Peat-Derived ZnCl2-Activated Ultramicroporous Carbon Materials for Hydrogen Adsorption
Abstract
:1. Introduction
2. Materials and Methods
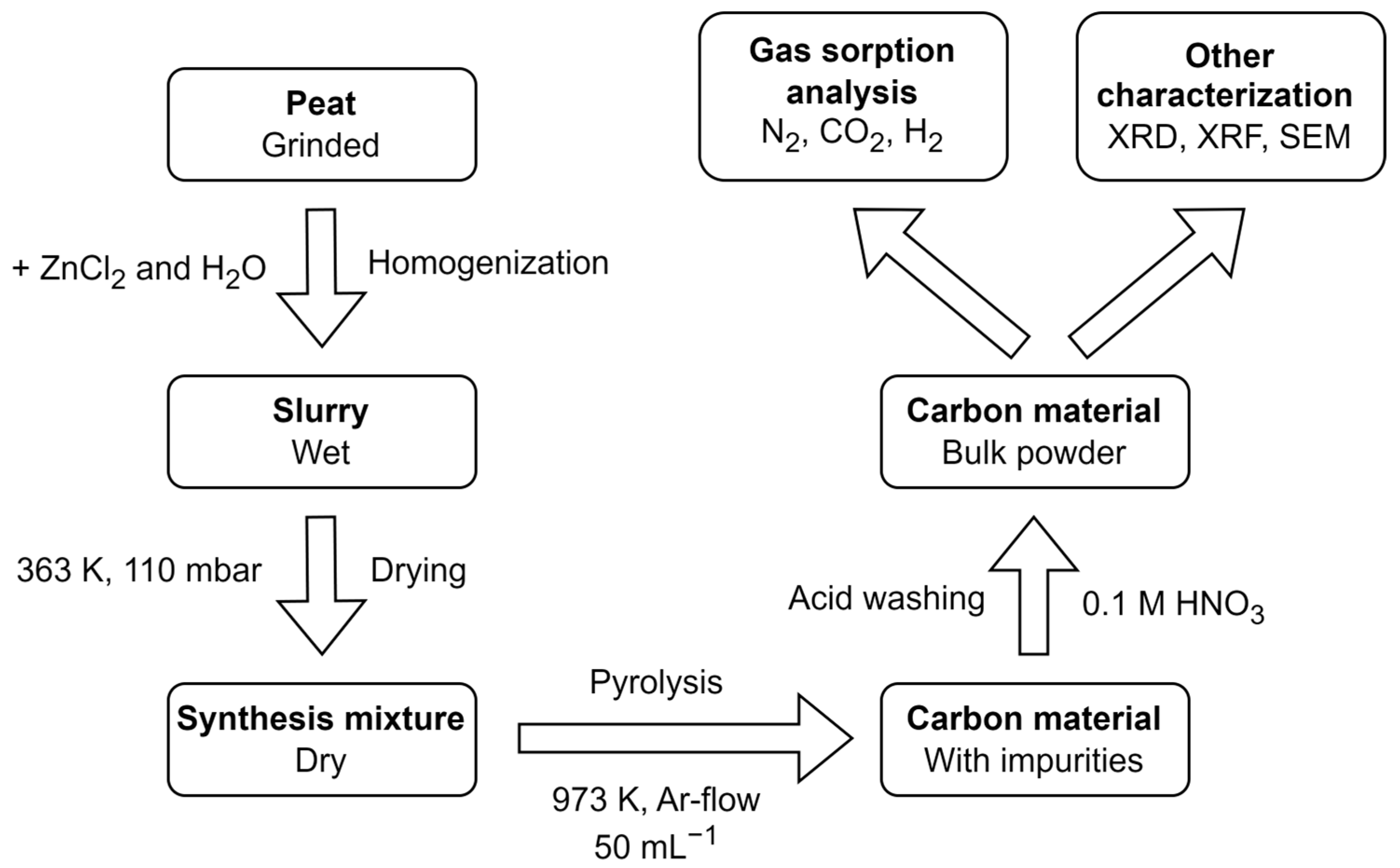
2.1. Synthesis
2.2. Physical Characterisation
2.3. Gas Adsorption and Porosity Characterisaion
3. Results
3.1. Physical Characterization
3.2. Specific Surface Area and Pore Size Distributions
3.3. Hydrogen Adsorption
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
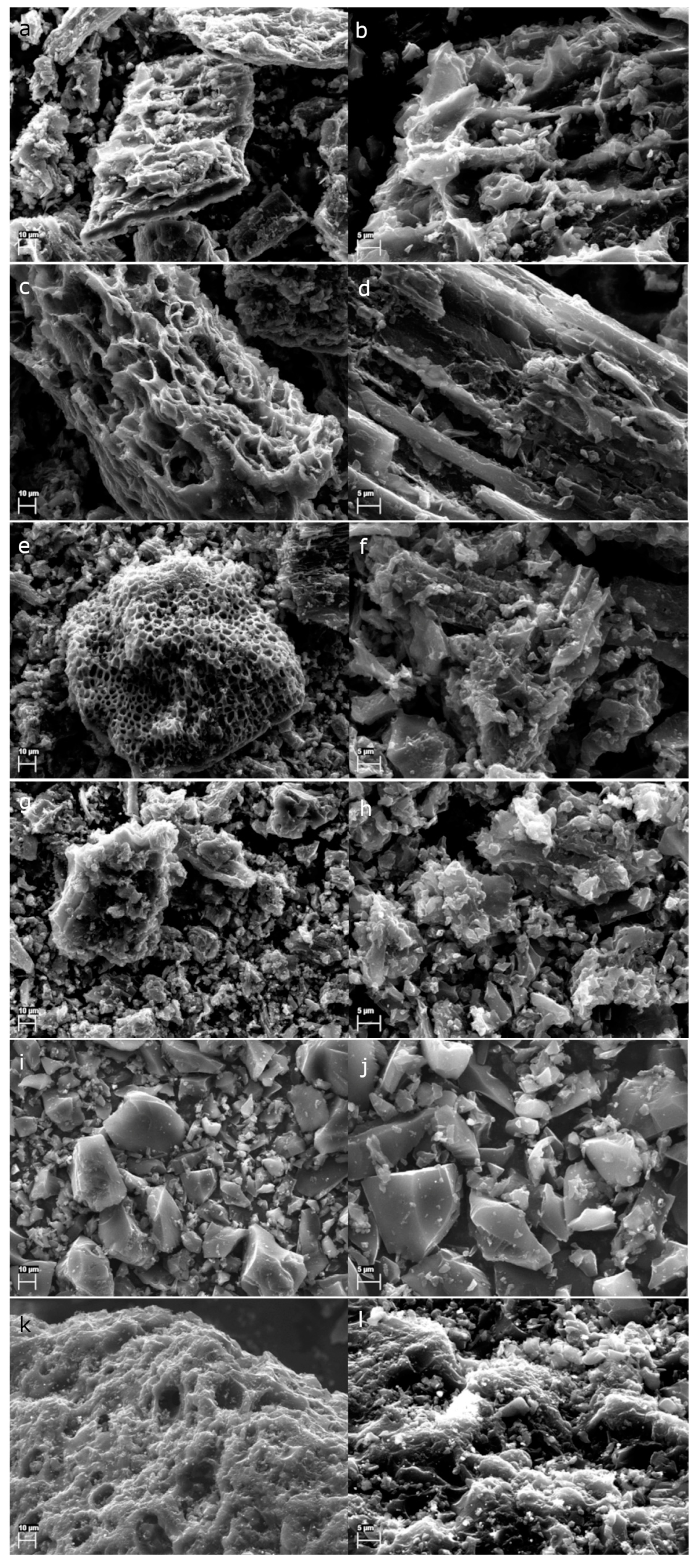
Appendix B
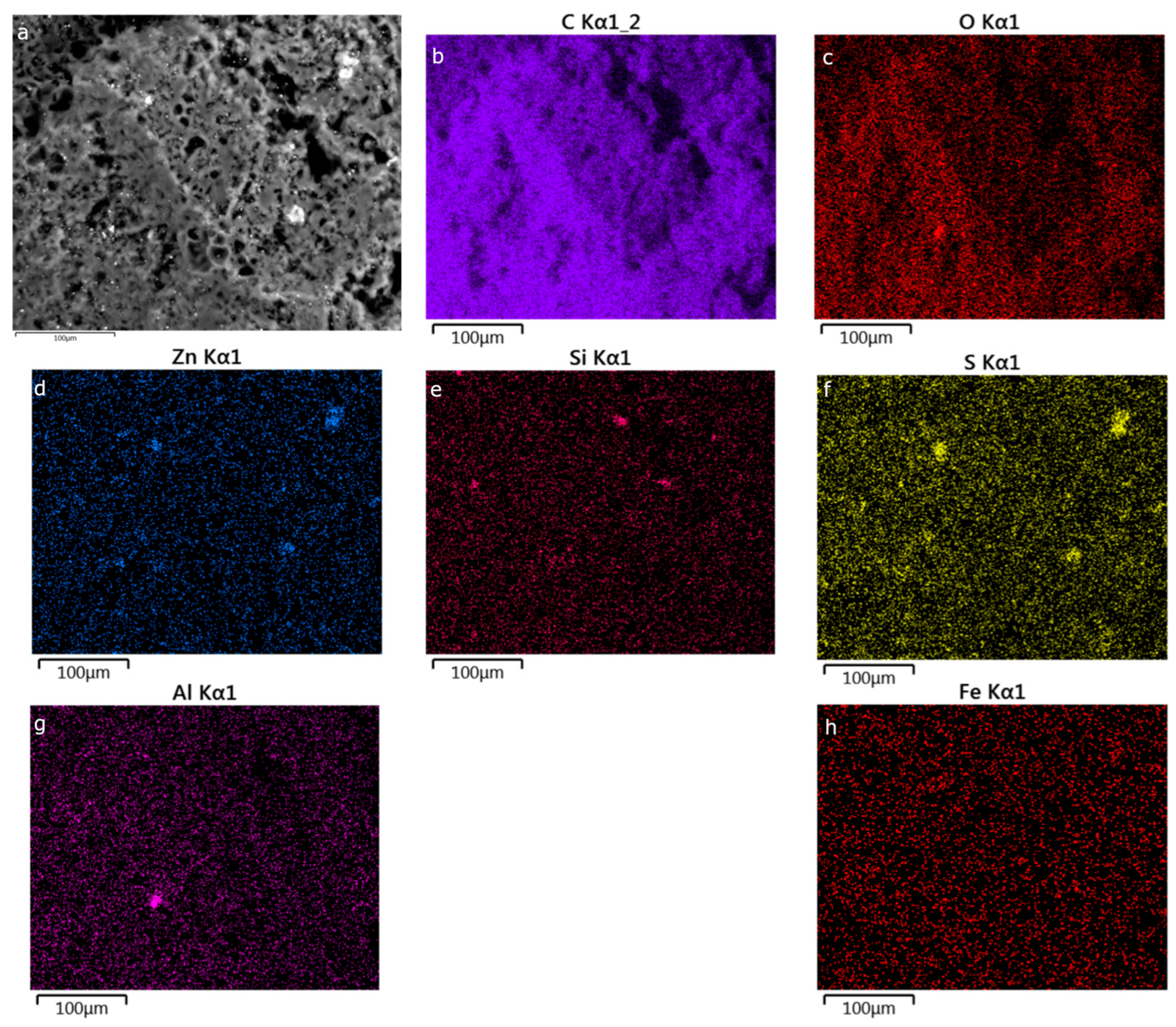


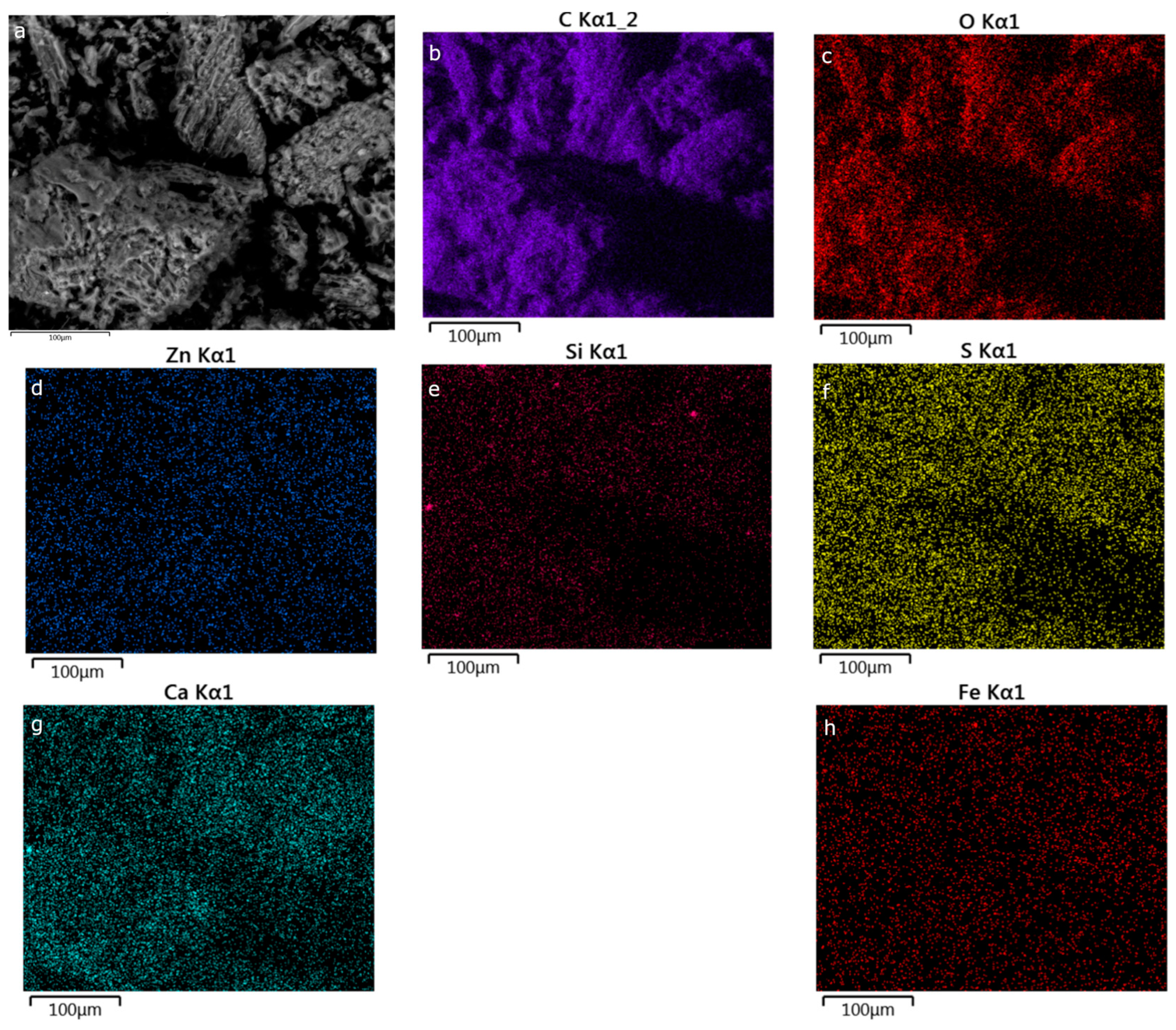

Appendix C
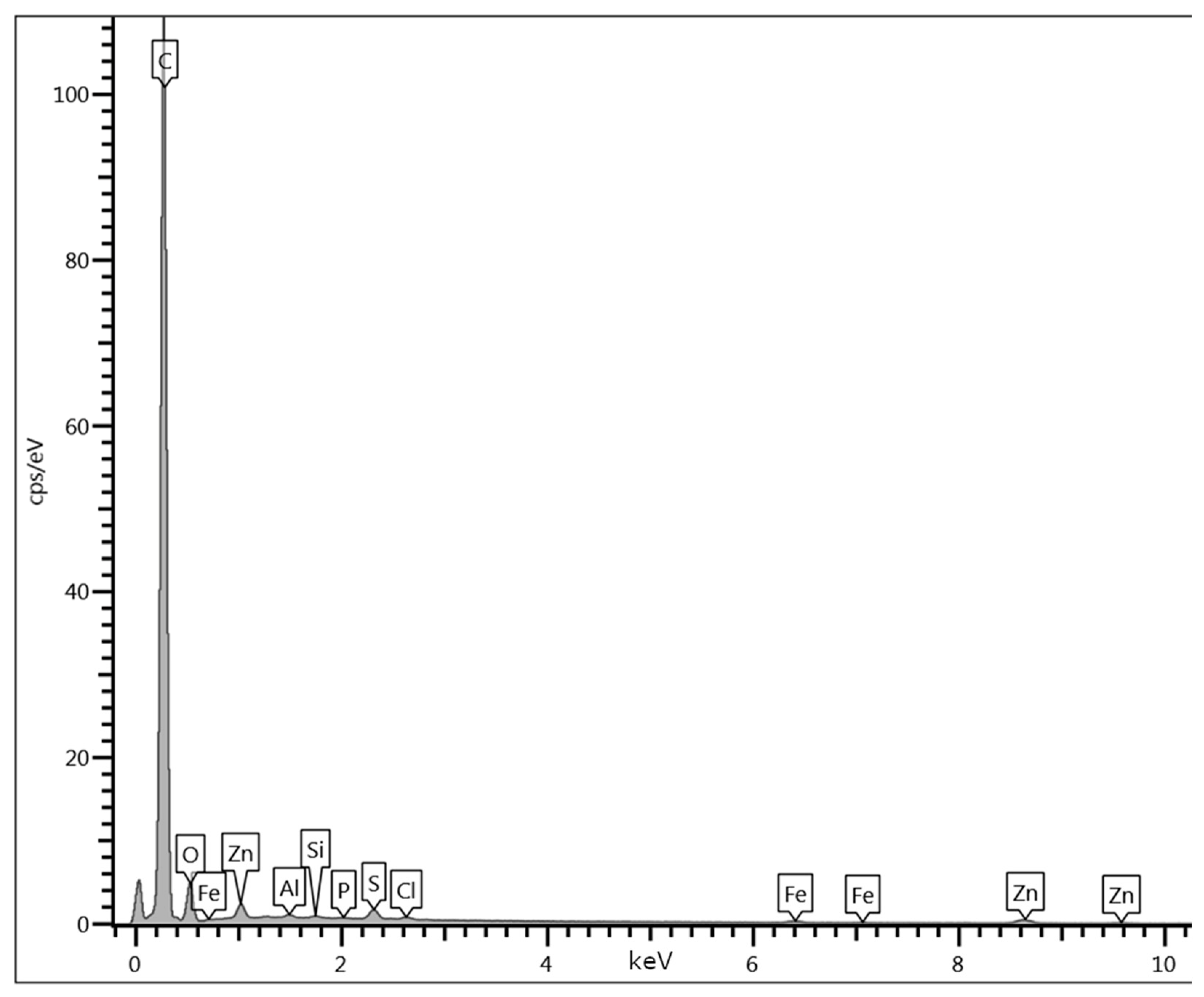
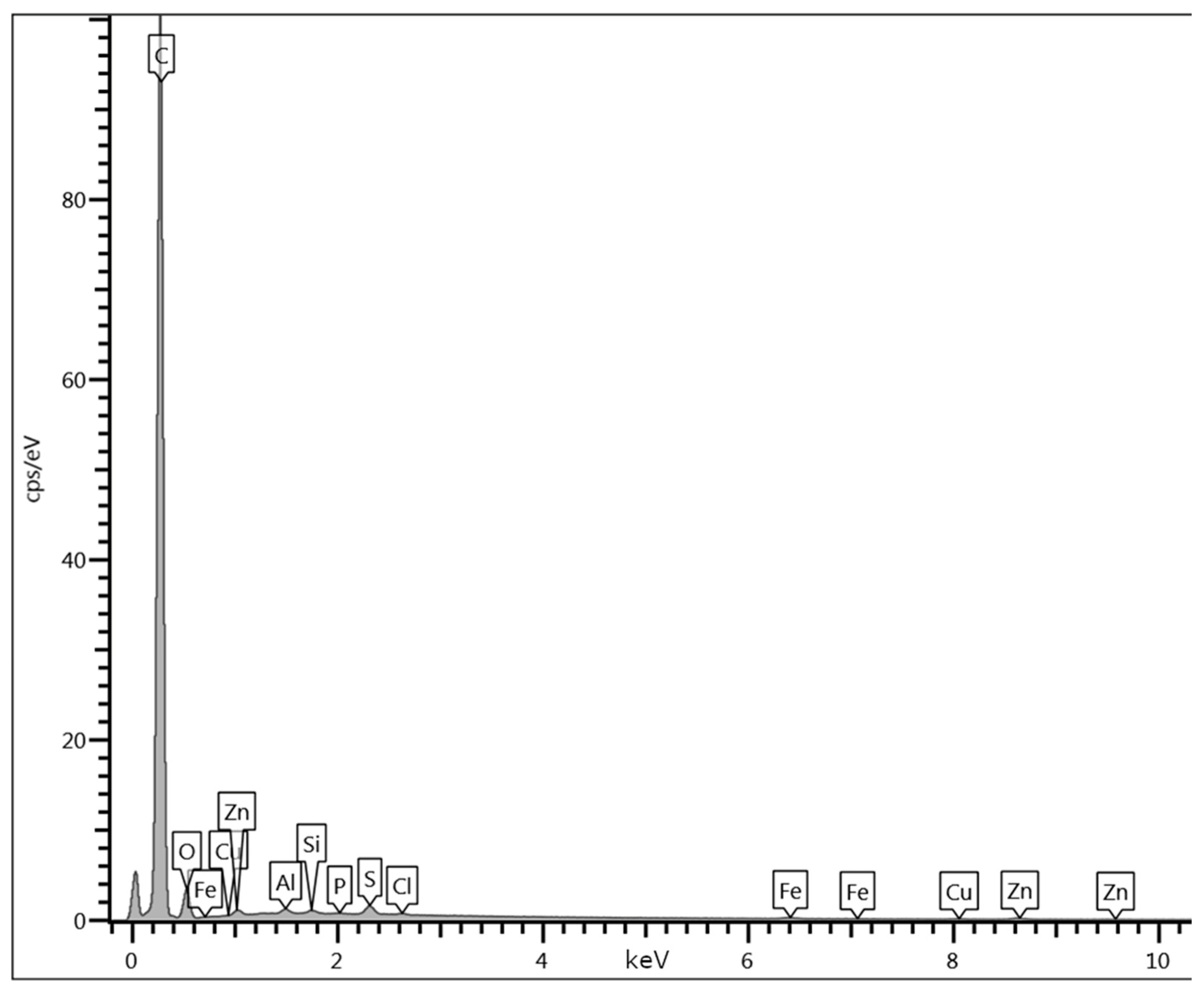



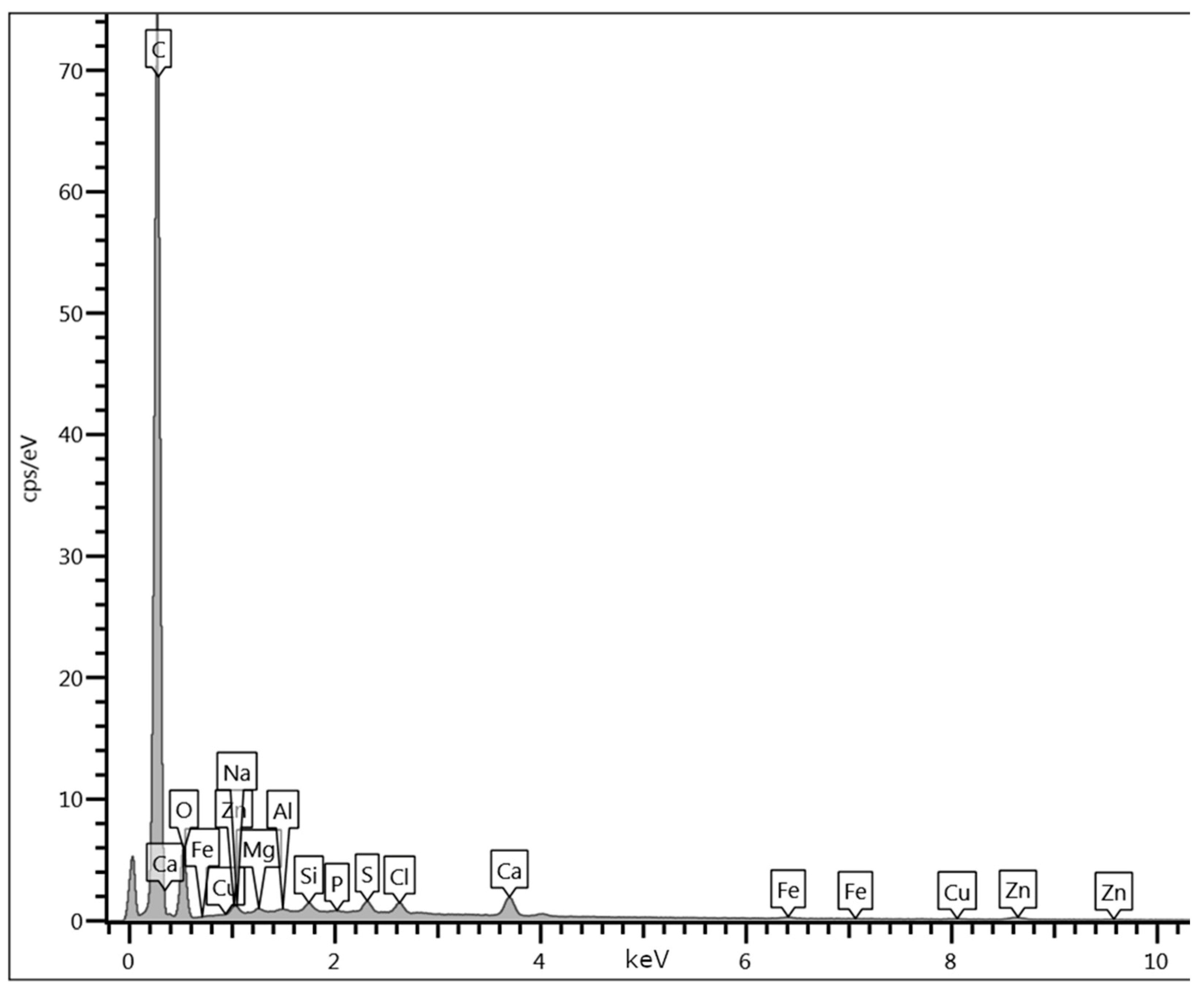
Appendix D
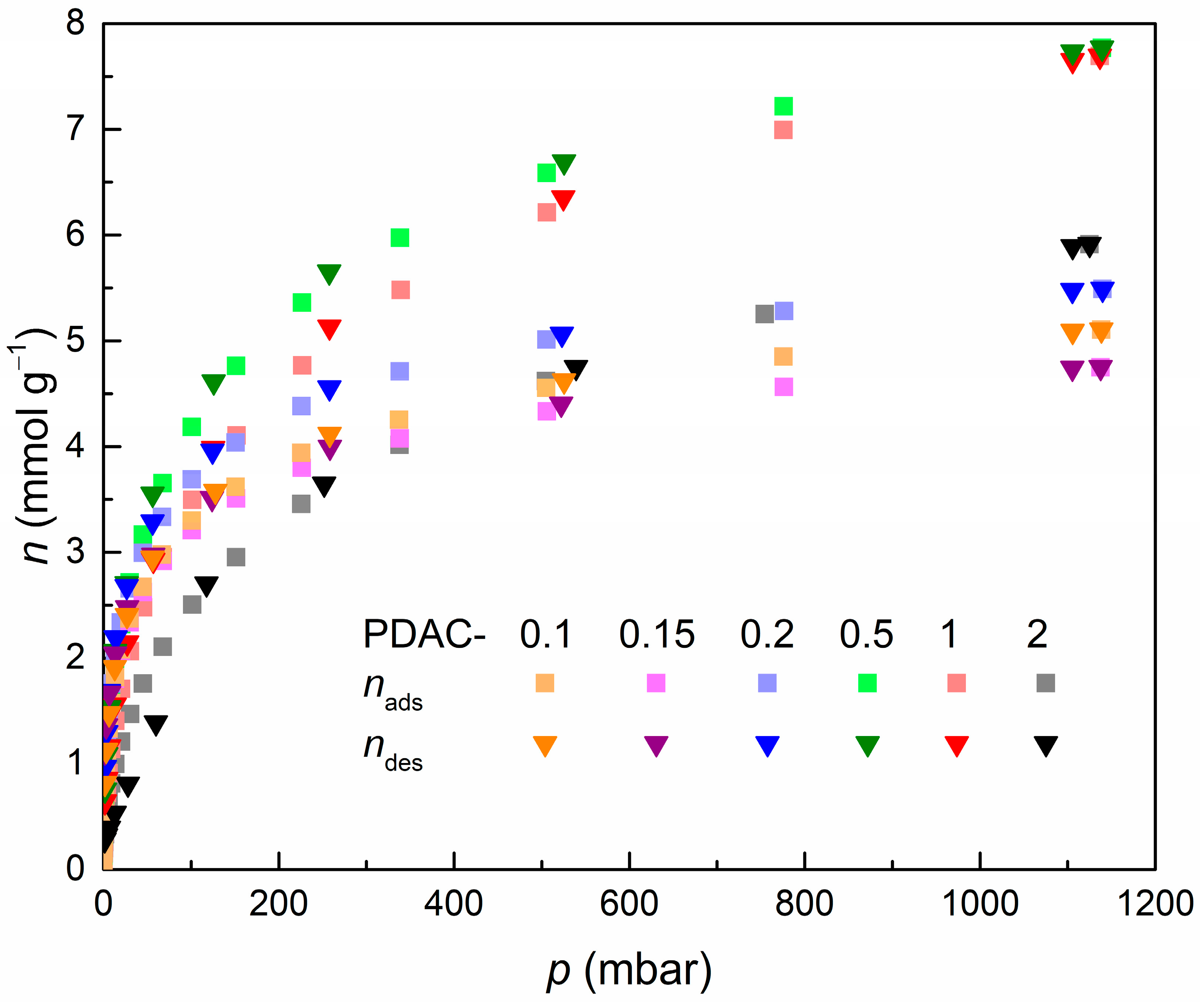
References
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The Role of Hydrogen and Fuel Cells in the Global Energy System. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef]
- Ramachandran, R. An Overview of Industrial Uses of Hydrogen. Int. J. Hydrogen Energy 1998, 23, 593–598. [Google Scholar] [CrossRef]
- Dawood, F.; Anda, M.; Shafiullah, G.M. Hydrogen Production for Energy: An Overview. Int. J. Hydrogen Energy 2020, 45, 3847–3869. [Google Scholar] [CrossRef]
- Nachtane, M.; Tarfaoui, M.; Abichou, M.A.; Vetcher, A.; Rouway, M.; Aâmir, A.; Mouadili, H.; Laaouidi, H.; Naanani, H. An Overview of the Recent Advances in Composite Materials and Artificial Intelligence for Hydrogen Storage Vessels Design. J. Compos. Sci. 2023, 7, 119. [Google Scholar] [CrossRef]
- Zheng, J.; Liu, X.; Xu, P.; Liu, P.; Zhao, Y.; Yang, J. Development of High Pressure Gaseous Hydrogen Storage Technologies. Int. J. Hydrogen Energy 2012, 37, 1048–1057. [Google Scholar] [CrossRef]
- Abohamzeh, E.; Salehi, F.; Sheikholeslami, M.; Abbassi, R.; Khan, F. Review of Hydrogen Safety during Storage, Transmission, and Applications Processes. J. Loss Prev. Process Ind. 2021, 72, 104569. [Google Scholar] [CrossRef]
- Palm, R.; Kurig, H.; Aruväli, J.; Lust, E. NaAlH4/Microporous Carbon Composite Materials for Reversible Hydrogen Storage. Microporous Mesoporous Mater. 2018, 264, 8–12. [Google Scholar] [CrossRef]
- Yushin, G.; Dash, R.; Jagiello, J.; Fischer, J.E.; Gogotsi, Y. Carbide-Derived Carbons: Effect of Pore Size on Hydrogen Uptake and Heat of Adsorption. Adv. Funct. Mater. 2006, 16, 2288–2293. [Google Scholar] [CrossRef]
