Sustainable Synthesis of Metal-Doped Lignin-Derived Electrospun Carbon Fibers for the Development of ORR Electrocatalysts
Abstract
:1. Introduction
2. Materials and Methods
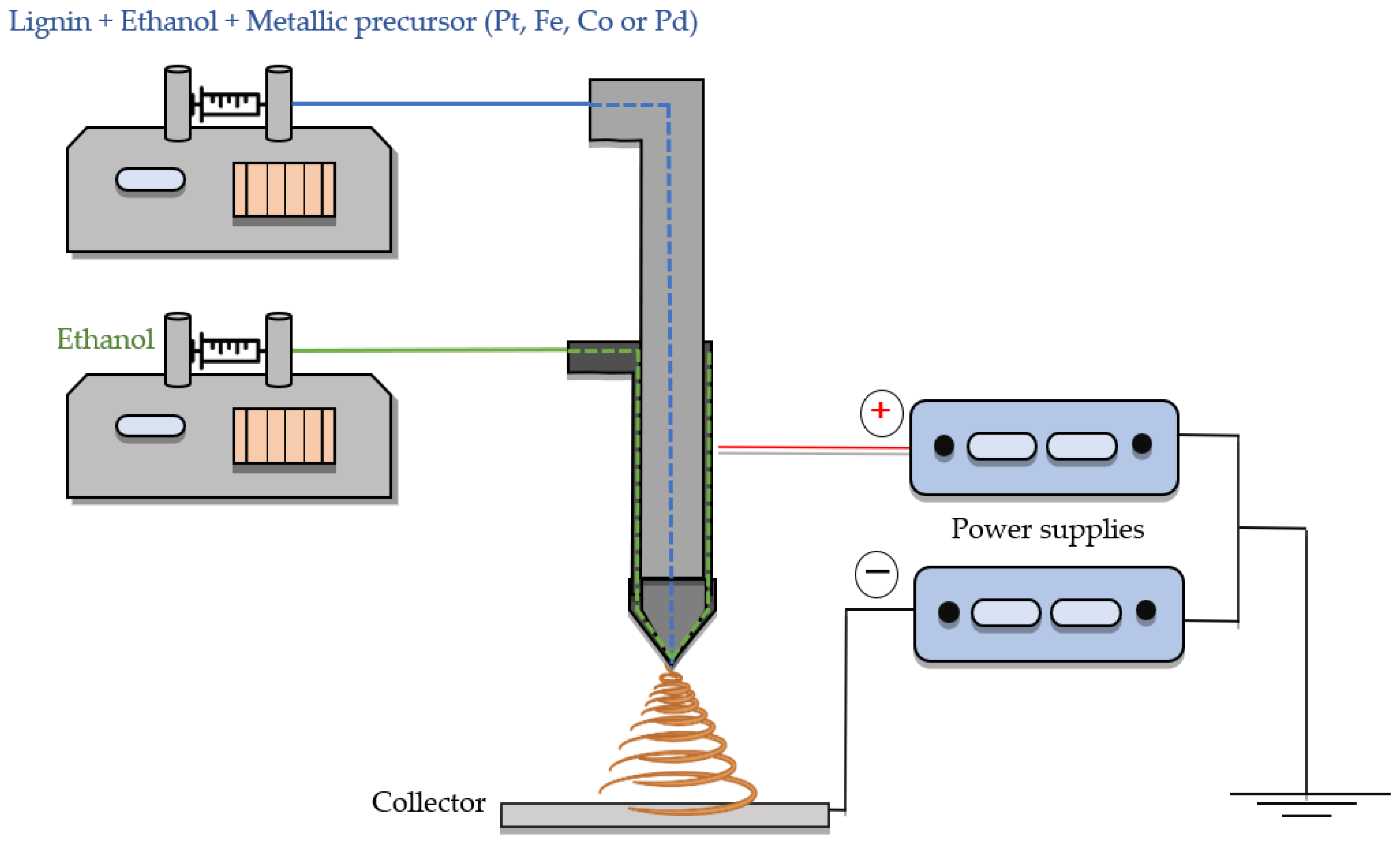
2.1. Electrocatalyst Preparation
2.2. Physicochemical Characterization Techniques
2.3. Electrochemical Characterization
3. Results and Discussion
3.1. Carbon Fiber Preparation Yields
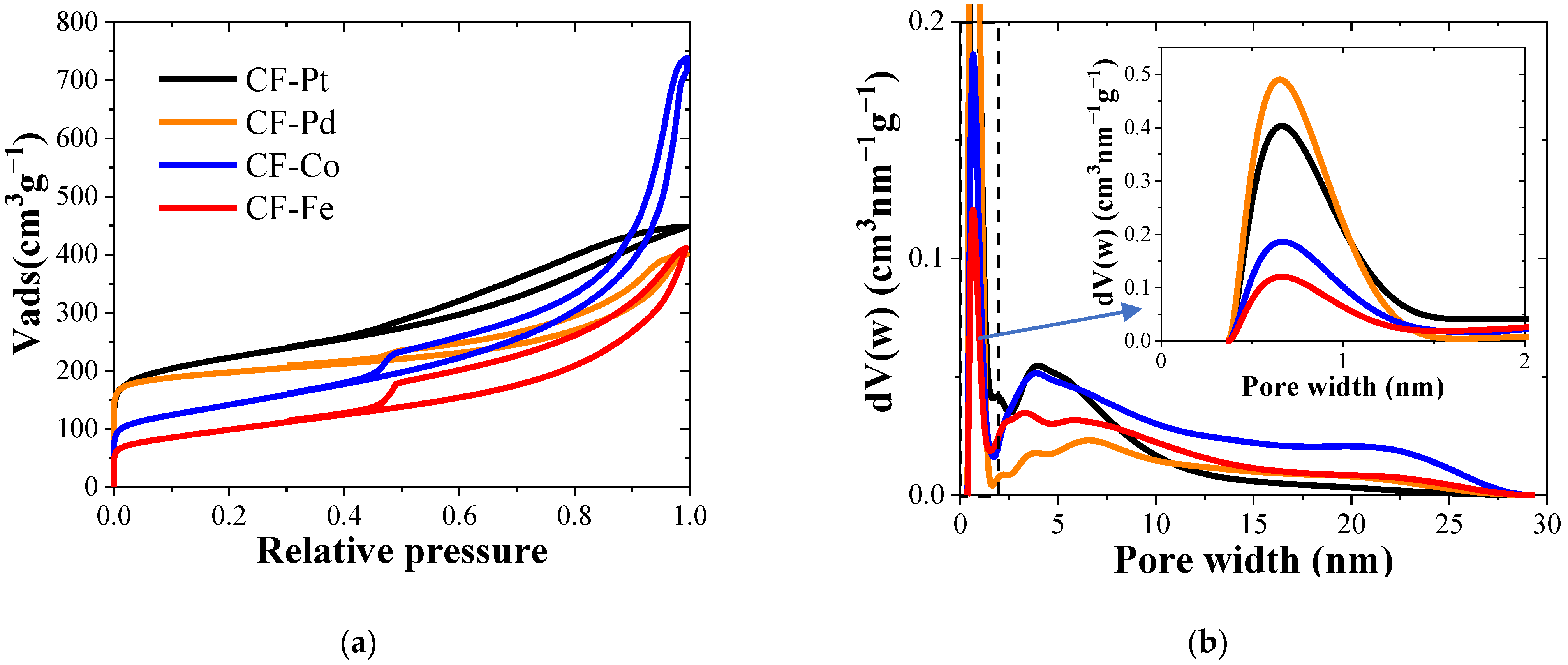
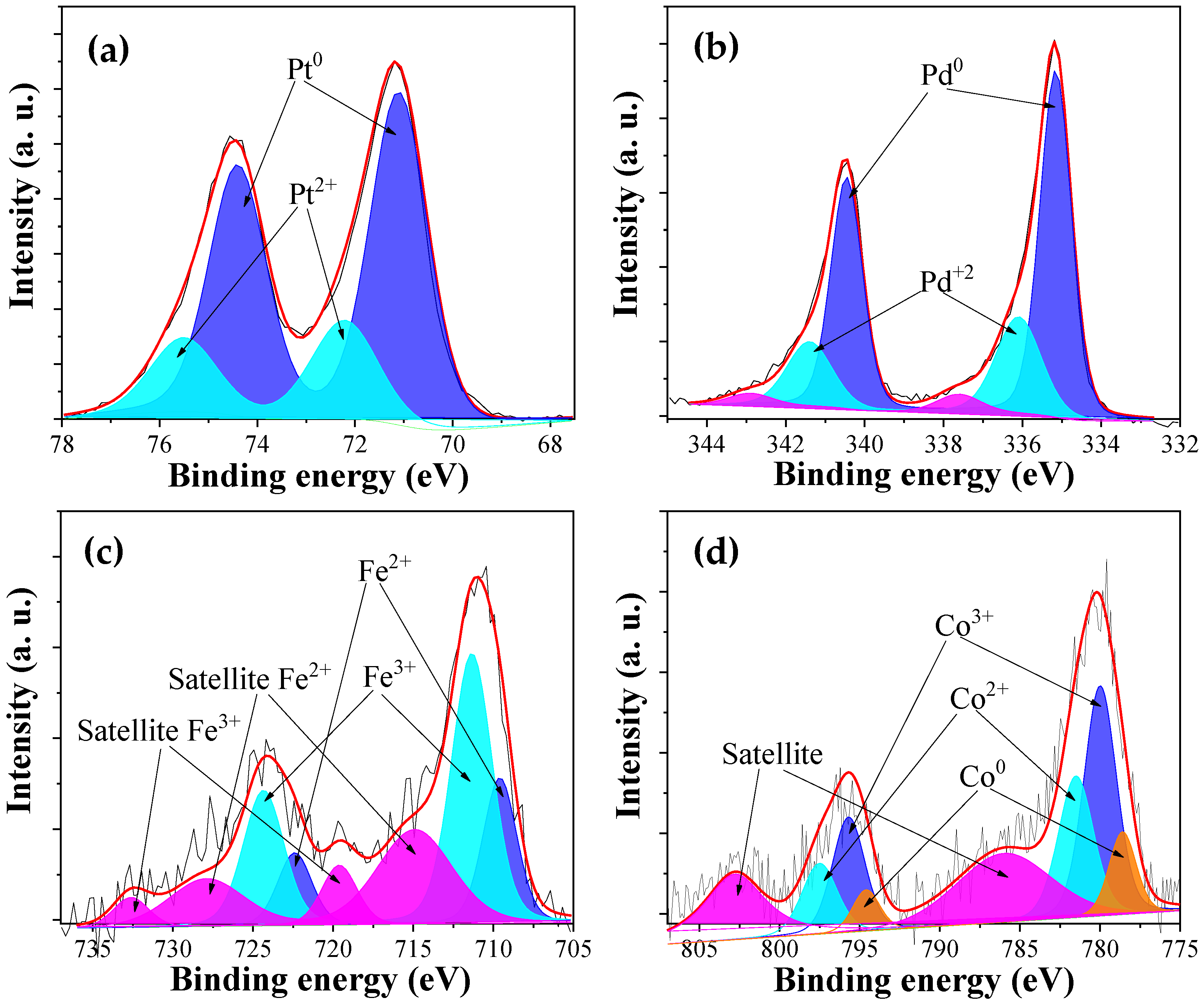
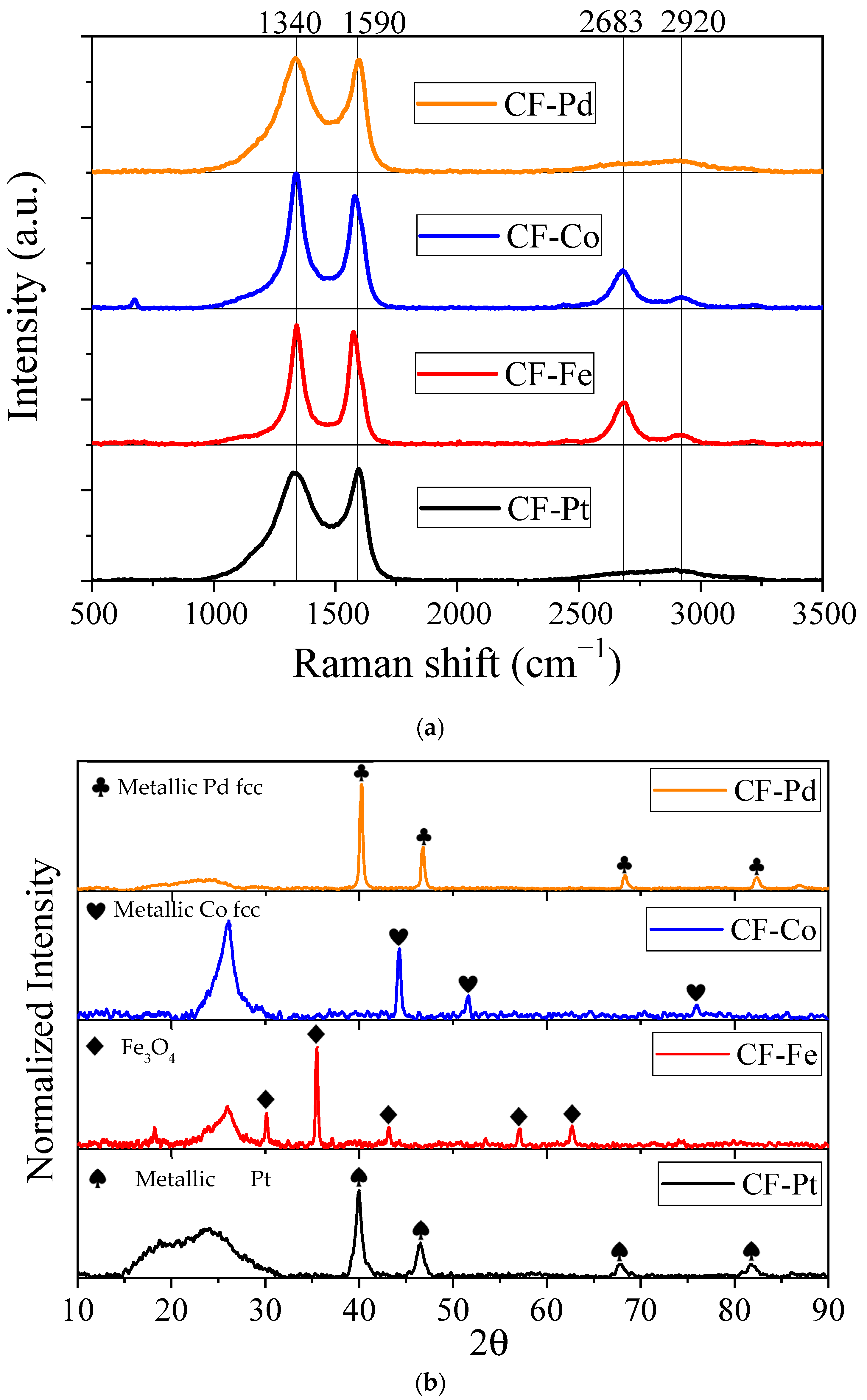
3.2. Physicochemical Characterization
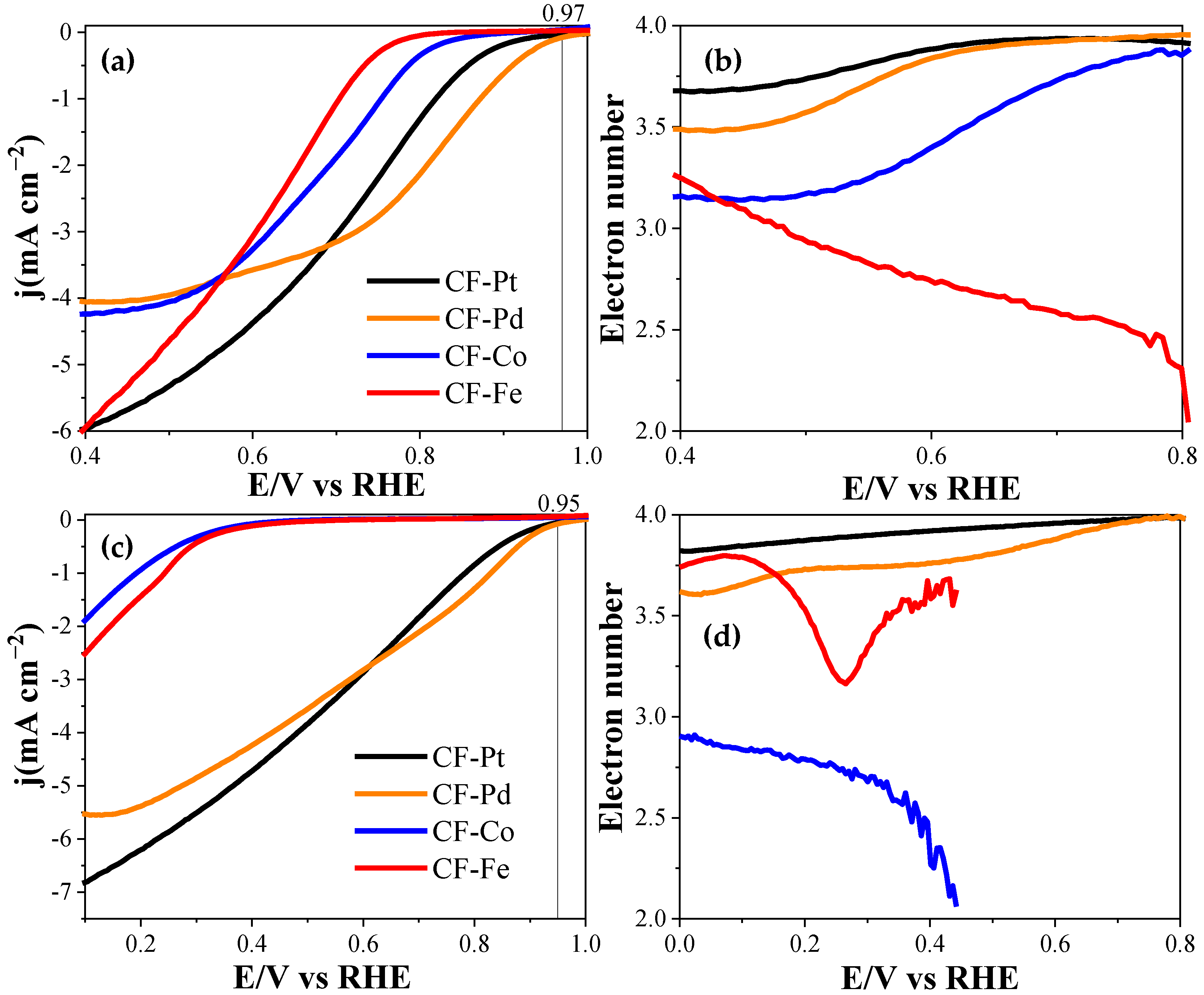
3.3. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sideri, I.K.; Tagmatarchis, N. Noble-Metal-Free Doped Carbon Nanomaterial Electrocatalysts. Chem.-A Eur. J. 2020, 26, 15397–15415. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Yang, J.; Ge, R.; Zhang, J.; Li, Y.; Zhu, M.; Dai, L.; Li, S.; Li, W. Carbon-based bifunctional electrocatalysts for oxygen reduction and oxygen evolution reactions: Optimization strategies and mechanistic analysis. J. Energy Chem. 2022, 71, 234–265. [Google Scholar] [CrossRef]
- Dai, L.; Chang, D.W.; Baek, J.-B.; Lu, W. Carbon Nanomaterials for Advanced Energy Conversion and Storage. Small 2012, 8, 1130–1166. [Google Scholar] [CrossRef]
- Gomez-Marín, A.M.; Feliu, J.M.; Edson, T. Reaction Mechanism for Oxygen Reduction on Platinum: Existence of a Fast Initial Chemical Step and a Soluble Species Different from H2O2. ACS Catal. 2018, 8, 7931–7943. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, H.; Liu, Y.; Pan, Y. Research progress of precise structural regulation of single atom catalyst for accelerating electrocatalytic oxygen reduction reaction. J. Energy Chem. 2022, 72, 56–72. [Google Scholar] [CrossRef]
- Niu, H.; Wan, X.; Wang, X.; Shao, C.; Robertson, J.; Zhang, Z.; Guo, Y. Single-Atom Rhodium on Defective g-C3N4: A Promising Bifunctional Oxygen Electrocatalyst. ACS Sustain. Chem. Eng. 2021, 9, 3590–3599. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, L.; Xie, Y.; Tong, M.; Tian, C.; Fu, H. The Fe3C-Nx Site Assists the Fe-Nx Site to Promote Activity of the Fe-N-C Electrocatalyst for Oxygen Reduction Reaction. ACS Sustain. Chem. Eng. 2022, 10, 3346–3354. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, K.; Cui, X.; Li, B.; Jiang, J. Defect-Rich, Graphenelike Carbon Sheets Derived from Biomass as Efficient Electrocatalysts for Rechargeable Zinc–Air Batteries. ACS Sustain. Chem. Eng. 2020, 8, 2981–2989. [Google Scholar] [CrossRef]
