Comparative Study of Different Pretreatment and Combustion Methods on the Grindability of Rice-Husk-Based SiO2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Ball Milling of RHA-SiO2
2.3. Characterization of RHA
2.4. Purity Analysis of Silica in RHA
- (1)
- The RHA sample was first dried in the blast dryer at 105 ± 5 °C for 12 h.
- (2)
- We weighed 2 g of the dried RHA sample (noted as m1, accurate to 0.1 g), placed it in a customized platinum crucible, and scorched it until the weight no longer changed, recording this mass as m2.
- (3)
- We prepared hydrofluoric acid 40 wt%, 98% concentrated sulfuric acid, diluted 1:1 with water.
- (4)
- A small amount of distilled water was used to moisten the cauterized sample, then 15 mL of hydrofluoric acid was measured using a measuring cylinder and 1 mL of sulfuric acid was pipetted into a platinum crucible, and then the sample was heated and cauterized to a slurry.
- (5)
- We cooled the crucible and collected the residue in the center of the crucible, continued to add 10 mL of hydrofluoric acid and heat evaporation to dry it, then placed it into the muffle furnace at 1000 °C for 15 min.
- (6)
- We removed the crucible and cooled it to room temperature, and measured and recorded the mass of the crucible and residue m3.
2.5. Nomenclature for Experimental Conditions
- (1)
- Pretreatment methods: acid leaching (H), boiling (Z), and water leaching (L).
- (2)
- Drying methods: oven drying (H) and microwave drying (W).
- (3)
- Torrefaction temperatures: 210 °C (210), 270 °C (270), and 330 °C (300).
- (4)
- Combustion temperatures: 600 °C (600), 700 °C (700), and 800 °C (800).
2.6. Parametric Optimization
3. Results
3.1. Data Analysis
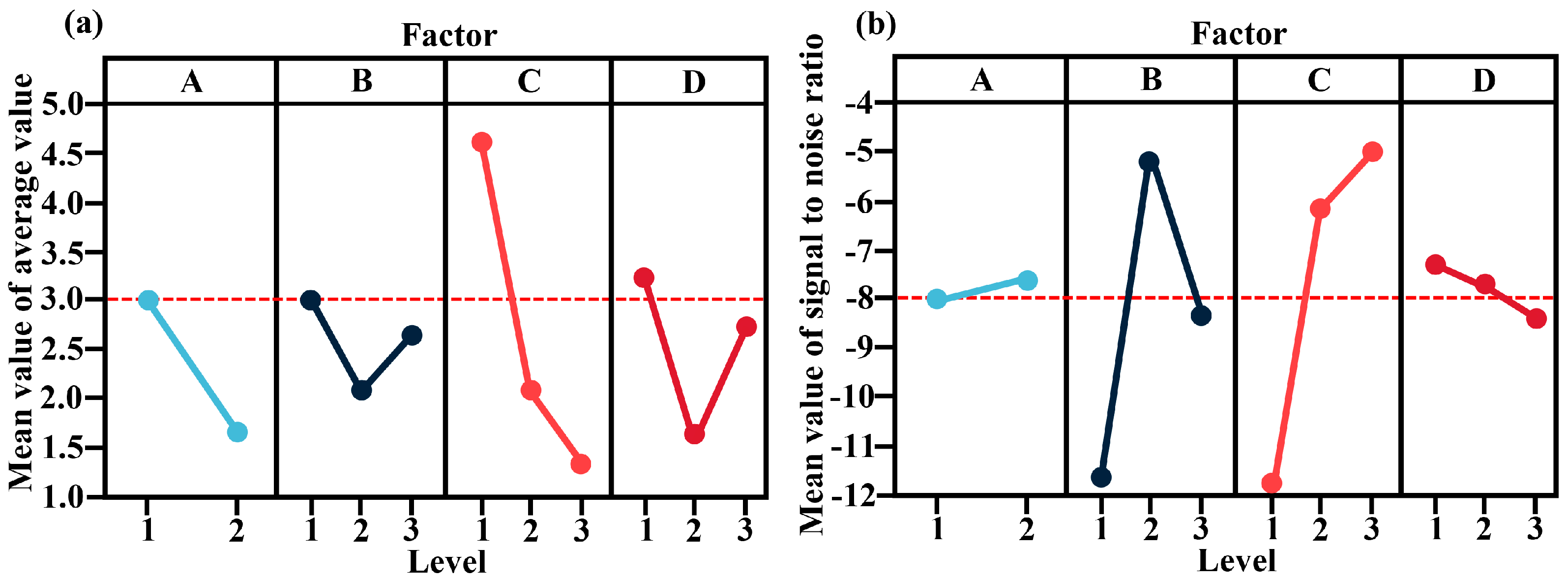
3.1.1. Visual Analysis
3.1.2. Range Analysis
