Harnessing Nuclear Energy to Gold Nanoparticles for the Concurrent Chemoradiotherapy of Glioblastoma
Abstract
1. Introduction
2. Materials and Methods
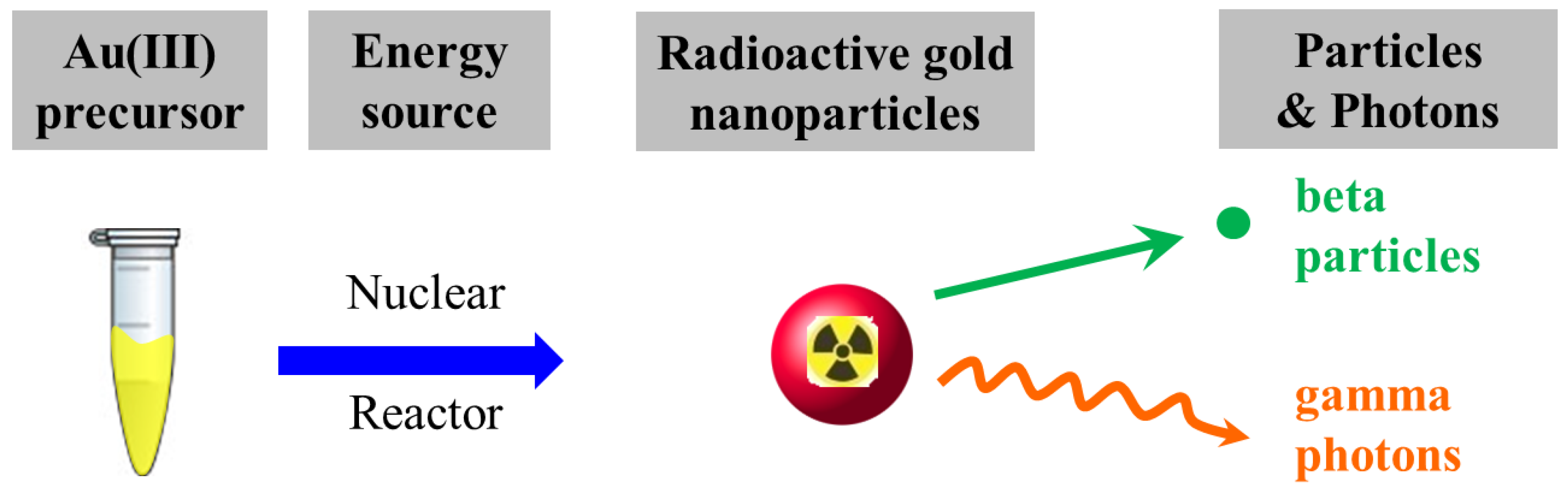
2.1. One-Pot/One-Step Synthesis of Radioactive Gold Nanoparticles
2.2. Physicochemical Characterization
2.3. Xenograft for Therapeutic Efficacy
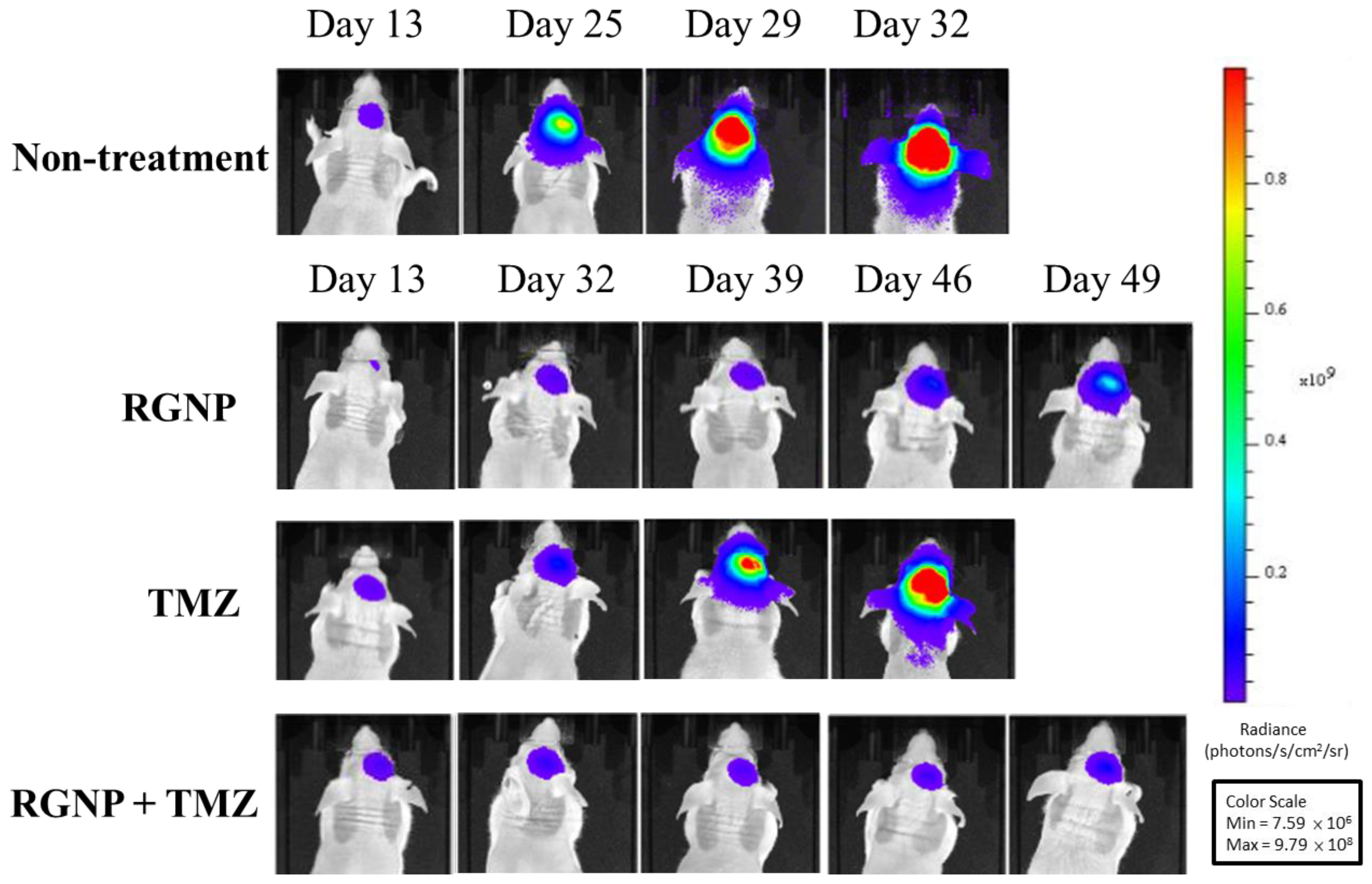
2.4. Bioluminescence Imaging
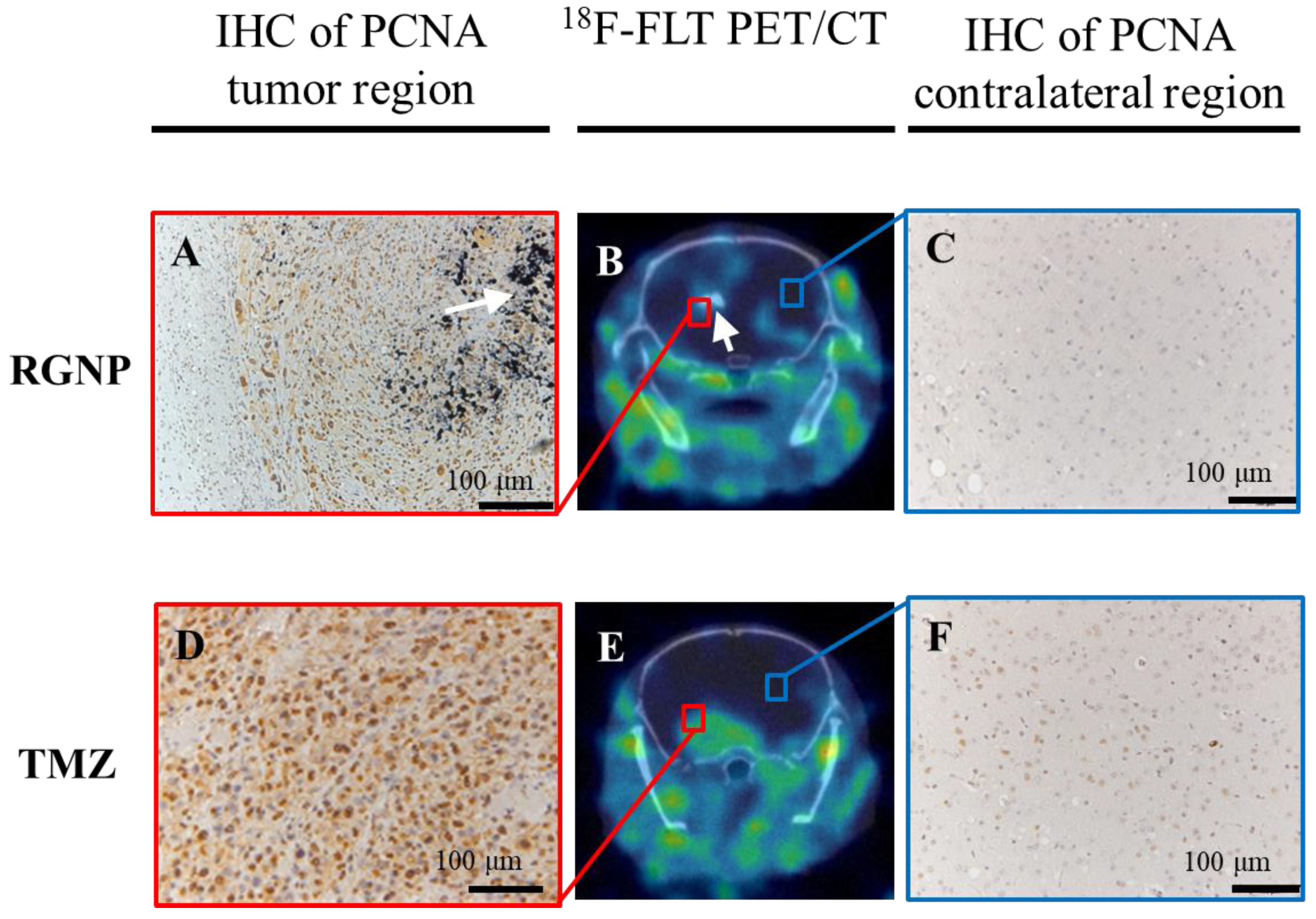
2.5. 18F-FLT PET/CT Imaging
2.6. Histopathological Inspections
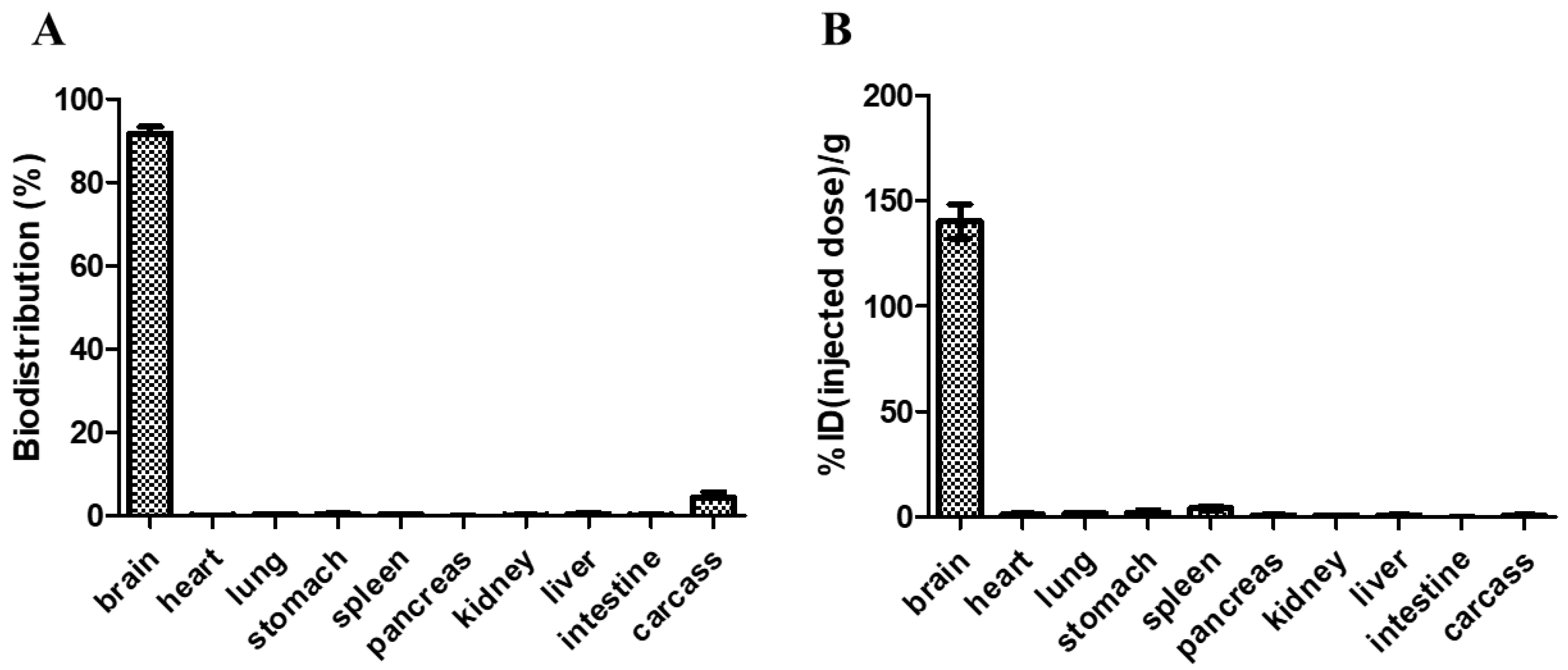
2.7. Biodistribution of Radioactive Gold Nanoparticles
3. Results and Discussion
3.1. One-Pot/One-Step Reactions in the Nuclear Reactor
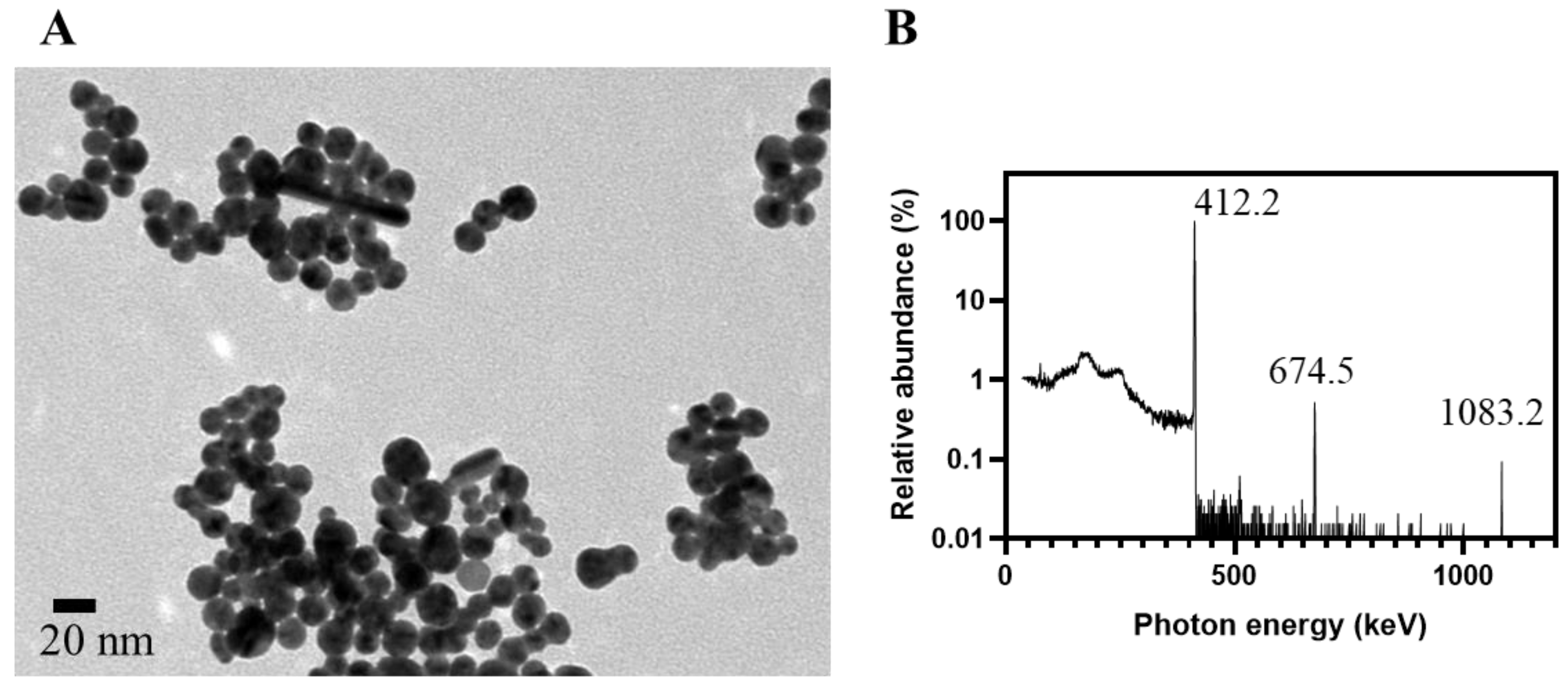
3.2. Determination of Particle Sizes
3.3. Harnessing Nuclear Energy to Generate Radioactive Gold Nanoparticles
3.4. Proposed Mechanism
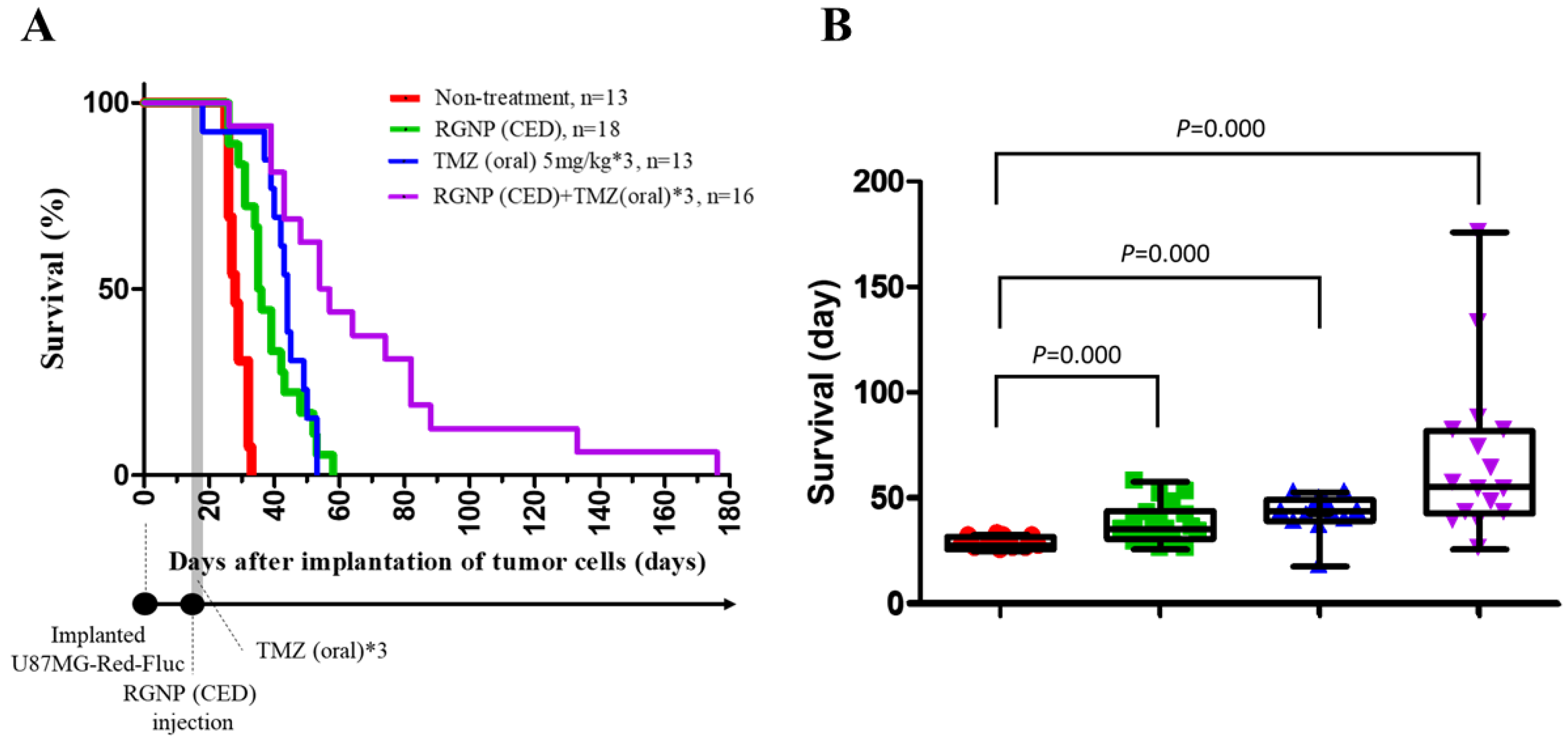
3.5. Therapeutic Efficacy
3.6. Biodistribution of RGNP after Convection-Enhanced Delivery
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Thompson, D. Michael Faraday’s recognition of ruby gold: The birth of modern nanotechnology. Gold Bull. 2007, 40, 267–269. [Google Scholar] [CrossRef]
- Boisselier, E.; Astruc, D. Gold nanoparticles in nanomedicine: Preparations, imaging, diagnostics, therapies and toxicity. Chem. Soc. Rev. 2009, 38, 1759–1782. [Google Scholar] [CrossRef] [PubMed]
- Faraday, M. The Bakerian Lecture.—Experimental relations of gold (and other metals) to light. Philos. Trans. R. Soc. Lond. 1857, 147, 145–181. [Google Scholar] [CrossRef]
- Faulk, W.P.; Taylor, G.M. An immunocolloid method for the electron microscope. Immunochemistry 1971, 8, 1081–1083. [Google Scholar] [CrossRef]
- Batten, T.F.; Hopkins, C.R. Use of protein A-coated colloidal gold particles for immunoelectronmicroscopic localization of ACTH on ultrathin sections. Histochemistry 1979, 60, 317–320. [Google Scholar] [CrossRef]
- Leuvering, J.H.; Thal, P.J.; Schuurs, A.H. Optimization of a sandwich sol particle immunoassay for human chorionic gonadotrophin. J. Immunol. Methods 1983, 62, 175–184. [Google Scholar] [CrossRef]
