The Improved Microwave Absorption Performance of the 3D Porous (Ni@NO-C)n/NO-C Composite Absorber
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ni Nanoparticle-Assembled Chains
2.3. Fabrication of 3D Porous (Ni@NO-C)n/NO-C Composite Absorber
2.4. Characterization Methods
2.5. Electromagnetic Parameter Test
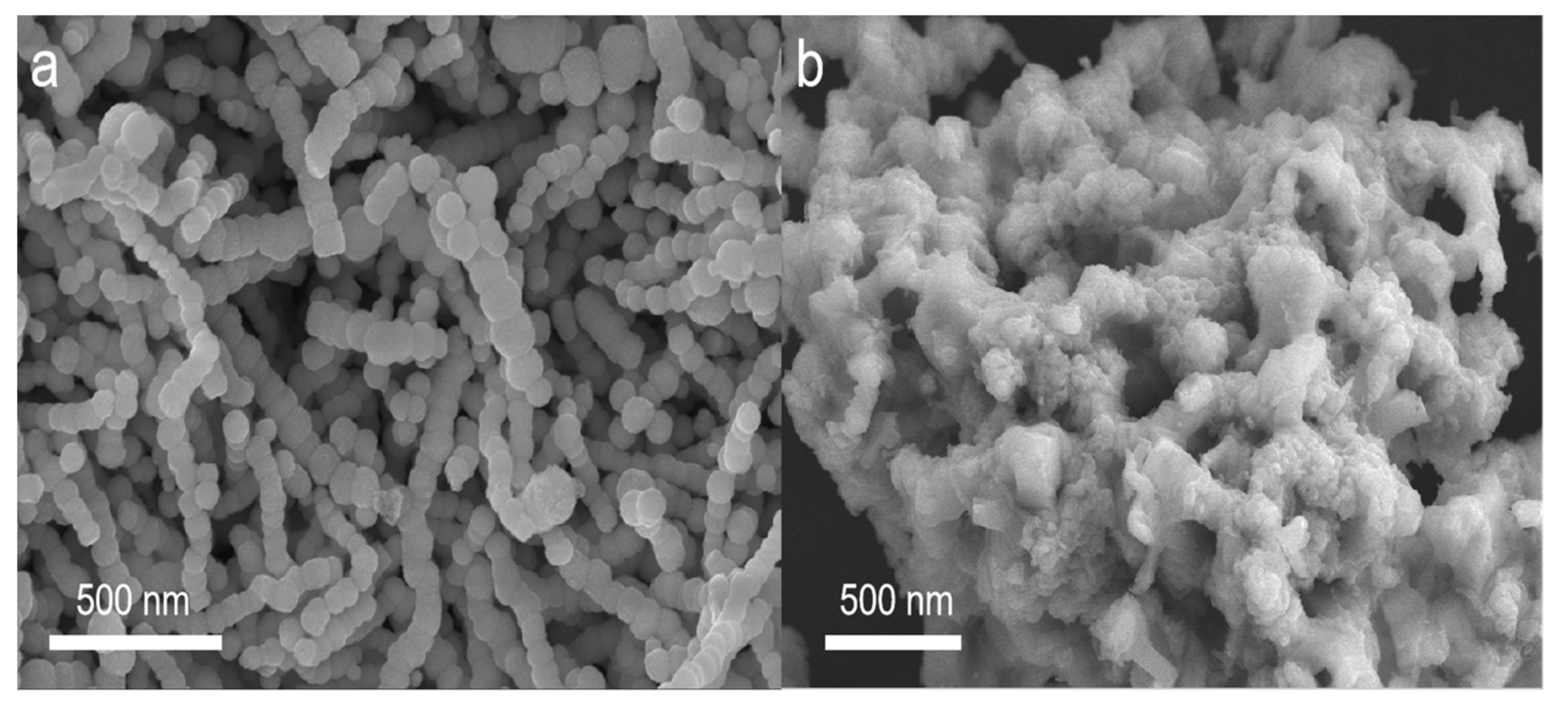
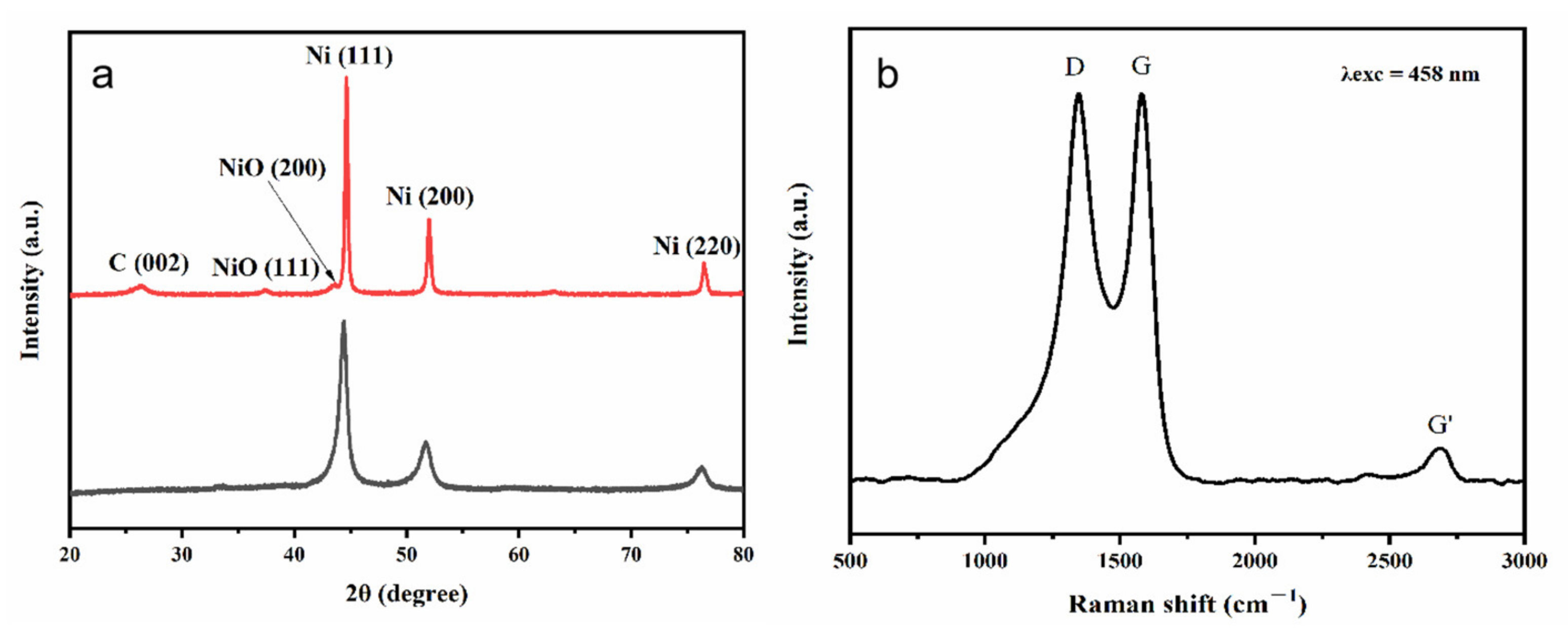
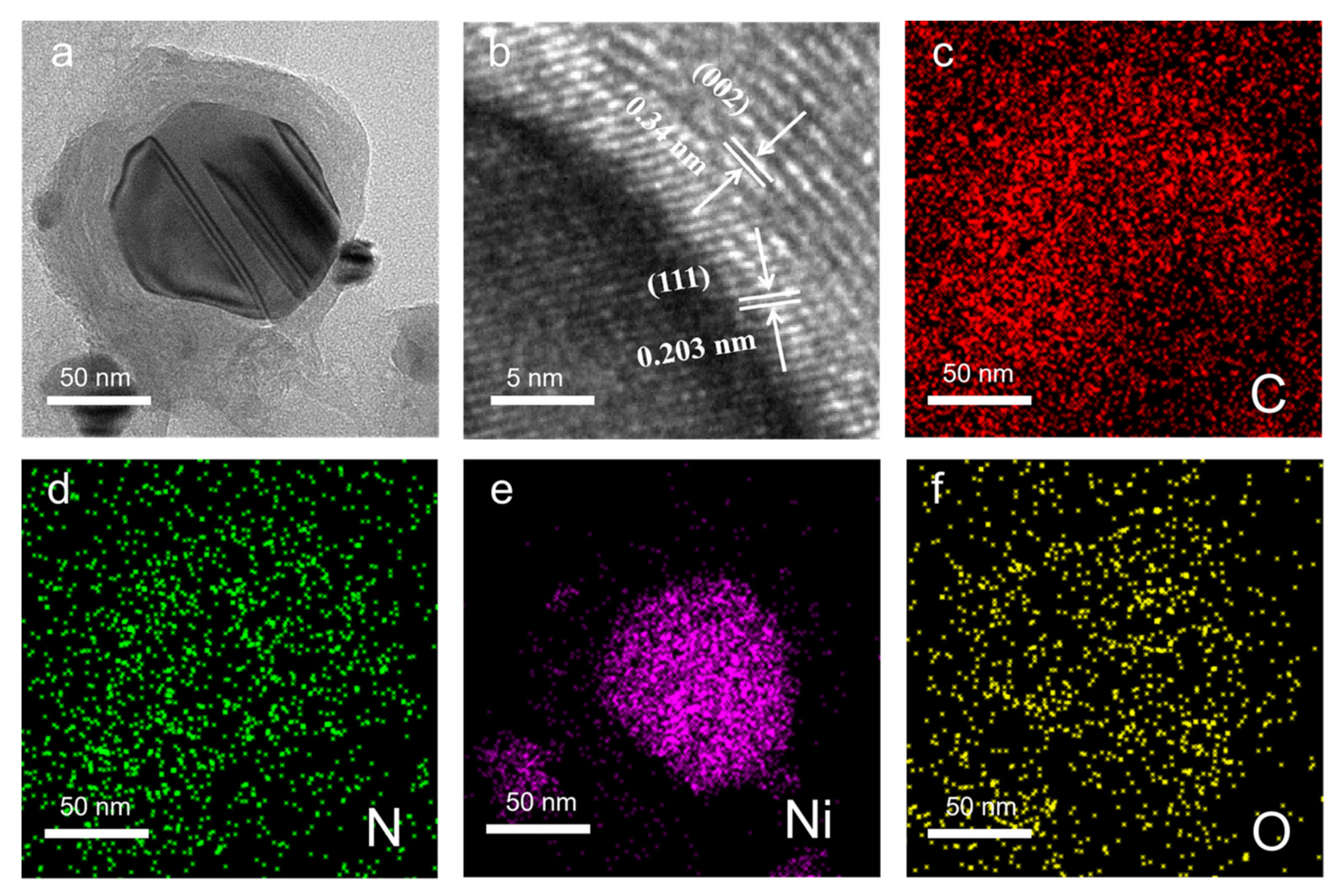
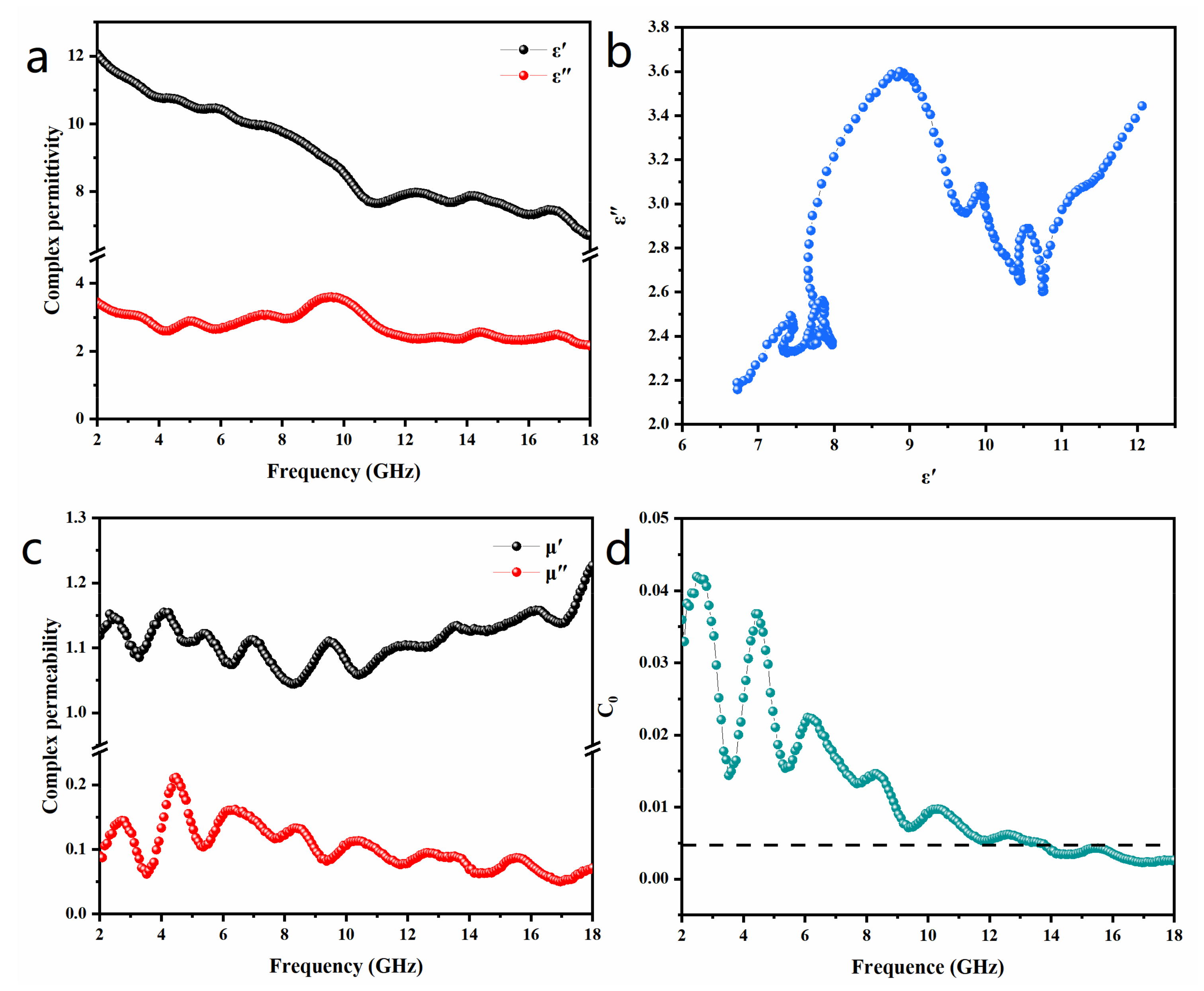
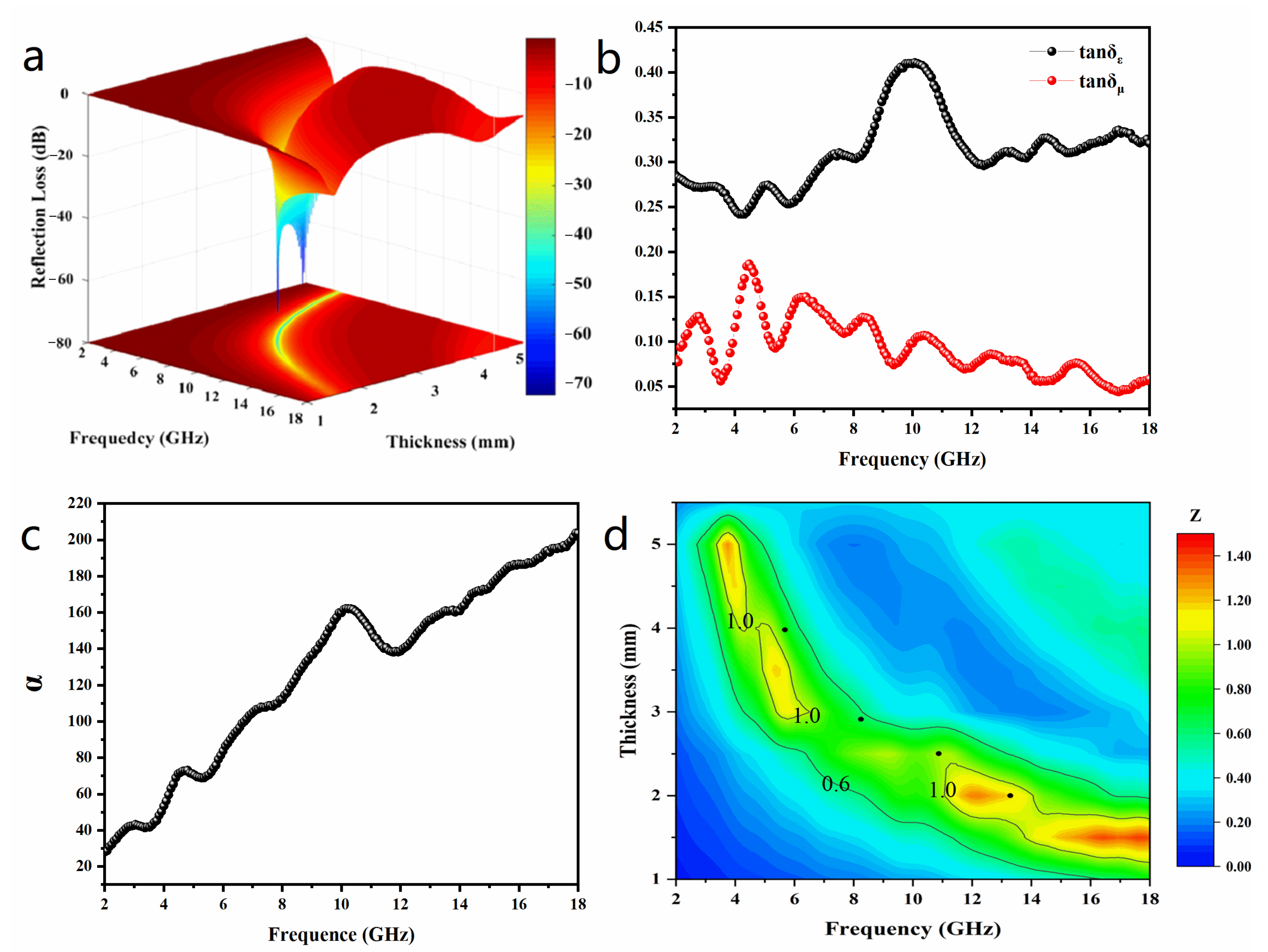
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, D.; Deng, Y.; Han, C.; Zhu, H.; Yan, C.; Zhang, H. Enhanced microwave absorption bandwidth in graphene-encapsulated iron nanoparticles with core-shell structure. Nanomaterials 2020, 10, 931. [Google Scholar] [CrossRef]
- Zhou, W.; Jiang, C.; Duan, X.; Song, J.; Yuan, Y.; Chen, N. Fe3O4/carbonized cellulose micro-nano hybrid for high-performance microwave absorber. Carbohydr. Polym. 2020, 245, 116531. [Google Scholar] [CrossRef]
- Deng, Z.; Li, Y.; Zhang, H.-B.; Zhang, Y.; Luo, J.-Q.; Liu, L.-X.; Yu, Z.-Z. Lightweight Fe@C hollow microspheres with tunable cavity for broadband microwave absorption. Compos. Part B Eng. 2019, 177, 107346. [Google Scholar] [CrossRef]
- Adam, M.; Hart, A.; Stevens, L.A.; Wood, J.; Robinson, J.P.; Rigby, S.P. Microwave synthesis of carbon onions in fractal aggregates using heavy oil as a precursor. Carbon 2018, 138, 427–435. [Google Scholar] [CrossRef]
- Cui, L.; Tian, C.; Tang, L.; Han, X.; Wang, Y.; Liu, D.; Xu, P.; Li, C.; Du, Y. Space-confined synthesis of core-shell BaTiO3@carbon microspheres as a high-performance binary dielectric system for microwave absorption. ACS Appl. Mater. Interfaces 2019, 11, 31182–31190. [Google Scholar] [CrossRef]
- Lv, X.; Ye, F.; Cheng, L.; Zhang, L. 3D printing “wire-on-sphere” hierarchical SiC nanowires/SiC whiskers foam for efficient high-temperature electromagnetic wave absorption. J. Mater. Sci. Technol. 2022, 109, 94–104. [Google Scholar] [CrossRef]
- Li, C.; Sui, J.; Jiang, X.; Zhang, Z.; Yu, L. A sustainable construction of an efficient lightweight microwave absorber from polymeric sponge. Ceram. Int. 2019, 45, 18572–18582. [Google Scholar] [CrossRef]
