Recent Advances of Composite Nanomaterials for Antibiofilm Application
Abstract
:1. Introduction
2. Organic Nanocomposites
2.1. Construction of Organic Nanocomposites by Non-Covalent Interaction
2.1.1. Host–Guest Interaction to Construct Organic Nanocomposites
2.1.2. Construction of Organic Nanocomposites Based on Other Non-Covalent Interactions
2.2. Construction of Organic Nanocomposites Formed by Covalent Interaction
3. Inorganic Nanomaterials
3.1. Gold Based Inorganic Nanomaterials
3.2. Silver-Based Inorganic Nanomaterials
3.3. Inorganic Nanomaterials Based on Other Metals
4. Organic/Inorganic Hybrid Nanomaterials
4.1. Binary Hybrid Nanomaterials
4.1.1. Hybrid Materials Based on Magnetic Nanoparticles
4.1.2. Hybrid Materials Based on Gold Nanomaterials
4.2. Ternary Hybrid Nanomaterials
5. Summary and Prospect
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hu, D.; Deng, Y.; Jia, F.; Jin, Q.; Ji, J. Surface Charge Switchable Supramolecular Nanocarriers for Nitric Oxide Synergistic Photodynamic Eradication of Biofilms. ACS Nano 2020, 14, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, D.; Xie, S. Current Progress and Prospects of Organic Nanoparticles against Bacterial Biofilm. Adv. Colloid Interface Sci. 2021, 294, 102475. [Google Scholar] [CrossRef]
- Abu Bakar, M.; McKimm, J.; Haque, S.Z.; Majumder, M.A.A.; Haque, M. Chronic Tonsillitis and Biofilms: A Brief Overview of Treatment Modalities. J. Inflamm. Res. 2018, 11, 329–337. [Google Scholar] [CrossRef]
- Niedzielski, A.; Chmielik, L.P.; Stankiewicz, T. The Formation of Biofilm and Bacteriology in Otitis Media with Effusion in Children: A Prospective Cross-Sectional Study. Int. J. Environ. Res. Public Health 2021, 18, 3555. [Google Scholar] [CrossRef]
- Lerche, C.J.; Schwartz, F.; Theut, M.; Fosbol, E.L.; Iversen, K.; Bundgaard, H.; Hoiby, N.; Moser, C. Anti-Biofilm Approach in Infective Endocarditis Exposes New Treatment Strategies for Improved Outcome. Front. Cell Dev. Biol. 2021, 9, 643335. [Google Scholar] [CrossRef]
- Martin, I.; Waters, V.; Grasemann, H. Approaches to Targeting Bacterial Biofilms in Cystic Fibrosis Airways. Int. J. Mol. Sci. 2021, 22, 2155. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wuertz, S. Bacteria and Archaea on Earth and Their Abundance in Biofilms. Nat. Rev. Microbiol. 2019, 17, 247–260. [Google Scholar] [CrossRef]
- Varma, A.; Warghane, A.; Dhiman, N.K.; Paserkar, N.; Upadhye, V.; Modi, A.; Saini, R. The Role of Nanocomposites against Biofilm Infections in Humans. Front. Cell. Infect. Microbiol. 2023, 13, 1104615. [Google Scholar] [CrossRef]
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial Biofilm: Formation, Architecture, Antibiotic Resistance, and Control Strategies. Braz. J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef]
- Sha, M.; Xu, W.; Wu, Z.; Gu, W.; Zhu, C. Recent Advances in Single-Atom Materials for Enzyme-Like Catalysis and Biomedical Applications. Chem. J. Chin. Univ.-Chin. 2022, 43, 20220077. [Google Scholar] [CrossRef]
- Maduna, L.; Patnaik, A. A Review of Wound Dressings Treated with Aloe Vera and Its Application on Natural Fabrics. J. Nat. Fibers 2023, 20, 2190190. [Google Scholar] [CrossRef]
- Ortiz, Y.; Garcia-Heredia, A.; Merino-Mascorro, A.; Garcia, S.; Solis-Soto, L.; Heredia, N. Natural and Synthetic Antimicrobials Reduce Adherence of Enteroaggregative and Enterohemorrhagic Escherichia coli to Epithelial Cells. PLoS ONE 2021, 16, e0251096. [Google Scholar] [CrossRef]
- Kalsy, M.; Tonk, M.; Hardt, M.; Dobrindt, U.; Zdybicka-Barabas, A.; Cytrynska, M.; Vilcinskas, A.; Mukherjee, K. The Insect Antimicrobial Peptide Cecropin a Disrupts Uropathogenic Escherichia Coli Biofilms. NPJ Biofilms Microbiomes 2020, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Portelinha, J.; Angeles-Boza, A.M. The Antimicrobial Peptide Gad-1 Clears Pseudomonas aeruginosa Biofilms under Cystic Fibrosis Conditions. ChemBioChem 2021, 22, 1646–1655. [Google Scholar] [CrossRef]