- Butler, S.A.; Khanlian, S.A.; Cole, L.A. Detection of early pregnancy forms of human chorionic gonadotropin by home pregnancy test devices. Clin. Chem. 2001, 47, 2131–2136. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Li, Y.-J.; Huang, C.-C. Information derived from cluster ions from DNA-modified gold nanoparticles under laser desorption/ionization: Analysis of coverage, structure, and single-nucleotide polymorphism. Anal. Chem. 2012, 85, 1021–1028. [Google Scholar] [CrossRef]
- Shehata, N.; Kandas, I.; Samir, E. In-situ gold-ceria nanoparticles: Superior optical fluorescence quenching sensor for dissolved oxygen. Nanomaterials 2020, 10, 314. [Google Scholar] [CrossRef]
- Zhou, N.; Li, J.; Wang, S.; Zhuang, X.; Ni, S.; Luan, F.; Wu, X.; Yu, S. An electrochemical sensor based on gold and bismuth bimetallic nanoparticles decorated L-cysteine functionalized graphene oxide nanocomposites for sensitive detection of iron ions in water samples. Nanomaterials 2021, 11, 2386. [Google Scholar] [CrossRef]
- Dörschug, A.; Schwanbeck, J.; Hahn, A.; Hillebrecht, A.; Blaschke, S.; Groß, U.; Heimesaat, M.M.; Frickmann, H.; Zautner, A.E. Evaluation of the Xiamen AmonMed Biotechnology rapid diagnostic test COVID-19 IgM/IgG test kit (Colloidal gold). Eur. J. Microbiol. Immunol. 2020, 10, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Terpos, E.; Ntanasis-Stathopoulos, I.; Skvarč, M. Clinical application of a new SARS-CoV-2 antigen detection kit (colloidal gold) in the detection of COVID-19. Diagnostics 2021, 11, 995. [Google Scholar] [CrossRef]
- Huo, S.; Ma, H.; Huang, K.; Liu, J.; Wei, T.; Jin, S.; Zhang, J.; He, S.; Liang, X.-J. Superior penetration and retention behavior of 50 nm gold nanoparticles in tumors. Cancer Res. 2013, 73, 319–330. [Google Scholar] [CrossRef]
- Lee, K.Y.J.; Lee, G.Y.; Lane, L.A.; Li, B.; Wang, J.; Lu, Q.; Wang, Y.; Nie, S. Functionalized, long-circulating, and ultrasmall gold nanocarriers for overcoming the barriers of low nanoparticle delivery efficiency and poor tumor penetration. Bioconjugate Chem. 2016, 28, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xu, M.; Sun, X.; Feliu, N.; Feng, L.; Parak, W.J.; Liu, S. Quantitative comparison of gold nanoparticle delivery via the enhanced permeation and retention (EPR) effect and mesenchymal stem cell (MSC)-based targeting. ACS Nano 2023, 17, 2039–2052. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.D.; Khanal, B.P.; Zubarev, E.R. Paclitaxel-functionalized gold nanoparticles. J. Am. Chem. Soc. 2007, 129, 11653–11661. [Google Scholar] [CrossRef] [PubMed]
