Recent Advances in Nanoscale Zero-Valent Iron (nZVI)-Based Advanced Oxidation Processes (AOPs): Applications, Mechanisms, and Future Prospects
Abstract
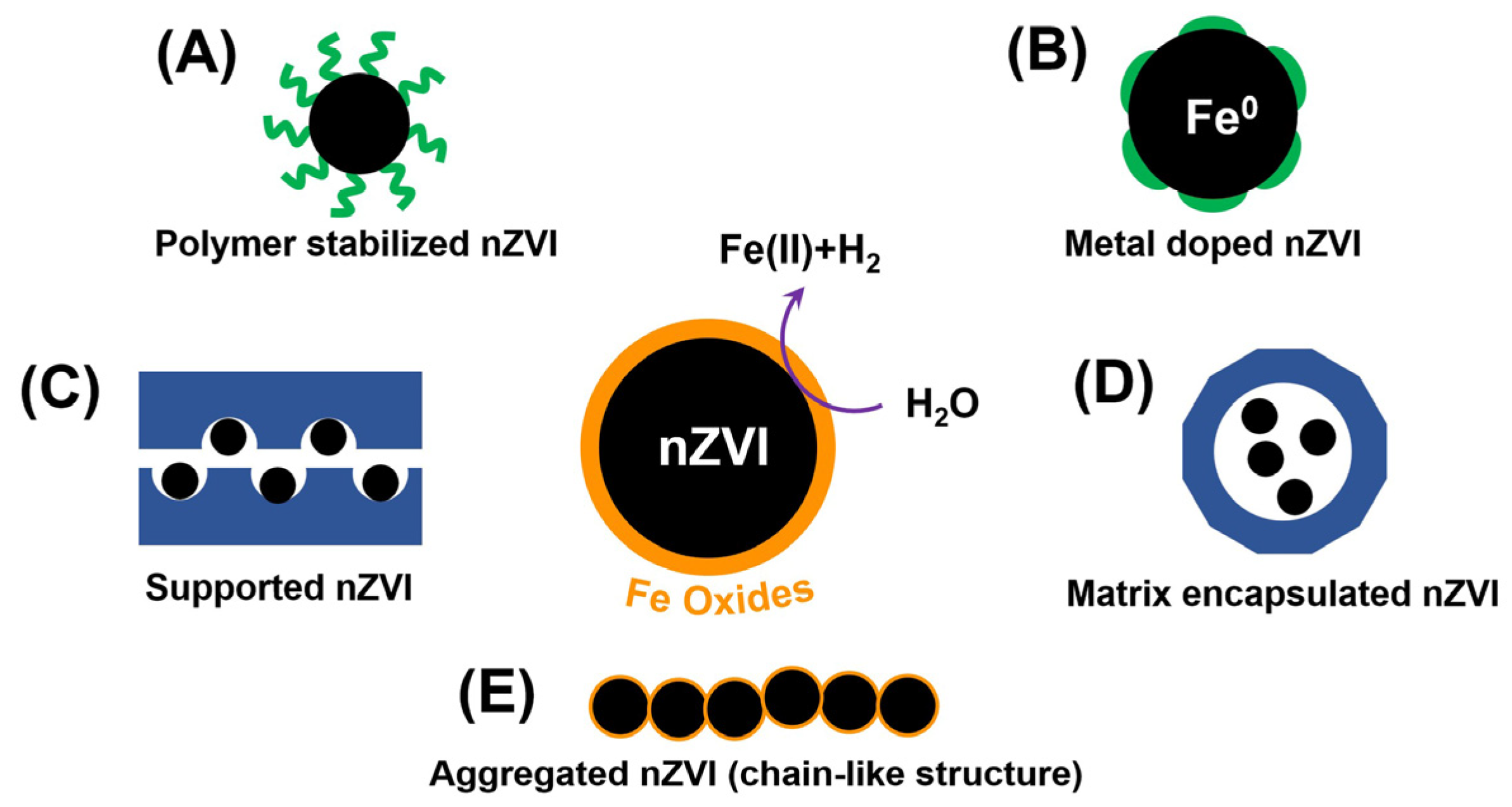
:1. Introduction
2. Persulfate-Based AOPs
2.1. Applications
2.2. Mechanisms
Cl− + •OH → OH− + •Cl
Br− + •OH → OH− + •Br
NO3− + •OH → OH− + •NO3
CO32− + •OH → OH− + •CO3−
HCO3− + •SO4− → SO42− + •CO3− + H+
HCO3− + •OH → H2O + •CO3−
2.3. Concepts for the Future
3. Hydrogen Peroxide-Based AOPs
3.1. Applications
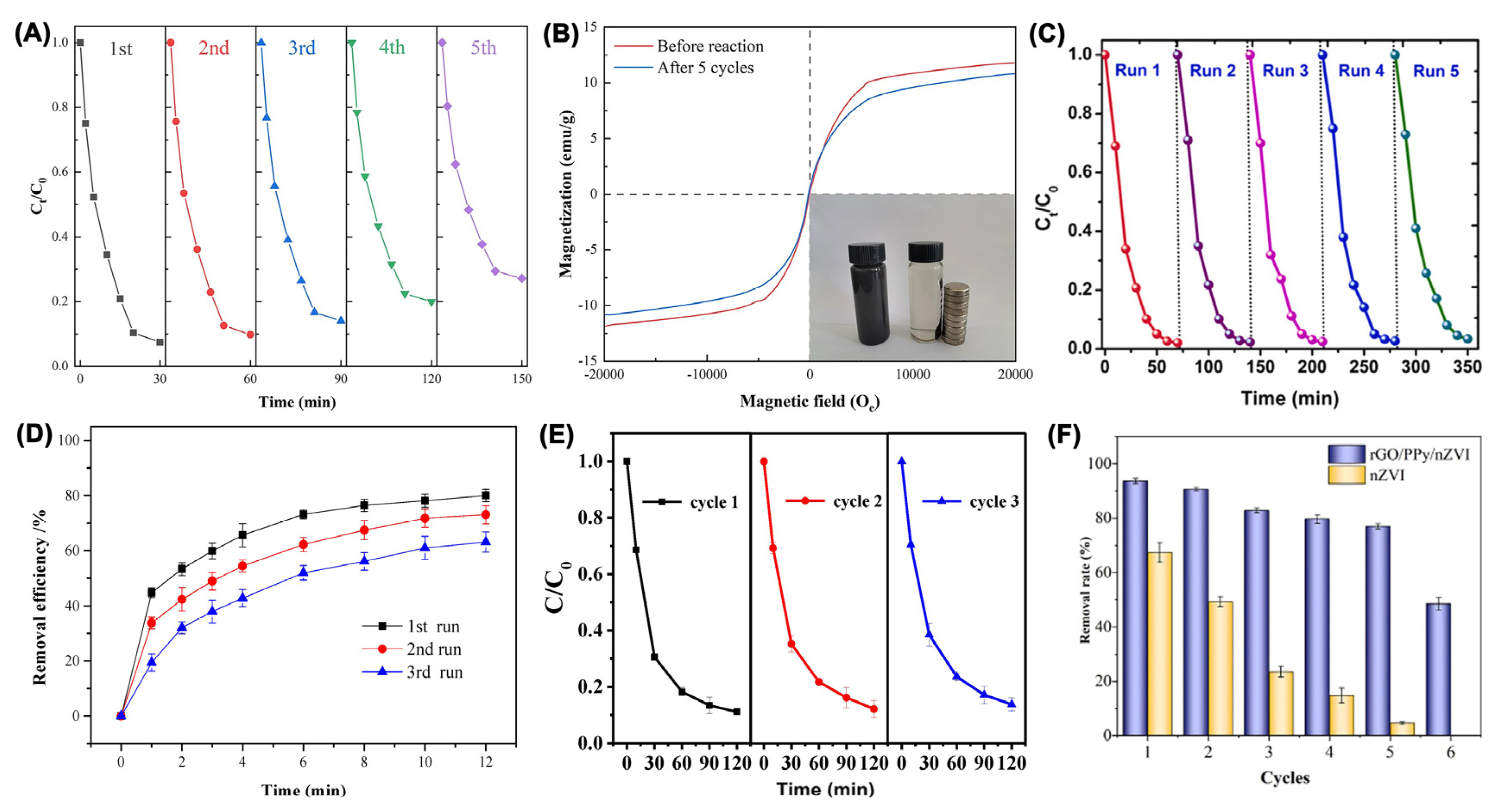
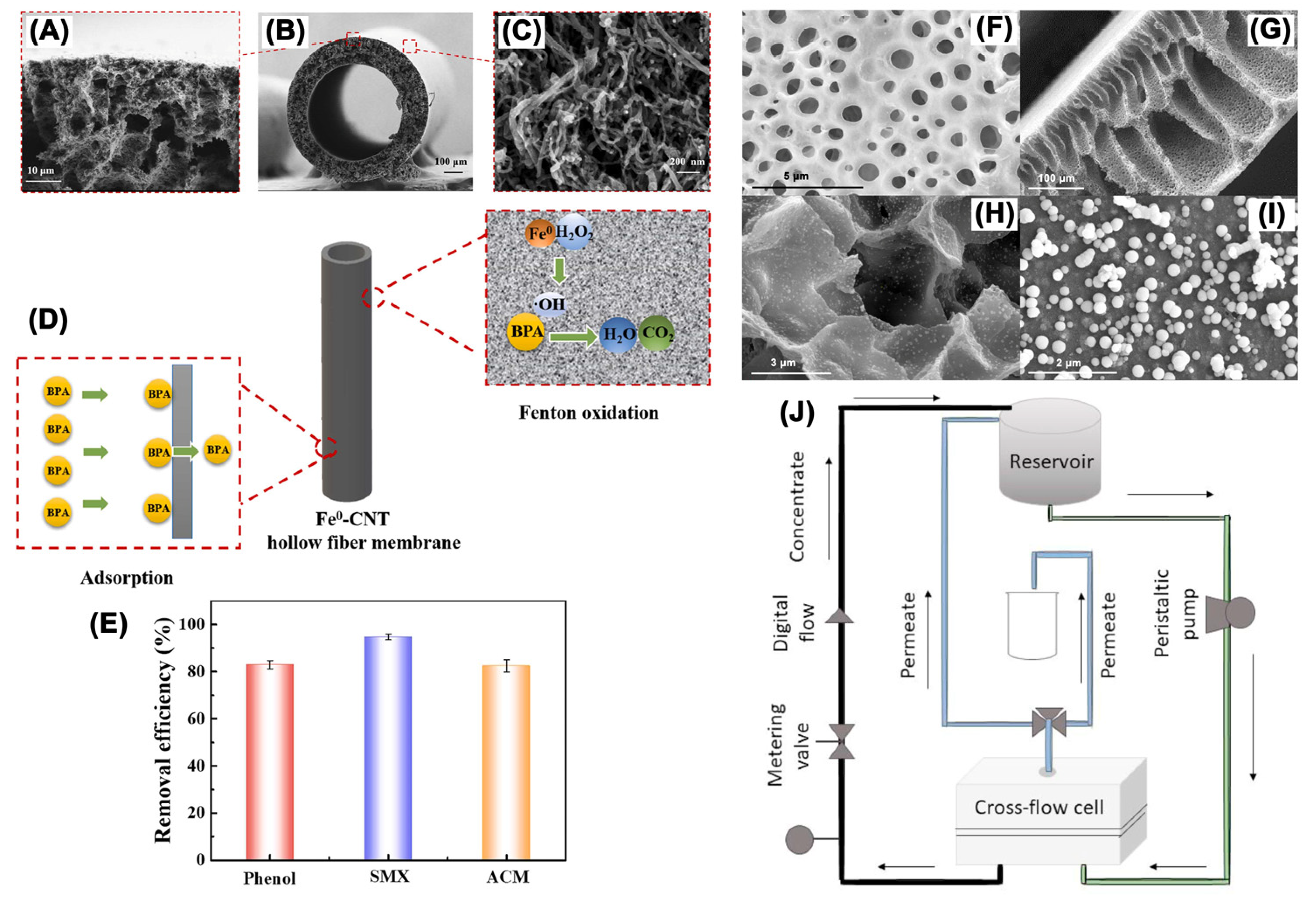

3.2. Mechanisms
Fe3+ + H2O2 → Fe2+ + •OOH + H+
3.3. Concepts for the Future
4. Oxygen-Based AOPs
4.1. Applications
4.2. Mechanisms
4.3. Concepts for the Future
5. Other Oxidant-Based AOPs
5.1. Peracetic Acid-Based AOPs
5.2. Periodate-Based AOPs
5.3. Percarbonate-Based AOPs
5.4. Concepts for the Future
6. Conclusions and Prospectives
- (1)
- The applications of nZVI-based materials in AOPs for the removal of organic pollutants still lack accurate models and reaction kinetics regarding the dissolution and catalyzation processes of nZVI. Quantitatively exploring and establishing these models and kinetics will provide theoretical guidance on the fabrication of more effective and sustainable nZVI materials, optimization of the reaction conditions, or a more reasonable dosage strategy of nZVI during AOPs.
- (2)
- A few research studies reported the combination of multiple oxidants in nZVI-based AOPs, in which the reaction mechanism involved is complicated and less understood. Therefore, employing multiple oxidants in AOPs conducted with nZVI calls for more studies to verify the potential enhancement effects on the oxidization ability toward contaminants, and would enable us to obtain in-depth knowledge of the interactions among various oxidants, uncharted degradation mechanisms, and their feasibility.
