Emerging Roles of Microrobots for Enhancing the Sensitivity of Biosensors
Abstract
:1. Introduction
2. Artificial Microrobots for Biosensing
| Detection Principle | Type of Microrobots a | Target | Propulsion Type | Limit of Detection | Function | Refs |
|---|---|---|---|---|---|---|
| Fluorescence | DNA-modified Au/Pt microrobots | HIV-1 RNA | Chemical | 1000 virus particles/mL | Expanding the range of target substances | [56] |
| rGO/Pt microrobots | Ricin | Chemical | 0.1 ng/mL | Enhance sensitivity | [57] | |
| rGO/Ni/PtNPs microrobots | Fumonizin B1 | Magnetic | 0.70 ng/mL | Reduce detection time | [58] | |
| Ochratoxin A | 4 ng/mL | |||||
| Graphdiyne tubular microrobots | Cholera toxin B | Chemical | 1.6 ng/mL | Reduce detection time | [41] | |
| PEDOT/Pt microrobots | Hg2+ | Chemical | 3 mg/L | Reduce detection time | [59] | |
| PEDOT/SiO2 microrobots | Poly(lactic-co-glycolic acid) | Acoustic | - | Enhance sensitivity | [48] | |
| SERS | Body of AgNW@SiO2 & tail of AgCl microrobots | Crystal violet & MCF-7 | Light | - | Enhance sensitivity | [42] |
| Au/SiO/Fe microrobots | Rhodamine 6G | Magnetic | 0.5 nM | Enhance sensitivity | [60] | |
| Locomotion | GO-wrapped/PtNPs Janus microrobots | Glutathione | Chemical | 0.89 µM | Expanding the range of target substances | [43] |
| EC | Ag–AuNRs microrobots | SARS-CoV-2 virus | Magnetic | 1.11 PFU/mL | Enhance sensitivity | [45] |
| Ag/Fe3O4 nanorobots | SARS-CoV-2 RNA | Magnetic | 6.1 ng/mL | Expanding the range of target substances | [44] | |
| Mg-Au Janus microrobots | Diphenyl phthalate | Chemical | 0.039 mM | Expanding the range of target substances | [46] | |
| EIS | MXene-derived γ-Fe2O3/Pt/TiO2 microrobots | Nanoplastics | Light | 106 nanoplastics/mL | Enhance sensitivity | [47] |
| Mg/Fe3O4/P/anti-E microrobots | MCF-7, MCF-10A & GL261 cells | Chemical | - | Expanding the range of target substances | [61] |
3. Biosensors with Microrobots
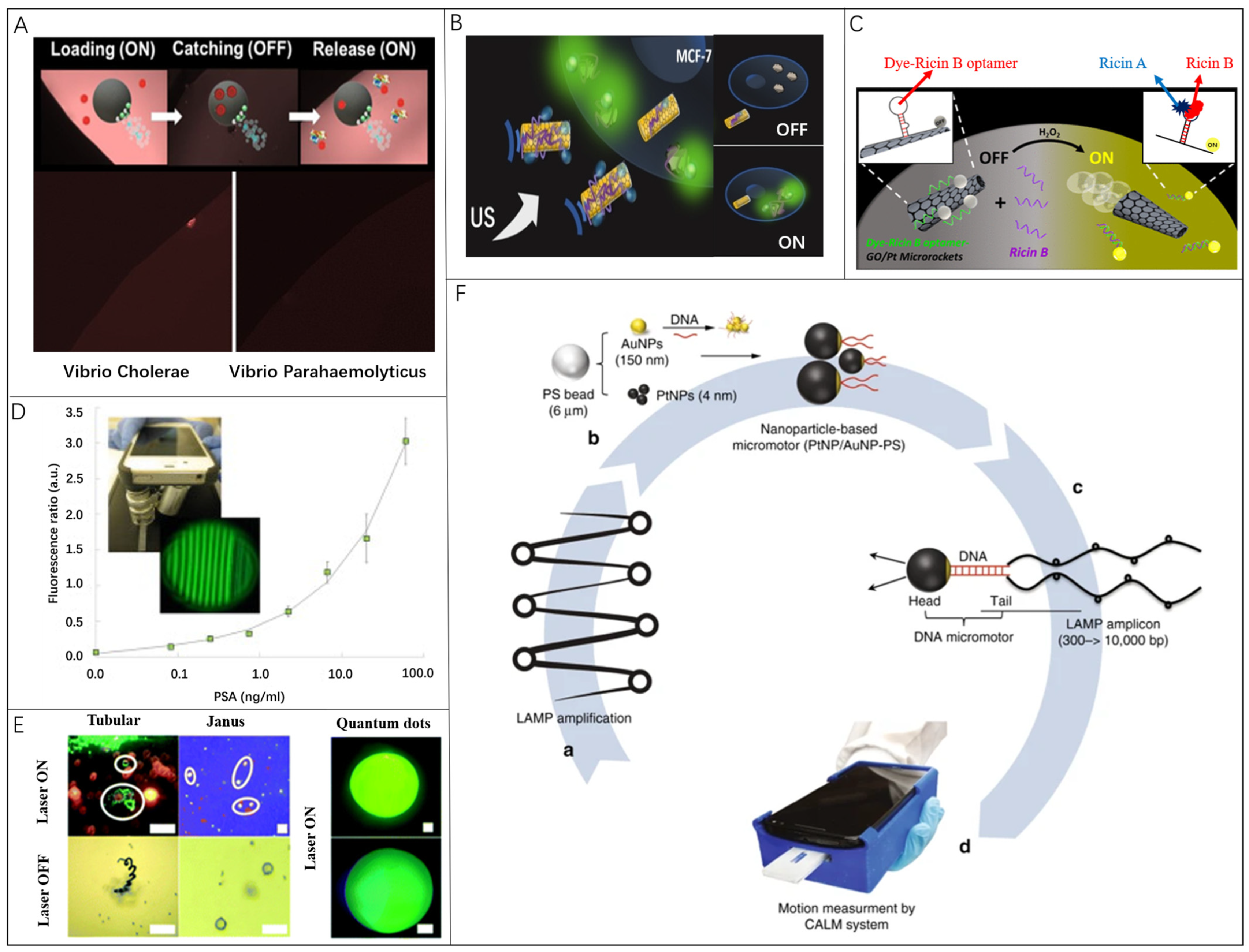
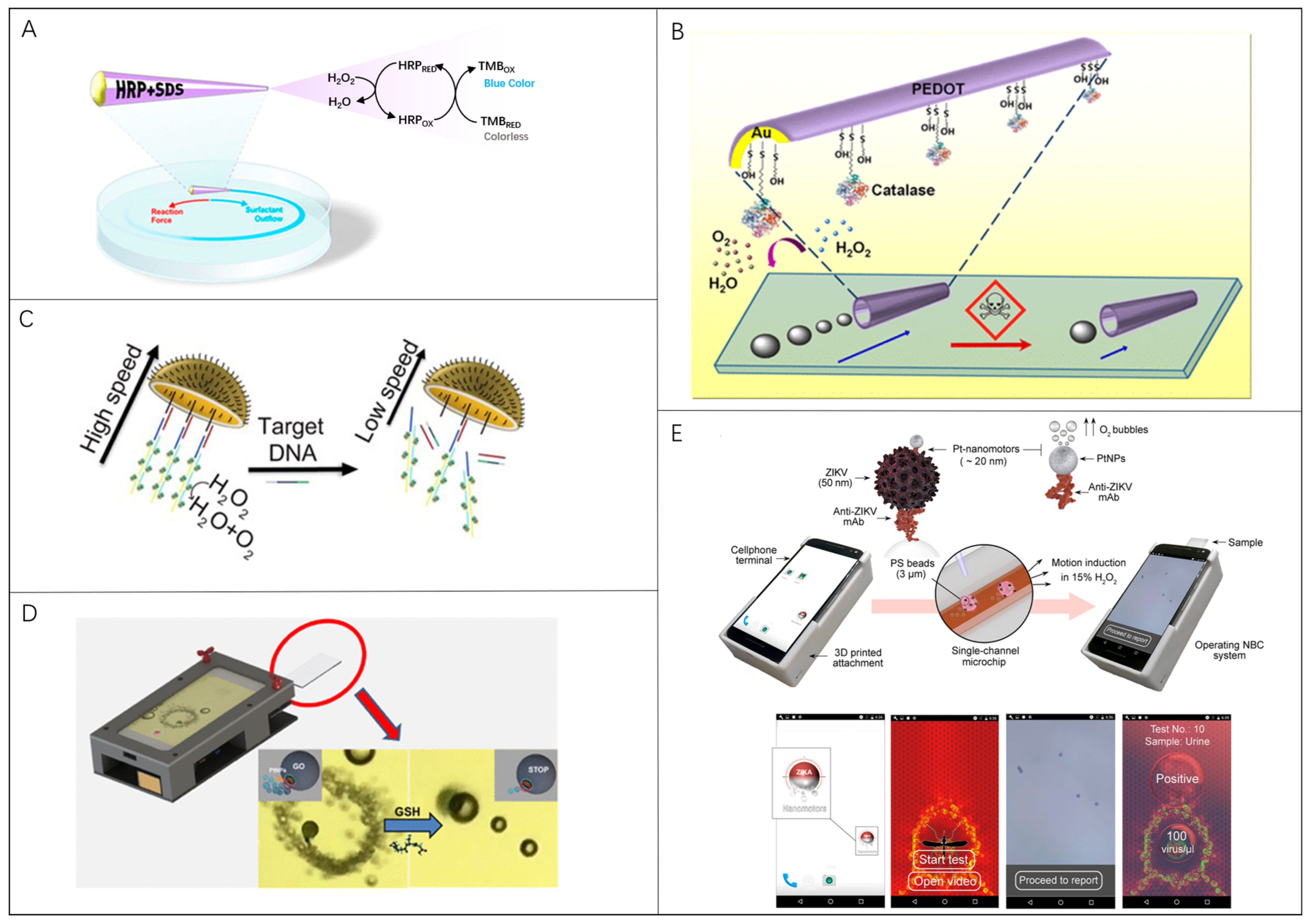
3.1. Microrobots in Fluorescent Biosensing
3.2. Microrobots in Surface-Enhanced Raman Scattering Biosensing
3.3. Microrobots in Locomotion-Based Biosensing
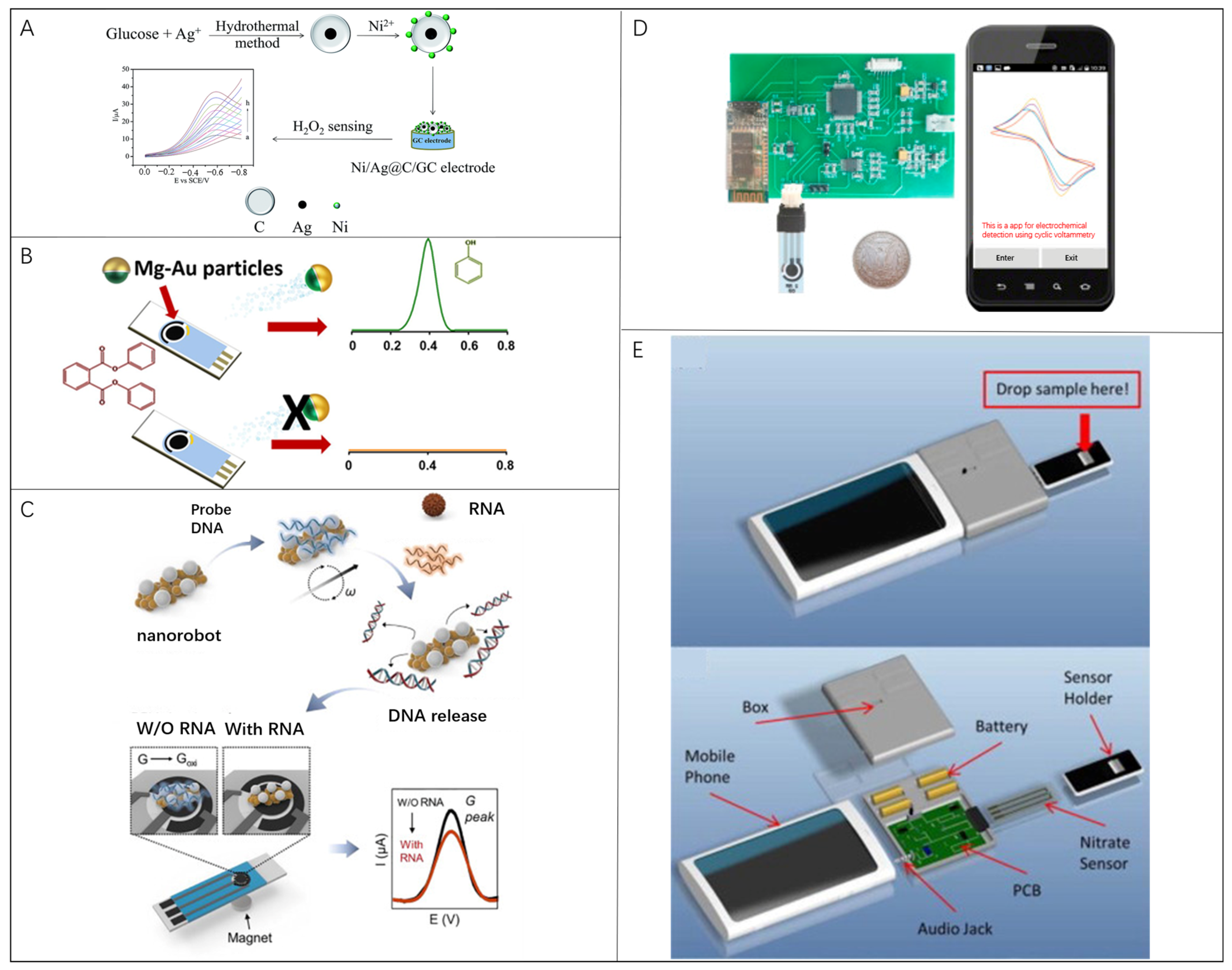
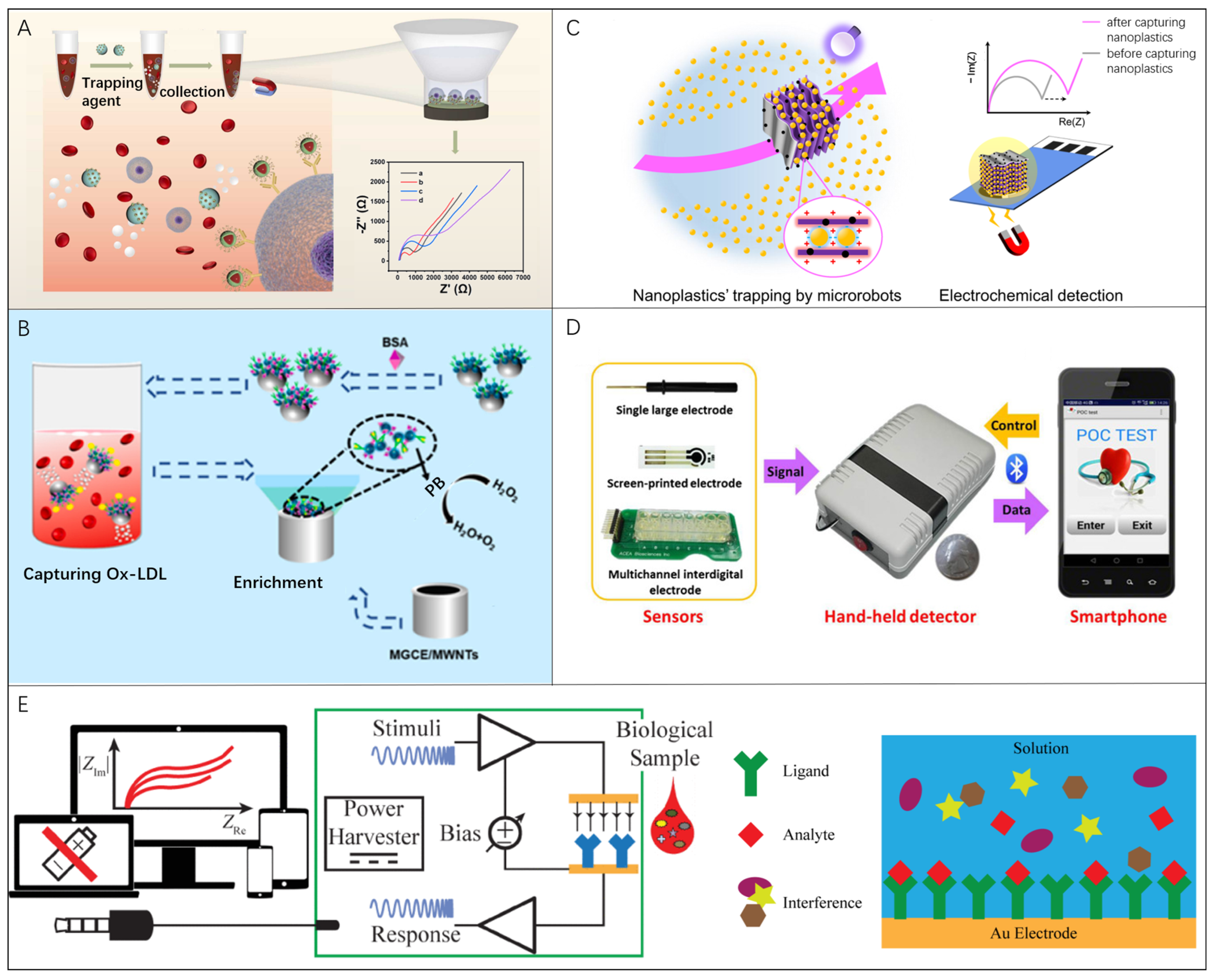
3.4. Microrobots in Electrochemical Current-Based Biosensing
3.5. Microrobots in Electrochemical Impedance Spectroscopy Biosensing
4. Conclusions and Perspective
- (i)
- Development of new synthesis strategy and surface modification to rise the loading capacity. Microrobots serving as active probes for biosensing tests are mostly fabricated with expensive materials and the surface modification is time consuming. Creating microrobots that use inorganic materials should be more efficient for mass production. Solvothermal/hydrothermal methods and template-assisted electrodeposition are adopted to synthesize microrobots just by one-step fabrication. Moreover, a large number of inorganic microrobots are featured with porous structures, which are better suited for rising the loading capacity. Based on the capability of mass production and enough space for surface modification, inorganic microrobots become excellent candidates to be applied in biosensor systems.
- (ii)
- Accurate swarm cooperation mechanism to overcome disturbance from harsh environments. Due to the high viscosity and large concentration of ions of tested specimens, microrobot individuals are difficult to effectively move because of the weak propulsion force. Based on swarm cooperation, one group of microrobots can aggregate together to output a large propulsion force together to overcome the disturbance. In general, the microrobot swarm powered by ultrasound maintains the highest locomotion speed and thus are primarily considered to be implemented for harsh environments in the biosensor platform. However, the method for accurately controlling the superfast movement of the ultrasonic microrobot swarm is lacking. With the development of metamaterials, unusual physical fields can be generated to modulate the spatial intensity and temporal variation, which can further improve the control accuracy of different microrobot swarms.
- (iii)
- Auxiliary instrument minimization for driving microrobots. It becomes easy for ordinary people to obtain commercialized biosensors, which are always designed to be portable or wearable for daily use. However, the operation of microrobots requires many auxiliary instruments closely related to the working principles. Most instruments for physically powered microrobots are bulky and expensive, and can only be implemented in the laboratory. With advanced electronic integrated circuit technology, it is possible to shrink these components to a minimized size, which can be well matched with the portable biosensing system. Regarding the costs for commercialization, detachable driving instruments are feasible if the microrobots can be effectively maneuvered to work inside biosensors.