- Hwu, J.R.; Lin, Y.S.; Josephrajan, T.; Hsu, M.-H.; Cheng, F.-Y.; Yeh, C.-S.; Su, W.-C.; Shieh, D.-B. Targeted Paclitaxel by conjugation to iron oxide and gold nanoparticles. J. Am. Chem. Soc. 2008, 131, 66–68. [Google Scholar] [CrossRef]
- Brown, S.D.; Nativo, P.; Smith, J.-A.; Stirling, D.; Edwards, P.R.; Venugopal, B.; Flint, D.J.; Plumb, J.A.; Graham, D.; Wheate, N.J. Gold nanoparticles for the improved anticancer drug delivery of the active component of oxaliplatin. J. Am. Chem. Soc. 2010, 132, 4678–4684. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.-C.; Dou, S.; Xiong, M.-H.; Sun, T.-M.; Wang, J. Doxorubicin-tethered responsive gold nanoparticles facilitate intracellular drug delivery for overcoming multidrug resistance in cancer cells. ACS Nano 2011, 5, 3679–3692. [Google Scholar] [CrossRef]
- Suarasan, S.; Focsan, M.; Potara, M.; Soritau, O.; Florea, A.; Maniu, D.; Astilean, S. Doxorubicin-incorporated nanotherapeutic delivery system based on gelatin-coated gold nanoparticles: Formulation, drug release, and multimodal imaging of cellular internalization. ACS Appl. Mater. Interfaces 2016, 8, 22900–22913. [Google Scholar] [CrossRef]
- Cui, T.; Liang, J.-J.; Chen, H.; Geng, D.-D.; Jiao, L.; Yang, J.-Y.; Qian, H.; Zhang, C.; Ding, Y. Performance of doxorubicin-conjugated gold nanoparticles: Regulation of drug location. ACS Appl. Mater. Interfaces 2017, 9, 8569–8580. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, D.; Joshi, N.; Tao, W.; Karp, J.M.; Peer, D. Progress and challenges towards targeted delivery of cancer therapeutics. Nat. Commun. 2018, 9, 1410. [Google Scholar] [CrossRef] [PubMed]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W.C.W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- van Vlerken, L.E.; Duan, Z.; Little, S.R.; Seiden, M.V.; Amiji, M.M. Biodistribution and pharmacokinetic analysis of Paclitaxel and ceramide administered in multifunctional polymer-blend nanoparticles in drug resistant breast cancer model. Mol. Pharm. 2008, 5, 516–526. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cassano, D.; Pocoví-Martínez, S.; Voliani, V. Ultrasmall-in-nano approach: Enabling the translation of metal nanomaterials to clinics. Bioconjugate Chem. 2018, 29, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Cassano, D.; Summa, M.; Pocoví-Martínez, S.; Mapanao, A.-K.; Catelani, T.; Bertorelli, R.; Voliani, V. Biodegradable ultrasmall-in-nano gold architectures: Mid-period in vivo distribution and excretion assessment. Part. Part. Syst. Charact. 2018, 36, 1800464. [Google Scholar] [CrossRef]