3.1.3. Variance Analysis
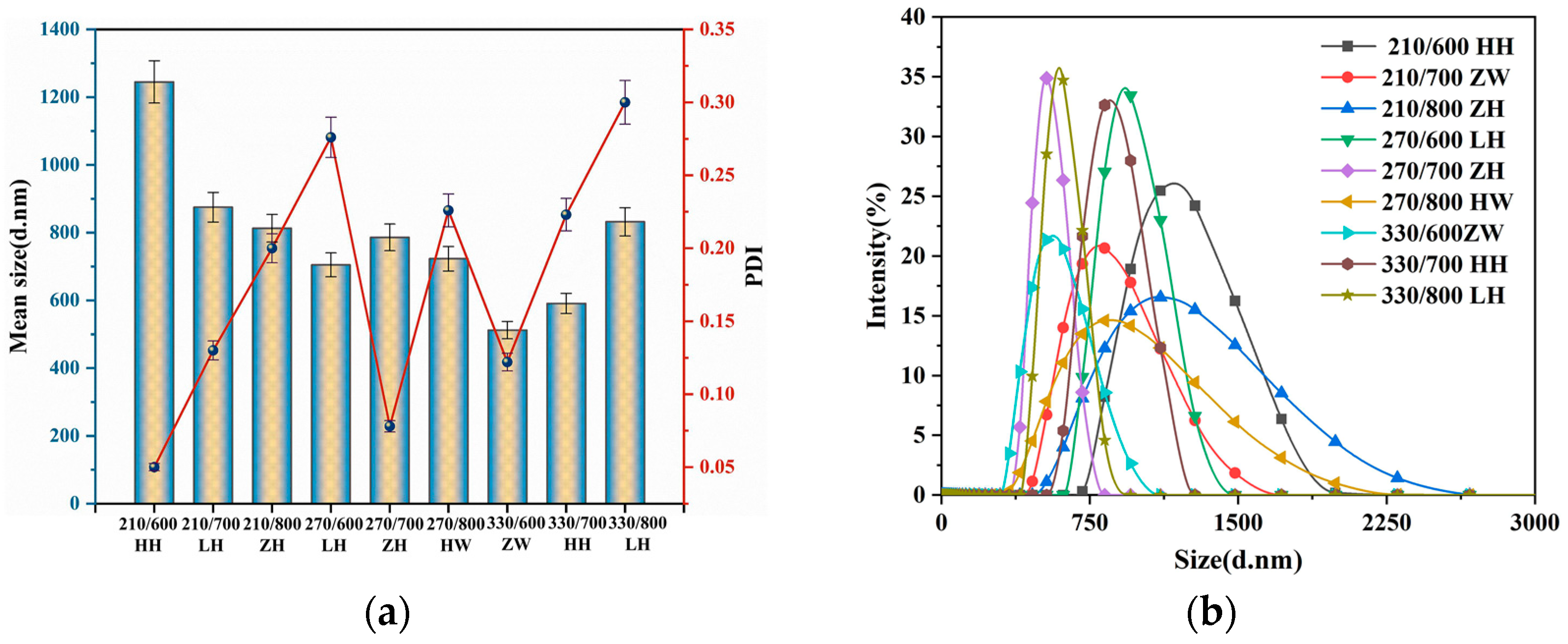
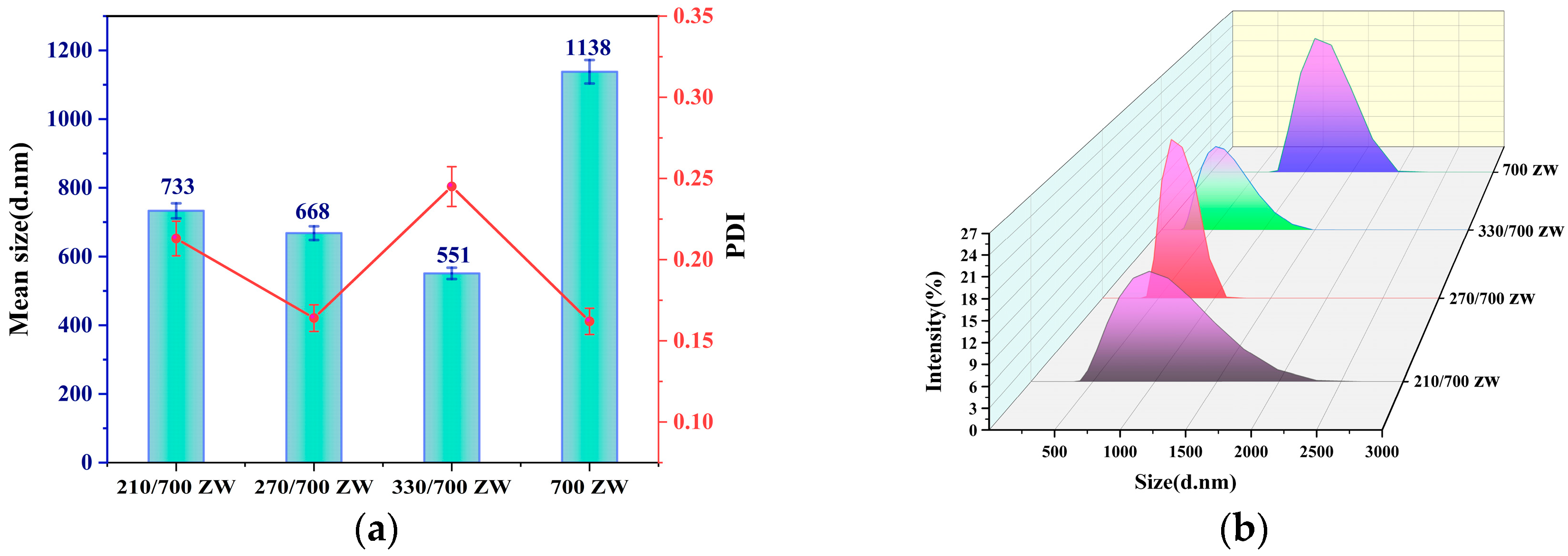
3.2. Effects of Different Treatment Methods on RHA Size and Dispersion
3.3. Verification of Significant Factor
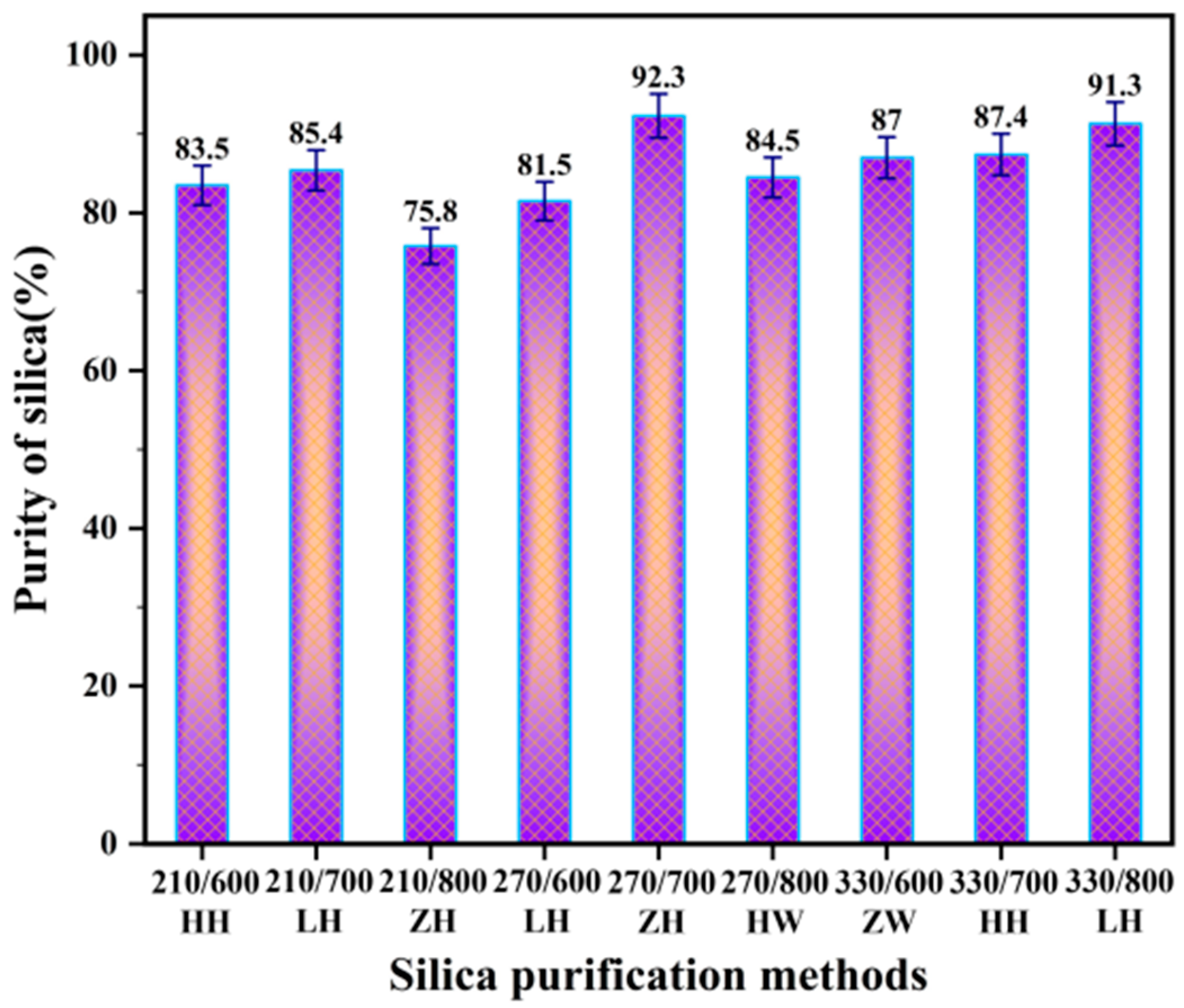
3.4. Effect of Different Treatment Methods on the Purity of RHA-SiO2
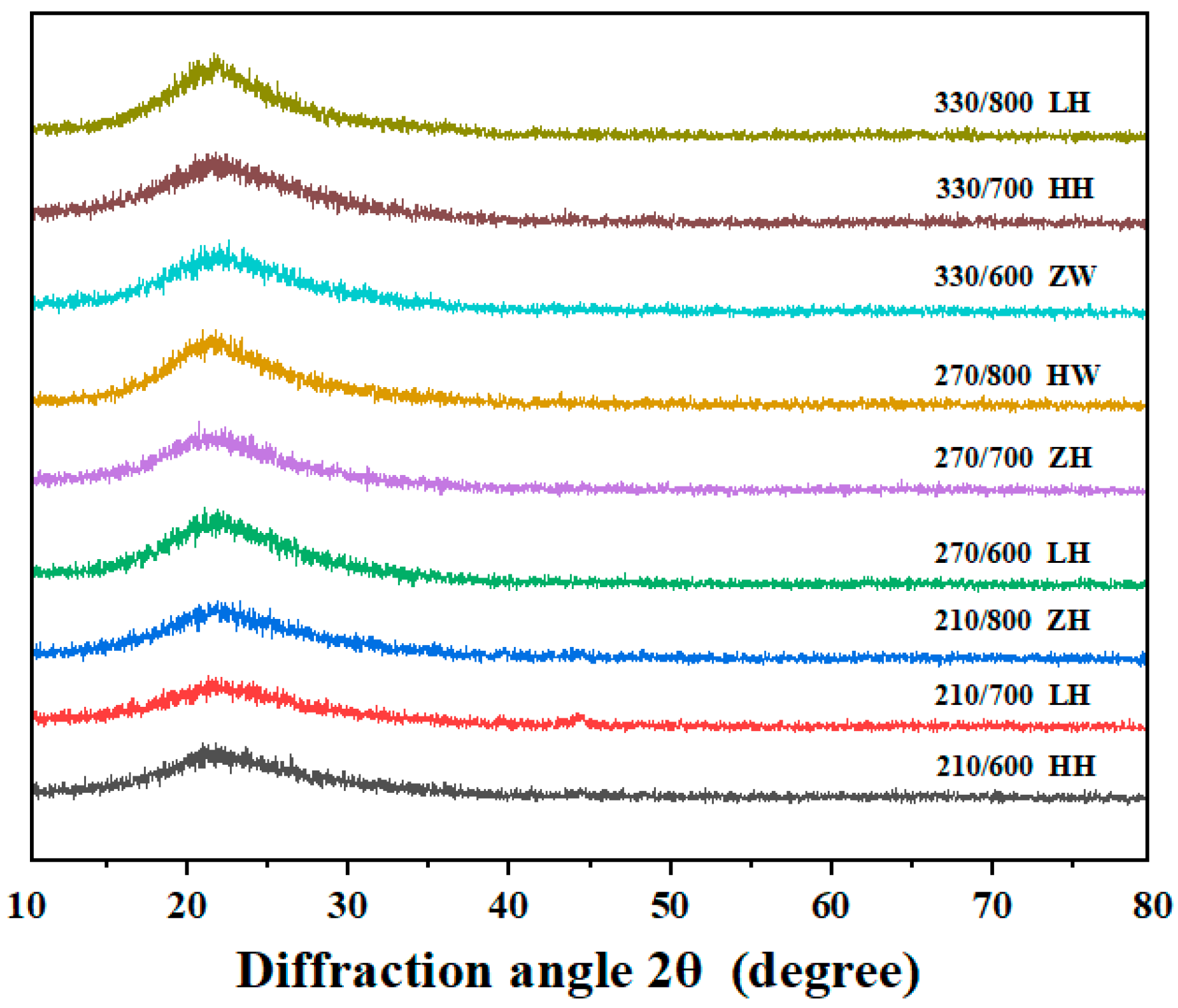
3.5. Effect of Different Treatment Methods on RHA’s Crystal Structure
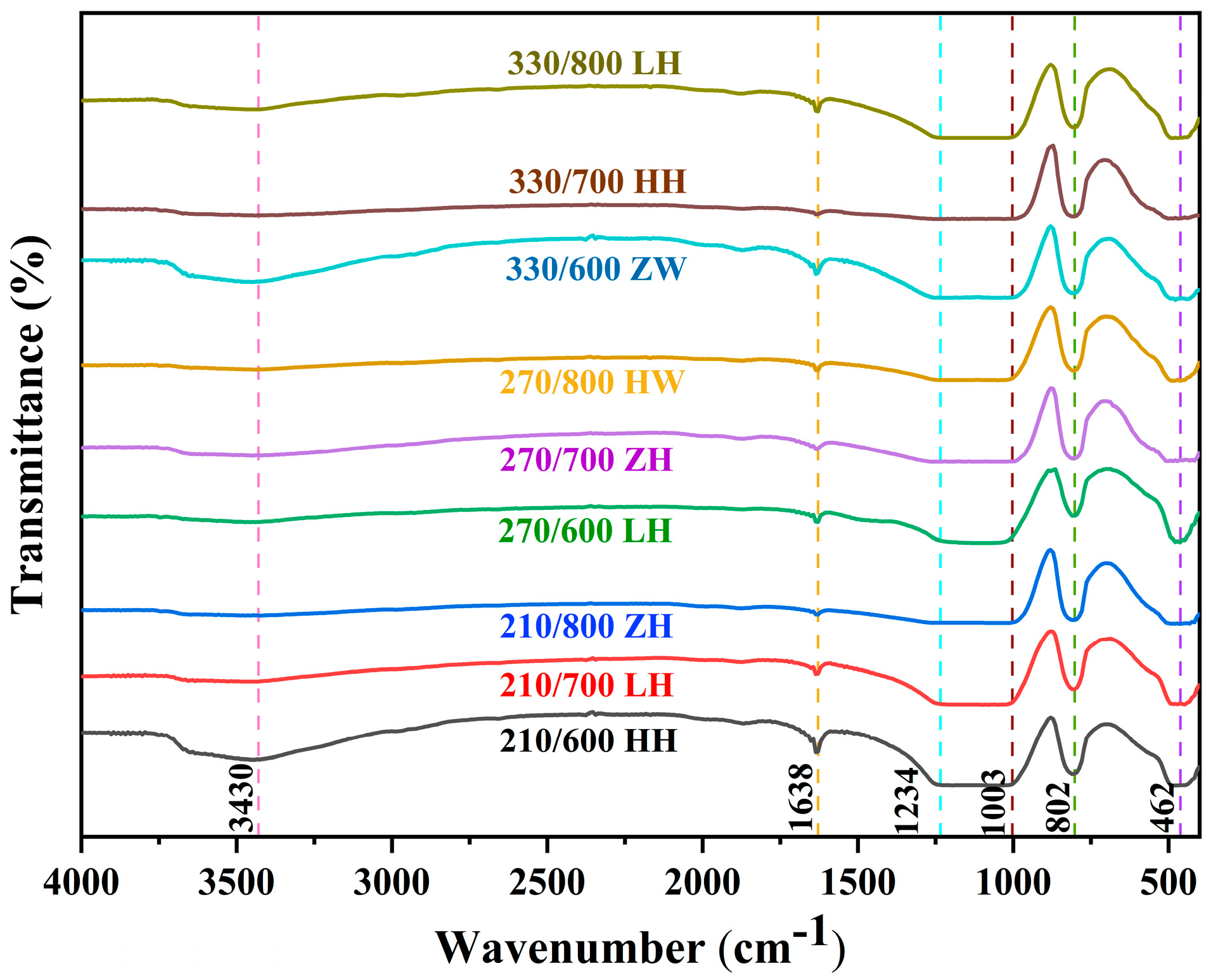
3.6. Effect of Different Treatment Methods on RHA Functional Groups
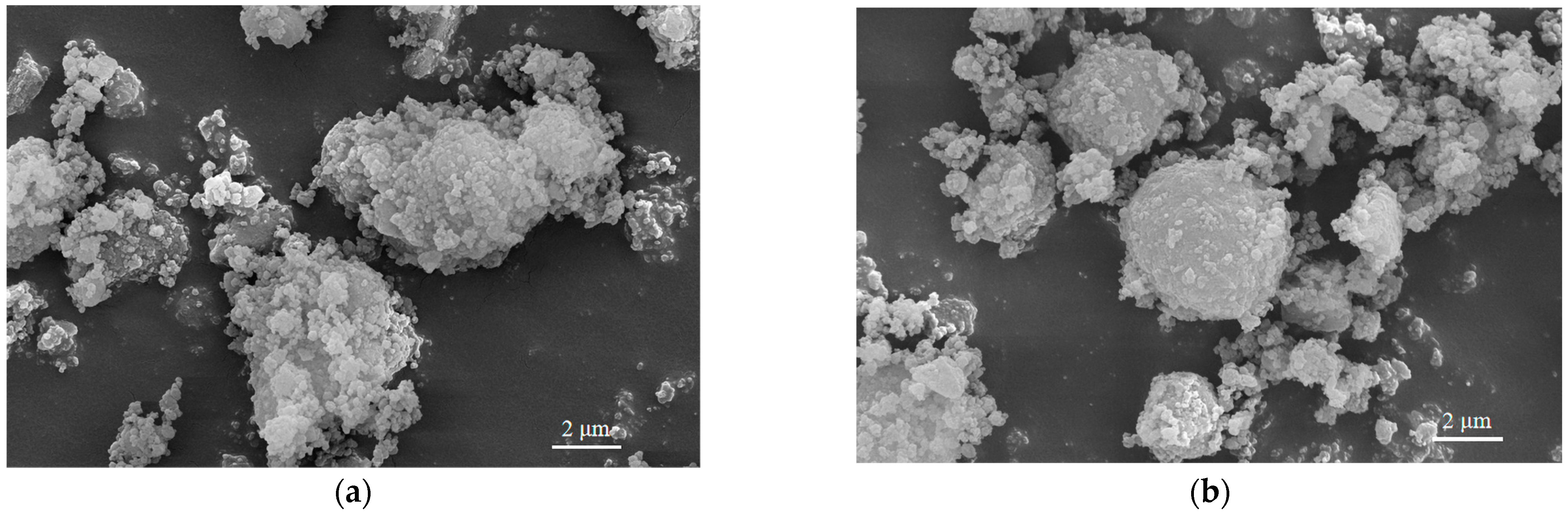
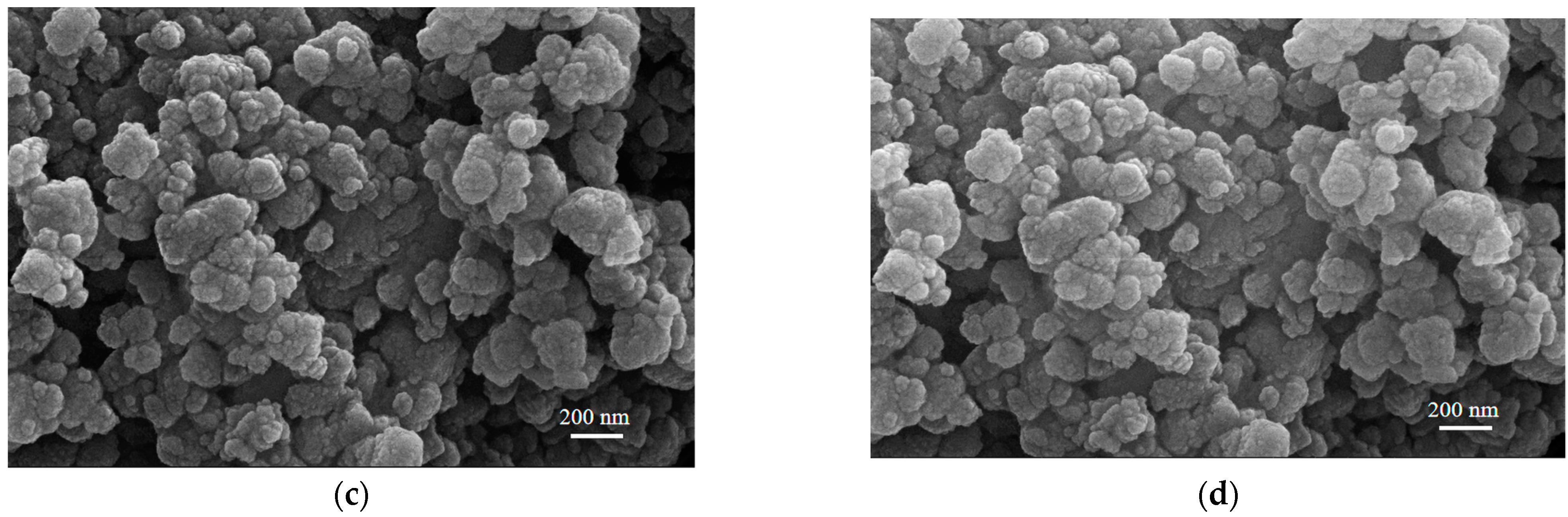
3.7. Effect of Different Treatment Methods on RHA Morphology
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Romasanta, L.J.; Lopez-Manchado, M.A.; Verdejo, R. Increasing the performance of dielectric elastomer actuators: A review from the materials perspective. Prog. Polym. Sci. 2015, 51, 188–211. [Google Scholar] [CrossRef]
- Zhou, H. Supply and demand situation and prospect of China’s rice market in 2021/2022. China Grain Econ. 2022, 3, 38–39. [Google Scholar]
- Luo, H.; Zhou, X.; Ellingford, C.; Zhang, Y.; Chen, S.; Zhou, K.; Zhang, D.; Bowen, C.R.; Wan, C. Interface design for high energy density polymer nanocomposites. Chem. Soc. Rev. 2019, 48, 4424–4465. [Google Scholar] [CrossRef] [PubMed]
- Kamari, S.; Ghorbani, F. Extraction of highly pure silica from rice husk as an agricultural by-product and its application in the production of magnetic mesoporous silica MCM–41. Biomass Convers. Biorefin. 2020, 11, 3001–3009. [Google Scholar] [CrossRef]
- Kumar Sharma, A.; Kumar Ghodke, P.; Goyal, N.; Nethaji, S.; Chen, W.-H. Machine learning technology in biohydrogen production from agriculture waste: Recent advances and future perspectives. Bioresour. Technol. 2022, 364, 128076. [Google Scholar] [CrossRef]