- Zubizarreta, L.; Arenillas, A.; Pis, J.J. Carbon Materials for H2 Storage. Int. J. Hydrogen Energy 2009, 34, 4575–4581. [Google Scholar] [CrossRef]
- Demiral, İ.; Aydın Şamdan, C.; Demiral, H. Production and Characterization of Activated Carbons from Pumpkin Seed Shell by Chemical Activation with ZnCl2. Desalination Water Treat. 2016, 57, 2446–2454. [Google Scholar] [CrossRef]
- Thomberg, T.; Härmas, M.; Romann, T.; Jänes, A.; Lust, E. Synthesis of Porous Carbon By Hydrothermal Carbonization and Zinc Chloride Activation of Granulated White Sugar for Supercapacitor Application. Meet. Abstr. 2018, MA2018-02, 132. [Google Scholar] [CrossRef]
- Deng, J.; Li, M.; Wang, Y. Biomass-Derived Carbon: Synthesis and Applications in Energy Storage and Conversion. Green Chem. 2016, 18, 4824–4854. [Google Scholar] [CrossRef]
- Tadda, M.A.; Ahsan, A.; Shitu, A.; Elsergany, M.; Thirugnanasambantham, A.; Jose, B.; Razzaque, M.; Norsyahariati, N. A Review on Activated Carbon: Process, Application and Prospects. J. Adv. Civ. Eng. Pract. Res. 2016, 2, 7–13. [Google Scholar]
- Teppor, P.; Jäger, R.; Paalo, M.; Adamson, A.; Härmas, M.; Volobujeva, O.; Aruväli, J.; Palm, R.; Lust, E. Peat as a Carbon Source for Non-Platinum Group Metal Oxygen Electrocatalysts and AEMFC Cathodes. Int. J. Hydrogen Energy 2022, 47, 16908–16920. [Google Scholar] [CrossRef]
- Bénard, P.; Chahine, R. Determination of the Adsorption Isotherms of Hydrogen on Activated Carbons above the Critical Temperature of the Adsorbate over Wide Temperature and Pressure Ranges. Langmuir 2001, 17, 1950–1955. [Google Scholar] [CrossRef]
- Härmas, R.; Palm, R.; Russina, M.; Kurig, H.; Grzimek, V.; Härk, E.; Koppel, M.; Tallo, I.; Paalo, M.; Oll, O.; et al. Transport Properties of H2 Confined in Carbide-Derived Carbons with Different Pore Shapes and Sizes. Carbon 2019, 155, 122–128. [Google Scholar] [CrossRef]
- Koppel, M.; Palm, R.; Härmas, R.; Russina, M.; Grzimek, V.; Jagiello, J.; Paalo, M.; Kurig, H.; Månsson, M.; Oll, O.; et al. Pore Wall Corrugation Effect on the Dynamics of Adsorbed H2 Studied by in Situ Quasi-Elastic Neutron Scattering: Observation of Two Timescaled Diffusion. Carbon 2022, 197, 359–367. [Google Scholar] [CrossRef]
- Koppel, M.; Palm, R.; Härmas, R.; Russina, M.; Matsubara, N.; Månsson, M.; Grzimek, V.; Paalo, M.; Aruväli, J.; Romann, T.; et al. In Situ Observation of Pressure Modulated Reversible Structural Changes in the Graphitic Domains of Carbide-Derived Carbons. Carbon 2021, 174, 190–200. [Google Scholar] [CrossRef]
