High-Rate One-Dimensional α-MnO2 Anode for Lithium-Ion Batteries: Impact of Polymorphic and Crystallographic Features on Lithium Storage
Abstract
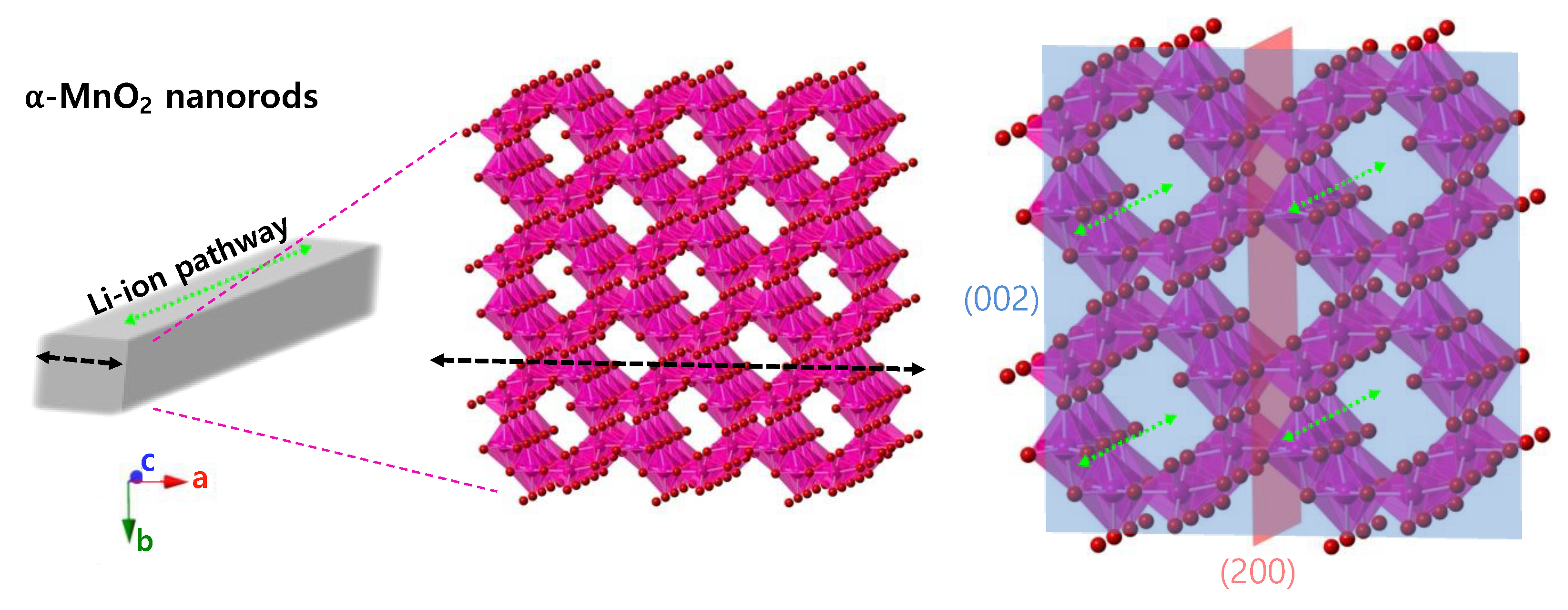
:1. Introduction
2. Materials and Methods
2.1. Preparation of δ-MnO2 and One-Dimensional α-MnO2
2.2. Material Characterization
2.3. Electrochemical Measurements
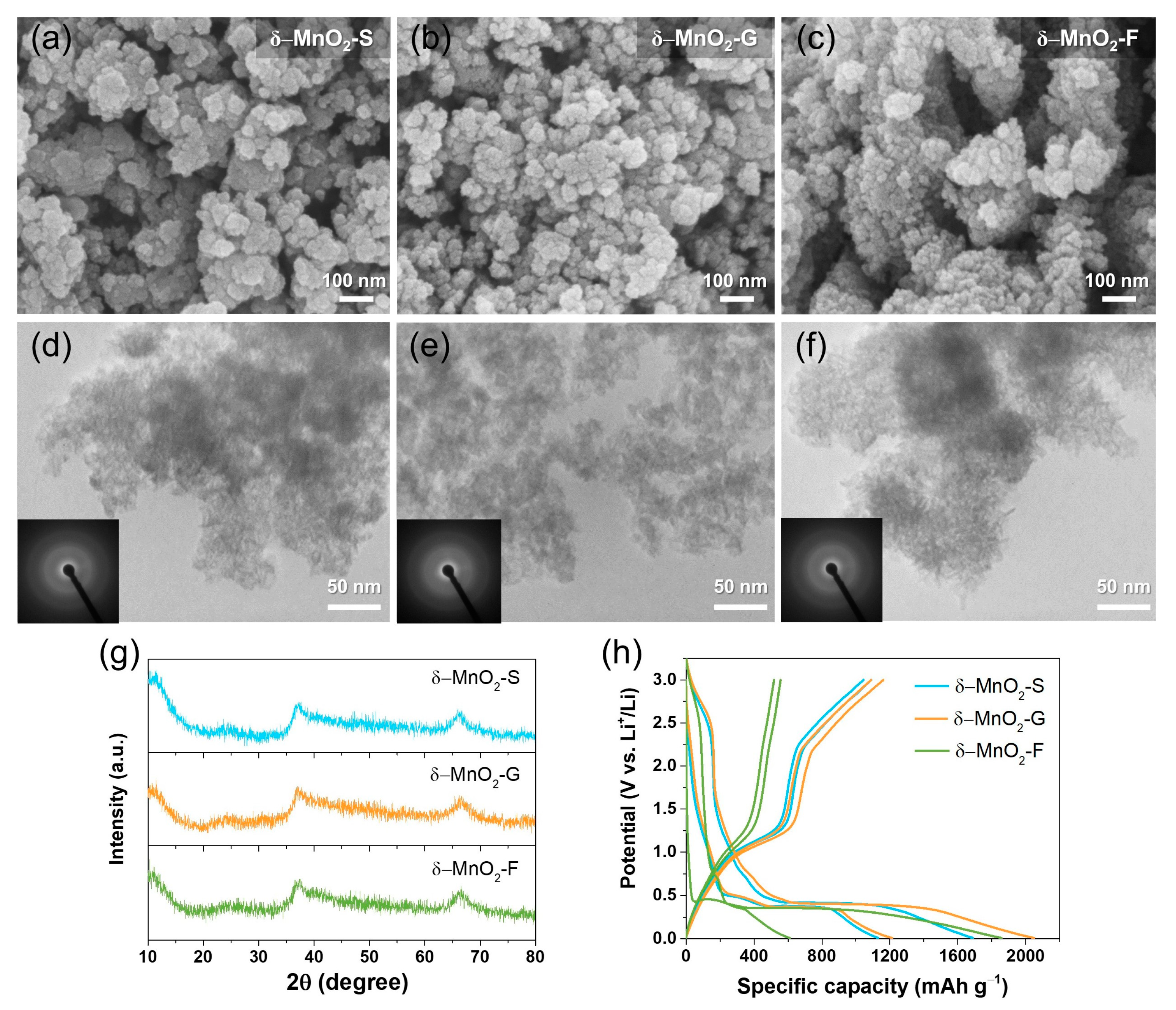
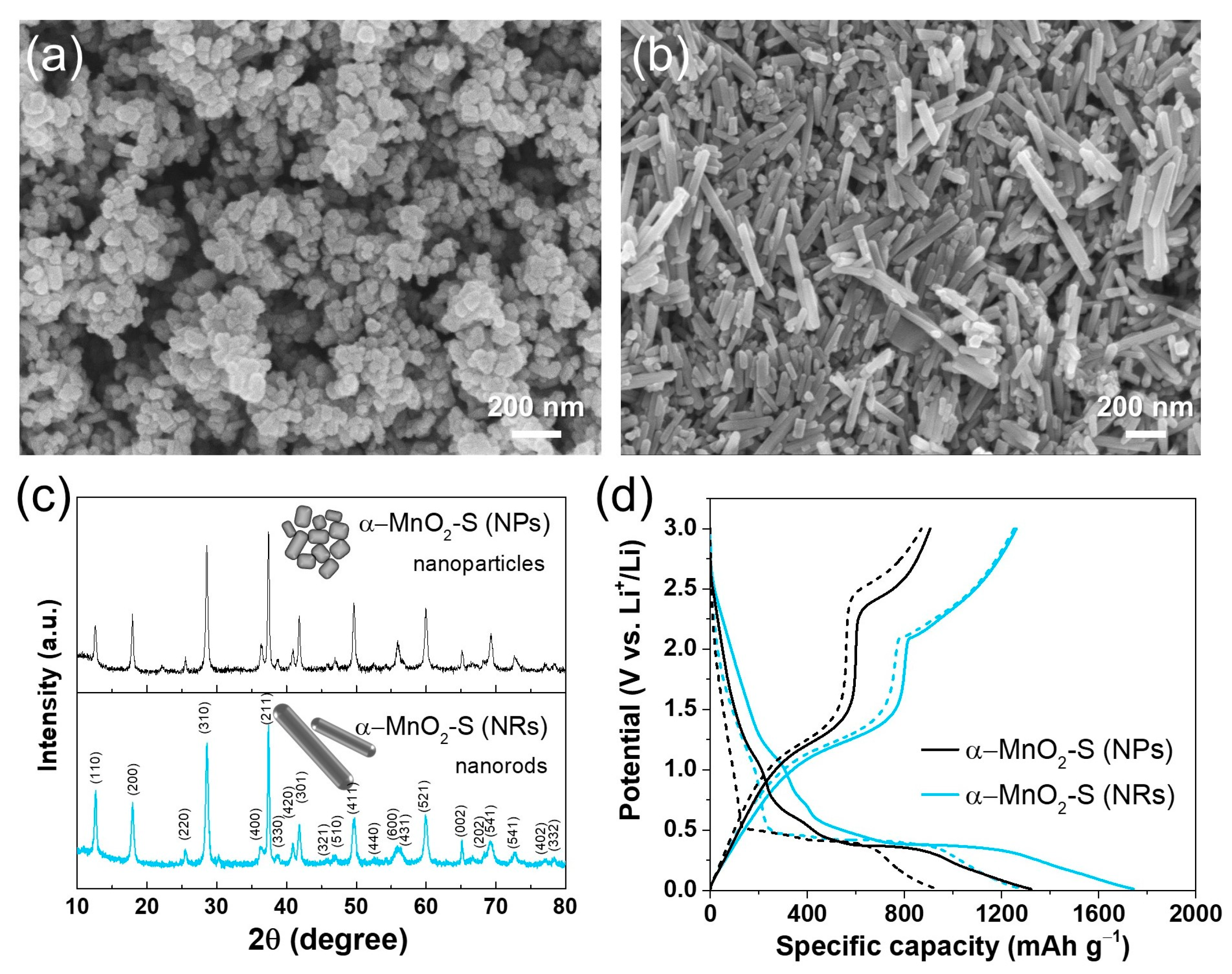
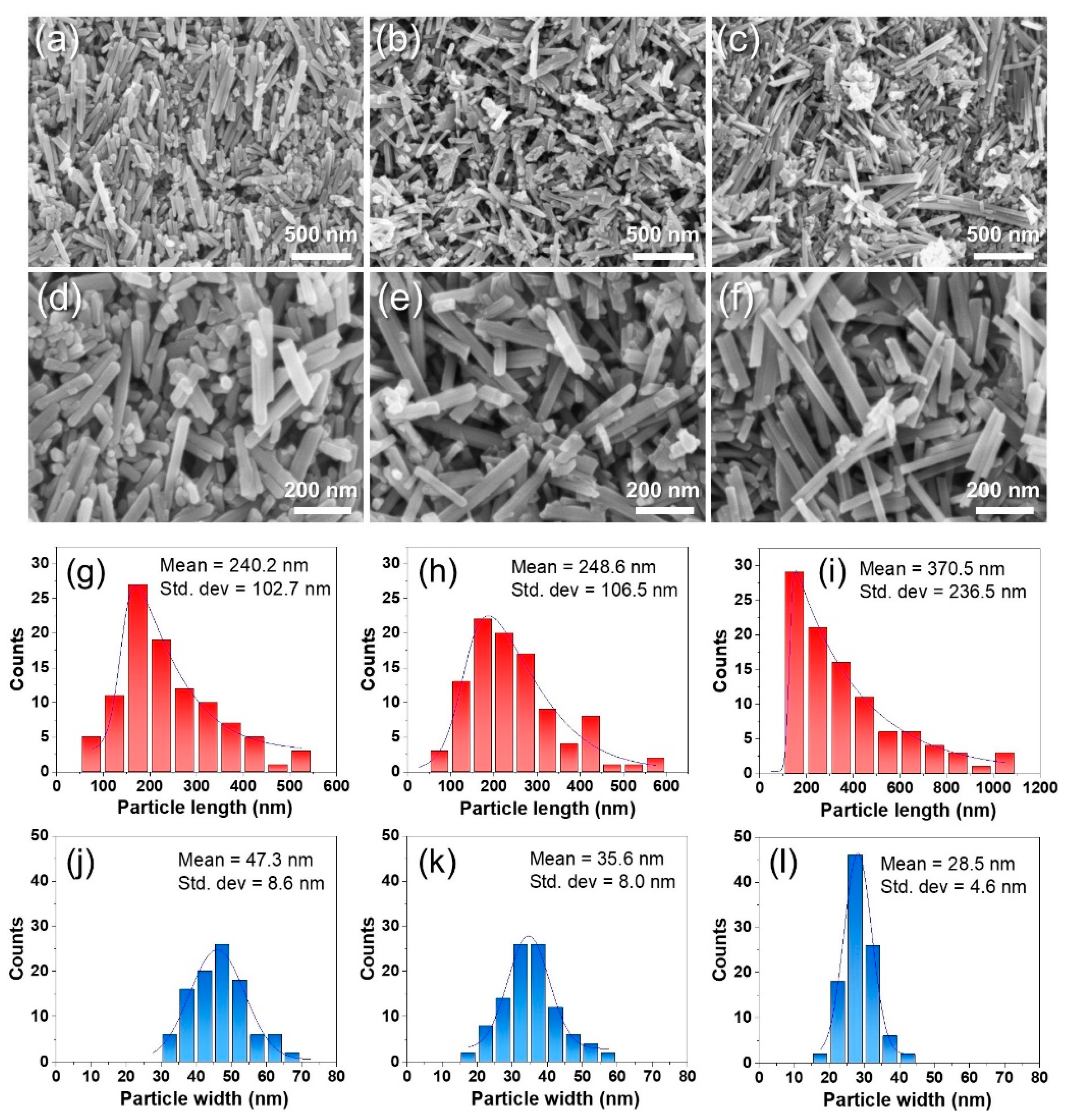
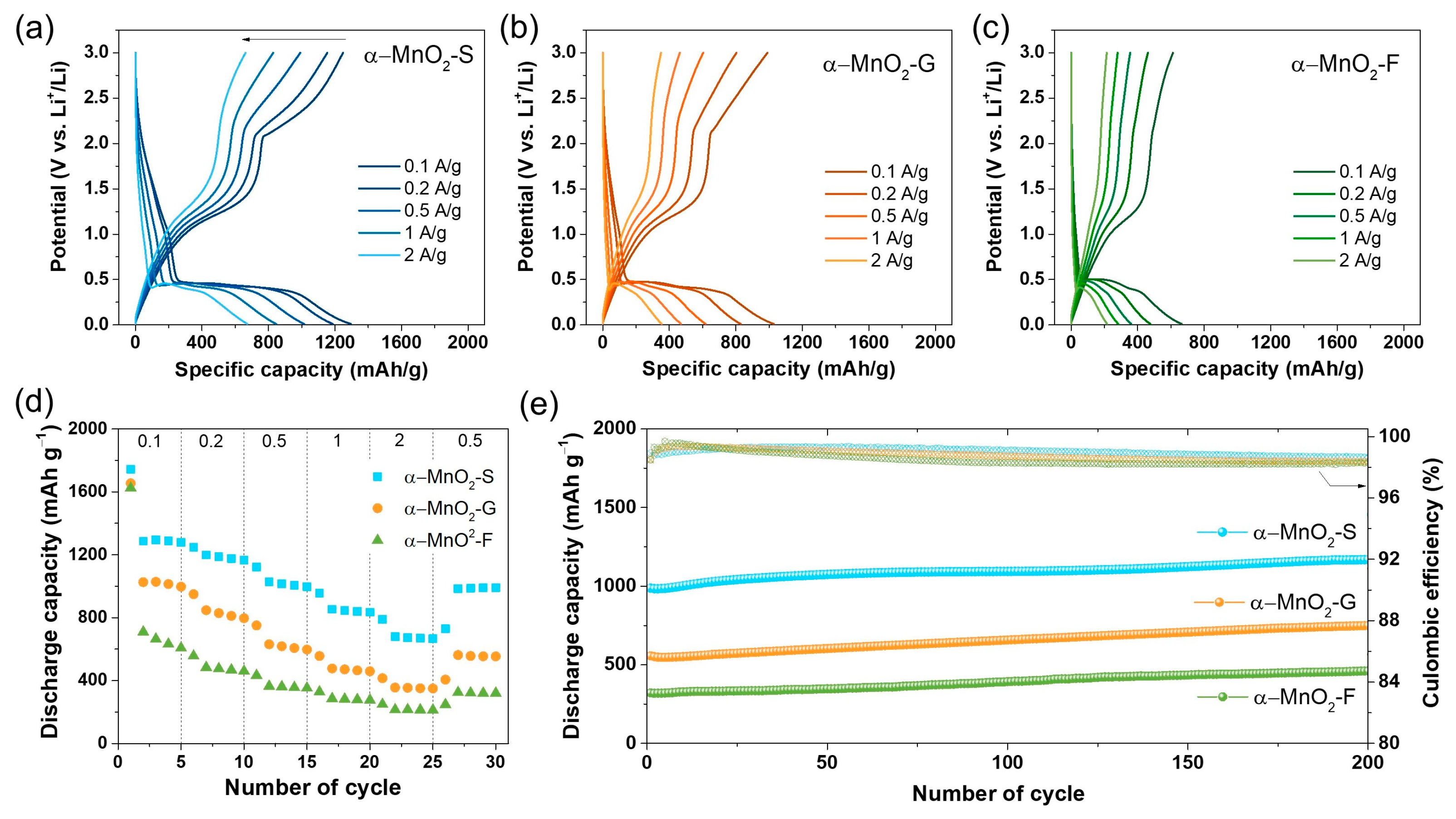
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brock, S.L.; Duan, N.; Tian, Z.R.; Giraldo, O.; Zhou, H.; Suib, S.L. A review of porous manganese oxide materials. Chem. Mater. 1998, 10, 2619–2628. [Google Scholar] [CrossRef]
- Ghosh, S.K. Diversity in the family of manganese oxides at the nanoscale: From fundamentals to applications. ACS Omega 2020, 5, 25493–25504. [Google Scholar] [CrossRef]
- Li, H.; Zhang, W.; Sun, K.; Guo, J.; Yuan, K.; Fu, J.; Zhang, T.; Zhang, X.; Long, H.; Zhang, Z. Manganese-based materials for rechargeable batteries beyond lithium-ion. Adv. Energy Mater. 2021, 11, 2100867. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, Y.; Zhang, X. Challenges and perspectives for manganese-based oxides for advanced aqueous zinc-ion batteries. InfoMat 2020, 2, 237–260. [Google Scholar] [CrossRef]
- Tian, L.; Zhai, X.; Wang, X.; Li, J.; Li, Z. Advances in manganese-based oxides for oxygen evolution reaction. J. Mater. Chem. A 2020, 8, 14400–14414. [Google Scholar] [CrossRef]
- Liu, Z.; He, H.B.; Luo, Z.X.; Wang, X.F.; Zeng, J. 3D printing of customized MnO2 cathode for aqueous zinc-ion batteries. Trans. Nonferrous Met. Soc. 2023, 33, 1193–1204. [Google Scholar] [CrossRef]
