A Mini Review of S-Nitrosoglutathione Loaded Nano/Micro-Formulation Strategies
Abstract
:1. Introduction
2. Direct GSNO Loaded Nano/Micro-Formulation
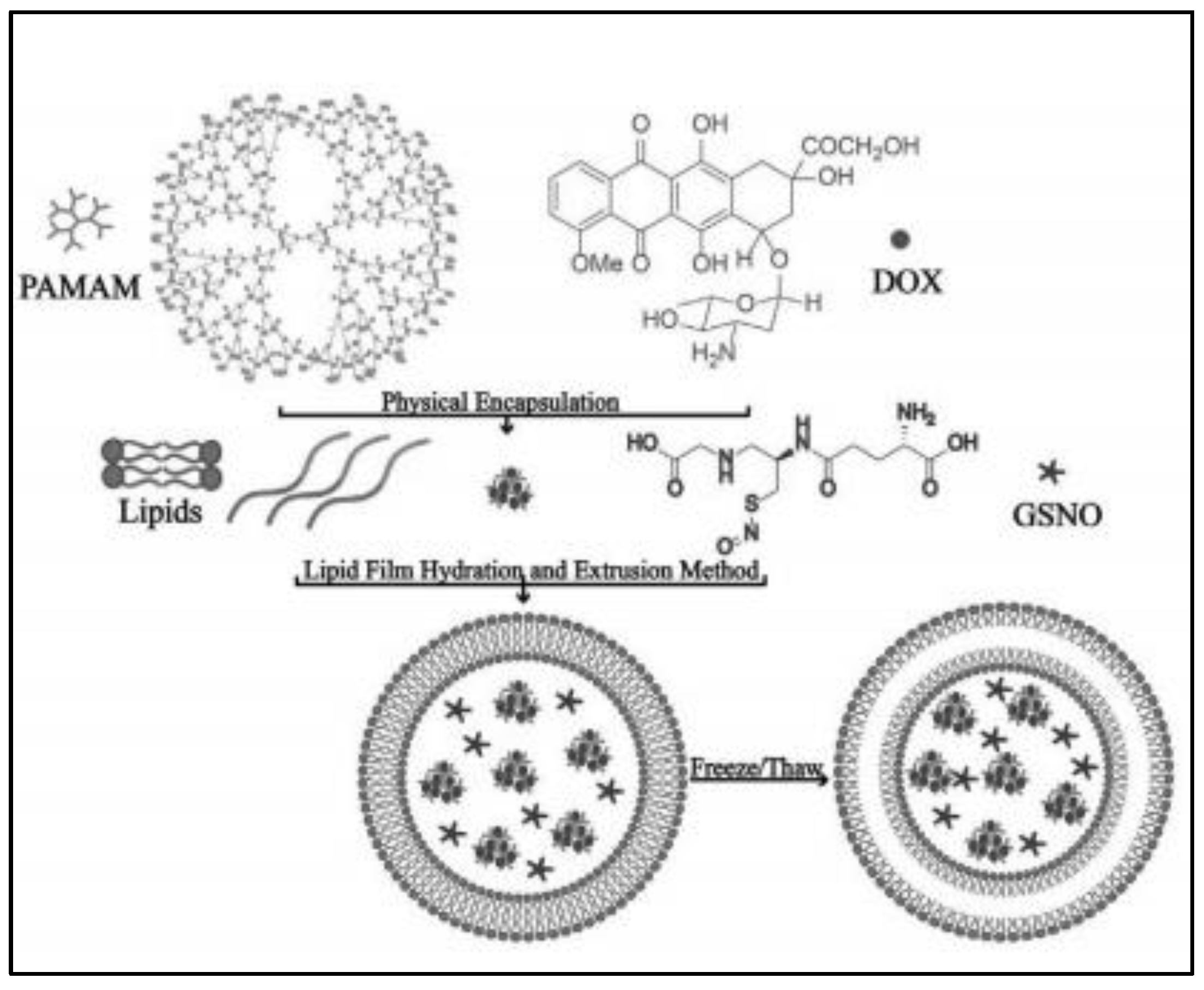
2.1. Liposomes
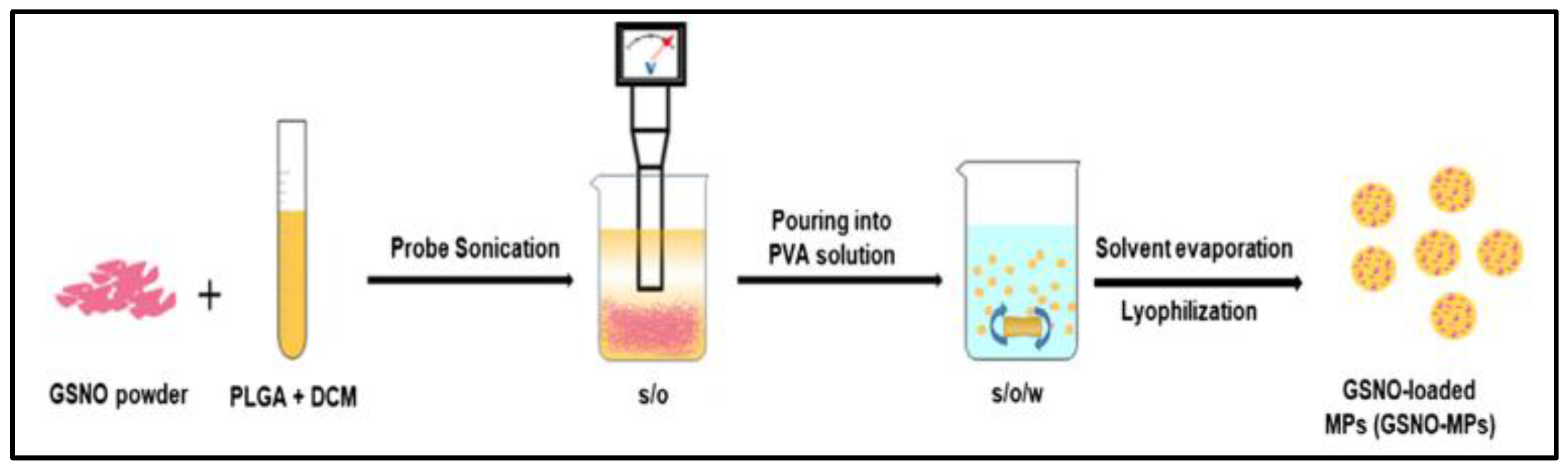
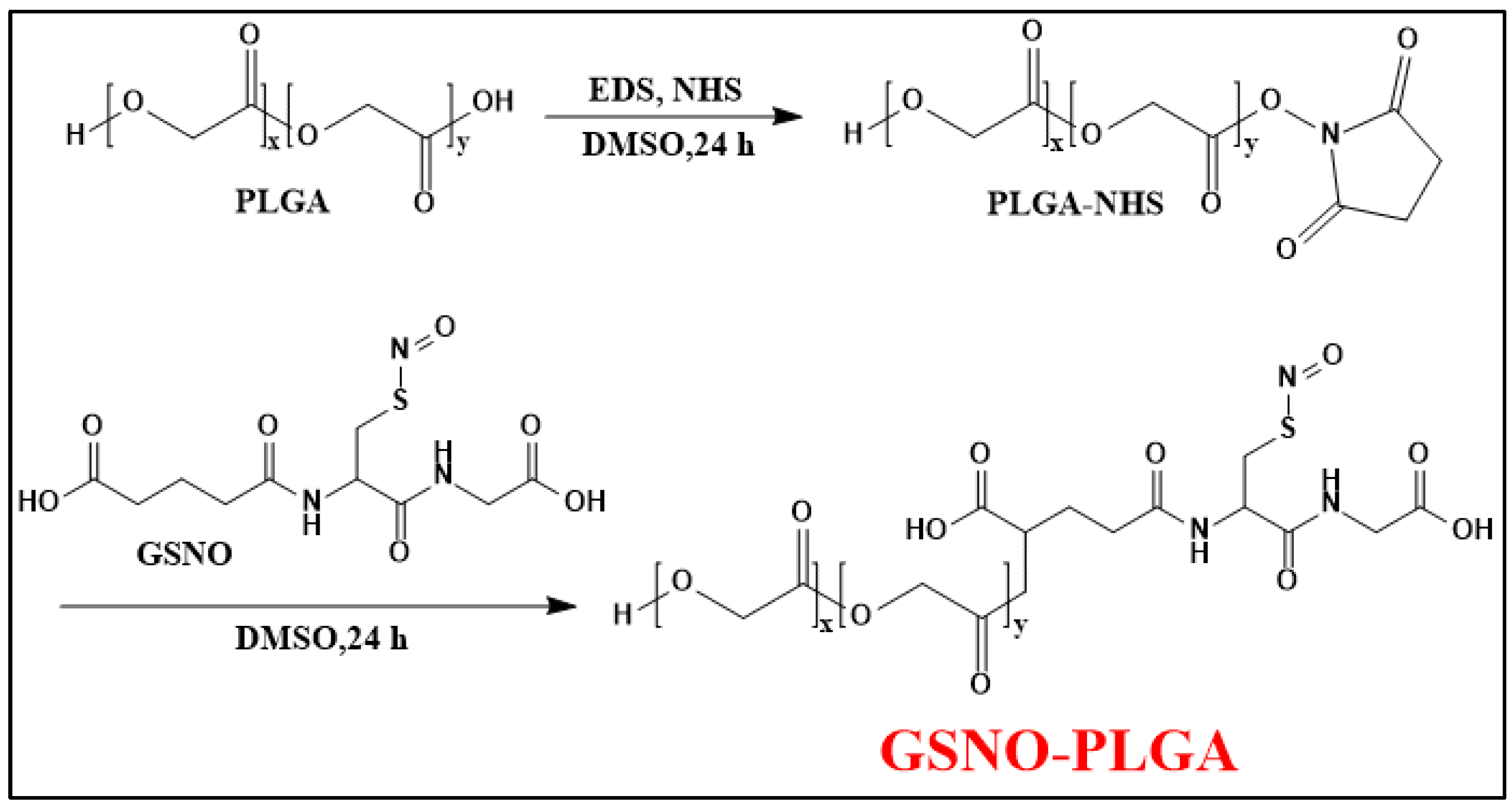
2.2. Polymeric Nano/Micro-Particles
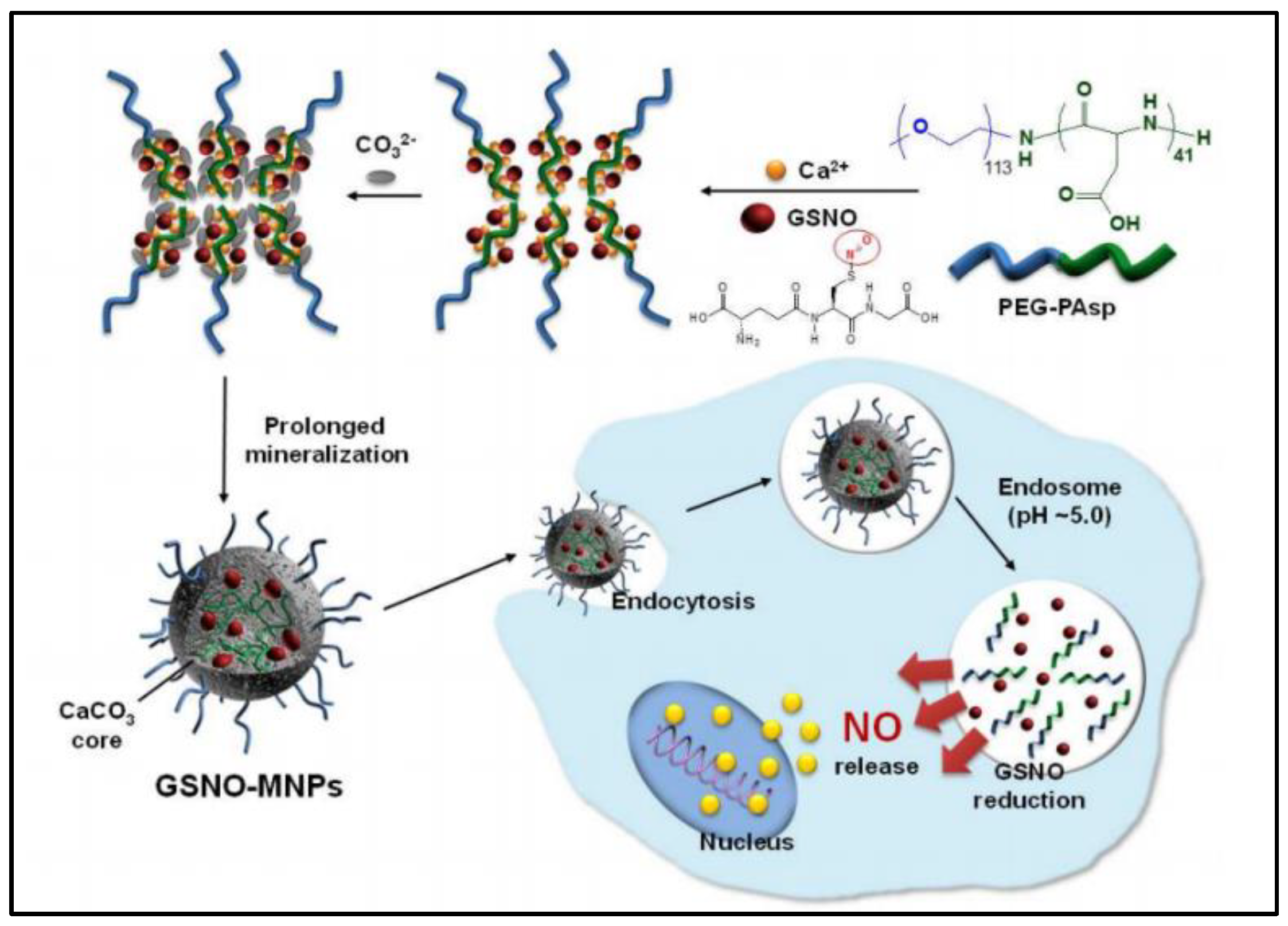
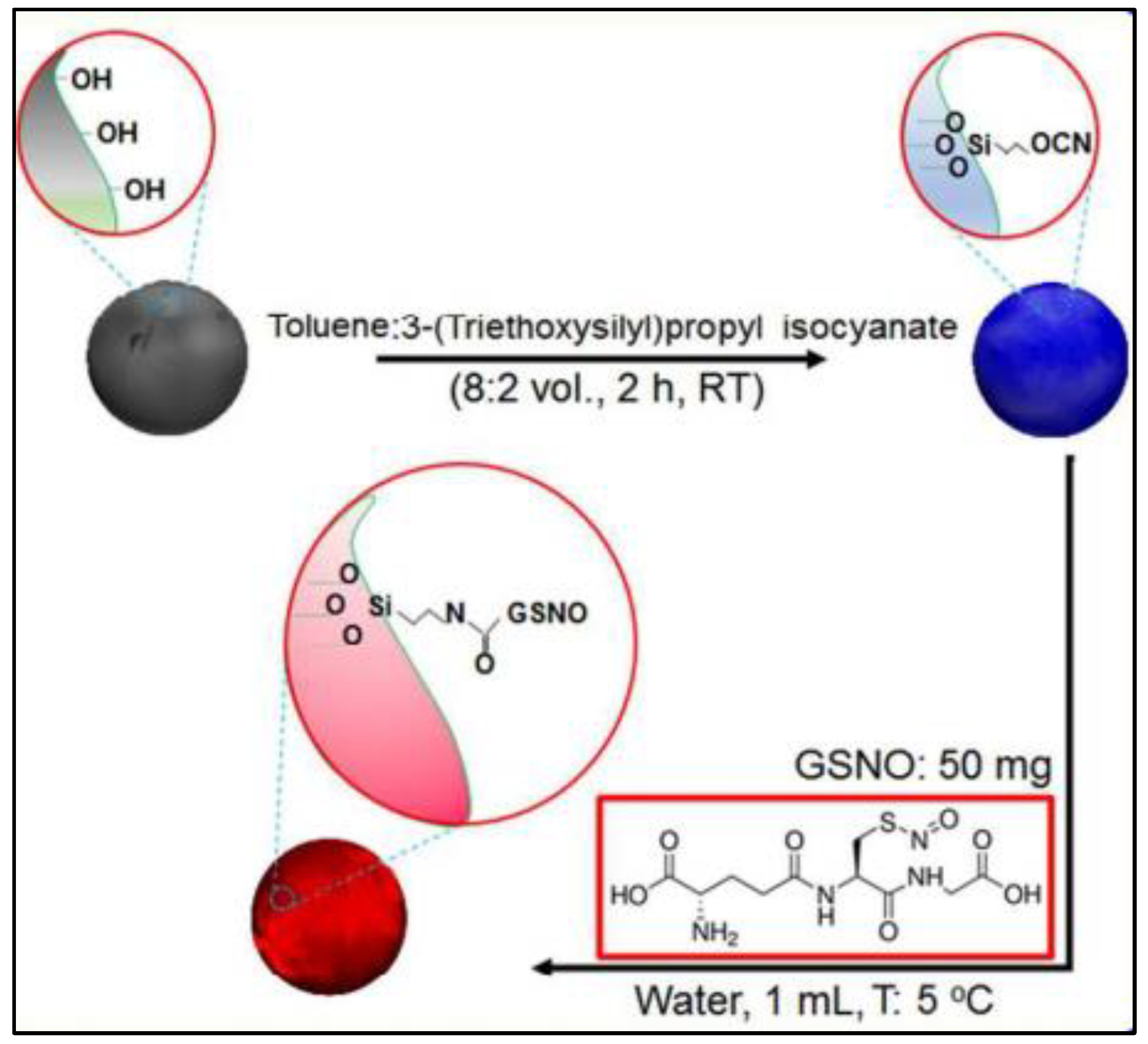
2.3. Inorganic Nanoparticles
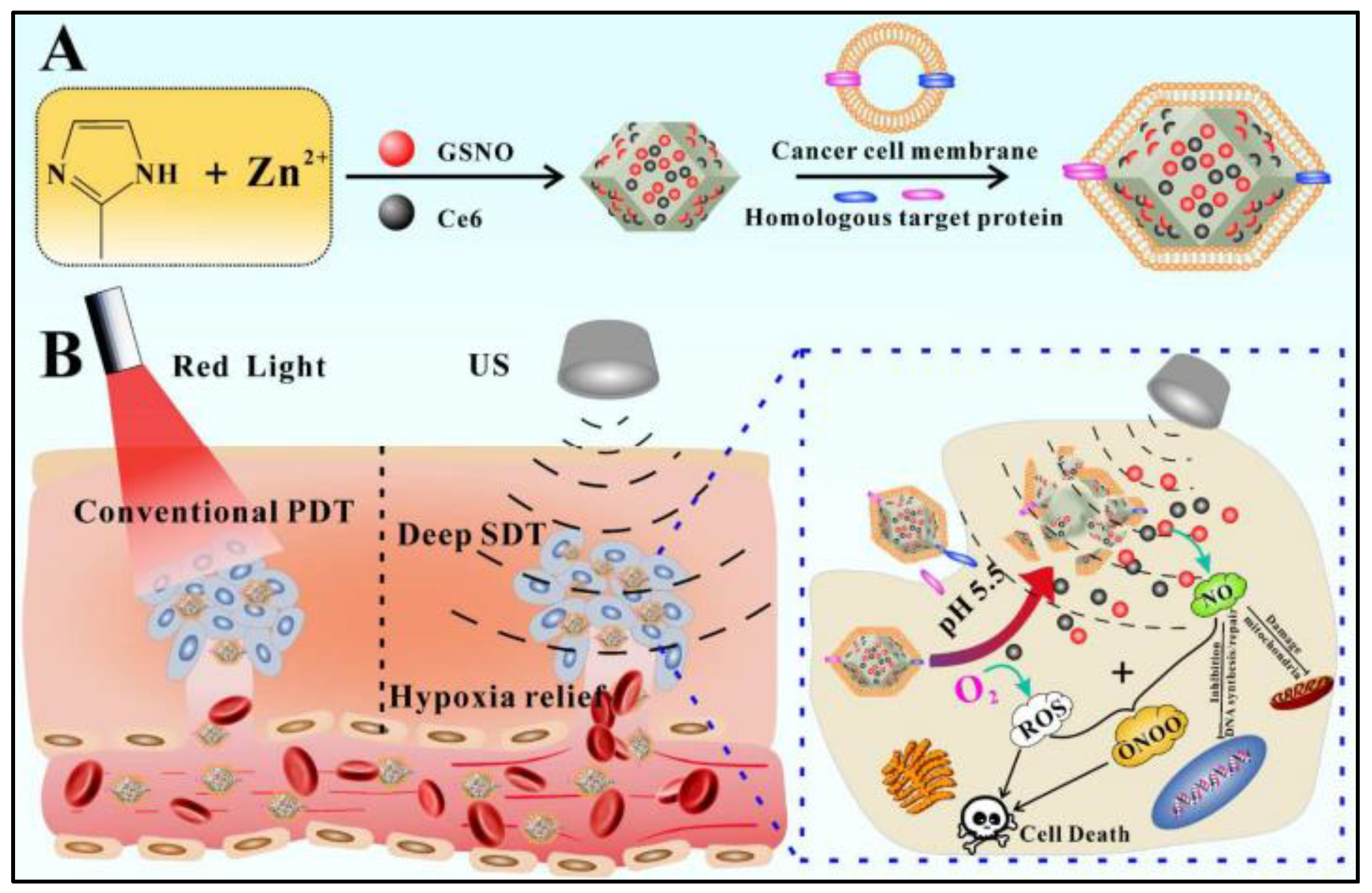
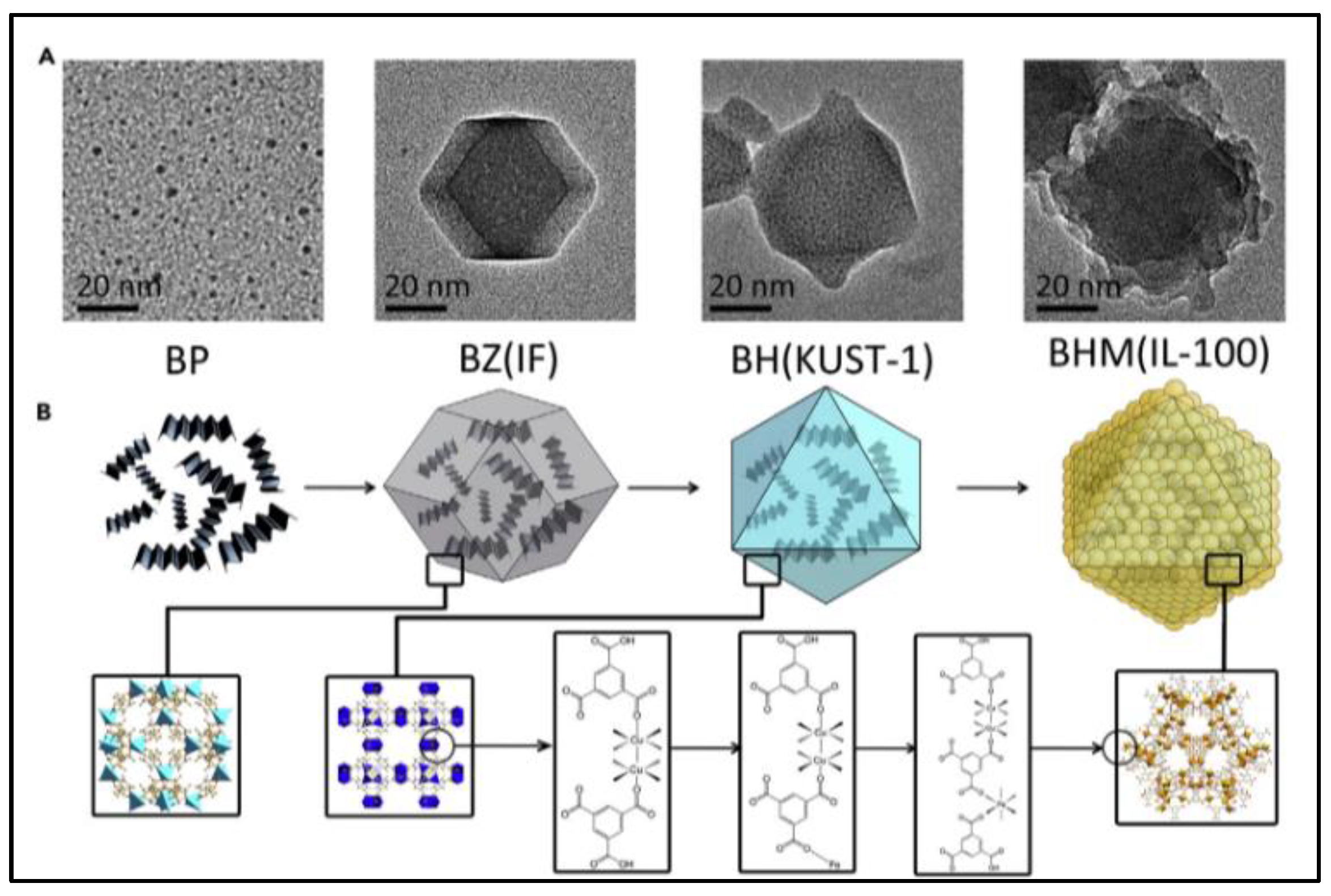
2.4. Nanocomposites
3. S-Nitrozation of GSH Loaded Nano/Micro-Formulation
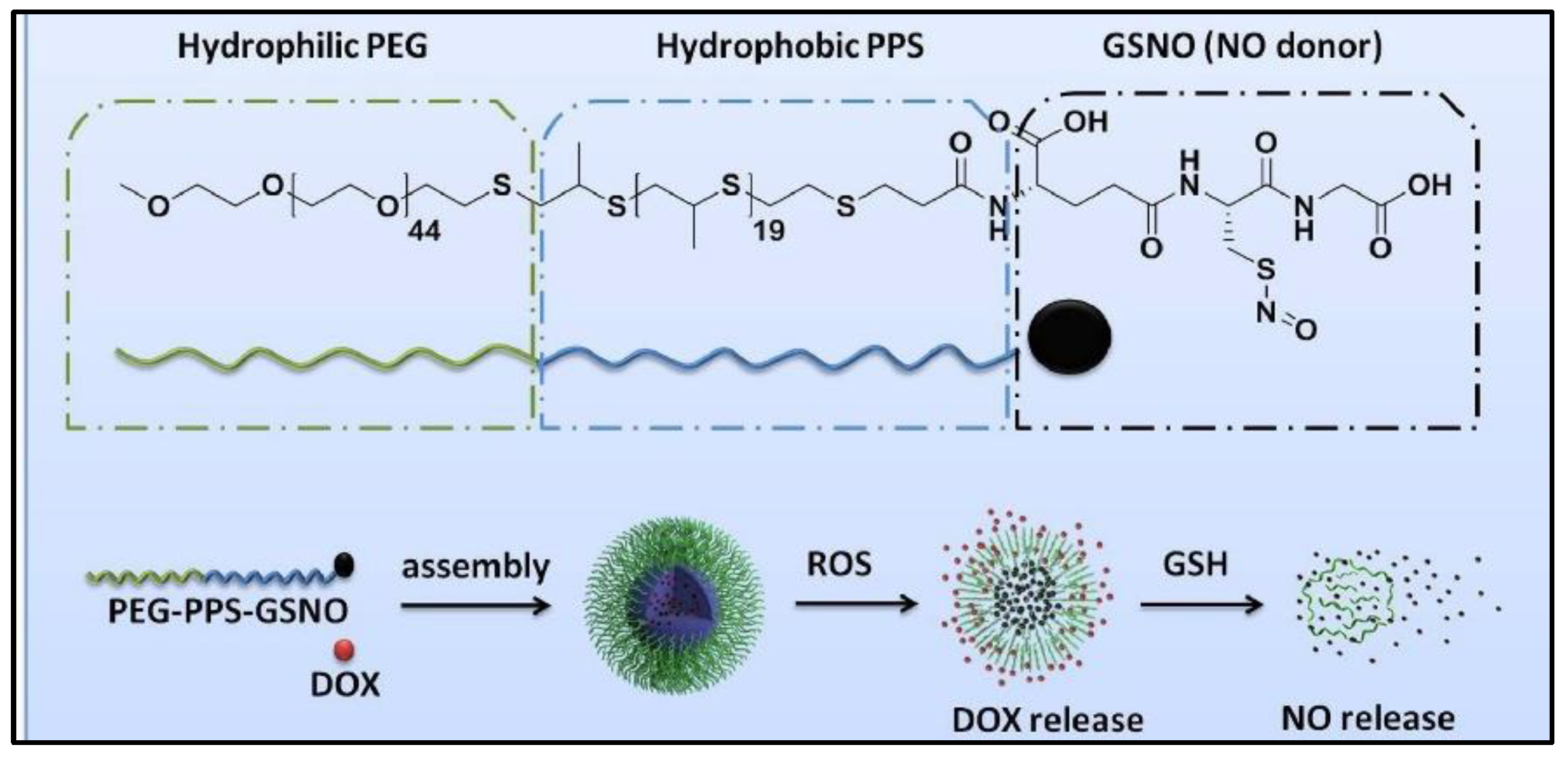
4. GSNO Conjugated Nano/Micro-Formulation
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Broniowska, K.A.; Diers, A.R.; Hogg, N. S-Nitrosoglutathione. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 3173–3181. [Google Scholar] [CrossRef] [Green Version]
- Kumar, G.; Dey, S.K.; Kundu, S. Functional implications of vascular endothelium in regulation of endothelial nitric oxide synthesis to control blood pressure and cardiac functions. Life Sci. 2020, 259, 118377. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E. Nitric oxide signaling in health and disease. Cell 2022, 185, 2853–2878. [Google Scholar] [CrossRef]
- Borase, H.; Patil, S.; Amin, D.; Dwivedi, M.K. In vitro Evaluation of the Nitric Oxide Pathway. In Biosafety Assessment of Probiotic Potential; Springer: Berlin/Heidelberg, Germany, 2022; pp. 149–155. [Google Scholar]
- Sharma, J.; Al-Omran, A.; Parvathy, S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Hummel, S.G.; Fischer, A.J.; Martin, S.M.; Schafer, F.Q.; Buettner, G.R. Nitric oxide as a cellular antioxidant: A little goes a long way. Free Radic. Biol. Med. 2006, 40, 501–506. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Fu, J.; Zhang, Y. Nitric oxide donor-based cancer therapy: Advances and prospects. J. Med. Chem. 2017, 60, 7617–7635. [Google Scholar] [CrossRef]
- Korde Choudhari, S.; Chaudhary, M.; Bagde, S.; Gadbail, A.R.; Joshi, V. Nitric oxide and cancer: A review. World J. Surg. Oncol. 2013, 11, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Huang, Z.; Li, L.L. Advanced nitric oxide donors: Chemical structure of NO drugs, NO nanomedicines and biomedical applications. Nanoscale 2021, 13, 444–459. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, J.; Feng, G.; Shen, J.; Kong, D.; Zhao, Q. Nitric oxide-releasing biomaterials for biomedical applications. Curr. Med. Chem. 2016, 23, 2579–2601. [Google Scholar] [CrossRef]