- (3)
- In nZVI-based AOPs, organic pollutants can be theoretically degraded and mineralized into harmful CO2 and H2O, although many reported research studies were mainly focused on the removal efficiency of the target pollutants. Future studies may need to stress the complete degradation of the pollutants, such as committing to reduction of the total organic carbon (TOC) value of the polluted water during AOPs, and clarifying the potential toxic aspects of intermediate products via toxicity studies.
- (4)
- Although this research results confirmed the feasibility of nZVI-based AOPs for the degradation of contaminants, there is a large gap between laboratory level research and the remediations aimed towards actual polluted water. In addition to actual polluted water containing a variety of pollutants, coexisting inorganic ions, and interfering matter, present works were usually carried out on simulated wastewater, and thus research studies that are dedicated to the applicability of nZVI-based AOPs in actual situations are necessary.
- (5)
- There is a need for further studies to obtain a greater understanding on the potential synergistic and antagonistic effects among contaminants, as well as their intermediate products during their oxidative degradation with ROS.
- (6)
- The recoverability and reusability of nZVI materials is an important characteristic that is crucial to the practical application on a large scale, since an appropriate catalyst that is applicable in AOPs under real conditions is required to be reasonable, durable, and convenient in operation. The recovery of the catalytic activity of nZVI after repeated use was commonly low in the existing research. Therefore, further exploration into nZVI materials with a controllable discharge of Fe2+, prolonged stable catalytic activity, favorable separation, and excellent recyclability is fascinating.
- (7)
- Extending the application of nZVI-based AOPs in high-value fields, such as preventing antimicrobial resistance emergence and biofilm formation [157], or biomedical applications, is also worthy of being studied.
- (8)
- Finally, studies on the techno-economic and environmental impact of nZVI-based AOPs by means of life cycle analysis are also worthwhile to conduct before undertaking practical applications.
Author Contributions
Funding
Conflicts of Interest
References
- Wang, Z.; Berbille, A.; Feng, Y.; Li, S.; Zhu, L.; Tang, W.; Wang, Z.L. Contact-electro-catalysis for the degradation of organic pollutants using pristine dielectric powders. Nat. Commun. 2022, 13, 130. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Xu, Z.; Hou, D.; Gao, B.; Cao, X.; Ok, Y.S.; Rinklebe, J.; Bolan, N.S.; Tsang, D.C. Waste-derived biochar for water pollution control and sustainable development. Nat. Rev. Earth Environ. 2022, 3, 444–460. [Google Scholar] [CrossRef]
- Sun, L.; Dong, H.; Lu, Y.; Zhang, L.; Yang, L.; Zhao, J.; Song, Y. A hydrate-based zero liquid discharge method for high-concentration organic wastewater: Resource recovery and water reclamation. NPJ Clean Water 2023, 6, 49. [Google Scholar] [CrossRef]
- Guo, Z.; Wang, D.; Yan, Z.; Qian, L.; Yang, L.; Yan, J.; Chen, M. Efficient remediation of p-chloroaniline contaminated soil by activated persulfate using ball milling nanosized zero valent iron/biochar composite: Performance and mechanisms. Nanomaterials 2023, 13, 1517. [Google Scholar] [CrossRef]
- Kong, E.D.H.; Chau, J.H.F.; Lai, C.W.; Khe, C.S.; Sharma, G.; Kumar, A.; Siengchin, S.; Sanjay, M.R. GO/TiO2-related nanocomposites as photocatalysts for pollutant removal in wastewater treatment. Nanomaterials 2022, 12, 3536. [Google Scholar] [CrossRef]
- Chen, G.; Wong, N.H.; Sunarso, J.; Wang, Y.; Huang, X.; Xiong, X.; Wang, F. Characterization of BiOBr/g-C3N4 heterostructures immobilized on flexible electrospun polyacrylonitrile nanofibers for photocatalytic applications. Appl. Surf. Sci. 2021, 569, 151011. [Google Scholar] [CrossRef]
- Xie, L.; Wang, P.; Li, Y.; Zhang, D.; Shang, D.; Zheng, W.; Xia, Y.; Zhan, S.; Hu, W. Pauling-type adsorption of O2 induced electrocatalytic singlet oxygen production on N–CuO for organic pollutants degradation. Nat. Commun. 2022, 13, 5560. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zheng, X.; Feng, Z.; Lu, Z.; Zhang, Z.; Huang, W.; Li, Y.; Vuckovic, D.; Li, Y.; Dai, S. Organic wastewater treatment by a single-atom catalyst and electrolytically produced H2O2. Nat. Sustain. 2021, 4, 233–241. [Google Scholar] [CrossRef]
- Liu, M.; Shan, C.; Chang, H.; Zhang, Z.; Huang, R.; Lee, D.W.; Qi, W.; He, Z.; Su, R. Nano-engineered natural sponge as a recyclable and deformable reactor for ultrafast conversion of pollutants from water. Chem. Eng. Sci. 2022, 247, 117049. [Google Scholar] [CrossRef]
- Choi, C.; Wang, X.; Kwon, S.; Hart, J.L.; Rooney, C.L.; Harmon, N.J.; Sam, Q.P.; Cha, J.J.; Goddard III, W.A.; Elimelech, M. Efficient electrocatalytic valorization of chlorinated organic water pollutant to ethylene. Nat. Nanotechnol. 2023, 18, 160–167. [Google Scholar] [CrossRef]
- Chen, G.; Wong, N.H.; Sunarso, J.; Wang, Y.; Liu, Z.; Chen, D.; Wang, D.; Dai, G. Flexible Bi2MoO6/S-C3N4/PAN heterojunction nanofibers made from electrospinning and solvothermal route for boosting visible-light photocatalytic performance. Appl. Surf. Sci. 2023, 612, 155893. [Google Scholar] [CrossRef]
- Liu, M.; Ye, Y.; Ye, J.; Gao, T.; Wang, D.; Chen, G.; Song, Z. Recent advances of magnetite (Fe3O4)-based magnetic materials in catalytic applications. Magnetochemistry 2023, 9, 110. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, C.; Yan, X.; Zhang, H.; Xiao, C.; Qi, J.; Zhu, Z.; Zhou, Y.; Sun, X.; Duan, X. Rational regulation of Co–N–C coordination for high-efficiency generation of 1O2 toward nearly 100% selective degradation of organic pollutants. Environ. Sci. Technol. 2022, 56, 8833–8843. [Google Scholar] [CrossRef] [PubMed]
- Pandis, P.K.; Kalogirou, C.; Kanellou, E.; Vaitsis, C.; Savvidou, M.G.; Sourkouni, G.; Zorpas, A.A.; Argirusis, C. Key points of advanced oxidation processes (AOPs) for wastewater, organic pollutants and pharmaceutical waste treatment: A mini review. ChemEngineering 2022, 6, 8. [Google Scholar] [CrossRef]
- Yu, Y.; Liu, C.; Yang, C.; Yu, Y.; Lu, L.; Ma, R.; Li, L. One-step synthesized iron-carbon core-shell nanoparticles to activate persulfate for effective degradation of tetrabromobisphenol A: Performance and activation mechanism. Nanomaterials 2022, 12, 4483. [Google Scholar] [CrossRef]
- Kessler, A.; Hedberg, J.; Blomberg, E.; Odnevall, I. Reactive oxygen species formed by metal and metal oxide nanoparticles in physiological media-a review of reactions of importance to nanotoxicity and proposal for categorization. Nanomaterials 2022, 12, 1922. [Google Scholar] [CrossRef] [PubMed]
- Richards, T.; Harrhy, J.H.; Lewis, R.J.; Howe, A.G.; Suldecki, G.M.; Folli, A.; Morgan, D.J.; Davies, T.E.; Loveridge, E.J.; Crole, D.A. A residue-free approach to water disinfection using catalytic in situ generation of reactive oxygen species. Nat. Catal. 2021, 4, 575–585. [Google Scholar] [CrossRef]
- Makhoul, E.; Boulos, M.; Cretin, M.; Lesage, G.; Miele, P.; Cornu, D.; Bechelany, M. CaCu3Ti4O12 perovskite materials for advanced oxidation processes for water treatment. Nanomaterials 2023, 13, 2119. [Google Scholar] [CrossRef]
- Barroso-Martínez, J.S.; Romo, A.I.B.; Pudar, S.; Putnam, S.T.; Bustos, E.; Rodríguez-López, J. Real-time detection of hydroxyl radical generated at operating electrodes via redox-active adduct formation using scanning electrochemical microscopy. J. Am. Chem. Soc. 2022, 144, 18896–18907. [Google Scholar] [CrossRef]
- Bai, X.; Yang, Q.; Guo, Y.; Hao, B.; Zhang, R.; Duan, R.; Li, J. Alkyl halide formation from degradation of carboxylic acids in the presence of Fe(III) and halides under light irradiation. Water Res. 2023, 235, 119842. [Google Scholar] [CrossRef]