- (iv)
- Reliable control algorithms to realize the self-adaptable microrobot locomotion. Currently, microrobots can be controlled to perform 2D in-plane movement, but long-distance locomotion along intricate routes is still not yet well demonstrated. For biological applications, multi-functional tests for different properties are sequentially accomplished on the same piece of biosensor with distributed testing regions. The fixed program setting is impossible to control microrobots to move effectively from one region to another in mazy microchannels. An artificial neural network control algorithm is a good option to be integrated with the manipulation system for dynamically optimizing the driving signals, enabling microrobots to be self-adaptable when performing multiple tasks in long-distance locomotion.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Rezazadeh, M.; Seidi, S.; Lid, M.; Pedersen-Bjergaard, S.; Yamini, Y. The modern role of smartphones in analytical chemistry. TrAC Trends Anal. Chem. 2019, 118, 548–555. [Google Scholar] [CrossRef]
- Olorunyomi, J.F.; Geh, S.T.; Caruso, R.A.; Doherty, C.M. Metal-organic frameworks for chemical sensing devices. Mater. Horiz. 2021, 8, 2387–2419. [Google Scholar] [CrossRef] [PubMed]
- Noviana, E.; Carrao, D.B.; Pratiwi, R.; Henry, C.S. Emerging applications of paper-based analytical devices for drug analysis: A review. Anal. Chim. Acta 2020, 1116, 70–90. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Chen, J.; Li, N.; Ji, X.; Yu, M.; He, Z. Microfluidic generation of magnetic-fluorescent Janus microparticles for biomolecular detection. Talanta 2016, 151, 126–131. [Google Scholar] [CrossRef]
- Liu, Y.; Hao, M.; Chen, Z.; Liu, L.; Liu, Y.; Yang, W.; Ramakrishna, S. A review on recent advances in application of electrospun nanofiber materials as biosensors. Curr. Opin. Biomed. Eng. 2020, 13, 174–189. [Google Scholar] [CrossRef]
- Mohankumar, P.; Ajayan, J.; Mohanraj, T.; Yasodharan, R. Recent developments in biosensors for healthcare and biomedical applications: A review. Measurement 2021, 167, 108293. [Google Scholar] [CrossRef]
- Rocchitta, G.; Spanu, A.; Babudieri, S.; Latte, G.; Madeddu, G.; Galleri, G.; Nuvoli, S.; Bagella, P.; Demartis, M.I.; Fiore, V.; et al. Enzyme Biosensors for Biomedical Applications: Strategies for Safeguarding Analytical Performances in Biological Fluids. Sensors 2016, 16, 780. [Google Scholar] [CrossRef]
- Kholafazad-Kordasht, H.; Hasanzadeh, M.; Seidi, F. Smartphone based immunosensors as next generation of healthcare tools: Technical and analytical overview towards improvement of personalized medicine. Trac-Trends Anal. Chem. 2021, 145, 116455. [Google Scholar] [CrossRef]
- Xiao, M.; Tian, F.; Liu, X.; Zhou, Q.; Pan, J.; Luo, Z.; Yang, M.; Yi, C. Virus Detection: From State-of-the-Art Laboratories to Smartphone-Based Point-of-Care Testing. Adv. Sci. 2022, 9, 2105904. [Google Scholar] [CrossRef]
- Jafari, S.; Guercetti, J.; Geballa-Koukoula, A.; Tsagkaris, A.S.; Nelis, J.L.D.; Marco, M.P.; Salvador, J.P.; Gerssen, A.; Hajslova, J.; Elliott, C.; et al. ASSURED Point-of-Need Food Safety Screening: A Critical Assessment of Portable Food Analyzers. Foods 2021, 10, 1399. [Google Scholar] [CrossRef]
- Zhao, W.; Tian, S.; Huang, L.; Liu, K.; Dong, L.; Guo, J. A smartphone-based biomedical sensory system. Analyst 2020, 145, 2873–2891. [Google Scholar] [CrossRef]
- Barragan, J.T.C.; Kubota, L.T. Minipotentiostat controlled by smartphone on a micropipette: A versatile, portable, agile and accurate tool for electroanalysis. Electrochim. Acta 2020, 341, 136048. [Google Scholar] [CrossRef]
- Danyliuk, N.; Tatarchuk, T.; Kannan, K.; Shyichuk, A. Optimization of TiO2-P25 photocatalyst dose and H2O2 concentration for advanced photooxidation using the smartphone-based colorimetry. Water Sci. Technol. 2021, 84, 469–483. [Google Scholar] [CrossRef]
- Golicz, K.; Hallett, S.; Sakrabani, R.; Ghosh, J. Adapting smartphone app used in water testing, for soil nutrient analysis. Comput. Electron. Agric. 2020, 175, 9. [Google Scholar] [CrossRef]
- Özdemir, G.K.; Bayram, A.; Kiliç, V.; Horzum, N.; Solmaz, M.E. Smartphone-based detection of dyes in water for environmental sustainability. Anal. Methods 2017, 9, 579–585. [Google Scholar] [CrossRef]
- Shogah, Z.A.C.; Bolshakov, D.S.; Amelin, V.G. Using Smartphones in Chemical Analysis. J. Anal. Chem. 2023, 78, 426–449. [Google Scholar] [CrossRef]