- Sun, J.-F.; Xu, Q.-Q.; Qi, J.-L.; Zhou, D.; Zhu, H.-Y.; Yin, J.-Z. Isolated Single Atoms Anchored on N-Doped Carbon Materials as a Highly Efficient Catalyst for Electrochemical and Organic Reactions. ACS Sustain. Chem. Eng. 2020, 8, 14630–14656. [Google Scholar] [CrossRef]
- García-Mateos, F.J.; Ruiz-Rosas, R.; Rosas, J.M.; Rodríguez-Mirasol, J.; Cordero, T. Controlling the Composition, Morphology, Porosity, and Surface Chemistry of Lignin-Based Electrospun Carbon Materials. Front. Mater. 2019, 6, 114. [Google Scholar] [CrossRef]
- Suhas; Carrott, P.J.M.; Ribeiro Carrott, M.M.L. Lignin—From natural adsorbent to activated carbon: A review. Bioresour. Technol. 2007, 98, 2301–2312. [Google Scholar] [CrossRef] [PubMed]
- Bedia, J.; Ruiz-Rosas, R.; Rodríguez-Mirasol, J.; Cordero, T. Kinetic study of the decomposition of 2-butanol on carbon-based acid catalyst. AIChE J. 2010, 56, 1557–1568. [Google Scholar] [CrossRef]
- García-Rollán, M.; García-Mateos, F.; Ruiz-Rosas, R.; Rosas, J.; Rodríguez-Mirasol, J.; Cordero, T. MgO-containing porous carbon spheres derived from magnesium lignosulfonate as sustainable basic catalysts. J. Environ. Chem. Eng. 2023, 11, 109060. [Google Scholar] [CrossRef]
- Meng, Y.; Liu, T.; Yu, S.; Cheng, Y.; Lu, J.; Wang, H. A lignin-based carbon aerogel enhanced by graphene oxide and application in oil/water separation. Fuel 2020, 278, 118376. [Google Scholar] [CrossRef]
- Thielemans, W.; Can, E.; Morye, S.S.; Wool, R.P. Novel applications of lignin in composite materials. J. Appl. Polym. Sci. 2002, 83, 323–331. [Google Scholar] [CrossRef]
- Snowdon, M.R.; Mohanty, A.K.; Misra, M. A Study of Carbonized Lignin as an Alternative to Carbon Black. ACS Sustain. Chem. Eng. 2014, 2, 1257–1263. [Google Scholar] [CrossRef]
- Gnedenkov, S.V.; Opra, D.P.; Zemnukhova, L.A.; Sinebryukhov, S.L.; Kedrinskii, I.A.; Patrusheva, O.V.; Sergienko, V.I. Electrochemical performance of Klason lignin as a low-cost cathode-active material for primary lithium battery. J. Energy Chem. 2015, 24, 346–352. [Google Scholar] [CrossRef]
