Synergistic Enhancement of Near-Infrared Emission in CsPbCl3 Host via Co-Doping with Yb3+ and Nd3+ for Perovskite Light Emitting Diodes
Abstract
:1. Introduction
2. Experimental Section
2.1. Chemical and Materials
2.2. Synthesis of Single Doped and Co-Doped PeNCs
2.3. PeLED Device Fabrication
2.4. Characterization
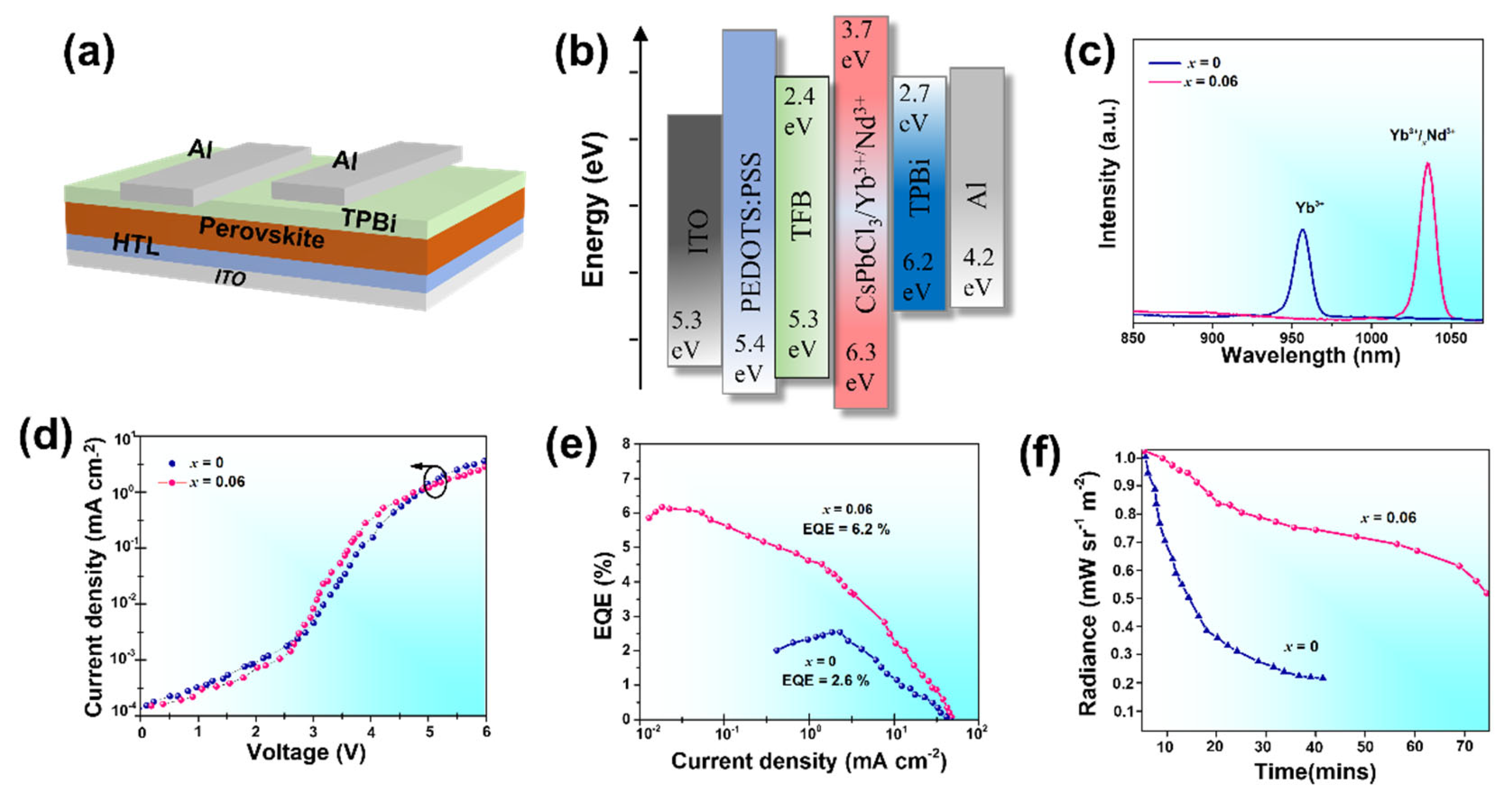
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of cesium lead halide perovskites (CsPbX3, X = Cl, Br, and I): Novel optoelectronic materials showing bright emission with wide color gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, X.; Song, J.; Xiao, L.; Zeng, H.; Sun, H. All-inorganic colloidal perovskite quantum dots: A new class of lasing materials with favorable characteristics. Adv. Mater. 2015, 27, 7101–7108. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, J.; Cai, B.; Song, J.; Zhang, F.; Fang, T.; Zeng, H. A bilateral interfacial passivation strategy promoting efficiency and stability of perovskite quantum dot light-emitting diodes. Nat. Commun. 2020, 11, 3902. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Huang, P.; Gong, Z.L.; Tu, D.; Xu, J.; Zou, Q.L.; Li, R.F.; You, W.W.; Bunzli, J.C.G.; Chen, X.Y. Near-infrared-triggered photon upconversion tuning in all-inorganic cesium lead halide perovskite quantum dots. Nat. Commun. 2018, 9, 3462. [Google Scholar] [CrossRef] [PubMed]
- Yong, Z.-J.; Guo, S.-Q.; Ma, J.-P.; Zhang, J.-Y.; Li, Z.-Y.; Chen, Y.-M.; Zhang, B.-B.; Zhou, Y.; Shu, J.; Gu, J.-L.; et al. Doping-enhanced short-range order of perovskite nanocrystals for near-unity violet luminescence quantum yield. J. Am. Chem. Soc. 2018, 140, 9942–9951. [Google Scholar] [CrossRef]
- Ravi, V.K.; Markad, G.B.; Nag, A. Band edge energies and excitonic transition probabilities of colloidal CsPbX3 (X = Cl, Br, I) perovskite nanocrystals. ACS Energy Lett. 2016, 1, 665–671. [Google Scholar] [CrossRef]
