Fe3O4-PVDF Composite Network for Dendrite-Free Lithium Metal Batteries
Abstract
:1. Introduction
2. Experiment
2.1. Fe3O4-PVDF Synthesis
2.2. Preparation of Sulfur Cathode
2.3. Structure Characterization
2.4. Electrochemical Measurement
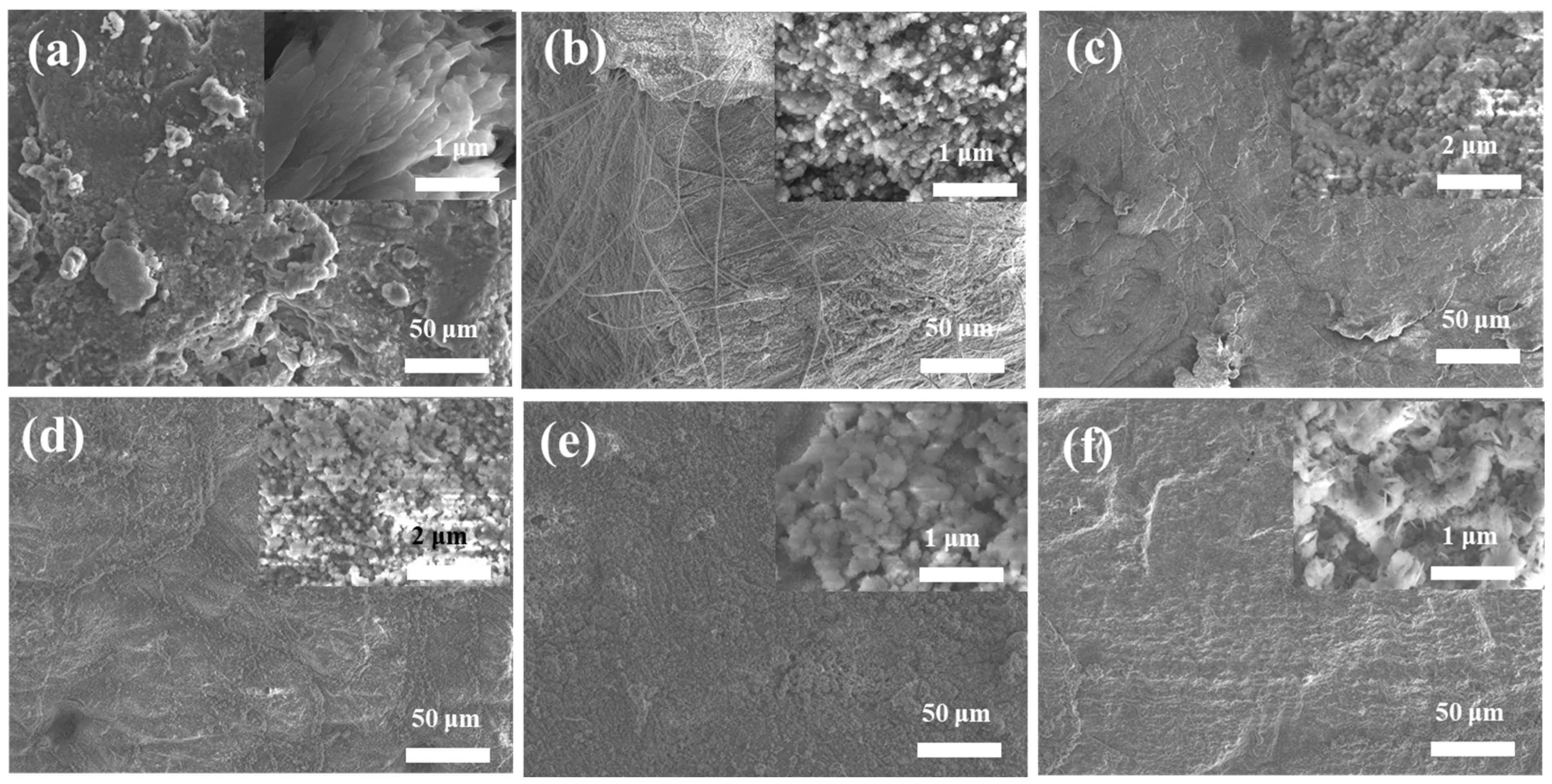
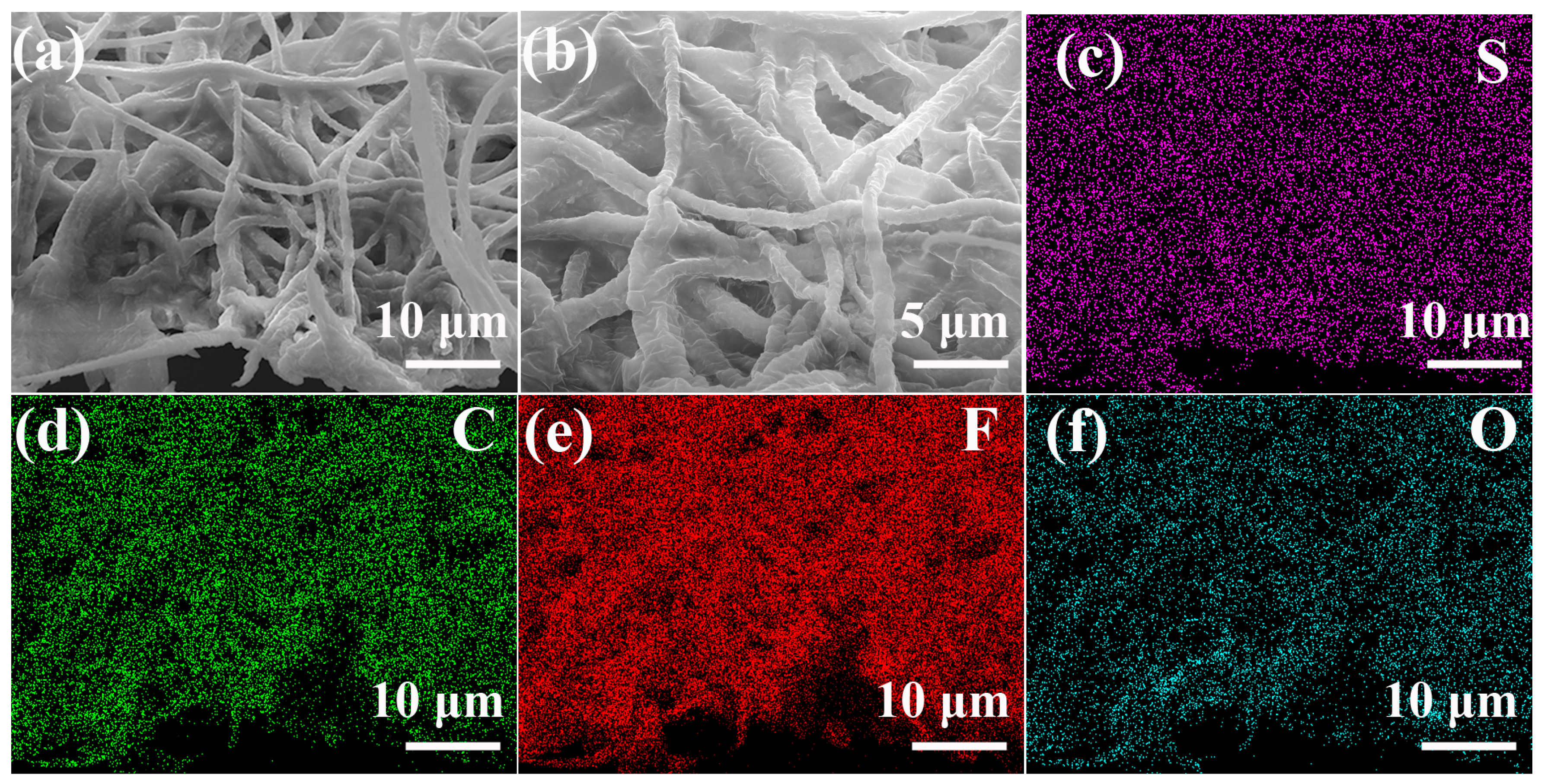
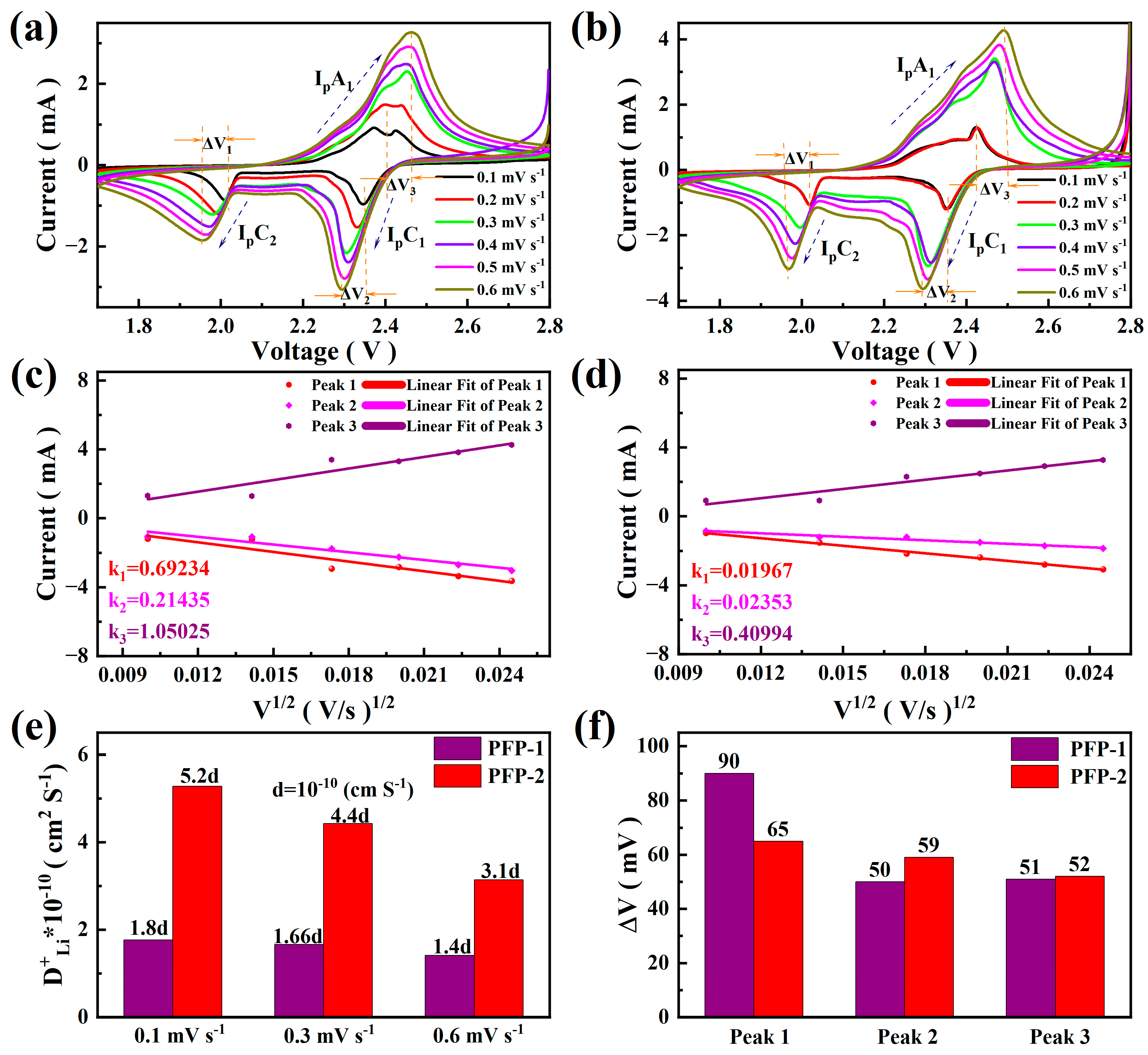
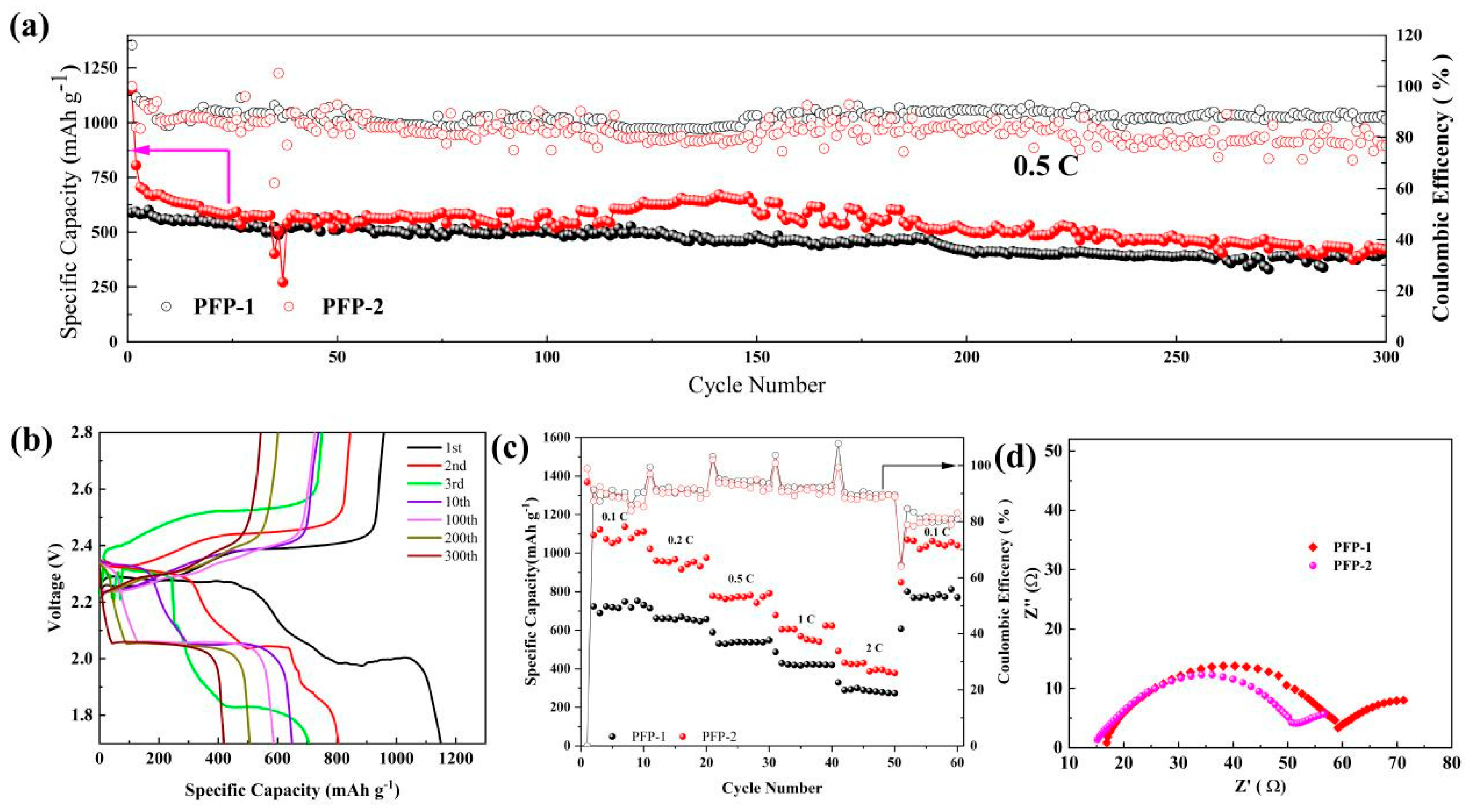
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pei, A.; Zheng, G.; Shi, F.; Li, Y.; Cui, Y. Nanoscale nucleation and growth of electrodeposited lithium metal. Nano Lett. 2017, 17, 1132–1139. [Google Scholar] [CrossRef]
- Wang, X.; Pawar, G.; Li, Y.; Ren, X.; Zhang, M.; Lu, B.; Banerjee, A.; Liu, P.; Dufek, E.J.; Zhang, J.-G. Glassy Li metal anode for high-performance rechargeable Li batteries. Nat. Mater. 2020, 19, 1339–1345. [Google Scholar] [CrossRef] [PubMed]
- Que, L.; Chen, W. Analysis of the lithium electrodeposition behavior in the charge process of lithium metal battery associated with overpotential. J. Power Sources 2023, 557, 232536. [Google Scholar] [CrossRef]
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Zhou, H.; Zhang, H.; Wang, H.; Zhang, S.; Feng, T.; Xu, Z.; Fang, Z.; Wu, M. Plasma grown fluoride-rich artificial SEI for stabilizing Li metal anodes. J. Alloys Compd. 2023, 935, 168081. [Google Scholar] [CrossRef]
- Fan, Y.; Wu, T.; He, M.; Chen, W.; Yan, C.; Li, F.; Hu, A.; Li, Y.; Wang, F.; Jiao, Y.; et al. Achieving Stable Lithium Metal Anode at 50 mA cm−2 Current Density by LiCl Enriched SEI. Small 2023, 19, 2301433. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Zhong, R.; Huang, Y.; Zhou, X.; Zhu, X.; Zhan, L.; Zhou, X.; Wang, Y.