- Liu, W.; Wu, Z.; Mao, C.; Guo, G.; Zeng, Z.; Fei, Y.; Wan, S.; Peng, J.; Wu, J. Antimicrobial Peptide Cec4 Eradicates the Bacteria of Clinical Carbapenem-Resistant Acinetobacter baumannii biofilm. Front. Microbiol. 2020, 11, 1532. [Google Scholar] [CrossRef]
- Prior, B.S.; Lange, M.D.; Salger, S.A.; Reading, B.J.; Peatman, E.; Beck, B.H. The Effect of Piscidin Antimicrobial Peptides on the Formation of Gram-Negative Bacterial Biofilms. J. Fish Dis. 2022, 45, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cheng, P.; Wang, S.; Li, X.; Peng, L.; Fang, R.; Xiong, J.; Li, H.; Mei, C.; Gao, J.; et al. Pseudomonas aeruginosa Biofilm Dispersion by the Mouse Antimicrobial Peptide Cramp. Vet. Res. 2022, 53, 605. [Google Scholar] [CrossRef]
- Yan, Y.; Li, Y.; Zhang, Z.; Wang, X.; Niu, Y.; Zhang, S.; Xu, W.; Ren, C. Advances of Peptides for Antibacterial Applications. Colloid Surf. B—Biointerfaces 2021, 202, 111682. [Google Scholar] [CrossRef]
- Fu, J.; Shen, T.; Wu, J.; Wang, C. Nanozyme: A New Strategy Combating Bacterial. J. Inorg. Mater. 2021, 36, 257–268. [Google Scholar] [CrossRef]
- Yue, L.; Wang, Z.; Zheng, M.; Wang, M.; Khan, I.M.; Ding, X.; Zhang, Y. Water-Soluble Chlorin e6-Hydroxypropyl Chitosan as a High-Efficiency Photoantimicrobial Agent against Staphylococcus aureus. Int. J. Biol. Macromol. 2022, 208, 669–677. [Google Scholar] [CrossRef]
- Yougbare, S.; Mutalik, C.; Krisnawati, D.I.; Kristanto, H.; Jazidie, A.; Nuh, M.; Cheng, T.-M.; Kuo, T.-R. Nanomaterials for the Photothermal Killing of Bacteria. Nanomaterials 2020, 10, 1123. [Google Scholar] [CrossRef]
- Ran, T.; Ning, Z.; Liangliang, Z.; Habumugisha, T.; Yicun, C.; Yin, L.; Yinjuan, W.; Kui, W.; Yangdong, W.; Jianchun, J. Characterization and Antivibrio Activity of Chitosan-Citral Schiff Base Calcium Complex for a Calcium Citrate Sustained Release Antibacterial Agent. Int. J. Biol. Macromol. 2023, 239, 124355. [Google Scholar] [CrossRef]
- Liu, Y.; Lixuan, R.; Yanzhen, Z.; Siqun, L.; Huifang, W.; Xianghua, G.; Baolong, N.; Wenfeng, L. Preparation and Characterization of PVA/Arginine Chitosan/ZnO NPs Composite Films. Int. J. Biol. Macromol. 2023, 226, 184–193. [Google Scholar] [CrossRef]
- Janani, B.; Okla, M.K.; Abdel-Maksoud, M.A.; AbdElgawad, H.; Thomas, A.M.; Raju, L.L.; Al-Qahtani, W.H.; Khan, S.S. CuO Loaded ZnS Nanoflower Entrapped on PVA-Chitosan Matrix for Boosted Visible Light Photocatalysis for Tetracycline Degradation and Anti-Bacterial Application. J. Environ. Manag. 2022, 306, 114396. [Google Scholar] [CrossRef]
- Zhang, J.; Sun, B.; Zhang, M.; Su, Y.; Xu, W.; Sun, Y.; Jiang, H.; Zhou, N.; Shen, J.; Wu, F. Modulating the Local Coordination Environment of Cobalt Single-Atomic Nanozymes for Enhanced Catalytic Therapy against Bacteria. Acta Biomater. 2023, 164, 563–576. [Google Scholar] [CrossRef]
- Shehabeldine, A.M.; Al-Askar, A.A.; AbdElgawad, H.; Hagras, F.A.; Ramadan, A.A.; Kamel, M.R.; Ahmed, M.A.; Atia, K.H.; Hashem, A.H. Wound Dressing Scaffold with High Anti-Biofilm Performance Based on Ciprofloxacin-Loaded Chitosan-Hydrolyzed Starch Nanocomposite: In Vitro and in Vivo Study. Appl. Biochem. Biotechnol. 2023, 195, 6421–6439. [Google Scholar] [CrossRef]
- Peng, X.; Han, Q.; Zhou, X.; Chen, Y.; Huang, X.; Guo, X.; Peng, R.; Wang, H.; Peng, X.; Cheng, L. Effect of pH-Sensitive Nanoparticles on Inhibiting Oral Biofilms. Drug Deliv. 2022, 29, 561–573. [Google Scholar] [CrossRef]
- Liu, D.; Xi, Y.; Yu, S.; Yang, K.; Zhang, F.; Yang, Y.; Wang, T.; He, S.; Zhu, Y.; Fan, Z.; et al. A Polypeptide Coating for Preventing Biofilm on Implants by Inhibiting Antibiotic Resistance Genes. Biomaterials 2023, 293, 121957. [Google Scholar] [CrossRef] [PubMed]
- Zmejkoski, D.Z.; Zdravkovic, N.M.; Trisic, D.D.; Budimir, M.D.; Markovic, Z.M.; Kozyrovska, N.O.; Markovic, B.M.T. Chronic Wound Dressings-Pathogenic Bacteria Anti-Biofilm Treatment with Bacterial Cellulose-Chitosan Polymer or Bacterial Cellulose-Chitosan Dots Composite Hydrogels. Int. J. Biol. Macromol. 2021, 191, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.A.; Abu-Elghait, M.; Ahmed, N.E.; Salem, S.S. Eco-Friendly Mycogenic Synthesis of ZnO and CuO Nanoparticles for in Vitro Antibacterial, Antibiofilm, and Antifungal Applications. Biol. Trace Elem. Res. 2021, 199, 2788–2799. [Google Scholar] [CrossRef]