- Inagaki, M.; Toyoda, M.; Soneda, Y.; Morishita, T. Nitrogen-doped carbon materials. Carbon 2018, 132, 104–140. [Google Scholar] [CrossRef]
- Bai, X.; Wang, L.; Lin, Y. Hierarchical N, S-codoped honeycomb-like porous C@Co9S8@CNTs structure as high-performance microwave absorber. Mater. Lett. 2020, 264, 27342. [Google Scholar] [CrossRef]
- Shen, Z.; Yang, H.; Xiong, Z.; Xie, Y.; Liu, C. Hollow core-shell CoNi@C and CoNi@NC composites as high-performance microwave absorbers. J. Alloys Compd. 2021, 871, 159574. [Google Scholar] [CrossRef]
- Park, J.-H.; Lee, S.; Chul, J.; Ro, S.-J. Suh, Yolk–shell Fe–Fe3O4@C nanoparticles with excellent reflection loss and wide bandwidth as electromagnetic wave absorbers in the high-frequency band. Appl. Surf. Sci. 2022, 573, 151469. [Google Scholar]
- Wang, B.; Wu, Q.; Fu, Y.; Liu, T. A review on carbon/magnetic metal composites for microwave absorption. J. Mater. Sci. Technol. 2021, 86, 91–109. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Z.; Qi, L.; Liao, Q.; Kang, Z.; Zhang, Y. Toward the application of high frequency electromagnetic wave absorption by carbon nanostructures. Adv. Sci. 2019, 6, 1801057. [Google Scholar] [CrossRef]
- Li, Y.; Chen, X.; Wei, Q.; Liu, W.; Zhang, Y.; Qin, G. Oxygen-sulfur Co-substitutional Fe@C nanocapsules for improving microwave absorption properties. Sci. Bull. 2020, 65, 623–630. [Google Scholar] [CrossRef]
- Wang, Y.; Di, X.; Gao, X.; Wu, X.; Wang, P. Rational construction of Co@C polyhedrons covalently-grafted on magnetic graphene as a superior microwave absorber. J. Alloys Compd. 2020, 843, 156031. [Google Scholar]
- Yan, J.; Huang, Y.; Yan, Y.; Zhao, X.; Liu, P. The composition design of MOF-derived Co-Fe bimetallic autocatalysis carbon nanotubes with controllable electromagnetic properties. Compos. Part A Appl. Sci. Manuf. 2020, 139, 106107. [Google Scholar] [CrossRef]
- Li, H.; Bao, S.; Li, Y.; Huang, Y.; Chen, J.; Zhao, H.; Jiang, Z.; Kuang, Q.; Xie, Z. Optimizing the electromagnetic wave absorption performances of designed Co3Fe7@C yolk-shell structures. ACS Appl. Mater. Interfaces 2018, 10, 28839–28849. [Google Scholar]