-H.; Wang, X. Oxygen defect-rich MnOOH nanorod as an effective modulator to boost polysulfide reaction kinetic for high-performance lithium sulfur battery. Appl. Surf. Sci 2023, 614, 155869. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Liang, Z.; Lee, H.-W.; Sun, J.; Wang, H.; Yan, K.; Xie, J.; Cui, Y. Layered reduced graphene oxide with nanoscale interlayer gaps as a stable host for lithium metal anodes. Nat. Nanotechnol. 2016, 11, 626. [Google Scholar] [CrossRef]
- Chi, S.S.; Liu, Y.; Song, W.L.; Fan, L.Z.; Zhang, Q. Prestoring lithium into sTable 3D nickel foam host as dendrite-free lithium metal anode. Adv. Funct. Mater. 2017, 27, 1700348. [Google Scholar] [CrossRef]
- Liu, L.; Yin, Y.X.; Li, J.Y.; Wang, S.H.; Guo, Y.G.; Wan, L.J. Uniform lithium nucleation/growth induced by lightweight nitrogen-doped graphitic carbon foams for high-performance lithium metal anodes. Adv. Mater. 2018, 30, 1706216. [Google Scholar] [CrossRef]
- Ren, W.; Zheng, Y.; Cui, Z.; Tao, Y.; Li, B.; Wang, W. Recent progress of functional separators in dendrite inhibition for lithium metal batteries. Energy Storage Mater. 2021, 35, 157. [Google Scholar] [CrossRef]
- Zhang, R.; Li, N.W.; Cheng, X.B.; Yin, Y.X.; Zhang, Q.; Guo, Y.G. Advanced micro/nanostructures for lithium metal anodes. Adv. Sci. 2017, 4, 1600445. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Deng, N.; Ju, J.; Kang, J.; Wang, X.; Ding, L.; Kang, W.; Cheng, B. A review on nanofiber materials for lithium-metal batteries to suppress the dendritic lithium growth. Chem. Eng. J. 2022, 433, 134392. [Google Scholar] [CrossRef]