- Liu, Y.P.; Xu, C.X.; Ren, W.Q.; Hu, L.Y.; Fu, W.B.; Wang, W.; Yin, H.; He, B.H.; Hou, Z.H.; Chen, L. Self-template synthesis of peapod-like MnO@N-doped hollow carbon nanotubes as an advanced anode for lithium-ion batteries. Rare Met. 2023, 42, 929–939. [Google Scholar] [CrossRef]
- Yao, Q.; Xiao, F.; Lin, C.; Xiong, P.; Lai, W.; Zhang, J.; Xue, H.; Sun, X.; Wei, M.; Qian, Q.; et al. Regeneration of spent lithium manganate into cation-doped and oxygen-deficient MnO2 cathodes toward ultralong lifespan and wide-temperature-tolerant aqueous Zn-ion batteries. Battery Energy 2023, 2, 20220065. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Luo, L.; Fan, Y.; Du, Z. A review on thermal management of lithium-ion batteries for electric vehicles. Energy 2022, 238, 121652. [Google Scholar] [CrossRef]
- Weiss, M.; Ruess, R.; Kasnatscheew, J.; Levartovsky, Y.; Levy, N.R.; Minnmann, P.; Stolz, L.; Waldmann, T.; Wohlfahrt-Mehrens, M.; Aurbach, D. Fast charging of lithium-ion batteries: A review of materials aspects. Adv. Energy Mater. 2021, 11, 2101126. [Google Scholar] [CrossRef]
- Hannan, M.A.; Lipu, M.H.; Hussain, A.; Mohamed, A. A review of lithium-ion battery state of charge estimation and management system in electric vehicle applications: Challenges and recommendations. Renew. Sust. Energy Rev. 2017, 78, 834–854. [Google Scholar] [CrossRef]
- Reddy, M.V.; Subba Rao, G.V.; Chowdari, B.V.R. Metal oxides and oxysalts as anode materials for Li ion batteries. Chem. Rev. 2013, 113, 5364–5457. [Google Scholar] [CrossRef]