- Sharma, P.; Prakash, J.; Kaushal, R. An insight into the green synthesis of SiO2 nanostructures as a novel adsorbent for removal of toxic water pollutants. Environ. Res. 2022, 212, 113328. [Google Scholar] [CrossRef]
- Golmohammadi, M.; Hanafi-Bojd, H.; Shiva, M. Photocatalytic degradation of ciprofloxacin antibiotic in water by biosynthesized silica supported silver nanoparticles. Ceram. Int. 2023, 49, 7717–7726. [Google Scholar] [CrossRef]
- Bakdash, R.S.; Aljundi, I.H.; Basheer, C.; Abdulazeez, I. Rice husk derived Aminated Silica for the efficient adsorption of different gases. Sci. Rep. 2020, 10, 19526. [Google Scholar] [CrossRef]
- Medina, C.; Sáez del Bosque, I.F.; Frías, M.; Sánchez de Rojas, M.I. Design and characterisation of ternary cements containing rice husk ash and fly ash. Constr. Build. Mater. 2018, 187, 65–76. [Google Scholar] [CrossRef]
- Wu, S.-Q.; Wang, J.-W.; Shao, J.; Wei, L.; Ge, R.-K.; Ren, H. An approach to developing enhanced dielectric property nanocomposites based on acrylate elastomer. Mater. Des. 2018, 146, 208–218. [Google Scholar] [CrossRef]
- Yang, D.; Ni, Y.; Kong, X.; Gao, D.; Wang, Y.; Hu, T.; Zhang, L. Mussel-inspired modification of boron nitride for natural rubber composites with high thermal conductivity and low dielectric constant. Compos. Sci. Technol. 2019, 177, 18–25. [Google Scholar] [CrossRef]
- Yang, D.; Ni, Y.; Liang, Y.; Li, B.; Ma, H.; Zhang, L. Improved thermal conductivity and electromechanical properties of natural rubber by constructing Al2O3-PDA-Ag hybrid nanoparticles. Compos. Sci. Technol. 2019, 180, 86–93. [Google Scholar] [CrossRef]