- Chen, S.; Dai, F.; Cai, M. Opportunities and challenges of high-energy lithium metal batteries for electric vehicle applications. ACS Energy Lett. 2020, 5, 3140. [Google Scholar] [CrossRef]
- Castillo, J.; Coca-Clemente, J.A.; Rikarte, J.; Sáenz de Buruaga, A.; Santiago, A.; Li, C. Recent progress on lithium anode protection for lithium–sulfur batteries: Review and perspective. APL Mater. 2023, 11, 010901. [Google Scholar] [CrossRef]
- Xu, R.; Zhang, X.Q.; Cheng, X.B.; Peng, H.J.; Zhao, C.Z.; Yan, C.; Huang, J.Q. Artificial Soft-Rigid Protective Layer for Dendrite-Free Lithium Metal Anode. Adv. Funct. Mater 2018, 28, 7. [Google Scholar] [CrossRef]
- Heine, J.; Hilbig, P.; Qi, X.; Niehoff, P.; Winter, M.; Bieker, P. Fluoroethylene carbonate as electrolyte additive in tetraethylene glycol dimethyl ether based electrolytes for application in lithium ion and lithium metal batteries. J. Electrochem. Soc. 2015, 162, A1094. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Cheng, X.B.; Chen, X.; Yan, C.; Zhang, Q. Fluoroethylene carbonate additives to render uniform Li deposits in lithium metal batteries. Adv. Funct. Mater. 2017, 27, 1605989. [Google Scholar] [CrossRef]
- Sahalie, N.A.; Assegie, A.A.; Su, W.-N.; Wondimkun, Z.T.; Jote, B.A.; Thirumalraj, B.; Huang, C.-J.; Yang, Y.-W.; Hwang, B.-J. Effect of bifunctional additive potassium nitrate on performance of anode free lithium metal battery in carbonate electrolyte. J. Power Sources 2019, 437, 226912. [Google Scholar] [CrossRef]
