Multi-Prismatic Hollow Cube CeVO4 with Adjustable Wall Thickness Directed towards Photocatalytic CO2 Reduction to CO
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
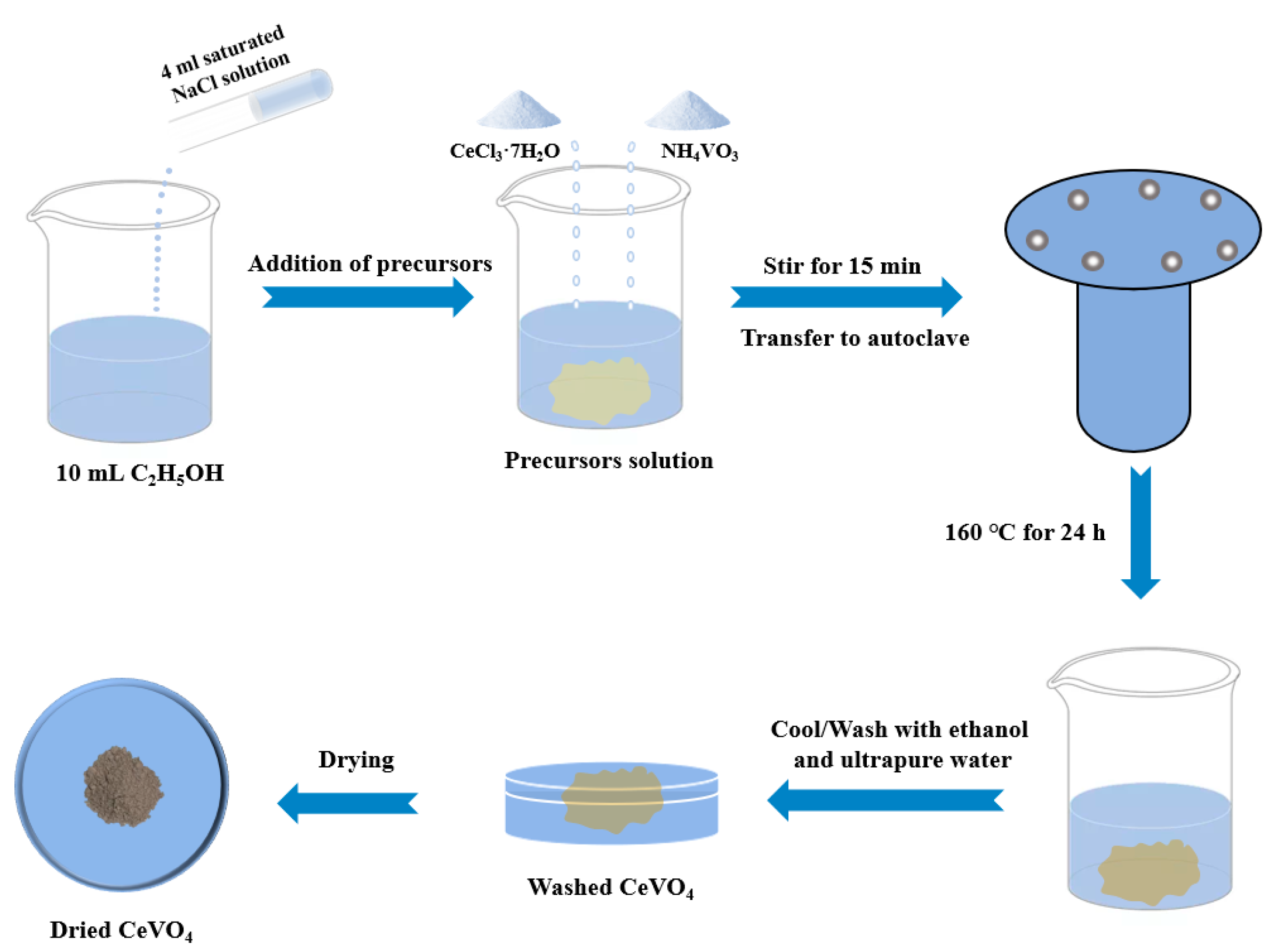
2.2. Synthesis of Hollow Cubic CeVO4 Microstructure
2.3. Characterization
2.4. Photocatalytic CO2 Reduction
3. Result and Discussion
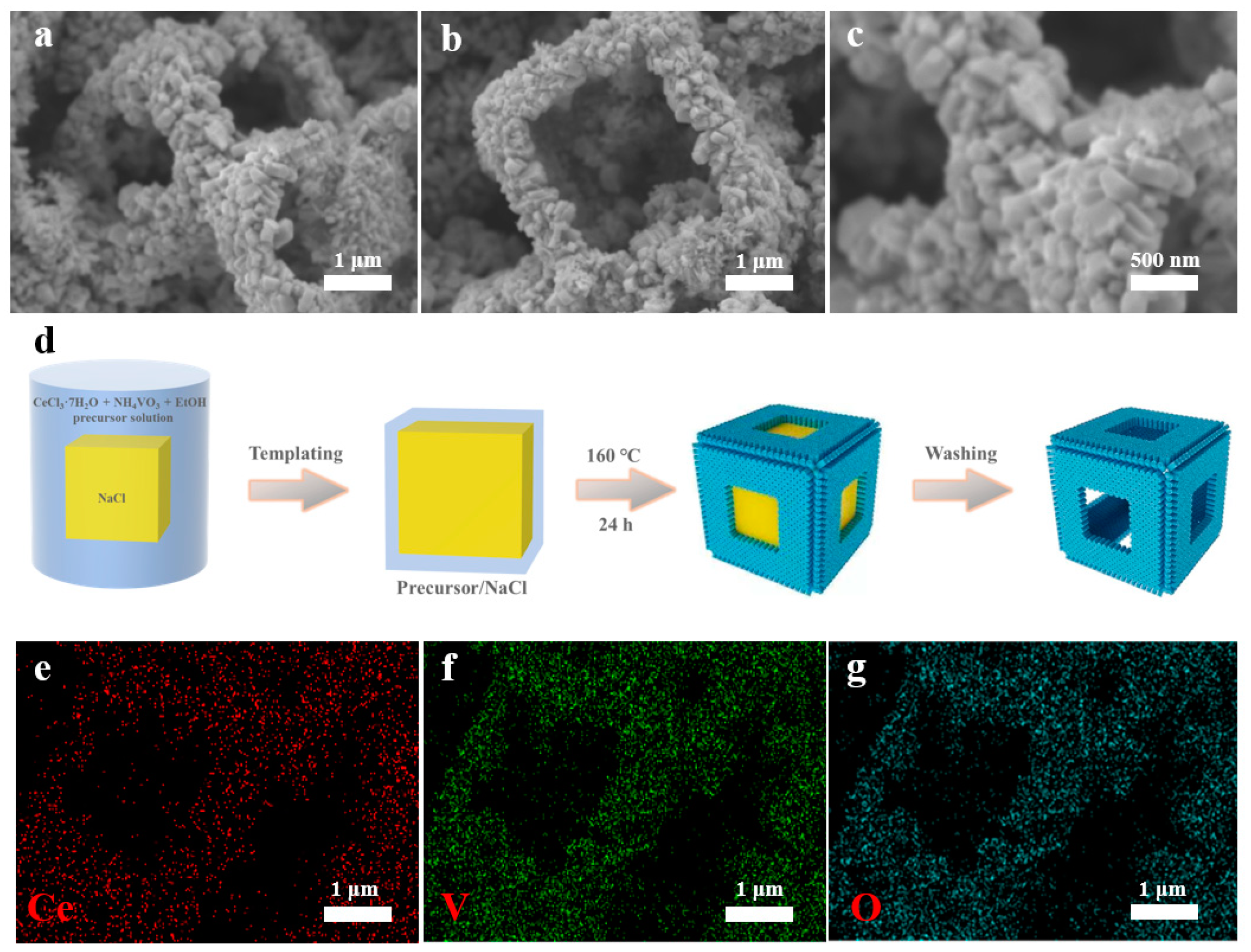
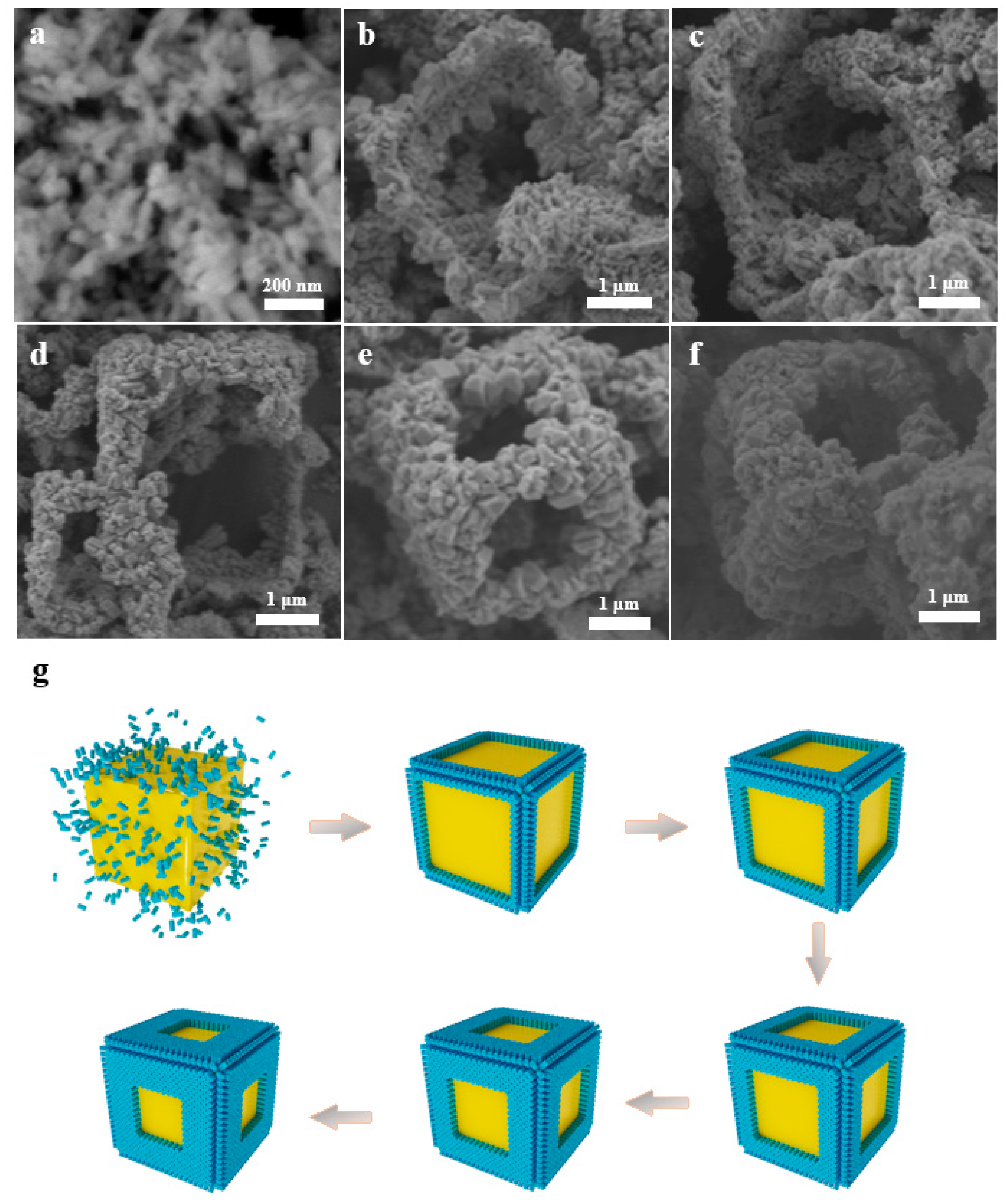
3.1. Catalyst Characterization
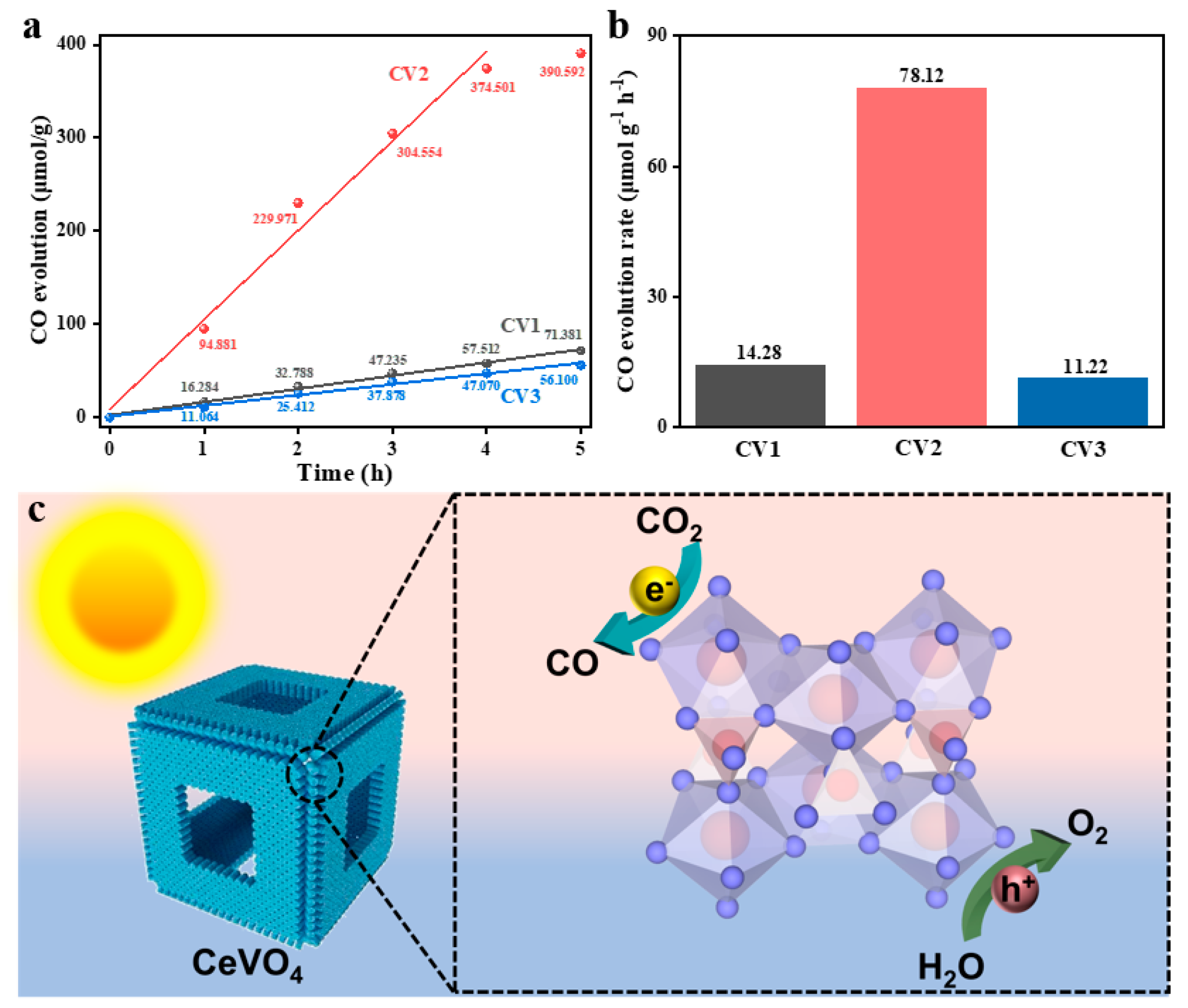
3.2. Photocatalytic Performance for CO2 Reduction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedlingstein, P.; Jones, M.W.; O’Sullivan, M.; Andrew, R.M.; Bakker, D.C.E.; Hauck, J.; Le Quéré, C.; Peters, G.P.; Peters, W.; Pongratz, J.; et al. Global Carbon Budget 2021. Earth Syst. Sci. Data 2022, 14, 1917–2005. [Google Scholar] [CrossRef]
- Li, D.; Zhou, J.; Zhang, Z.; Jiang, Y.; Dong, Z.; Xu, J.; Yao, C. Enhanced Photocatalytic Activity for CO2 Reduction over a CsPbBr3/CoAl-LDH Composite: Insight into the S-Scheme Charge Transfer Mechanism. ACS Appl. Energy Mater. 2022, 5, 6238–6247. [Google Scholar] [CrossRef]
- Zhang, F.; Li, Y.-H.; Qi, M.-Y.; Yamada, Y.M.; Anpo, M.; Tang, Z.-R.; Xu, Y.-J. Photothermal catalytic CO2 reduction over nanomaterials. Chem Catal. 2021, 1, 272–297. [Google Scholar] [CrossRef]
- Bi, Z.-X.; Guo, R.-T.; Hu, X.; Wang, J.; Chen, X.; Pan, W.-G. Research progress on photocatalytic reduction of CO2 based on LDH materials. Nanoscale 2022, 14, 3367–3386. [Google Scholar] [CrossRef]