- Jeong, H.; Park, J.-H.; Shin, J.H.; Yoo, J.-C.; Park, C.Y.; Hong, J. Prolonged release period of nitric oxide gas for treatment of bacterial keratitis by amine-rich polymer decoration of nanoparticles. Chem. Mater. 2018, 30, 8528–8537. [Google Scholar] [CrossRef]
- Miller, M.; Megson, I. Recent developments in nitric oxide donor drugs. Br. J. Pharmacol. 2007, 151, 305–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franca-Silva, M.S.; Balarini, C.M.; Cruz, J.C.; Khan, B.A.; Rampelotto, P.H.; Braga, V.A. Organic Nitrates: Past, Present and Future. Molecules 2014, 19, 15314–15323. [Google Scholar] [CrossRef] [PubMed]
- Burov, O.N.; Kletskii, M.E.; Kurbatov, S.V.; Lisovin, A.V.; Fedik, N.S. Mechanisms of nitric oxide generation in living systems. Nitric Oxide 2022, 118, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Pieretti, J.C.; Pelegrino, M.T.; Boudier, A.; Seabra, A.B. Recent progress in the toxicity of nitric oxide-releasing nanomaterials. Mater. Adv. 2021, 2, 7530–7542. [Google Scholar] [CrossRef]
- Li, W.; Wang, D.; Lao, K.U.; Wang, X. Buffer concentration dramatically affects the stability of S-nitrosothiols in aqueous solutions. Nitric Oxide 2022, 118, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.Z.; Yang, T.; Namivandi-Zangeneh, R.; Boyer, C.; Liang, K.; Chandrawati, R. Copper-doped metal-organic frameworks for the controlled generation of nitric oxide from endogenous S-nitrosothiols. J. Mater. Chem. B 2021, 9, 1059–1068. [Google Scholar] [CrossRef]
- Filipczak, N.; Pan, J.; Yalamarty, S.S.K.; Torchilin, V.P. Recent advancements in liposome technology. Adv. Drug Deliv. Rev. 2020, 156, 4–22. [Google Scholar] [CrossRef]
- Diab, R.; Virriat, A.S.; Ronzani, C.; Fontanay, S.; Grandemange, S.; Elaissari, A.; Foliguet, B.; Maincent, P.; Leroy, P.; Duval, R.E.; et al. Elaboration of Sterically Stabilized Liposomes for S-Nitrosoglutathione Targeting to Macrophages. J. Biomed. Nanotechnol. 2016, 12, 217–230. [Google Scholar] [CrossRef]
- Wang, B.; Zhai, Y.; Shi, J.; Zhuang, L.; Liu, W.; Zhang, H.; Zhang, H.; Zhang, Z. Simultaneously overcome tumor vascular endothelium and extracellular matrix barriers via a non-destructive size-controlled nanomedicine. J. Control. Release 2017, 268, 225–236. [Google Scholar] [CrossRef]
- Wu, W.; Gaucher, C.; Diab, R.; Fries, I.; Xiao, Y.L.; Hu, X.M.; Maincent, P.; Sapin-Minet, A. Time lasting S-nitrosoglutathione polymeric nanoparticles delay cellular protein S-nitrosation. Eur. J. Pharm. Biopharm. 2015, 89, 1–8. [Google Scholar] [CrossRef]
