow-spun nanofibrous composite Self-cleaning membrane for enhanced purification of oily wastewater. J. Colloid Interface Sci. 2022, 608, 2860–2869. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, S.; Tian, Z.; Duan, G.; Pan, H.; Yue, Y.; Li, S.; Jian, S.; Yang, W.; Liu, K. MOFs meet wood: Reusable magnetic hydrophilic composites toward efficient water treatment with super-high dye adsorption capacity at high dye concentration. Chem. Eng. J. 2022, 446, 136851. [Google Scholar] [CrossRef]
- Zhu, X.; Fan, Z.; Zhang, X.F.; Yao, J. Metal-organic frameworks decorated wood aerogels for efficient particulate matter removal. J. Colloid Interface Sci. 2023, 629, 182–188. [Google Scholar] [CrossRef]
- Wang, S.; Wang, C.; Zhou, Q. Strong foam-like composites from highly mesoporous wood and metal-organic frameworks for efficient CO2 capture. ACS Appl. Mater. Interfaces 2021, 13, 29949–29959. [Google Scholar] [CrossRef]
- Tu, K.; Puértolas, B.; Adobes-Vidal, M.; Wang, Y.; Sun, J.; Traber, J.; Burgert, I.; Pérez-Ramírez, J.; Keplinger, T. Green synthesis of hierarchical metal–organic framework/wood functional composites with superior mechanical properties. Adv. Sci. 2022, 7, 1902897. [Google Scholar] [CrossRef] [PubMed]
- Santoso, E.; Ediati, R.; Istiqomah, Z.; Sulistiono, D.O.; Nugraha, R.E.; Kusumawati, Y.; Bahruji, H.; Prasetyoko, D. Facile syn-thesis of ZIF-8 nanoparticles using polar acetic acid solvent for enhanced adsorption of methylene blue. Microporous Mesoporous Mater. 2021, 310, 110620. [Google Scholar] [CrossRef]
- Fu, Q.; Ansari, F.; Zhou, Q.; Berglund, L.A. Wood nanotechnology for strong, mesoporous, and hydrophobic biocomposites for selective separation of oil/water mixtures. ACS Nano 2018, 12, 2222–2230. [Google Scholar] [CrossRef] [PubMed]
- Frey, M.; Widner, D.; Segmehl, J.S.; Casdorff, K.; Keplinger, T.; Burgert, I. Delignified and densified cellulose bulk materials with excellent tensile properties for sustainable engineering. ACS Appl. Mater. Interfaces 2018, 10, 5030–5037. [Google Scholar] [CrossRef]
- Khakalo, A.; Tanaka, A.; Korpela, A.; Orelma, H. Delignification and ionic liquid treatment of wood toward multifunctional high-performance structural materials. ACS Appl. Mater. Interfaces 2020, 12, 23532–23542. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, M.; Zhong, L.; Huang, J.; Liu, M. A family of MOFs@ Wood-Derived hierarchical porous composites as free-standing thick electrodes of solid supercapacitors with enhanced areal capacitances and energy densities. Mater. Today Energy 2022, 24, 100951. [Google Scholar] [CrossRef]
- Abbasi, Z.; Shamsaei, E.; Leong, S.K.; Ladewig, B.; Zhang, X.; Wang, H. Effect of carbonization temperature on adsorption property of ZIF-8 derived nanoporous carbon for water treatment. Microporous Mesoporous Mater. 2016, 236, 28–37. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, M.; Li, Y.; Zhang, M.; Xue, X.; Shi, Y.; Dai, B.; Guo, X.; Yu, F. Heteroatom-doped porous carbon from methyl orange dye wastewater for oxygen reduction. Green Energy Environ. 2018, 3, 172–178. [Google Scholar] [CrossRef]