- Palm, R.; Tallo, I.; Romann, T.; Kurig, H. Methane Adsorption on Specially Designed TiC and Mo2C Derived Carbons with Different Pore Size and Surface Morphology. Microporous Mesoporous Mater. 2015, 218, 167–173. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, Z.; Zhang, H.; Liu, Z. Nitrogen-Doped Microporous Carbon Materials with Uniform Pore Diameters: Design and Applications in CO2 and H2 Adsorption. Microporous Mesoporous Mater. 2020, 296, 109992. [Google Scholar] [CrossRef]
- Tian, M.; Lennox, M.J.; O’Malley, A.J.; Porter, A.J.; Krüner, B.; Rudić, S.; Mays, T.J.; Düren, T.; Presser, V.; Terry, L.R.; et al. Effect of Pore Geometry on Ultra-Densified Hydrogen in Microporous Carbons. Carbon 2021, 173, 968–979. [Google Scholar] [CrossRef]
- Nguyen, T.X.; Bae, J.-S.; Wang, Y.; Bhatia, S.K. On the Strength of the Hydrogen−Carbon Interaction as Deduced from Physisorption. Langmuir 2009, 25, 4314–4319. [Google Scholar] [CrossRef] [PubMed]
- Gogotsi, Y.; Portet, C.; Osswald, S.; Simmons, J.M.; Yildirim, T.; Laudisio, G.; Fischer, J.E. Importance of Pore Size in High-Pressure Hydrogen Storage by Porous Carbons. Int. J. Hydrogen Energy 2009, 34, 6314–6319. [Google Scholar] [CrossRef]
- Yurduşen, A.; Yürüm, A.; Yürüm, Y. A Remarkable Increase in the Adsorbed H2 Amount: Influence of Pore Size Distribution on the H2 Adsorption Capacity of Fe-BTC. Int. J. Hydrogen Energy 2020, 45, 12394–12407. [Google Scholar] [CrossRef]
- Yan, T.; Yang, J.; Lu, J.; Zhou, L.; Zhang, Y.; He, G. Facile Synthesis of Ultra-Microporous Pillar-Layered Metal–Organic Framework Membranes for Highly H2 -Selective Separation. ACS Appl. Mater. Interfaces 2023, 15, 20571–20582. [Google Scholar] [CrossRef]
- Yan, J.; Zhang, B.; Wang, Z. Ultramicroporous Carbons Derived from Semi-Cycloaliphatic Polyimide with Outstanding Adsorption Properties for H2, CO2, and Organic Vapors. J. Phys. Chem. C 2017, 121, 22753–22761. [Google Scholar] [CrossRef]
- Almanassra, I.W.; Kochkodan, V.; Ponnusamy, G.; Mckay, G.; Ali Atieh, M.; Al-Ansari, T. Carbide Derived Carbon (CDC) as Novel Adsorbent for Ibuprofen Removal from Synthetic Water and Treated Sewage Effluent. J. Environ. Health Sci. Eng. 2020, 18, 1375–1390. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, X.; Li, Z.; Yang, C.; Zhong, W.; Li, W.; Zhang, C.; Yang, N.; Zhang, Q.; Li, X. Toward High-Performance Capacitive Potassium-Ion Storage: A Superior Anode Material from Silicon Carbide-Derived Carbon with a Well-Developed Pore Structure. Adv. Funct. Mater. 2020, 30, 2004348. [Google Scholar] [CrossRef]