- Ribadeneyra, M.C.; Grogan, L.; Au, H.; Schlee, P.; Herou, S.; Neville, T.; Cullen, P.L.; Kok, M.D.; Hosseinaei, O.; Danielsson, S.; et al. Lignin-derived electrospun freestanding carbons as alternative electrodes for redox flow batteries. Carbon 2020, 157, 847–856. [Google Scholar] [CrossRef]
- Kienberger, M.; Maitz, S.; Pichler, T.; Demmelmayer, P. Systematic Review on Isolation Processes for Technical Lignin. Processes 2021, 9, 804. [Google Scholar] [CrossRef]
- Wen, Y.; Kok, M.D.; Tafoya, J.P.V.; Sobrido, A.B.J.; Bell, E.; Gostick, J.T.; Herou, S.; Schlee, P.; Titirici, M.-M.; Brett, D.J.; et al. Electrospinning as a route to advanced carbon fibre materials for selected low-temperature electrochemical devices: A review. J. Energy Chem. 2021, 59, 492–529. [Google Scholar] [CrossRef]
- Gao, K.; Shen, M.; Duan, C.; Xiong, C.; Dai, L.; Zhao, W.; Lu, W.; Ding, S.; Ni, Y. Co-N-Doped Directional Multichannel PAN/CA-Based Electrospun Carbon Fibers as High-Efficiency Bifunctional Oxygen Electrocatalysts for Zn–Air Batteries. ACS Sustain. Chem. Eng. 2021, 9, 17068–17077. [Google Scholar] [CrossRef]
- Yang, L.; Feng, S.; Xu, G.; Wei, B.; Zhang, L. Electrospun MOF-Based FeCo Nanoparticles Embedded in Nitrogen-Doped Mesoporous Carbon Fibers as an Efficient Bifunctional Catalyst for Oxygen Reduction and Oxygen Evolution Reactions in Zinc-Air Batteries. ACS Sustain. Chem. Eng. 2019, 7, 5462–5475. [Google Scholar] [CrossRef]
- Dallmeyer, I.; Ko, F.; Kadla, J.F. Electrospinning of Technical Lignins for the Production of Fibrous Networks. J. Wood Chem. Technol. 2010, 30, 315–329. [Google Scholar] [CrossRef]
- Dallmeyer, I.; Ko, F.; Kadla, J.F. Correlation of Elongational Fluid Properties to Fiber Diameter in Electrospinning of Softwood Kraft Lignin Solutions. Ind. Eng. Chem. Res. 2014, 53, 2697–2705. [Google Scholar] [CrossRef]
- Valero-Romero, M.; García-Mateos, F.; Kapteijn, F.; Rodríguez-Mirasol, J.; Cordero, T. Fischer-Tropsch synthesis over lignin-derived cobalt-containing porous carbon fiber catalysts. Appl. Catal. B Environ. 2023, 321, 122078. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, H.; Liang, J.; Wang, Y.; Li, T.; Luo, Y.; Shi, X.; Lu, S.; Feng, Z.; Wu, Q.; et al. Noble-metal-free electrospun nanomaterials as electrocatalysts for oxygen reduction reaction. Mater. Today Phys. 2020, 15, 100280. [Google Scholar] [CrossRef]