- Liu, W.; Lin, Q.; Li, H.; Wu, K.; Robel, I.; Pietryga, J.M.; Klimov, V.I. Mn2+-doped lead halide perovskite nanocrystals with dual-color emission controlled by halide content. J. Am. Chem. Soc. 2016, 138, 14954–14961. [Google Scholar] [CrossRef]
- Imran, M.; Caligiuri, V.; Wang, M.; Goldoni, L.; Prato, M.; Krahne, R.; De Trizio, L.; Manna, L. Benzoyl Halides as alternative precursors for the colloidal synthesis of lead-based halide perovskite nanocrystals. J. Am. Chem. Soc. 2018, 140, 2656–2664. [Google Scholar] [CrossRef]
- Luo, X.; Liang, G.; Han, Y.; Li, Y.; Ding, T.; He, S.; Liu, X.; Wu, K. Triplet energy transfer from perovskite nanocrystals mediated by electron transfer. J. Am. Chem. Soc. 2020, 142, 11270–11278. [Google Scholar] [CrossRef]
- Liu, Y.; Molokeev, M.S.; Xia, Z. Lattice doping of lanthanide ions in Cs2AgInCl6 nanocrystals enabling tunable photoluminescence. Energy Mater. Adv. 2021, 585274, 9. [Google Scholar] [CrossRef]
- Song, Z.; Zhao, J.; Liu, Q. Luminescent perovskites: Recent advances in theory and experiments. Inorg. Chem. Front. 2019, 6, 2969–3011. [Google Scholar] [CrossRef]
- Zhou, D.; Sun, R.; Xu, W.; Ding, N.; Li, D.; Chen, X.; Pan, G.; Bai, X.; Song, H. Impact of host composition, codoping, or tridoping on quantum-cutting emission of ytterbium in halide perovskite quantum dots and solar cell applications. Nano Lett. 2019, 19, 6904–6913. [Google Scholar] [CrossRef]
- Pan, G.; Bai, X.; Yang, D.; Chen, X.; Jing, P.; Qu, S.; Zhang, L.; Zhou, D.; Zhu, J.; Xu, W.; et al. Doping lanthanide into perovskite nanocrystals: Highly improved and expanded optical properties. Nano Lett. 2017, 17, 8005–8011. [Google Scholar] [CrossRef] [PubMed]
- Bünzli, J.-C.G.; Eliseeva, S.V. Lanthanide NIR luminescence for telecommunications, bioanalyses and solar energy conversion. J. Rare Earths 2010, 28, 824–842. [Google Scholar] [CrossRef]