- Xin, S.; Ma, B.; Zhang, C.; Ma, X.; Xu, P.; Zhang, G.; Gao, M.; Xin, Y. Catalytic activation of peroxydisulfate by alfalfa-derived nitrogen self-doped porous carbon supported CuFeO2 for nimesulide degradation: Performance, mechanism and DFT calculation. Appl. Catal. B 2021, 294, 120247. [Google Scholar] [CrossRef]
- Sayed, M.; Ren, B.; Ali, A.M.; Al-Anazi, A.; Nadagouda, M.N.; Ismail, A.A.; Dionysiou, D.D. Solar light induced photocatalytic activation of peroxymonosulfate by ultra-thin Ti3+ self-doped Fe2O3/TiO2 nanoflakes for the degradation of naphthalene. Appl. Catal. B 2022, 315, 121532. [Google Scholar] [CrossRef]
- Zhang, H.; Xiong, R.; Peng, S.; Xu, D.; Ke, J. Highly active manganese oxide from electrolytic manganese anode slime for efficient removal of antibiotics induced by dissociation of peroxymonosulfate. Nanomaterials 2023, 13, 1600. [Google Scholar] [CrossRef] [PubMed]
- Oyekunle, D.T.; Gendy, E.A.; Ifthikar, J.; Chen, Z. Heterogeneous activation of persulfate by metal and non-metal catalyst for the degradation of sulfamethoxazole: A review. Chem. Eng. J. 2022, 437, 135277. [Google Scholar] [CrossRef]
- Dong, C.; Zheng, Z.; Wang, Z.; He, J.; Ye, Z.; Gong, X.; Lo, I.M. N-doped graphitic C3N4 nanosheets decorated with CoP nanoparticles: A highly efficient activator in singlet oxygen dominated visible-light-driven peroxymonosulfate activation for degradation of pharmaceuticals and personal care products. J. Hazard. Mater. 2021, 416, 125891. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Chen, L.; Sun, P.; Zhang, Q.; Chen, Y.; Zheng, X. Heterogeneous catalytic reactions of in-situ generated bromide ions via hydrodehalogenation of tetrabromobisphenol A in advanced oxidation processes over palladium nanoparticles. Appl. Catal. B 2023, 340, 123213. [Google Scholar] [CrossRef]
- Hodges, B.C.; Cates, E.L.; Kim, J.-H. Challenges and prospects of advanced oxidation water treatment processes using catalytic nanomaterials. Nat. Nanotechnol. 2018, 13, 642–650. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Mofijur, M.; Nuzhat, S.; Chowdhury, A.T.; Rafa, N.; Uddin, M.A.; Inayat, A.; Mahlia, T.; Ong, H.C.; Chia, W.Y. Recent developments in physical, biological, chemical, and hybrid treatment techniques for removing emerging contaminants from wastewater. J. Hazard. Mater. 2021, 416, 125912. [Google Scholar] [CrossRef] [PubMed]
- Davarazar, M.; Kamali, M.; Venâncio, C.; Gabriel, A.; Aminabhavi, T.M.; Lopes, I. Activation of persulfate using copper oxide nanoparticles for the degradation of Rhodamine B containing effluents: Degradation efficiency and ecotoxicological studies. Chem. Eng. J. 2023, 453, 139799. [Google Scholar] [CrossRef]
- Liu, T.; Yao, B.; Luo, Z.; Li, W.; Li, C.; Ye, Z.; Gong, X.; Yang, J.; Zhou, Y. Applications and influencing factors of the biochar-persulfate based advanced oxidation processes for the remediation of groundwater and soil contaminated with organic compounds. Sci. Total Environ. 2022, 836, 155421. [Google Scholar] [CrossRef]
- Sun, P.; Wang, Z.; An, S.; Zhao, J.; Yan, Y.; Zhang, D.; Wu, Z.; Shen, B.; Lyu, H. Biochar-supported nZVI for the removal of Cr(VI) from soil and water: Advances in experimental research and engineering applications. J. Environ. Manag. 2022, 316, 115211. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Peng, C.; Shi, X. Preparation, characterization, and applications of Fe-based catalysts in advanced oxidation processes for organics removal: A review. Environ. Pollut. 2022, 293, 118565. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, S.; Li, S.; Tang, J.; Hua, T.; Li, F. Mechanism of the application of single-atom catalyst-activated PMS/PDS to the degradation of organic pollutants in water environment: A review. J. Clean. Prod. 2023, 397, 136468. [Google Scholar] [CrossRef]
- Duan, X.; Yang, S.; Wacławek, S.; Fang, G.; Xiao, R.; Dionysiou, D.D. Limitations and prospects of sulfate-radical based advanced oxidation processes. J. Environ. Chem. Eng. 2020, 8, 103849. [Google Scholar] [CrossRef]
- Zhao, G.; Zou, J.; Chen, X.; Liu, L.; Wang, Y.; Zhou, S.; Long, X.; Yu, J.; Jiao, F. Iron-based catalysts for persulfate-based advanced oxidation process: Microstructure, property and tailoring. Chem. Eng. J. 2021, 421, 127845. [Google Scholar] [CrossRef]
- Wang, J.; Tang, J. Fe-based Fenton-like catalysts for water treatment: Preparation, characterization and modification. Chemosphere 2021, 276, 130177. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Zhang, Y.; Zhang, Z.; Zhou, L.; Yu, G.; Wen, X.; Chi, T.; Wang, G.; Su, Y.; Deng, F. Fe-based metal organic frameworks (Fe-MOFs) for organic pollutants removal via photo-Fenton: A review. Chem. Eng. J. 2022, 431, 133932. [Google Scholar] [CrossRef]
- Cardoso, I.M.F.; Cardoso, R.M.F.; da Silva, J. Advanced oxidation processes coupled with nanomaterials for water treatment. Nanomaterials 2021, 11, 2045. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, X.; Li, X.; Jiang, L.; Wang, H. Burgeoning prospects of biochar and its composite in persulfate-advanced oxidation process. J. Hazard. Mater. 2021, 409, 124893. [Google Scholar] [CrossRef]
- Zhou, Y.-H.; Yang, S.-Y.; Wang, M.-X.; Guan, Y.-H.; Ma, J. Fast degradation of atrazine by nZVI-Cu0/PMS: Re-evaluation and quantification of reactive species, generation pathways, and application feasibility. Water Res. 2023, 243, 120311. [Google Scholar] [CrossRef]
- Wei, X.; Yuan, H.; Li, J.; Chen, T.; Yuan, Y.; Chen, W.; Guan, C.; Wang, Z.; Guo, Q.; Han, B. Reactive oxygen species generated in iron sulfide mediated advanced oxidation systems: A critical review of mechanisms and implications for geochemistry and environmental remediation. J. Environ. Chem. Eng. 2022, 10, 108841. [Google Scholar] [CrossRef]
- Wang, P.; Fu, F.; Liu, T. A review of the new multifunctional nano zero-valent iron composites for wastewater treatment: Emergence, preparation, optimization and mechanism. Chemosphere 2021, 285, 131435. [Google Scholar] [CrossRef] [PubMed]
- Priyadarshini, A.; Kumar Sahoo, P.; Ghosal, A.; Kumar Sahoo, N. Stabilization of zero-valent iron for wastewater treatment: Challenges and future prospective. Mater. Today Proc. 2022, 67, 1073–1079. [Google Scholar] [CrossRef]
- Liu, M.; Huang, R.; Che, M.; Su, R.; Qi, W.; He, Z. Tannic acid-assisted fabrication of Fe-Pd nanoparticles for stable rapid dechlorination of two organochlorides. Chem. Eng. J. 2018, 352, 716–721. [Google Scholar] [CrossRef]
- Liu, M.; Huang, R.; Li, C.; Che, M.; Su, R.; Li, S.; Yu, J.; Qi, W.; He, Z. Continuous rapid dechlorination of p-chlorophenol by Fe-Pd nanoparticles promoted by procyanidin. Chem. Eng. Sci. 2019, 201, 121–131. [Google Scholar] [CrossRef]
- Zhou, L.; Li, A.; Ma, F.; Zhao, H.; Deng, F.; Pi, S.; Tang, A.; Yang, J. Combining high electron transfer efficiency and oxidation resistance in nZVI with coatings of microbial extracellular polymeric substances to enhance Sb(V) reduction and adsorption. Chem. Eng. J. 2020, 395, 125168. [Google Scholar] [CrossRef]
- Raji, M.; Mirbagheri, S.A.; Ye, F.; Dutta, J. Nano zero-valent iron on activated carbon cloth support as Fenton-like catalyst for efficient color and COD removal from melanoidin wastewater. Chemosphere 2021, 263, 127945. [Google Scholar] [CrossRef] [PubMed]
- Ansari, A.; Siddiqui, V.U.; Akram, M.K.; Siddiqi, W.A.; Khan, A.; Al-Romaizan, A.N.; Hussein, M.A.; Puttegowda, M. Synthesis of atmospherically stable zero-valent iron nanoparticles (nZVI) for the efficient catalytic treatment of high-strength domestic wastewater. Catalysts 2021, 12, 26. [Google Scholar] [CrossRef]
