Structural, Optical, Electrical, and Thermoelectric Properties of Bi2Se3 Films Deposited at a High Se/Bi Flow Rate
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of Bi2Se3 Thin Films
2.2. Characterization of Prepared Thin Films
2.2.1. Structural Characterization
2.2.2. Electrical and Thermoelectric Characterization
2.2.3. Optical Characterization
3. Results and Discussion
3.1. XRD and Raman Analysis
3.2. XPS Analysis
3.3. AFM and HRTEM Analysis
3.4. Optical Characteristics of Films
3.5. Electrical Characteristics
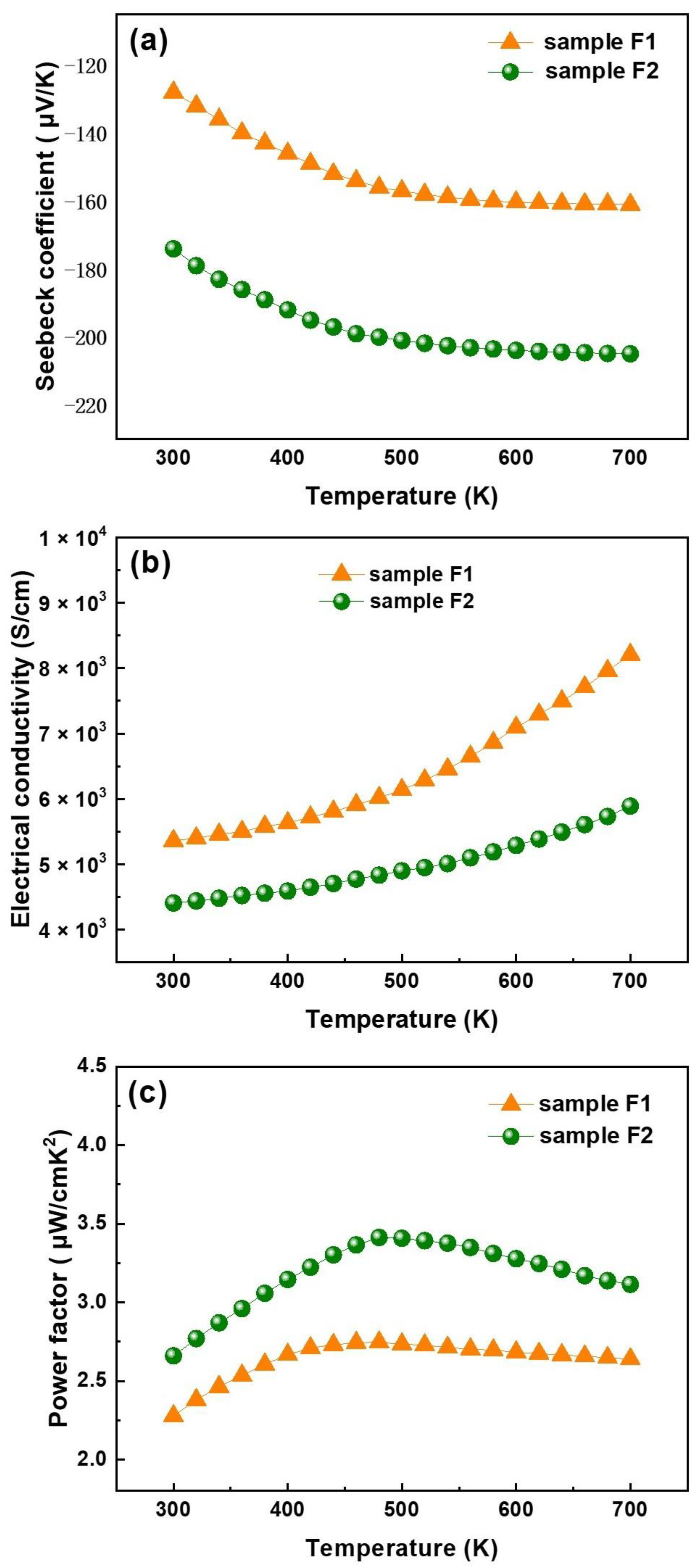
3.6. Thermoelectric Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Li, M.; Liu, Y.; Zhang, Y.; Han, X.; Zhang, T.; Zuo, Y.; Xie, C.; Xiao, K.; Arbiol, J.; Llorca, J.; et al. Effect of the annealing atmosphere on crystal phase and thermoelectric properties of copper sulfide. ACS Nano 2021, 15, 4967–4978. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, Y.; Zhang, Y.; Zuo, Y.; Li, J.; Lim, K.H.; Cadavid, D.; Ng, K.M.; Cabot, A. Crystallographically textured SnSe nanomaterials produced from the liquid phase sintering of nanocrystals. Dalton Trans. 2019, 48, 3641–3647. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Ortega, S.; Ibáñez, M.; Lim, K.H.; Grau-Carbonell, A.; Martí-Sánchez, S.; Ng, K.M.; Arbiol, J.; Kovalenko, M.V.; et al. Crystallographically textured nanomaterials produced from the liquid phase sintering of BixSb2−xTe3 nanocrystal building blocks. Nano Lett. 2018, 18, 2557–2563. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, Y.; Lim, K.H.; Ibáñez, M.; Ortega, S.; Li, M.; David, J.; Martí-Sànchez, S.; Ng, K.M.; Arbiol, J.; et al. High thermoelectric performance in crystallographically textured n type Bi2Te3−xSex produced from asymmetric colloidal nanocrystals. ACS Nano 2018, 12, 7174–7184. [Google Scholar] [CrossRef]
- DiSalvo, F.J. Thermoelectric cooling and power generation. Science 1999, 285, 703–706. [Google Scholar] [CrossRef]
- Ali, Z.; Butt, S.; Cao, C.; Butt, F.K.; Tahir, M.; Tanveer, M.; Aslam, I.; Rizwan, M.; Idrees, F.; Khalid, S. Thermochemically evolved nanoplatelets of bismuth selenide with enhanced thermoelectric figure of merit. AIP Adv. 2014, 4, 117129. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Z.G.; Hong, M.; Han, G.; Zou, J. Enhanced thermoelectric performance of nanostructured Bi2Te3 through significant phonon scattering. ACS Appl. Mater. Interfaces 2015, 7, 23694–23699. [Google Scholar] [CrossRef]
