Dynamically Tunable Assemblies of Superparamagnetic Nanoparticles Stabilized with Liquid Crystal-like Ligands in Organic Thin Films
Abstract
:1. Introduction
2. Materials and Methods
Synthetic Procedures
3. Results and Discussion
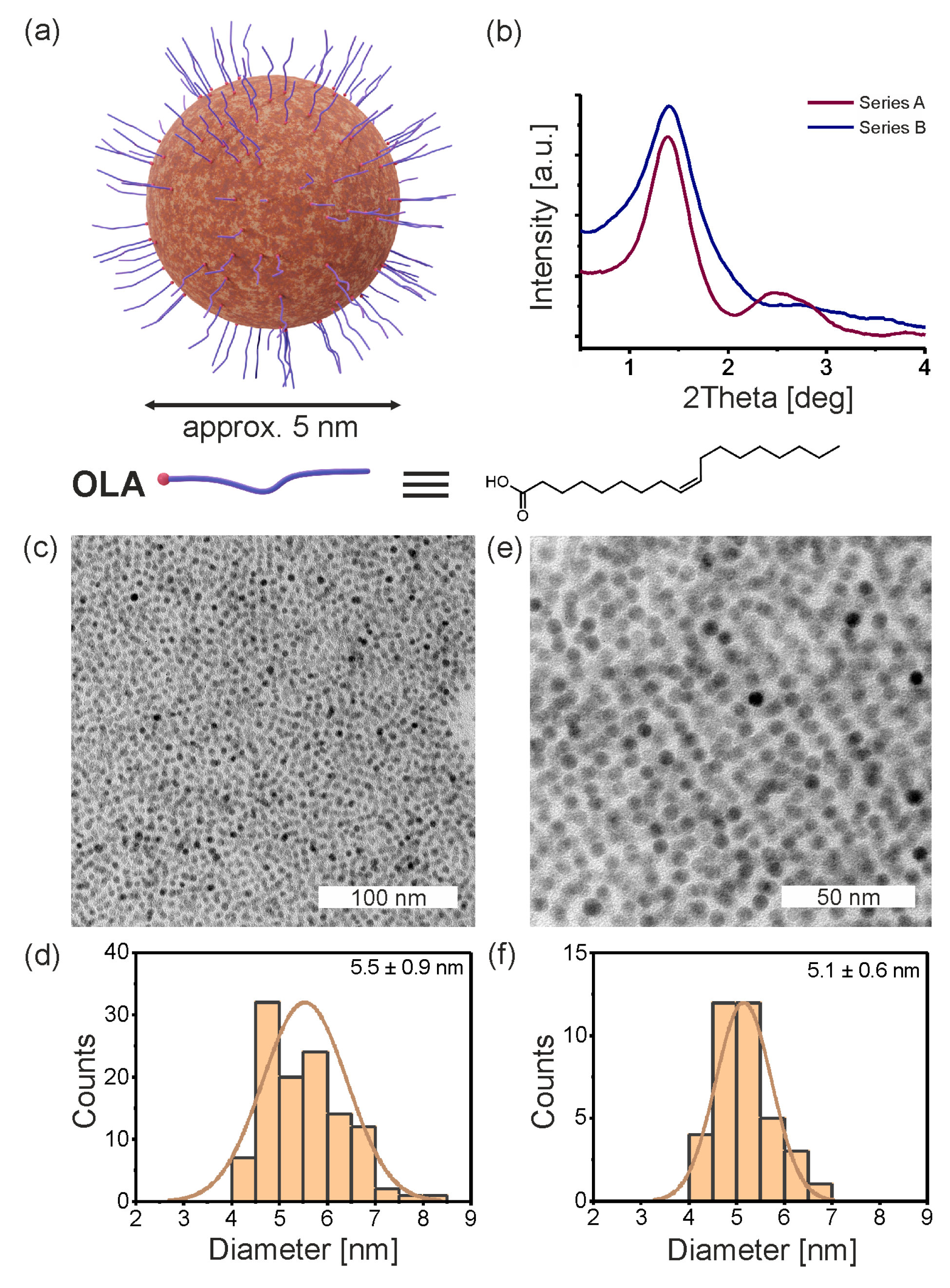
3.1. Primary Iron Oxide Nanoparticles
3.2. Synthesis of Promesogenic Ligand (L)
3.3. Hybrid Iron Oxide Nanoparticles
3.4. Composites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wu, L.; Mendoza-Garcia, A.; Li, Q.; Sun, S. Organic Phase Syntheses of Magnetic Nanoparticles and Their Applications. Chem. Rev. 2016, 116, 10473–10512. [Google Scholar] [CrossRef] [PubMed]
- Grzelczak, M.; Liz-Marzán, L.M.; Klajn, R. Stimuli-Responsive Self-Assembly of Nanoparticles. Chem. Soc. Rev. 2019, 48, 1342–1361. [Google Scholar] [CrossRef] [PubMed]
- Saif, S.; Tahir, A.; Chen, Y. Green Synthesis of Iron Nanoparticles and Their Environmental Applications and Implications. Nanomaterials 2016, 6, 209. [Google Scholar] [CrossRef]
- Dukenbayev, K.; Korolkov, I.V.; Tishkevich, D.I.; Kozlovskiy, A.L.; Trukhanov, S.V.; Gorin, Y.G.; Shumskaya, E.E.; Kaniukov, E.Y.; Vinnik, D.A.; Zdorovets, M.V.; et al. Fe 3 O 4 Nanoparticles for Complex Targeted Delivery and Boron Neutron Capture Therapy. Nanomaterials 2019, 9, 494. [Google Scholar] [CrossRef] [PubMed]
- Vinardell, M.P.; Mitjans, M. Antitumor Activities of Metal Oxide Nanoparticles. Nanomaterials 2015, 5, 1004–1021. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Han, L.; Wang, J.; Wan, J.; Song, G.; Rao, J. Engineering of Magnetic Nanoparticles as Magnetic Particle Imaging Tracers. Chem. Soc. Rev. 2021, 50, 8102–8146. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.; Cervadoro, A.; Ramirez, M.R.; Stigliano, C.; Brazdeikis, A.; Colvin, V.L.; Civera, P.; Key, J.; Decuzzi, P. Assembly of Iron Oxide Nanocubes for Enhanced Cancer Hyperthermia and Magnetic Resonance Imaging. Nanomaterials 2017, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Ye, Y.; Wan, X.; Liu, J.; Yang, S.; Xia, Y.; Li, G.; He, Q. Morphology–Dependent Electrochemical Sensing Properties of Iron Oxide–Graphene Oxide Nanohybrids for Dopamine and Uric Acid. Nanomaterials 2019, 9, 835. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Yang, C.; Chen, X.; Xue, W.; Hutchins-Crawford, H.J.; Yu, Q.; Topham, P.D.; Wang, L. A Review on Electrospun Magnetic Nanomaterials: Methods, Properties and Applications. J. Mater. Chem. C 2021, 9, 9042–9082. [Google Scholar] [CrossRef]
- Reiss, G.; Hütten, A. Magnetic Nanoparticles: Applications beyond Data Storage. Nat. Mater. 2005, 4, 725–726. [Google Scholar] [CrossRef] [PubMed]