- Wu, M.; Wen, Z.; Jin, J.; Cui, Y. Effects of combinatorial AlCl3 and pyrrole on the SEI formation and electrochemical performance of Li electrode. Electrochim. Acta 2013, 103, 199. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, F.; Bai, Y.; Li, Y.; Chen, G.; Wang, Z.; Wu, C. Regulating Li deposition by constructing LiF-rich host for dendrite-free lithium metal anode. Energy Storage Mater. 2019, 16, 411. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Li, Y.; Cui, Y. Lithium metal anode materials design: Interphase and host. Electrochem. Energy Rev. 2019, 2, 509. [Google Scholar] [CrossRef]
- Huang, Y.; Wu, X.; Nie, L.; Chen, S.; Sun, Z.; He, Y.; Liu, W. Mechanism of lithium electrodeposition in a magnetic field. Solid State Ion. 2020, 345, 115171. [Google Scholar] [CrossRef]
- Shen, K.; Wang, Z.; Bi, X.; Ying, Y.; Zhang, D.; Jin, C.; Hou, G.; Cao, H.; Wu, L.; Zheng, G. Magnetic Field–Suppressed Lithium Dendrite Growth for Stable Lithium-Metal Batteries. Adv. Energy Mater. 2019, 9, 1900260. [Google Scholar] [CrossRef]
- Wang, A.; Deng, Q.; Deng, L.; Guan, X.; Luo, J. Eliminating tip dendrite growth by Lorentz force for stable lithium metal anodes. Adv. Funct. Mater. 2019, 29, 1902630. [Google Scholar] [CrossRef]
- He, J.; Luo, L.; Chen, Y.; Manthiram, A. Yolk–shelled C@ Fe3O4 nanoboxes as efficient sulfur hosts for high-performance lithium–sulfur batteries. Adv. Mater. 2017, 29, 1702707. [Google Scholar] [CrossRef]
- Shen, Y.-Q.; Zeng, F.-L.; Zhou, X.-Y.; Wang, A.-b.; Wang, W.-k.; Yuan, N.-Y.; Ding, J.-N. A novel permselective organo-polysulfides/PVDF gel polymer electrolyte enables stable lithium anode for lithium–sulfur batteries. J. Energy Chem. 2020, 48, 267. [Google Scholar] [CrossRef]