- Wang, Y.; Gan, R.; Zhao, S.; Ma, W.; Zhang, X.; Song, Y.; Ma, C.; Shi, J. B, N, F tri-doped lignin-derived carbon nanofibers as an efficient metal-free bifunctional electrocatalyst for ORR and OER in rechargeable liquid/solid-state Zn-air batteries. Appl. Surf. Sci. 2022, 598, 153891. [Google Scholar] [CrossRef]
- Zhou, Y.; Yuan, J.; Dong, R.; Fu, Y.; Zhou, J.; Qin, H. High-performance electrospun carbon fiber derived from lignin and metal composite. Ionics 2022, 28, 1119–1127. [Google Scholar] [CrossRef]
- Lai, C.; Kolla, P.; Zhao, Y.; Fong, H.; Smirnova, A.L. Lignin-derived electrospun carbon nanofiber mats with supercritically deposited Ag nanoparticles for oxygen reduction reaction in alkaline fuel cells. Electrochim. Acta 2014, 130, 431–438. [Google Scholar] [CrossRef]
- Rois, M.F.; Sasono, S.R.A.; Widiyastuti, W.; Nurtono, T.; Setyawan, H. High-performance electrocatalyst made from lignosulfonate nanofiber composited with manganese dioxide without carbonation process. Adv. Powder Technol. 2022, 33, 103572. [Google Scholar] [CrossRef]
- García-Mateos, F.J.; Rosas, J.M.; Ruiz-Rosas, R.; Rodríguez-Mirasol, J.; Cordero, T. Highly porous and conductive functional carbon fibers from electrospun phosphorus-containing lignin fibers. Carbon 2022, 200, 134–148. [Google Scholar] [CrossRef]
- Kaneko, K.; Ishii, C. Superhigh surface area determination of microporous solids. Colloids Surfaces 1992, 67, 203–212. [Google Scholar] [CrossRef]
- Jagiello, J.; Olivier, J.P. 2D-NLDFT adsorption models for carbon slit-shaped pores with surface energetical heterogeneity and geometrical corrugation. Carbon 2013, 55, 70–80. [Google Scholar] [CrossRef]
- Waseda, Y.; Matsubara, E.; Shinoda, K. X-ray Diffraction Crystallography; Springer: Berlin/Heidelberg, Germany, 2011; ISBN 978-3-642-16634-1. [Google Scholar]
- Flores-Lasluisa, J.; Huerta, F.; Cazorla-Amorós, D.; Morallón, E. Manganese oxides/LaMnO3 perovskite materials and their application in the oxygen reduction reaction. Energy 2022, 247, 123456. [Google Scholar] [CrossRef]
- Braun, J.; Holtman, K.; Kadla, J. Lignin-based carbon fibers: Oxidative thermostabilization of kraft lignin. Carbon 2005, 43, 385–394. [Google Scholar] [CrossRef]