- Su, X.; Yang, Z.; Han, G.; Wang, Y.; Wen, M.; Pan, S. Role of the metal cation types around VO4groups on the nonlinear optical behavior of materials: Experimental and theoretical analysis. Dalton Trans. 2016, 45, 14394–14402. [Google Scholar] [CrossRef]
- Lu, G.; Song, B.; Li, Z.; Liang, H.; Zou, X. Photocatalytic degradation of naphthalene on CeVO4 nanoparticles under visible light. Chem. Eng. J. 2020, 402, 125645. [Google Scholar] [CrossRef]
- Pellizzeri, T.M.S.; Morrison, G.; McMillen, C.D.; Loye, H.Z.; Kolis, J.W. Sodium Transition Metal Vanadates from Hydrothermal Brines: Synthesis and Characterization of NaMn4(VO4)3, Na2Mn3(VO4)3, and Na2Co3(VO4)2(OH)2. Eur. J. Inorg. Chem. 2020, 2020, 3408–3415. [Google Scholar] [CrossRef]
- Wu, L.; Dai, P.; Wen, D. New Structural Design Strategy: Optical Center VO4-Activated Broadband Yellow Phosphate Phosphors for High-Color-Rendering WLEDs. ACS Sustain. Chem. Eng. 2022, 10, 3757–3765. [Google Scholar] [CrossRef]
- Liu, M.; Lv, Z.-L.; Cheng, Y.; Ji, G.-F.; Gong, M. Structural, elastic and electronic properties of CeVO4 via first-principles calculations. Comput. Mater. Sci. 2013, 79, 811–816. [Google Scholar] [CrossRef]
- Ameri, V.; Eghbali-Arani, M.; Pourmasoud, S. New route for preparation of cerium vanadate nanoparticles with different morphology and investigation of optical and photocatalytic properties. J. Mater. Sci. Mater. Electron. 2017, 28, 18835–18841. [Google Scholar] [CrossRef]