- Penilla, E.H.; Devia-Cruz, L.F.; Duarte, M.A.; Hardin, C.L.; Kodera, Y.; Garay, J.E. Gain in polycrystalline Nd-doped alumina: Leveraging length scales to create a new class of high-energy, short pulse, tunable laser materials. Light, science & applications. Light Sci. Appl. 2018, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Sinha, N.; Jiménez, J.-R.; Pfund, B.; Prescimone, A.; Piguet, C.; Wenger, O.S. A near-infrared-II emissive chromium(III) Complex. Angew. Chem. 2021, 60, 23722–23728. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Zhao, X.; Ong, E.W.Y.; Tan, Z.-K. Transparent near-infrared perovskite light-emitting diodes. Nat. Commun. 2020, 11, 4213. [Google Scholar] [CrossRef] [PubMed]
- Bao, C.; Xu, W.; Yang, J.; Bai, S.; Teng, P.; Yang, Y.; Wang, J.; Zhao, N.; Zhang, W.; Huang, W.; et al. Bidirectional optical signal transmission between two identical devices using perovskite diodes. Nat. Electron. 2020, 3, 156–164. [Google Scholar] [CrossRef]
- Qiao, J.; Zhou, G.; Zhou, Y.; Zhang, Q.; Xia, Z.J. Divalent europium-doped near-infrared-emitting phosphor for light-emitting diodes. Nat. Commun. 2019, 10, 5267. [Google Scholar] [CrossRef]
- Milstein, T.J.; Kluherz, K.T.; Kroupa, D.M.; Erickson, C.S.; De Yoreo, J.J.; Gamelin, D.R. Anion exchange and the quantum-cutting energy threshold in ytterbium-doped CsPb(Cl1–xBrx)3 perovskite nanocrystals. Nano Lett. 2019, 19, 1931–1937. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Kumar, S.; Bär, J.; Bertolotti, F.; Masciocchi, N.; Guagliardi, A.; Grotevent, M.; Shorubalko, I.; Bodnarchuk, M.I.; et al. Dismantling the “red wall” of colloidal perovskites: Highly luminescent formamidinium and formamidinium-cesium lead iodide nanocrystals. ACS Nano 2017, 11, 3119–3134. [Google Scholar] [CrossRef] [PubMed]
- Locardi, F.; Cirignano, M.; Baranov, D.; Dang, Z.; Prato, M.; Drago, F.; Ferretti, M.; Pinchetti, V.; Fanciulli, M.; Brovelli, S.; et al. Colloidal synthesis of double perovskite Cs2AgInCl6 and Mn-Doped Cs2AgInCl6 nanocrystals. J. Am. Chem. Soc. 2018, 140, 12989–12995. [Google Scholar] [CrossRef] [PubMed]
- Mahor, Y.; Mir, W.J.; Nag, A. Synthesis and Near-Infrared Emission of Yb-Doped Cs2AgInCl6 double perovskite microcrystals and nanocrystals. J. Phys. Chem. C 2019, 123, 15787–15793. [Google Scholar] [CrossRef]