- Kumar, R.; Yadav, A.K.; Biswas, A.; Nand, M.; Bahadur, J.; Ghosh, S.; Jha, S.N.; Bhattacharyya, D. Crystalline Bi2Se3 topological insulator flms prepared by dc magnetron sputtering. Vacuum 2020, 177, 109366. [Google Scholar] [CrossRef]
- Zhang, M.; Wei, Z.; Jin, R.; Ji, Y.; Yan, Y.; Pu, X.; Yang, X.; Zhao, Y. Electrical transport properties and morphology of topological insulator Bi2Se3 thin flms with diferent thickness prepared by magnetron sputtering. Thin Solid Films 2016, 603, 289–293. [Google Scholar] [CrossRef]
- Wang, W.J.; Gao, K.H.; Li, Z.Q. Thickness-dependent transport channels in topological insulator Bi2Se3 thin flms grown by magnetron sputtering. Sci. Rep. 2016, 6, 25291. [Google Scholar] [CrossRef] [PubMed]
- Andzane, A.; Buks, K.; Strakova, M.N.; Zubkins, M.; Bechelany, M.; Marnauza, M.; Baitimirova, M.; Erts, D. Structure and doping determined thermoelectric properties of Bi2Se3 thin flms deposited by vapour-solid technique. IEEE Trans. Nanotechnol. 2019, 18, 948–954. [Google Scholar] [CrossRef]
- Gautam, S.; Verma, A.K.; Balapure, A.; Singh, B.; Ganesan, R.; Kumar, M.S.; Singh, V.N.; Gahtori, B.; Kushvaha, S.S. Structural, electronic and thermoelectric properties of Bi2Se3 thin films deposited by RF magnetron sputtering. J. Electron Mater. 2022, 51, 2500–2509. [Google Scholar] [CrossRef]
- Hong, M.; Chen, Z.G.; Yang, L.; Han, G.; Zou, J. Enhanced thermoelectric performance of ultrathin Bi2Se3 nanosheets through thickness control. Adv. Electron. Mater. 2015, 1, 1500025. [Google Scholar] [CrossRef]
- Bahk, J.H.; Bian, Z.X.; Shakouri, A. Electron energy filtering by a nonplanar potential to enhance the thermoelectric power factor in bulk materials. Phys. Rev. B 2013, 87, 075204. [Google Scholar] [CrossRef]
- Kulsi, C.; Kargupta, K.; Ganguly, S.; Banerjee, D. Enhanced thermoelectric performance of n-type bismuth selenide doped with nickel. Curr. Appl. Phys. 2017, 17, 1609–1615. [Google Scholar] [CrossRef]
- Sun, G.; Qin, X.; Li, D.; Zhang, J.; Ren, B.; Zou, T.; Xin, H.; Paschen, S.B.; Yan, X. Enhanced thermoelectric performance of n-type Bi2Se3 doped with Cu. J. Alloys Compd. 2015, 639, 9–14. [Google Scholar] [CrossRef]