- Wu, W.; Gaucher, C.; Fries, I.; Hu, X.M.; Maincent, P.; Sapin-Minet, A. Polymer nanocomposite particles of S-nitrosoglutathione: A suitable formulation for protection and sustained oral delivery. Int. J. Pharm. 2015, 495, 354–361. [Google Scholar] [PubMed]
- Wu, W.; Perrin-Sarrado, C.; Ming, H.; Lartaud, I.; Maincent, P.; Hu, X.M.; Sapin-Minet, A.; Gaucher, C. Polymer nanocomposites enhance S-nitrosoglutathione intestinal absorption and promote the formation of releasable nitric oxide stores in rat aorta. Nanomed.-Nanotechnol. Biol. Med. 2016, 12, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Gaucher, C.; Fries, I.; Hobekkaya, M.-A.; Martin, C.; Leonard, C.; Deschamps, F.; Sapin-Minet, A.; Parent, M. Challenging development of storable particles for oral delivery of a physiological nitric oxide donor. Nitric Oxide 2020, 104–105, 1–10. [Google Scholar]
- Bonetti, J.; Zhou, Y.; Parent, M.; Clarot, I.; Yu, H.; Fries-Raeth, I.; Leroy, P.; Lartaud, I.; Gaucher, C. Intestinal absorption of S-nitrosothiols: Permeability and transport mechanisms. Biochem. Pharmacol. 2018, 155, 21–31. [Google Scholar]
- Kim, S.-M.; Huh, J.-W.; Kim, E.-Y.; Shin, M.-K.; Park, J.-E.; Kim, S.W.; Lee, W.; Choi, B.; Chang, E.-J. Endothelial dysfunction induces atherosclerosis: Increased aggrecan expression promotes apoptosis in vascular smooth muscle cells. BMB Rep. 2019, 52, 145. [Google Scholar]
- Murugesan, P.; Zhang, Y.; Youn, J.Y.; Cai, H. Novel and robust attenuating effects on pulmonary hypertension of Netrin-1 and netrin-1-derived small peptides. Redox Biol. 2022, 55, 102348. [Google Scholar] [CrossRef]
- Freedman, J.; Loscalzo, J. Nitric oxide and its relationship to thrombotic disorders. J. Thromb. Haemost. 2003, 1, 1183–1188. [Google Scholar]
- Najafi, H.; Abolmaali, S.S.; Heidari, R.; Valizadeh, H.; Jafari, M.; Tamaddon, A.M.; Azarpira, N. Nitric oxide releasing nanofibrous Fmoc-dipeptide hydrogels for amelioration of renal ischemia/reperfusion injury. J. Control. Release 2021, 337, 1–13. [Google Scholar] [CrossRef]
- Safar, R.; Ronzani, C.; Diab, R.; Chevrier, J.; Bensoussan, D.; Grandemange, S.; Le Faou, A.; Rihn, B.H.; Joubert, O. Human monocyte response to S-nitrosoglutathione-loaded nanoparticles: Uptake, viability, and transcriptome. Mol. Pharm. 2015, 12, 554–561. [Google Scholar]
- Shah, S.U.; Socha, M.; Sejil, C.; Gibaud, S. Spray-dried microparticles of glutathione and S-nitrosoglutathione based on Eudragit® FS 30D polymer. Ann. Pharm. Françaises 2017, 75, 95–104. [Google Scholar] [CrossRef]