- Bossini, D.; Pancaldi, M.; Soumah, L.; Basini, M.; Mertens, F.; Cinchetti, M.; Satoh, T.; Gomonay, O.; Bonetti, S. Ultrafast Amplification and Nonlinear Magnetoelastic Coupling of Coherent Magnon Modes in an Antiferromagnet. Phys. Rev. Lett. 2021, 127, 77202. [Google Scholar] [CrossRef]
- Wang, X.; Huang, X.; Chen, Z.; Liao, X.; Liu, C.; Shi, B. Ferromagnetic Hierarchical Carbon Nanofiber Bundles Derived from Natural Collagen Fibers: Truly Lightweight and High-Performance Microwave Absorption Materials. J. Mater. Chem. C 2015, 3, 10146–10153. [Google Scholar] [CrossRef]
- Grzelczak, M.; Vermant, J.; Furst, E.M.; Liz-marza, L.M. Directed Self-Assembly of Nanoparticles. ACS Nano 2010, 4, 3591–3605. [Google Scholar] [CrossRef] [PubMed]
- Theis-Bröhl, K.; Saini, A.; Wolff, M.; Dura, J.A.; Maranville, B.B.; Borchers, J.A. Self-Assembly of Magnetic Nanoparticles in Ferrofluids on Different Templates Investigated by Neutron Reflectometry. Nanomaterials 2020, 10, 1231. [Google Scholar] [CrossRef] [PubMed]
- Saini, A.; Theis-Bröhl, K.; Koutsioubas, A.; Krycka, K.L.; Borchers, J.A.; Wolff, M. Magnetic Particle Self-Assembly at Functionalized Interfaces. Langmuir 2021, 37, 4064–4071. [Google Scholar] [CrossRef]
- Singh, G.; Chan, H.; Udayabhaskararao, T.; Gelman, E.; Peddis, D.; Baskin, A.; Leitus, G.; Král, P.; Klajn, R. Magnetic Field-Induced Self-Assembly of Iron Oxide Nanocubes. Faraday Discuss. 2015, 181, 403–421. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Pearson, T.; Cordeau, Y.; Mefford, O.T.; Crawford, T.M. Triggered Self-Assembly of Magnetic Nanoparticles. Sci. Rep. 2016, 6, 23145. [Google Scholar] [CrossRef] [PubMed]
- Kundu, P.K.; Samanta, D.; Leizrowice, R.; Margulis, B.; Zhao, H.; Börner, M.; Udayabhaskararao, T.; Manna, D.; Klajn, R. Light-Controlled Self-Assembly of Non-Photoresponsive Nanoparticles. Nat. Chem. 2015, 7, 646–652. [Google Scholar] [CrossRef]
- Kundu, P.K.; Olsen, G.L.; Kiss, V.; Klajn, R. Nanoporous Frameworks Exhibiting Multiple Stimuli Responsiveness. Nat. Commun. 2014, 5, 3588. [Google Scholar] [CrossRef]
- Dierking, I.; Heberle, M.; Osipov, M.A.; Giesselmann, F. Ordering of Ferromagnetic Nanoparticles in Nematic Liquid Crystals. Soft Matter 2017, 13, 4636–4643. [Google Scholar] [CrossRef]
- Zhao, H.; Sen, S.; Udayabhaskararao, T.; Sawczyk, M.; Kucanda, K.; Manna, D.; Kundu, P.K.; Lee, J.W.; Král, P.; Klajn, R. Reversible Trapping and Reaction Acceleration within Dynamically Self-Assembling Nanoflasks. Nat. Nanotechnol. 2016, 11, 82–88. [Google Scholar] [CrossRef]