- Zhang, J.; Yu, A. Nanostructured transition metal oxides as advanced anodes for lithium-ion batteries. Sci. Bull. 2015, 60, 823–838. [Google Scholar] [CrossRef]
- Tang, Y.; Zheng, S.; Xu, Y.; Xiao, X.; Xue, H.; Pang, H. Advanced batteries based on manganese dioxide and its composites. Energy Storage Mater. 2018, 12, 284–309. [Google Scholar] [CrossRef]
- Li, J.; Hwang, S.; Guo, F.; Li, S.; Chen, Z.; Kou, R.; Sun, K.; Sun, C.-J.; Gan, H.; Yu, A. Phase evolution of conversion-type electrode for lithium ion batteries. Nat. Commun. 2019, 10, 2224. [Google Scholar] [CrossRef]
- Yoon, J.; Choi, W.; Kim, T.; Kim, H.; Choi, Y.S.; Kim, J.M.; Yoon, W.S. Reaction mechanism and additional lithium storage of mesoporous MnO2 anode in Li batteries. J. Energy Chem. 2021, 53, 276–284. [Google Scholar] [CrossRef]
- Guo, X.; Yang, S.; Wang, D.; Chen, A.; Wang, Y.; Li, P.; Liang, G.; Zhi, C. The energy storage mechanisms of MnO2 in batteries. Curr. Opin. Electrochem. 2021, 30, 100769. [Google Scholar] [CrossRef]
- Liu, H.; Hu, Z.; Su, Y.; Ruan, H.; Hu, R.; Zhang, L. MnO2 nanorods/3D-rGO composite as high performance anode materials for Li-ion batteries. Appl. Surf. Sci. 2017, 392, 777–784. [Google Scholar] [CrossRef]
- Débart, A.; Paterson, A.J.; Bao, J.; Bruce, P.G. MnO2 nanowires: A catalyst for the O2 electrode in rechargeable lithium batteries. Angew. Chem. Int. Ed. 2008, 47, 4521–4524. [Google Scholar] [CrossRef]
- Devaraj, S.; Munichandraiah, N. Effect of crystallographic structure of MnO2 on its electrochemical capacitance properties. J. Phys. Chem. C 2008, 112, 4406–4417. [Google Scholar] [CrossRef]
- Kitchaev, D.A.; Peng, H.; Liu, Y.; Sun, J.; Perdew, J.P.; Ceder, G. Energetics of MnO2 polymorphs in density functional theory. Phys. Rev. B 2016, 93, 045132. [Google Scholar] [CrossRef]