- Ma, C.; Yao, C.; Tang, Z.; Wang, Y.; Ou, Y.; Liu, L.; Song, H.; Wang, F.; Cheng, J. Lithium–sulfur battery cathode design: Sulfur-infiltrated PVDF nanofiber-based Fe3O4 network for polysulfide adsorption and volume expansion suppression. Colloids Surf. A Physicochem. Eng. Asp. 2023, 666, 131331. [Google Scholar] [CrossRef]
- Zhang, J.L.; Chen, H.B.; Wen, M.; Shen, K.; Chen, Q.; Hou, G.Y.; Tang, Y.P. Lithiophilic 3D Copper-Based Magnetic Current Collector for Lithium-Free Anode to Realize Deep Lithium Deposition. Adv. Funct. Mater. 2022, 32, 8. [Google Scholar] [CrossRef]
- Sagane, F.; Shimokawa, R.; Sano, H.; Sakaebe, H.; Iriyama, Y. In-situ scanning electron microscopy observations of Li plating and stripping reactions at the lithium phosphorus oxynitride glass electrolyte/Cu interface. J. Power Sources 2013, 225, 245. [Google Scholar] [CrossRef]
- Yang, C.-P.; Yin, Y.-X.; Zhang, S.-F.; Li, N.-W.; Guo, Y.-G. Accommodating lithium into 3D current collectors with a submicron skeleton towards long-life lithium metal anodes. Nat. Commun. 2015, 6, 8058. [Google Scholar] [CrossRef]
- Tang, Y.; Shen, K.; Lv, Z.; Xu, X.; Hou, G.; Cao, H.; Wu, L.; Zheng, G.; Deng, Y. Three-dimensional ordered macroporous Cu current collector for lithium metal anode: Uniform nucleation by seed crystal. J. Power Sources 2018, 403, 82. [Google Scholar] [CrossRef]
- Zou, P.; Sui, Y.; Zhan, H.; Wang, C.; Xin, H.L.; Cheng, H.-M.; Kang, F.; Yang, C. Polymorph evolution mechanisms and regulation strategies of lithium metal anode under multiphysical fields. Chem. Rev. 2021, 121, 5986. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhang, H.W.; Liang, L.Y.; Liu, Z.; Qi, Y.; Lu, P.; Chen, J.; Chen, L.-Q. Modulation of dendritic patterns during electrodeposition: A nonlinear phase-field model. J. Power Sources 2015, 300, 376. [Google Scholar] [CrossRef]