- Schmitz, F.; Lago, N.; Fagiolari, L.; Burkhart, J.; Cester, A.; Polo, A.; Prato, M.; Meneghesso, G.; Gross, S.; Bella, F.; et al. High open-circuit voltage Cs2AgBiBr6 carbon-based perovskite solar cells via green processing of ultrasonic spray-coated carbon electrodes from waste tire sources. ChemSusChem 2022, 15, e202201590. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, G.J.A.C. Codoping of lead-free double perovskites promotes near-infrared photoluminescence. Angew. Chem. 2021, 60, 540–542. [Google Scholar] [CrossRef]
- Chen, N.; Cai, T.; Li, W.; Hills-Kimball, K.; Yang, H.; Que, M.; Nagaoka, Y.; Liu, Z.; Yang, D.; Dong, A.; et al. Yb- and Mn-doped lead-free double Perovskite Cs2AgBiX6 (X = Cl–, Br–) nanocrystals. ACS Appl. Mater. Interfaces 2019, 11, 16855–16863. [Google Scholar] [CrossRef]
- Ishii, A.; Miyasaka, T. Sensitized Yb3+ Luminescence in CsPbCl3 film for highly efficient near-infrared light-emitting diodes. Adv. Sci. 2020, 7, 1903142. [Google Scholar] [CrossRef]
- Arfin, H.; Kaur, J.; Sheikh, T.; Chakraborty, S.; Nag, A. Bi(3+) -Er(3+) and Bi(3+) -Yb(3+) Codoped Cs(2) AgInCl(6) double perovskite near-infrared emitters. Angew. Chem. 2020, 59, 11307–11311. [Google Scholar] [CrossRef]
- Jin, S.; Li, R.; Huang, H.; Jiang, N.; Lin, J.; Wang, S.; Zheng, Y.; Chen, X.; Chen, D. Compact ultrabroadband light-emitting diodes based on lanthanide-doped lead-free double perovskites. Light Sci. Appl. 2022, 11, 52. [Google Scholar] [CrossRef]
- Liu, N.; Zheng, W.; Sun, R.; Li, X.; Xie, X.; Wang, L.; Zhang, Y. Near-infrared afterglow and related photochromism from solution-grown perovskite Crystal. Adv. Funct. Mater. 2022, 32, 2110663. [Google Scholar] [CrossRef]