- Savidge, T.C.; Newman, P.; Pothoulakis, C.; Ruhl, A.; Neunlist, M.; Bourreille, A.; Hurst, R.; Sofroniew, M.V. Enteric glia regulate intestinal barrier function and inflammation via release of S-nitrosoglutathione. Gastroenterology 2007, 132, 1344–1358. [Google Scholar] [CrossRef]
- Cheadle, G.A.; Costantini, T.W.; Lopez, N.; Bansal, V.; Eliceiri, B.P.; Coimbra, R. Enteric glia cells attenuate cytomix-induced intestinal epithelial barrier breakdown. PLoS ONE 2013, 8, e69042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Zhang, X.; Zhou, H.; Liu, W.; Li, J. Exogenous S-nitrosoglutathione attenuates inflammatory response and intestinal epithelial barrier injury in endotoxemic rats. J. Trauma Acute Care Surg. 2016, 80, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Hagbom, M.; Faria, F.M.D.; Winberg, M.E.; Westerberg, S.; Nordgren, J.; Sharma, S.; Keita, Å.V.; Loitto, V.; Magnusson, K.-E.; Svensson, L. Neurotrophic Factors Protect the Intestinal Barrier from Rotavirus Insult in Mice. mBio 2020, 11, e02834-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hlaing, S.P.; Kim, J.; Lee, J.; Hasan, N.; Cao, J.; Naeem, M.; Lee, E.H.; Shin, J.H.; Jung, Y.; Lee, B.L.; et al. S-Nitrosoglutathione loaded poly(lactic-co-glycolic acid) microparticles for prolonged nitric oxide release and enhanced healing of methicillin-resistant Staphylococcus aureus-infected wounds. Eur. J. Pharm. Biopharm. 2018, 132, 94–102. [Google Scholar] [CrossRef]
- Parent, M.; Boudier, A.; Dupuis, F.; Nouvel, C.; Sapin, A.; Lartaud, I.; Six, J.-L.; Leroy, P.; Maincent, P. Are in situ formulations the keys for the therapeutic future of S-nitrosothiols? Eur. J. Pharm. Biopharm. 2013, 85, 640–649. [Google Scholar] [CrossRef]
- Parent, M.; Boudier, A.; Perrin, J.; Vigneron, C.; Maincent, P.; Violle, N.; Bisson, J.F.; Lartaud, I.; Dupuis, F. In Situ Microparticles Loaded with S-Nitrosoglutathione Protect from Stroke. PLoS ONE 2015, 10, e0144659. [Google Scholar] [CrossRef]
- Gordge, M.; Xiao, F. S-nitrosothiols as selective antithrombotic agents-possible mechanisms. Br. J. Pharmacol. 2010, 159, 1572–1580. [Google Scholar] [CrossRef] [Green Version]
- Radomski, M.W.; Rees, D.D.; Dutra, A.; Moncada, S. S-nitroso-glutathione inhibits platelet activation in vitro and in vivo. Br. J. Pharmacol. 1992, 107, 745. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.J.; Park, D.J.; Choi, G.H.; Yang, D.-N.; Heo, J.S.; Lee, S.C. pH-Responsive mineralized nanoparticles as stable nanocarriers for intracellular nitric oxide delivery. Colloids Surf. B Biointerfaces 2016, 146, 1–8. [Google Scholar] [CrossRef]