- Zhang, L.; Tu, L.-Y.; Liang, Y.; Chen, Q.; Li, Z.-S.; Li, C.-H.; Wang, Z.-H.; Li, W. Coconut-based activated carbon fibers for efficient adsorption of various organic dyes. RSC Adv. 2018, 8, 42280–42291. [Google Scholar] [CrossRef]
- Treeweranuwat, P.; Boonyoung, P.; Chareonpanich, M.; Nueangnoraj, K. Role of nitrogen on the porosity, surface, and electrochemical characteristics of activated carbon. ACS Omega 2020, 5, 1911–1918. [Google Scholar] [CrossRef]
- Xiao, W.; Garba, Z.N.; Sun, S.; Lawan, I.; Wang, L.; Lin, M.; Yuan, Z. Preparation and evaluation of an effective activated carbon from white sugar for the adsorption of rhodamine B dye. J. Clean. Prod. 2020, 253, 119989. [Google Scholar] [CrossRef]
- Üner, O.; Geçgel, Ü.; Kolancilar, H.; Bayrak, Y. Adsorptive removal of rhodamine B with activated carbon obtained from okra wastes. Chem. Eng. Commun. 2017, 204, 772–783. [Google Scholar] [CrossRef]
- Li, Y.; Yan, X.; Hu, X.; Feng, R.; Zhou, M. Trace pyrolyzed ZIF-67 loaded activated carbon pellets for enhanced adsorption and catalytic degradation of Rhodamine B in water. Chem. Eng. J. 2019, 375, 122003. [Google Scholar] [CrossRef]
- Hou, M.-F.; Ma, C.-X.; Zhang, W.-D.; Tang, X.-Y.; Fan, Y.-N.; Wan, H.-F. Removal of rhodamine B using iron-pillared bentonite. J. Hazard. Mater. 2011, 186, 1118–1123. [Google Scholar] [CrossRef]
- Oyetade, O.A.; Nyamori, V.O.; Martincigh, B.S.; Jonnalagadda, S.B. Effectiveness of carbon nanotube–cobalt ferrite nanocomposites for the adsorption of rhodamine B from aqueous solutions. RSC Adv. 2015, 5, 22724–22739. [Google Scholar] [CrossRef]
- Peng, L.; Qin, P.; Lei, M.; Zeng, Q.; Song, H.; Yang, J.; Shao, J.; Liao, B.; Gu, J. Modifying Fe3O4 nanoparticles with humic acid for removal of Rhodamine B in water. J. Hazard. Mater. 2012, 209, 193–198. [Google Scholar] [CrossRef]
- Nazir, M.A.; Najam, T.; Shahzad, K.; Wattoo, M.A.; Hussain, T.; Tufail, M.K.; Shah, S.S.A.; Rehman, A.-U. Heterointerface engineering of water stable ZIF-8@ZIF-67: Adsorption of rhodamine B from water. Surf. Interfaces 2022, 34, 102324. [Google Scholar] [CrossRef]
- Koniarz, M.P.; Goscianska, J.R.; Pietrzak, R. Removal of rhodamine B from water by modified carbon xerogels. Colloid Surf. A Physicochem. Eng. Asp. 2018, 543, 109–117. [Google Scholar] [CrossRef]
- Chen, H.M.; Lau, W.M.; Zhou, D. Waste-Coffee-Derived activated carbon as efficient adsorbent for water treatment. Materials 2022, 15, 8684. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.T.; Nguyen, T.T.; Nguyen, H.P.T.; Khuat, H.B.; Nguyen, T.H.; Tran, V.K.; Chang, S.W.; Nguyen-Tri, P.; Nguyen, D.D.; La, D.D. Activated carbon with ultrahigh surface area derived from sawdust biowaste for the removal of rhodamine B in water. Environ. Technol. Innov. 2021, 24, 101811. [Google Scholar] [CrossRef]