- Li, Y.F.; Zhu, S.C.; Liu, Z.P. Reaction network of layer-to-tunnel transition of MnO2. J. Am. Chem. Soc. 2016, 138, 5371–5379. [Google Scholar] [CrossRef]
- Yuan, Y.; Liu, C.; Byles, B.W.; Yao, W.; Song, B.; Cheng, M.; Huang, Z.; Amine, K.; Pomerantseva, E.; Shahbazian-Yassar, R. Ordering heterogeneity of [MnO6] octahedra in tunnel-structured MnO2 and its influence on ion storage. Joule 2019, 3, 471–484. [Google Scholar] [CrossRef]
- Byles, B.W.; Palapati, N.K.R.; Subramanian, A.; Pomerantseva, E. The role of electronic and ionic conductivities in the rate performance of tunnel structured manganese oxides in Li-ion batteries. APL Mater. 2016, 4, 04610. [Google Scholar] [CrossRef]
- Dawadi, S.; Gupta, A.; Khatri, M.; Budhathoki, B.; Lamichhane, G.; Parajuli, N. Manganese dioxide nanoparticles: Synthesis, application and challenges. Bull. Mater. Sci. 2020, 43, 277. [Google Scholar] [CrossRef]
- Walanda, D.K.; Lawrance, G.A.; Donne, S.W. Hydrothermal MnO2: Synthesis, structure, morphology and discharge performance. J. Power Sources 2005, 139, 325–341. [Google Scholar] [CrossRef]
- Chokradjaroen, C.; Wang, X.; Niu, J.; Fan, T.; Saito, N. Fundamentals of solution plasma for advanced materials synthesis. Mater. Today Adv. 2022, 14, 100244. [Google Scholar] [CrossRef]
- Saito, N.; Hieda, J.; Takai, O. Synthesis process of gold nanoparticles in solution plasma. Thin Solid Film. 2009, 518, 912–917. [Google Scholar] [CrossRef]
- Kim, H.; Watthanaphanit, A.; Saito, N. Synthesis of colloidal MnO2 with a sheet-like structure by one-pot plasma discharge in permanganate aqueous solution. RSC Adv. 2016, 6, 2826–2834. [Google Scholar] [CrossRef]