- Nishihara, H.; Hou, P.-X.; Li, L.-X.; Ito, M.; Uchiyama, M.; Kaburagi, T.; Ikura, A.; Katamura, J.; Kawarada, T.; Mizuuchi, K.; et al. High-Pressure Hydrogen Storage in Zeolite-Templated Carbon. J. Phys. Chem. C 2009, 113, 3189–3196. [Google Scholar] [CrossRef]
- Sevilla, M.; Alam, N.; Mokaya, R. Enhancement of Hydrogen Storage Capacity of Zeolite-Templated Carbons by Chemical Activation. J. Phys. Chem. C 2010, 114, 11314–11319. [Google Scholar] [CrossRef]
- Stadie, N.P.; Vajo, J.J.; Cumberland, R.W.; Wilson, A.A.; Ahn, C.C.; Fultz, B. Zeolite-Templated Carbon Materials for High-Pressure Hydrogen Storage. Langmuir 2012, 28, 10057–10063. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Li, L.; Liu, Y.; Geng, X.; Yang, H.; Sun, C.; An, B. Synthesis and ORR Performance of Nitrogen-Doped Ordered Microporous Carbon by CVD of Acetonitrile Vapor Using Silanized Zeolite as Template. Appl. Surf. Sci. 2020, 504, 144438. [Google Scholar] [CrossRef]
- Sdanghi, G.; Canevesi, R.L.S.; Celzard, A.; Thommes, M.; Fierro, V. Characterization of Carbon Materials for Hydrogen Storage and Compression. C 2020, 6, 46. [Google Scholar] [CrossRef]
- Heidarinejad, Z.; Dehghani, M.H.; Heidari, M.; Javedan, G.; Ali, I.; Sillanpää, M. Methods for Preparation and Activation of Activated Carbon: A Review. Environ. Chem. Lett. 2020, 18, 393–415. [Google Scholar] [CrossRef]
- Härmas, M.; Thomberg, T.; Kurig, H.; Romann, T.; Jänes, A.; Lust, E. Microporous–Mesoporous Carbons for Energy Storage Synthesized by Activation of Carbonaceous Material by Zinc Chloride, Potassium Hydroxide or Mixture of Them. J. Power Sources 2016, 326, 624–634. [Google Scholar] [CrossRef]
- Varila, T.; Bergna, D.; Lahti, R.; Romar, H.; Hu, T.; Lassi, U. Activated Carbon Production from Peat Using ZnCl2: Characterization and Applications. BioResources 2017, 12, 8078–8092. [Google Scholar] [CrossRef]
- Saramas, D.; Ekgasit, S. Nano-Zinc Oxide-Doped Activated Carbon from Popped Rice and Its Application for Feed Additive. Eng. J. 2021, 25, 41–50. [Google Scholar] [CrossRef]