- Othman, I.; Zain, J.H.; Abu Haija, M.; Banat, F. Catalytic activation of peroxymonosulfate using CeVO4 for phenol degradation: An insight into the reaction pathway. Appl. Catal. B Environ. 2020, 266, 118601. [Google Scholar] [CrossRef]
- Ju, P.; Yu, Y.; Wang, M.; Zhao, Y.; Zhang, D.; Sun, C.; Han, X. Synthesis of EDTA-assisted CeVO4nanorods as robust peroxidase mimics towards colorimetric detection of H2O2. J. Mater. Chem. B 2016, 4, 6316–6325. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, W.; Jian, P.; Wang, L.; Yan, X. Promoted selective oxidation of ethylbenzene in liquid phase achieved by hollow CeVO4 microspheres. J. Colloid Interface Sci. 2022, 614, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Hu, X.; Zhan, Z.; Sun, T.; Tang, Y. Facile fabrication of CeVO4 hierarchical hollow microspheres with enhanced photocatalytic activity. Mater. Lett. 2019, 253, 259–262. [Google Scholar] [CrossRef]
- Wang, Q.; Zhou, R.; Liu, S.; Sethupathy, S.; Liu, J.; Sun, J.; Zou, L.; Zhu, Q. Templated synthesis and assembly with sustainable cellulose nanomaterial for functional nanostructure. Cellulose 2022, 29, 4287–4321. [Google Scholar] [CrossRef]
- Ashirov, T.; Song, K.S.; Coskun, A. Salt-Templated Solvothermal Synthesis of Dioxane-Linked Three-Dimensional Nanoporous Organic Polymers for Carbon Dioxide and Iodine Capture. ACS Appl. Nano Mater. 2022, 5, 13711–13719. [Google Scholar] [CrossRef]