- Zhu, C.; Guo, S.; Fang, Y.; Dong, S. Reducing sugar: New functional molecules for the green synthesis of graphene nanosheets. ACS Nano 2010, 4, 2429–2437. [Google Scholar] [CrossRef]
- Panigrahi, S.; Kundu, S.; Ghosh, S.; Nath, S.; Pal, T. General method of synthesis for metal nanoparticles. J. Nanopart. Res. 2004, 6, 411–414. [Google Scholar] [CrossRef]
- Pratt, C.W.; Cornely, K. Essential Biochemistry, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2014; pp. 290–303. [Google Scholar]
- Makarov, V.; Love, A.; Sinitsyna, O.; Makarova, S.; Yaminsky, I.; Taliansky, M.; Kalinina, N. “Green” nanotechnologies: Synthesis of metal nanoparticles using plants. Acta Nat. 2014, 6, 35–44. [Google Scholar] [CrossRef]
- Durmazel, S.; Uzer, A.; Erbil, B.; Sayın, B.; Apak, R. Silver nanoparticle formation-based colorimetric determination of reducing sugars in food extracts via Tollens’ reagent. ACS Omega 2019, 4, 7596–7604. [Google Scholar] [CrossRef]
- Thanh, N.T.; Maclean, N.; Mahiddine, S. Mechanisms of nucleation and growth of nanoparticles in solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef]
- Prasertsung, I.; Aroonraj, K.; Kamwilaisak, K.; Saito, N.; Damrongsakkul, S. Production of reducing sugar from cassava starch waste (CSW) using solution plasma process (SPP). Carbohydr. Polym. 2019, 205, 472–479. [Google Scholar] [CrossRef]
- Dills, W.L., Jr. Protein fructosylation: Fructose and the Maillard reaction. Am. J. Clin. Nutr. 1993, 58, 779S–787S. [Google Scholar] [CrossRef]