- Salinas, G.; Pavel, I.A.; Sojic, N.; Kuhn, A. Electrochemistry-Based Light-Emitting Mobile Systems. ChemElectroChem 2020, 7, 4853–4862. [Google Scholar] [CrossRef]
- Karshalev, E.; Esteban-Fernandez de Avila, B.; Wang, J. Micromotors for “Chemistry-on-the-Fly”. J. Am. Chem. Soc. 2018, 140, 3810–3820. [Google Scholar] [CrossRef]
- Joshi, S.; Sharma, P.; Siddiqui, R.; Kaushal, K.; Sharma, S.; Verma, G.; Saini, A. A review on peptide functionalized graphene derivatives as nanotools for biosensing. Microchim. Acta 2019, 187, 27. [Google Scholar] [CrossRef] [PubMed]
- Campuzano, S.; Kagan, D.; Orozco, J.; Wang, J. Motion-driven sensing and biosensing using electrochemically propelled nanomotors. Analyst 2011, 136, 4621–4630. [Google Scholar] [CrossRef]
- Jurado-Sánchez, B.; Escarpa, A. Milli, micro and nanomotors: Novel analytical tools for real-world applications. TrAC Trends Anal. Chem. 2016, 84, 48–59. [Google Scholar] [CrossRef]
- Jurado-Sánchez, B.; Escarpa, A. Janus Micromotors for Electrochemical Sensing and Biosensing Applications: A Review. Electroanalysis 2017, 29, 14–23. [Google Scholar] [CrossRef]
- Wang, J. Self-propelled affinity biosensors: Moving the receptor around the sample. Biosens. Bioelectron. 2016, 76, 234–242. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Shen, H.; Zhao, K.; Wang, Z.; Peng, H.; Liu, W. Micro-/Nanomachines Driven by Ultrasonic Power Sources. Chem. Asian J. 2019, 14, 2406–2416. [Google Scholar] [CrossRef]
- Zhu, C.; Yang, G.; Li, H.; Du, D.; Lin, Y. Electrochemical sensors and biosensors based on nanomaterials and nanostructures. Anal. Chem. 2015, 87, 230–249. [Google Scholar] [CrossRef]
- Campuzano, S.; Esteban-Fernandez de Avila, B.; Yanez-Sedeno, P.; Pingarron, J.M.; Wang, J. Nano/microvehicles for efficient delivery and (bio)sensing at the cellular level. Chem. Sci. 2017, 8, 6750–6763. [Google Scholar] [CrossRef]
- Wu, J.; Balasubramanian, S.; Kagan, D.; Manesh, K.M.; Campuzano, S.; Wang, J. Motion-based DNA detection using catalytic nanomotors. Nat. Commun. 2010, 1, 36. [Google Scholar] [CrossRef]
- Kong, L.; Guan, J.; Pumera, M. Micro- and nanorobots based sensing and biosensing. Curr. Opin. Electrochem. 2018, 10, 174–182. [Google Scholar] [CrossRef]
- Lu, X.; Shen, H.; Wei, Y.; Ge, H.; Wang, J.; Peng, H.; Liu, W. Ultrafast Growth and Locomotion of Dandelion-Like Microswarms with Tubular Micromotors. Small 2020, 16, 2003678. [Google Scholar] [CrossRef]
- Lu, X.; Wei, Y.; Ou, H.; Zhao, C.; Shi, L.; Liu, W. Universal Control for Micromotor Swarms with a Hybrid Sonoelectrode. Small 2021, 17, 2104516. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Zhao, K.; Liu, W.; Yang, D.; Shen, H.; Peng, H.; Guo, X.; Li, J.; Wang, J. A Human Microrobot Interface Based on Acoustic Manipulation. ACS Nano 2019, 13, 11443–11452. [Google Scholar] [CrossRef]
- Moo, J.G.; Wang, H.; Zhao, G.; Pumera, M. Biomimetic artificial inorganic enzyme-free self-propelled microfish robot for selective detection of Pb(2+) in water. Chemistry 2014, 20, 4292–4296. [Google Scholar] [CrossRef] [PubMed]
- Bunea, A.I.; Pavel, I.A.; David, S.; Gaspar, S. Sensing based on the motion of enzyme-modified nanorods. Biosens. Bioelectron. 2015, 67, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Li, J.X.; de Avila, B.E.F.; Gao, W.; Zhang, L.F.; Wang, J. Micro/nanorobots for biomedicine: Delivery, surgery, sensing, and detoxification. Sci. Robot. 2017, 2, eaam6431. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Chen, X.; Lu, X.; Wang, J.; Zhang, Y.; Gu, Z. From Passive Inorganic Oxides to Active Matters of Micro/Nanomotors. Adv. Funct. Mater. 2020, 30, 2003195. [Google Scholar] [CrossRef]
- Soto, F.; Karshalev, E.; Zhang, F.; de Avila, B.E.F.; Nourhani, A.; Wang, J. Smart Materials for Microrobots. Chem. Rev. 2022, 122, 5365–5403. [Google Scholar] [CrossRef]
- Wang, B.; Kostarelos, K.; Nelson, B.J.; Zhang, L. Trends in Micro-/Nanorobotics: Materials Development, Actuation, Localization, and System Integration for Biomedical Applications. Adv. Mater. 2021, 33, 2002047. [Google Scholar] [CrossRef]
- Patra, D.; Sengupta, S.; Duan, W.T.; Zhang, H.; Pavlick, R.; Sen, A. Intelligent, self-powered, drug delivery systems. Nanoscale 2013, 5, 1273–1283. [Google Scholar] [CrossRef]
- Chatzipirpiridis, G.; Ergeneman, O.; Pokki, J.; Ullrich, F.; Fusco, S.; Ortega, J.A.; Sivaraman, K.M.; Nelson, B.J.; Pane, S. Electroforming of Implantable Tubular Magnetic Microrobots for Wireless Ophthalmologic Applications. Adv. Healthc. Mater. 2015, 4, 209–214. [Google Scholar] [CrossRef]
- Kagan, D.; Campuzano, S.; Balasubramanian, S.; Kuralay, F.; Flechsig, G.U.; Wang, J. Functionalized Micromachines for Selective and Rapid Isolation of Nucleic Acid Targets from Complex Samples. Nano Lett. 2011, 11, 2083–2087. [Google Scholar] [CrossRef]