- Lv, L.; Cheng, P.; Zhang, Y.; Zhang, Y.; Lei, Z.; Wang, Y.; Xu, L.; Weng, Z.; Li, C. Ultra-high response acetone gas sensor based on ZnFe2O4 pleated hollow microspheres prepared by green NaCl template. Sens. Actuators B Chem. 2022, 358, 131490. [Google Scholar] [CrossRef]
- Li, Y.; Wu, Z.; Lu, P.; Wang, X.; Liu, W.; Liu, Z.; Ma, J.; Ren, W.; Jiang, Z.; Bao, X. High-Valence Nickel Single-Atom Catalysts Coordinated to Oxygen Sites for Extraordinarily Activating Oxygen Evolution Reaction. Adv. Sci. 2020, 7, 1903089. [Google Scholar] [CrossRef] [Green Version]
- Huan, Y.; Shi, J.; Zou, X.; Gong, Y.; Xie, C.; Yang, Z.; Zhang, Z.; Gao, Y.; Shi, Y.; Li, M.; et al. Scalable Production of Two-Dimensional Metallic Transition Metal Dichalcogenide Nanosheet Powders Using NaCl Templates toward Electrocatalytic Applications. J. Am. Chem. Soc. 2019, 141, 18694–18703. [Google Scholar] [CrossRef]
- Juvanen, S.; Sarapuu, A.; Vlassov, S.; Kook, M.; Kisand, V.; Käärik, M.; Treshchalov, A.; Aruväli, J.; Kozlova, J.; Tamm, A.; et al. Iron-Containing Nitrogen-Doped Carbon Nanomaterials Prepared via NaCl Template as Efficient Electrocatalysts for the Oxygen Reduction Reaction. Chemelectrochem 2021, 8, 2288–2297. [Google Scholar] [CrossRef]
- Schaffner, R.D.A.; Borba, C.E.; Tavares, F.; Oliveira, L.G.; Castaño, P.; Alves, H.J. Green Synthesis of Templated Porous Carbons. Can. J. Chem. Eng. 2022, 2, 24739. [Google Scholar] [CrossRef]