- Liou, T.-H. Development of Mesoporous Structure and High Adsorption Capacity of Biomass-Based Activated Carbon by Phosphoric Acid and Zinc Chloride Activation. Chem. Eng. J. 2010, 158, 129–142. [Google Scholar] [CrossRef]
- Yorgun, S.; Vural, N.; Demiral, H. Preparation of High-Surface Area Activated Carbons from Paulownia Wood by ZnCl2 Activation. Microporous Mesoporous Mater. 2009, 122, 189–194. [Google Scholar] [CrossRef]
- Agbozu, I.E.; Wategire, O.P. Comparative Carbon Synthesis of Peat Using ZnCl2 and H3PO4 for Heavy Metal Adsorption in Oilfield Produced Water. Int. Res. J. Pure Appl. Chem. 2023, 24, 81–94. [Google Scholar] [CrossRef]
- Azwar, E.; Wan Mahari, W.A.; Chuah, J.H.; Vo, D.-V.N.; Ma, N.L.; Lam, W.H.; Lam, S.S. Transformation of Biomass into Carbon Nanofiber for Supercapacitor Application–A Review. Int. J. Hydrogen Energy 2018, 43, 20811–20821. [Google Scholar] [CrossRef]
- Contescu, C.; Adhikari, S.; Gallego, N.; Evans, N.; Biss, B. Activated Carbons Derived from High-Temperature Pyrolysis of Lignocellulosic Biomass. C 2018, 4, 51. [Google Scholar] [CrossRef]
- Kosheleva, R.I.; Mitropoulos, A.C.; Kyzas, G.Z. Synthesis of Activated Carbon from Food Waste. Environ. Chem. Lett. 2019, 17, 429–438. [Google Scholar] [CrossRef]
- Juárez-Galán, J.M.; Silvestre-Albero, A.; Silvestre-Albero, J.; Rodríguez-Reinoso, F. Synthesis of Activated Carbon with Highly Developed “Mesoporosity”. Microporous Mesoporous Mater. 2009, 117, 519–521. [Google Scholar] [CrossRef]
- Ogungbenro, A.E.; Quang, D.V.; Al-Ali, K.A.; Vega, L.F.; Abu-Zahra, M.R.M. Synthesis and Characterization of Activated Carbon from Biomass Date Seeds for Carbon Dioxide Adsorption. J. Environ. Chem. Eng. 2020, 8, 104257. [Google Scholar] [CrossRef]
- Ren, Z.; Romar, H.; Varila, T.; Xu, X.; Wang, Z.; Sillanpää, M.; Leiviskä, T. Ibuprofen Degradation Using a Co-Doped Carbon Matrix Derived from Peat as a Peroxymonosulphate Activator. Environ. Res. 2021, 193, 110564. [Google Scholar] [CrossRef]
- Varghese, S.M.; Chowdhury, A.R.; Arnepalli, D.N.; Ranga Rao, G. Delineating the Effects of Pore Structure and N-Doping on CO2 Adsorption Using Coco Peat Derived Carbon. Carbon Trends 2023, 10, 100250. [Google Scholar] [CrossRef]
- Shkolin, A.V.; Strizhenov, E.M.; Chugaev, S.S.; Men’shchikov, I.E.; Gaidamavichute, V.V.; Grinchenko, A.E.; Zherdev, A.A. Natural Gas Storage Filled with Peat-Derived Carbon Adsorbent: Influence of Nonisothermal Effects and Ethane Impurities on the Storage Cycle. Nanomaterials 2022, 12, 4066. [Google Scholar] [CrossRef]