- Rendón, J.; Giraldo, C.H.C.; Monyake, K.C.; Alagha, L.; Colorado, H.A. Experimental investigation on composites incorporating rice husk nanoparticles for environmental noise management. J. Environ. Manag. 2023, 325, 116477. [Google Scholar] [CrossRef] [PubMed]
- Sankar, S.; Sharma, S.K.; Kaur, N.; Lee, B.; Kim, D.Y.; Lee, S.; Jung, H. Biogenerated silica nanoparticles synthesized from sticky, red, and brown rice husk ashes by a chemical method. Ceram. Int. 2016, 42, 4875–4885. [Google Scholar] [CrossRef]
- Cen, K.; Zhang, J.; Ma, Z.; Chen, D.; Zhou, J.; Ma, H. Investigation of the relevance between biomass pyrolysis polygeneration and washing pretreatment under different severities: Water, dilute acid solution and aqueous phase bio-oil. Bioresour. Technol. 2019, 278, 26–33. [Google Scholar] [CrossRef]
- Azat, S.; Korobeinyk, A.V.; Moustakas, K.; Inglezakis, V.J. Sustainable production of pure silica from rice husk waste in Kazakhstan. J. Clean. Prod. 2019, 217, 352–359. [Google Scholar] [CrossRef]
- Fapohunda, C.; Akinbile, B.; Shittu, A. Structure and properties of mortar and concrete with rice husk ash as partial replacement of ordinary Portland cement—A review. Int. J. Sustain. Built Environ. 2017, 6, 675–692. [Google Scholar] [CrossRef]
- Dominic, M.C.D.; Neenu, K.V.; Begum, P.M.S.; Joseph, R.; Rosa, D.d.S.; Duan, Y.; Balan, A.; Ajithkumar, T.G.; Soumya, M.; Shelke, A.; et al. Nanosilica from Averrhoa bilimbi juice pre-treated rice husk: Preparation and characterization. J. Clean. Prod. 2023, 413, 137476. [Google Scholar] [CrossRef]
- Chun, J.; Mo Gu, Y.; Hwang, J.; Oh, K.K.; Lee, J.H. Synthesis of ordered mesoporous silica with various pore structures using high-purity silica extracted from rice husk. J. Ind. Eng. Chem. 2020, 81, 135–143. [Google Scholar] [CrossRef]