- Křenek, T.; Kovářík, T.; Pola, M.; Jakubec, I.; Bezdička, P.; Bastl, Z.; Pokorná, D.; Urbanová, M.; Galíková, A.; Pola, J. Enhancement of thermal stability of silver(I) acetylacetonate by platinum(II) acetylacetonate. Thermochim. Acta 2013, 554, 1–7. [Google Scholar] [CrossRef]
- Melnikov, P.; Nascimento, V.A.; Arkhangelsky, I.V.; Consolo, L.Z.Z.; de Oliveira, L.C.S. Thermal decomposition mechanism of iron(III) nitrate and characterization of intermediate products by the technique of computerized modeling. J. Therm. Anal. Calorim. 2014, 115, 145–151. [Google Scholar] [CrossRef]
- Szłyk, E.; Barwiołek, M. Studies of thermal decomposition of palladium(II) complexes with olefin ligands. Thermochim. Acta 2009, 495, 85–89. [Google Scholar] [CrossRef]
- Cao, J.; Xiao, G.; Xu, X.; Shen, D.; Jin, B. Study on carbonization of lignin by TG-FTIR and high-temperature carbonization reactor. Fuel Process. Technol. 2013, 106, 41–47. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Huba, Z.J.; Carpenter, E.E. Monitoring the formation of carbide crystal phases during the thermal decomposition of 3d transition metal dicarboxylate complexes. Dalton Trans. 2014, 43, 12236–12242. [Google Scholar] [CrossRef] [PubMed]
- Berenguer, R.; García-Mateos, F.J.; Ruiz-Rosas, R.; Cazorla-Amorós, D.; Morallón, E.; Rodríguez-Mirasol, J.; Cordero, T. Biomass-derived binderless fibrous carbon electrodes for ultrafast energy storage. Green Chem. 2016, 18, 1506–1515. [Google Scholar] [CrossRef]
- Munuera, J.M.; Paredes, J.I.; Villar-Rodil, S.; Ayán-Varela, M.; Martínez-Alonso, A.; Tascón, J.M.D. Electrolytic exfoliation of graphite in water with multifunctional electrolytes: En route towards high quality, oxide-free graphene flakes. Nanoscale 2016, 8, 2982–2998. [Google Scholar] [CrossRef] [PubMed]
- Fleisch, T.; Zajac, G.; Schreiner, J.; Mains, G. An XPS study of the UV photoreduction of transition and noble metal oxides. Appl. Surf. Sci. 1986, 26, 488–497. [Google Scholar] [CrossRef]
- Yamashita, T.; Hayes, P. Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl. Surf. Sci. 2008, 254, 2441–2449. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Liu, Z.-Q.; Cheng, H.; Li, N.; Ma, T.Y.; Su, Y.-Z. ZnCo2O4 Quantum Dots Anchored on Nitrogen-Doped Carbon Nanotubes as Reversible Oxygen Reduction/Evolution Electrocatalysts. Adv. Mater. 2016, 28, 3777–3784. [Google Scholar] [CrossRef]