- Yuan, K.; de la Asunción-Nadal, V.; Cuntin-Abal, C.; Jurado-Sánchez, B.; Escarpa, A. On-board smartphone micromotor-based fluorescence assays. Lab Chip 2022, 22, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhou, C.; Wang, W.; Xu, D.; Zeng, F.; Zhan, C.; Gu, J.; Li, M.; Zhao, W.; Zhang, J.; et al. Photocatalytically Powered Matchlike Nanomotor for Light-Guided Active SERS Sensing. Angew. Chem. Int. Ed. Engl. 2018, 57, 13110–13113. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; Cuntín-Abal, C.; Jurado-Sánchez, B.; Escarpa, A. Smartphone-Based Janus Micromotors Strategy for Motion-Based Detection of Glutathione. Anal. Chem. 2021, 93, 16385–16392. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Mayorga-Martinez, C.C.; Vyskocil, J.; Ruzek, D.; Pumera, M. Plasmonic-magnetic nanorobots for SARS-CoV-2 RNA detection through electronic readout. Appl. Mater. Today 2022, 27, 101402. [Google Scholar] [CrossRef]
- Mayorga-Martinez, C.C.; Vyskočil, J.; Novotný, F.; Bednar, P.; Ruzek, D.; Alduhaishe, O.; Pumera, M. Collective behavior of magnetic microrobots through immuno-sandwich assay: On-the-fly COVID-19 sensing. Appl. Mater. Today 2022, 26, 101337. [Google Scholar] [CrossRef]
- Rojas, D.; Jurado-Sánchez, B.; Escarpa, A. “Shoot and Sense” Janus Micromotors-Based Strategy for the Simultaneous Degradation and Detection of Persistent Organic Pollutants in Food and Biological Samples. Anal. Chem. 2016, 88, 4153–4160. [Google Scholar] [CrossRef]
- Urso, M.; Ussia, M.; Novotny, F.; Pumera, M. Trapping and detecting nanoplastics by MXene-derived oxide microrobots. Nat. Commun. 2022, 13, 3573. [Google Scholar] [CrossRef]
- Wei, Y.; Lu, X.L.; Ou, H.; Li, Z.Y.; Liu, Y.L.; Bao, J.H.; Yin, J.; Liu, W.J. Acoustically powered micro-sonobots for enhanced fluorescence biodetection. Int. J. Mech. Sci. 2023, 248, 11. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, W.; Sun, Y. Self-Propelled Micro-/Nanomotors as “On-the-Move” Platforms: Cleaners, Sensors, and Reactors. Adv. Funct. Mater. 2021, 32, 2109181. [Google Scholar] [CrossRef]
- Jurado-Sanchez, B.; Campuzano, S.; Pingarron, J.M.; Escarpa, A. Janus particles and motors: Unrivaled devices for mastering (bio)sensing. Microchim. Acta 2021, 188, 416. [Google Scholar] [CrossRef] [PubMed]
- Moo, J.G.S.; Mayorga-Martinez, C.C.; Wang, H.; Khezri, B.; Teo, W.Z.; Pumera, M. Nano/Microrobots Meet Electrochemistry. Adv. Funct. Mater. 2017, 27, 1604759. [Google Scholar] [CrossRef]
- Oja, S.M.; Fan, Y.; Armstrong, C.M.; Defnet, P.; Zhang, B. Nanoscale Electrochemistry Revisited. Anal. Chem. 2016, 88, 414–430. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, M.; Lopez, M.A.; Jurado-Sanchez, B.; Escarpa, A. Self-propelled micromachines for analytical sensing: A critical review. Anal. Bioanal. Chem. 2019, 411, 6561–6573. [Google Scholar] [CrossRef]
- Yánez-Sedeño, P.; Campuzano, S.; Pingarrón, J.M. Janus particles for (bio)sensing. Appl. Mater. Today 2017, 9, 276–288. [Google Scholar] [CrossRef]
- Yi, Y.; Sanchez, L.; Gao, Y.; Yu, Y. Janus particles for biological imaging and sensing. Analyst 2016, 141, 3526–3539. [Google Scholar] [CrossRef]
- Draz, M.S.; Kochehbyoki, K.M.; Vasan, A.; Battalapalli, D.; Sreeram, A.; Kanakasabapathy, M.K.; Kallakuri, S.; Tsibris, A.; Kuritzkes, D.R.; Shafiee, H. DNA engineered micromotors powered by metal nanoparticles for motion based cellphone diagnostics. Nat. Commun. 2018, 9, 4282. [Google Scholar] [CrossRef] [PubMed]
- Esteban-Fernández de Ávila, B.; Lopez-Ramirez, M.A.; Báez, D.F.; Jodra, A.; Singh, V.V.; Kaufmann, K.; Wang, J. Aptamer-Modified Graphene-Based Catalytic Micromotors: Off–On Fluorescent Detection of Ricin. ACS Sens. 2016, 1, 217–221. [Google Scholar] [CrossRef]
- Molinero-Fernández, Á.; Jodra, A.; Moreno-Guzmán, M.; Lopez, M.Á.; Escarpa, A. Magnetic Reduced Graphene Oxide/Nickel/Platinum Nanoparticles Micromotors for Mycotoxin Analysis. Chemistry 2018, 24, 7172–7176. [Google Scholar] [CrossRef]
- Jurado-Sánchez, B.; Escarpa, A.; Wang, J. Lighting up micromotors with quantum dots for smart chemical sensing. Chem. Commun. 2015, 51, 14088–14091. [Google Scholar] [CrossRef]
- Fan, X.; Hao, Q.; Li, M.; Zhang, X.; Yang, X.; Mei, Y.; Qiu, T. Hotspots on the Move: Active Molecular Enrichment by Hierarchically Structured Micromotors for Ultrasensitive SERS Sensing. ACS Appl. Mater. Interfaces 2020, 12, 28783–28791. [Google Scholar] [CrossRef]
- Chen, Q.; Guo, W.Y.; Fang, D.; Li, T.; Chen, L.; Mao, C.; Wan, M.M.; Shen, J. Mg-based micromotors for efficient electrochemical detection of circulating tumor cells. Sens. Actuator B-Chem. 2023, 390, 10. [Google Scholar] [CrossRef]