- Da Silva Lacerda, V.; López-Sotelo, J.B.; Correa-Guimarães, A.; Hernández-Navarro, S.; Sánchez-Báscones, M.; Navas-Gracia, L.M.; Martín-Ramos, P.; Martín-Gil, J. Rhodamine B removal with activated carbons obtained from lignocellulosic waste. J. Environ. Manag. 2015, 155, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Bhadusha, N.; Ananthabaskaran, T. Kinetic, thermodynamic and equilibrium studies on uptake of rhodamine B onto ZnCl2 activated low cost carbon. J. Chem. 2012, 9, 137–144. [Google Scholar]
| Samples | SBET (m2 g−1) | Vm (cm3 g−1) | Pore Size (nm) |
|---|---|---|---|
| Balsa wood | 1.5 | 0.02 | 46.0 |
| ZIF-8/DW-CA | 662.1 | 0.30 | 1.9 |
| ZIF-8/TW-CA | 785.8 | 0.46 | 2.4 |
| Samples | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|
| qt (mg/g) | K1 | R2 | qt (mg/g) | K1 | R2 | |
| ZIF-8/DW-CA-293 K | 8.6 | 0.13 | 0.94 | 8.7 | 0.01 | 0.95 |
| ZIF-8/TW-CA-293 K | 9.2 | 0.20 | 0.96 | 9.5 | 0.02 | 0.98 |
| ZIF-8/TW-CA-308 K | 9.7 | 0.32 | 0.98 | 10.1 | 0.04 | 0.99 |
| ZIF-8/TW-CA-318 K | 9.6 | 0.30 | 0.99 | 10.0 | 0.03 | 0.99 |
| Adsorbent | Adsorbent (mg) | Adsorbate (mL) | qe (mg/g) | References |
|---|---|---|---|---|
| Sugar-based carbon | 100 | 1000 | 123.5 | [71] |
| RGO-Ni nanocomposite | 0.5 | 50 | 65.3 | [72] |
| Cal-ZIF-67/AC | 20 | 100 | 46.2 | [73] |
| Iron-pillared bentonite | 10 | 20 | 98.6 | [74] |
| MWCNT-COOH | 50 | 25 | 42.7 | [75] |
| Fe3O4/Humic acid | 50 | 100 | 161.8 | [76] |
| ZIF-8@ZIF-67 | 0.4 | 20 | 143.3 | [77] |
| Carbon xerogel | 20 | 50 | 132.0 | [78] |
| Coffee-activated carbon | 200 | 200 | 83.4 | [79] |
| Sawdust-activated carbon | 10 | 10 | 35.7 | [80] |
| Lignocellulosic-activated carbon | 2000 | 1000 | 39.98 | [81] |
| ZnCl2-activated carbon | 200 | 100 | 46.7 | [82] |
| ZIF-8/TW-CA | 10 | 10 | 169.4 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeon, Y.; Kim, D.; Lee, S.; Lee, K.; Ko, Y.; Kwon, G.; Park, J.; Kim, U.-J.; Hwang, S.Y.; Kim, J.; et al. Multiscale Porous Carbon Materials by In Situ Growth of Metal–Organic Framework in the Micro-Channel of Delignified Wood for High-Performance Water Purification. Nanomaterials 2023, 13, 2695. https://doi.org/10.3390/nano13192695
Jeon Y, Kim D, Lee S, Lee K, Ko Y, Kwon G, Park J, Kim U-J, Hwang SY, Kim J, et al. Multiscale Porous Carbon Materials by In Situ Growth of Metal–Organic Framework in the Micro-Channel of Delignified Wood for High-Performance Water Purification. Nanomaterials. 2023; 13(19):2695. https://doi.org/10.3390/nano13192695
Chicago/Turabian StyleJeon, Youngho, Dabum Kim, Suji Lee, Kangyun Lee, Youngsang Ko, Goomin Kwon, Jisoo Park, Ung-Jin Kim, Sung Yeon Hwang, Jeonghun Kim, and et al. 2023. "Multiscale Porous Carbon Materials by In Situ Growth of Metal–Organic Framework in the Micro-Channel of Delignified Wood for High-Performance Water Purification" Nanomaterials 13, no. 19: 2695. https://doi.org/10.3390/nano13192695
APA StyleJeon, Y., Kim, D., Lee, S., Lee, K., Ko, Y., Kwon, G., Park, J., Kim, U.-J., Hwang, S. Y., Kim, J., & You, J. (2023). Multiscale Porous Carbon Materials by In Situ Growth of Metal–Organic Framework in the Micro-Channel of Delignified Wood for High-Performance Water Purification. Nanomaterials, 13(19), 2695. https://doi.org/10.3390/nano13192695