- Kundu, P.K.; Klajn, R. Watching Single Molecules Move in Response to Light. ACSnano 2014, 8, 11913–11916. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Tardy, B.L.; Christofferson, A.J.; Dai, Y.; Richardson, J.J.; Zhu, W.; Hu, M.; Ju, Y.; Cui, J.; Dagastine, R.R.; et al. Modular Assembly of Superstructures from Polyphenol-Functionalized Building Blocks. Nat. Nanotechnol. 2016, 11, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Marichez, V.; Sato, A.; Dunne, P.A.; Leira-Iglesias, J.; Formon, G.J.M.; Schicho, M.K.; De Feijter, I.; Hébraud, P.; Bailleul, M.; Besenius, P.; et al. Magnetic Control over the Fractal Dimension of Supramolecular Rod Networks. J. Am. Chem. Soc. 2021, 143, 11914–11918. [Google Scholar] [CrossRef]
- Hu, C.; Wu, J.; Wei, T.; Zhan, W.; Qu, Y.; Pan, Y.; Yu, Q.; Chen, H. A Supramolecular Approach for Versatile Biofunctionalization of Magnetic Nanoparticles. J. Mater. Chem. B 2018, 6, 2198–2203. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Seo, K.S.; Lee, C.W.; Kim, J.M. A Polymerizable Supramolecular Approach for the Fabrication of Patterned Magnetic Nanoparticles. Chem. Commun. 2015, 51, 10734–10737. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Dierking, I. Perspectives in Liquid-Crystal-Aided Nanotechnology and Nanoscience. Appl. Sci. 2019, 9, 2512. [Google Scholar] [CrossRef]
- Bisoyi, H.K.; Li, Q. Light-Driven Liquid Crystalline Materials: From Photo-Induced Phase Transitions and Property Modulations to Applications. Chem. Rev. 2016, 116, 15089–15166. [Google Scholar] [CrossRef] [PubMed]
- Nemati, A.; Shadpour, S.; Querciagrossa, L.; Mori, T.; Zannoni, C.; Hegmann, T. Highly Sensitive, Tunable Chirality Amplification through Space Visualized for Gold Nanorods Capped with Axially Chiral Binaphthyl Derivatives. ACS Nano 2019, 13, 10312–10326. [Google Scholar] [CrossRef]
- Ji, Y.; Fan, F.; Xu, S.; Yu, J.; Chang, S. Manipulation Enhancement of Terahertz Liquid Crystal Phase Shifter Magnetically Induced by Ferromagnetic Nanoparticles. Nanoscale 2019, 11, 4933–4941. [Google Scholar] [CrossRef]
- Hähsler, M.; Nádasi, H.; Feneberg, M.; Marino, S.; Giesselmann, F.; Behrens, S.; Eremin, A. Magnetic Tilting in Nematic Liquid Crystals Driven by Self-Assembly. Adv. Funct. Mater. 2021, 31, 2101847. [Google Scholar] [CrossRef]
- Mendozza, M.; Montis, C.; Caselli, L.; Wolf, M.; Baglioni, P.; Berti, D. On the Thermotropic and Magnetotropic Phase Behavior of Lipid Liquid Crystals Containing Magnetic Nanoparticles. Nanoscale 2018, 10, 3480–3488. [Google Scholar] [CrossRef] [PubMed]
- Zep, A.; Wojcik, M.M.; Lewandowski, W.; Sitkowska, K.; Prominski, A.; Mieczkowski, J.; Pociecha, D.; Gorecka, E. Phototunable Liquid-Crystalline Phases Made of Nanoparticles. Angew. Chemie 2014, 126, 13945–13948. [Google Scholar] [CrossRef]
- Yu, H.; Qu, W.; Liu, F.; Mehl, G.H. Two Helices from One Chiral Centre—Self Organization of Disc Shaped Chiral Nanoparticles. Chem. Sci. 2021, 12, 1778–1782. [Google Scholar] [CrossRef]