- Chen, B.; Huang, Q.L.; Qu, Z.B.; Li, C.; Li, Q.; Shi, J.Y.; Fan, C.H.; Wang, L.H.; Zuo, X.L.; Shen, J.L.; et al. Probing Transient DNA Conformation Changes with an Intercalative Fluorescent Excimer. Angew. Chem.-Int. Edit. 2021, 60, 6624–6630. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.H.; Hu, C.G.; Zhao, Y.; Qu, L.T. Graphene fiber: A new material platform for unique applications. Npg Asia Mater. 2014, 6, 13. [Google Scholar] [CrossRef]
- Shao, Y.Y.; Wang, J.; Engelhard, M.; Wang, C.M.; Lin, Y.H. Facile and controllable electrochemical reduction of graphene oxide and its applications. J. Mater. Chem. 2010, 20, 743–748. [Google Scholar] [CrossRef]
- Lu, C.H.; Yang, H.H.; Zhu, C.L.; Chen, X.; Chen, G.N. A Graphene Platform for Sensing Biomolecules. Angew. Chem. Int. Edit. 2009, 48, 4785–4787. [Google Scholar] [CrossRef] [PubMed]
- Morales-Narváez, E.; Merkoçi, A. Graphene Oxide as an Optical Biosensing Platform. Adv. Mater. 2012, 24, 3298–3308. [Google Scholar] [CrossRef]
- Pérez-López, B.; Merkoçi, A. Carbon nanotubes and graphene in analytical sciences. Microchim. Acta 2012, 179, 1–16. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Gehlot, P.; Sidapra, K.; Edwards, A.D.; Reis, N.M. Portable smartphone quantitation of prostate specific antigen (PSA) in a fluoropolymer microfluidic device. Biosens. Bioelectron. 2015, 70, 5–14. [Google Scholar] [CrossRef]
- Kim, Y.S.; Raston, N.H.; Gu, M.B. Aptamer-based nanobiosensors. Biosens. Bioelectron. 2016, 76, 2–19. [Google Scholar]
- Beltrán-Gasttélum, M.; Esteban-Fernández de Ávila, B.; Gong, H.; Venugopalan, P.L.; Hianik, T.; Wang, J.; Subjakova, V. Rapid Detection of AIB1 in Breast Cancer Cells Based on Aptamer-Functionalized Nanomotors. ChemPhysChem 2019, 20, 3177–3180. [Google Scholar] [CrossRef]
- de Ávila, B.E.F.; Martin, A.; Soto, F.; Lopez-Ramirez, M.A.; Campuzano, S.; Vasquez-Machado, G.M.; Gao, W.W.; Zhang, L.F.; Wang, J. Single Cell Real-Time miRNAs Sensing Based on Nanomotors. ACS Nano 2015, 9, 6756–6764. [Google Scholar] [CrossRef]
- Khoshbin, Z.; Zamanian, J.; Davoodian, N.; Mohammad Danesh, N.; Ramezani, M.; Alibolandi, M.; Abnous, K.; Taghdisi, S.M. A simple and ultrasensitive metal-organic framework-based aptasensor for fluorescence detection of ethanolamine. Spectrochim. Acta Part A 2022, 267, 120488. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, M.; Jurado-Sánchez, B.; Escarpa, A. Sensitive Monitoring of Enterobacterial Contamination of Food Using Self-Propelled Janus Microsensors. Anal. Chem. 2018, 90, 2912–2917. [Google Scholar] [CrossRef]
- Molinero-Fernández, Á.; Moreno-Guzmán, M.; López, M.Á.; Escarpa, A. Biosensing Strategy for Simultaneous and Accurate Quantitative Analysis of Mycotoxins in Food Samples Using Unmodified Graphene Micromotors. Anal. Chem. 2017, 89, 10850–10857. [Google Scholar] [CrossRef]
- Song, S.; Wang, L.; Li, J.; Fan, C.; Zhao, J. Aptamer-based biosensors. TrAC Trends Anal. Chem. 2008, 27, 108–117. [Google Scholar] [CrossRef]
- Zhu, C.; Zeng, Z.; Li, H.; Li, F.; Fan, C.; Zhang, H. Single-Layer MoS2-Based Nanoprobes for Homogeneous Detection of Biomolecules. J. Am. Chem. Soc. 2013, 135, 5998–6001. [Google Scholar] [CrossRef]
- Kou, H.-S.; Lo, S.-T.; Wang, C.-C. One Single Tube Reaction of Aptasensor-Based Magnetic Sensing System for Selective Fluorescent Detection of VEGF in Plasma. Biosensors 2023, 13, 574. [Google Scholar] [CrossRef]
- Azzouz, A.; Kumar, V.; Hejji, L.; Kim, K.-H. Advancements in nanomaterial-based aptasensors for the detection of emerging organic pollutants in environmental and biological samples. Biotechnol. Adv. 2023, 66, 108156. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, L.; Qiao, L.; Deng, F. Universal CRISPR/Cas12a-associated aptasensor suitable for rapid detection of small proteins with a plate reader. Front. Bioeng. Biotechnol. 2023, 11, 1201175. [Google Scholar] [CrossRef]
- Song, P.; Fan, C. Selecting aptamers with programmed affinities. Nat. Chem. 2023, 15, 747–748. [Google Scholar] [CrossRef] [PubMed]
- Kara, N.; Ayoub, N.; Ilgu, H.; Fotiadis, D.; Ilgu, M. Aptamers Targeting Membrane Proteins for Sensor and Diagnostic Applications. Molecules 2023, 28, 3728. [Google Scholar] [CrossRef]
- Mahmud, A.; Chang, D.; Das, J.; Gomis, S.; Foroutan, F.; Chen, J.B.; Pandey, L.; Flynn, C.D.; Yousefi, H.; Geraili, A.; et al. Monitoring Cardiac Biomarkers with Aptamer-Based Molecular Pendulum Sensors. Angew. Chem. Int. Edit. 2023, 62, e202213567. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Luo, J.; Jing, L.; Wang, Y.; Shen, H.; Yu, R.; Sun, S.; Xing, Y.; Ming, T.; Liu, M.; et al. Reduced Graphene Oxide and Gold Nanoparticles-Modified Electrochemical Aptasensor for Highly Sensitive Detection of Doxorubicin. Nanomaterials 2023, 13, 1223. [Google Scholar] [CrossRef] [PubMed]