- Zhang, G.; Wei, Y.; Dang, P.; Xiao, H.; Liu, D.; Li, X.; Cheng, Z.; Lin, J. Facile solution synthesis of Bi3+/Yb3+ ions co-doped Cs2Na0.6Ag0.4 InCl6 double perovskites with near-infrared emission. Dalton Trans. 2020, 49, 15231–15237. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, J.; Shizu, K.; Huang, S.; Hirata, S.; Miyazaki, H.; Adachi, C. Design of efficient thermally activated delayed fluorescence materials for pure blue organic light emitting diodes. J. Am. Chem. Soc. 2012, 134, 14706–14709. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-J.; Zou, C.; Shen, W.-S.; Zheng, X.; Tian, Q.-S.; Yu, Y.-J.; Chen, C.-H.; Zhao, B.; Wang, Z.-K.; Di, D.; et al. Efficient near-infrared electroluminescence from lanthanide-doped perovskite quantum cutters. Angew. Chem. 2023, 62, e202302005. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Artizzu, F.; Liu, J.; Singh, S.; Locardi, F.; Mara, D.; Hens, Z.; Van Deun, R. Boosting the Er3+ 1.5 μm luminescence in CsPbCl3 perovskite nanocrystals for photonic devices operating at telecommunication wavelengths. ACS Appl. Nano Mater. 2020, 3, 4699–4707. [Google Scholar] [CrossRef]
- Dagnall, K.A.; Conley, A.M.; Yoon, L.U.; Rajeev, H.S.; Lee, S.-H.; Choi, J.J. Ytterbium-doped cesium lead chloride perovskite as an X-ray scintillator with high light yield. ACS Omega 2022, 7, 20968–20974. [Google Scholar] [CrossRef]
- Pan, G.; Bai, X.; Xu, W.; Chen, X.; Zhou, D.; Zhu, J.; Shao, H.; Zhai, Y.; Dong, B.; Xu, L.; et al. Impurity ions codoped cesium lead halide perovskite nanocrystals with bright white light emission toward ultraviolet–white light-emitting diode. ACS Appl. Mater. Interfaces 2018, 10, 39040–39048. [Google Scholar] [CrossRef]
- Cao, L.; Jia, X.; Gan, W.; Ma, C.-G.; Zhang, J.; Lou, B.; Wang, J. Strong self-trapped exciton emission and highly efficient near-infrared luminescence in Sb3+-Yb3+ co-doped Cs2AgInCl6 double perovskite. Adv. Funct. Mater. 2023, 33, 2212135. [Google Scholar] [CrossRef]
- Li, D.; Chen, G. Near-Infrared Photoluminescence from Ytterbium-and Erbium-Codoped CsPbCl3 Perovskite quantum dots with negative thermal quenching. J. Phys. Chem. Lett. 2023, 14, 2837–2844. [Google Scholar] [CrossRef]