- Velgosova, O.; Mudra, E.; Vojtko, M. Preparing, Characterization and Anti-Biofilm Activity of Polymer Fibers Doped by Green Synthesized AgNPs. Polymers 2021, 13, 605. [Google Scholar] [CrossRef] [PubMed]
- Adnan, R.; Abdallah, A.M.; Mezher, M.; Noun, M.; Khalil, M.; Awad, R. Impact of Mg-Doping on the Structural, Optical, and Magnetic Properties of CuO Nanoparticles and Their Antibiofilm Activity. Phys. Scr. 2023, 98, 055935. [Google Scholar] [CrossRef]
- Wang, W.; Luo, Q.; Li, J.; Li, L.; Li, Y.; Huo, X.; Du, X.; Li, Z.; Wang, N. Photothermal-Amplified Single Atom Nanozyme for Biofouling Control in Seawater. Adv. Funct. Mater. 2022, 32, 2205461. [Google Scholar] [CrossRef]
- Wang, X.; Hu, W.; Xia, X.-H.; Wang, C. Implanting of Single Zinc Sites into 2D Metal-Organic Framework Nanozymes for Boosted Antibiofilm Therapy. Adv. Funct. Mater. 2023, 33, 212798. [Google Scholar] [CrossRef]
- He, J.; Hong, M.; Xie, W.; Chen, Z.; Chen, D.; Xie, S. Progress and Prospects of Nanomaterials against Resistant Bacteria. J. Control. Release 2022, 351, 301–323. [Google Scholar] [CrossRef]
- Zhang, J.; Tang, W.; Zhang, X.; Song, Z.; Tong, T. An Overview of Stimuli-Responsive Intelligent Antibacterial Nanomaterials. Pharmaceutics 2023, 15, 2113. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Ukidve, A.; Krishnan, V.; Mitragotri, S. Effect of Physicochemical and Surface Properties on in Vivo Fate of Drug Nanocarriers. Adv. Drug Deliv. Rev. 2019, 143, 3–21. [Google Scholar] [CrossRef]
- Xie, Y.-Y.; Zhang, Y.-W.; Liu, X.-Z.; Ma, X.-F.; Qin, X.-T.; Jia, S.-R.; Zhong, C. Aggregation-Induced Emission-Active Amino Acid/Berberine Hydrogels with Enhanced Photodynamic Antibacterial and Anti-Biofilm Activity. Chem. Eng. J. 2021, 413, 127542. [Google Scholar] [CrossRef]
- Shi, J.; Wang, Y.; He, W.; Ye, Z.; Liu, M.; Zhao, Z.; Lam, J.W.Y.; Zhang, P.; Kwok, R.T.K.; Tang, B.Z. Precise Molecular Engineering of Type I Photosensitizer with Aggregation-Induced Emission for Image-Guided Photodynamic Eradication of Biofilm. Molecules 2023, 28, 5368. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhao, W.; Cao, B.; Wang, Z.; Zhou, Q.; Lu, S.; Lu, L.; Zhan, M.; Hu, X. Biofilm-Responsive Polymeric Nanoparticles with Self-Adaptive Deep Penetration for in Vivo Photothermal Treatment of Implant Infection. Chem. Mat. 2020, 32, 7725–7738. [Google Scholar] [CrossRef]
- Bernal-Mercado, A.T.; Juarez, J.; Valdez, M.A.; Ayala-Zavala, J.F.; Del-Toro-Sanchez, C.L.; Encinas-Basurto, D. Hydrophobic Chitosan Nanoparticles Loaded with Carvacrol against Pseudomonas aeruginosa Biofilms. Molecules 2022, 27, 699. [Google Scholar] [CrossRef]
- Nwabuife, J.C.; Pant, A.M.; Govender, T. Liposomal Delivery Systems and Their Applications against Staphylococcus aureus and Methicillin-Resistant Staphylococcus aureus. Adv. Drug Deliv. Rev. 2021, 178, 113861. [Google Scholar] [CrossRef]
- Karim, A.A.; Dou, Q.; Li, Z.; Loh, X.J. Emerging Supramolecular Therapeutic Carriers Based on Host-Guest Interactions. Chem.—Asian J. 2016, 11, 1300–1321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Qian, X.; Zhang, Z.; Li, C.; Xie, C.; Wu, W.; Jiang, X. Supramolecular Amphiphilic Polymer-Based Micelles with Seven-Armed Polyoxazoline Coating for Drug Delivery. ACS Appl. Mater. Interfaces 2017, 9, 5768–5777. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Huang, D.; Deng, Y.; Yu, W.; Jin, Q.; Ji, J.; Fu, G. Chlorin e6 (Ce6)-Loaded Supramolecular Polypeptide Micelles with Enhanced Photodynamic Therapy Effect against Pseudomonas aeruginosa. Chem. Eng. J. 2021, 417, 129334. [Google Scholar] [CrossRef]
- Chen, M.; Qiu, B.; Zhang, Z.; Xie, S.; Liu, Y.; Xia, T.; Li, X. Light-Triggerable and Ph/Lipase-Responsive Release of Antibiotics and Β-Lactamase Inhibitors from Host-Guest Self-Assembled Micelles to Combat Biofilms and Resistant Bacteria. Chem. Eng. J. 2021, 424, 130330. [Google Scholar] [CrossRef]