- Jin, Q.; Zhang, C.; Wang, W.; Chen, B.; Ruan, J.; Qian, H. Recent Development on Controlled Synthesis of Metal Sulfides Hollow Nanostructures via Hard Template Engaged Strategy: A Mini-Review. Chem. Rec. 2020, 20, 882–892. [Google Scholar] [CrossRef]
- Ghotekar, S.; Pansambal, S.; Lin, K.-Y.A.; Pore, D.; Oza, R. Recent Advances in Synthesis of CeVO4 Nanoparticles and Their Potential Scaffold for Photocatalytic Applications. Top. Catal. 2022, 7, 1–15. [Google Scholar] [CrossRef]
- Chen, J.; Zhou, K.; Wang, Y.; Gao, J.; Yuan, T.; Pang, J.; Tang, S.; Chen, H.-Y.; Wang, W. Measuring the activation energy barrier for the nucleation of single nanosized vapor bubbles. Proc. Natl. Acad. Sci. USA 2019, 116, 12678–12683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Cui, Z.; Xue, Y. Determination of Interfacial Tension of Nanomaterials and the Effect of Particle Size on Interfacial Tension. Langmuir 2021, 37, 14463–14471. [Google Scholar] [CrossRef]
- Freitas, R.; Reed, E.J. Uncovering the effects of interface-induced ordering of liquid on crystal growth using machine learning. Nat. Commun. 2020, 11, 3260. [Google Scholar] [CrossRef]
- Phuruangrat, A.; Kuntalue, B.; Thongtem, S.; Thongtem, T. Effect of PEG on phase, morphology and photocatalytic activity of CeVO4 nanostructures. Mater. Lett. 2016, 174, 138–141. [Google Scholar] [CrossRef]