- Chiba, T.; Sato, J.; Ishikawa, S.; Takahashi, Y.; Ebe, H.; Sumikoshi, S.; Ohisa, S.; Kido, J. Neodymium chloride-doped perovskite nanocrystals for efficient blue light-emitting devices. ACS Appl. Mater. Interfaces 2020, 12, 53891–53898. [Google Scholar] [CrossRef]
- Shao, H.; Bai, X.; Cui, H.; Pan, G.; Jing, P.; Qu, S.; Zhu, J.; Zhai, Y.; Dong, B.; Song, H. White light emission in Bi3+/Mn2+ ion co-doped CsPbCl3 perovskite nanocrystals. Nanoscale 2018, 10, 1023–1029. [Google Scholar] [CrossRef]
- Xie, Y.; Peng, B.; Bravić, I.; Yu, Y.; Dong, Y.; Liang, R.; Ou, Q.; Monserrat, B.; Zhang, S. Highly efficient blue-emitting CsPbBr3 perovskite nanocrystals through neodymium doping. Adv. Sci. 2020, 7, 2001698. [Google Scholar] [CrossRef]
- Lyu, J.; Dong, B.; Pan, G.; Sun, L.; Bai, X.; Hu, S.; Shen, B.; Zhou, B.; Wang, L.; Xu, W.; et al. Ni2+ and Pr3+ Co-doped CsPbCl3 perovskite quantum dots with efficient infrared emission at 1300 nm. Nanoscale 2021, 13, 16598–16607. [Google Scholar] [CrossRef]
- Vinothkumar, G.; Rengaraj, S.; Arunkumar, P.; Cha, S.W.; Suresh Babu, K. Ionic radii and concentration dependency of Re3+ (Eu3+, Nd3+, Pr3+, and La3+)-doped cerium oxide nanoparticles for enhanced multienzyme-mimetic and hydroxyl radical scav-enging activity. J. Phys. Chem. C 2019, 123, 541–553. [Google Scholar] [CrossRef]
- Dias, S.; Kumawat, K.L.; Biswas, S.; Krupanidhi, S.B. Heat-up synthesis of Cu2SnS3 quantum dots for near infrared photodetection. RSC Adv. 2017, 7, 23301–23308. [Google Scholar] [CrossRef]
- Padhiar, M.A.; Ji, Y.; Wang, M.; Pan, S.; Ali khan, S.; Khan, N.Z.; Zhao, L.; Qin, F.; Zhao, Z.; Zhang, S. Sr2+ doped CsPbBrI2 perovskite nanocrystals coated with ZrO2 for applications as white LEDs. Nanotechnology 2023, 34, 275201. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Wang, M.; Yang, Z.; Wang, H.; Padhiar, M.A.; Qiu, H.; Dang, J.; Miao, Y.; Zhou, Y.; Bhatti, A.S. Strong violet emission from ultra-stable strontium doped CsPbCl3 superlattices. Nanoscale 2022, 14, 2359–2366. [Google Scholar] [CrossRef]