- Moreno-Barcenas, A.; Arizpe-Zapata, J.A.; Rivera Haro, J.A.; Sepulveda, P.; Garcia-Garcia, A. Jute fibers synergy with nZVI/GO: Superficial properties enhancement for arsenic removal in water with possible application in dynamic flow filtration systems. Nanomaterials 2022, 12, 3974. [Google Scholar] [CrossRef]
- Pasinszki, T.; Krebsz, M. Synthesis and application of zero-valent iron nanoparticles in water treatment, environmental remediation, catalysis, and their biological effects. Nanomaterials 2020, 10, 917. [Google Scholar] [CrossRef]
- Suazo-Hernández, J.; Sepúlveda, P.; Cáceres-Jensen, L.; Castro-Rojas, J.; Poblete-Grant, P.; Bolan, N.; Mora, M.d.l.L. nZVI-based nanomaterials used for phosphate removal from aquatic systems. Nanomaterials 2023, 13, 399. [Google Scholar] [CrossRef]
- Ahmad, S.; Liu, X.; Tang, J.; Zhang, S. Biochar-supported nanosized zero-valent iron (nZVI/BC) composites for removal of nitro and chlorinated contaminants. Chem. Eng. J. 2022, 431, 133187. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, X.; Fu, L.; Peng, X.; Pan, C.; Mao, Q.; Wang, C.; Yan, J. Nonradicals induced degradation of organic pollutants by peroxydisulfate (PDS) and peroxymonosulfate (PMS): Recent advances and perspective. Sci. Total Environ. 2021, 765, 142794. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhang, J.; Hou, Z.; Chen, P.; Zhou, X.; Wang, W.; Tan, F.; Wang, X.; Qiao, X. Improvement of carbonyl groups and surface defects in carbon nanotubes to activate peroxydisulfate for tetracycline degradation. Nanomaterials 2023, 13, 216. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Cheng, M.; Zhong, H.; Liu, Z.; Liu, Y.; Yang, X.; Liang, Q. Iron-mediated activation of persulfate and peroxymonosulfate in both homogeneous and heterogeneous ways: A review. Chem. Eng. J. 2020, 384, 123265. [Google Scholar] [CrossRef]
- Zhang, B.; Guo, Y.; Huo, J.; Xie, H.; Xu, C.; Liang, S. Combining chemical oxidation and bioremediation for petroleum polluted soil remediation by BC-nZVI activated persulfate. Chem. Eng. J. 2020, 382, 123055. [Google Scholar] [CrossRef]
- Li, S.; Tang, J.; Liu, Q.; Liu, X.; Gao, B. A novel stabilized carbon-coated nZVI as heterogeneous persulfate catalyst for enhanced degradation of 4-chlorophenol. Environ. Int. 2020, 138, 105639. [Google Scholar] [CrossRef] [PubMed]
- Rahmani, A.R.; Salari, M.; Shabanloo, A.; Shabanloo, N.; Bajalan, S.; Vaziri, Y. Sono-catalytic activation of persulfate by nZVI-reduced graphene oxide for degradation of nonylphenol in aqueous solution: Process optimization, synergistic effect and degradation pathway. J. Environ. Chem. Eng. 2020, 8, 104202. [Google Scholar] [CrossRef]
- Rahmani, A.; Salari, M.; Tari, K.; Shabanloo, A.; Shabanloo, N.; Bajalan, S. Enhanced degradation of furfural by heat-activated persulfate/nZVI-rGO oxidation system: Degradation pathway and improving the biodegradability of oil refinery wastewater. J. Environ. Chem. Eng. 2020, 8, 104468. [Google Scholar] [CrossRef]
- Huang, X.; Zhu, N.; Wei, X.; Ding, Y.; Ke, Y.; Wu, P.; Liu, Z. Mechanism insight into efficient peroxydisulfate activation by novel nano zero-valent iron anchored yCo3O4 (nZVI/yCo3O4) composites. J. Hazard. Mater. 2020, 400, 123157. [Google Scholar] [CrossRef]
- Chen, R.; Yin, H.; Peng, H.; Wei, X.; Yu, X.; Xie, D.; Lu, G.; Dang, Z. Removal of triphenyl phosphate by nanoscale zerovalent iron (nZVI) activated bisulfite: Performance, surface reaction mechanism and sulfate radical-mediated degradation pathway. Environ. Pollut. 2020, 260, 113983. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Wu, Y.; Liang, Y.; Li, H.; Liang, W. Degradation mechanism of norfloxacin in water using persulfate activated by BC@nZVI/Ni. Chem. Eng. J. 2020, 389, 124276. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, B.-T.; Teng, Y.; Zhao, J. Activated carbon supported nanoscale zero valent iron for cooperative adsorption and persulfate-driven oxidation of ampicillin. Environ. Technol. Innov. 2020, 19, 100956. [Google Scholar] [CrossRef]
- Wu, J.; Wang, B.; Cagnetta, G.; Huang, J.; Wang, Y.; Deng, S.; Yu, G. Nanoscale zero valent iron-activated persulfate coupled with Fenton oxidation process for typical pharmaceuticals and personal care products degradation. Sep. Purif. Technol. 2020, 239, 116534. [Google Scholar] [CrossRef]
- Du, L.; Xu, W.; Liu, Y.; Li, X.; Huang, D.; Wu, S. Removal of sulfamethoxazole in aqueous solutions by iron-based advanced oxidation processes: Performances and mechanisms. Water Air Soil Pollut. 2020, 231, 159. [Google Scholar] [CrossRef]
- Bajagain, R.; Jeong, S.W. Degradation of petroleum hydrocarbons in soil via advanced oxidation process using peroxymonosulfate activated by nanoscale zero-valent iron. Chemosphere 2021, 270, 128627. [Google Scholar] [CrossRef]
- Diao, Z.-H.; Jin, J.-C.; Zou, M.-Y.; Liu, H.; Qin, J.-Q.; Zhou, X.-H.; Qian, W.; Guo, P.-R.; Kong, L.-J.; Chu, W. Simultaneous degradation of amoxicillin and norfloxacin by TiO2@nZVI composites coupling with persulfate: Synergistic effect, products and mechanism. Sep. Purif. Technol. 2021, 278, 119620. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, Y.; Jiang, S.; Wang, Y.; Li, H.; Han, W.; Qu, J.; Wang, L.; Hu, Y. Graphene-like carbon sheet-supported nZVI for efficient atrazine oxidation degradation by persulfate activation. Chem. Eng. J. 2021, 403, 126309. [Google Scholar] [CrossRef]
- Tan, W.; Ruan, Y.; Diao, Z.; Song, G.; Su, M.; Hou, L.; Chen, D.; Kong, L.; Deng, H. Removal of levofloxacin through adsorption and peroxymonosulfate activation using carbothermal reduction synthesized nZVI/carbon fiber. Chemosphere 2021, 280, 130626. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, Q.; Jiang, S.; Li, H.; Zhang, R.; Qu, J.; Zhang, S.; Han, W. One-step synthesis of biochar supported nZVI composites for highly efficient activating persulfate to oxidatively degrade atrazine. Chem. Eng. J. 2021, 420, 129868. [Google Scholar] [CrossRef]
- Idrees, A.; Shan, A.; Ali, M.; Abbas, Z.; Shahzad, T.; Hussain, S.; Mahmood, F.; Farooq, U.; Danish, M.; Lyu, S. Highly efficient degradation of trichloroethylene in groundwater based on persulfate activation by polyvinylpyrrolidone functionalized Fe/Cu bimetallic nanoparticles. J. Environ. Chem. Eng. 2021, 9, 105341. [Google Scholar] [CrossRef]
- Shan, A.; Idrees, A.; Zaman, W.Q.; Abbas, Z.; Ali, M.; Rehman, M.S.U.; Hussain, S.; Danish, M.; Gu, X.; Lyu, S. Synthesis of nZVI-Ni@BC composite as a stable catalyst to activate persulfate: Trichloroethylene degradation and insight mechanism. J. Environ. Chem. Eng. 2021, 9, 104808. [Google Scholar] [CrossRef]
- Rao, Z.; Zhu, N.; Wei, X.; Li, F.; Wu, P.; Dang, Z.; Cui, B. Efficient peroxydisulfate activation with nZVI/CuO@BC nanocomposite derived from wastes for degradation of tetrabromobisphenol A in alkaline environment. J. Hazard. Mater. 2021, 417, 126029. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, D.; Wacławek, S.; Sobel, B.; Torres-Mendieta, R.; Pawlyta, M.; Padil, V.V.T.; Filip, J.; Černík, M. Modification of nZVI with a bio-conjugate containing amine and carbonyl functional groups for catalytic activation of persulfate. Sep. Purif. Technol. 2021, 257, 117880. [Google Scholar] [CrossRef]
- Xiao, J.; Xiao, S.; Dong, H.; Jin, Z.; Li, Y.; Li, L.; Tian, R.; Li, R.; Chen, J.; Xie, Q. Degradation of sulfamethazine by amorphous zero-valent iron microspheres (A-mZVI) activated peroxydisulfate in groundwater. J. Cleaner Prod. 2022, 346, 131276. [Google Scholar] [CrossRef]
- Ma, B.; Yao, J.; Knudsen, T.S.; Chen, Z.; Liu, B.; Zhao, C.; Zhu, X. Simultaneous removal of typical flotation reagent 8-hydroxyquinoline and Cr(VI) through heterogeneous Fenton-like processes mediated by polydopamine functionalized ATP supported nZVI. J. Hazard. Mater. 2022, 424, 126698. [Google Scholar] [CrossRef]