- Byakodi, M.; Shrikrishna, N.S.; Sharma, R.; Bhansali, S.; Mishra, Y.; Kaushik, A.; Gandhi, S. Emerging 0D, 1D, 2D, and 3D nanostructures for efficient point-of-care biosensing. Biosens. Bioelectron. X 2022, 12, 100284. [Google Scholar] [CrossRef]
- Khalifa, H.; El-Safty, S.A.; Reda, A.; Selim, M.M.; Shenashen, M.A. One-dimensional hierarchical anode/cathode materials engineering for high-performance lithium ion batteries. Energy Stor. Mater. 2021, 37, 363–377. [Google Scholar] [CrossRef]
- Abràmoff, M.D.; Magalhães, P.J.; Ram, S. Image processing with ImageJ. J. Biophotonics Int. 2004, 11, 36–42. [Google Scholar]
- Pearce, A.K.; Wilks, T.R.; Arno, M.C.; O’Reilly, R.K. Synthesis and applications of anisotropic nanoparticles with precisely defined dimensions. Nat. Rev. Chem. 2021, 5, 21–45. [Google Scholar] [CrossRef]
- Xue, X.; Penn, R.L.; Leite, E.R.; Huang, F.; Lin, Z. Crystal growth by oriented attachment: Kinetic models and control factors. CrystEngComm 2014, 16, 1419–1429. [Google Scholar] [CrossRef]
- Qi, S.; Feng, J.; Xu, X.; Wang, J.; Hou, X.; Zhang, M. The growth process of rod-shaped La0.7Sr0.3MnO3 in solid state method. J. Alloys Compd. 2009, 478, 317–320. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Sing, Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution. Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Fujiwara, M.; Matsushita, T.; Ikeda, S. Evaluation of Mn3s X-ray photoelectron spectroscopy for characterization of manganese complexes. J. Electron. Spectrosc. Relat. Phenom. 1995, 74, 201–206. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Z.; Li, B.; Zhang, Z.; Wei, J.; Wang, B.; Huang, Y. Controlling the Aspect Ratio of α-MnO2 via the Confined-Space Growth Effect to Enhance the Performance in Aqueous Zinc-Ion Storage. ACS Appl. Energy Mater. 2023, 6, 3329–3336. [Google Scholar] [CrossRef]
