Unveiling Oxygen K-Edge and Cobalt L-Edge Electron Energy Loss Spectra of Cobalt Hydroxide and Their Evolution under Electron Beam Irradiation
Abstract
1. Introduction
2. Experimental Methods
2.1. Materials Synthesis
2.2. Electron Microscopy
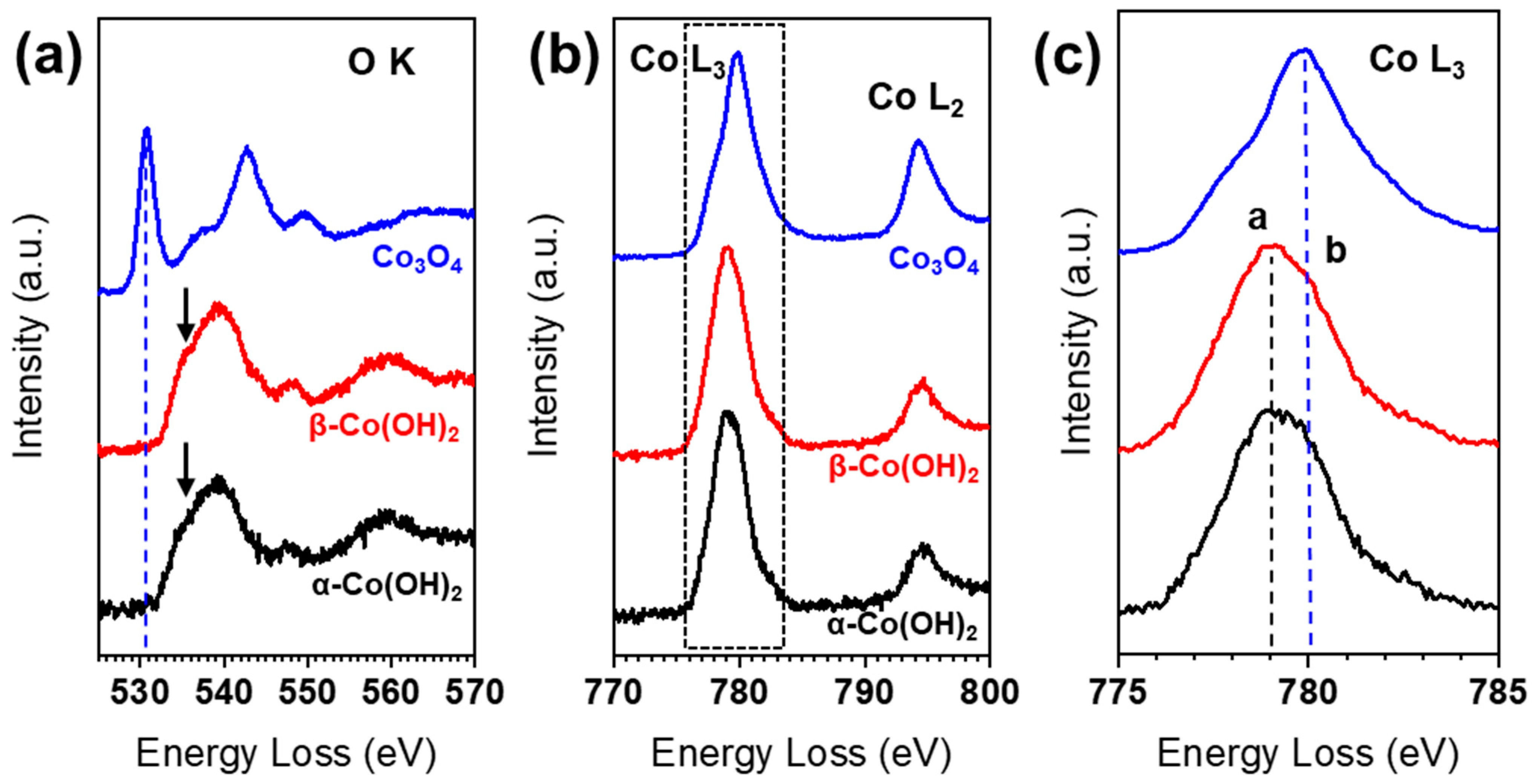
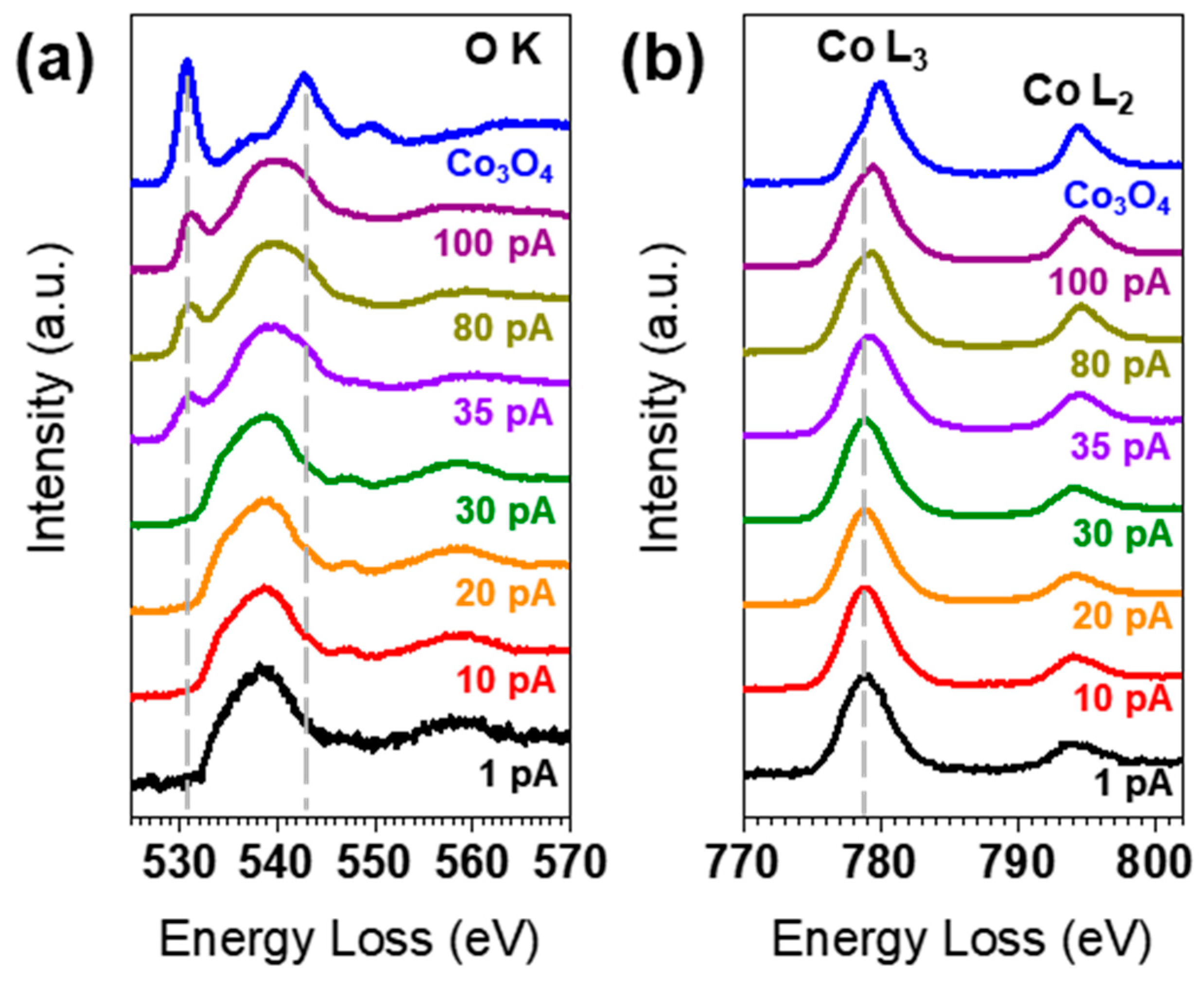
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, W.; Yin, R.; Shi, W.; Xu, X.; Shen, X.; Yin, Q.; Xu, L.; Cao, X. Gram-Scale Preparation of 2D Transition Metal Hydroxide/Oxide Assembled Structures for Oxygen Evolution and Zn-Air Battery. ACS Appl. Energy Mater. 2019, 2, 579–586. [Google Scholar] [CrossRef]
- Dionigi, F.; Zhu, J.; Zeng, Z.; Merzdorf, T.; Sarodnik, H.; Gliech, M.; Pan, L.; Li, W.; Greeley, J.; Strasser, P. Intrinsic Electrocatalytic Activity for Oxygen Evolution of Crystalline 3d-Transition Metal Layered Double Hydroxides. Angew. Chem. 2021, 133, 14567–14578. [Google Scholar] [CrossRef]
- Park, M.-C.; Kim, H.; Park, D.-H.; Yang, J.-H.; Choy, J.-H. Ketoprofen-LDH Nanohybrid for Transdermal Drug Delivery System. Bull. Korean Chem. Soc. 2012, 33, 1827–1828. [Google Scholar] [CrossRef][Green Version]
- Huang, L.; Jiang, J.; Ai, L. Interlayer Expansion of Layered Cobalt Hydroxide Nanobelts to Highly Improve Oxygen Evolution Electrocatalysis. ACS Appl. Mater. Interfaces 2017, 9, 7059–7067. [Google Scholar] [CrossRef]
- Liu, P.F.; Yang, S.; Zheng, L.R.; Zhang, B.; Yang, H.G. Electrochemical Etching of α-Cobalt Hydroxide for Improvement of Oxygen Evolution Reaction. J. Mater. Chem. A 2016, 4, 9578–9584. [Google Scholar] [CrossRef]