- Wei, Y.-C.; Wang, S.F.; Hu, Y.; Liao, L.-S.; Chen, D.-G.; Chang, K.-H.; Wang, C.-W.; Liu, S.-H.; Chan, W.-H.; Liao, J.-L.; et al. Overcoming the energy gap law in near-infrared OLEDs by exciton–vibration decoupling. Nat. Photonics 2020, 14, 570–577. [Google Scholar] [CrossRef]
- Shahalizad, A.; Malinge, A.; Hu, L.; Laflamme, G.; Haeberlé, L.; Myers, D.M.; Mao, J.; Skene, W.G.; Kéna-Cohen, S. Efficient Solution-Processed Hyperfluorescent OLEDs with Spectrally Narrow Emission at 840 nm. Adv. Funct. Mater. 2021, 31, 2007119. [Google Scholar] [CrossRef]
- Ding, J.; Dong, S.; Zhang, M.; Li, F. Efficient pure near-infrared organic light-emitting diodes based on tris(2,4,6-trichlorophenyl)methyl radical derivatives. J. Mater. Chem. C 2022, 10, 14116–14121. [Google Scholar] [CrossRef]
- Qiu, W.; Xiao, Z.; Roh, K.; Noel, N.K.; Shapiro, A.; Heremans, P.; Rand, B.P. Mixed Lead-Tin Halide Perovskites for Efficient and Wavelength-Tunable Near-Infrared Light-Emitting Diodes. Adv. Mater. 2019, 31, e1806105. [Google Scholar] [CrossRef]
- Lai, M.L.; Tay, T.Y.; Sadhanala, A.; Dutton, S.E.; Li, G.; Friend, R.H.; Tan, Z.K. Tunable Near-Infrared Luminescence in Tin Halide Perovskite Devices. J. Phys. Chem. Lett. 2016, 7, 2653–2658. [Google Scholar] [CrossRef] [PubMed]
- Hong, W.L.; Huang, Y.C.; Chang, C.Y.; Zhang, Z.C.; Tsai, H.R.; Chang, N.Y.; Chao, Y.C. Efficient Low-Temperature Solution-Processed Lead-Free Perovskite Infrared Light-Emitting Diodes. Adv. Mater. 2016, 28, 8029–8036. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Guan, X.; Li, Y.; Lin, K.; Feng, W.; Zhao, Y.; Yan, C.; Li, M.; Shen, Y.; Qin, X.; et al. Dendritic CsSnI3 for efficient and flexible near-infrared perovskite light-emitting diodes. Adv. Mater. 2021, 33, 2104414. [Google Scholar] [CrossRef] [PubMed]

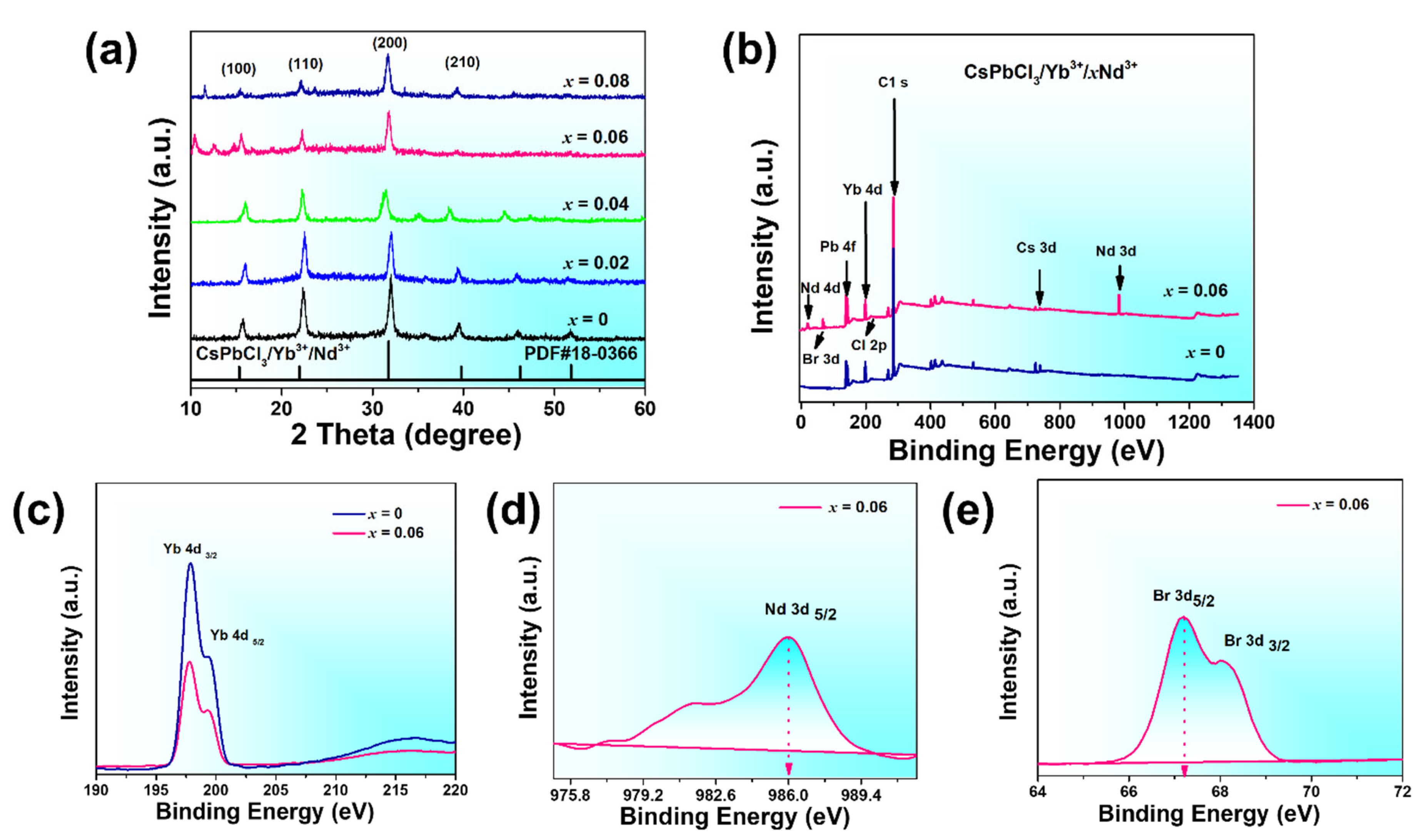
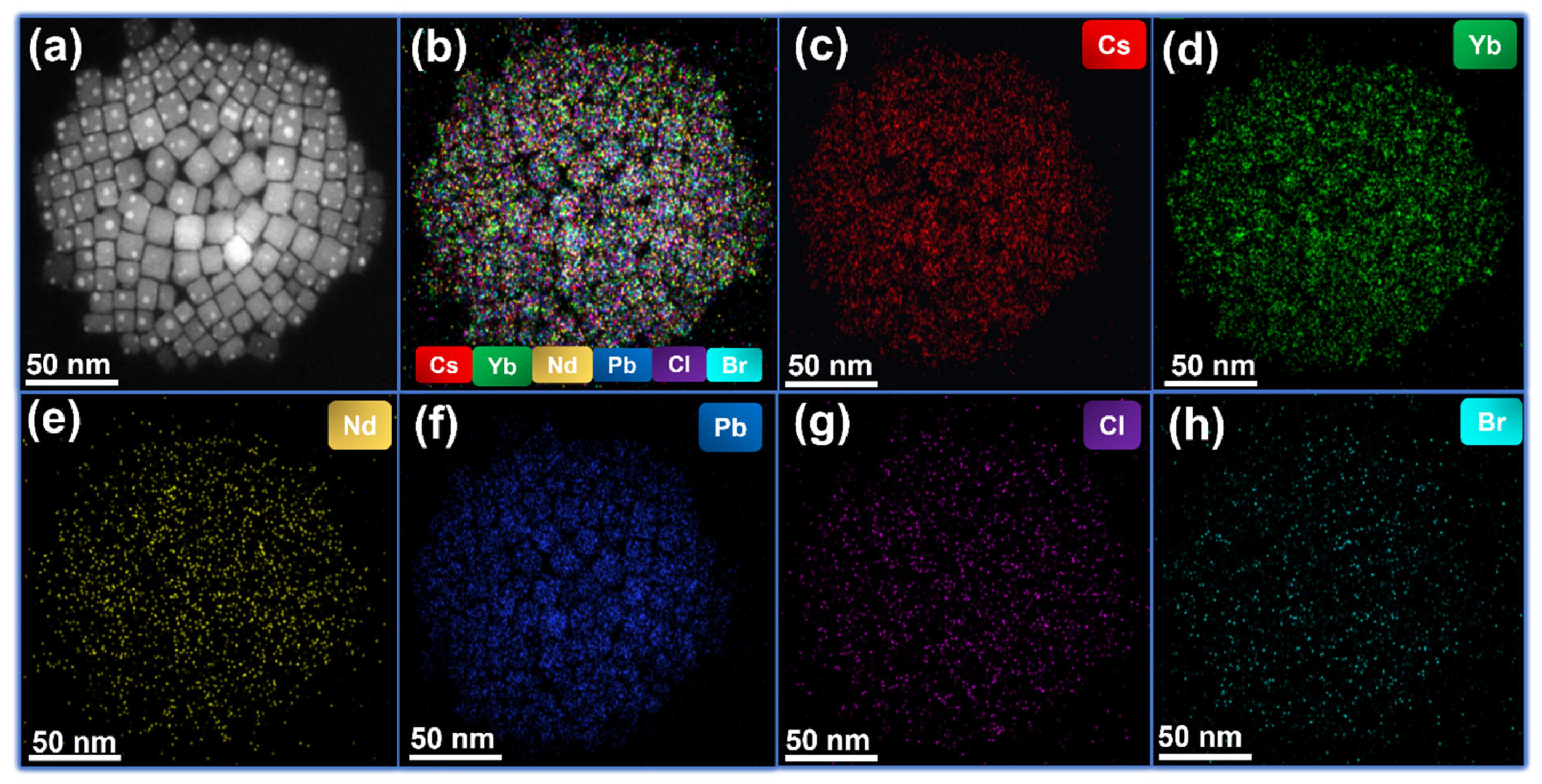
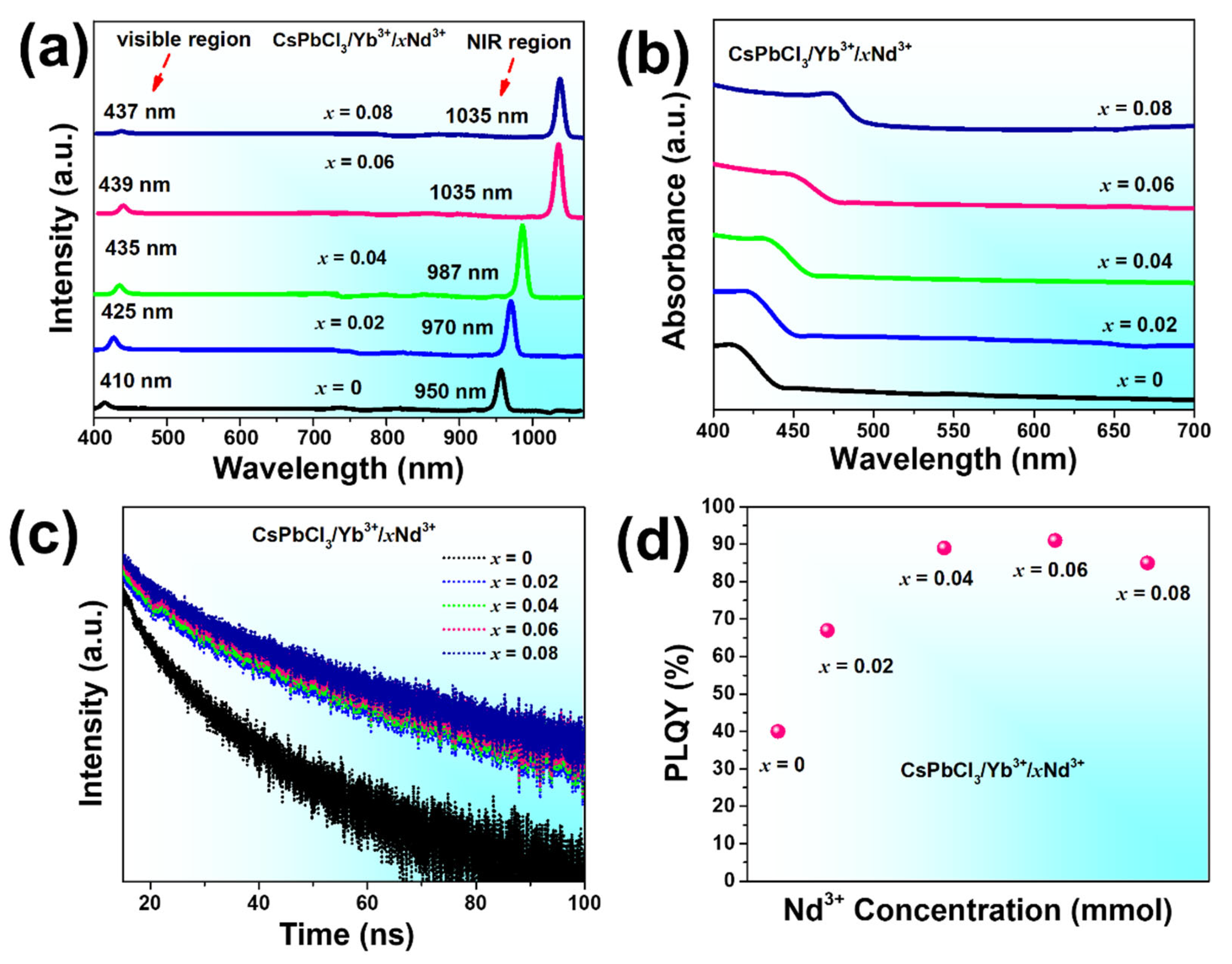
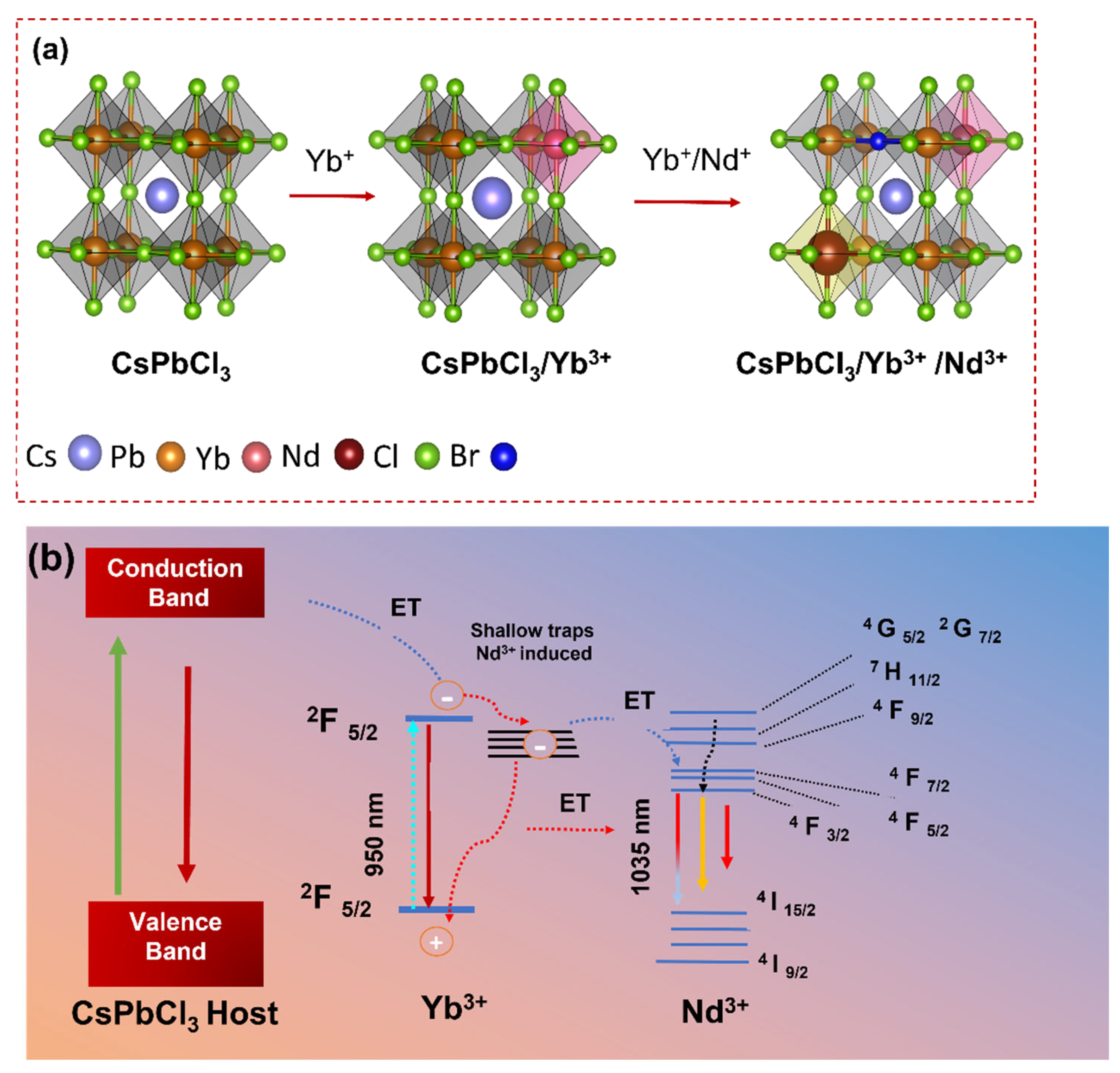
| Crystal Plane | Spacing Distance/Å PDF#18-0366 | Spacing Distance/Å x = 0 | Spacing Distance/Å x = 0.2 | Spacing Distance/Å x = 0.4 | Spacing Distance/Å x = 0.06 | Spacing Distance/Å x = 0.08 |