- Gdovinová, V.; Schroer, M.A.; Tomašovičová, N.; Appel, I.; Behrens, S.; Majorošová, J.; Kováč, J.; Svergun, D.I.; Kopčanský, P. Structuralization of Magnetic Nanoparticles in 5CB Liquid Crystals. Soft Matter 2017, 13, 7890–7896. [Google Scholar] [CrossRef]
- Yu, H.; Welch, C.; Qu, W.; Schubert, C.J.; Liu, F.; Siligardi, G.; Mehl, G.H. Chirality Enhancement in Macro-Chiral Liquid Crystal Nanoparticles. Mater. Horizons 2020, 7, 3021–3027. [Google Scholar] [CrossRef]
- Mundoor, H.; Senyuk, B.; Smalyukh, I.I. Triclinic Nematic Colloidal Crystals from Competing Elastic and Electrostatic Interactions. Science 2016, 352, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, P.J.; Van De Lagemaat, J.; Smalyukh, I.I. Self-Assembly and Electrostriction of Arrays and Chains of Hopfion Particles in Chiral Liquid Crystals. Nat. Commun. 2015, 6, 6012. [Google Scholar] [CrossRef]
- Lesiak, P.; Bednarska, K.; Lewandowski, W.; Wójcik, M.; Polakiewicz, S.; Bagiński, M.; Osuch, T.; Markowski, K.; Orzechowski, K.; Makowski, M.; et al. Self-Organized, One-Dimensional Periodic Structures in a Gold Nanoparticle-Doped Nematic Liquid Crystal Composite. ACS Nano 2019, 13, 10154–10160. [Google Scholar] [CrossRef]
- Coursault, D.; Blach, J.F.; Grand, J.; Coati, A.; Vlad, A.; Zappone, B.; Babonneau, D.; Lévi, G.; Félidj, N.; Donnio, B.; et al. Tailoring Anisotropic Interactions between Soft Nanospheres Using Dense Arrays of Smectic Liquid Crystal Edge Dislocations. ACS Nano 2015, 9, 11678–11689. [Google Scholar] [CrossRef]
- Pelliser, L.; Manceau, M.; Lethiec, C.; Coursault, D.; Vezzoli, S.; Leménager, G.; Coolen, L.; DeVittorio, M.; Pisanello, F.; Carbone, L.; et al. Alignment of Rod-Shaped Single-Photon Emitters Driven by Line Defects in Liquid Crystals. Adv. Funct. Mater. 2015, 25, 1719–1726. [Google Scholar] [CrossRef]
- Qi, H.; Hegmann, T. Liquid Crystal-Gold Nanoparticle Composites. Liq. Cryst. Today 2011, 20, 102–114. [Google Scholar] [CrossRef]
- Saliba, S.; Mingotaud, C.; Kahn, M.L.; Marty, J.D. Liquid Crystalline Thermotropic and Lyotropic Nanohybrids. Nanoscale 2013, 5, 6641–6661. [Google Scholar] [CrossRef] [PubMed]
- Bagiński, M.; Tomczyk, E.; Vetter, A.; Suryadharma, R.N.S.; Rockstuhl, C.; Lewandowski, W. Achieving Highly Stable, Reversibly Reconfigurable Plasmonic Nanocrystal Superlattices through the Use of Semifluorinated Surface Ligands. Chem. Mater. 2018, 30, 8201–8210. [Google Scholar] [CrossRef]
- Bagiński, M.; Tupikowska, M.; González-Rubio, G.; Wójcik, M.; Lewandowski, W. Shaping Liquid Crystals with Gold Nanoparticles: Helical Assemblies with Tunable and Hierarchical Structures Via Thin-Film Cooperative Interactions. Adv. Mater. 2020, 32, 1904581. [Google Scholar] [CrossRef]