- Shen, Y.; Zhang, F.; Song, P.; Zhang, Y.; Zhang, T.; Wen, X.; Ma, J.; Zhang, D.; Du, X. Design and synthesis of magnetic porous carbon nanofibers with excellent microwave absorption. J. Alloys Compd. 2022, 903, 163971. [Google Scholar] [CrossRef]
- Sha, L.; Gao, P.; Wu, T.; Chen, Y. Chemical Ni-C bonding in Ni-carbon nanotube composite by a microwave welding method and its induced high-frequency radar frequency electromagnetic wave absorption. ACS Appl. Mater. Interfaces 2017, 9, 40412–40419. [Google Scholar] [CrossRef]
- Ding, Y.; Bai, J.; Liu, H.; Zhang, Y.; Li, K.; Yang, P.-a.; Zhang, Y.; Bao, Z. Nitrogen-doped carbon nanosheets homogeneously embedded with Co nanoparticles via biostructure confinement as highly efficient microwave absorbers. Appl. Surf. Sci. 2022, 590, 153119. [Google Scholar] [CrossRef]
- Qi, Y.; Qin, Y.; Kimura, H.; Wang, Y.; Yang, Y.; Ni, C.; Yu, X.; Huang, C.; Tian, J.; Liu, R.; et al. Co/CoO/lotus seedpod nanoporous carbon composites reduced from Co3O4 for high-performance microwave absorbers. ACS Appl. Nano Mater. 2023, 6, 4681–4692. [Google Scholar] [CrossRef]
- Zhao, B.; Li, Y.; Ji, H.; Bai, P.; Wang, S.; Fan, B.; Guo, X.; Zhang, R. Lightweight graphene aerogels by decoration of 1D CoNi chains and CNTs to achieve ultra-wide microwave absorption. Carbon 2021, 176, 411–420. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, C.; Xian, G.; Zhu, Z.; Liu, Y.; Kong, L.B. Synthesis of nitrogen-doped polyaniline-derived carbon/Ni3Fe nanocomposites as high-performance microwave absorbers. J. Alloys Compd. 2022, 924, 166585. [Google Scholar] [CrossRef]
- Ning, M.; Li, J.; Kuang, B.; Wang, C.; Su, D.; Zhao, Y.; Jin, H.; Cao, M. One-step fabrication of N-doped CNTs encapsulating M nanoparticles (M = Fe, Co, Ni) for efficient microwave absorption. Appl. Surf. Sci. 2018, 447, 244–253. [Google Scholar] [CrossRef]
- Dai, W.; Chen, F.; Luo, H.; Xiong, Y.; Wang, X.; Cheng, Y.; Gong, R. Synthesis of yolk-shell structured carbonyl iron@void@nitrogen doped carbon for enhanced microwave absorption performance. J. Alloys Compd. 2020, 812, 152083. [Google Scholar] [CrossRef]