- Karim, M.; Hossain, M.; Khan, M.; Zain, M.; Jamil, M.; Lai, F. On the Utilization of Pozzolanic Wastes as an Alternative Resource of Cement. Materials 2014, 7, 7809–7827. [Google Scholar] [CrossRef]
- Granado, M.P.P.; Gadelha, A.M.T.; Rodrigues, D.S.; Antonio, G.C.; De Conti, A.C. Effect of torrefaction on the properties of briquettes produced from agricultural waste. Bioresour. Technol. Rep. 2023, 21, 101340. [Google Scholar] [CrossRef]
- Ataie, F.F. Utilization of Treated Agricultural Residue Ash as Sodium Silicate in Alkali Activated Slag Systems. Materials 2021, 14, 329. [Google Scholar] [CrossRef] [PubMed]
- Ni, Z.; Bi, H.; Jiang, C.; Sun, H.; Zhou, W.; Qiu, Z.; He, L.; Lin, Q. Influence of biomass on coal slime combustion characteristics based on TG-FTIR, principal component analysis, and artificial neural network. Sci. Total Environ. 2022, 843, 156983. [Google Scholar] [CrossRef] [PubMed]
- Duman, G.; Balmuk, G.; Cay, H.; Kantarli, I.C.; Yanik, J. Comparative Evaluation of Torrefaction and Hydrothermal Carbonization: Effect on Fuel Properties and Combustion Behavior of Agricultural Wastes. Energy Fuels 2020, 34, 11175–11185. [Google Scholar] [CrossRef]
- Safar, M.; Lin, B.-J.; Chen, W.-H.; Langauer, D.; Chang, J.-S.; Raclavska, H.; Pétrissans, A.; Rousset, P.; Pétrissans, M. Catalytic effects of potassium on biomass pyrolysis, combustion and torrefaction. Appl. Energy 2019, 235, 346–355. [Google Scholar] [CrossRef]
- Shabbirahmed, A.M.; Joel, J.; Gomez, A.; Patel, A.K.; Singhania, R.R.; Haldar, D. Environment friendly emerging techniques for the treatment of waste biomass: A focus on microwave and ultrasonication processes. Environ. Sci. Pollut. Res. 2023, 30, 79706–79723. [Google Scholar] [CrossRef]