- Men’shchikov, I.; Shkolin, A.; Khozina, E.; Fomkin, A. Thermodynamics of Adsorbed Methane Storage Systems Based on Peat-Derived Activated Carbons. Nanomaterials 2020, 10, 1379. [Google Scholar] [CrossRef]
- Adamson, A.; Väli, R.; Paalo, M.; Aruväli, J.; Koppel, M.; Palm, R.; Härk, E.; Nerut, J.; Romann, T.; Lust, E.; et al. Peat-Derived Hard Carbon Electrodes with Superior Capacity for Sodium-Ion Batteries. RSC Adv. 2020, 10, 20145–20154. [Google Scholar] [CrossRef]
- Paalo, M.; Härmas, M.; Romann, T.; Jänes, A.; Lust, E. Modification of Micro/Mesoporous Carbon Synthesis Method from Well Decomposed Peat Using ZnCl2 Additional Activation Step. Electrochem. Commun. 2023, 153, 107543. [Google Scholar] [CrossRef]
- Ma, Y. Comparison of Activated Carbons Prepared from Wheat Straw via ZnCl2 and KOH Activation. Waste Biomass Valor. 2017, 8, 549–559. [Google Scholar] [CrossRef]
- Nguyen, T.-B.; Truong, Q.-M.; Chen, C.-W.; Doong, R.; Chen, W.-H.; Dong, C.-D. Mesoporous and Adsorption Behavior of Algal Biochar Prepared via Sequential Hydrothermal Carbonization and ZnCl2 Activation. Bioresour. Technol. 2022, 346, 126351. [Google Scholar] [CrossRef]
- Gates-Rector, S.; Blanton, T. The Powder Diffraction File: A Quality Materials Characterization Database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Jagiello, J.; Ania, C.; Parra, J.B.; Cook, C. Dual Gas Analysis of Microporous Carbons Using 2D-NLDFT Heterogeneous Surface Model and Combined Adsorption Data of N2 and CO2. Carbon 2015, 91, 330–337. [Google Scholar] [CrossRef]
- Jagiello, J.; Olivier, J.P. Carbon Slit Pore Model Incorporating Surface Energetical Heterogeneity and Geometrical Corrugation. Adsorption 2013, 19, 777–783. [Google Scholar] [CrossRef]
- Do, D.D. Adsorption Analysis: Equilibria and Kinetics; Series on chemical engineering; Imperial College Press: London, UK, 1998; ISBN 978-1-86094-130-6. [Google Scholar]
- Karthikeyan, R.; Wang, B.; Xuan, J.; Wong, J.W.C.; Lee, P.K.H.; Leung, M.K.H. Interfacial Electron Transfer and Bioelectrocatalysis of Carbonized Plant Material as Effective Anode of Microbial Fuel Cell. Electrochim. Acta 2015, 157, 314–323. [Google Scholar] [CrossRef]