- Li, J.; Lin, Q.; Luo, H.; Fu, H.; Wu, L.; Chen, Y.; Ma, Y. The effect of nanoscale zero-valent iron-loaded N-doped biochar on the generation of free radicals and nonradicals by peroxydisulfate activation. J. Water Process Eng. 2022, 47, 102681. [Google Scholar] [CrossRef]
- Xiao, J.; Li, R.; Dong, H.; Li, Y.; Li, L.; Xiao, S.; Jin, Z. Activation of sulfite via zero-valent iron-manganese bimetallic nanomaterials for enhanced sulfamethazine removal in aqueous solution: Key roles of Fe/Mn molar ratio and solution pH. Sep. Purif. Technol. 2022, 297, 121479. [Google Scholar] [CrossRef]
- Zhang, C.; Tang, J.; Gao, F.; Yu, C.; Li, S.; Lyu, H.; Sun, H. Tetrahydrofuran aided self-assembly synthesis of nZVI@gBC composite as persulfate activator for degradation of 2,4-dichlorophenol. Chem. Eng. J. 2022, 431, 134063. [Google Scholar] [CrossRef]
- Fan, M.; Zhang, P.; Wang, C.; Tang, J.; Sun, H. Tailored design of three-dimensional rGOA-nZVI catalyst as an activator of persulfate for degradation of organophosphorus pesticides. J. Hazard. Mater. 2022, 428, 128254. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Ni, J.; Liu, D.; Shi, W.; Yuan, Y.; Cui, F.; Tian, J.; Wang, W. Molybdenum disulfide as excellent Co-catalyst boosting catalytic degradation of sulfamethoxazole by nZVI/PDS process. Sep. Purif. Technol. 2022, 285, 120398. [Google Scholar] [CrossRef]
- Guo, J.; Jiang, J.; Chen, Y.; Wen, X.; Chen, W.; Wang, Y.; Su, L.; Cao, J. Synthesis of nZVI-BC composite for persulfate activation to degrade pyrene: Performance, correlative mechanisms and degradation pathways. Process Saf. Environ. Prot. 2022, 162, 733–745. [Google Scholar] [CrossRef]
- Wang, P.; Xu, Z.; Zeng, G.; Lyu, S. Efficient degradation of trichloroethene with the existence of surfactants by peroxymonosulfate activated by nano-zero-valent iron: Performance and mechanism investigation. Environ. Sci. Pollut. Res. Int. 2023, 30, 48351–48362. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, B.; Wang, N.; Zhang, N.; Ma, Y.; Zang, L.; Li, Z.; Xue, R. Refractory organics removal in PMS and H2O2/PMS oxidation system activated by biochar/nZVI/MoS2 composite: Synthesis, performance, mechanism and dosing methods. J. Environ. Chem. Eng. 2023, 11, 109134. [Google Scholar] [CrossRef]
- Huang, P.; Zhang, P.; Wang, C.; Du, X.; Jia, H.; Sun, H. P-doped biochar regulates nZVI nanocracks formation for superefficient persulfate activation. J. Hazard. Mater. 2023, 450, 130999. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, J.; Zeng, Q.; Zeng, X.; Zhang, G.; Niu, Y. Enhanced activation of peroxydisulfate by regulating pyrolysis temperature of biochar supported nZVI for the degradation of oxytetracycline. J. Taiwan Inst. Chem. Eng. 2023, 145, 104775. [Google Scholar] [CrossRef]
- Cao, B.; Qu, J.; Chu, Y.; Zhu, Y.; Jiang, Y.; Zhang, X.; Sun, M.; Jiang, Z.; Ma, S.; Zhang, Y. One-step self-assembly of Fe-biochar composite for enhanced persulfate activation to phenol degradation: Different active sites-induced radical/non-radical mechanism. Chemosphere 2023, 322, 138168. [Google Scholar] [CrossRef]
- Cai, M.; Cheng, P.; Li, J.; Wu, F.; Sarakha, M.; Mailhot, G.; Brigante, M. Toward a better understanding of peroxymonosulfate and peroxydisulfate activation using a nano zero-valent iron catalyst supported on graphitized carbon: Mechanisms and application to the degradation of estrogenic compounds in different water matrix. J. Clean. Prod. 2023, 414, 137702. [Google Scholar] [CrossRef]
- Liu, M.; Yu, T.; Huang, R.; Qi, W.; He, Z.; Su, R. Fabrication of nanohybrids assisted by protein-based materials for catalytic applications. Catal. Sci. Technol. 2020, 10, 3515–3531. [Google Scholar] [CrossRef]
- Liang, W.; Wang, G.; Peng, C.; Tan, J.; Wan, J.; Sun, P.; Li, Q.; Ji, X.; Zhang, Q.; Wu, Y. Recent advances of carbon-based nano zero valent iron for heavy metals remediation in soil and water: A critical review. J. Hazard. Mater. 2022, 426, 127993. [Google Scholar] [CrossRef]
- Li, M.; Shang, H.; Li, H.; Hong, Y.; Ling, C.; Wei, K.; Zhou, B.; Mao, C.; Ai, Z.; Zhang, L. Kirkendall effect boosts phosphorylated nZVI for efficient heavy metal wastewater treatment. Angew. Chem. Int. Ed. 2021, 60, 17115–17122. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, X.; Sun, K.; Lin, C.; Ma, J.; He, M.; Ouyang, W. Persulfate-based advanced oxidation processes (AOPs) for organic-contaminated soil remediation: A review. Chem. Eng. J. 2019, 372, 836–851. [Google Scholar] [CrossRef]
- Yuan, X.; Li, T.; He, Y.; Xue, N. Degradation of TBBPA by nZVI activated persulfate in soil systems. Chemosphere 2021, 284, 131166. [Google Scholar] [CrossRef]
- Sun, M.; Zou, L.; Wang, P.; Fan, X.; Pan, Z.; Liu, Y.; Song, C. Nano valent zero iron (nZVI) immobilized CNTs hollow fiber membrane for flow-through heterogeneous Fenton process. J. Environ. Chem. Eng. 2022, 10, 107806. [Google Scholar] [CrossRef]
- Cao, Y.; Yuan, X.; Zhao, Y.; Wang, H. In-situ soil remediation via heterogeneous iron-based catalysts activated persulfate process: A review. Chem. Eng. J. 2022, 431, 133833. [Google Scholar] [CrossRef]
- Calenciuc, C.; Fdez-Sanromán, A.; Lama, G.; Annamalai, S.; Sanromán, A.; Pazos, M. Recent developments in advanced oxidation processes for organics-polluted soil reclamation. Catalysts 2022, 12, 64. [Google Scholar] [CrossRef]
- Li, S.; Tang, J.; Yu, C.; Liu, Q.; Wang, L. Efficient degradation of anthracene in soil by carbon-coated nZVI activated persulfate. J. Hazard. Mater. 2022, 431, 128581. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Zhao, P.; Yu, R.; Jiang, J.; Liang, R.; Liu, G. Cost-efficient collagen fibrous aerogel cross-linked by Fe(III)/silver nanoparticle complexes for simultaneously degrading antibiotics, eliminating antibiotic-resistant bacteria, and adsorbing heavy metal ions from wastewater. Sep. Purif. Technol. 2022, 303, 122209. [Google Scholar] [CrossRef]
- Duan, W.; Gao, J.; Li, D.; Dai, H.; Wang, Z.; Zhang, W.; Wang, Y.; Liu, J. Unravelling the roles of Ginkgo biloba L. for modification of nanoscale zero valent iron in persulfate system to remove antibiotic resistance genes by the tool of metabonomic analysis. Chem. Eng. J. 2021, 417, 128038. [Google Scholar] [CrossRef]
- Duan, W.-J.; Gao, J.-F.; Zhang, W.-Z.; Wang, Y.-W.; Liu, J. Elimination of antibiotic resistance genes in waste activated sludge by persulfate treatment during the process of sludge dewatering. Bioresour. Technol. 2020, 311, 123509. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, Y.; Li, S.; Wei, Z.; Huang, H.; Xie, T. Remediation and optimisation of petroleum hydrocarbon degradation in contaminated water by persulfate activated with bagasse biochar-supported nanoscale zerovalent iron. Sustainability 2022, 14, 9324. [Google Scholar] [CrossRef]
- Lee, Y.; Kwon, Y.; Kim, Y.; Yu, C.; Feng, S.; Park, J.; Doh, J.; Wannemacher, R.; Koo, B.; Gierschner, J. A water-soluble organic photocatalyst discovered for highly efficient additive-free visible-light-driven grafting of polymers from proteins at ambient and aqueous environments. Adv. Mater. 2022, 34, 2108446. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Tian, J.; Ma, J.; Ni, J.; Liu, D.; Wang, W.; Shi, W.; Yuan, Y.; Cui, F.; Chen, Z. Peroxydisulfate activation by a versatile ball-milled nZVI@MoS2 composite: Performance and potential activation mechanism. Chem. Eng. J. 2023, 453, 139830. [Google Scholar] [CrossRef]
- Wen, Q.; Liu, N.; Qu, R.; Ge, F. High salinity promotes the photoaging of polystyrene microplastics with humic acid in seawater. Sci. Total Environ. 2023, 901, 165741. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Huang, J.; Xu, Z.; Ali, M.; Shan, A.; Fu, R.; Lyu, S. Mechanism of contaminants degradation in aqueous solution by persulfate in different Fe(II)-based synergistic activation environments: Taking chlorinated organic compounds and benzene series as the targets. Sep. Purif. Technol. 2021, 273, 118990. [Google Scholar] [CrossRef]