- Kamm, A.; Przychodzen, P.; Kuban-Jankowska, A.; Jacewicz, D.; Dabrowska, A.M.; Nussberger, S.; Wozniak, M.; Gorska-Ponikowska, M. Nitric oxide and its derivatives in the cancer battlefield. Nitric Oxide 2019, 93, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Muhammed Shameem, M.; Sasikanth, S.M.; Annamalai, R.; Ganapathi Raman, R. A brief review on polymer nanocomposites and its applications. Mater. Today Proc. 2021, 45, 2536–2539. [Google Scholar] [CrossRef]
- An, J.; Hu, Y.G.; Li, C.; Hou, X.L.; Cheng, K.; Zhang, B.; Zhang, R.Y.; Li, D.Y.; Liu, S.J.; Liu, B.; et al. A pH/Ultrasound dual-response biomimetic nanoplatform for nitric oxide gas-sonodynamic combined therapy and repeated ultrasound for relieving hypoxia. Biomaterials 2020, 230, 119636. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Qin, Z.; Zheng, Y.; Jiang, H.; Weizmann, Y.; Wang, X. The “Framework Exchange”-Strategy-Based MOF Platform for Biodegradable Multimodal Therapy. Chem 2019, 5, 2942–2954. [Google Scholar] [CrossRef]
- Lee, I.J.; Kao, P.-T.; Hung, S.-A.; Wang, Z.-W.; Lin, H.-J.; Chang, W.-T.; Yeh, C.-S.; Liau, I. Light triggering goldsomes enable local NO-generation and alleviate pathological vasoconstriction. Nanomed. Nanotechnol. Biol. Med. 2020, 30, 102282. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, X.; Shi, D.; Wang, Z. Zeolitic imidazolate framework-8 (ZIF-8) for drug delivery: A critical review. Front. Chem. Sci. Eng. 2020, 15, 221–237. [Google Scholar] [CrossRef]
- Xie, L.; Chen, W.; Chen, Q.; Jiang, Y.; Song, E.; Zhu, X.; Song, Y. Synergistic hydroxyl radical formation, system XC-inhibition and heat shock protein crosslinking tango in ferrotherapy: A prove-of-concept study of “sword and shield” theory. Mater. Today Bio. 2022, 16, 100353. [Google Scholar] [CrossRef]
- Lenzen, S.; Lushchak, V.I.; Scholz, F. The pro-radical hydrogen peroxide as a stable hydroxyl radical distributor: Lessons from pancreatic beta cells. Arch. Toxicol. 2022, 96, 1915–1920. [Google Scholar] [CrossRef]
- Vong, L.B.; Nagasaki, Y. Nitric oxide nano-delivery systems for cancer therapeutics: Advances and challenges. Antioxidants 2020, 9, 791. [Google Scholar] [CrossRef]
- Yang, T.; Zelikin, A.N.; Chandrawati, R. Progress and promise of nitric oxide-releasing platforms. Adv. Sci. 2018, 5, 1701043. [Google Scholar] [CrossRef] [Green Version]