- Ke, C.; Zhang, Y.; Gao, Y.; Pan, Y.; Li, B.; Wang, Y.; Ruan, R. Syngas production from microwave-assisted air gasification of biomass: Part 1 model development. Renew. Energy 2019, 140, 772–778. [Google Scholar] [CrossRef]
- Bundhoo, Z.M.A. Microwave-assisted conversion of biomass and waste materials to biofuels. Renew. Sustain. Energy Rev. 2018, 82, 1149–1177. [Google Scholar] [CrossRef]
- Duran-Jimenez, G.; Monti, T.; Titman, J.J.; Hernandez-Montoya, V.; Kingman, S.W.; Binner, E.R. New insights into microwave pyrolysis of biomass: Preparation of carbon-based products from pecan nutshells and their application in wastewater treatment. J. Anal. Appl. Pyrolysis 2017, 124, 113–121. [Google Scholar] [CrossRef]
- Chen, W.-H.; Carrera Uribe, M.; Kwon, E.E.; Lin, K.-Y.A.; Park, Y.-K.; Ding, L.; Saw, L.H. A comprehensive review of thermoelectric generation optimization by statistical approach: Taguchi method, analysis of variance (ANOVA), and response surface methodology (RSM). Renew. Sustain. Energy Rev. 2022, 169, 112917. [Google Scholar] [CrossRef]
- Sorkhabi, T.S.; Samberan, M.F.; Ostrowski, K.A.; Zajdel, P.; Stempkowska, A.; Gawenda, T. Electrospinning of Poly (Acrylamide), Poly (Acrylic Acid) and Poly (Vinyl Alcohol) Nanofibers: Characterization and Optimization Study on the Effect of Different Parameters on Mean Diameter Using Taguchi Design of Experiment Method. Materials 2022, 15, 5876. [Google Scholar] [CrossRef] [PubMed]
- Patra, S.N.; Easteal, A.J.; Bhattacharyya, D. Parametric study of manufacturing poly(lactic) acid nanofibrous mat by electrospinning. J. Mater. Sci. 2009, 44, 647–654. [Google Scholar] [CrossRef]
- ASTM D6866-22; Standard Test Methods for Determining the Biobased Content of Solid, Liquid, and Gaseous Samples Using Radiocarbon Analysis. American Society for Testing and Materials(ASTM): New York, NY, USA, 2022.