- Krause, A.; Uhlemann, M.; Gebert, A.; Schultz, L. A study of nucleation, growth, texture and phase formation of electrodeposited cobalt layers and the influence of magnetic fields. Thin Solid Film. 2006, 515, 1694. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Xu, P.; Liu, Q.B.; Yuan, D.; Long, X.; Zhu, S.K. Cobalt embedded in porous carbon fiber membranes for high-performance lithium-sulfur batteries. Carbon 2022, 187, 187. [Google Scholar] [CrossRef]
- Razzaq, A.; Yao, Y.; Shah, R.; Qi, P.; Miao, L.; Chen, M.; Zhao, X.; Peng, Y.; Deng, Z. High-Performance Lithium Sulfur Batteries Enabled by a Synergy between Sulfur and Carbon Nanotubes. Energy Storage Mater. 2019, 16, 194–202. [Google Scholar] [CrossRef]
- Kim, J.R.; Choi, S.W.; Jo, S.M.; Lee, W.S.; Kim, B.C. Electrospun PVdF-based fibrous polymer electrolytes for lithium ion polymer batteries. Electrochim. Acta 2004, 50, 69. [Google Scholar] [CrossRef]
- Su, Y.-S.; Manthiram, A. Lithium–sulphur batteries with a microporous carbon paper as a bifunctional interlayer. Nat. Commun. 2012, 3, 1166. [Google Scholar] [CrossRef]
- Lei, T.; Xie, Y.; Wang, X.; Miao, S.; Xiong, J.; Yan, C. TiO2 feather duster as effective polysulfides restrictor for enhanced electrochemical kinetics in lithium–sulfur batteries. Small 2017, 13, 1701013. [Google Scholar] [CrossRef]