- Shi, Y.; Cao, Y.; Cheng, J.; Yu, W.; Liu, M.; Yin, J.; Huang, C.; Liang, X.; Zhou, H.; Liu, H.; et al. Construction of Self-Activated Nanoreactors for Cascade Catalytic Anti-Biofilm Therapy Based on H2O2 Self-Generation and Switch-on NO Release. Adv. Funct. Mater. 2022, 32, 2111148. [Google Scholar] [CrossRef]
- Raj, V.; Kim, Y.; Kim, Y.-G.; Lee, J.-H.; Lee, J. Chitosan-Gum Arabic Embedded Alizarin Nanocarriers Inhibit Biofilm Formation of Multispecies Microorganisms. Carbohydr. Polym. 2022, 284, 118959. [Google Scholar] [CrossRef]
- Zou, L.; Hu, D.; Wang, F.; Jin, Q.; Ji, J. The Relief of Hypoxic Microenvironment Using an O2 Self-Sufficient Fluorinated Nanoplatform for Enhanced Photodynamic Eradication of Bacterial Biofilms. Nano Res. 2021, 15, 1636–1644. [Google Scholar] [CrossRef]
- Wu, S.; Xu, C.; Zhu, Y.; Zheng, L.; Zhang, L.; Hu, Y.; Yu, B.; Wang, Y.; Xu, F.-J. Biofilm-Sensitive Photodynamic Nanoparticles for Enhanced Penetration and Antibacterial Efficiency. Adv. Funct. Mater. 2021, 31, 2103591. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, J.; Chai, M.; Li, X.; Deng, Y.; Jin, Q.; Ji, J. Size and Charge Adaptive Clustered Nanoparticles Targeting the Biofilm Microenvironment for Chronic Lung Infection Management. ACS Nano 2020, 14, 5686–5699. [Google Scholar] [CrossRef]
- Ding, M.; Zhao, W.; Zhang, X.; Song, L.; Luan, S. Charge-Switchable MOF Nanocomplex for Enhanced Biofilm Penetration and Eradication. J. Hazard. Mater. 2022, 439, 129594. [Google Scholar] [CrossRef]
- Yuan, Z.; Lin, C.; He, Y.; Tao, B.; Chen, M.; Zhang, J.; Liu, P.; Cai, K. Near-Infrared Light-Triggered Nitric-Oxide-Enhanced Photodynamic Therapy and Low-Temperature Photothermal Therapy for Biofilm Elimination. ACS Nano 2020, 14, 3546–3562. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Xue, K.; Zhang, Y.; Xiao, M.; Wu, K.; Shi, L.; Zhu, C. Thermoresponsive Hydrogel-Enabled Thermostatic Photothermal Therapy for Enhanced Healing of Bacteria-Infected Wounds. Adv. Sci. 2023, 10, 2206865. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Tian, J.; Zhu, J.; Chen, J.; Li, L.; Yang, C.; Chen, J.; Chen, D. Photodynamic and Photothermal co-Driven CO-Enhanced Multi-Mode Synergistic Antibacterial Nanoplatform to Effectively Fight against Biofilm Infections. Chem. Eng. J. 2021, 426, 131919. [Google Scholar] [CrossRef]
- Vidakis, N.; Petousis, M.; Michailidis, N.; Papadakis, V.; Korlos, A.; Mountakis, N.; Argyros, A. Multi-Functional 3D-Printed Vat Photopolymerization Biomedical-Grade Resin Reinforced with Binary Nano Inclusions: The Effect of Cellulose Nanofibers and Antimicrobial Nanoparticle Agents. Polymers 2022, 14, 1903. [Google Scholar] [CrossRef]
- Zhou, C.; Zhou, Y.; Zheng, Y.; Yu, Y.; Yang, K.; Chen, Z.; Chen, X.; Wen, K.; Chen, Y.; Bai, S.; et al. Amphiphilic Nano-Swords for Direct Penetration and Eradication of Pathogenic Bacterial Biofilms. ACS Appl. Mater. Interfaces 2023, 15, 20458–20473. [Google Scholar] [CrossRef]
- Wan, P.; Guo, W.; Wang, Y.; Deng, M.; Xiao, C.; Chen, X. Photosensitizer-Polypeptide Conjugate for Effective Elimination of Candida albicans Biofilm. Adv. Healthc. Mater. 2022, 11, e2200268. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, Y.F. Modern Developments in the Application and Function of Metal/Metal Oxide Nanocomposite-Based Antibacterial Agents. BioNanoScience 2023, 13, 840–852. [Google Scholar] [CrossRef]
- Maric, T.; Lovind, A.; Zhang, Z.; Geng, J.; Boisen, A. Near-Infrared Light-Driven Mesoporous SiO2/Au Nanomotors for Eradication of Pseudomonas aeruginosa Biofilm. Adv. Healthc. Mater. 2023, 12, 2203018. [Google Scholar] [CrossRef]
- Zheng, X.; Sun, J.; Li, W.; Dong, B.; Song, Y.; Xu, W.; Zhou, Y.; Wang, L. Engineering Nanotubular Titania with Gold Nanoparticles for Antibiofilm Enhancement and Soft Tissue Healing Promotion. J. Electroanal. Chem. 2020, 871, 114362. [Google Scholar] [CrossRef]
- Cao, M.; Chang, Z.; Tan, J.; Wang, X.; Zhang, P.; Lin, S.; Liu, J.; Li, A. Superoxide Radical-Mediated Self-Synthesized Au/MoO(3-X) Hybrids with Enhanced Peroxidase-Like Activity and Photothermal Effect for Anti-Mrsa Therapy. ACS Appl. Mater. Interfaces 2022, 14, 13025–13037. [Google Scholar] [CrossRef]