- Hojamberdiev, M.; Kadirova, Z.C.; Makinose, Y.; Zhu, G.; Emin, S.; Matsushita, N.; Hasegawa, M.; Okada, K. Involving CeVO4 in improving the photocatalytic activity of a Bi2WO6/allophane composite for the degradation of gaseous acetaldehyde under visible light. Coll. Surf. A Physicochem. Eng. Asp. 2017, 529, 600–612. [Google Scholar] [CrossRef]
- Sun, Y.; Su, G.; He, Z.; Wei, Y.; Hu, J.; Liu, H.; Liu, G.; Liu, J. Porous Carbons Derived from Desiliconized Rice Husk Char and Their Applications as an Adsorbent in Multivalent Ions Recycling for Spent Battery. J. Chem. 2022, 2022, 1–12. [Google Scholar] [CrossRef]
- Ding, L.; Han, Q.; Lu, H.; Yang, Y.; Lu, G.; Zhang, H.; Ran, X.; Xia, Y.; Li, P.; Chen, Y.; et al. Valence Regulation of Ultrathin Cerium Vanadate Nanosheets for Enhanced Photocatalytic CO2 Reduction to CO. Catalysts 2021, 11, 1115. [Google Scholar] [CrossRef]
- Yu, H.; Huang, J.; Jiang, L.; Leng, L.; Yi, K.; Zhang, W.; Zhang, C.; Yuan, X. In situ construction of Sn-doped structurally compatible heterojunction with enhanced interfacial electric field for photocatalytic pollutants removal and CO2 reduction. Appl. Catal. B Environ. 2021, 298, 120618. [Google Scholar] [CrossRef]
- Liu, L.; Huang, J.; Yu, H.; Wan, J.; Liu, L.; Yi, K.; Zhang, W.; Zhang, C. Construction of MoO3 nanopaticles/g-C3N4 nanosheets 0D/2D heterojuntion photocatalysts for enhanced photocatalytic degradation of antibiotic pollutant. Chemosphere 2021, 282, 131049. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.L. Reticular Materials for Artificial Photoreduction of CO2. Adv. Energy Mater. 2020, 10, 2091. [Google Scholar] [CrossRef]