- Flores-Lasluisa, J.X.; Huerta, F.; Cazorla-Amorós, D.; Morallon, E. Structural and morphological alterations induced by cobalt substitution in LaMnO3 perovskites. J. Colloid Interface Sci. 2019, 556, 658–666. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman spectra of disordered and amorphous carbon. Phys. Rev. B Condens. Matter Mater. Phys. 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Torres-Canas, F.; Bentaleb, A.; Föllmer, M.; Roman, J.; Neri, W.; Ly, I.; Derré, A.; Poulin, P. Improved structure and highly conductive lignin-carbon fibers through graphene oxide liquid crystal. Carbon 2020, 163, 120–127. [Google Scholar] [CrossRef]
- Schwan, J.; Ulrich, S.; Batori, V.; Ehrhardt, H.; Silva, S.R.P. Raman spectroscopy on amorphous carbon films. J. Appl. Phys. 1996, 80, 440–447. [Google Scholar] [CrossRef]
- Gabe, A.; Ruiz-Rosas, R.; Morallón, E.; Cazorla-Amorós, D. Understanding of oxygen reduction reaction by examining carbon-oxygen gasification reaction and carbon active sites on metal and heteroatoms free carbon materials of different porosities and structures. Carbon 2019, 148, 430–440. [Google Scholar] [CrossRef]
- Yoon, D.; Moon, H.; Cheong, H.; Choi, J.; Choi, J.; Park, B. Variations in the Raman Spectrum as a Function of the Number ofGraphene Layers. J. Korean Phys. Soc. 2009, 55, 1299–1303. [Google Scholar] [CrossRef]
- Vautard, F.; Ozcan, S.; Paulauskas, F.; Spruiell, J.; Meyer, H.; Lance, M. Influence of the carbon fiber surface microstructure on the surface chemistry generated by a thermo-chemical surface treatment. Appl. Surf. Sci. 2012, 261, 473–480. [Google Scholar] [CrossRef]
- Hoekstra, J.; Beale, A.M.; Soulimani, F.; Versluijs-Helder, M.; Geus, J.W.; Jenneskens, L.W. Base Metal Catalyzed Graphitization of Cellulose: A Combined Raman Spectroscopy, Temperature-Dependent X-ray Diffraction and High-Resolution Transmission Electron Microscopy Study. J. Phys. Chem. C 2015, 119, 10653–10661. [Google Scholar] [CrossRef]
- Krivoruchko, O.P.; Zaikovskii, V.I. A new phenomenon involving the formation of liquid mobile metal–carbon particles in the low-temperature catalytic graphitisation of amorphous carbon by metallic Fe, Co and Ni. Mendeleev Commun. 1998, 8, 97–99. [Google Scholar] [CrossRef]