- Yuan, K.; López, M.A.; Jurado-Sánchez, B.; Escarpa, A. Janus Micromotors Coated with 2D Nanomaterials as Dynamic Interfaces for (Bio)-Sensing. ACS Appl. Mater. Interfaces 2020, 12, 46588–46597. [Google Scholar] [CrossRef]
- Cardinal, M.F.; Vander Ende, E.; Hackler, R.A.; McAnally, M.O.; Stair, P.C.; Schatz, G.C.; Van Duyne, R.P. Expanding applications of SERS through versatile nanomaterials engineering. Chem. Soc. Rev. 2017, 46, 3886–3903. [Google Scholar] [CrossRef] [PubMed]
- Duan, B.; Hou, S.; Wang, P.; Chen, Y.; Xiong, Q.; Das, P.; Duan, H. Chemical processing of interfacially assembled metal nanowires for surface-enhanced Raman scattering detection of food contaminants. J. Raman Spectrosc. 2020, 52, 532–540. [Google Scholar] [CrossRef]
- Gahlaut, S.K.; Savargaonkar, D.; Sharan, C.; Yadav, S.; Mishra, P.; Singh, J.P. SERS Platform for Dengue Diagnosis from Clinical Samples Employing a Hand Held Raman Spectrometer. Anal. Chem. 2020, 92, 2527–2534. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; He, X.; Bensong, C. Urchin-like ZnO-nanorod arrays templated growth of ordered hierarchical Ag/ZnO hybrid arrays for surface-enhanced Raman scattering. Nanotechnology 2020, 31, 165301. [Google Scholar] [CrossRef]
- Feng, E.; Tian, Y. Surface-enhanced Raman Scattering of Self-assembled Superstructures. Chem. Res. Chin. Univ. 2021, 37, 989–1007. [Google Scholar] [CrossRef]
- García-Lojo, D.; Gómez-Graña, S.; Martin, V.F.; Solís, D.M.; Taboada, J.M.; Pérez-Juste, J.; Pastoriza-Santos, I. Integrating Plasmonic Supercrystals in Microfluidics for Ultrasensitive, Label-Free, and Selective Surface-Enhanced Raman Spectroscopy Detection. ACS Appl. Mater. Interfaces 2020, 12, 46557–46564. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Li, Y.; Xu, D.; Pan, X.; Chen, Y.; Zhou, D.; Wang, B.; Feng, H.; Ma, X. Magnetic Nanomotor-Based Maneuverable SERS Probe. Research 2020, 2020, 7962024. [Google Scholar] [CrossRef]
- Jia, X.; Liu, Z.; Peng, Y.; Hou, G.; Chen, W.; Xiao, R. Automatic and sensitive detection of West Nile virus non-structural protein 1 with a portable SERS-LFIA detector. Microchim. Acta 2021, 188, 206. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liu, Z.; Wang, W.; Tian, Y. Real-time Tracking and Sensing of Cu(+) and Cu(2+) with a Single SERS Probe in the Live Brain: Toward Understanding Why Copper Ions Were Increased upon Ischemia. Angew. Chem. Int. Ed. Engl. 2021, 60, 21351–21359. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Bai, Y.C.; Zhang, L.; Yang, Z.B.; Fan, Q.K.; Zheng, H.Q.; Yin, Y.D.; Gao, C.B. Porous Au-Ag Nanospheres with High-Density and Highly Accessible Hotspots for SERS Analysis. Nano Lett. 2016, 16, 3675–3681. [Google Scholar] [CrossRef]
- Liu, X.; Yang, S.; Lyu, X.; Liu, S.; Wang, Y.; Li, Y.; Wang, B.; Chen, W.; Wang, W.; Guo, J.; et al. Instant Preparation of Ultraclean Gold Nanothorns under Ambient Conditions for SERS Kit-Enabled Mobile Diagnosis. Anal. Chem. 2021, 93, 16628–16637. [Google Scholar] [CrossRef]
- Qian, X.M.; Nie, S.M. Single-molecule and single-nanoparticle SERS: From fundamental mechanisms to biomedical applications. Chem. Soc. Rev. 2008, 37, 912–920. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Shen, Y.; Xie, A. An assembled ordered W18O49 nanowire film with high SERS sensitivity and stability for the detection of RB. Appl. Surf. Sci. 2020, 504, 144073. [Google Scholar] [CrossRef]
- Lu, Y.; Lin, L.; Ye, J. Human metabolite detection by surface-enhanced Raman spectroscopy. Mater. Today Bio 2022, 13, 100205. [Google Scholar] [CrossRef]
- Shan, F.; Zhang, X.Y.; Fu, X.C.; Zhang, L.J.; Su, D.; Wang, S.J.; Wu, J.Y.; Zhang, T. Investigation of simultaneously existed Raman scattering enhancement and inhibiting fluorescence using surface modified gold nanostars as SERS probes. Sci. Rep. 2017, 7, 6813. [Google Scholar] [CrossRef]
- Srivastav, S.; Dankov, A.; Adanalic, M.; Grzeschik, R.; Tran, V.; Pagel-Wieder, S.; Gessler, F.; Spreitzer, I.; Scholz, T.; Schnierle, B.; et al. Rapid and Sensitive SERS-Based Lateral Flow Test for SARS-CoV2-Specific IgM/IgG Antibodies. Anal. Chem. 2021, 93, 12391–12399. [Google Scholar] [CrossRef]
- Wang, W.; Li, Y.; Nie, A.; Fan, G.C.; Han, H. A portable SERS reader coupled with catalytic hairpin assembly for sensitive microRNA-21 lateral flow sensing. Analyst 2021, 146, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Dai, E.; Xiao, R.; Zhou, Z.; Zhang, M.; Bai, Z.; Shao, Y.; Qi, K.; Tu, J.; Wang, C.; et al. Development of a SERS-based lateral flow immunoassay for rapid and ultra-sensitive detection of anti-SARS-CoV-2 IgM/IgG in clinical samples. Sens. Actuators B 2021, 329, 129196. [Google Scholar] [CrossRef]