- Wojcik, M.; Kolpaczynska, M.; Pociecha, D.; Mieczkowski, J.; Gorecka, E. Multidimensional Structures Made by Gold Nanoparticles with Shape-Adaptive Grafting Layers. Soft Matter 2010, 6, 5397–5400. [Google Scholar] [CrossRef]
- Klöckner, B.; Daniel, P.; Brehmer, M.; Tremel, W.; Zentel, R. Liquid Crystalline Phases from Polymer Functionalized Ferri-Magnetic Fe3O4 Nanorods. J. Mater. Chem. C 2017, 5, 6688–6696. [Google Scholar] [CrossRef]
- Tomczyk, E.; Promiński, A.; Bagiński, M.; Górecka, E.; Wójcik, M. Gold Nanoparticles Thin Films with Thermo- and Photoresponsive Plasmonic Properties Realized with Liquid-Crystalline Ligands. Small 2019, 15, 1902807. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, W.; Szustakiewicz, P.; Kowalska, N.; Grzelak, D.; Narushima, T.; Góra, M.; Bagiński, M.; Pociecha, D.; Okamoto, H.; Liz-Marzán, L.M. Supramolecular Chirality Synchronization in Thin Films of Plasmonic Nanocomposites. ACS Nano 2020, 14, 12918–12928. [Google Scholar] [CrossRef]
- Jedrych, A.; Pawlak, M.; Gorecka, E.; Lewandowski, W.; Wojcik, M.M. Light-Responsive Supramolecular Nanotubes-Based Chiral Plasmonic Assemblies. ACS Nano 2023, 17, 5548–5560. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zeng, H. Size-Controlled Synthesis of Magnetite Nanoparticles. J. Am. Chem. Soc. 2002, 124, 8204–8205. [Google Scholar] [CrossRef]
- Park, J.; An, K.; Hwang, Y.; Park, J.E.G.; Noh, H.J.; Kim, J.Y.; Park, J.H.; Hwang, N.M.; Hyeon, T. Ultra-Large-Scale Syntheses of Monodisperse Nanocrystals. Nat. Mater. 2004, 3, 891–895. [Google Scholar] [CrossRef]
- Wójcik, M.M.; Wróbel, J.; Jańczuk, Z.Z.; Mieczkowski, J.; Górecka, E.; Choi, J.; Cho, M.; Pociecha, D. Liquid-Crystalline Elastomers with Gold Nanoparticle Cross-Linkers. Chem. Eur. J. 2017, 23, 8912–8920. [Google Scholar] [CrossRef] [PubMed]
- Wojcik, M.M.; Gora, M.; Mieczkowski, J.; Romiszewski, J.; Gorecka, E.; Pociecha, D. Temperature-Controlled Liquid Crystalline Polymorphism of Gold Nanoparticles. Soft Matter 2011, 7, 10561–10564. [Google Scholar] [CrossRef]
- Zhang, L.; He, R.; Gu, H.C. Oleic Acid Coating on the Monodisperse Magnetite Nanoparticles. Appl. Surf. Sci. 2006, 253, 2611–2617. [Google Scholar] [CrossRef]
- Bagiński, M.; Pedrazo-Tardajos, A.; Altantzis, T.; Tupikowska, M.; Vetter, A.; Tomczyk, E.; Suryadharma, R.N.S.; Pawlak, M.; Andruszkiewicz, A.; Górecka, E.; et al. Understanding and Controlling the Crystallization Process in Reconfigurable Plasmonic Superlattices. ACS Nano 2021, 15, 4916–4926. [Google Scholar] [CrossRef] [PubMed]
- Bisoyi, H.K.; Kumar, S. Liquid-Crystal Nanoscience: An Emerging Avenue of Soft Self-Assembly. Chem. Soc. Rev. 2011, 40, 306–319. [Google Scholar] [CrossRef]