- Seher, J.; Froba, M. Shape matters: The effect of particle morphology on the fast-charging performance of LiFePO4/C nanoparticle composite electrodes. ACS Omega 2021, 6, 24062–24069. [Google Scholar] [CrossRef]
- Guo, J.; Liu, Q.; Wang, C.; Zachariah, M.R. Interdispersed amorphous MnOx–carbon nanocomposites with superior electrochemical performance as lithium-storage material. Adv. Funct. Mater. 2012, 22, 803–811. [Google Scholar] [CrossRef]
- Ponrouch, A.; Taberna, P.L.; Simon, P.; Palacin, M.R. On the origin of the extra capacity at low potential in materials for Li batteries reacting through conversion reaction. Electrochim. Acta 2012, 61, 13–18. [Google Scholar] [CrossRef]
- Rana, M.; Avvaru, V.S.; Boaretto, N.; Marcilla, R.; Etacheri, V.; Vilatela, J.J. High rate hybrid MnO2@CNT fabric anodes for Li-ion batteries: Properties and a lithium storage mechanism study by in situ synchrotron X-ray scattering. J. Mater. Chem. A 2019, 7, 26596–26606. [Google Scholar] [CrossRef]
- Shon, J.K.; Lee, H.S.; Park, G.O.; Yoon, J.; Park, E.; Park, G.S.; Kong, S.S.; Jin, M.; Choi, J.-M.; Chang, H. Discovery of abnormal lithium-storage sites in molybdenum dioxide electrodes. Nat. Commun. 2016, 7, 11049. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, X.; Shen, X.; Shi, Y.; Zhu, C.; Zeng, S.; Xu, H.; Cao, P.; Wang, Y.; Di, J. 3D confined zinc plating/stripping with high discharge depth and excellent high-rate reversibility. J. Mater. Chem. A 2020, 8, 11719–11727. [Google Scholar] [CrossRef]