- Hu, Z.-A.; Xie, Y.-L.; Wang, Y.-X.; Xie, L.-J.; Fu, G.-R.; Jin, X.-Q.; Zhang, Z.-Y.; Yang, Y.-Y.; Wu, H.-Y. Synthesis of α-Cobalt Hydroxides with Different Intercalated Anions and Effects of Intercalated Anions on Their Morphology, Basal Plane Spacing, and Capacitive Property. J. Phys. Chem. C 2009, 113, 12502–12508. [Google Scholar] [CrossRef]
- Morales, F.; Grandjean, D.; De Groot, F.M.F.; Stephan, O.; Weckhuysen, B.M. Combined EXAFS and STEM-EELS Study of the Electronic State and Location of Mn as Promoter in Co-Based Fischer–Tropsch Catalysts. Phys. Chem. Chem. Phys. 2005, 7, 568–572. [Google Scholar] [CrossRef]
- Muto, S.; Yamamoto, Y.; Sakakura, M.; Tian, H.-K.; Tateyama, Y.; Iriyama, Y. STEM-EELS Spectrum Imaging of an Aerosol-Deposited NASICON-Type LATP Solid Electrolyte and LCO Cathode Interface. ACS Appl. Energy Mater. 2022, 5, 98–107. [Google Scholar] [CrossRef]
- Cypriano, J.; Werckmann, J.; Vargas, G.; Lopes Dos Santos, A.; Silva, K.T.; Leão, P.; Almeida, F.P.; Bazylinski, D.A.; Farina, M.; Lins, U.; et al. Uptake and Persistence of Bacterial Magnetite Magnetosomes in a Mammalian Cell Line: Implications for Medical and Biotechnological Applications. PLoS ONE 2019, 14, e0215657. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N. Electron Beam Damage in Oxides: A Review. Rep. Prog. Phys. 2016, 79, 016501. [Google Scholar] [CrossRef]
- Lin, F.; Markus, I.M.; Doeff, M.M.; Xin, H.L. Chemical and Structural Stability of Lithium-Ion Battery Electrode Materials under Electron Beam. Sci. Rep. 2014, 4, 5694. [Google Scholar] [CrossRef]