- Pelegrino, M.T.; de Araújo, D.R.; Seabra, A.B. S-nitrosoglutathione-containing chitosan nanoparticles dispersed in Pluronic F-127 hydrogel: Potential uses in topical applications. J. Drug Deliv. Sci. Technol. 2018, 43, 211–220. [Google Scholar] [CrossRef]
- Rolim, W.R.; Pieretti, J.C.; Reno, D.L.S.; Lima, B.A.; Nascimento, M.H.M.; Ambrosio, F.N.; Lombello, C.B.; Brocchi, M.; de Souza, A.C.S.; Seabra, A.B. Antimicrobial Activity and Cytotoxicity to Tumor Cells of Nitric Oxide Donor and Silver Nanoparticles Containing PVA/PEG Films for Topical Applications. ACS Appl. Mater. Interfaces 2019, 11, 6589–6604. [Google Scholar] [CrossRef]
- Singh, R.J.; Hogg, N.; Joseph, J.; Kalyanaraman, B. Photosensitized decomposition of S-nitrosothiols and 2-methyl-2-nitrosopropane Possible use for site-directed nitric oxide production. FEBS Lett. 1995, 360, 47–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grossi, L.; Montevecchi, P.C. A Kinetic Study of S-Nitrosothiol Decomposition. Chem.–A Eur. J. 2002, 8, 380–387. [Google Scholar] [CrossRef]
- Marcato, D.P.; Adami, F.L.; de Melo Barbosa, R.; Melo, S.P.; Ferreira, R.I.; de Paula, L.; Duran, N.; Seabra, B.A. Development of a Sustained-release System for Nitric Oxide Delivery using Alginate/Chitosan Nanoparticles. Curr. Nanosci. 2013, 9, 1–7. [Google Scholar]
- Marcato, P.D.; Adami, L.F.; Melo, P.S.; de Paula, L.; Durán, N.; Seabra, A. Glutathione and S-nitrosoglutathione in alginate/chitosan nanoparticles: Cytotoxicity. Proc. J.Phys. Conf. Ser. 2011, 304, 012045. [Google Scholar] [CrossRef]
- Pereira, A.E.S.; Narciso, A.M.; Seabra, A.B.; Fraceto, L.F. Evaluation of the effects of nitric oxide-releasing nanoparticles on plants. J. Phys. Conf. Ser. 2015, 617, 012025. [Google Scholar] [CrossRef]
- Silveira, N.M.; Seabra, A.B.; Marcos, F.C.C.; Pelegrino, M.T.; Machado, E.C.; Ribeiro, R.V. Encapsulation of S-nitrosoglutathione into chitosan nanoparticles improves drought tolerance of sugarcane plants. Nitric Oxide 2019, 84, 38–44. [Google Scholar] [CrossRef]
- Pelegrino, M.T.; Weller, R.B.; Paganotti, A.; Seabra, A.B. Delivering nitric oxide into human skin from encapsulated S-nitrosoglutathione under UV light: An in vitro and ex vivo study. Nitric Oxide-Biol. Chem. 2020, 94, 108–113. [Google Scholar] [CrossRef]
- Santos, M.C.; Seabra, A.B.; Pelegrino, M.T.; Haddad, P.S. Synthesis, characterization and cytotoxicity of glutathione- and PEG-glutathione-superparamagnetic iron oxide nanoparticles for nitric oxide delivery. Appl. Surf. Sci. 2016, 367, 26–35. [Google Scholar] [CrossRef]