- Chang, M.; Wang, M.; Chen, Y.; Shu, M.; Zhao, Y.; Ding, B.; Hou, Z.; Lin, J. Self-Assembled CeVO4/Ag Nanohybrid as Photoconversion Agents with Enhanced Solar-Driven Photocatalysis and Nir-Responsive Photothermal/Photodynamic Synergistic Therapy Performance. Nanoscale 2019, 11, 10129–10136. [Google Scholar] [CrossRef] [PubMed]
- D’Agostino, A.; Taglietti, A.; Desando, R.; Bini, M.; Patrini, M.; Dacarro, G.; Cucca, L.; Pallavicini, P.; Grisoli, P. Bulk Surfaces Coated with Triangular Silver Nanoplates: Antibacterial Action Based on Silver Release and Photo-Thermal Effect. Nanomaterials 2017, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Fernandez, S.; Lozano-Iturbe, V.; Garcia, B.; Andres, L.J.; Menendez, M.F.; Rodriguez, D.; Vazquez, F.; Martin, C.; Quiros, L.M. Antibacterial Effect of Silver Nanorings. BMC Microbiol. 2020, 20, 172. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Ma, J.; Jo, S.; Lee, S.; Kim, C.S. Enhancement of Antibacterial Performance of Silver Nanowire Transparent Film by Post-Heat Treatment. Nanomaterials 2020, 10, 938. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Bai, Q.; Liang, M.; Yang, D.; Li, S.; Wang, L.; Liu, J.; Yu, W.W.; Sui, N.; Zhu, Z. Silver Peroxide Nanoparticles for Combined Antibacterial Sonodynamic and Photothermal Therapy. Small 2022, 18, 2104160. [Google Scholar] [CrossRef]
- Obeng, E.; Feng, J.; Wang, D.; Zheng, D.; Xiang, B.; Shen, J. Multifunctional Phototheranostic Agent ZnO@Ag for Anti-Infection through Photothermal/Photodynamic Therapy. Front. Chem. 2022, 10, 1054739. [Google Scholar] [CrossRef]
- Elyamny, S.; Eltarahony, M.; Abu-Serie, M.; Nabil, M.M.; Kashyout, A.E.-H.B. One-Pot Fabrication of Ag@Ag2O Core-Shell Nanostructures for Biosafe Antimicrobial and Antibiofilm Applications. Sci. Rep. 2021, 11, 22543. [Google Scholar] [CrossRef]
- Ye, L.; Cao, Z.; Liu, X.; Cui, Z.; Li, Z.; Liang, Y.; Zhu, S.; Wu, S. Noble Metal-Based Nanomaterials as Antibacterial Agents. J. Alloys Compd. 2022, 904, 164091. [Google Scholar] [CrossRef]
- Sonbol, H.; Ameen, F.; AlYahya, S.; Almansob, A.; Alwakeel, S. Padina boryana Mediated Green Synthesis of Crystalline Palladium Nanoparticles as Potential Nanodrug against Multidrug Resistant Bacteria and Cancer Cells. Sci. Rep. 2021, 11, 5444. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Zhou, Z.; Wu, X.; Wang, Z.; Qi, W.; Yang, J.; Qing, L.; Tang, J.; Deng, L. Down-Regulation of Hsp by Pd-Cu Nanozymes for Nir Light Triggered Mild-Temperature Photothermal Therapy against Wound Bacterial Infection: In Vitro and in Vivo Assessments. Int. J. Nanomed. 2023, 18, 4805–4819. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Lee, J.-W.; Pham, D.N.T.; Khan, M.M.; Park, S.-K.; Shin, I.-S.; Kim, Y.-M. Antibiofilm Action of ZnO, SnO2 and CeO2 Nanoparticles towards Gram-Positive Biofilm Forming Pathogenic Bacteria. Recent Pat. Nanotechnol. 2020, 14, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Togawa, G.; Takahashi, M.; Tada, H.; Takada, Y. Development of Ternary Ti-Ag-Cu Alloys with Excellent Mechanical Properties and Antibiofilm Activity. Materials 2022, 15, 9011. [Google Scholar] [CrossRef]
- Chen, Q.; Qi, M.; Shi, F.; Liu, C.; Shi, Y.; Sun, Y.; Bai, X.; Wang, L.; Sun, X.; Dong, B.; et al. Novel Twin-Crystal Nanosheets with MnO2 Modification to Combat Bacterial Biofilm against Periodontal Infections via Multipattern Strategies. Adv. Healthc. Mater. 2023, 12, 2300313. [Google Scholar] [CrossRef]
- Aziz, S.N.; Al-Kadmy, I.M.S.; Rheima, A.M.; Al-Sallami, K.J.; Abd Ellah, N.H.; El-Saber Batiha, G.; El-Bouseary, M.M.; Algammal, A.M.; Hetta, H.F. Binary CuO\CoO Nanoparticles Inhibit Biofilm Formation and Reduce the Expression of Papc and Fimh Genes in Multidrug-Resistant Klebsiella Oxytoca. Mol. Biol. Rep. 2023, 50, 5969–5976. [Google Scholar] [CrossRef]
- Leung, Y.H.; Xu, X.; Ma, A.P.Y.; Liu, F.; Ng, A.M.C.; Shen, Z.; Gethings, L.A.; Guo, M.Y.; Djurisic, A.B.; Lee, P.K.H.; et al. Toxicity of ZnO and TiO2 to Escherichia Coli Cells. Sci. Rep. 2016, 6, 35243. [Google Scholar] [CrossRef]