- Li, Y.; Liu, X.; Guo, J.; Zhang, Y.; Guo, J.; Wu, X.; Wang, B.; Ma, X. Simultaneous Detection of Inflammatory Biomarkers by SERS Nanotag-Based Lateral Flow Assay with Portable Cloud Raman Spectrometer. Nanomaterials 2021, 11, 1496. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.; Walkenfort, B.; König, M.; Salehi, M.; Schlücker, S. Rapid, Quantitative, and Ultrasensitive Point-of-Care Testing: A Portable SERS Reader for Lateral Flow Assays in Clinical Chemistry. Angew. Chem. Int. Ed. Engl. 2019, 58, 442–446. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhao, H.; Cai, Y.; Jurado-Sanchez, B.; Dong, R. Recent Advances in One-Dimensional Micro/Nanomotors: Fabrication, Propulsion and Application. Nano-Micro Lett. 2023, 15, 20. [Google Scholar] [CrossRef]
- Thome, C.P.; Hoertdoerfer, W.S.; Bendorf, J.R.; Lee, J.G.; Shields, C.W. Electrokinetic Active Particles for Motion-Based Biomolecule Detection. Nano Lett. 2023, 23, 2379–2387. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Xu, D.; Zhang, Z.; Li, N.; Zhao, Y. Tailoring Functional Micromotors for Sensing. Research 2023, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Popescu, M.N.; Gaspar, S. Analyte Sensing with Catalytic Micromotors. Biosensors 2023, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, P.; Zhang, S.; Zhu, K.; Shangguan, X.; Liu, L.; Zhang, S. Application of Janus Particles in Point-of-Care Testing. Biosensors 2022, 12, 689. [Google Scholar] [CrossRef]
- Chang, S.H. Micro/nanomotors for metal ion detection and removal from water: A review. Mater. Today Sustain. 2022, 19, 100196. [Google Scholar] [CrossRef]
- Moreno-Guzman, M.; Jodra, A.; López, M.A.; Escarpa, A. Self-Propelled Enzyme-Based Motors for Smart Mobile Electrochemical and Optical Biosensing. Anal. Chem. 2015, 87, 12380–12386. [Google Scholar] [CrossRef]
- Orozco, J.; Garcia-Gradilla, V.; D’Agostino, M.; Gao, W.; Cortés, A.; Wang, J. Artificial Enzyme-Powered Microfish for Water-Quality Testing. ACS Nano 2013, 7, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, C.; Wu, J.; Ju, H. Bubble-Propelled Jellyfish-like Micromotors for DNA Sensing. ACS Appl. Mater. Interfaces 2019, 11, 13581–13588. [Google Scholar] [CrossRef] [PubMed]
- Draz, M.S.; Lakshminaraasimulu, N.K.; Krishnakumar, S.; Battalapalli, D.; Vasan, A.; Kanakasabapathy, M.K.; Sreeram, A.; Kallakuri, S.; Thirumalaraju, P.; Li, Y.; et al. Motion-Based Immunological Detection of Zika Virus Using Pt-Nanomotors and a Cellphone. ACS Nano 2018, 12, 5709–5718. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.C.; Ataide, V.N.; Silva Chagas, C.L.; Angnes, L.; Tomazelli Coltro, W.K.; Longo Cesar Paixao, T.R.; de Araujo, W.R. Wearable electrochemical sensors for forensic and clinical applications. Trac-Trends Anal. Chem. 2019, 119, 115622. [Google Scholar] [CrossRef]
- Naghdi, T.; Ardalan, S.; Adib, Z.A.; Sharifi, A.R.; Golmohammadi, H. Moving toward smart biomedical sensing. Biosens. Bioelectron. 2023, 223, 115009. [Google Scholar] [CrossRef]
- Madrid, R.E.; Ramallo, F.A.; Barraza, D.E.; Chaile, R.E. Smartphone-Based Biosensor Devices for Healthcare: Technologies, Trends, and Adoption by End-Users. Bioengineering 2022, 9, 101. [Google Scholar] [CrossRef]
- Lyu, Q.; Gong, S.; Dyson, J.M.; Cheng, W. Soft, Disruptive and Wearable Electrochemical Biosensors. Curr. Anal. Chem. 2022, 18, 689–704. [Google Scholar] [CrossRef]
- Sinha, G.N.; Subramanyam, P.; Sivaramakrishna, V.; Subrahmanyam, C. Electrodeposited copper bismuth oxide as a low-cost, non-enzymatic electrochemical sensor for sensitive detection of uric acid and hydrogen peroxide. Inorg. Chem. Commun. 2021, 129, 108627. [Google Scholar] [CrossRef]
- Abellán-Llobregat, A.; Jeerapan, I.; Bandodkar, A.; Vidal, L.; Canals, A.; Wang, J.; Morallón, E. A stretchable and screen-printed electrochemical sensor for glucose determination in human perspiration. Biosens. Bioelectron. 2017, 91, 885–891. [Google Scholar] [CrossRef]
- Chia, H.L.; Mayorga-Martinez, C.C.; Gusmao, R.; Novotny, F.; Webster, R.D.; Pumera, M. A highly sensitive enzyme-less glucose sensor based on pnictogens and silver shell-gold core nanorod composites. Chem. Commun. 2020, 56, 7909–7912. [Google Scholar] [CrossRef]
- Jain, U.; Chauhan, N. Glycated hemoglobin detection with electrochemical sensing amplified by gold nanoparticles embedded N-doped graphene nanosheet. Biosens. Bioelectron. 2017, 89, 578–584. [Google Scholar] [CrossRef]
- Ji, D.; Liu, L.; Li, S.; Chen, C.; Lu, Y.; Wu, J.; Liu, Q. Smartphone-based cyclic voltammetry system with graphene modified screen printed electrodes for glucose detection. Biosens. Bioelectron. 2017, 98, 449–456. [Google Scholar] [CrossRef]