- Kong, Y.; Ji, L.; Wang, Y.; Li, J.; Lu, H.; Mo, S.; Wang, X.; Zhu, L.; Xu, X.; Zheng, X. Combined effect of nZVI and H2O2 on the cyanobacterium microcystis aeruginosa: Performance and mechanism. Nanomaterials 2022, 12, 3017. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Zeng, Y.; Jiang, T.; Chen, J.; Du, Q. Efficient removal of ciprofloxacin from contaminated water via polystyrene anion exchange resin with nanoconfined zero-valent iron. Nanomaterials 2022, 13, 116. [Google Scholar] [CrossRef]
- Masud, A.; Chavez Soria, N.G.; Aga, D.S.; Aich, N. Adsorption and advanced oxidation of diverse pharmaceuticals and personal care products (PPCPs) from water using highly efficient rGO-nZVI nanohybrids. Environ. Sci. Water Res. Technol. 2020, 6, 2223–2238. [Google Scholar] [CrossRef]
- Silwana, N.; Calderon, B.; Ntwampe, S.K.O.; Fullana, A. Heterogeneous fenton degradation of patulin in apple juice using carbon-encapsulated nano zero-valent iron (CE-nZVI). Foods 2020, 9, 674. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, L.; Yang, Y.; Sun, P. Fenton-like oxidation of antibiotic ornidazole using biochar-supported nanoscale zero-valent iron as heterogeneous hydrogen peroxide activator. Int. J. Environ. Res. Public Health 2020, 17, 1324. [Google Scholar] [CrossRef]
- Samarghandi, M.R.; Dargahi, A.; Zolghadr Nasab, H.; Ghahramani, E.; Salehi, S. Degradation of azo dye Acid Red 14 (AR14) from aqueous solution using H2O2/nZVI and S2O82–/nZVI processes in the presence of UV irradiation. Water Environ. Res. 2020, 92, 1173–1183. [Google Scholar] [CrossRef]
- Haneef, T.; Ul Mustafa, M.R.; Rasool, K.; Ho, Y.C.; Mohamed Kutty, S.R. Removal of polycyclic aromatic hydrocarbons in a heterogeneous fenton like oxidation system using nanoscale zero-valent iron as a catalyst. Water 2020, 12, 2430. [Google Scholar] [CrossRef]
- Sun, Y.M.; Feng, L.; Yang, L. Degradation of PCB67 in soil using the heterogenous Fenton process induced by montmorillonite supported nanoscale zero-valent iron. J. Hazard. Mater. 2021, 406, 124305. [Google Scholar] [CrossRef]
- Xiang, M.; Huang, M.; Li, H.; Wang, W.; Huang, Y.; Lu, Z.; Wang, C.; Si, R.; Cao, W. Nanoscale zero-valent iron/cobalt@mesoporous hydrated silica core-shell particles as a highly active heterogeneous Fenton catalyst for the degradation of tetrabromobisphenol A. Chem. Eng. J. 2021, 417, 129208. [Google Scholar] [CrossRef]
- Le, S.T.; Israpanich, A.; Phenrat, T. Using sequential H2O2 addition to sustain 1,2-dichloroethane detoxification by a nanoscale zerovalent iron-induced Fenton’s system at a natural pH. Chemosphere 2022, 305, 135376. [Google Scholar] [CrossRef]
- Bashir, A.; Pandith, A.H.; Qureashi, A.; Malik, L.A.; Gani, M.; Perez, J.M. Catalytic propensity of biochar decorated with core-shell nZVI@Fe3O4: A sustainable photo-Fenton catalysis of methylene blue dye and reduction of 4-nitrophenol. J. Environ. Chem. Eng. 2022, 10, 107401. [Google Scholar] [CrossRef]
- Puiatti, G.A.; de Carvalho, J.P.; de Matos, A.T.; Lopes, R.P. Green synthesis of Fe0 nanoparticles using Eucalyptus grandis leaf extract: Characterization and application for dye degradation by a (Photo) Fenton-like process. J. Environ. Manag. 2022, 311, 114828. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, L.; Kamani, H.; Asghari, A.; Mohammadpour, A.; Golaki, M.; Rahdar, A.; Kyzas, G.Z. Removal of amoxicillin from aqueous media by Fenton-like sonolysis/H2O2 process using zero-valent iron nanoparticles. Molecules 2022, 27, 6308. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Zeng, G.; Xu, Z.; Zhou, Z.; Zhou, Z.; Ali, M.; Sun, Y.; Sun, X.; Huang, J.; Lyu, S. Insights into the role of nanoscale zero-valent iron in Fenton oxidation and its application in naphthalene degradation from water and slurry systems. Water Environ. Res. 2022, 94, 10710. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, M.; Chen, T.; Zhang, G.; Xu, H.; Sun, H.; Zhang, L. Facile fabrication of rGO/PPy/nZVI catalytic microreactor for ultrafast removal of p-nitrophenol from water. Appl. Catal. B 2023, 324, 122270. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, X.; Zhao, X.; Ma, R.; Du, Y.; Zuo, S.; Dong, K.; Wang, R.; Zhang, Y.; Gu, Y.; et al. Heterogeneous Fenton oxidation of 2,4-dichlorophenol catalyzed by PEGylated nanoscale zero-valent iron supported by biochar. Environ. Sci. Pollut. Res. Int. 2023, 30, 41333–41347. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Tang, J.; Zhang, X.; Zhang, A. Degradation of refractory organic matter in MBR effluent from treating landfill leachate by the UV-nZVI-H2O2 system. Environ. Sci. Pollut. Res. Int. 2023, 30, 50295–50308. [Google Scholar] [CrossRef]
- Ahmed, N.; Vione, D.; Rivoira, L.; Castiglioni, M.; Beldean-Galea, M.S.; Bruzzoniti, M.C. Feasibility of a heterogeneous nanoscale zero-valent iron Fenton-like process for the removal of glyphosate from water. Molecules 2023, 28, 2214. [Google Scholar] [CrossRef] [PubMed]
- Leovac Macerak, A.; Kulic Mandic, A.; Pesic, V.; Tomasevic Pilipovic, D.; Becelic-Tomin, M.; Kerkez, D. “Gree” nZVI-biochar as fenton catalyst: Perspective of closing-the-loop in wastewater treatment. Molecules 2023, 28, 1425. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Zhang, L.; Xu, Z.; Sheng, X.; Li, D.; Chen, Y.; Habib, M.; Lyu, S. Degradation of 1,2,3-trichloropropane in aqueous solution by ZVI-enhanced Fenton system: Performances and mechanisms. J. Environ. Chem. Eng. 2023, 11, 110432. [Google Scholar] [CrossRef]
- Sosamony, K.J.; Soloman, P.A. Comparison in the performance of magnetic field-induced MBBR-coupled with advanced oxidation processes for textile effluent treatment with cost estimation. Environ. Qual. Manag. 2021, 30, 145–157. [Google Scholar]
- Zhang, C.; Li, F.; Wen, R.; Zhang, H.; Elumalai, P.; Zheng, Q.; Chen, H.; Yang, Y.; Huang, M.; Ying, G. Heterogeneous electro-Fenton using three-dimension nZVI-BC electrodes for degradation of neonicotinoid wastewater. Water Res. 2020, 182, 115975. [Google Scholar] [CrossRef]
- Silva, L.L.S.; Abdelraheem, W.; Nadagouda, M.N.; Rocco, A.M.; Dionysiou, D.D.; Fonseca, F.V.; Borges, C.P. Novel microwave-driven synthesis of hydrophilic polyvinylidene fluoride/polyacrylic acid (PVDF/PAA) membranes and decoration with nano zero-valent-iron (nZVI) for water treatment applications. J. Membr. Sci. 2021, 620, 118817. [Google Scholar] [CrossRef]
- Su, X.; Lv, H.; Gong, J.; Zhou, M. Bi/mZVI combined with citric acid and sodium citrate to mineralize multiple sulfa antibiotics: Performance and mechanism. Antibiotics 2022, 11, 51. [Google Scholar] [CrossRef]
- Mouele, E.S.M.; Tijani, J.O.; Badmus, K.O.; Pereao, O.; Babajide, O.; Fatoba, O.O.; Zhang, C.; Shao, T.; Sosnin, E.; Tarasenko, V. A critical review on ozone and co-species, generation and reaction mechanisms in plasma induced by dielectric barrier discharge technologies for wastewater remediation. J. Environ. Chem. Eng. 2021, 9, 105758. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhu, R.; Xi, Y.; Zhu, J.; He, H. Strategies for enhancing the heterogeneous fenton catalytic reactivity: A review. Appl. Catal. B 2019, 255, 117739. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, L.; Wang, J. An enhancement of singlet oxygen generation from dissolved oxygen activated by three-dimensional graphene wrapped nZVI-doped amorphous Al species for chloramphenicol removal in the Fenton-like system. Chem. Eng. J. 2021, 425, 131497. [Google Scholar] [CrossRef]