|---|---|---|---|---|---|---|
| 100 | 5.6000 | 5.484 | 5.518 | 5.53 | 5.510 | 5.601 |
| 101 | 3.9600 | 3.80 | 3.846 | 3.875 | 3.945 | 3.967 |
| 200 | 2.7940 | 2.621 | 2.656 | 2.681 | 2.765 | 2.796 |
| Device Type | Wavelength (nm) | EQE (%) | Reference |
|---|---|---|---|
| OLED | 930 | 2.14% | [47] |
| OLED | 840 | 3.8% | [48] |
| OLED | 830 | 3.1% | [49] |
| PeLED | 917 | 5.0% | [50] |
| PeLED | 945 | 0.72 | [51] |
| PeLED | 950 | 3.8% | [52] |
| PeLED | 1000 | 5.9% | [53] |
| PeLED | 940 | 5.4% | [27] |
| PeLED | 990 | 7.7% | [33] |
| PeLED | 950 | 2.6% | This work |
| PeLED | 1035 | 6.2% | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Padhiar, M.A.; Zhang, S.; Wang, M.; Zamin Khan, N.; Iqbal, S.; Ji, Y.; Muhammad, N.; Khan, S.A.; Pan, S. Synergistic Enhancement of Near-Infrared Emission in CsPbCl3 Host via Co-Doping with Yb3+ and Nd3+ for Perovskite Light Emitting Diodes. Nanomaterials 2023, 13, 2703. https://doi.org/10.3390/nano13192703
Padhiar MA, Zhang S, Wang M, Zamin Khan N, Iqbal S, Ji Y, Muhammad N, Khan SA, Pan S. Synergistic Enhancement of Near-Infrared Emission in CsPbCl3 Host via Co-Doping with Yb3+ and Nd3+ for Perovskite Light Emitting Diodes. Nanomaterials. 2023; 13(19):2703. https://doi.org/10.3390/nano13192703
Chicago/Turabian StylePadhiar, Muhammad Amin, Shaolin Zhang, Minqiang Wang, Noor Zamin Khan, Shoaib Iqbal, Yongqiang Ji, Nisar Muhammad, Sayed Ali Khan, and Shusheng Pan. 2023. "Synergistic Enhancement of Near-Infrared Emission in CsPbCl3 Host via Co-Doping with Yb3+ and Nd3+ for Perovskite Light Emitting Diodes" Nanomaterials 13, no. 19: 2703. https://doi.org/10.3390/nano13192703
APA StylePadhiar, M. A., Zhang, S., Wang, M., Zamin Khan, N., Iqbal, S., Ji, Y., Muhammad, N., Khan, S. A., & Pan, S. (2023). Synergistic Enhancement of Near-Infrared Emission in CsPbCl3 Host via Co-Doping with Yb3+ and Nd3+ for Perovskite Light Emitting Diodes. Nanomaterials, 13(19), 2703. https://doi.org/10.3390/nano13192703