- Redondo, E.; Pumera, M. Fully metallic copper 3D-printed electrodes via sintering for electrocatalytic biosensing. Appl. Mater. Today 2021, 25, 101253. [Google Scholar] [CrossRef]
- Shen, X.; Ju, F.; Li, G.; Ma, L. Smartphone-Based Electrochemical Potentiostat Detection System Using PEDOT: PSS/Chitosan/Graphene Modified Screen-Printed Electrodes for Dopamine Detection. Sensors 2020, 20, 2781. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Q.; Shen, Y.; Zhang, J.; Zheng, J. Ni doped Ag@C core–shell nanomaterials and their application in electrochemical H2O2 sensing. Anal. Methods 2017, 9, 163–169. [Google Scholar] [CrossRef]
- Shi, L.; Layani, M.; Cai, X.; Zhao, H.; Magdassi, S.; Lan, M. An inkjet printed Ag electrode fabricated on plastic substrate with a chemical sintering approach for the electrochemical sensing of hydrogen peroxide. Sens. Actuators B 2018, 256, 938–945. [Google Scholar] [CrossRef]
- Wang, X.; Gartia, M.R.; Jiang, J.; Chang, T.-W.; Qian, J.; Liu, Y.; Liu, X.; Liu, G.L. Audio jack based miniaturized mobile phone electrochemical sensing platform. Sens. Actuators B 2015, 209, 677–685. [Google Scholar] [CrossRef]
- Xu, Y.; Dai, Y.; Li, C.; Zhang, H.; Guo, M.; Yang, Y. PC software-based portable cyclic voltammetry system with PB-MCNT-GNPs-modified electrodes for E. coli detection. Rev. Sci. Instrum. 2020, 91, 014103. [Google Scholar] [CrossRef]
- Yusoff, N.; Rameshkumar, P.; Mehmood, M.S.; Pandikumar, A.; Lee, H.W.; Huang, N.M. Ternary nanohybrid of reduced graphene oxide-nafion@silver nanoparticles for boosting the sensor performance in non-enzymatic amperometric detection of hydrogen peroxide. Biosens. Bioelectron. 2017, 87, 1020–1028. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tian, Z.; Yang, S.; Rich, J.; Zhao, S.; Klingeborn, M.; Huang, P.H.; Li, Z.; Stout, A.; Murphy, Q.; et al. Electrochemical micro-aptasensors for exosome detection based on hybridization chain reaction amplification. Microsyst. Nanoeng. 2021, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Amouzadeh Tabrizi, M.; Shamsipur, M.; Saber, R.; Sarkar, S. Isolation of HL-60 cancer cells from the human serum sample using MnO2-PEI/Ni/Au/aptamer as a novel nanomotor and electrochemical determination of thereof by aptamer/gold nanoparticles-poly(3,4-ethylene dioxythiophene) modified GC electrode. Biosens. Bioelectron. 2018, 110, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Grossi, M.; Parolin, C.; Vitali, B.; Riccò, B. Electrical Impedance Spectroscopy (EIS) characterization of saline solutions with a low-cost portable measurement system. Eng. Sci. Technol. 2019, 22, 102–108. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, X.; Chao, R.; Ren, Y.; Hu, C.; Xu, Z.; Liu, G.L. Smartphone based portable bacteria pre-concentrating microfluidic sensor and impedance sensing system. Sens. Actuators B 2014, 193, 653–659. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, X.; Zhou, Z.; Han, Y.; Xiang, N.; Ni, Z. Microfluidic impedance cytometry for single-cell sensing: Review on electrode configurations. Talanta 2021, 233, 122571. [Google Scholar] [CrossRef]
- Fang, D.; Tang, S.W.; Wu, Z.Y.; Chen, C.L.; Wan, M.M.; Mao, C.; Zhou, M. Electrochemical sensor based on micromotor technology for detection of Ox-LDL in whole blood. Biosens. Bioelectron. 2022, 217, 6. [Google Scholar] [CrossRef]
- Zhang, D.; Lu, Y.; Zhang, Q.; Liu, L.; Li, S.; Yao, Y.; Jiang, J.; Liu, G.L.; Liu, Q. Protein detecting with smartphone-controlled electrochemical impedance spectroscopy for point-of-care applications. Sens. Actuators B 2016, 222, 994–1002. [Google Scholar] [CrossRef]
- Jiang, H.; Sun, A.; Venkatesh, A.G.; Hall, D.A. An Audio Jack-Based Electrochemical Impedance Spectroscopy Sensor for Point-of-Care Diagnostics. IEEE Sens. J. 2017, 17, 589–597. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Bao, J.; Wei, Y.; Zhang, S.; Liu, W.; Wu, J. Emerging Roles of Microrobots for Enhancing the Sensitivity of Biosensors. Nanomaterials 2023, 13, 2902. https://doi.org/10.3390/nano13212902
Lu X, Bao J, Wei Y, Zhang S, Liu W, Wu J. Emerging Roles of Microrobots for Enhancing the Sensitivity of Biosensors. Nanomaterials. 2023; 13(21):2902. https://doi.org/10.3390/nano13212902
Chicago/Turabian StyleLu, Xiaolong, Jinhui Bao, Ying Wei, Shuting Zhang, Wenjuan Liu, and Jie Wu. 2023. "Emerging Roles of Microrobots for Enhancing the Sensitivity of Biosensors" Nanomaterials 13, no. 21: 2902. https://doi.org/10.3390/nano13212902
APA StyleLu, X., Bao, J., Wei, Y., Zhang, S., Liu, W., & Wu, J. (2023). Emerging Roles of Microrobots for Enhancing the Sensitivity of Biosensors. Nanomaterials, 13(21), 2902. https://doi.org/10.3390/nano13212902