- Wang, J.; Li, Q.; Ren, J.; Zhang, A.; Zhang, Q.; Zhang, B. Synthesis of bowknot-like N-doped Co@C magnetic nanoparticles constituted by acicular structural units for excellent microwave absorption. Carbon 2021, 181, 28–39. [Google Scholar] [CrossRef]
- Zhang, X.; Yan, F.; Zhang, S.; Yuan, H.; Zhu, C.; Zhang, X.; Chen, Y. Hollow N-doped carbon polyhedron containing CoNi alloy nanoparticles embedded within few-layer N-doped graphene as high-performance electromagnetic wave absorbing material. ACS Appl. Mater. Interfaces 2018, 10, 24920–24929. [Google Scholar] [CrossRef]
- Liu, H.; Li, Y.; Yuan, M.; Sun, G.; Li, H.; Ma, S.; Liao, Q.; Zhang, Y. In situ preparation of cobalt nanoparticles decorated in N-doped carbon nanofibers as excellent electromagnetic wave absorbers. ACS Appl. Mater. Interfaces 2018, 10, 22591–22601. [Google Scholar] [CrossRef]
- Kang, Y.; Chu, Z.; Zhang, D.; Li, G.; Jiang, Z.; Cheng, H.; Li, X. Incorporate boron and nitrogen into graphene to make BCN hybrid nanosheets with enhanced microwave absorbing properties. Carbon 2013, 61, 200–208. [Google Scholar] [CrossRef]
- Tan, L.; Zhu, M.; Li, X.; Feng, H.; Chen, N.; Zhao, D. Lightweight excellent microwave absorption properties based on sulfur doped graphene. J. Saudi Chem. Soc. 2020, 24, 9–19. [Google Scholar] [CrossRef]
- Liu, J.; Cao, M.S.; Luo, Q.; Shi, H.L.; Wang, W.Z.; Yuan, J. Electromagnetic property and tunable microwave absorption of 3D nets from nickel chains at elevated temperature. ACS Appl. Mater. Interfaces 2016, 8, 22615–22622. [Google Scholar] [CrossRef] [PubMed]
- Heckmann, A.; Fromm, O.; Rodehorst, U.; Münster, P.; Winter, M.; Placke, T. New insights into electrochemical anion intercalation into carbonaceous materials for dual-ion batteries: Impact of the graphitization degree. Carbon 2018, 131, 201–212. [Google Scholar] [CrossRef]
- Yi, P.; Yao, Z.; Zhou, J.; Wei, B.; Lei, L.; Tan, R.; Fan, H. Facile synthesis of 3D Ni@C nanocomposites derived from two kinds of petal-like Ni-based MOFs towards lightweight and efficient microwave absorbers. Nanoscale 2021, 13, 3119–3135. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Duan, Y.; Pang, H. Microwave absorption of crystalline Fe/MnO@C nanocapsules embedded in amorphous carbon. Nano-Micro Lett. 2020, 12, 57. [Google Scholar] [CrossRef] [PubMed]