- Mapanao, A.K.; Giannone, G.; Summa, M.; Ermini, M.L.; Zamborlin, A.; Santi, M.; Cassano, D.; Bertorelli, R.; Voliani, V. Biokinetics and clearance of inhaled gold ultrasmall-in-nano architectures. Nanoscale Adv. 2020, 2, 3815–3820. [Google Scholar] [CrossRef]
- Zhang, R.; Kiessling, F.; Lammers, T.; Pallares, R.M. Clinical translation of gold nanoparticles. Drug Deliv. Transl. Res. 2022, 13, 378–385. [Google Scholar] [CrossRef]
- Paciotti, G.F.; Myer, L.; Weinreich, D.; Goia, D.; Pavel, N.; McLaughlin, R.E.; Tamarkin, L. Colloidal gold: A novel nanoparticle vector for tumor directed drug delivery. Drug Deliv. 2004, 11, 169–183. [Google Scholar] [CrossRef]
- Goel, R.; Shah, N.B.; Visaria, R.; Paciotti, G.F.; Bischof, J.C.; Ibrahim, K.E.; Bakhiet, A.O.; Awadalla, M.E.; Khan, H.A.; Mazur, J.; et al. Biodistribution of TNF-alpha-coated gold nanoparticles in an in vivo model system. Nanomedicine 2009, 4, 401–410. [Google Scholar] [CrossRef]
- Libutti, S.K.; Paciotti, G.F.; Byrnes, A.A.; Alexander, H.R.; Gannon, W.E.; Walker, M.; Seidel, G.D.; Yuldasheva, N.; Tamarkin, L. Phase I and pharmacokinetic studies of CYT-6091, a novel PEGylated colloidal gold-rhTNF nanomedicine. Clin. Cancer Res. 2010, 16, 6139–6149. [Google Scholar] [CrossRef] [PubMed]
- Walsh, A.A. Chemisorption of iodine-125 to gold nanoparticles allows for real-time quantitation and potential use in nanomedicine. J. Nanoparticle Res. 2017, 19, 152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yang, Z.; Lu, W.; Zhang, R.; Huang, Q.; Tian, M.; Li, L.; Liang, D.; Li, C. Influence of anchoring ligands and particle size on the colloidal stability and in vivo biodistribution of polyethylene glycol-coated gold nanoparticles in tumor-xenografted mice. Biomaterials 2009, 30, 1928–1936. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.B.; Lee, J.-E.; Cho, S.J.; Chin, J.; Kim, S.K.; Lee, I.-K.; Lee, J.; Jeon, Y.H. Crushed gold shell nanoparticles labeled with radioactive iodine as a theranostic nanoplatform for macrophage-mediated photothermal therapy. Nano-Micro Lett. 2019, 11, 36. [Google Scholar] [CrossRef] [PubMed]