- Brodowski, S.; Amelung, W.; Haumaier, L.; Abetz, C.; Zech, W. Morphological and Chemical Properties of Black Carbon in Physical Soil Fractions as Revealed by Scanning Electron Microscopy and Energy-Dispersive X-Ray Spectroscopy. Geoderma 2005, 128, 116–129. [Google Scholar] [CrossRef]
- Steinmann, P.; Shotyk, W. Chemical Composition, pH, and Redox State of Sulfur and Iron in Complete Vertical Porewater Profiles from Two Sphagnum Peat Bogs, Jura Mountains, Switzerland. Geochim. Cosmochim. Acta 1997, 61, 1143–1163. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, R.; Wang, J.; Wang, B.; Liang, B.; Yildirim, T.; Zhang, J.; Zhou, W.; Chen, B. Optimization of the Pore Structures of MOFs for Record High Hydrogen Volumetric Working Capacity. Adv. Mater. 2020, 32, 1907995. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Rogacka, J.; Firlej, L.; Kuchta, B. Modeling of Low Temperature Adsorption of Hydrogen in Carbon Nanopores. J. Mol. Model. 2017, 23, 20. [Google Scholar] [CrossRef] [PubMed]
- Beenakker, J.J.M.; Borman, V.D.; Krylov, S.Y. Molecular Transport in Subnanometer Pores: Zero-Point Energy, Reduced Dimensionality and Quantum Sieving. Chem. Phys. Lett. 1995, 232, 379–382. [Google Scholar] [CrossRef]
- Jagiello, J.; Kenvin, J.; Ania, C.O.; Parra, J.B.; Celzard, A.; Fierro, V. Exploiting the Adsorption of Simple Gases O2 and H2 with Minimal Quadrupole Moments for the Dual Gas Characterization of Nanoporous Carbons Using 2D-NLDFT Models. Carbon 2020, 160, 164–175. [Google Scholar] [CrossRef]
- Contescu, C.I.; Saha, D.; Gallego, N.C.; Mamontov, E.; Kolesnikov, A.I.; Bhat, V.V. Restricted Dynamics of Molecular Hydrogen Confined in Activated Carbon Nanopores. Carbon 2012, 50, 1071–1082. [Google Scholar] [CrossRef]
- Ting, V.P.; Ramirez-Cuesta, A.J.; Bimbo, N.; Sharpe, J.E.; Noguera-Diaz, A.; Presser, V.; Rudic, S.; Mays, T.J. Direct Evidence for Solid-like Hydrogen in a Nanoporous Carbon Hydrogen Storage Material at Supercritical Temperatures. ACS Nano 2015, 9, 8249–8254. [Google Scholar] [CrossRef]
- Gallego, N.C.; He, L.; Saha, D.; Contescu, C.I.; Melnichenko, Y.B. Hydrogen Confinement in Carbon Nanopores: Extreme Densification at Ambient Temperature. J. Am. Chem. Soc. 2011, 133, 13794–13797. [Google Scholar] [CrossRef]
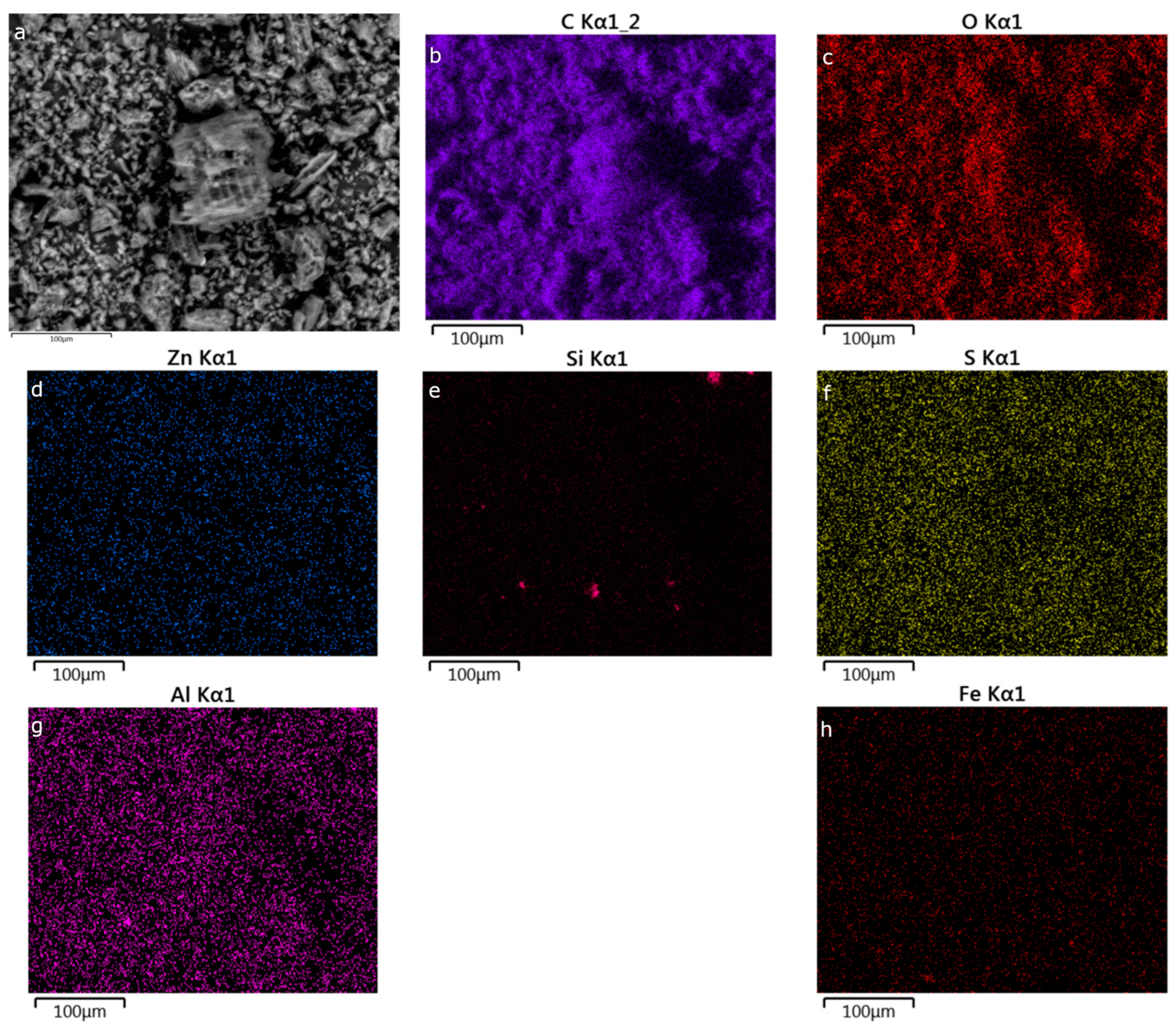
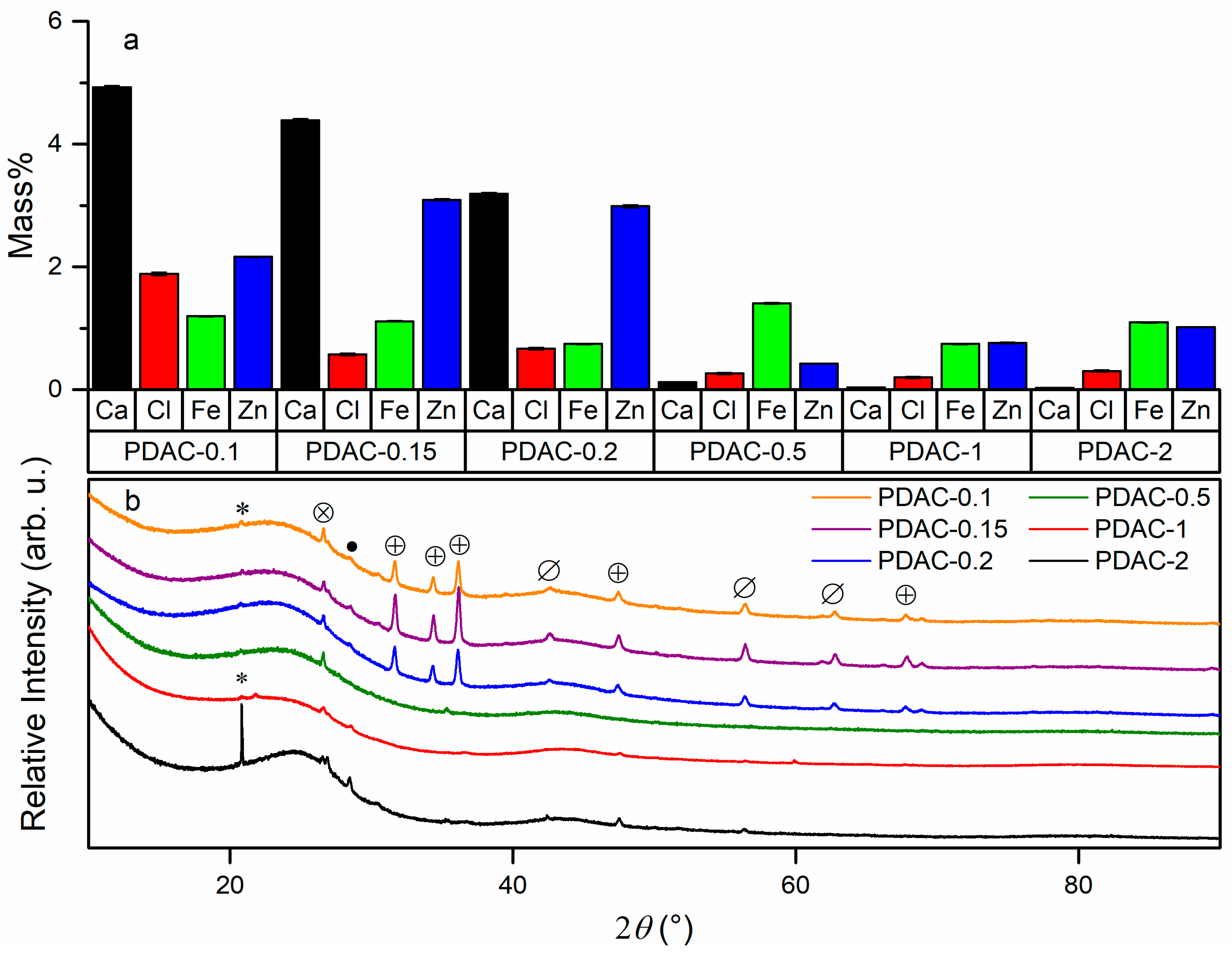
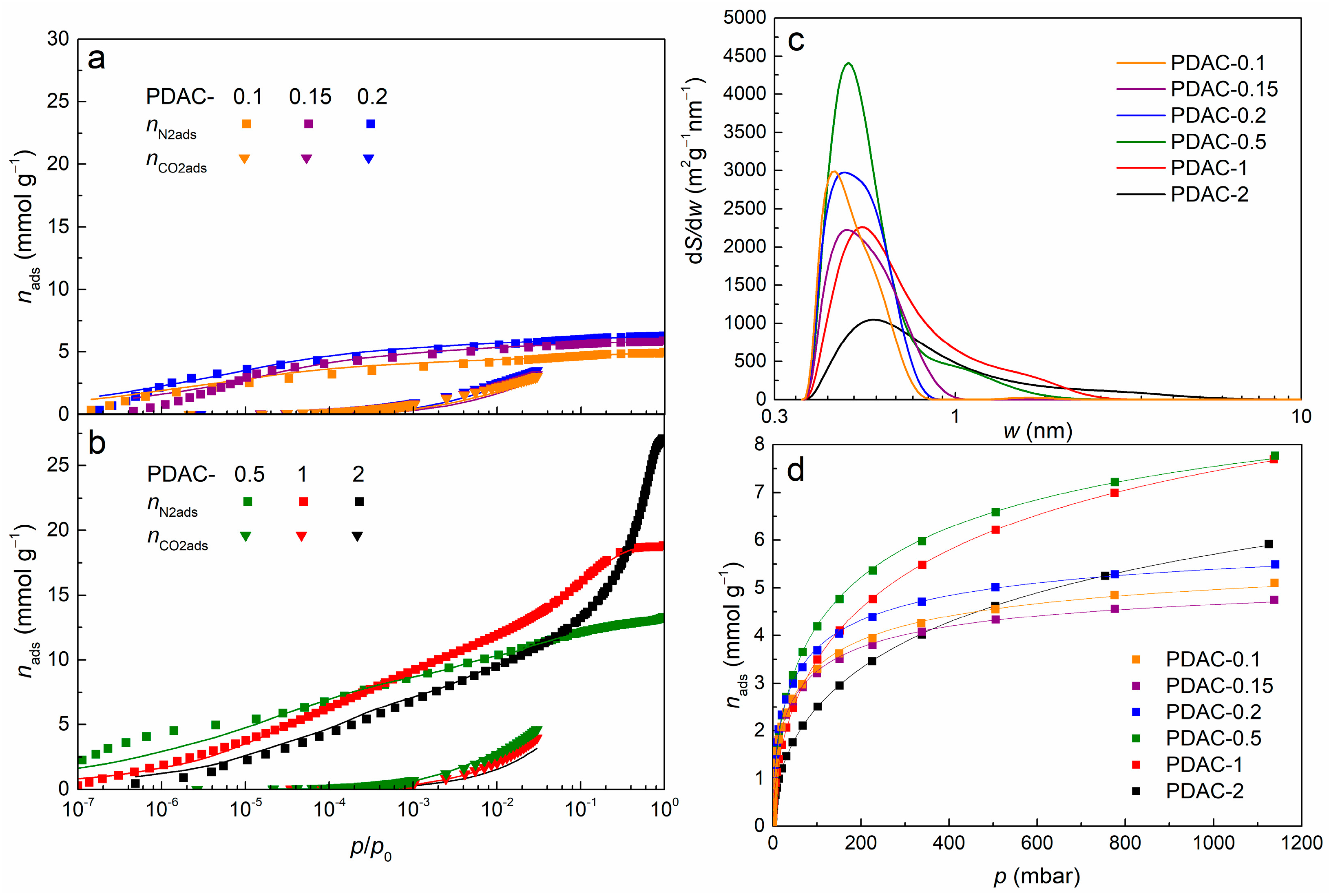
| Carbon | ZnCl2:Peat Mass Ratio a | Zn Mass% b | SBET (m2 g−1) c |
|---|---|---|---|
| PDAC-0.1 | 1:10 | 2.16 (1) | 370 |