- Di, X.; Wang, Y.; Fu, Y.; Wu, X.; Wang, P. Wheat flour-derived nanoporous carbon@ZnFe2O4 hierarchical composite as an outstanding microwave absorber. Carbon 2021, 173, 174–184. [Google Scholar] [CrossRef]
- Yang, R.; Yuan, J.; Yu, C.; Yan, K.; Fu, Y.; Xie, H.; Chen, J.; Chu, P.; Wu, X. Efficient electromagnetic wave absorption by SiC/Ni/NiO/C nanocomposites. J. Alloys Compd. 2020, 816, 152519. [Google Scholar] [CrossRef]
- Pan, F.; Cai, L.; Dong, Y.; Zhu, X.; Shi, Y.; Lu, W. Mixed-dimensional hierarchical configuration of 2D Ni2P nanosheets anchored on 1D silk-derived carbon fiber for extraordinary electromagnetic wave absorption. J. Mater. Sci. Technol. 2022, 101, 85–94. [Google Scholar] [CrossRef]
- Shang, Q.; Feng, H.; Liu, J.; Lian, Q.; Feng, Z.; Chen, N.; Qiu, J.; Wu, H. Constructing and optimizing hollow ZnxFe3-xO4@polyaniline composites as high-performance microwave absorbers. J. Colloid Interface Sci. 2021, 584, 80–91. [Google Scholar] [CrossRef]
- Masoudpanah, S. PVP-assisted hydrothermal synthesis of rod-like NiCo2O4 powders as high-performance microwave absorbers. J. Mater Res Technol. 2022, 20, 3264–3274. [Google Scholar] [CrossRef]
- Li, P.; Zhao, Y.; Zhao, Y.; Yan, J.; Zhao, H.; Zhao, W.; Yun, J.; Chen, C.; Deng, Z.; Zhang, Z. Trimetallic prussian blue analogue derived FeCo/FeCoNi@NPC composites for highly efficient microwave absorption. Compos. B Eng. 2022, 246, 110268. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Xiang, Z.; Zhu, X.; Dong, Y.; Huang, C.; Cai, L.; Lu, W. Biconical prisms Ni@C composites derived from metal-organic frameworks with an enhanced electromagnetic wave absorption. Carbon 2021, 184, 115–126. [Google Scholar] [CrossRef]
- Wu, N.; Liu, D.; Xu, J.; Liu, W.; Shao, Q.; Guo, Z. Enhanced electromagnetic wave absorption of three-dimensional porous Fe3O4/C composite flowers. ACS Sustain. Chem. Eng. 2018, 6, 12471–12480. [Google Scholar] [CrossRef]