- Chanda, N.; Kan, P.; Watkinson, L.D.; Shukla, R.; Zambre, A.; Carmack, T.L.; Engelbrecht, H.; Lever, J.R.; Katti, K.; Fent, G.M.; et al. Radioactive gold nanoparticles in cancer therapy: Therapeutic efficacy studies of GA-198AuNP nanoconstruct in prostate tumor-bearing mice. Nanomedicine 2010, 6, 201–209. [Google Scholar] [CrossRef]
- Kannan, R.; Zambre, A.; Chanda, N.; Kulkarni, R.; Shukla, R.; Katti, K.; Upendran, A.; Cutler, C.; Boote, E.; Katti, K.V. Functionalized radioactive gold nanoparticles in tumor therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2011, 4, 42–51. [Google Scholar] [CrossRef]
- Axiak-Bechtel, S.M.; Upendran, A.; Lattimer, J.C.; Kelsey, J.; Cutler, C.S.; Selting, K.A.; Bryan, J.N.; Henry, C.J.; Boote, E.; Tate, D.J.; et al. Gum arabic-coated radioactive gold nanoparticles cause no short-term local or systemic toxicity in the clinically relevant canine model of prostate cancer. Int. J. Nanomed. 2014, 9, 5001–5011. [Google Scholar] [CrossRef]
- Zhou, C.; Hao, G.; Thomas, P.; Liu, J.; Yu, M.; Sun, S.; Öz, O.K.; Sun, X.; Zheng, J. Near-infrared emitting radioactive gold nanoparticles with molecular pharmacokinetics. Angew. Chem. Int. Ed. 2012, 51, 10118–10122. [Google Scholar] [CrossRef]
- Chen, C.-H.; Lin, F.-S.; Liao, W.-N.; Liang, S.L.; Chen, M.-H.; Chen, Y.-W.; Lin, W.-Y.; Hsu, M.-H.; Wang, M.-Y.; Peir, J.-J.; et al. Establishment of a trimodality analytical platform for tracing, imaging and quantification of gold nanoparticles in animals by radiotracer techniques. Anal. Chem. 2014, 87, 601–608. [Google Scholar] [CrossRef]
- Wang, C.-H.; Chien, C.-C.; Yu, Y.-L.; Liu, C.-J.; Lee, C.-F.; Chen, C.-H.; Hwu, Y.; Yang, C.-S.; Je, J.-H.; Margaritondo, G. Structural properties of naked gold nanoparticles formed by synchrotron X-ray irradiation. J. Synchrotron Radiat. 2007, 14, 477–482. [Google Scholar] [CrossRef]
- Huang, X.L. Nuclear data sheets for A=198. Nucl. Data Sheets 2009, 110, 2533–2688. [Google Scholar] [CrossRef]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Patabendige, A.A.; Dolman, D.E.; Yusof, S.R.; Begley, D.J. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010, 37, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Huo, T.; Barth, R.F.; Gupta, N.; Weldon, M.; Grecula, J.C.; Ross, B.D.; Hoff, B.A.; Chou, T.