- Yuan, G.; Zhang, S.; Yang, Z.; Wu, S.; Chen, H.; Tian, X.; Cheng, S.; Pan, Y.; Zhou, R. Precisely Modulated 2D PdCu Alloy Nanodendrites as Highly Active Peroxidase Mimics for the Elimination of Biofilms. Biomater. Sci. 2022, 10, 7067–7076. [Google Scholar] [CrossRef]
- Li, Y.; Yang, L.; Liao, Y.; Zhao, R.; Ji, L.; Su, R.; Xu, D.; Wang, F. Photothermal Heating-Assisted Superior Antibacterial and Antibiofilm Activity of High-Entropy-Alloy Nanoparticles. Adv. Funct. Mater. 2023, 33, 2302712. [Google Scholar] [CrossRef]
- Mamani, J.B.; Borges, J.P.; Rossi, A.M.; Gamarra, L.F. Magnetic Nanoparticles for Therapy and Diagnosis in Nanomedicine. Pharmaceutics 2023, 15, 1663. [Google Scholar] [CrossRef]
- Liu, W.; Pei, W.; Moradi, M.; Zhao, D.; Li, Z.; Zhang, M.; Xu, D.; Wang, F. Polyethyleneimine Functionalized Mesoporous Magnetic Nanoparticles with Enhanced Antibacterial and Antibiofilm Activity in an Alternating Magnetic Field. ACS Appl. Mater. Interfaces 2022, 14, 18794–18805. [Google Scholar] [CrossRef]
- Ji, Y.; Han, Z.; Ding, H.; Xu, X.; Wang, D.; Zhu, Y.; An, F.; Tang, S.; Zhang, H.; Deng, J.; et al. Enhanced Eradication of Bacterial/Fungi Biofilms by Glucose Oxidase-Modified Magnetic Nanoparticles as a Potential Treatment for Persistent Endodontic Infections. ACS Appl. Mater. Interfaces 2021, 13, 17289–17299. [Google Scholar] [CrossRef] [PubMed]
- Mutalik, C.; Saukani, M.; Khafid, M.; Krisnawati, D.I.; Widodo, R.; Darmayanti, R.; Puspitasari, B.; Cheng, T.-M.; Kuo, T.-R. Gold-Based Nanostructures for Antibacterial Application. Int. J. Mol. Sci. 2023, 24, 10006. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.; Qiao, Z.; Yan, D.; Yang, M.; Yang, L.; Wan, X.; Chen, H.; Luo, J.; Xiao, H. Ciprofloxacin Conjugated Gold Nanorods with pH Induced Surface Charge Transformable Activities to Combat Drug Resistant Bacteria and Their Biofilms. Mater. Sci. Eng. C-Mater. Biol. Appl. 2021, 128, 112292. [Google Scholar] [CrossRef]
- Aguilera-Correa, J.J.; Garcia-Alvarez, R.; Mediero, A.; Esteban, J.; Vallet-Regi, M. Effect of Gold Nanostars plus Amikacin against Carbapenem-Resistant Klebsiella pneumoniae Biofilms. Biology 2022, 11, 162. [Google Scholar] [CrossRef]
- He, X.; Dai, L.; Ye, L.; Sun, X.; Enoch, O.; Hu, R.; Zan, X.; Lin, F.; Shen, J. A Vehicle-Free Antimicrobial Polymer Hybrid Gold Nanoparticle as Synergistically Therapeutic Platforms for Staphylococcus aureus Infected Wound Healing. Adv. Sci. 2022, 9, 2105223. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.Z.; Wang, T.J.; Feng, J.H.; Rong, F.; Wang, K.; Li, P.; Huang, W. Photoactivatable Nitric Oxide-Releasing Gold Nanocages for Enhanced Hyperthermia Treatment of Biofilm-Associated Infections. ACS Appl. Mater. Interfaces 2021, 13, 50668–50681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhang, H.; Feng, J.; Zhou, Y.; Wang, B. Synergistic Chemotherapy, Physiotherapy and Photothermal Therapy against Bacterial and Biofilms Infections through Construction of Chiral Glutamic Acid Functionalized Gold Nanobipyramids. Chem. Eng. J. 2020, 393, 124778. [Google Scholar] [CrossRef]
- Liu, C.; Wang, J.; Wan, J.; Yu, C. MOF-on-MOF Hybrids: Synthesis and Applications. Coord. Chem. Rev. 2021, 432, 213743. [Google Scholar] [CrossRef]
- He, Y.; Wang, X.; Zhang, C.; Sun, J.; Xu, J.; Li, D. Near-Infrared Light-Mediated Cyclodextrin Metal-Organic Frameworks for Synergistic Antibacterial and Anti-Biofilm Therapies. Small 2023, 19, 2300199. [Google Scholar] [CrossRef]
- Fan, X.; Wu, X.; Yang, F.; Wang, L.; Ludwig, K.; Ma, L.; Trampuz, A.; Cheng, C.; Haag, R. A Nanohook-Equipped Bionanocatalyst for Localized Near-Infrared-Enhanced Catalytic Bacterial Disinfection. Angew. Chem. Int. Ed. 2022, 61, e202113833. [Google Scholar] [CrossRef] [PubMed]
- Concepcion, O.; de Melo, O. The Versatile Family of Molybdenum Oxides: Synthesis, Properties, and Recent Applications. J. Phys.—Condens. Matter 2023, 35, 143002. [Google Scholar] [CrossRef]