- Fu, X.; Wang, Y.; Wu, N.; Gui, L.; Tang, Y. Preparation of colloidal solutions of thin platinum nanowires. J. Mater. Chem. 2003, 13, 1192–1195. [Google Scholar] [CrossRef]
- Maity, D.; Agrawal, D. Synthesis of iron oxide nanoparticles under oxidizing environment and their stabilization in aqueous and non-aqueous media. J. Magn. Magn. Mater. 2007, 308, 46–55. [Google Scholar] [CrossRef]
- Manjunatha, M.; Reddy, G.S.; Mallikarjunaiah, K.J.; Damle, R.; Ramesh, K.P. Determination of Phase Composition of Cobalt Nanoparticles Using 59Co Internal Field Nuclear Magnetic Resonance. J. Supercond. Nov. Magn. 2019, 32, 3201–3209. [Google Scholar] [CrossRef]
- Xu, L.; Wu, X.-C.; Zhu, J.-J. Green preparation and catalytic application of Pd nanoparticles. Nanotechnology 2008, 19, 305603. [Google Scholar] [CrossRef]
- García-Mateos, F.J.; Cordero-Lanzac, T.; Berenguer, R.; Morallón, E.; Cazorla-Amorós, D.; Rodríguez-Mirasol, J.; Cordero, T. Lignin-derived Pt supported carbon (submicron)fiber electrocatalysts for alcohol electro-oxidation. Appl. Catal. B Environ. 2017, 211, 18–30. [Google Scholar] [CrossRef]
- Raymundo-Piñero, E.; Kierzek, K.; Machnikowski, J.; Béguin, F. Relationship between the nanoporous texture of activated carbons and their capacitance properties in different electrolytes. Carbon 2006, 44, 2498–2507. [Google Scholar] [CrossRef]
- Wang, D.; Lee, S.H.; Kim, J.; Park, C.B. “Waste to Wealth”: Lignin as a Renewable Building Block for Energy Harvesting/Storage and Environmental Remediation. ChemSusChem 2020, 13, 2807–2827. [Google Scholar] [CrossRef] [PubMed]

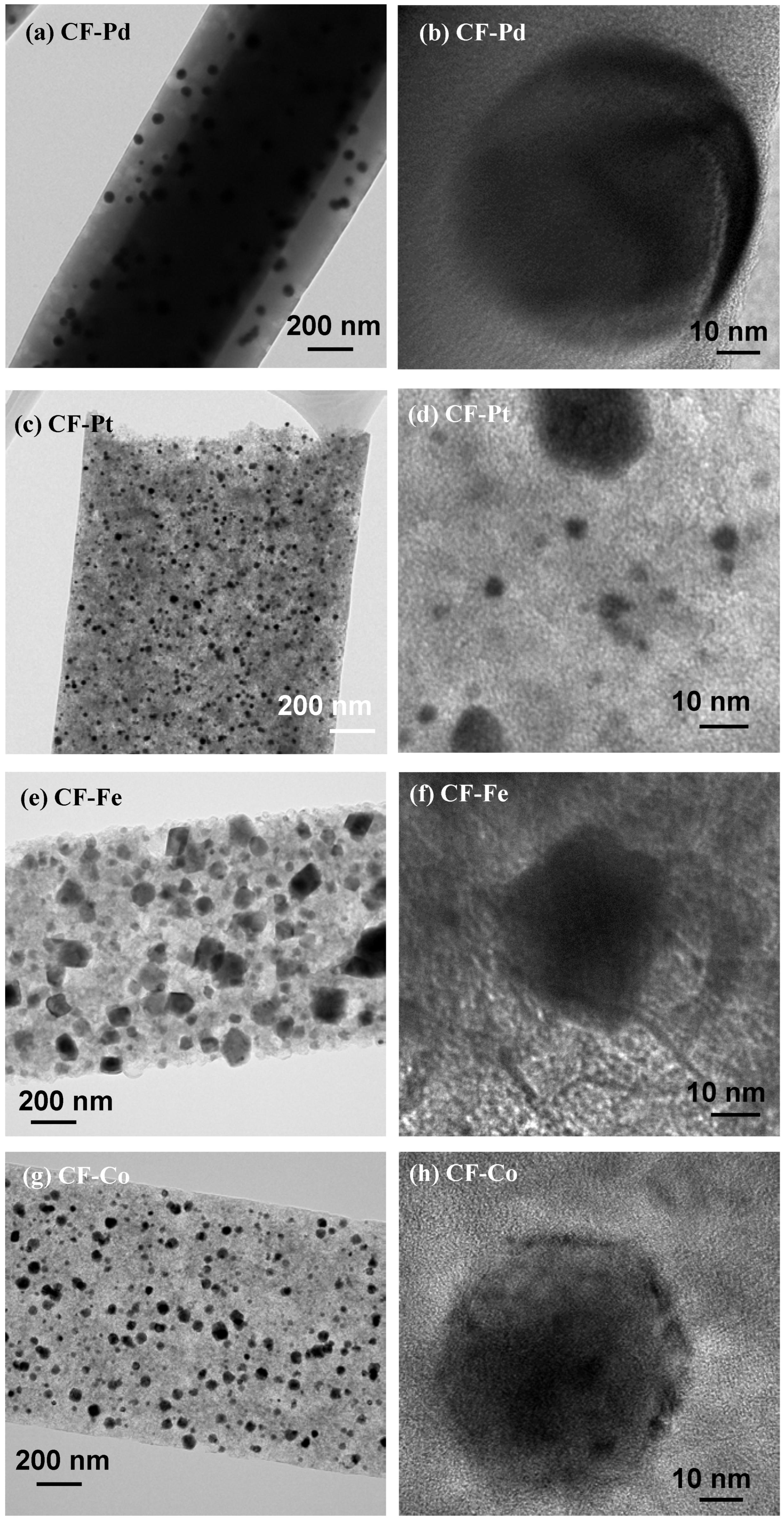
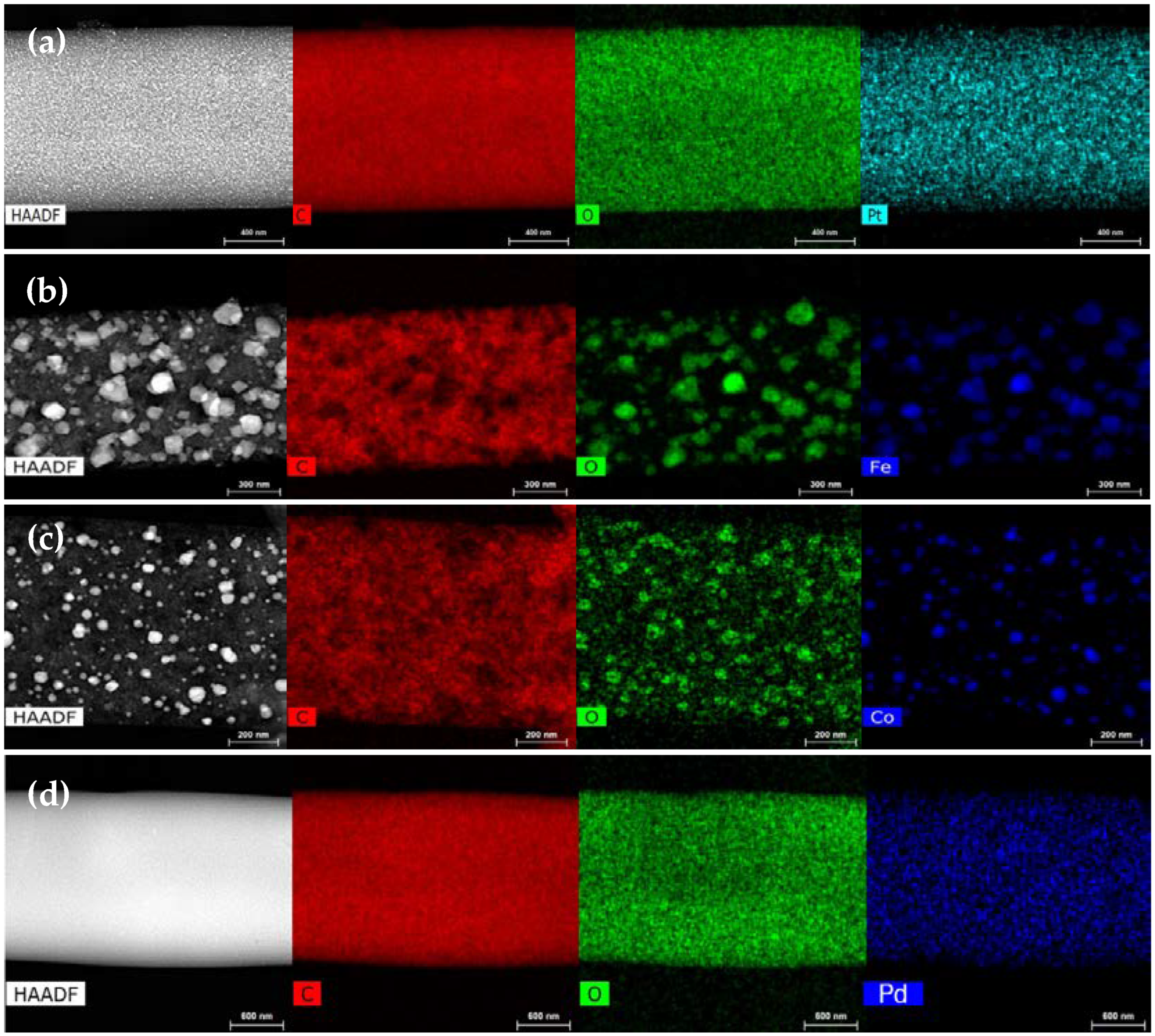
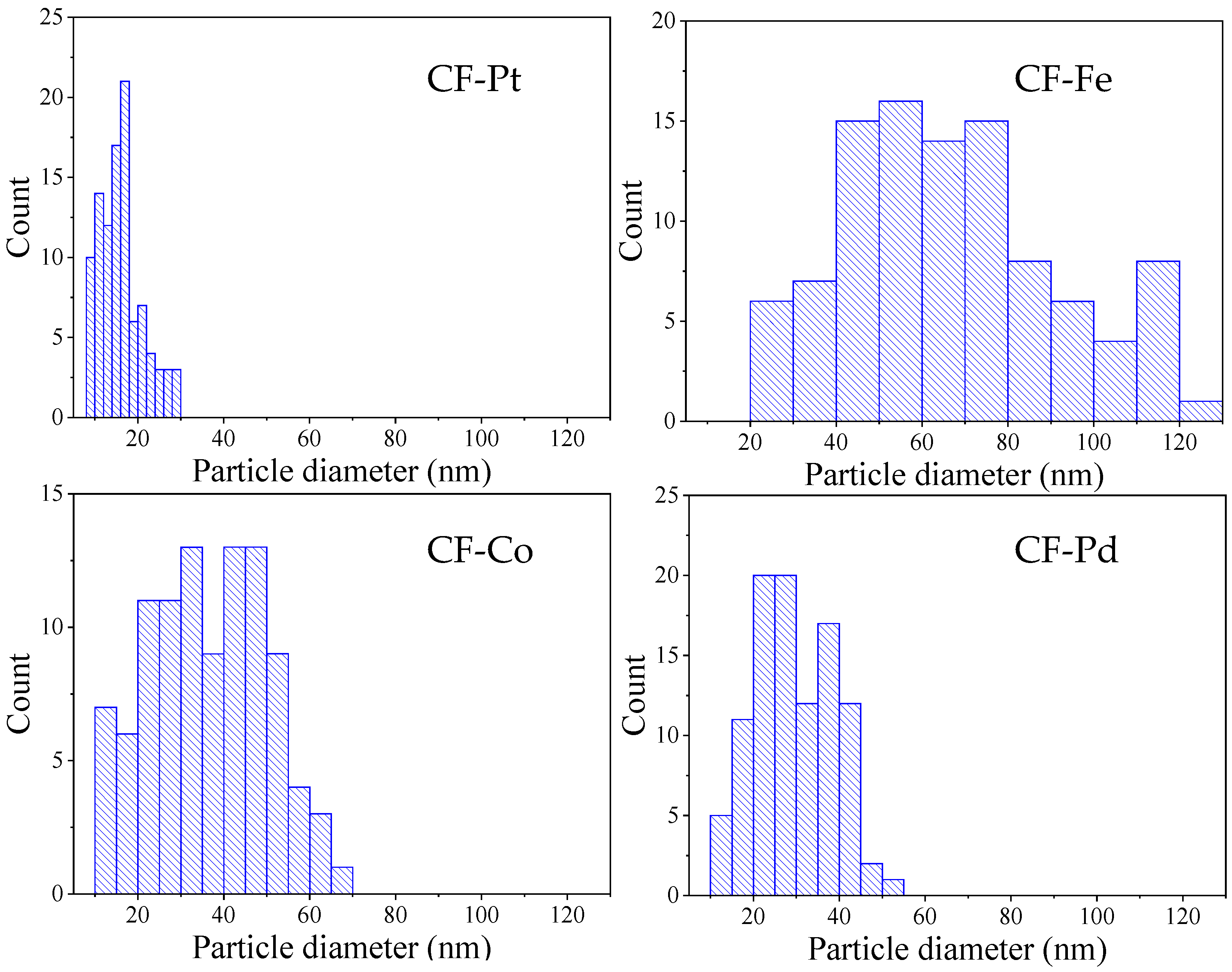

| Sample | Stabilization Yield (wt%) | Carbonization Yield (wt%) | Overall Yield (wt%) |
|---|---|---|---|
| CF-Pt | 45 | 22 | 9.8 |
| CF-Co | 53 | 16 | 8.5 |
| CF-Fe | 33 | 25 | 8.3 |
| CF-Pd | 56 | 30 | 16.8 |
| N2 Adsorption Isotherm | XPS (wt%) | Bulk Metal Loading (XRF) wt% | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | ABET m2 g−1 | Vt cm3 g−1 | Vmes cm3 g−1 | C | O | Pt | Fe | Co | Pd | |