-C.; Rousseau, J.; et al. Convection enhanced delivery of carboplatin in combination with radiotherapy for the treatment of brain tumors. J. Neuro-Oncol. 2010, 101, 379–390. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Elleaume, H.; Barth, R.F.; Rousseau, J.; Bobyk, L.; Balosso, J.; Yang, W.; Huo, T.; Nakkula, R. Radiation therapy combined with intracerebral convection-enhanced delivery of cisplatin or carboplatin for treatment of the F98 rat glioma. J. Neuro-Oncol. 2020, 149, 193–208. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Ozawa, T.; Drummond, D.C.; Kalra, A.; Fitzgerald, J.B.; Kirpotin, D.B.; Wei, K.-C.; Butowski, N.; Prados, M.D.; Berger, M.S.; et al. Comparing routes of delivery for nanoliposomal irinotecan shows superior anti-tumor activity of local administration in treating intracranial glioblastoma xenografts. Neuro-Oncol. 2013, 15, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Pang, H.-H.; Chen, P.-Y.; Wei, K.-C.; Huang, C.-W.; Shiue, Y.-L.; Huang, C.-Y.; Yang, H.-W. Convection-enhanced delivery of a virus-like nanotherapeutic agent with dual-modal imaging for besiegement and eradication of brain tumors. Theranostics 2019, 9, 1752–1763. [Google Scholar] [CrossRef]
- Finbloom, J.A.; Aanei, I.L.; Bernard, J.M.; Klass, S.H.; Elledge, S.K.; Han, K.; Ozawa, T.; Nicolaides, T.P.; Berger, M.S.; Francis, M.B. Evaluation of three morphologically distinct virus-like particles as nanocarriers for convection-enhanced drug delivery to glioblastoma. Nanomaterials 2018, 8, 1007. [Google Scholar] [CrossRef]
- Glisic, B.D.; Rajkovic, S.; Stanic, Z.D.; Djuran, M.I. A spectroscopic and electrochemical investigation of the oxidation pathway of glycyl-D,L-methionine and its N-acetyl derivative induced by gold(III). Gold Bull. 2011, 44, 91–98. [Google Scholar] [CrossRef][Green Version]
- Link, S.; El-Sayed, M.A. Size and temperature dependence of the plasmon absorption of colloidal gold nanoparticles. J. Phys. Chem. B 1999, 103, 4212–4217. [Google Scholar] [CrossRef]
- Link, S.; El-Sayed, M.A. Spectral properties and relaxation dynamics of surface plasmon electronic oscillations in gold and silver nanodots and nanorods. J. Phys. Chem. B 1999, 103, 8410–8426. [Google Scholar] [CrossRef]