- HG/T 3062-3072-2008; Rubber Compounding Ingredients. Silica, Precipitated, Hydrated. Determination of Silica Content on Dried Sample. Rubber and Rubber Products: Shenyang, China, 2008.
- Albetran, H.; Dong, Y.; Low, I.M. Characterization and optimization of electrospun TiO2/PVP nanofibers using Taguchi design of experiment method. J. Asian Ceram. Soc. 2018, 3, 292–300. [Google Scholar] [CrossRef]
- Ge, H. Mechanism and influencing factors of bacterial cellulose synthesis based on Minitab. J. Comput. Methods Sci. Eng. 2022, 22, 1333–1348. [Google Scholar] [CrossRef]
- Aghajani, H.; Soflaei, F.G. Introduction and calculation of interaction coefficients in solvent extraction of rhenium from molybdenite. Miner. Eng. 2022, 190, 107869. [Google Scholar] [CrossRef]
- Zhang, S.; Dong, Q.; Zhang, L.; Xiong, Y. Effects of water washing and torrefaction on the pyrolysis behavior and kinetics of rice husk through TGA and Py-GC/MS. Bioresour. Technol. 2016, 199, 352–361. [Google Scholar] [CrossRef]
- Bianchini, P.; Merlo, F.; Maraschi, F.; Brescia, R.; Prato, M.; Profumo, A.; Speltini, A. From Rice Husk Ash to Silica-Supported Carbon Nanomaterials: Characterization and Analytical Application for Pre-Concentration of Steroid Hormones from Environmental Waters. Molecules 2023, 28, 745. [Google Scholar] [CrossRef]
- Maroušek, J.; Maroušková, A.; Periakaruppan, R.; Gokul, G.M.; Anbukumaran, A.; Bohatá, A.; Kříž, P.; Bárta, J.; Černý, P.; Olšan, P. Silica Nanoparticles from Coir Pith Synthesized by Acidic Sol-Gel Method Improve Germination Economics. Polymers 2022, 14, 266. [Google Scholar] [CrossRef]
- Durairaj, K.; Senthilkumar, P.; Velmurugan, P.; Dhamodaran, K.; Kadirvelu, K.; Kumaran, S. Sol-gel mediated synthesis of silica nanoparticle from Bambusa vulgaris leaves and its environmental applications: Kinetics and isotherms studies. J. Sol-Gel Sci. Technol. 2019, 90, 653–664. [Google Scholar] [CrossRef]
- Nayantara; Kaur, P. Biosynthesis of nanoparticles using eco-friendly factories and their role in plant pathogenicity: A review. Biotechnol. Res. Innov. 2018, 2, 63–73. [Google Scholar] [CrossRef]
- Heng, Z.W.; Tan, Y.Y.; Chong, W.C.; Mahmoudi, E.; Mohammad, A.W.; Teoh, H.C.; Sim, L.C.; Koo, C.H. Preparation of a novel polysulfone membrane by incorporated with carbon dots grafted silica from rice husk for dye removal. J. Water Process Eng. 2021, 40, 101805. [Google Scholar] [CrossRef]
- Homagai, P.L.; Poudel, R.; Poudel, S.; Bhattarai, A. Adsorption and removal of crystal violet dye from aqueous solution by modified rice husk. Heliyon 2022, 8, e09261. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wei, J.; Chen, J.; Zhang, B.; Xu, P.; Ren, J.; Yu, Q. Comparative Study of Water-Leaching and Acid-Leaching Pretreatment on the Thermal Stability and Reactivity of Biomass Silica for Viability as a Pozzolanic Additive in Cement. Materials 2018, 11, 1697. [Google Scholar] [CrossRef] [PubMed]
- Amer, M.; Nour, M.; Ahmed, M.; Ookawara, S.; Nada, S.; Elwardany, A. The effect of microwave drying pretreatment on dry torrefaction of agricultural biomasses. Bioresour. Technol. 2019, 286, 121400. [Google Scholar] [CrossRef] [PubMed]
| Name | Proximate Analysis (wt%) | Ultimate Analysis (wt%) | ||||||
|---|---|---|---|---|---|---|---|---|
| V | A | FC | C | H | N | S | O | |
| RH | 59.6 | 24.8 | 15.6 | 43.5 | 6.4 | 0.6 | 0.1 | 35.2 |
| Processing Method | Raw Material | Instrument/Reagent | Process |
|---|---|---|---|
| Acid leaching | RH | H2SO4 0.5 mol/L | RH at room temperature with a solid–liquid ratio of 1:20, soak for 2.5 h, and then rinse with water to neutral, drain, bag for standby |
| Boiling | RH | Electric cooker/water | Boil 1 L of water in an electric pot, add 50 g of RH, boil continuously for 2.5 h (keep adding water during this time), and then rinse with water, drain, bag, and set aside |
| Water leaching | RH | Water | Rinse RH with running water at room temperature, drain, and bag |
| Microwave | Pretreated RH | PM2003 microwave oven | Take 50 g of the treated RH each time, put it in a microwave oven, heat it for 7 min, and adjust the power to 450 W |