- Okamoto, N.L.; Shimokawa, K.; Tanimura, H.; Ichitsubo, T. Feasible Transformation of MgCo2O4 from Spinel to Defect Rocksalt Structure under Electron Irradiation. Scr. Mater. 2019, 167, 26–30. [Google Scholar] [CrossRef]
- Parajuli, P.; Park, H.; Kwon, B.J.; Guo, J.; Key, B.; Vaughey, J.T.; Zapol, P.; Klie, R.F. Direct Observation of Electron Beam-Induced Phase Transition in MgCrMnO4. Chem. Mater. 2020, 32, 10456–10462. [Google Scholar] [CrossRef]
- Egerton, R.F.; Li, P.; Malac, M. Radiation Damage in the TEM and SEM. Micron 2004, 35, 399–409. [Google Scholar] [CrossRef]
- Seo, J.H.; Park, J.Y.; Kim, Y.-I.; Nam, K.M.; Jang, J.H.; Kwon, J.-H. Real-Time Observation of Phase Transition from Layered to Spinel Phase under Electron Beam Irradiation. J. Anal. Sci. Technol. 2023, 14, 31. [Google Scholar] [CrossRef]
- Chen, J.; Li, H.; Chen, S.; Fei, J.; Liu, C.; Yu, Z.; Shin, K.; Liu, Z.; Song, L.; Henkelman, G.; et al. Co–Fe–Cr (Oxy)Hydroxides as Efficient Oxygen Evolution Reaction Catalysts. Adv. Energy Mater. 2021, 11, 2003412. [Google Scholar] [CrossRef]
- Wender, H.; Gonçalves, R.V.; Dias, C.S.B.; Zapata, M.J.M.; Zagonel, L.F.; Mendonça, E.C.; Teixeira, S.R.; Garcia, F. Photocatalytic Hydrogen Production of Co(OH)2 Nanoparticle-Coated α-Fe2O3 Nanorings. Nanoscale 2013, 5, 9310. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, H.Y.; Kim, Y.-I.; Jo, S.Y.; Abbas, S.A.; Seo, D.; Ma, A.; Nam, K.M. Chemical and Electrochemical Synthesis of Cobalt Hydroxides: Selective Phase Transformation and Application to Distinct Electrocatalytic Reactions. J. Mater. Chem. A 2022, 10, 12047–12054. [Google Scholar] [CrossRef]