- Haiss, W.; Thanh, N.T.; Aveyard, J.; Fernig, D.G. Determination of size and concentration of gold nanoparticles from UV-Vis spectra. Anal. Chem. 2007, 79, 4215–4221. [Google Scholar] [CrossRef] [PubMed]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- Frens, G. Controlled nucleation for regulation of particle-size in monodisperse gold suspensions. Nat. Phys. Sci. 1973, 241, 20–22. [Google Scholar] [CrossRef]
- Aslan, K.; Perez-Luna, V.H. Surface modification of colloidal gold by chemisorption of alkanethiols in the presence of a nonionic surfactant. Langmuir 2002, 18, 6059–6065. [Google Scholar] [CrossRef]
- Erdtmann, G. Neutron Activation Tables; Verlag Chemie: Weinheim, Germany, 1976. [Google Scholar]
- Katti, K.V.; Kannan, R.; Kattumori, V.; Pandrapraganda, R.; Rahing, V.; Cutler, C.; Boote, E.J.; Casteel, S.W.; Smith, C.J.; Robertson, J.D.; et al. Hybrid gold nanoparticles in molecular imaging and radiotherapy. Czechoslov. J. Phys. 2006, 56, D23–D34. [Google Scholar] [CrossRef]
- Haygarth, K.; Bartels, D.M. Neutron and beta/γ radiolysis of water up to supercritical conditions. 2. SF(6) as a scavenger for hydrated electron. J. Phys. Chem. A 2010, 114, 7479–7484. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-H.; Lin, Y.-C.; Nievaart, S.; Chou, W.-T.; Liu, H.-M.; Jiang, S.-H. Determination of the γ-ray spectrum in a strong neutron/γ-ray mixed field. Nucl. Instrum. Methods Phys. Res. Sect. A 2010, 652, 554–558. [Google Scholar] [CrossRef]
- Friedlander, G.; Kennedy, J.W.; Macias, E.S.; Miller, J.M. Nuclear and Radiochemistry; John Wiley & Sons, Inc.: New York, NY, USA, 1981. [Google Scholar]
- Belloni, J.; Mostafavi, M.; Remita, H.; Marignier, J.L.; Delcourt, M.O. Radiation-induced synthesis of mono- and multi-metallic clusters and nanocolloids. New J. Chem. 1998, 22, 1239–1255. [Google Scholar] [CrossRef]
- Van Eldik, R.; Meyerstein, D. High-pressure pulse radiolysis as a tool in the study of transition metal reaction mechanisms. Accounts Chem. Res. 2000, 33, 207–214. [Google Scholar] [CrossRef]
- Kayaselcuk, F.; Zorludemir, S.; Gumurduhu, D.; Zeren, H.; Erman, T. PCNA and Ki-67 in central nervous system tumors: Correlation with the histological type and grade. J. Neuro-Oncol. 2002, 57, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Zeng, X.; Wu, X.; Hu, J.; Zhu, Y.; Yang, X. Hyperbaric oxygen as an adjuvant to temozolomide nanoparticle inhibits glioma growth by inducing G2/M phase arrest. Nanomedicine 2018, 13, 887–898. [Google Scholar] [CrossRef] [PubMed]