- Hui, X.; Zhao, R.; Zhang, P.; Li, C.; Wang, C.; Yin, L. Low-temperature reduction strategy synthesized Si/Ti3C2 MXene composite anodes for high-performance Li-ion batteries. Adv. Energy Mater. 2019, 9, 1901065. [Google Scholar] [CrossRef]
- Tompsett, D.A.; Islam, M.S. Electrochemistry of hollandite α-MnO2: Li-ion and Na-ion insertion and Li2O incorporation. Chem. Mater. 2013, 25, 2515–2526. [Google Scholar] [CrossRef]
- Kim, H.; Kim, D.J.; Seo, D.H.; Yeom, M.S.; Kang, K.; Kim, D.K.; Jung, Y. Ab initio study of the sodium intercalation and intermediate phases in Na0.44MnO2 for sodium-ion battery. Chem. Mater. 2012, 24, 1205–1211. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-m.; Cha, B.-c.; Kim, D.-w. High-Rate One-Dimensional α-MnO2 Anode for Lithium-Ion Batteries: Impact of Polymorphic and Crystallographic Features on Lithium Storage. Nanomaterials 2023, 13, 2808. https://doi.org/10.3390/nano13202808
Kim H-m, Cha B-c, Kim D-w. High-Rate One-Dimensional α-MnO2 Anode for Lithium-Ion Batteries: Impact of Polymorphic and Crystallographic Features on Lithium Storage. Nanomaterials. 2023; 13(20):2808. https://doi.org/10.3390/nano13202808
Chicago/Turabian StyleKim, Hye-min, Byung-chul Cha, and Dae-wook Kim. 2023. "High-Rate One-Dimensional α-MnO2 Anode for Lithium-Ion Batteries: Impact of Polymorphic and Crystallographic Features on Lithium Storage" Nanomaterials 13, no. 20: 2808. https://doi.org/10.3390/nano13202808
APA StyleKim, H.-m., Cha, B.-c., & Kim, D.-w. (2023). High-Rate One-Dimensional α-MnO2 Anode for Lithium-Ion Batteries: Impact of Polymorphic and Crystallographic Features on Lithium Storage. Nanomaterials, 13(20), 2808. https://doi.org/10.3390/nano13202808