- Noradoun, C.; Engelmann, M.D.; McLaughlin, M.; Hutcheson, R.; Breen, K.; Paszczynski, A.; Cheng, I.F. Destruction of chlorinated phenols by dioxygen activation under aqueous room temperature and pressure conditions. Ind. Eng. Chem. Res. 2003, 42, 5024–5030. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, L.; Shen, H.; Wang, J. Construction of three-dimensional reduced graphene oxide wrapped nZVI doped with Al2O3 as the ternary Fenton-like catalyst: Optimization, characterization and catalytic mechanism. Sci. Total Environ. 2021, 780, 146576. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Jiang, T.; Wu, J.; Chen, J.; Du, Q. Effect of dissolved silicate on the degradation of sulfamethoxazole by nZVI@D201 nanocomposite. J. Mol. Liq. 2022, 368, 120767. [Google Scholar] [CrossRef]
- Lei, C.; Song, Y.; Meng, F.; Sun, Y.; Tsang, D.C.W.; Yang, K.; Lin, D. Iron-crosslinked alginate derived Fe/C composites for atrazine removal from water. Sci. Total Environ. 2021, 756, 143866. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Xu, P.; Chen, J.; Zhang, R.; Huang, J.; Xu, W.; Xiao, S. Immobilization of nZVI particles on cotton fibers for rapid decolorization of organic dyes. Cellulose 2021, 28, 7925–7940. [Google Scholar] [CrossRef]
- Cheng, Q.; Li, Q.; Huang, X.; Li, X.; Wang, Y.; Liu, W.; Lin, Z. The high efficient Sb(III) removal by cauliflower like amorphous nanoscale zero-valent iron (A-nZVI). J. Hazard. Mater. 2022, 436, 129056. [Google Scholar] [CrossRef]
- Chen, L.; Ni, R.; Yuan, T.; Gao, Y.; Kong, W.; Zhang, P.; Yue, Q.; Gao, B. Effects of green synthesis, magnetization, and regeneration on ciprofloxacin removal by bimetallic nZVI/Cu composites and insights of degradation mechanism. J. Hazard. Mater. 2020, 382, 121008. [Google Scholar] [CrossRef]
- Tran, M.L.; Nguyen, C.H.; Tran, T.T.V.; Juang, R.-S. One-pot synthesis of bimetallic Pt/nZVI nanocomposites for enhanced removal of oxytetracycline: Roles of morphology changes and Pt catalysis. J. Taiwan Inst. Chem. Eng. 2020, 111, 130–140. [Google Scholar] [CrossRef]
- Nguyen, C.H.; Tran, M.L.; Van Tran, T.T.; Juang, R.-S. Efficient removal of antibiotic oxytetracycline from water by Fenton-like reactions using reduced graphene oxide-supported bimetallic Pd/nZVI nanocomposites. J. Taiwan Inst. Chem. Eng. 2021, 119, 80–89. [Google Scholar] [CrossRef]
- Li, S.; Tang, J.; Wang, L.; Liu, X. Carbon coating enhances single-electron oxygen reduction reaction on nZVI surface for oxidative degradation of nitrobenzene. Sci. Total Environ. 2021, 770, 144680. [Google Scholar] [CrossRef]
- Yang, Y.; Shen, H.; Xu, L. Three-dimensional graphene anchored nZVI hybrid MnO2 as a dissolved oxygen activated Fenton-like catalyst for efficient mineralization of oxytetracycline. Chem. Eng. J. 2023, 464, 142781. [Google Scholar] [CrossRef]
- Xue, C.; Peng, Y.; Chen, A.; Peng, L.; Luo, S. Drastically inhibited nZVI-Fenton oxidation of organic pollutants by cysteine: Multiple roles in the nZVI/O2/hv system. J. Colloid Interface Sci. 2021, 582, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Fu, Y.; Wang, Z.; Zhou, G.; Zhou, R.; Liu, Y. Removal of diclofenac in water using peracetic acid activated by zero valent copper. Sep. Purif. Technol. 2021, 276, 119319. [Google Scholar] [CrossRef]
- Yang, L.-W.; She, L.-H.; Xie, Z.-H.; He, Y.-L.; Tian, X.-Y.; Zhao, C.-L.; Guo, Y.-Q.; Hai, C.; He, C.-S.; Lai, B. Boosting activation of peracetic acid by Co@mZVI for efficient degradation of sulfamethoxazole: Interesting two-phase generation of reactive oxidized species. Chem. Eng. J. 2022, 448, 137667. [Google Scholar] [CrossRef]
- Wang, L.; Yan, T.; Tang, R.; Ping, Q.; Li, Y.; Wang, J. Motivation of reactive oxidation species in peracetic acid by adding nanoscale zero-valent iron to synergic removal of spiramycin under ultraviolet irradiation: Mechanism and N-nitrosodimethylamine formation potential assessment. Water Res. 2021, 205, 117684. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, X.; Zhao, X.; Jing, G.; Zhou, Z. Activation of peracetic acid with zero-valent iron for tetracycline abatement: The role of Fe(II) complexation with tetracycline. J. Hazard. Mater. 2022, 424, 127653. [Google Scholar] [CrossRef] [PubMed]
- Zong, Y.; Zhang, H.; Shao, Y.; Ji, W.; Zeng, Y.; Xu, L.; Wu, D. Surface-mediated periodate activation by nano zero-valent iron for the enhanced abatement of organic contaminants. J. Hazard. Mater. 2022, 423, 126991. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Lv, L.; Pillai, S.C.; Wang, H.; Xue, J.; Ma, Y.; Liu, Y.; Chen, Y.; Wu, L.; Zhang, Z.; et al. Efficient degradation of diclofenac sodium by periodate activation using Fe/Cu bimetallic modified sewage sludge biochar/UV system. Sci. Total Environ. 2021, 783, 146974. [Google Scholar] [CrossRef]
- Che, M.; Xiao, J.; Shan, C.; Chen, S.; Huang, R.; Zhou, Y.; Cui, M.; Qi, W.; Su, R. Efficient removal of chloroform from groundwater using activated percarbonate by cellulose nanofiber-supported Fe/Cu nanocomposites. Water Res. 2023, 243, 120420. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Liu, X.; Huang, Y.; Kang, W.; Wang, Z.; Zheng, H. Roles of hydroxyl and carbonate radicals in bisphenol a degradation via a nanoscale zero-valent iron/percarbonate system: Influencing factors and mechanisms. RSC Adv. 2021, 11, 3636–3644. [Google Scholar] [CrossRef]
- Rashid, T.; Iqbal, D.; Hazafa, A.; Hussain, S.; Sher, F.; Sher, F. Formulation of zeolite supported nano-metallic catalyst and applications in textile effluent treatment. J. Environ. Chem. Eng. 2020, 8, 104023. [Google Scholar] [CrossRef]
- Wang, H.; Xiao, W.; Zhang, C.; Sun, Y.; Wang, Y.-n.; Gong, Z.; Zhan, M.; Fu, Y.; Liu, K. Effective removal of refractory organic contaminants from reverse osmosis concentrated leachate using PFS-nZVI/PMS/O3 process. Waste Manag. 2021, 128, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Guerra-Rodríguez, S.; Rodríguez, E.; Rodríguez-Chueca, J. Pilot-scale regeneration of wastewater through intensified sulfate radical-based advanced oxidation processes (PMS/UV-A, PMS/H2O2/UV-A, and PMS/O3): Inactivation of bacteria and mechanistic considerations. Chem. Eng. J. 2023, 469, 143859. [Google Scholar] [CrossRef]
- Jiang, W.; Dionysiou, D.D.; Kong, M.; Liu, Z.; Sui, Q.; Lyu, S. Utilization of formic acid in nanoscale zero valent iron-catalyzed Fenton system for carbon tetrachloride degradation. Chem. Eng. J. 2020, 380, 122537. [Google Scholar] [CrossRef]
- Yu, Z.; Li, X.; Guo, J. Combat antimicrobial resistance emergence and biofilm formation through nanoscale zero-valent iron particles. Chem. Eng. J. 2022, 444, 136569. [Google Scholar] [CrossRef]
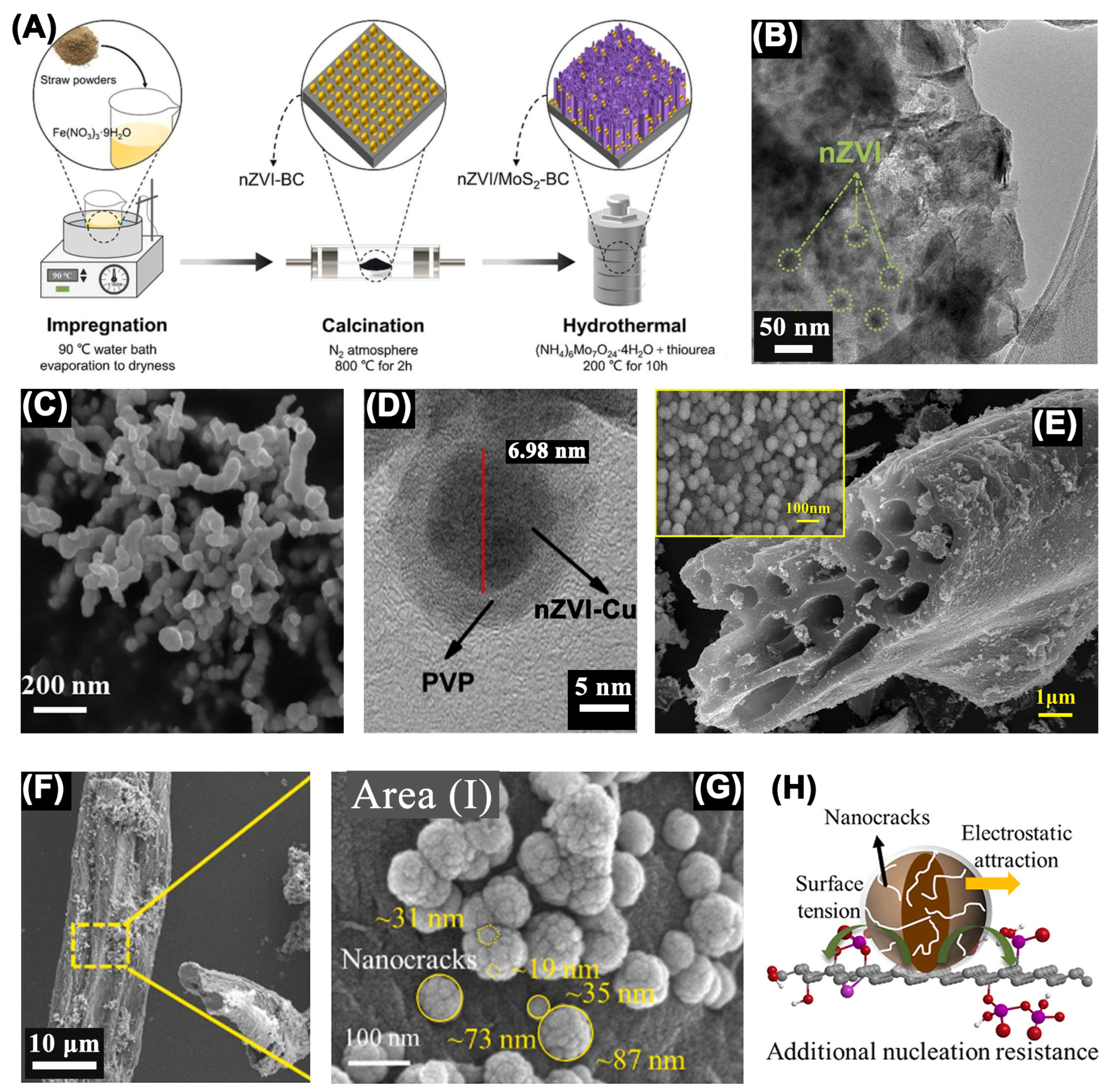
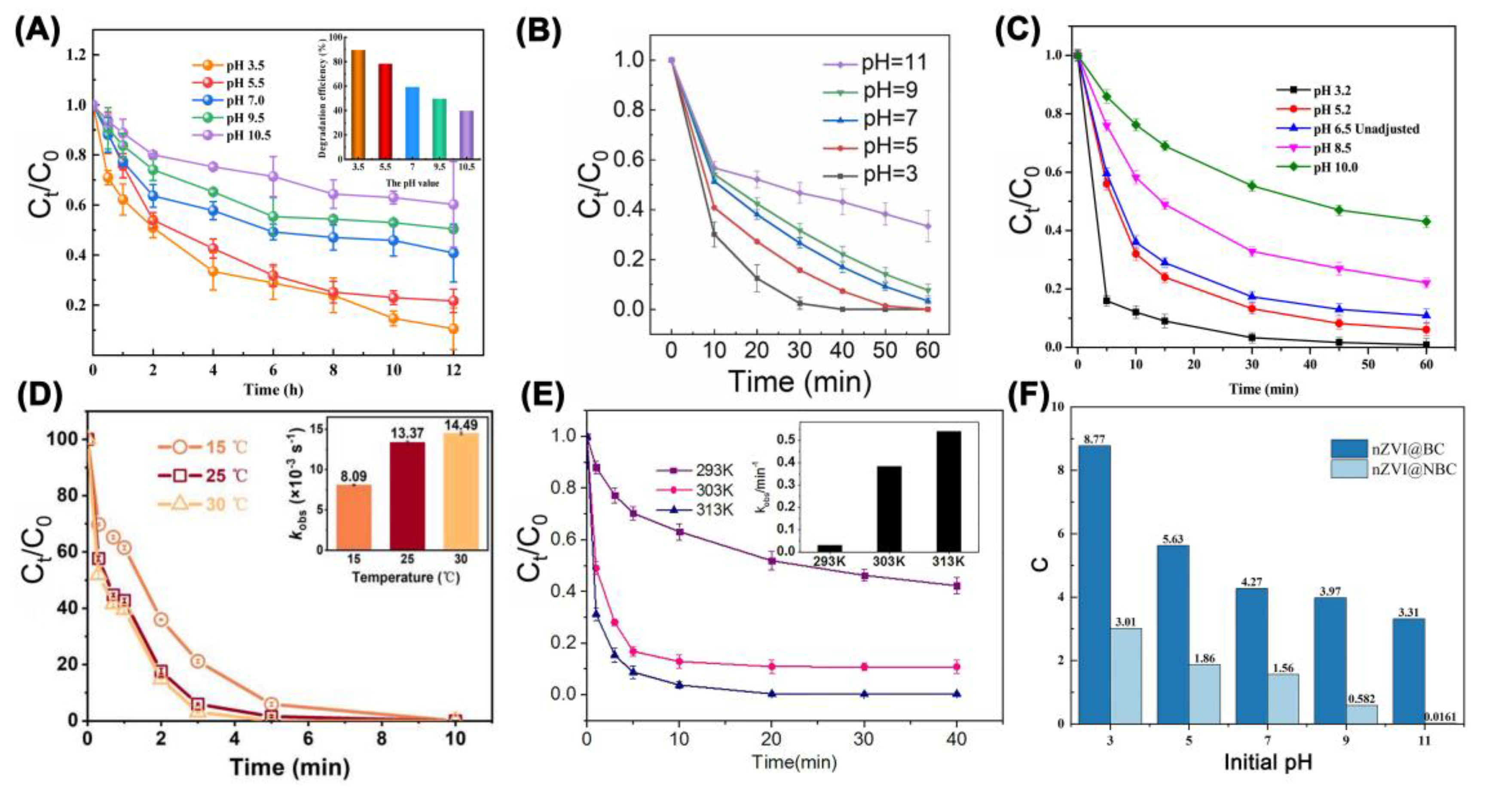
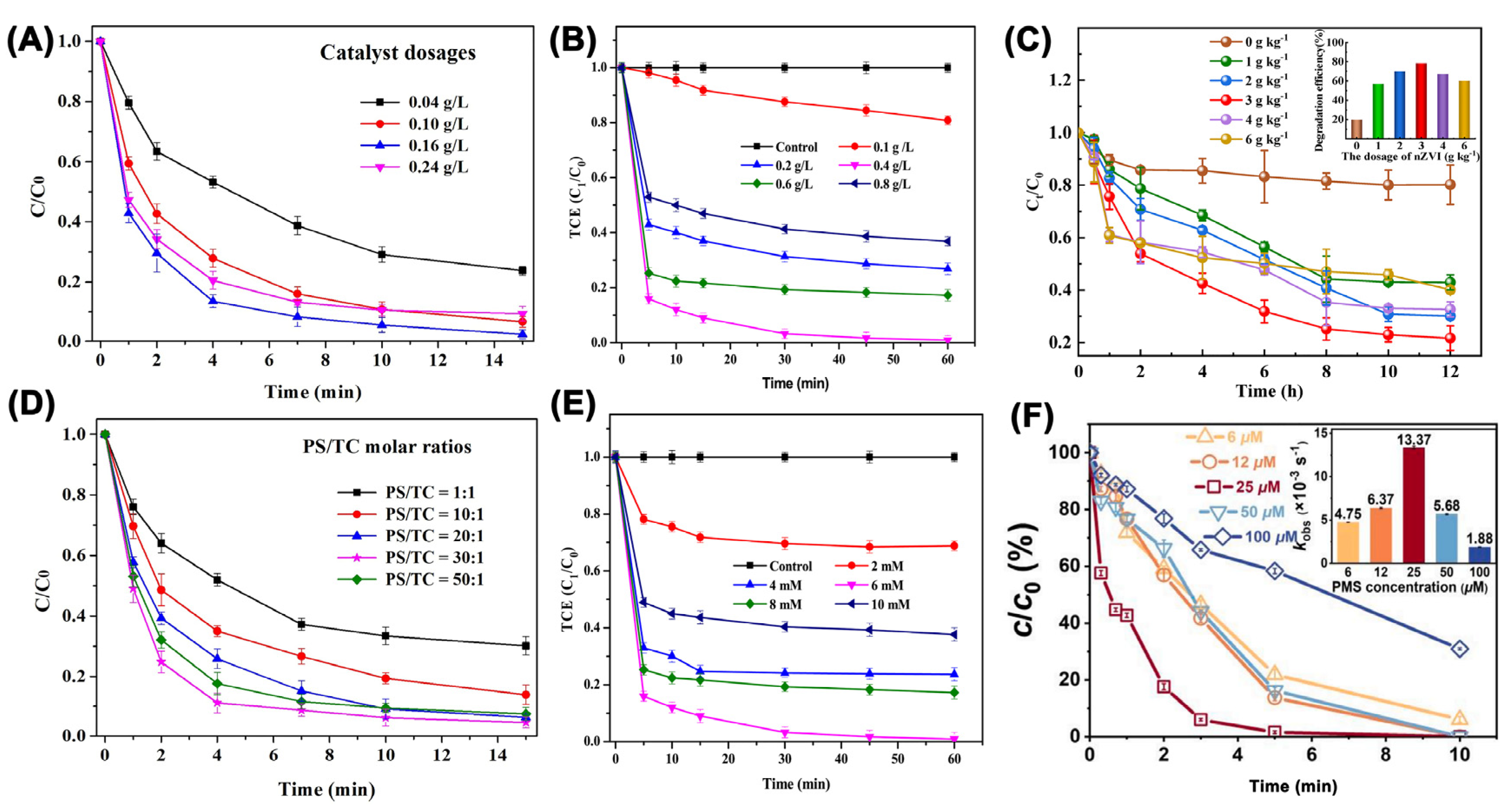
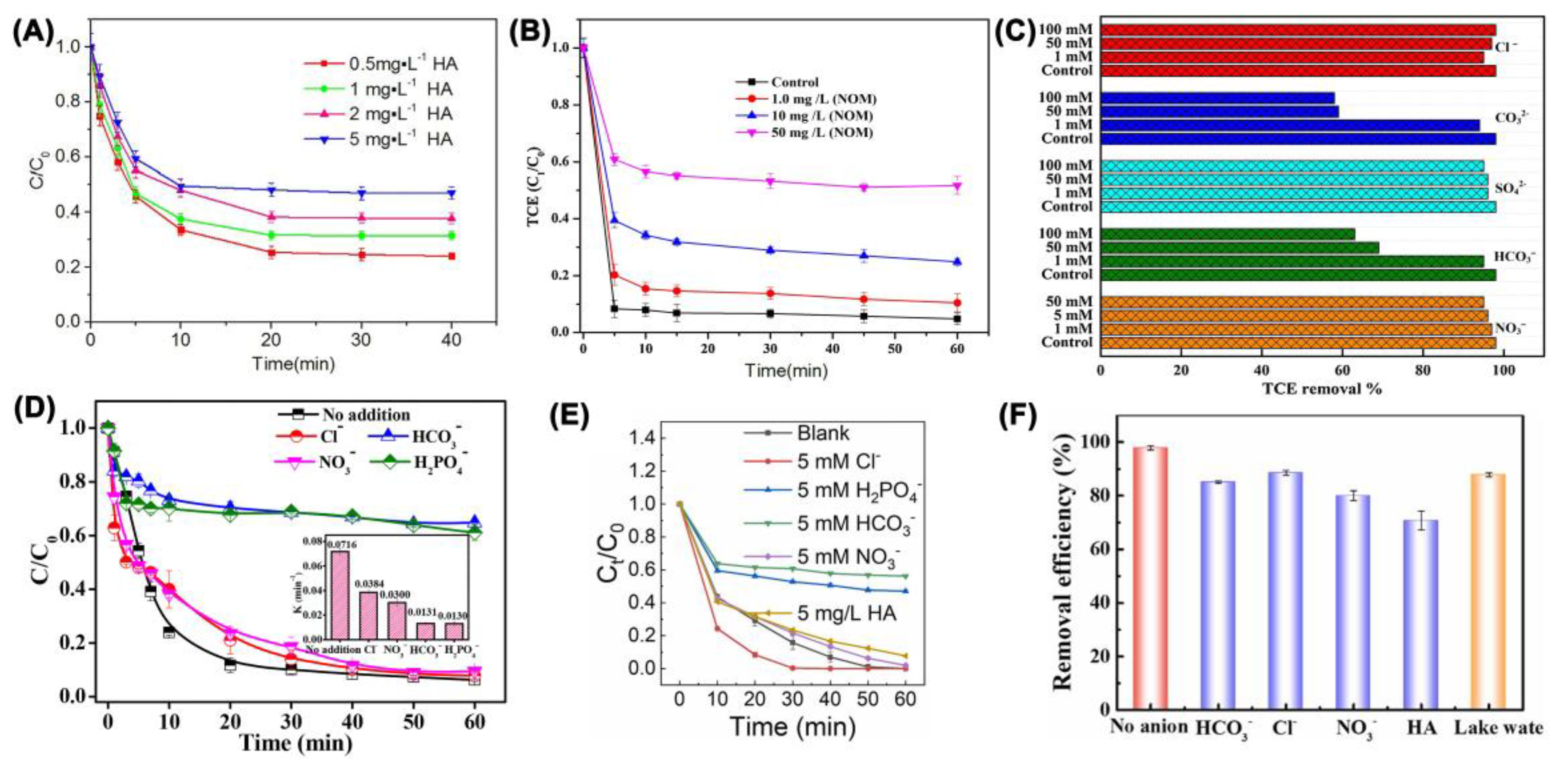
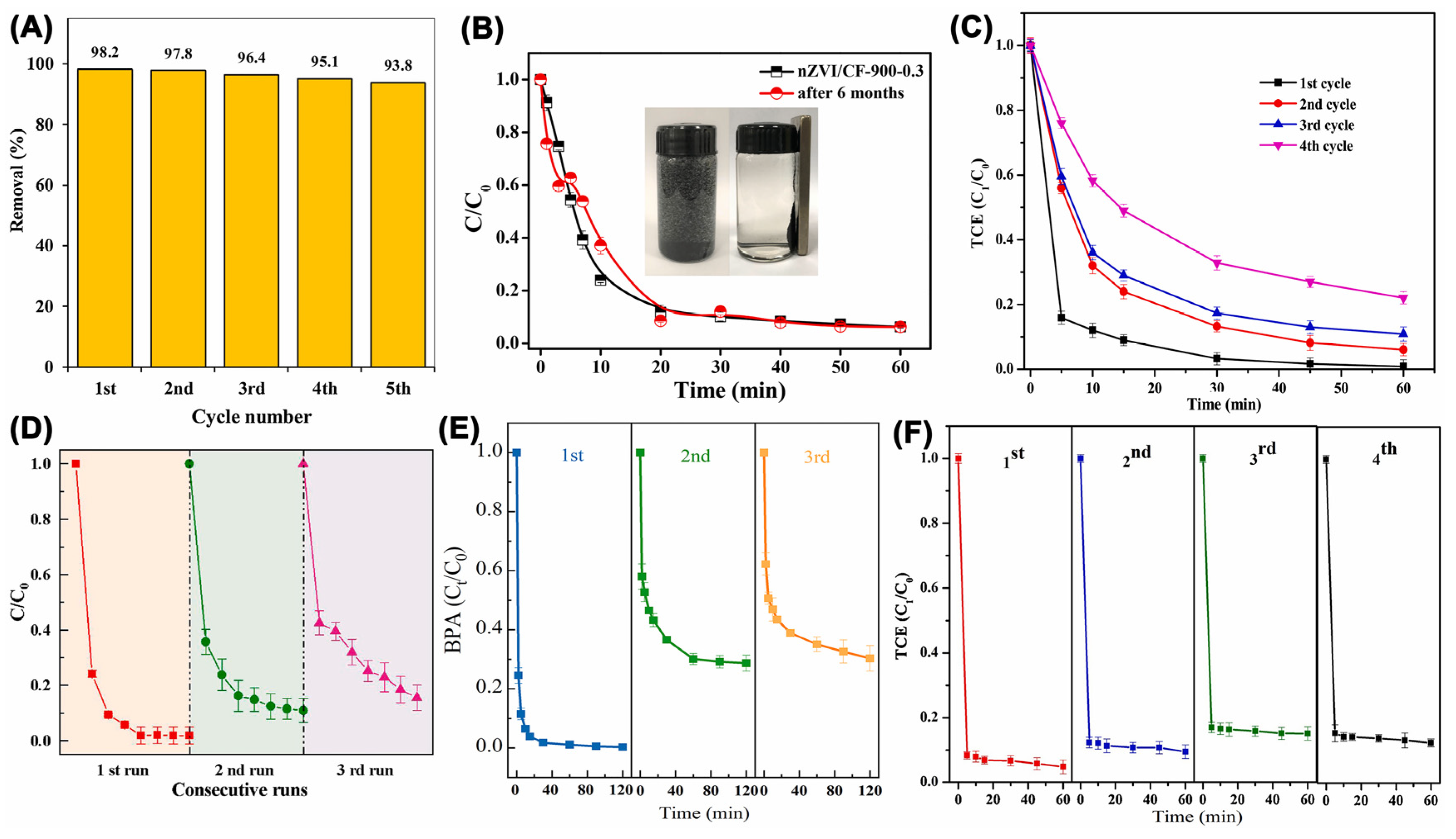





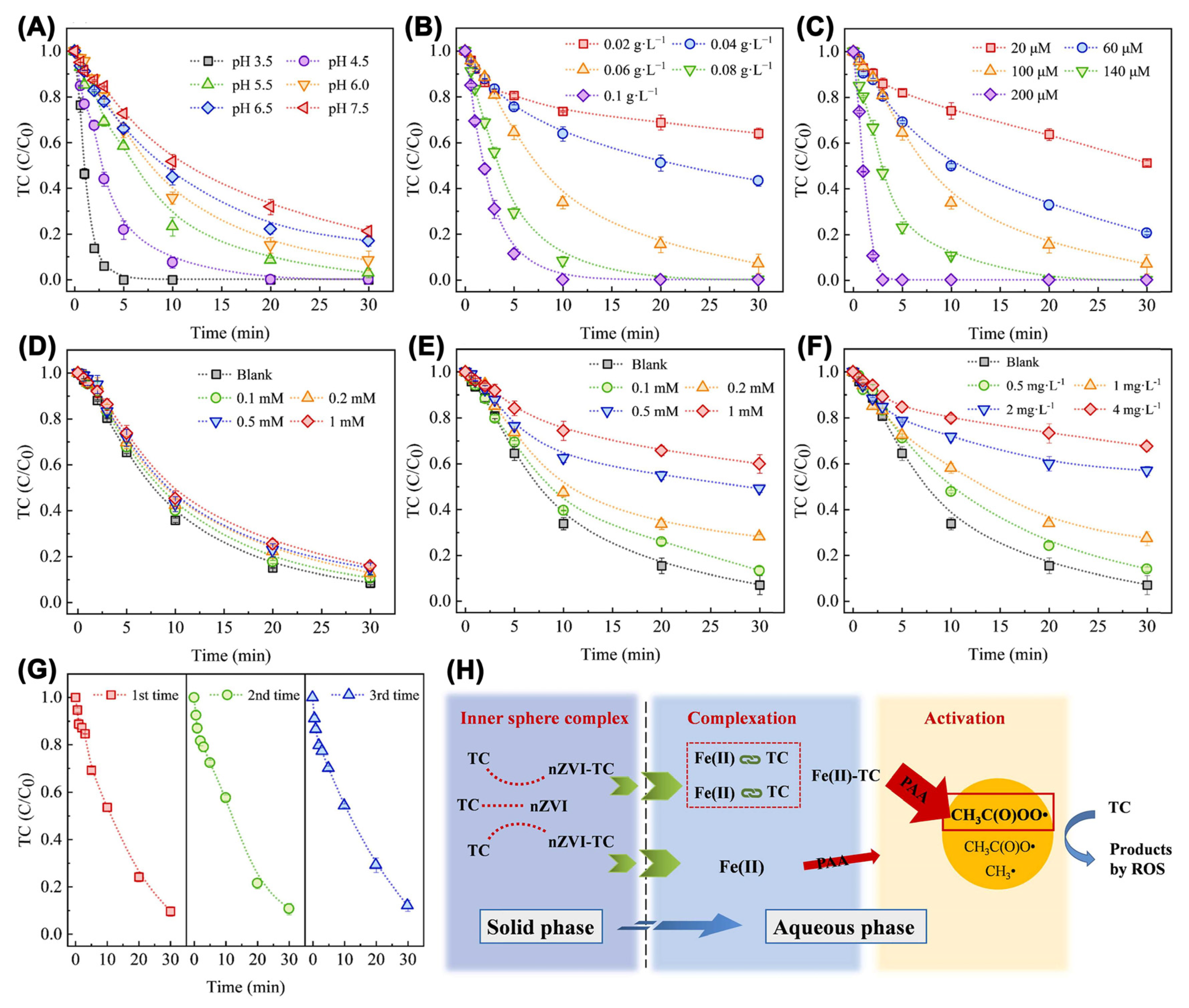
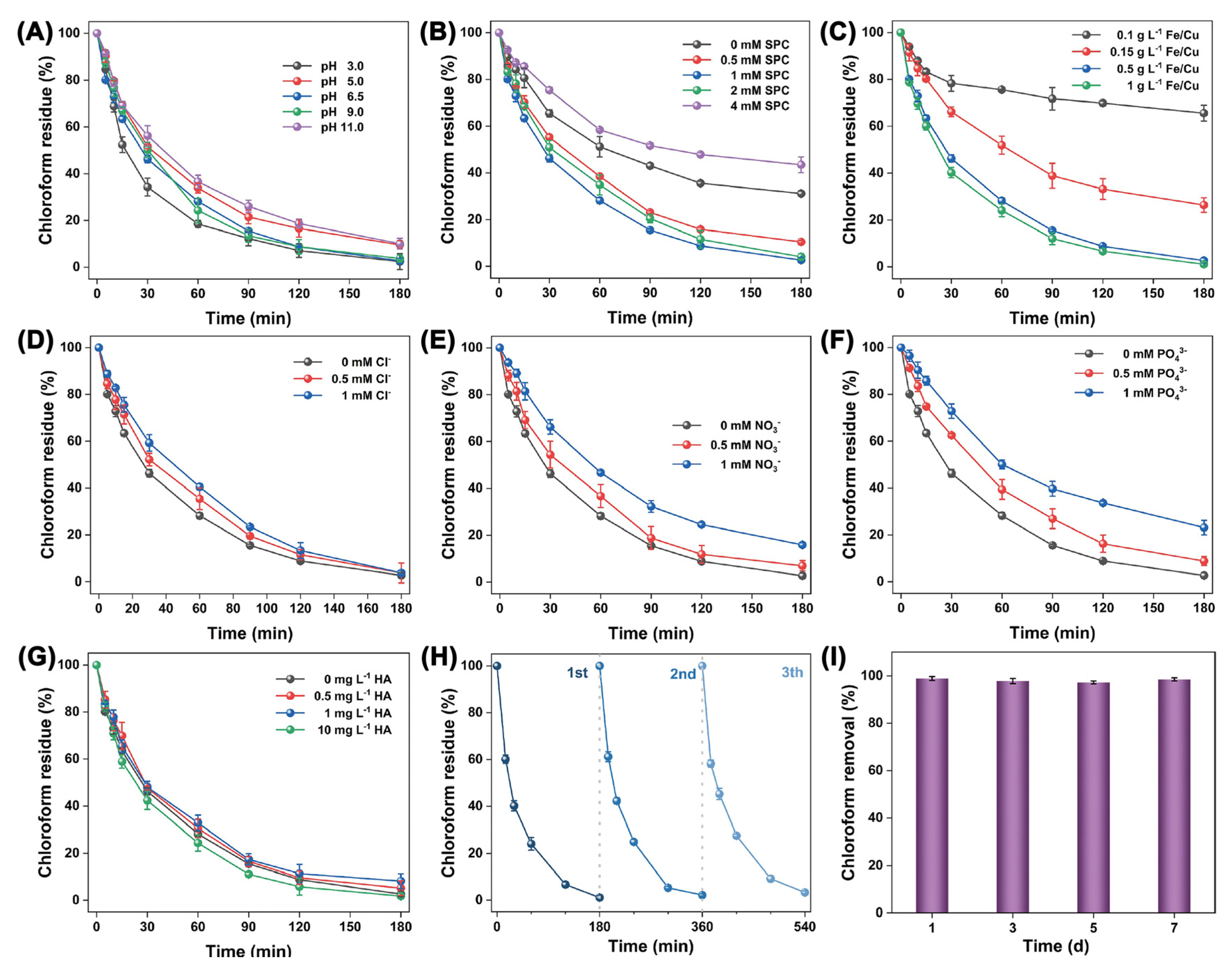
| Catalyst | Oxidant (Concentration, mM) | Pollutant (Concentration, mM) | Operation Conditions (°C/Mole Ratio/pH) | Dominant Radicals | Removal Efficiency | Catalyst Reusability | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BC-nZVI | -- | -- | Petroleum | -- | 25 | 1:1 | -- | •SO4− | 50% (40 h) | -- | [56] |
| Fe0@C | Na2S2O8 | 1.0 | 4-CP | 0.15 | 25 | 1.75:1 | 5.7 | •OH > •SO4− | 82% (15 min) | -- | [57] |
| nZVI-rGO | Na2S2O8 | 6.5 | Nonylphenol | 0.09 | 30 | 1.26:1 | 4.2 | •SO4− | 98.2% (50 min) | 5 | [58] |
| nZVI-rGO | Na2S2O8 | 4.28 | Furfural | 2.6 | 70 | 3.97:1 | 5.5 | •SO4− > •OH | 97.8% (50 min) | 5 | [59] |
| nZVI/Co3O4 | K2S2O8 | 0.90 | TC | 0.045 | 25 | 3.98:1 | 3.0 | •SO4− | 97.6% (15 min) | 4 | [60] |
| nZVI | -- | 2.0 | TPHP | 0.006 | 30 | 1:4 | 3.0 | •SO4− | 98.2% (60 min) | -- | [61] |
| BC@nZVI/Ni | Na2S2O8 | 0.6 | Norfloxacin | 0.031 | 30 | 0.82:1 | 3.0 | •SO4− | 99% (30 min) | -- | [62] |
| AC-nZVI | -- | 1.0 | Ampicillin | 0.05 | 60 | 0.25:1 | -- | •OH > •SO4− | ~99% (60 min) | 5 | [63] |
| nZVI | Na2S2O8 | 1.0 | SMZ | 0.19 | 25 | 2:1 | 6.8 | •SO4− and •OH | 96% (30 min) | -- | [64] |
| nZVI | Na2S2O8 | 1.0 | SMX | 0.0395 | 25 | 1.79:1 | 5.53 | •SO4− and •OH | 88.4% (2 h) | -- | [65] |