- Albero, J.; Peng, Y.; García, H. Photocatalytic CO2 Reduction to C2+ Products. ACS Catal. 2020, 10, 5734–5749. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Wang, G.; Wu, J.; Chen, Z.; Zhang, C.; Li, P.; Zhou, Y.; Huang, W. Multi-Prismatic Hollow Cube CeVO4 with Adjustable Wall Thickness Directed towards Photocatalytic CO2 Reduction to CO. Nanomaterials 2023, 13, 283. https://doi.org/10.3390/nano13020283
Zhou Y, Wang G, Wu J, Chen Z, Zhang C, Li P, Zhou Y, Huang W. Multi-Prismatic Hollow Cube CeVO4 with Adjustable Wall Thickness Directed towards Photocatalytic CO2 Reduction to CO. Nanomaterials. 2023; 13(2):283. https://doi.org/10.3390/nano13020283
Chicago/Turabian StyleZhou, Yong, Guan Wang, Jiahao Wu, Zihao Chen, Chen Zhang, Ping Li, Yong Zhou, and Wei Huang. 2023. "Multi-Prismatic Hollow Cube CeVO4 with Adjustable Wall Thickness Directed towards Photocatalytic CO2 Reduction to CO" Nanomaterials 13, no. 2: 283. https://doi.org/10.3390/nano13020283
APA StyleZhou, Y., Wang, G., Wu, J., Chen, Z., Zhang, C., Li, P., Zhou, Y., & Huang, W. (2023). Multi-Prismatic Hollow Cube CeVO4 with Adjustable Wall Thickness Directed towards Photocatalytic CO2 Reduction to CO. Nanomaterials, 13(2), 283. https://doi.org/10.3390/nano13020283