| Drying | Pretreated RH | Blast dryers | Take out 50 g of treated RH each time, put it into a blast-drying oven at 105 ± 5 °C, and dry continuously for 24 h until a constant weight is reached |
| Torrefaction | RH after secondary drying | Muffle furnace/crucible | A 15 g sample was placed in a crucible and, when the muffle temperature was raised to 50 °C, the crucible was placed, and then the temperature was raised to 210 °C, 270 °C, and 330 °C for 40 min of torrefaction, with a temperature rise rate of 10 °C/min |
| Control Factor | Level | |||
|---|---|---|---|---|
| Ⅰ | Ⅱ | Ⅲ | Units | |
| A: Drying method | Oven drying (H) | Microwave drying (W) | — | — |
| B: Combustion treatment | 600 | 700 | 800 | °C |
| C: Torrefaction treatment | 210 | 270 | 330 | °C |
| D: Pretreatment method | Acid leaching (H) | Boiling (Z) | Water leaching (L) | — |
| Exp. No. | Drying Method A (—) | Combustion Treatment B (°C) | Torrefaction Process C (°C) | Pretreatment Method D (—) | Original Data D50 (nm) | Treated Data D50 (nm) | PDI |
|---|---|---|---|---|---|---|---|
| 1 | 1 (H) | 1 (600) | 1 (210) | 1 (H) | 1316 | 7 | 0.098 |
| 2 | 1 (H) | 2 (700) | 2 (270) | 2 (Z) | 849 | 2 | 0.135 |
| 3 | 1 (H) | 3 (800) | 3 (330) | 3 (L) | 902 | 3 | 0.331 |
| 4 | 1 (H) | 1 (600) | 2 (270) | 3 (L) | 782 | 2 | 0.310 |
| 5 | 1 (H) | 2 (700) | 3 (330) | 1 (H) | 653 | 1 | 0.247 |
| 6 | 1 (H) | 3 (800) | 1 (210) | 2 (Z) | 876 | 3 | 0.231 |
| 7 | 2 (W) | 1 (600) | 3 (330) | 2 (Z) | 576 | 0 | 0.161 |
| 8 | 2 (W) | 2 (700) | 1 (210) | 3 (L) | 931 | 3 | 0.164 |
| 9 | 2 (W) | 3 (800) | 2 (270) | 1 (H) | 810 | 2 | 0.260 |
| Exp. No. | Drying Method A (—) | Combustion Treatment B (°C) | Torrefaction Process C (°C) | Pretreatment Method D (—) | Treated Data D50 (nm) |
|---|---|---|---|---|---|
| 1 | 1 (H) | 1 (600) | 1 (210) | 1 (H) | 7 |
| 2 | 1 (H) | 2 (700) | 2 (270) | 2 (Z) | 2 |
| 3 | 1 (H) | 3 (800) | 3 (330) | 3 (L) | 3 |
| 4 | 1 (H) | 1 (600) | 2 (270) | 3 (L) | 2 |
| 5 | 1 (H) | 2 (700) | 3 (330) | 1 (H) | 1 |
| 6 | 1 (H) | 3 (800) | 1 (210) | 2 (Z) | 3 |
| 7 | 2 (W) | 1 (600) | 3 (330) | 2 (Z) | 0 |
| 8 | 2 (W) | 2 (700) | 1 (210) | 3 (L) | 3 |
| 9 | 2 (W) | 3 (800) | 2 (270) | 1 (H) | 2 |
| K1 | 18 | 9 | 13 | 10 | |
| K2 | 5 | 6 | 9 | 5 | |
| K3 | / | 8 | 4 | 8 | |
| k1 | 3.00 | 3.00 | 4.33 | 3.33 | |
| k2 | 1.67 | 2.00 | 3.00 | 1.67 | |
| k3 | / | 2.67 | 1.33 | 2.67 | |
| R | 1.33 | 1.00 | 3.00 | 1.67 | |
| Primary and secondary factors | C > D > A > B | ||||
| Optimal combination | A2 | B2 | C3 | D2 | |
| Variance Source | DF | Seq SS | Adj SS | Adj MS | F | p |
|---|---|---|---|---|---|---|
| Drying method A | 1 | 3.556 | 3.556 | 3.556 | 0.59 | 0.582 |
| Combustion treatment B | 2 | 1.556 | 1.556 | 0.778 | 0.13 | 0.891 |
| Torrefaction process C | 2 | 14.889 | 14.889 | 7.448 | 1.24 | 0.536 |
| Pretreatment method D | 2 | 4.222 | 4.222 | 2.118 | 0.35 | 0.766 |
| Error | 1 | 6.000 | 6.000 | 6.000 | ||
| Amount | 8 | 30.222 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, Y.; Yuan, S.; Ma, Z.; Hou, Y.; Niu, S.; Lekai, L.; Liu, G.; Cao, F. Comparative Study of Different Pretreatment and Combustion Methods on the Grindability of Rice-Husk-Based SiO2. Nanomaterials 2023, 13, 2951. https://doi.org/10.3390/nano13222951
Ma Y, Yuan S, Ma Z, Hou Y, Niu S, Lekai L, Liu G, Cao F. Comparative Study of Different Pretreatment and Combustion Methods on the Grindability of Rice-Husk-Based SiO2. Nanomaterials. 2023; 13(22):2951. https://doi.org/10.3390/nano13222951
Chicago/Turabian StyleMa, Yunhai, Shengwang Yuan, Zichao Ma, Yihao Hou, Shichao Niu, Li Lekai, Guoqin Liu, and Feipeng Cao. 2023. "Comparative Study of Different Pretreatment and Combustion Methods on the Grindability of Rice-Husk-Based SiO2" Nanomaterials 13, no. 22: 2951. https://doi.org/10.3390/nano13222951
APA StyleMa, Y., Yuan, S., Ma, Z., Hou, Y., Niu, S., Lekai, L., Liu, G., & Cao, F. (2023). Comparative Study of Different Pretreatment and Combustion Methods on the Grindability of Rice-Husk-Based SiO2. Nanomaterials, 13(22), 2951. https://doi.org/10.3390/nano13222951