- Obaid, N.M.; Al-Nafiey, A.; Al-Dahash, G. Graphene/Molybdenum Disulfide Nanocomposites: Characterization and Optoelectronic Application. J. Nanophoton. 2023, 17, 010901. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Guo, C.; Wang, Y. Molybdenum Carbide-Based Photocatalysts: Synthesis, Functionalization, and Applications. Langmuir 2022, 38, 12739–12756. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Gong, M.; Xiao, J.; Hai, L.; Luo, Y.; He, L.; Wang, Z.; Deng, L.; He, D. Photothermally Activated Multifunctional MoS2 Bactericidal Nanoplatform for Combined Chemo/Photothermal/Photodynamic Triple-Mode Therapy of Bacterial and Biofilm Infections. Chem. Eng. J. 2022, 429, 132600. [Google Scholar] [CrossRef]
- Li, H.; Yang, K.; Hai, L.; Wang, Z.; Luo, Y.; He, L.; Yi, W.; Li, J.; Xu, C.; Deng, L.; et al. Photothermal-Triggered Release of Alkyl Radicals and Cascade Generation of Hydroxyl Radicals via a Versatile Hybrid Nanocatalyst for Hypoxia-Irrelevant Synergistic Antibiofilm Therapy. Chem. Eng. J. 2023, 455, 140903. [Google Scholar] [CrossRef]
- Tasia, W.; Lei, C.; Cao, Y.; Ye, Q.; He, Y.; Xu, C. Enhanced Eradication of Bacterial Biofilms with DNase I-Loaded Silver-Doped Mesoporous Silica Nanoparticles. Nanoscale 2020, 12, 2328–2332. [Google Scholar] [CrossRef]
- Mohammadpour, R.; Dobrovolskaia, M.A.; Cheney, D.L.; Greish, K.F.; Ghandehari, H. Subchronic and Chronic Toxicity Evaluation of Inorganic Nanoparticles for Delivery Applications. Adv. Drug Deliv. Rev. 2019, 144, 112–132. [Google Scholar] [CrossRef]
- Xia, L.; Park, J.H.; Biggs, K.; Lee, C.G.; Liao, L.; Shannahan, J.H. Compositional Variations in Metal Nanoparticle Components of Welding Fumes Impact Lung Epithelial Cell Toxicity. J. Toxicol. Environ. Health Part A 2023, 86, 735–757. [Google Scholar] [CrossRef]
- Dianova, L.; Tirpak, F.; Halo, M.; Slanina, T.; Massanyi, M.; Stawarz, R.; Formicki, G.; Madeddu, R.; Massanyi, P. Effects of Selected Metal Nanoparticles (Ag, ZnO, TiO2) on the Structure and Function of Reproductive Organs. Toxics 2022, 10, 459. [Google Scholar] [CrossRef]
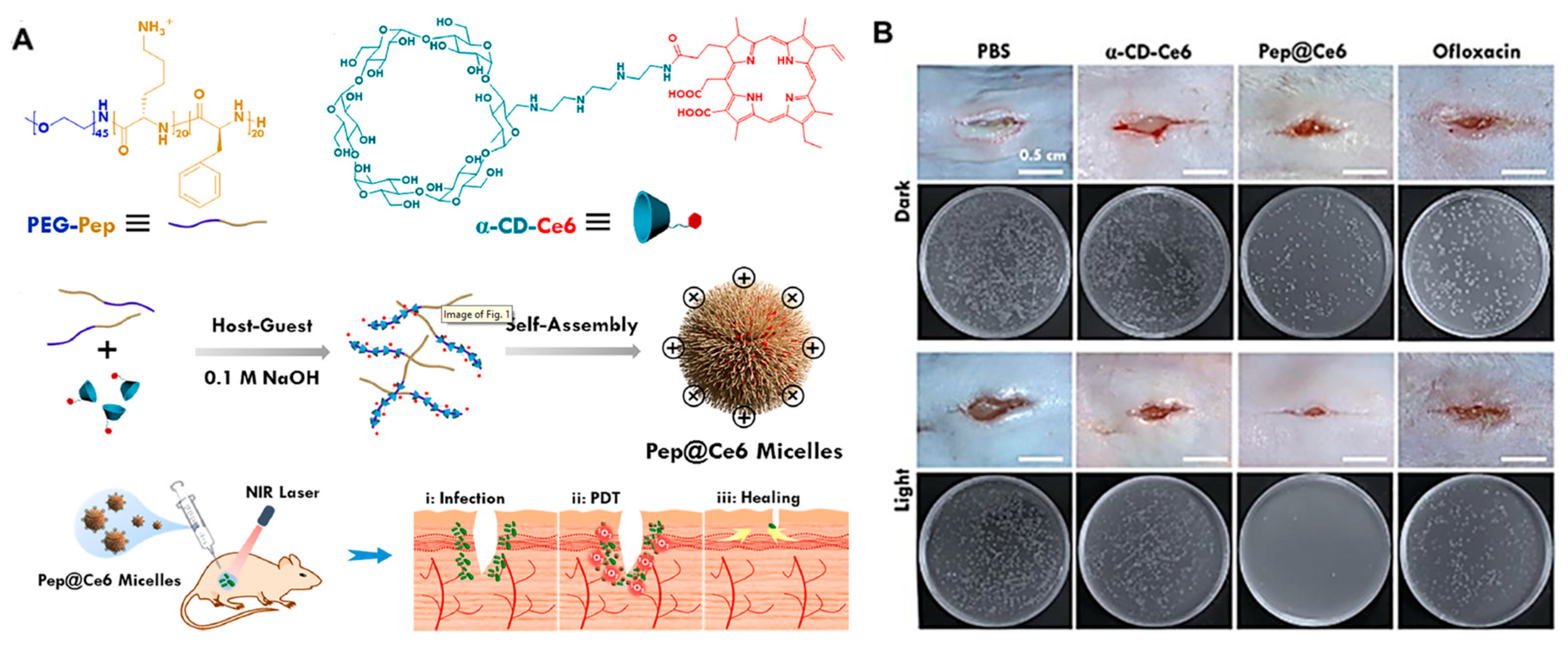

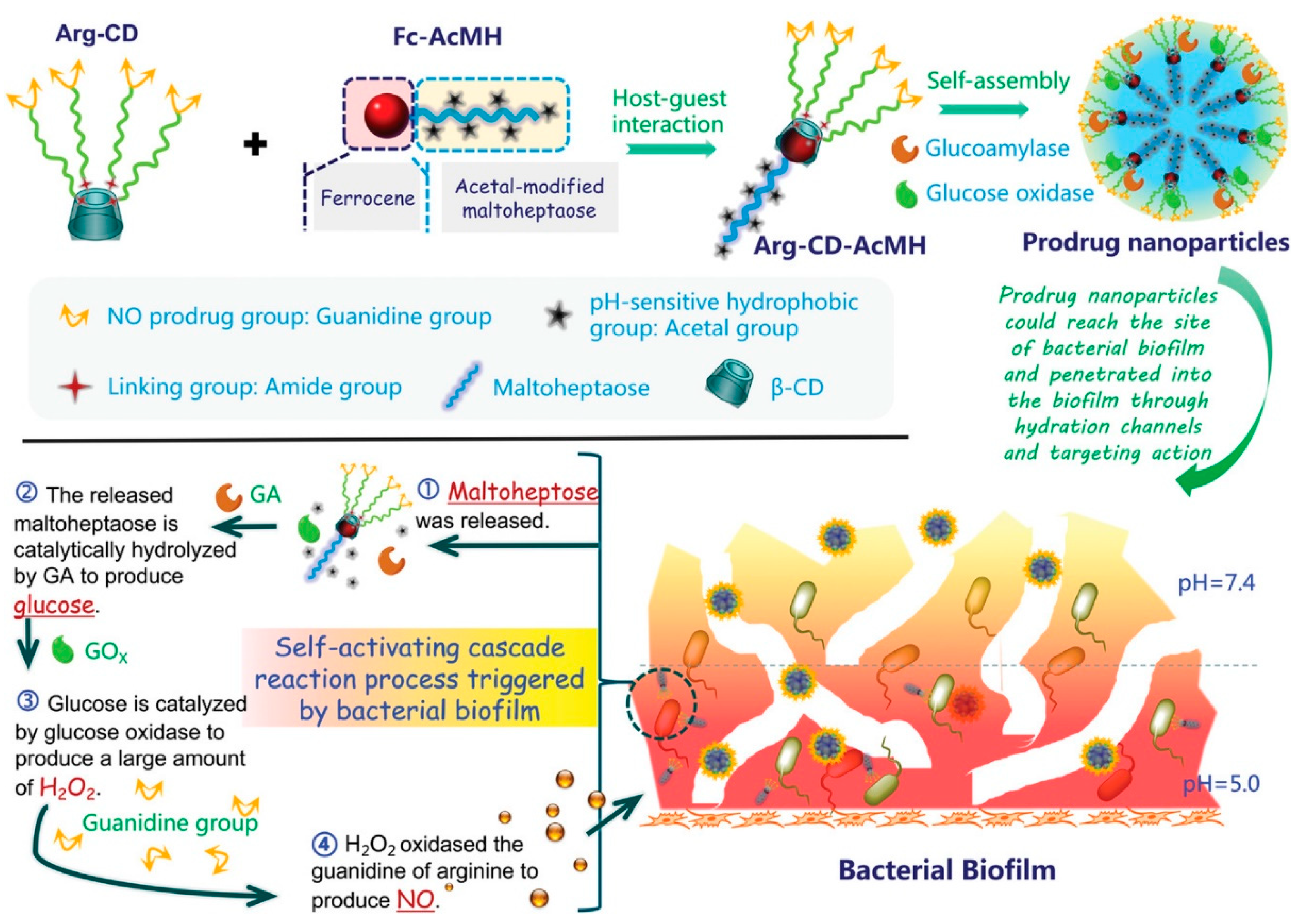

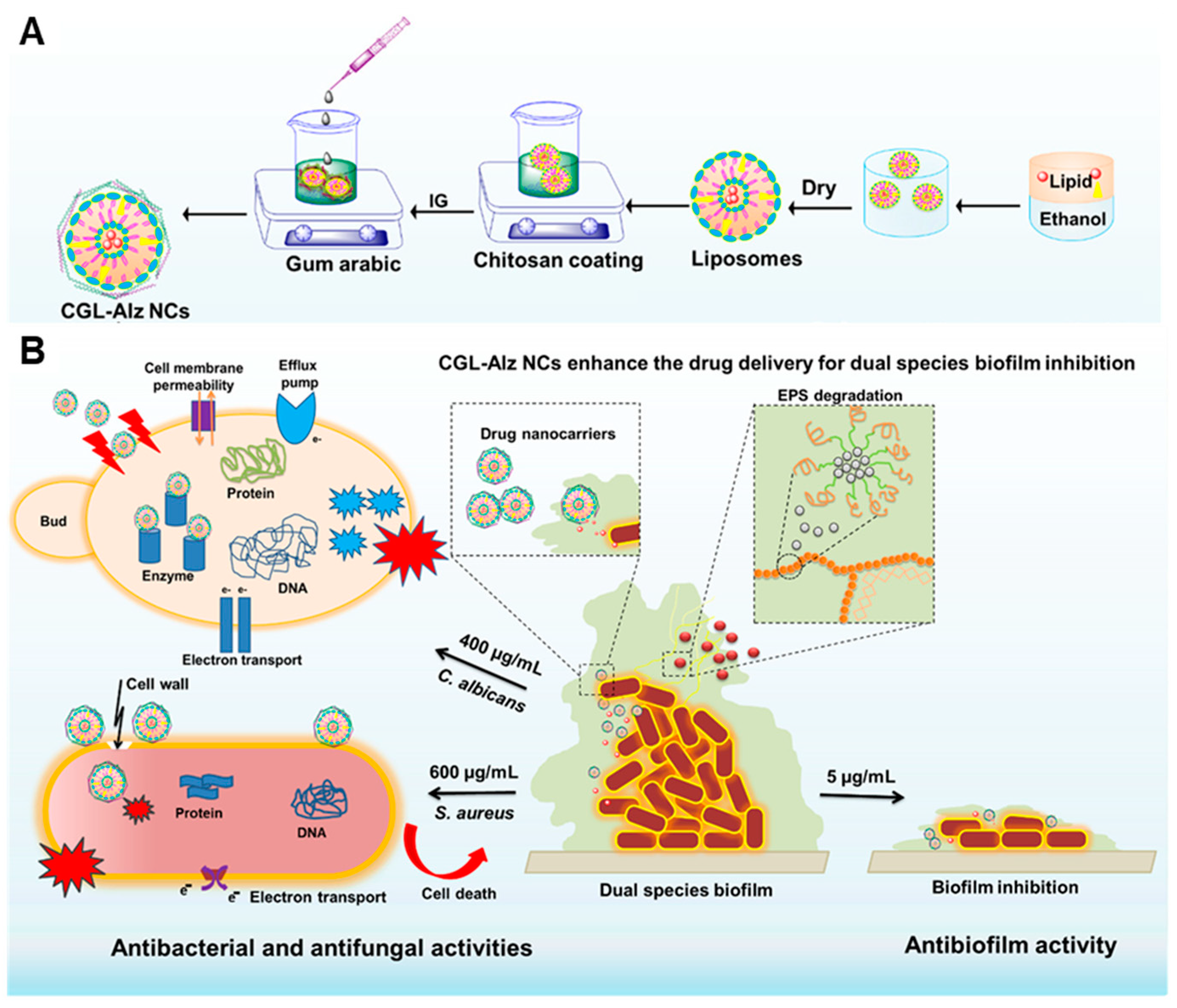



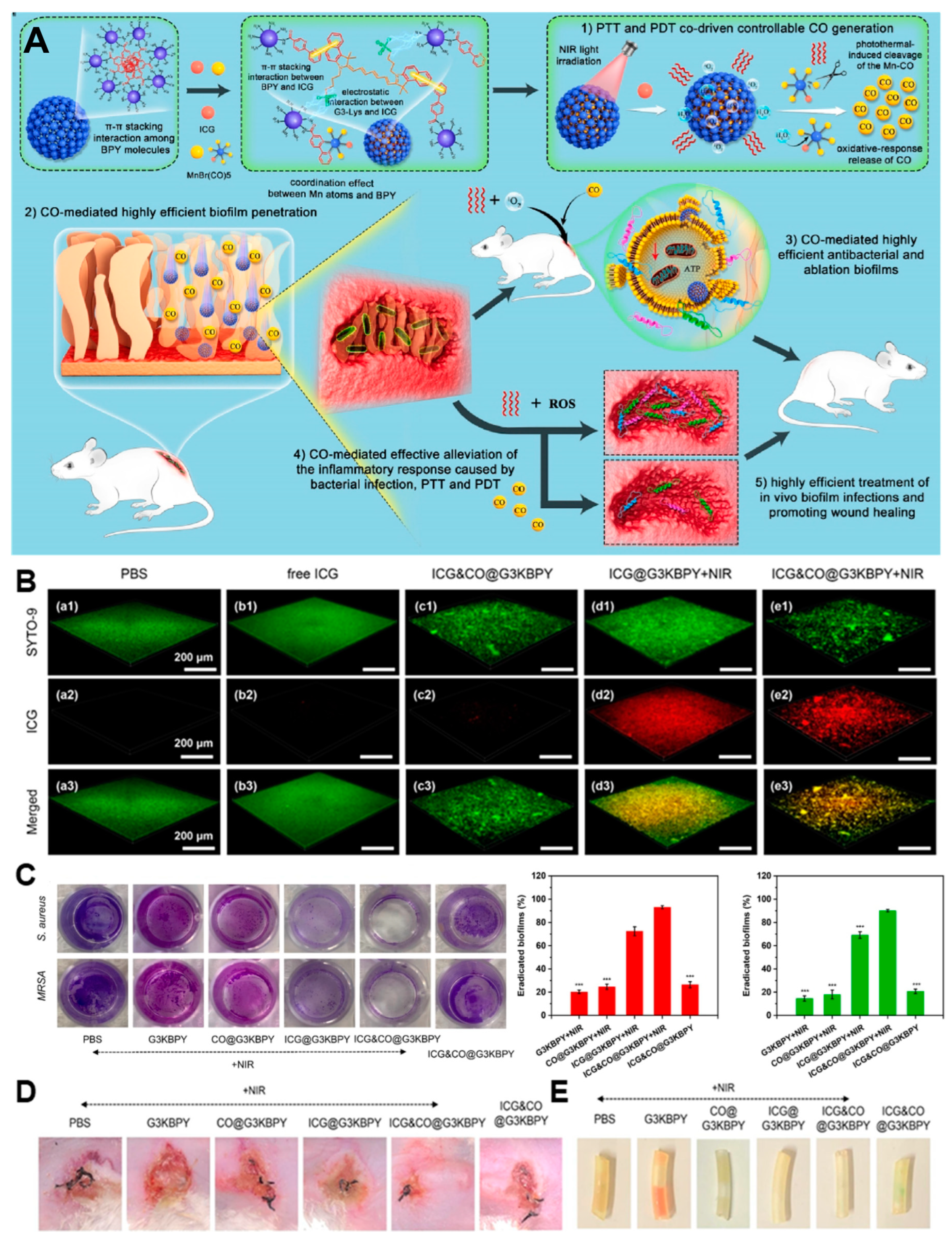

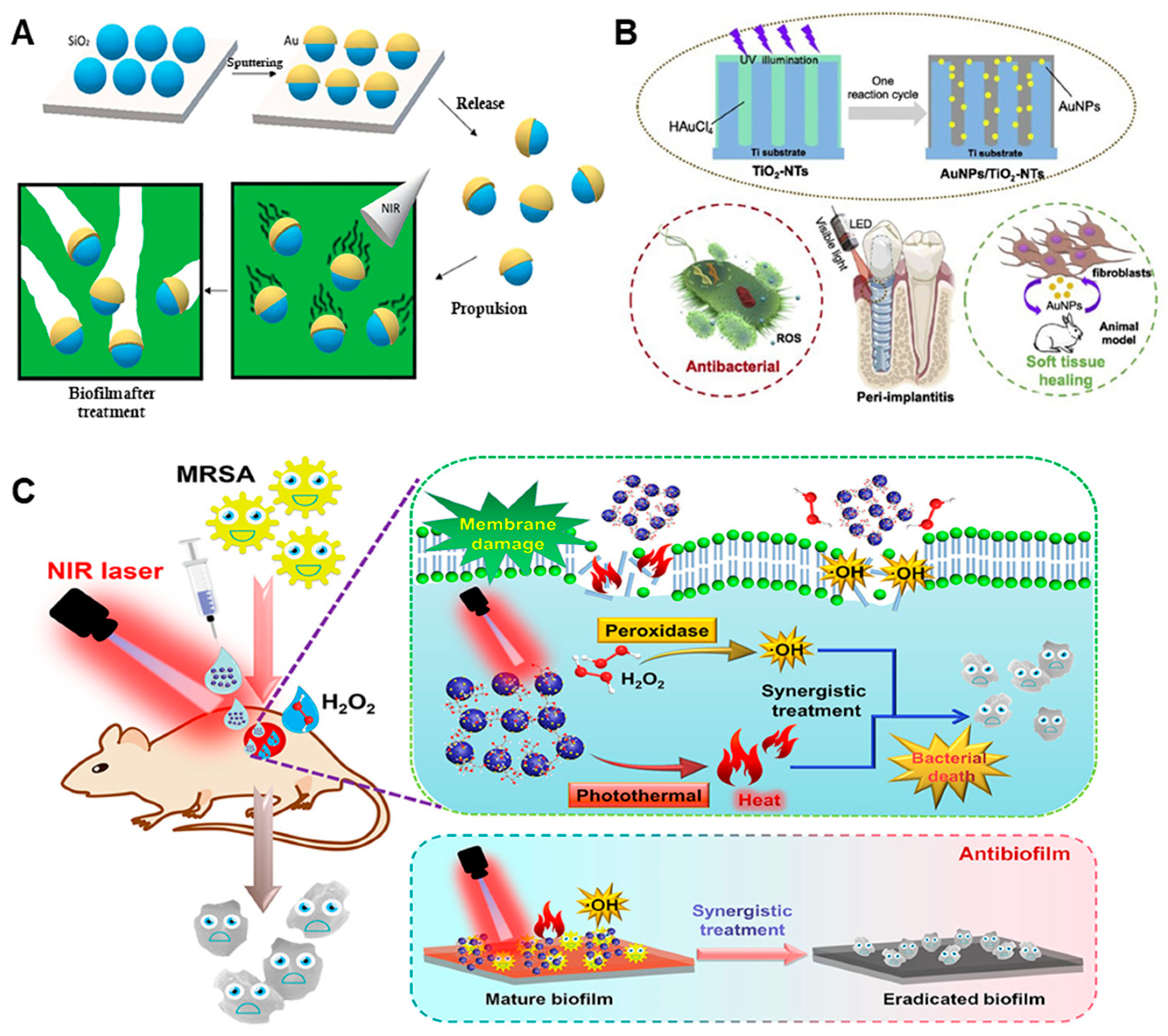


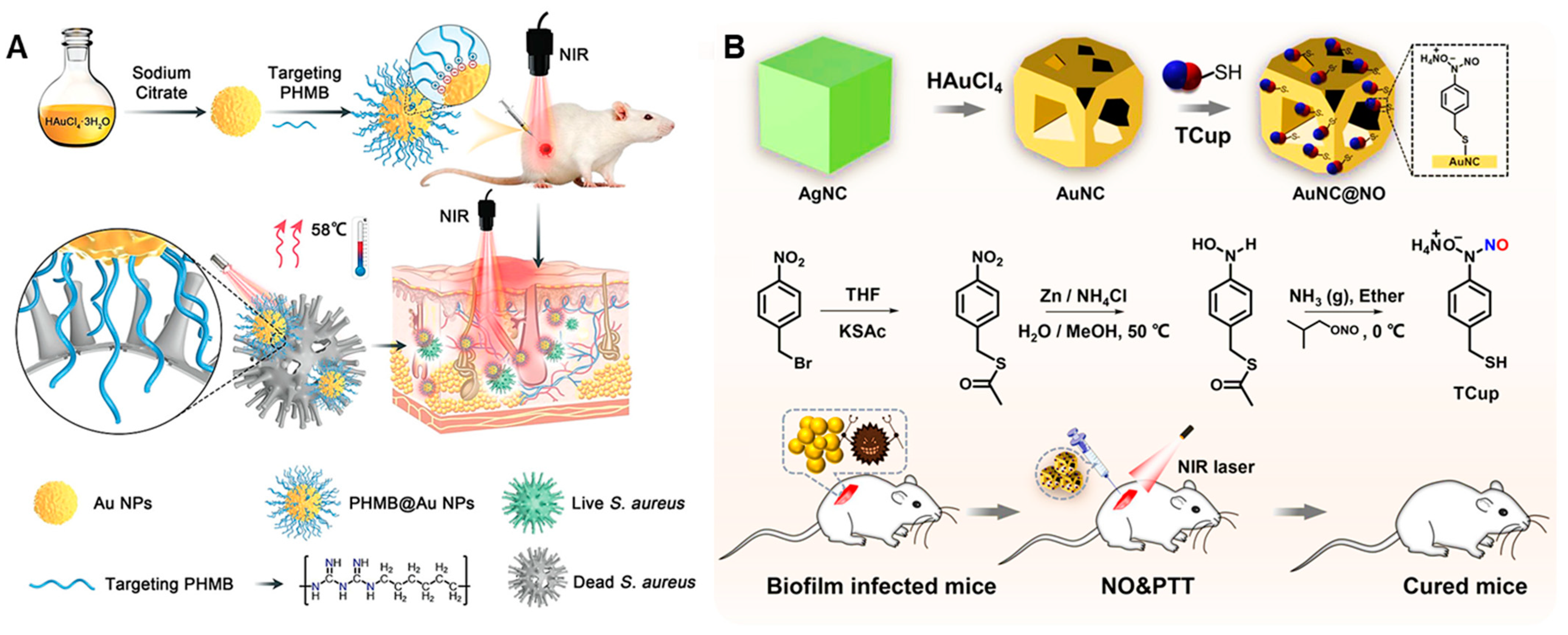



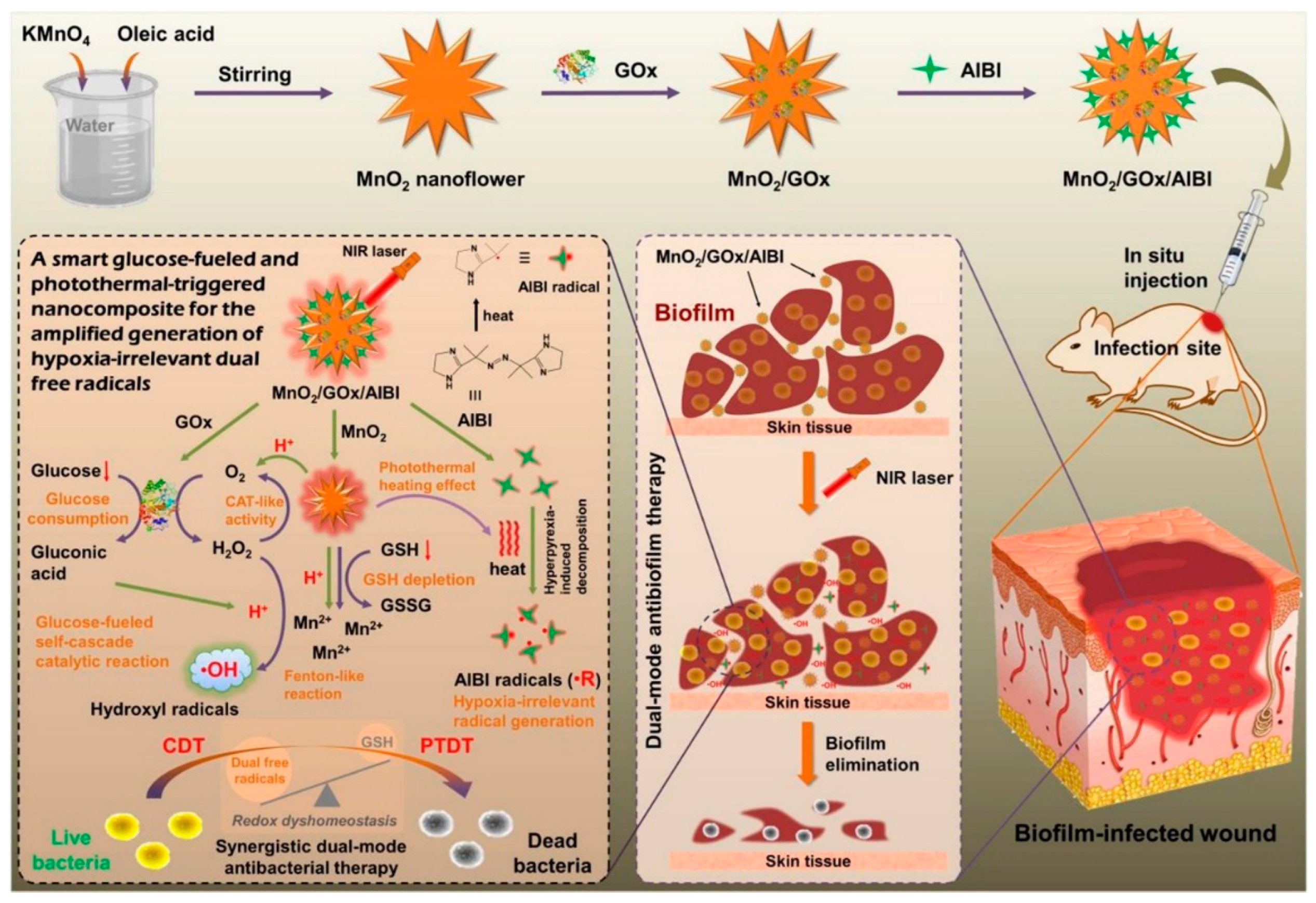
| Anti-Biological Film Agent | Functional Unit | Types of Anti-Biofilm | Mechanism | References |
|---|---|---|---|---|
| Pep@Ce6 micelle | Coupling photosensitizing agent Ce6 (α-CD-Ce6), PEG-Pep | Reduced the thickness of P. aeruginosa biofilm from 25 μM to 14 μM. | PDT | [45] |
| PECL@PTTA micelle | The hydrophilic fragment PECL-ad, hydrophobic fragment cd-PTTA includes β-cyclodextrin, PBA, photosensitizer TPE, thione linker and Amp | The eradication rate of MRSA biofilm was 83%. | Antibiotic Amp, PDT | [46] |
| Arg-CD-AcMH | L-arginine modified with β-CD (hydrophilic fragment), acetalized maltoheptaose modified with ferrocene (hydrophobic fragment), GOx and GA | The biofilm formed by E. coli and S. aureus could be almost completely eliminated. | Enzymatic cascade catalysis continuously releases NO and damages biofilm. | [47] |
| α-CD-Ce6-NO-DA | NO-prodrug (α-CD-NO), photosensitizer Ce6-prodrug (α-CD-Ce6) and pH-sensitive PEG-(KLAKLAK)2-DA | The MRSA biofilm could be almost completely eliminated. | NO/PDT | [1] |
| CGL-Alz NCs | Alizarin, liposome and chitosan–gum arabic-coated. | The formation rate of C. albicans and E. coli biofilm were 90–95% | Leading to protein inactivation, interaction with nucleic acids (DNA and RNA), alteration of efflux pumps, and destruction of membrane integrity. | [48] |
| Lip-Ce6-PFH@O2 | O2 carrier PFH, photosensitizer Ce6 and liposome | The eradication rate of P. aeruginosa biofilm is 90.1%. | O2 enhanced PDT | [49] |
| RB@PMB@GA NPs | RB-NH2, PDA, PMB and gluconic acid | At pH 5.0, the penetration and eradication rate of P. aeruginosa biofilm were, respectively, 93% and 100%. | PDT/PTT synergistic antibacterial activity. | [50] |
| AZM-DA NPs | PAMAM-AZM NPs, PEG-b-PLys | The bactericidal rate of AZM-DA NPs for bacteria in P. aeruginosa biofilm was 99.998%. | Antibiotic AZM | [51] |