- Chang, C.; Yang, C.; Wu, Q.; Wang, X.; Nie, H.; Zhou, X.; Xie, X.; Hwang, B.; Ye, Y. All-in-one Janus separator for lithium–sulfur batteries with lithium polysulfide and dendrite growth suppressed at temperature gradient effect. J. Power Sources 2022, 550, 232115. [Google Scholar] [CrossRef]
- Cheng, P.; Guo, P.Q.; Liu, D.Q.; Wang, Y.R.; Sun, K.; Zhao, Y.G.; He, D.Y. Fe3O4/RGO modified separators to suppress the shuttle effect for advanced lithium-sulfur batteries. J. Alloys Compd. 2019, 784, 149. [Google Scholar] [CrossRef]
- Zhang, S. Role of LiNO3 in rechargeable lithium/sulfur battery. Electrochim. Acta 2012, 70, 344. [Google Scholar] [CrossRef]
- Zhang, S. Effect of discharge cutoff voltage on reversibility of lithium/sulfur batteries with LiNO3-contained electrolyte. J. Electrochem. Soc. 2012, 159, A920. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ou, Y.; Ma, C.; Tang, Z.; Yao, C.; Zhao, Y.; Cheng, J. Fe3O4-PVDF Composite Network for Dendrite-Free Lithium Metal Batteries. Nanomaterials 2023, 13, 2782. https://doi.org/10.3390/nano13202782
Ou Y, Ma C, Tang Z, Yao C, Zhao Y, Cheng J. Fe3O4-PVDF Composite Network for Dendrite-Free Lithium Metal Batteries. Nanomaterials. 2023; 13(20):2782. https://doi.org/10.3390/nano13202782
Chicago/Turabian StyleOu, Yun, Chaoyong Ma, Zhiyong Tang, Chenqi Yao, Yunzhuo Zhao, and Juanjuan Cheng. 2023. "Fe3O4-PVDF Composite Network for Dendrite-Free Lithium Metal Batteries" Nanomaterials 13, no. 20: 2782. https://doi.org/10.3390/nano13202782
APA StyleOu, Y., Ma, C., Tang, Z., Yao, C., Zhao, Y., & Cheng, J. (2023). Fe3O4-PVDF Composite Network for Dendrite-Free Lithium Metal Batteries. Nanomaterials, 13(20), 2782. https://doi.org/10.3390/nano13202782