- Kim, H.S.; Hong, S.J. Thermoelectric properties of n-type 95%Bi2Te3-5%Bi2Se3 compounds fabricated by gas-atomization and spark plasma sintering. J. Alloys Compd. 2014, 586, S428–S431. [Google Scholar] [CrossRef]
- Lee, G.E. Preparation and thermoelectric properties of Bi2Te3-Bi2Se3 solid solutions. J. Korean Phys. Soc. 2014, 64, 1416–1420. [Google Scholar] [CrossRef]
- Jimenez, S.; Perez, J.G.; Tritt, T.M.; Zhu, S.; Lopez, O. Synthesis and thermoelectric performance of a p-type Bi0.4Sb1.6Te3 material developed via mechanical alloying. Energy Convers. Manag. 2014, 87, 868–873. [Google Scholar] [CrossRef]
- Wang, C.C.; Shieu, F.S.; Shih, H.C. Photosensing and characterizing of the pristine and In-, Sn-Doped Bi2Se3 nanoplatelets fabricated by thermal V–S process. Nanomaterials 2021, 11, 1352. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zhang, Y.; Zhang, T.; Zuo, Y.; Xiao, K.; Arbiol, J.; Liorca, J.; Liu, Y.; Cabot, A. Enhanced thermoelectric performance of n-type Bi2Se3 nanosheets through Sn doping. Nanomaterials 2021, 11, 1827. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Sharma, P.; Sharma, M.M.; Kumar, Y.; Awana, V.P.S. Growth and characterization of Ni substituted Bi2Se3 single crystals. J. Supercond Nov. Magn. 2022, 35, 1017–1024. [Google Scholar] [CrossRef]
- Mohyedin, M.Z.; Malik, N.A.; Taib, M.F.M.; Yahya, M.Z.A. First principles study of structural, electronic and optical properties of orthorhombic phase Ni-doped Bi2Se3 using density functional theory. Comput. Condens. Matter. 2020, 25, e00510. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.; Woo, J.; Lee, G. Growth of Bi2Se3 topological insulator thin film on Ge(111) substrate. Appl. Surf. Sci. 2018, 432, 152–155. [Google Scholar] [CrossRef]
- Zhang, J.; Jiang, T.; Zhou, T.; Ouyang, H.; Zhang, C.; Xin, Z.; Wang, Z. Saturated absorption of different layered Bi2Se3 films in the resonance zone. Photonics Res. 2018, 6, C8–C14. [Google Scholar] [CrossRef]
- Antonova, I.V.; Nebogatikova, N.A.; Kokh, K.A.; Kustov, D.A.; Soots, R.A.; Golyashov, V.A.; Tereshchenko, O.E. Electrochemically Exfoliated Thin Bi2Se3 Films and Van Der Waals Heterostructures Bi2Se3/Graphene. Nanotechnology 2020, 31, 125602. [Google Scholar] [CrossRef]