| PRZ nanocomplex | Protease K, photosensitizer RB and ZIF-8. | The eradication rate of S. aureus biofilm was 95%. | Protease K decomposes the protein, PDT | [52] |
| AI-MPDA | L-Arg, MPDA and photosensitizer ICG | The eradication rate of S. aureus biofilm was 99%. | NO/PDT/PTT | [53] |
| ICG&CO@G3KBPY | ICG, CO precursor MnBr(CO)5 and nanogel G3KBPY. | The penetration and eradication rate of S. aureus biofilm were, respectively, 99% and 93%. | CO/PDT/PTT | [55] |
| Amphiphilic oligamine(3a) | p-ethylphthalimidate and various diamines | MBIC (S. aureus, E. coli, P. aeruginosa) = 16 μg·mL−1, MBEC (S. aureus, E.coli, P. aeruginosa) = 64, 128 and 16 μg·mL−1. | Membrane penetration, DNA destruction, and ROS oxidative stress were synergistic in antibacterial activity. | [57] |
| PPa-cP | Pyrophosphoramide (PPa), cationic polypeptide (cP) | The eradication rate of C. albicans biofilm was 87.2%. | PDT | [58] |
| Mesoporous SiO2/Au nanomotors | Mesoporous SiO2, Au NPs | The eradication rate of P. aeruginosa biofilm was 71% at 3 min. | Physical damage | [60] |
| Au NPs/TiO2-NTs | Au NPs, TiO2-NTs | The inhibition rate of multispecies biofilms was 99%. | ROS | [61] |
| Au/MoO3−x Nanoenzyme | MoO3−x, Au NPs | The MRSA biofilm could be almost completely eliminated. | POD-like/PTT | [62] |
| Ag2O2 NPs | Ag2O2 NPs | The eradication rate of MRSA biofilm was 95%. | Ag/SDT/PTT | [67] |
| ZnO@Ag nanocomposites | Different proportions of Ag NPs (0%, 0.5%, 2%, 8%), ZnO | The formation of S. aureus biofilm could be almost completely inhibited. | Ag/PDT/PTT | [68] |
| 2D PdCu alloy nanodendrites | Cu, Pd | The eradication rate of P. aeruginosa biofilm was 60%. | POD-like | [78] |
| FeNiTiCrMnCux HEA-NPs | Cu | The eradication rate of P. aeruginosa biofilm was 97.4%. | PTT | [79] |
| Fe3O4@PEI NPs | Fe3O4 NPs, PEI | The eradication rates of S. aureus, E. coli and P. aeruginosa biofilms were, respectively, 87.4%, 84.9% and 85.8%. | Physical damage/PTT | [81] |
| GMNPs | GOx, magnetic nanoparticles | The E. faecalis and C. albicans biofilms could be almost completely eliminated. | Cascade catalysis/physical damage | [82] |
| PHMB@Au NPs | Antibacterial agent PHMB, Au NPs | The inhibition rate of S. aureus biofilm was 85%. | Chemotherapeutic/PDT | [86] |
| AuNC@NO | AuNC, NO donor Tcup | Dispersing MRSA biofilm, resulting in a decrease of 6 orders of magnitude in residual bacteria within the biofilm. | NO/PDT | [87] |
| D/L-Glu-Au NBPs | D-Glu, L-Glu and Au NBPs | The eradication rate of the S. epidermidis biofilm was, respectively, 80% and 90% by L-Glu-Au NBPs and D-Glu-Au NBPs. | PTT | [88] |
| Ag@MOF@PDA | CD-MOF, PDA and Ag NPs | The eradication rate of S. aureus biofilm was 75%. | Ag/PDT/PTT | [90] |
| Ni@Co-NC | MOF, Co/Ni NPs | The MRSA biofilms could be almost completely eliminated. | POD-like/PTT | [91] |
| MoS2/ICG/Ag | Ag NPs, MoS2 and ICG | The inhibition and eradication rates of S. aureus biofilm were, respectively, 77.9% and 96.8%. | chemical/PTT/PDT | [95] |
| MnO2/GOx/AIBI | MnO2,·R precursor AIBI and GOx | The inhibition and eradication rates of S. aureus biofilm were, respectively, 64.7% and 88.5%. | PDT/PTT related to hypoxia. | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, R.; Cui, Y.; Liu, J.; Wang, X.; Yuan, H. Recent Advances of Composite Nanomaterials for Antibiofilm Application. Nanomaterials 2023, 13, 2725. https://doi.org/10.3390/nano13192725
Qi R, Cui Y, Liu J, Wang X, Yuan H. Recent Advances of Composite Nanomaterials for Antibiofilm Application. Nanomaterials. 2023; 13(19):2725. https://doi.org/10.3390/nano13192725
Chicago/Turabian StyleQi, Ruilian, Yuanyuan Cui, Jian Liu, Xiaoyu Wang, and Huanxiang Yuan. 2023. "Recent Advances of Composite Nanomaterials for Antibiofilm Application" Nanomaterials 13, no. 19: 2725. https://doi.org/10.3390/nano13192725
APA StyleQi, R., Cui, Y., Liu, J., Wang, X., & Yuan, H. (2023). Recent Advances of Composite Nanomaterials for Antibiofilm Application. Nanomaterials, 13(19), 2725. https://doi.org/10.3390/nano13192725