- Yu, Y.; Jain, A.; Guillaussier, A.; Voggu, V.R.; Truskett, T.M.; Smilgies, D.M.; Korgel, B.A. Nanocrystal Superlattices That Exhibit Improved Order on Heating: An Example of Inverse Melting? Faraday Discuss. 2015, 181, 181–192. [Google Scholar] [CrossRef]
- Lewandowski, W.; Wojcik, M.; Gorecka, E. Metal Nanoparticles with Liquid-Crystalline Ligands: Controlling Nanoparticle Superlattice Structure and Properties. ChemPhysChem 2014, 15, 1283–1295. [Google Scholar] [CrossRef]





| Primary IONPs | Hybrid IONPs | Free Ligand to IONPs Ratio (by Weight) | Composites with Ligand Excess |
|---|---|---|---|
| Serie A | HA1 | 1:1 | EA1 |
| 2:1 | EA2 | ||
| 4:1 | EA3 | ||
| Serie B | HB1 | 1:1 | EB1 |
| 2:1 | EB2 | ||
| 4:1 | EB3 |
| Space Group | Peaks Positions [nm] | Cell Parameters [nm] | Cell Volume [nm3] | Cell Volume per Nps [nm3] | ||
|---|---|---|---|---|---|---|
| EB1 | SRO (30 °C) | P63/mmc | ||||
| FCC (150 °C) | Fm-3 m | (111) − 10.3 (002) − 8.9 (022) − 6.3 (311) − 5.4 (222) − 5.1 | a = 17.8 | 5709.0 | 1427.3 | |
| EB2 | SRO (30 °C) | P63/mmc | ||||
| SRO (110 °C) | P63/mmc | |||||
| FCC (220 °C) | Fm-3 m | (111) − 9.9 (002) − 8.6 (022) − 6.1 (311) − 5.2 (222) − 5.0 | a = 17.2 | 5047.4 | 1261.9 | |
| EB3 | SRO (30 °C) | P63/mmc | ||||
| FCC1 (65 °C) | Fm-3 m | (111) − 10.0 (002) − 8.7 (022) − 6.1 (311) − 5.2 (222) − 5.0 | a = 17.3 | 5218.0 | 1304.5 | |
| FCC2 (110 °C) | Fm-3 m | (111) − 10.5 (002) − 9.1 (022) − 6.4 (311) − 5.5 (222) − 5.3 | a = 18.2 | 6010.4 | 1502.6 | |
| FCC3 (150 °C) | Fm-3 m | (111) − 10.6 (002) − 9.2 (022) − 6.5 (311) − 5.5 (222) − 5.3 | a = 18.3 | 6157.8 | 1539.5 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jańczuk, Z.Z.; Jedrych, A.; Parzyszek, S.; Gardias, A.; Szczytko, J.; Wojcik, M. Dynamically Tunable Assemblies of Superparamagnetic Nanoparticles Stabilized with Liquid Crystal-like Ligands in Organic Thin Films. Nanomaterials 2023, 13, 2908. https://doi.org/10.3390/nano13212908
Jańczuk ZZ, Jedrych A, Parzyszek S, Gardias A, Szczytko J, Wojcik M. Dynamically Tunable Assemblies of Superparamagnetic Nanoparticles Stabilized with Liquid Crystal-like Ligands in Organic Thin Films. Nanomaterials. 2023; 13(21):2908. https://doi.org/10.3390/nano13212908
Chicago/Turabian StyleJańczuk, Zuzanna Z., Agnieszka Jedrych, Sylwia Parzyszek, Anita Gardias, Jacek Szczytko, and Michal Wojcik. 2023. "Dynamically Tunable Assemblies of Superparamagnetic Nanoparticles Stabilized with Liquid Crystal-like Ligands in Organic Thin Films" Nanomaterials 13, no. 21: 2908. https://doi.org/10.3390/nano13212908
APA StyleJańczuk, Z. Z., Jedrych, A., Parzyszek, S., Gardias, A., Szczytko, J., & Wojcik, M. (2023). Dynamically Tunable Assemblies of Superparamagnetic Nanoparticles Stabilized with Liquid Crystal-like Ligands in Organic Thin Films. Nanomaterials, 13(21), 2908. https://doi.org/10.3390/nano13212908