| CF-Pt | 785 ± 2 | 0.18 ± 0.04 | 0.51 ± 0.06 | 90.4 | 2.5 | 7.1 | -- | -- | -- | 10 |
| CF-Co | 500 ± 1 | 0.04 ± 0.01 | 1.10 ± 0.10 | 93.2 | 3.2 | -- | -- | 3.4 | -- | 16 |
| CF-Fe | 350 ± 1 | 0.04 ± 0.01 | 0.59 ± 0.05 | 91.5 | 3.8 | -- | 4.3 | -- | -- | 16 |
| CF-Pd | 755 ± 2 | 0.23 ± 0.03 | 0.39 ± 0.04 | 90.2 | 2.6 | -- | -- | -- | 7.2 | 8 |
| Sample | 2θ | Crystallite Size (Scherrer eq, nm) | Average Nanoparticle Size Determined via TEM (nm) | Nanoparticle Size Range Determined via TEM (nm) |
|---|---|---|---|---|
| CF-Pd | 40.22 | 25.2 | 29.4 | 13.2–52.4 |
| CF-Co | 44.26 | 24.0 | 36.2 | 12.7–65.8 |
| CF-Fe | 35.48 | 35.8 | 67.3 | 20.1–122.1 |
| CF-Pt | 39.93 | 10.6 | 16.1 | 8.2–29.6 |
| Alkaline Medium | |||
|---|---|---|---|
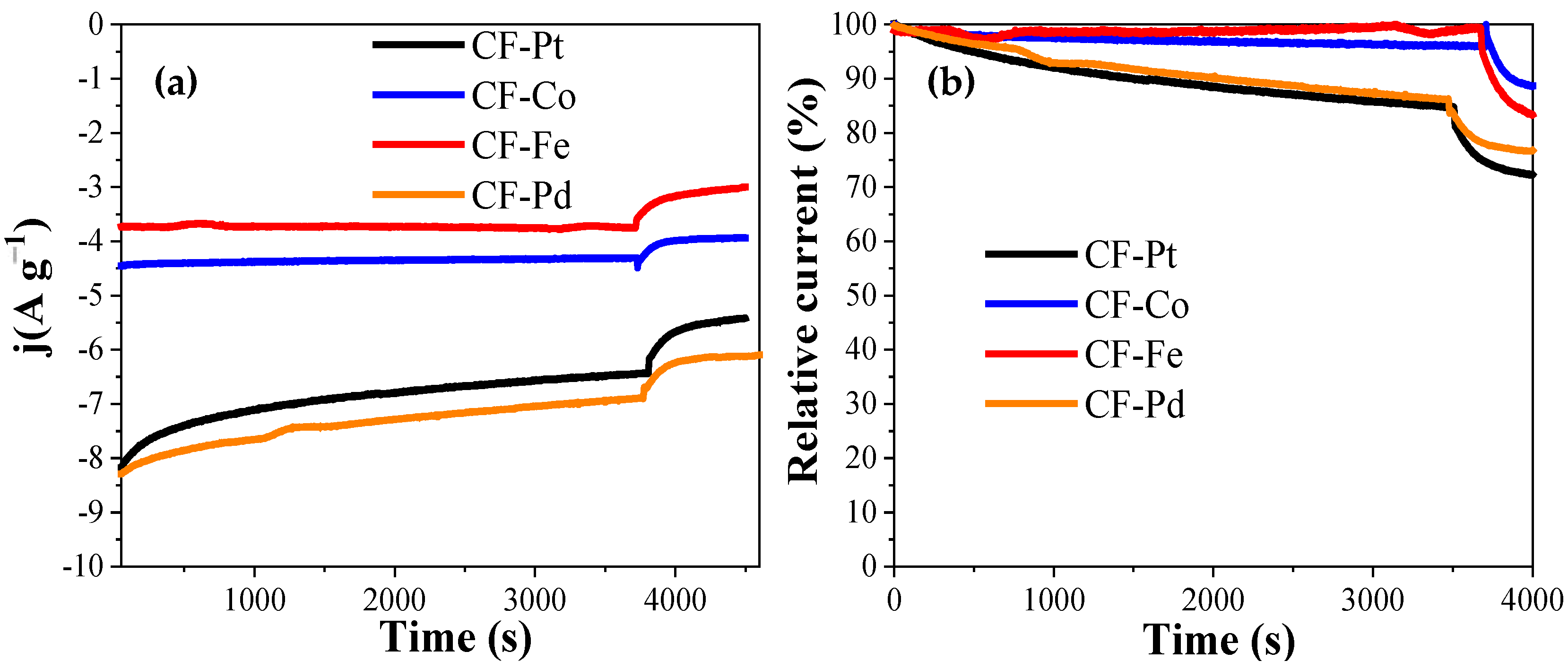
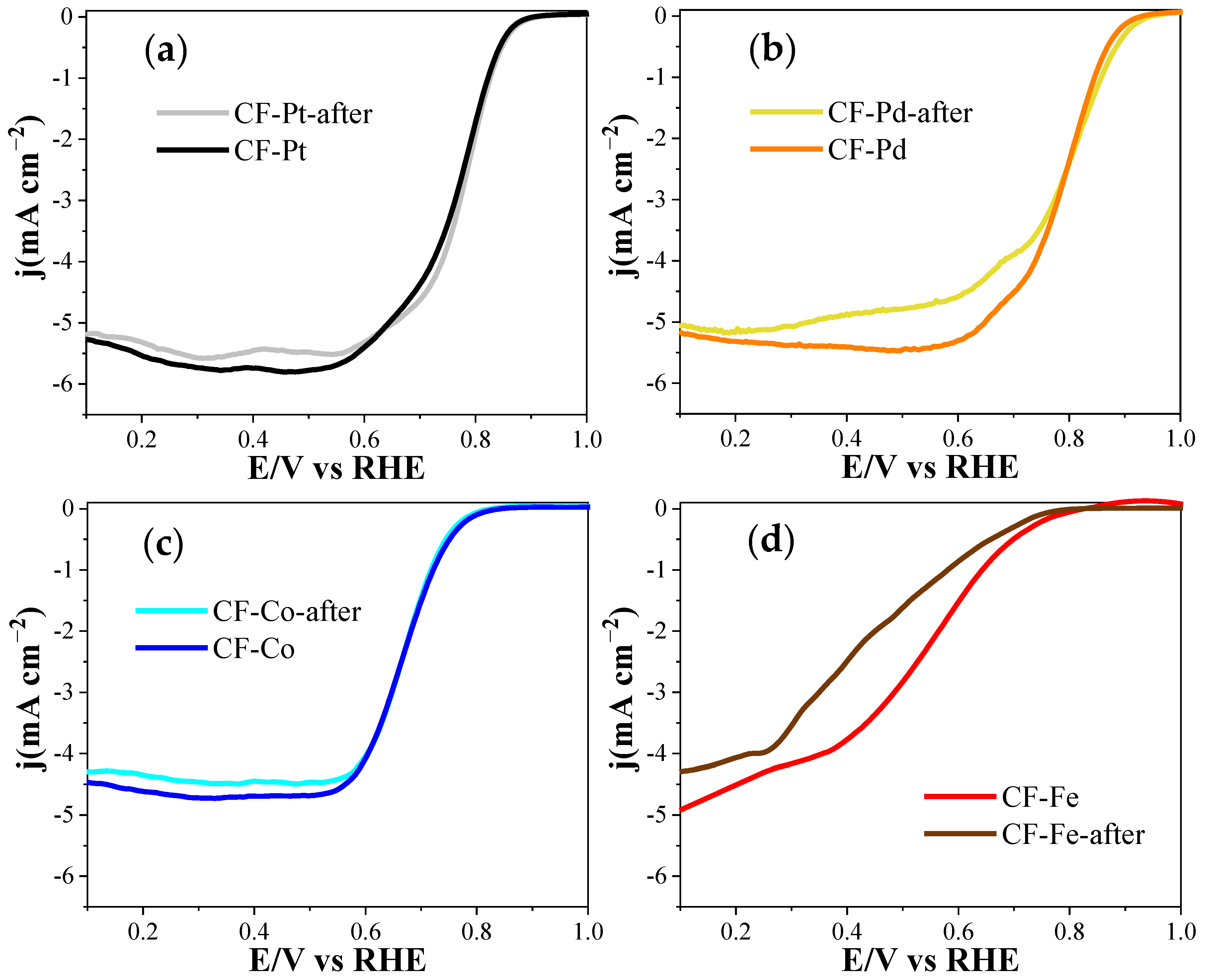
| Electrocatalyst | E (Onset) (V) (at j = 0.1 mA cm−2) | n (at 0.7 V) | Current After 30 min (A g−1) |
| CF-Pd | 0.97 | 4.0 | 7.3 |
| CF-Co | 0.85 | 3.8 | 4.4 |
| CF-Fe | 0.79 | 2.6 | 3.7 |
| CF-Pt | 0.93 | 3.9 | 6.8 |
| Acidic Medium | |||
| Electrocatalyst | E (Onset) (V) (at j = 0.1 mA cm−2) | n | |
| CF-Pd | 0.95 | 4.0 (at 0.7 V) | |
| CF-Co | 0.42 | 2.7 (at 0.3 V) | |
| CF-Fe | 0.46 | 3.3 (at 0.3 V) | |
| CF-Pt | 0.95 | 4.0 (at 0.7 V) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaimes-Paez, C.D.; García-Mateos, F.J.; Ruiz-Rosas, R.; Rodríguez-Mirasol, J.; Cordero, T.; Morallón, E.; Cazorla-Amorós, D. Sustainable Synthesis of Metal-Doped Lignin-Derived Electrospun Carbon Fibers for the Development of ORR Electrocatalysts. Nanomaterials 2023, 13, 2921. https://doi.org/10.3390/nano13222921
Jaimes-Paez CD, García-Mateos FJ, Ruiz-Rosas R, Rodríguez-Mirasol J, Cordero T, Morallón E, Cazorla-Amorós D. Sustainable Synthesis of Metal-Doped Lignin-Derived Electrospun Carbon Fibers for the Development of ORR Electrocatalysts. Nanomaterials. 2023; 13(22):2921. https://doi.org/10.3390/nano13222921
Chicago/Turabian StyleJaimes-Paez, Cristian Daniel, Francisco José García-Mateos, Ramiro Ruiz-Rosas, José Rodríguez-Mirasol, Tomás Cordero, Emilia Morallón, and Diego Cazorla-Amorós. 2023. "Sustainable Synthesis of Metal-Doped Lignin-Derived Electrospun Carbon Fibers for the Development of ORR Electrocatalysts" Nanomaterials 13, no. 22: 2921. https://doi.org/10.3390/nano13222921