- Su, X.G.; Han, M.J.; Wang, J.; Wu, Q.L.; Duan, H.J.; Liang, C.B.; Zhang, S.C.; Pu, Z.Z.; Liu, Y.Q. Regulated dielectric loss based on core-sheath carbon-carbon hierarchical nanofibers toward the high-performance microwave absorption. J. Colloid Interface Sci. 2022, 624, 619–628. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhou, Q.; Zhao, H.; Ruan, C.; Wang, Y.; Li, Z.; Lian, Y. The arc-discharged Ni-cored carbon onions with enhanced microwave absorption performances. Mater. Lett. 2020, 265, 16374–16385. [Google Scholar] [CrossRef]
- Qiu, Y.; Lin, Y.; Yang, H.; Wang, L.; Wang, M.; Wen, B. Hollow Ni/C microspheres derived from Ni-metal organic framework for electromagnetic wave absorption. Chem. Eng. J. 2020, 383, 123207. [Google Scholar] [CrossRef]
- Li, J.; Zhang, F.; Lu, H.; Guo, W.; He, X.; Yuan, Y. Heterogeneous rod-like Ni@C composites toward strong and stable microwave absorption performance. Carbon 2021, 181, 358–369. [Google Scholar] [CrossRef]
- Li, S.-T.; Shi, G.-M.; Li, Q.; Shi, F.-N.; Wang, X.-L.; Yang, L.-M. One-step synthesis and performances of Ni@CN nanocapsules with superior dual-function as electrocatalyst and microwave absorbent. Colloids Surf. Physicochem. Eng. Asp. 2021, 615, 126162. [Google Scholar] [CrossRef]
- Hu, S.; Zhou, Y.; He, M.; Liao, Q.; Yang, H.; Li, H.; Xu, R.; Ding, Q. Hollow Ni-Co layered double hydroxides-derived NiCo-alloy@g-C3N4 microtubule with high-performance microwave absorption. Mater. Lett. 2018, 231, 171–174. [Google Scholar] [CrossRef]
- Xie, P.; Li, H.; He, B.; Dang, F.; Lin, J.; Fan, R.; Hou, C.; Liu, H.; Zhang, J.; Ma, Y.; et al. Bio-gel derived nickel/carbon nanocomposites with enhanced microwave absorption. J. Mater. Chem. C 2018, 6, 8812–8822. [Google Scholar] [CrossRef]
- Qiu, S.; Lyu, H.; Liu, J.; Liu, Y.; Wu, N.; Liu, W. Facile synthesis of porous nickel/carbon composite, icrospheres with enhanced electromagnetic wave absorption by magnetic and dielectric Losses. ACS Appl. Mater. Interfaces 2016, 8, 20258–20266. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Quan, B.; Ji, G.; Liang, X.; Liu, W.; Du, Y. A facile self-template strategy for synthesizing 1D porous Ni@C nanorods towards efficient microwave absorption. Nanotechnology 2017, 28, 115704. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Shao, Q.; Ji, G.; Liang, X.; Cheng, Y.; Quan, B.; Du, Y. Metal–organic-frameworks derived porous carbon-wrapped Ni composites with optimized impedance matching as excellent lightweight electromagnetic wave absorber. Chem. Eng. J. 2017, 313, 734–744. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, G.; Luo, J.; Hu, Y.; Hao, G.; Guo, H.; Guo, F.; Wang, S.; Jiang, W. A MOFs-derived 3D superstructure nanocomposite as excellent microwave absorber. Chem. Eng. J. 2021, 426, 130725. [Google Scholar] [CrossRef]
- Han, X.; Huang, Y.; Ding, L.; Song, Y.; Li, T.; Liu, P. Ti3C2Tx MXene nanosheet/metal–organic framework composites for microwave absorption. ACS Appl. Nano Mater 2020, 4, 691–701. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, S.-J.; Li, D.-S.; Jiang, L. Lightweight and recoverable ANF/rGO/PI composite aerogels for broad and high-performance microwave absorption. Compos. B Eng. 2021, 213, 108701. [Google Scholar] [CrossRef]
- Feng, J.; Pu, F.; Li, Z.; Li, X.; Hu, X.; Bai, J. Interfacial interactions and synergistic effect of CoNi nanocrystals and nitrogen-doped graphene in a composite microwave absorber. Carbon 2016, 104, 214–225. [Google Scholar] [CrossRef]
- Li, X.-P.; Deng, Z.; Li, Y.; Zhang, H.-B.; Zhao, S.; Zhang, Y.; Wu, X.-Y.; Yu, Z.-Z. Controllable synthesis of hollow microspheres with Fe@carbon dual-shells for broad bandwidth microwave absorption. Carbon 2019, 147, 172–181. [Google Scholar] [CrossRef]
- Li, W.; Li, W.C.; Ying, Y.; Yu, J.; Zheng, J.; Qiao, L.; Li, J.; Che, S. Multifunctional flower-like core-shell Fe/Fe4N@SiO2 composites for broadband and high-efficiency ultrathin electromagnetic wave absorber. J. Mater. Sci. Technol. 2023, 132, 90–99. [Google Scholar] [CrossRef]
- Liu, D.; Du, Y.; Xu, P.; Wang, F.; Wang, Y.; Cui, L.; Zhao, H.; Han, X. Rationally designed hierarchical N-doped carbon nanotubes wrapping waxberry-like Ni@C microspheres for efficient microwave absorption. J. Mater. Chem. A 2021, 9, 5086–5096. [Google Scholar] [CrossRef]