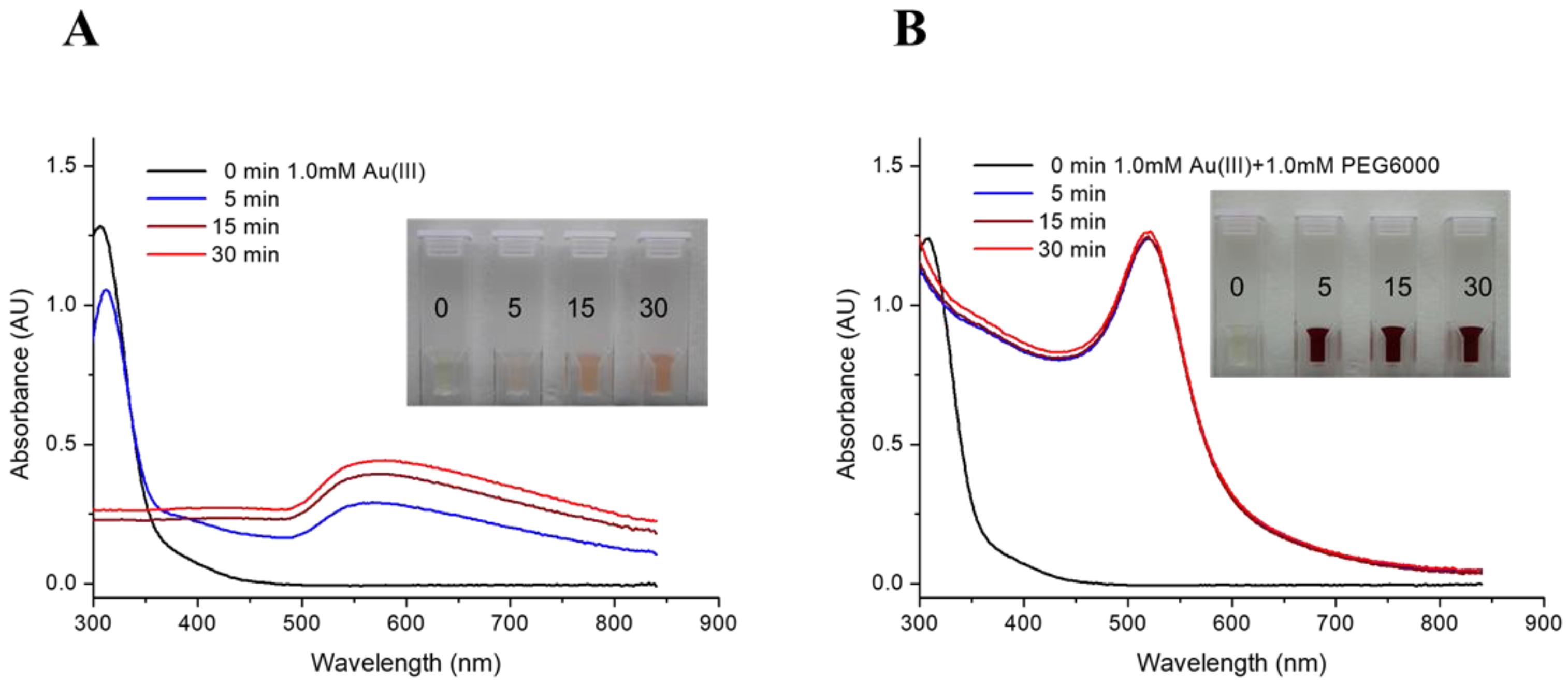

| Irradiation Time @THOR (min) | HAuCl4 | HAuCl4/PEG6000 | ||||
|---|---|---|---|---|---|---|
| 5 | 15 | 30 | 5 | 15 | 30 | |
| λmax of UV-Vis (nm) | 568–575 | 575–580 | 578–580 | 520 | 519–520 | 521 |
| Absorbance of λmax (AU) | 0.291 | 0.392 | 0.441 | 1.239 | 1.246 | 1.264 |
| Radioactivity (106 CPM) † | 0.78 | 2.22 | 4.30 | 0.70 | 2.05 | 3.83 |
| Yields of RGNP (%) | 53.6 | 61.5 | 100 | 100 | 100 | 100 |
| Sizes by prediction (nm) * | 99–105 | 105–108 | 107–108 | 14.8 | 14.8 | 21.7 |
| Sizes by TEM (nm) # | 8.6 ± 2.1 105.9 ± 29.6 | 146.3 ± 24.9 | 140.7 ± 26.8 | 14.6 ± 3.7 | 14.1 ± 4.3 | 15.9 ± 3.7 |
| Groups | Median Survival (Days) | Mean Survival (Days) | p-Value | p-Value | p-Value | p-Value |
|---|---|---|---|---|---|---|
| non-treatment | 28 | 28.6 ± 0.8 | 0.000 | 0.000 | 0.000 | |
| RGNP(CED) | 35 | 38.4 ± 2.2 | 0.000 | 0.158 | 0.000 | |
| TMZ(oral) | 44 | 42.8 ± 2.5 | 0.000 | 0.158 | 0.003 | |
| RGNP(CED) + TMZ(oral) | 54 | 68.9 ± 9.7 | 0.000 | 0.000 | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.-P.; Kuo, Y.-C.; Liao, W.-N.; Yang, Y.-T.; Chen, S.-Y.; Chien, Y.-T.; Wu, K.-H.; Wang, M.-Y.; Chou, F.-I.; Yang, M.-H.; et al. Harnessing Nuclear Energy to Gold Nanoparticles for the Concurrent Chemoradiotherapy of Glioblastoma. Nanomaterials 2023, 13, 2821. https://doi.org/10.3390/nano13212821
Li J-P, Kuo Y-C, Liao W-N, Yang Y-T, Chen S-Y, Chien Y-T, Wu K-H, Wang M-Y, Chou F-I, Yang M-H, et al. Harnessing Nuclear Energy to Gold Nanoparticles for the Concurrent Chemoradiotherapy of Glioblastoma. Nanomaterials. 2023; 13(21):2821. https://doi.org/10.3390/nano13212821
Chicago/Turabian StyleLi, Jui-Ping, Yu-Cheng Kuo, Wei-Neng Liao, Ya-Ting Yang, Sih-Yu Chen, Yu-Ting Chien, Kuo-Hung Wu, Mei-Ya Wang, Fong-In Chou, Mo-Hsiung Yang, and et al. 2023. "Harnessing Nuclear Energy to Gold Nanoparticles for the Concurrent Chemoradiotherapy of Glioblastoma" Nanomaterials 13, no. 21: 2821. https://doi.org/10.3390/nano13212821
APA StyleLi, J.-P., Kuo, Y.-C., Liao, W.-N., Yang, Y.-T., Chen, S.-Y., Chien, Y.-T., Wu, K.-H., Wang, M.-Y., Chou, F.-I., Yang, M.-H., Hueng, D.-Y., Yang, C.-S., & Chen, J.-K. (2023). Harnessing Nuclear Energy to Gold Nanoparticles for the Concurrent Chemoradiotherapy of Glioblastoma. Nanomaterials, 13(21), 2821. https://doi.org/10.3390/nano13212821