| PDAC-0.15 | 1:7 | 3.09 (2) | 380 |
| PDAC-0.2 | 1:5 | 2.99 (3) | 470 |
| PDAC-0.5 | 1:2 | 0.43 (1) | 1030 |
| PDAC-1 | 1:1 | 0.76 (2) | 1400 |
| PDAC-2 | 1:0.5 | 1.02 (1) | 1240 |
| Carbon Sample | SDFT (m2 g−1) | Smicro (m2 g−1) | Smicro/SDFT (%) | VDFT (cm3 g−1) | Vmicro (cm3 g−1) | V0.8nm (cm3 g−1) |
|---|---|---|---|---|---|---|
| PDAC-0.1 | 630 | 630 | 100 | 0.177 | 0.173 | 0.161 |
| PDAC-0.15 | 680 | 680 | 100 | 0.201 | 0.200 | 0.185 |
| PDAC-0.2 | 790 | 790 | 100 | 0.221 | 0.220 | 0.212 |
| PDAC-0.5 | 1260 | 1250 | 99 | 0.446 | 0.428 | 0.270 |
| PDAC-1 | 1280 | 1220 | 95 | 0.608 | 0.539 | 0.207 |
| PDAC-2 | 1020 | 720 | 71 | 0.879 | 0.340 | 0.109 |
| Carbon Sample | b (mbar−1) | x | nH2,max (mmol g−1) | nH2,max/SBET (Mass%/500 m2) | nH2,max/SDFT (Mass%/500 m2) | nH2,1bar (mmol g−1) | nH2,1bar/nH2,max |
|---|---|---|---|---|---|---|---|
| PDAC-0.1 | 1.35 × 10−2 | 1.86 | 6.18 | 1.68 | 0.99 | 4.96 | 0.803 |
| PDAC-0.15 | 1.83 × 10−2 | 1.81 | 5.58 | 1.48 | 0.83 | 4.65 | 0.833 |
| PDAC-0.2 | 1.72 × 10−2 | 1.79 | 6.49 | 1.39 | 0.83 | 5.39 | 0.831 |
| PDAC-0.5 | 4.12 × 10−3 | 1.82 | 10.99 | 1.08 | 0.88 | 7.54 | 0.686 |
| PDAC-1 | 1.87 × 10−3 | 1.74 | 12.63 | 0.91 | 0.99 | 7.44 | 0.589 |
| PDAC-2 | 9.38 × 10−4 | 1.82 | 11.61 | 0.94 | 1.15 | 5.70 | 0.491 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Möller, E.; Palm, R.; Tuul, K.; Härmas, M.; Koppel, M.; Aruväli, J.; Külaviir, M.; Lust, E. Peat-Derived ZnCl2-Activated Ultramicroporous Carbon Materials for Hydrogen Adsorption. Nanomaterials 2023, 13, 2883. https://doi.org/10.3390/nano13212883
Möller E, Palm R, Tuul K, Härmas M, Koppel M, Aruväli J, Külaviir M, Lust E. Peat-Derived ZnCl2-Activated Ultramicroporous Carbon Materials for Hydrogen Adsorption. Nanomaterials. 2023; 13(21):2883. https://doi.org/10.3390/nano13212883
Chicago/Turabian StyleMöller, Egert, Rasmus Palm, Kenneth Tuul, Meelis Härmas, Miriam Koppel, Jaan Aruväli, Marian Külaviir, and Enn Lust. 2023. "Peat-Derived ZnCl2-Activated Ultramicroporous Carbon Materials for Hydrogen Adsorption" Nanomaterials 13, no. 21: 2883. https://doi.org/10.3390/nano13212883
APA StyleMöller, E., Palm, R., Tuul, K., Härmas, M., Koppel, M., Aruväli, J., Külaviir, M., & Lust, E. (2023). Peat-Derived ZnCl2-Activated Ultramicroporous Carbon Materials for Hydrogen Adsorption. Nanomaterials, 13(21), 2883. https://doi.org/10.3390/nano13212883