- Haddad, P.S.; Santos, M.C.; de Guzzi Cassago, C.A.; Bernardes, J.S.; de Jesus, M.B.; Seabra, A.B. Synthesis, characterization, and cytotoxicity of glutathione-PEG-iron oxide magnetic nanoparticles. J. Nanoparticle Res. 2016, 18, 1–13. [Google Scholar] [CrossRef]
- Ignarro, L.J. Nitric Oxide: Biology and Pathobiology; Academic Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Molina, M.M.; Seabra, A.B.; de Oliveira, M.G.; Itri, R.; Haddad, P.S. Nitric oxide donor superparamagnetic iron oxide nanoparticles. Mater. Sci. Eng. C 2013, 33, 746–751. [Google Scholar] [CrossRef]
- Georgii, J.L.; Amadeu, T.P.; Seabra, A.B.; de Oliveira, M.G.; Monte-Alto-Costa, A. Topical S-nitrosoglutathione-releasing hydrogel improves healing of rat ischaemic wounds. J. Tissue Eng. Regen. Med. 2011, 5, 612–619. [Google Scholar] [CrossRef]
- Shah, S.U.; Martinho, N.; Socha, M.; Pinto Reis, C.; Gibaud, S. Synthesis and characterization of S-nitrosoglutathione-oligosaccharide-chitosan as a nitric oxide donor. Expert Opin. Drug Deliv. 2015, 12, 1209–1223. [Google Scholar] [CrossRef]
- Shah, S.U.; Socha, M.; Fries, I.; Gibaud, S. Synthesis of S-nitrosoglutathione-alginate for prolonged delivery of nitric oxide in intestines. Drug Deliv. 2016, 23, 2927–2935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duong, H.T.; Kamarudin, Z.M.; Erlich, R.B.; Li, Y.; Jones, M.W.; Kavallaris, M.; Boyer, C.; Davis, T.P. Intracellular nitric oxide delivery from stable NO-polymeric nanoparticle carriers. Chem Commun (Camb) 2013, 49, 4190–4192. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kwak, D.; Kim, H.; Kim, J.; Hlaing, S.P.; Hasan, N.; Cao, J.; Yoo, J.-W. Nitric Oxide-Releasing S-Nitrosoglutathione-Conjugated Poly(Lactic-Co-Glycolic Acid) Nanoparticles for the Treatment of MRSA-Infected Cutaneous Wounds. Pharmaceutics 2020, 12, 618. [Google Scholar] [CrossRef]
- Kafshgari, M.H.; Delalat, B.; Harding, F.; Cavallaro, A.; Mäkilä, E.; Salonen, J.; Vasilev, K.; Voelcker, N. Antibacterial properties of nitric oxide-releasing porous silicon nanoparticles. J. Mater. Chem. B 2016, 4, 2051–2058. [Google Scholar] [CrossRef]
- Wu, W.; Chen, M.; Luo, T.; Fan, Y.; Zhang, J.; Zhang, Y.; Zhang, Q.; Sapin-Minet, A.; Gaucher, C.; Xia, X. ROS and GSH-responsive S-nitrosoglutathione functionalized polymeric nanoparticles to overcome multidrug resistance in cancer. Acta Biomater. 2020, 103, 259–271. [Google Scholar] [CrossRef]