| nZVI | Na2S2O8 | 1.9 | Petroleum | -- | 25 | 0.28:1 | 4.2 | •SO4− > •OH | 61.2% (2 h) | 5 | [66] |
| TiO2@nZVI | K2S2O8 | 0.5 | Amoxicillin | 0.055 | -- | -- | 5.0 | •SO4− and •OH | 99% (60 min) | 4 | [67] |
| nZVI@CS | -- | 1.0 | Atrazine | 0.0464 | 25 | 1.125:1 | 7.0 | •SO4− > •OH | 96.65% (60 min) | 4 | [68] |
| nZVI/CF | KHSO5 | 1.0 | Levofloxacin | 0.05 | 25 | 2.86:1 | 7.0 | •SO4− > •OH | 93.83% (60 min) | -- | [69] |
| nZVI@BC | Na2S2O8 | 1.0 | Atrazine | 0.046 | 25 | 0.2:1 | 5.0 | •SO4− | 93.8% (30 min) | 4 | [70] |
| PVP-nZVI-Cu | Na2S2O8 | 6.0 | TCE | 0.15 | 25 | 0.25:1 | 3.2 | •SO4− and •OH | 99.6% (60 min) | 4 | [71] |
| nZVI-Ni@BC | Na2S2O8 | 4.0 | TCE | 0.15 | -- | 0.18:1 | ~3.49 | •SO4− and •OH | ~99.0% (60 min) | 4 | [72] |
| nZVI/CuO@BC | K2S2O8 | 0.2 | TBBPA | 0.0184 | 25 | 2.2:1 | 8.0 | •SO4− and •OH | 98.46% (45 min) | -- | [73] |
| CS-PHB-nZVI | K2S2O8 | 2.0 | MO | 0.15 | 25 | 0.29:1 | 7.0 | •SO4− and •OH | ~100% (30 min) | 4 | [74] |
| C-nZVI | Na2S2O8 | 1.0 | SMZ | 0.072 | 20 | 1:1 | 8.3 | •SO4− and •OH | 45.3% (120min) | -- | [75] |
| PDA/ATP-nZVI | Na2S2O8 | 2.0 | 8-HQ | 0.172 | 25 | 2.2:1 | 3.0 | •SO4− and •OH | 96.6% (35min) | -- | [76] |
| nZVI@NBC | Na2S2O8 | 1.0 | BPA | 0.1 | 25 | 0.55:1 | 7.0 | SO4−, •OH, 1O2 | 95% (120 min) | 3 | [77] |
| nZVI/Mn | -- | 1.0 | SMZ | 0.018 | 30 | 1.25:1 | 3.0 | •OH | 95% (60 min) | -- | [78] |
| nZVI@gBC | Na2S2O8 | 2.0 | 2,4-DCP | 0.12 | 26 | 0.53:1 | 6.54 | •SO4− | ~100% (20 min) | 3 | [79] |
| rGOA-nZVI | -- | 4.0 | OPPs | 0.038 | 25 | -- | 5.0 | •SO4− > •OH | 99.5% (5 min) | -- | [80] |
| Fe@MC | Na2S2O8 | 1.0 | TBBPA | 0.0184 | -- | 1.79:1 | 7.0 | •SO4− and •OH | 94.9% (30 min) | 4 | [15] |
| MoS2/nZVI | K2S2O8 | 1.2 | SMX | 0.02 | -- | 1.49:1 | 4.0 | •SO4− and •OH | 98.6% (30 min) | 5 | [81] |
| nZVI-BC | Na2S2O8 | 6.0 | Pyrene | 0.049 | 25 | 1.79:1 | 3.0 | •SO4− and •OH | 99.4% (60 min) | -- | [82] |
| nZVI | KHSO5 | 0.9 | TCE | 0.15 | 20 | 2.38:1 | 5.0 | •SO4− > •OH | 97.8% (15 min) | -- | [83] |
| nZVI/MoS2-BC | KHSO5 | 0.325 | RhB | 0.021 | -- | -- | 6.0 | •SO4− > •OH | 90.88% (60 min) | 5 | [84] |
| nZVI@P-BC | Na2S2O8 | 4.0 | γ-HCH | 0.0344 | 25 | 2.8:1 | -- | •OH and 1O2 | 92.6% (10 min) | 5 | [85] |
| nZVI-BC | Na2S2O8 | 0.5 | OTC | 1.3 | 25 | 24:1 | -- | •SO4−, •OH, 1O2 | 98.34% (5 h) | -- | [86] |
| nZVI-BC | -- | 3.0 | Phenol | 0.53 | 25 | 0.95:1 | 7.0 | •O2− and 1O2 | 100% (60 min) | 3 | [87] |
| GC-nZVI | KHSO5 | 0.25 | BPA | 0.025 | 25 | 0.25:1 | 7.0 | •SO4− > •OH | 100% (60 min) | 3 | [88] |
| nZVI-Cu0 | KHSO5 | 0.025 | Atrazine | 0.0021 | 25 | 36:1 | 4.8 | •SO4− > •OH | 100% (10 min) | 5 | [40] |
| Catalyst | Oxidant (Concentration, mM) | Pollutant (Concentration, mM) | Operation Conditions (°C/Mole Ratio/pH) | Dominant Radicals | Removal Efficiency | Catalyst Reusability | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| rGO–nZVI | H2O2 | 10 | Venlafaxine | 0.0007 | -- | 0.73:1 | 3.0 | •OH | 93.2% (30 min) | -- | [108] |
| Citalopram | 0.0006 | 98.51% (30 min) | |||||||||
| Paroxetine | 0.0005 | 99.34% (30 min) | |||||||||
| Naproxen | 0.0009 | 99.56% (30 min) | |||||||||
| Lamotrigine | 0.0008 | 97.9% (30 min) | |||||||||
| CE-nZVI | H2O2 | 0.5 | Patulin | 0.0026 | -- | 1.79:1 | 3.5 | •OH | 99.1% (1 min) | -- | [109] |
| 4.5 | 98.0% (240 min) | ||||||||||
| 5.0 | 87% (240 min) | ||||||||||
| 6.0 | 27.8% (240 min) | ||||||||||
| nZVI-BC | H2O2 | 12 | Ornidazole | 0.455 | 25 | 0.15:1 | 3.0 | •OH | 80.1% (12 min) | 3 | [110] |
| nZVI | H2O2 | 10 | Acid red 14 | 0.199 | 22 | 0.09:1 | 3.0 | •OH | 89.3% (90 min) | -- | [111] |
| nZVI | H2O2 | 47 | PAHs | -- | 25 | 1.65:1 | 2.9 | •OH | 89.3% (200 min) | -- | [112] |
| MMT-nZVI | H2O2 | 676 | PCB67 | 0.0028 | 28 | -- | 3.5 | •OH | 76.38% (80 min) | -- | [113] |
| nZVI/Co@mHS | H2O2 | 20 | TBBPA | 0.0184 | 30 | 0.11:1 | 5.0 | •OH | 97.13% (6 h) | 6 | [114] |
| nZVI | H2O2 | 25 | 1,2-DCA | 20.21 | -- | 7.2:1 | 7.0 | •OH | 99% (16 h) | 3 | [115] |
| nZVI-FBC | H2O2 | 7 | MB | 0.0625 | 25 | 0.04:1 | 4.5 | •OH | 99% (40 min) | 5 | [116] |
| EG-nZVI | H2O2 | 1.5 | Direct red 80 | 0.0728 | 25 | 0.01:1 | 5.5 | •OH | 90% (180 min) | -- | [117] |
| 3.5 | 0.05:1 | 5.5 | ~95% (180 min) | ||||||||
| nZVI | H2O2 | 888 | Amoxicillin | 0.274 | -- | 0.01:1 | 3.0 | •OH | 99.7% (120 min) | -- | [118] |
| Fe(II)/nZVI | H2O2 | 1.0 | Naphthalene | 0.1 | 20 | 0.5:1 | 5.52 | •OH | 99% (120 min) | -- | [119] |
| rGO/PPy/nZVI | H2O2 | 6.52 | p-NP | 0.072 | -- | -- | 3.0 | •OH | 99.6% (50 s) | -- | [120] |
| PEG-nZVI@BC | H2O2 | 4.0 | 2,4-DCP | 0.307 | 25 | 2.4:1 | 3.0 | •OH, •O2−, 1O2 | 92.94% (30 min) | 5 | [121] |
| nZVI | H2O2 | 30 | Refractory organic matter | -- | -- | 0.3:1 | 3.0 | •OH | 85.79% (60 min) | -- | [122] |
| nZVI | H2O2 | 0.3 | Glyphosate | 0.012 | 20 | 0.6:1 | 3.0 | •OH | ~100% (30 min) | -- | [123] |
| nZVI-BC | H2O2 | 10 | Reactive blue 4 | 0.0785 | 20 | 0.09:1 | 3.2 | •OH | 99.56% (30 min) | -- | [124] |
| Fe(II)/nZVI | H2O2 | 3.0 | TCP | 0.15 | 20 | 0.75:1 | 5.71 | •OH | 95.4% (120 min) | -- | [125] |
| Catalyst | Oxidant | Pollutant (Concentration, mM) | Operation Conditions (°C/CFe/pH) | Dominant Radicals | Removal Efficiency | Catalyst Reusability | Ref. | |||
|---|---|---|---|---|---|---|---|---|---|---|
| GT-nZVI/Cu | DO | Ciprofloxacin | 0.151 | 20 | 8.3 | 6.0 | •OH | 86.9% (90 min) | 4 | [139] |
| 30 | 92.9% (90 min) | |||||||||
| 40 | ~100% (90 min) | |||||||||
| Pt/nZVI | DO | OTC | 0.217 | 25 | 8.9 | 5.0 | •OH | ~100% (20 min) | 5 | [140] |
| 3D-GN@Fe/Al | DO | Chloramphenicol | 0.062 | 25 | 7.6 | 8.2 | 1O2, •O2− | 90% (120 min) | -- | [132] |
| 12.6 | 100% (120 min) | |||||||||
| nZVI@CF | DO | MB | 0.188 | 25 | ~9.5 | 6.68 | •OH | 96.8% (5 min) | -- | [137] |
| Brilliant green | 0.124 | 96.4% (5 min) | ||||||||
| Pd/nZVI/rGO | DO | OTC | 0.217 | 25 | 1.7 | 5.0 | •OH | 96.5% (60 min) | 5 | [141] |
| nZVI@C | DO | Nitrobenzene | 0.325 | 25 | 4.6 | 7.05 | •OH, •O2− | 91% (120 min) | 4 | [142] |
| 3D-rGO@nZVI/Al2O3 | DO | Chloramphenicol | 0.047 | 25 | 2.6 | 3.2 | 1O2, •O2− | 100% (min) | -- | [134] |
| 5.0 | 99% (min) | |||||||||
| 7.5 | 99% (min) | |||||||||
| 10.1 | 85% (min) | |||||||||
| nZVI/C | DO | Atrazine | 0.046 | 25 | 1.1 | 3−9 | •OH | 93.5% (24 h) | 4 | [136] |
| A-nZVI | DO | Sb(III) | 0.821 | 25 | 8.9 | 5.0 | •OH | 99% (30 min) | -- | [138] |
| nZVI@D201 | DO | SMX | 0.14 | 25 | ~7.6 | 5.0 | •OH | ~98% (48 h) | -- | [135] |
| nZVI/PA | DO | Ciprofloxacin | 0.151 | 25 | 7.6 | 5.0 | •OH, •O2− | 98.5% (360 min) | 5 | [107] |
| 3D-rGO@nZVI/MnO2 | DO | OTC | 0.109 | 25 | -- | 3.0-6.5 | •OH, 1O2 | 100% (120 min) | 3 | [143] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, M.; Ye, Y.; Xu, L.; Gao, T.; Zhong, A.; Song, Z. Recent Advances in Nanoscale Zero-Valent Iron (nZVI)-Based Advanced Oxidation Processes (AOPs): Applications, Mechanisms, and Future Prospects. Nanomaterials 2023, 13, 2830. https://doi.org/10.3390/nano13212830
Liu M, Ye Y, Xu L, Gao T, Zhong A, Song Z. Recent Advances in Nanoscale Zero-Valent Iron (nZVI)-Based Advanced Oxidation Processes (AOPs): Applications, Mechanisms, and Future Prospects. Nanomaterials. 2023; 13(21):2830. https://doi.org/10.3390/nano13212830
Chicago/Turabian StyleLiu, Mingyue, Yuyuan Ye, Linli Xu, Ting Gao, Aiguo Zhong, and Zhenjun Song. 2023. "Recent Advances in Nanoscale Zero-Valent Iron (nZVI)-Based Advanced Oxidation Processes (AOPs): Applications, Mechanisms, and Future Prospects" Nanomaterials 13, no. 21: 2830. https://doi.org/10.3390/nano13212830
APA StyleLiu, M., Ye, Y., Xu, L., Gao, T., Zhong, A., & Song, Z. (2023). Recent Advances in Nanoscale Zero-Valent Iron (nZVI)-Based Advanced Oxidation Processes (AOPs): Applications, Mechanisms, and Future Prospects. Nanomaterials, 13(21), 2830. https://doi.org/10.3390/nano13212830