- Badalova, Z.I.; Abdullayev, N.A. Anharmonicity of lattice vibrations in Bi2Se3 single crystals. Semiconductors 2019, 53, 291–295. [Google Scholar] [CrossRef]
- Chuai, Y.H.; Hu, B.; Li, Y.; Shen, H.; Zheng, C.; Wang, Y.D. Effect of Sn substitution on the structure, morphology and photoelectricity properties of high c-axis oriented CuFe1-xSnxO2 thin film. J. Alloys Compd. 2015, 627, 299–306. [Google Scholar] [CrossRef]
- Fang, M.; Wang, Z.; Gu, H.; Song, B.; Guo, Z.; Zhu, J.; Chen, X.; Zhang, C.; Jiang, H.; Liu, S. Complex optical conductivity of Bi2Se3 thin film: Approaching two-dimensional limit. Appl. Phys. Lett. 2021, 118, 191101. [Google Scholar] [CrossRef]
- Guillet, T.; Marty, A.; Beigné, C.; Vergnaud, C.; Dau, M.T.; Noël, P.; Frigerio, J.; Isella, G.; Jamet, M. Magnetotransport in Bi2Se3 thin films epitaxially grown on Ge (111). AIP Adv. 2018, 8, 115125. [Google Scholar] [CrossRef]
- Li, D.; Fang, X.; Zhao, A.; Deng, Z.; Dong, W.; Tao, R. Physical properties of CuCrO2 films prepared by pulsed laser deposition. Vacuum 2010, 84, 851–856. [Google Scholar] [CrossRef]
- Chuai, Y.H.; Zhu, C.; Yue, D.; Bai, Y. Epitaxial growth of Bi2Se3 infrared transparent conductive film and heterojunction diode by molecular beam epitaxy. Front Chem. 2022, 10, 847972. [Google Scholar] [CrossRef]
- Pan, J.J.; Wang, W.W.; Wu, D.Q.; Fu, Q.; Ma, D. Tungsten doped indium oxide thin films deposited at room temperature by radio frequency magnetron sputtering. J. Mater. Sci. Technol. 2014, 30, 644–648. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Zang, D.; Feng, L. Structural and opto-electrical properties of Cu–Al–O thin films prepared by magnetron sputtering method. Vacuum 2014, 99, 160–165. [Google Scholar] [CrossRef]
- Yang, L.; Han, J.C.; Zhu, J.; Zhu, Y.; Schlaberg, H.I. Chemical bonding and optoelectrical properties of ruthenium doped yttrium oxide thin films. Mater. Res. Bull. 2013, 48, 4486–4490. [Google Scholar] [CrossRef]
- Hasan, M.Z.; Kane, C.L. Colloquium: Topological insulators. Rev. Mod. Phys. 2010, 82, 3045. [Google Scholar] [CrossRef]
- Sun, Y.; Cheng, H.; Gao, S.; Liu, Q.; Sun, Z.; Xiao, C.; Wu, C.; Wei, S.; Xie, Y. Atomically thick bismuth selenide freestanding single layers achieving enhanced thermoelectric energy harvesting. J. Am. Chem. Soc. 2012, 134, 20294–20297. [Google Scholar] [CrossRef]
 : Bi).
: Bi).
 : Bi).
: Bi).