| Formulation Type | Preparation Methods | Formulation Efficiency | Applications | Limitations | References |
|---|---|---|---|---|---|
| liposomes | solvent-spherule evaporation | Sustained release, targeted to macrophage, enhanced NO uptake and antibacterial effect | Anti-bacteria | Low encapsulation efficiency, unclear stability and toxicity of liposome | [19] |
| liposomes | thin film evaporation combined frozen-thaw method | Controlled release (ultrasound responsive), promoted cellular internalization and co-delivery with DOX enhanced anti-tumor effect | Anti-cancer | Unclear encapsulation efficiency | [20] |
| polymer nano/micro-particles | W(S)/O/W double emulsion-evaporation process | Slightly sustained release and increased intestinal permeability | / | Unclear effectiveness | [24] |
| polymer nanoparticles | W/O/W double emulsion-evaporation process | Sustained release and enhanced intracellular delivery of GSNO | Immune defenses | Not long enough prolonged release | [22,30] |
| polymer microparticles | spray-drying method | High encapsulation efficiency, controlled release (pH-sensitive) | Anti-inflammation | Non-sustained release | [31] |
| polymer microparticles | S/O/W emulsion-solvent evaporation method | Sustained release and enhanced antibacterial effect | Anti-bacteria | Difficult to scale up | [36] |
| polymer microparticles | in-situ O/W emulsion | Sustained release, reduced burst release and | Reduce arterial pressures | Uncontrollable size and potential side effects | [37,38] |
| CaCO3 nanoparticles | anionic block copolymer-templated mineralization | Increase GSNO stability, controlled release (pH-sensitive) and co-delivery with DOX enhanced anti-tumor effect | Anti-cancer | Not able to release NO quickly enough at physiological pH and unclear in vivo efficacy and safety | [41] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ming, H.; Zhang, K.; Ge, S.; Shi, Y.; Du, C.; Guo, X.; Zhang, L. A Mini Review of S-Nitrosoglutathione Loaded Nano/Micro-Formulation Strategies. Nanomaterials 2023, 13, 224. https://doi.org/10.3390/nano13020224
Ming H, Zhang K, Ge S, Shi Y, Du C, Guo X, Zhang L. A Mini Review of S-Nitrosoglutathione Loaded Nano/Micro-Formulation Strategies. Nanomaterials. 2023; 13(2):224. https://doi.org/10.3390/nano13020224
Chicago/Turabian StyleMing, Hui, Kunpeng Zhang, Shengbo Ge, Yang Shi, Chunan Du, Xuqiang Guo, and Libo Zhang. 2023. "A Mini Review of S-Nitrosoglutathione Loaded Nano/Micro-Formulation Strategies" Nanomaterials 13, no. 2: 224. https://doi.org/10.3390/nano13020224