- Liu, X.; Hao, C.; Jiang, H.; Zeng, M.; Yu, R. Hierarchical NiCo2O4/Co3O4/NiO porous composite: A lightweight electromagnetic wave absorber with tunable absorbing performance. J. Mater. Chem. C 2017, 5, 3770–3778. [Google Scholar] [CrossRef]
- Liu, S.; Yu, M.; Zheng, Q.; Liang, X.; Xie, S.; Xu, Y.; Wang, C. Optimized impedance matching and enhanced microwave absorbing performance of porous flaky Fe4N wrapped with SiO2. J. Magn. Magn. Mater. 2021, 536, 168119. [Google Scholar] [CrossRef]
- Wang, Y.; Cheng, R.; Cui, W.-G.; Lu, Z.; Yang, Y.; Pan, H.; Che, R. Heterostructure design of 3D hydrangea-like Fe3O4/Fe7S8@C core-shell composite as a high-efficiency microwave absorber. Carbon 2023, 210, 118043. [Google Scholar] [CrossRef]

| Materials | Filler Load (wt. %) | Matching Thickness (mm) | RLmax (dB) | EAB (GHz) | Refs. |
|---|---|---|---|---|---|
| Ni/C hollow microspheres | 30 | 1.80 | −57.25 | 5.10 | [45] |
| Ni@C nanorods | 30 | 1.66 | −58.7 | 4.4 | [46] |
| Ni@CN nanocapsules | - | 2.3 | −35.8 | ≈3.5 | [47] |
| NiCo@g-C3N4 | 20 | 2.0 | −35.05 | 4.80 | [48] |
| Ni/C | 30 | 1.5 | ≈−17.6 | 4.8 | [49] |
| Ni/C microsphere | 75 | 1.8 | −28.40 | 4.90 | [50] |
| Ni@C nanorods | 40 | 1.7 | ≈−22 | 5.2 | [51] |
| Ni/C composite (s500) | 40 | 2.60 | −51.80 | 3.48 | [52] |
| MXene/Ni/N-CNT (HM1) | - | 1.49 | −57.78 | 2.08 | [53] |
| MXene@Ni-CZIF | 50 | 3.4 | −64.11 | ≈1.7 | [54] |
| MXene@Ni-CZIF | 33 | 4.8 | −34.52 | 1.48 | [54] |
| (Ni@NO-C)n/NO-C | 30 | 2.5 | −57.9 | 4.0 | Herein |
| (Ni@NO-C)n/NO-C | 30 | 2.9 | −72.3 | 4.2 | Herein |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, X.; Li, Z.; Ruan, C.; Lian, Y. The Improved Microwave Absorption Performance of the 3D Porous (Ni@NO-C)n/NO-C Composite Absorber. Nanomaterials 2023, 13, 2772. https://doi.org/10.3390/nano13202772
Jia X, Li Z, Ruan C, Lian Y. The Improved Microwave Absorption Performance of the 3D Porous (Ni@NO-C)n/NO-C Composite Absorber. Nanomaterials. 2023; 13(20):2772. https://doi.org/10.3390/nano13202772
Chicago/Turabian StyleJia, Xinmeng, Zhigang Li, Chao Ruan, and Yongfu Lian. 2023. "The Improved Microwave Absorption Performance of the 3D Porous (Ni@NO-C)n/NO-C Composite Absorber" Nanomaterials 13, no. 20: 2772. https://doi.org/10.3390/nano13202772