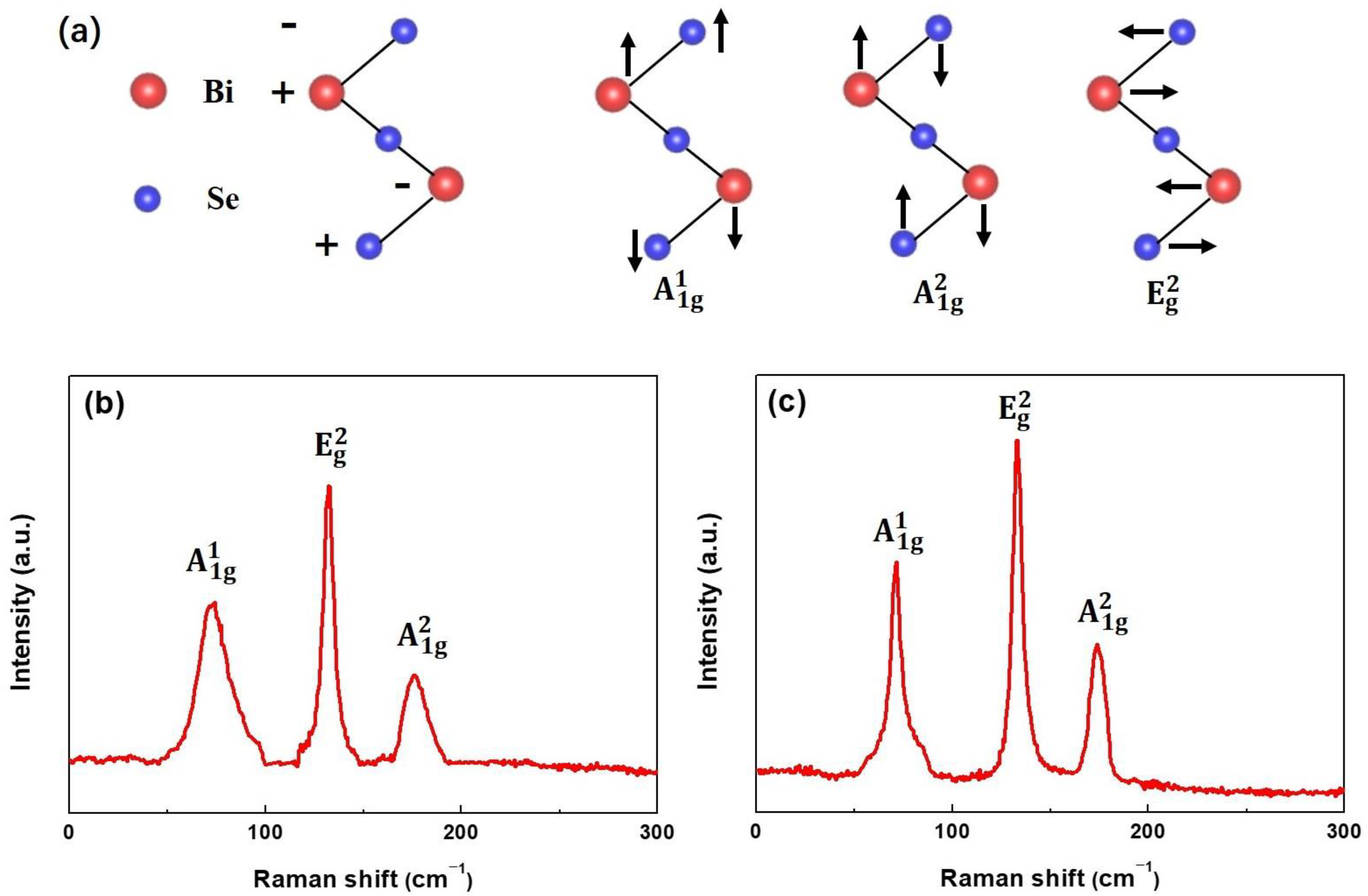

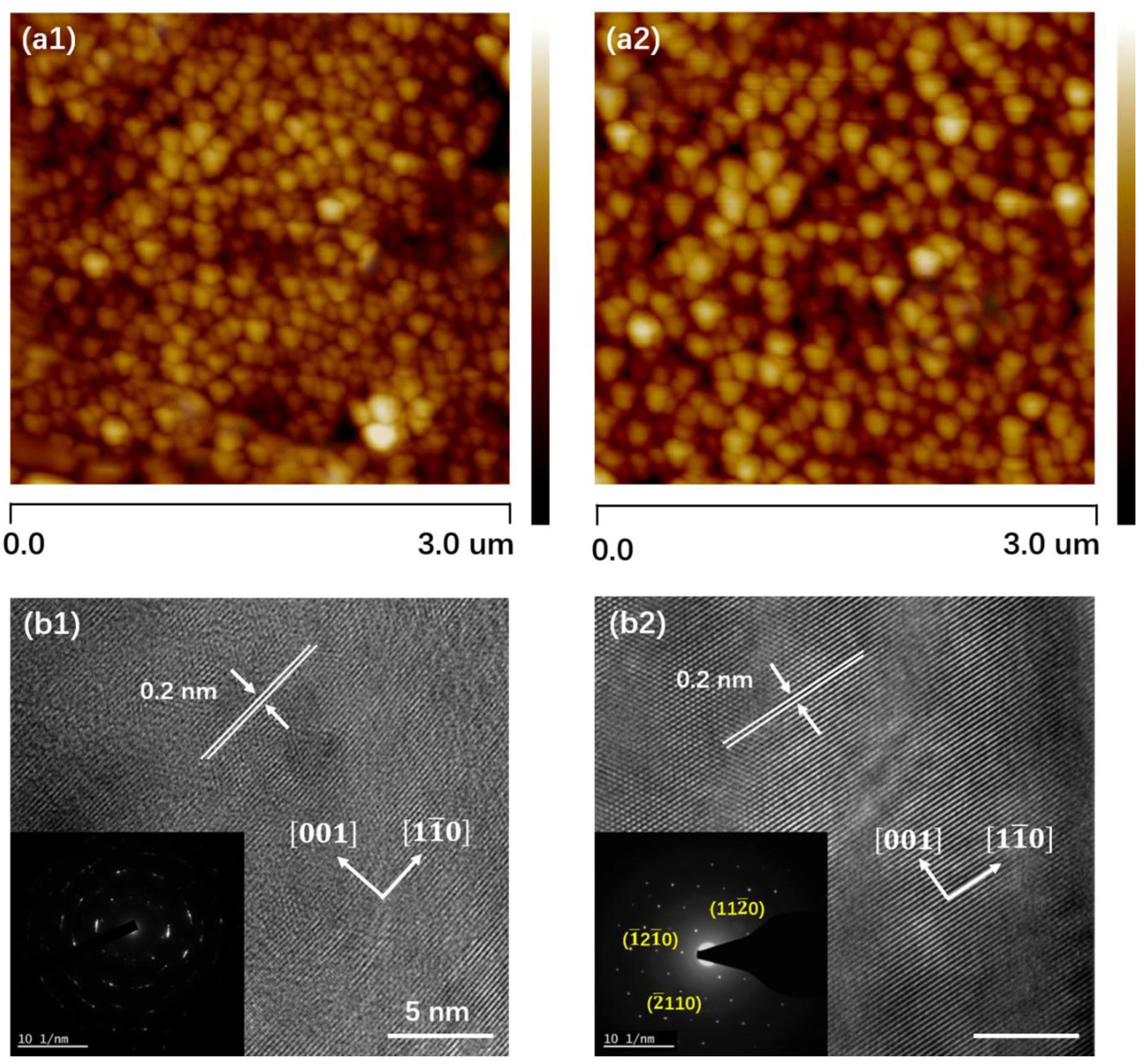
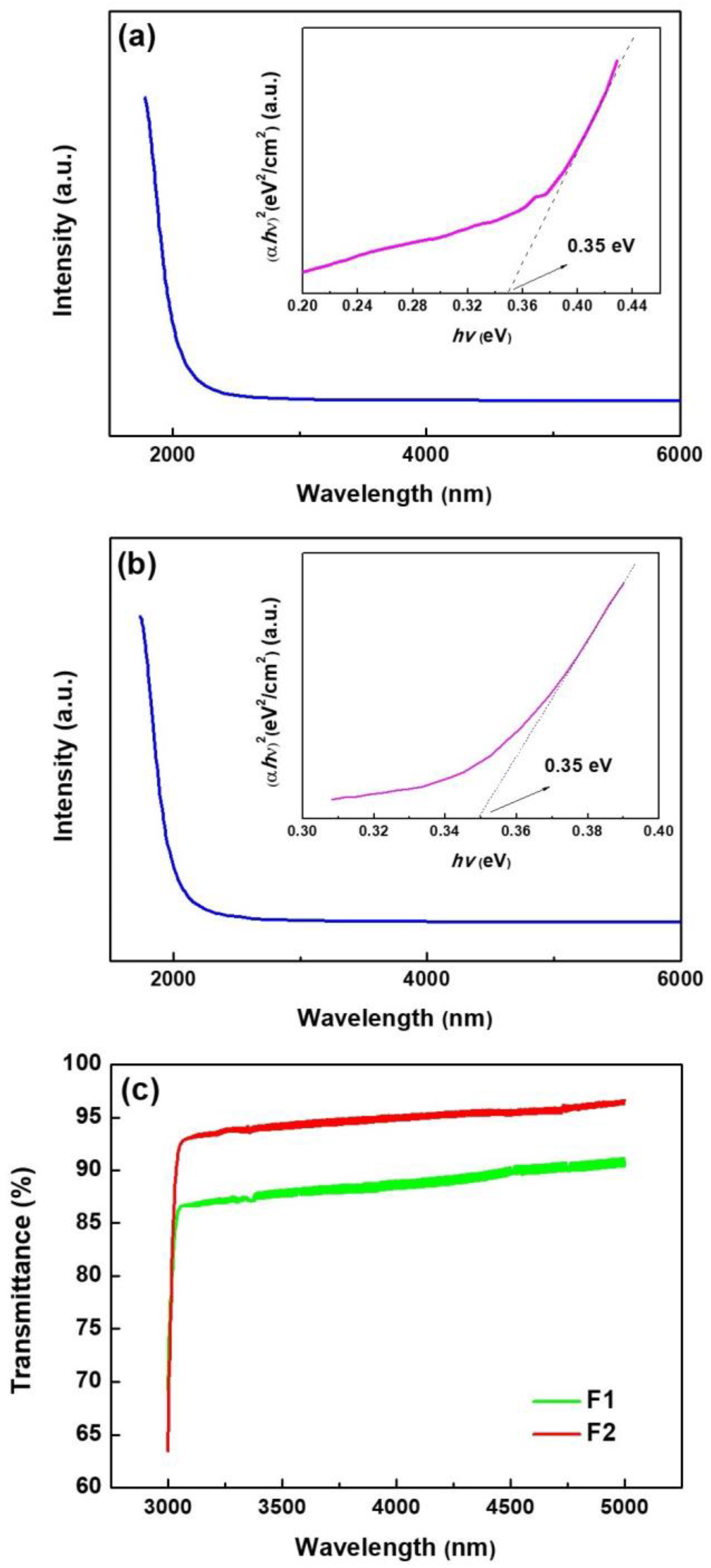
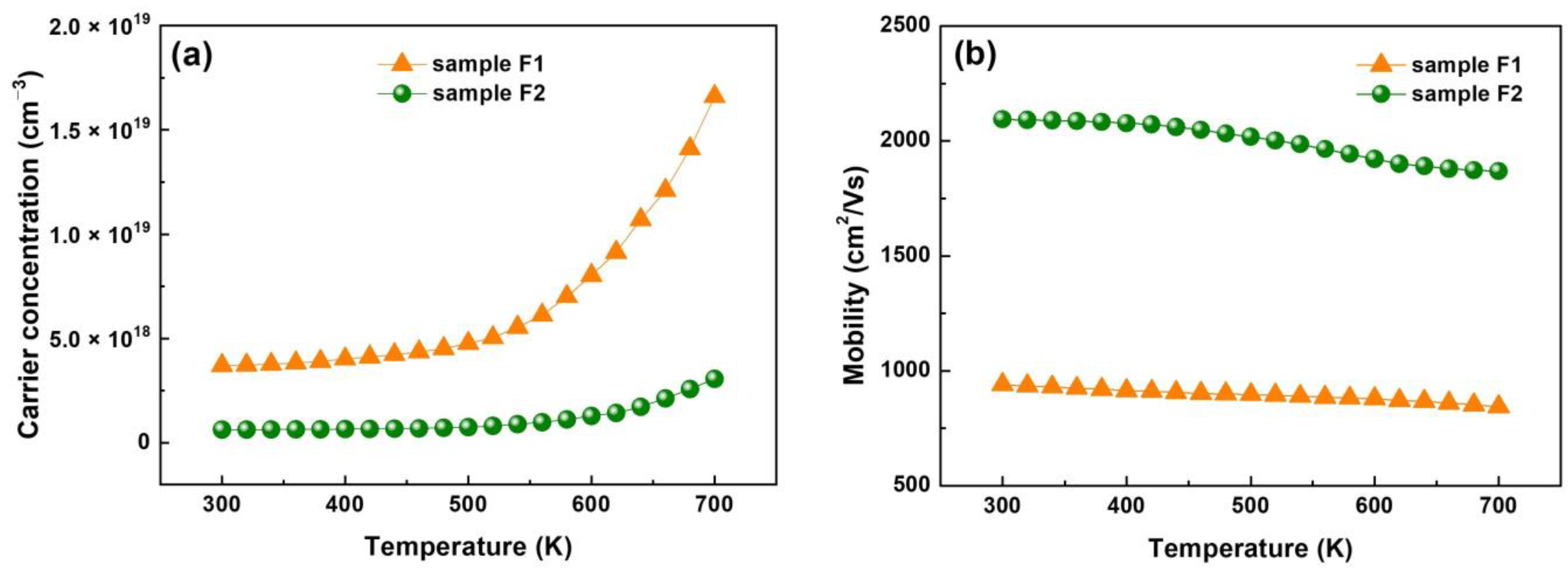
| Sample | a (Å) | b (Å) | c (Å) |
|---|---|---|---|
| F1 | 4.121 (3) | 4.121 (3) | 28.605 (2) |
| F2 | 4.138 (2) | 4.138 (2) | 28.669 (3) |
| Sample | Bi | Se |
|---|---|---|
| F1 | 48.91 | 51.09 |
| F2 | 40.77 | 59.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuai, Y.-H.; Wang, Y.-F.; Bai, Y. Structural, Optical, Electrical, and Thermoelectric Properties of Bi2Se3 Films Deposited at a High Se/Bi Flow Rate. Nanomaterials 2023, 13, 2785. https://doi.org/10.3390/nano13202785
Chuai Y-H, Wang Y-F, Bai Y. Structural, Optical, Electrical, and Thermoelectric Properties of Bi2Se3 Films Deposited at a High Se/Bi Flow Rate. Nanomaterials. 2023; 13(20):2785. https://doi.org/10.3390/nano13202785
Chicago/Turabian StyleChuai, Ya-Hui, Yun-Fan Wang, and Yu Bai. 2023. "Structural, Optical, Electrical, and Thermoelectric Properties of Bi2Se3 Films Deposited at a High Se/Bi Flow Rate" Nanomaterials 13, no. 20: 2785. https://doi.org/10.3390/nano13202785