- Frati, F.; Hunault, M.O.J.Y.; De Groot, F.M.F. Oxygen K-Edge X-Ray Absorption Spectra. Chem. Rev. 2020, 120, 4056–4110. [Google Scholar] [CrossRef]
- Zhao, Y.; Feltes, T.E.; Regalbuto, J.R.; Meyer, R.J.; Klie, R.F. In Situ Electron Energy Loss Spectroscopy Study of Metallic Co and Co Oxides. J. Appl. Phys. 2010, 108, 063704. [Google Scholar] [CrossRef]
- Ramesh, T.N. Polytypic Transformations during the Thermal Decomposition of Cobalt Hydroxide and Cobalt Hydroxynitrate. J. Solid State Chem. 2010, 183, 1433–1436. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, J.H.; Kwon, J.-H. Unveiling Oxygen K-Edge and Cobalt L-Edge Electron Energy Loss Spectra of Cobalt Hydroxide and Their Evolution under Electron Beam Irradiation. Nanomaterials 2023, 13, 2767. https://doi.org/10.3390/nano13202767
Seo JH, Kwon J-H. Unveiling Oxygen K-Edge and Cobalt L-Edge Electron Energy Loss Spectra of Cobalt Hydroxide and Their Evolution under Electron Beam Irradiation. Nanomaterials. 2023; 13(20):2767. https://doi.org/10.3390/nano13202767
Chicago/Turabian StyleSeo, Jong Hyeok, and Ji-Hwan Kwon. 2023. "Unveiling Oxygen K-Edge and Cobalt L-Edge Electron Energy Loss Spectra of Cobalt Hydroxide and Their Evolution under Electron Beam Irradiation" Nanomaterials 13, no. 20: 2767. https://doi.org/10.3390/nano13202767
APA StyleSeo, J. H., & Kwon, J.-H. (2023). Unveiling Oxygen K-Edge and Cobalt L-Edge Electron Energy Loss Spectra of Cobalt Hydroxide and Their Evolution under Electron Beam Irradiation. Nanomaterials, 13(20), 2767. https://doi.org/10.3390/nano13202767






