Construction of Binary RGO/TiO2 Fibrous Membranes with Enhanced Mechanical Properties for E. coli Inactivation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
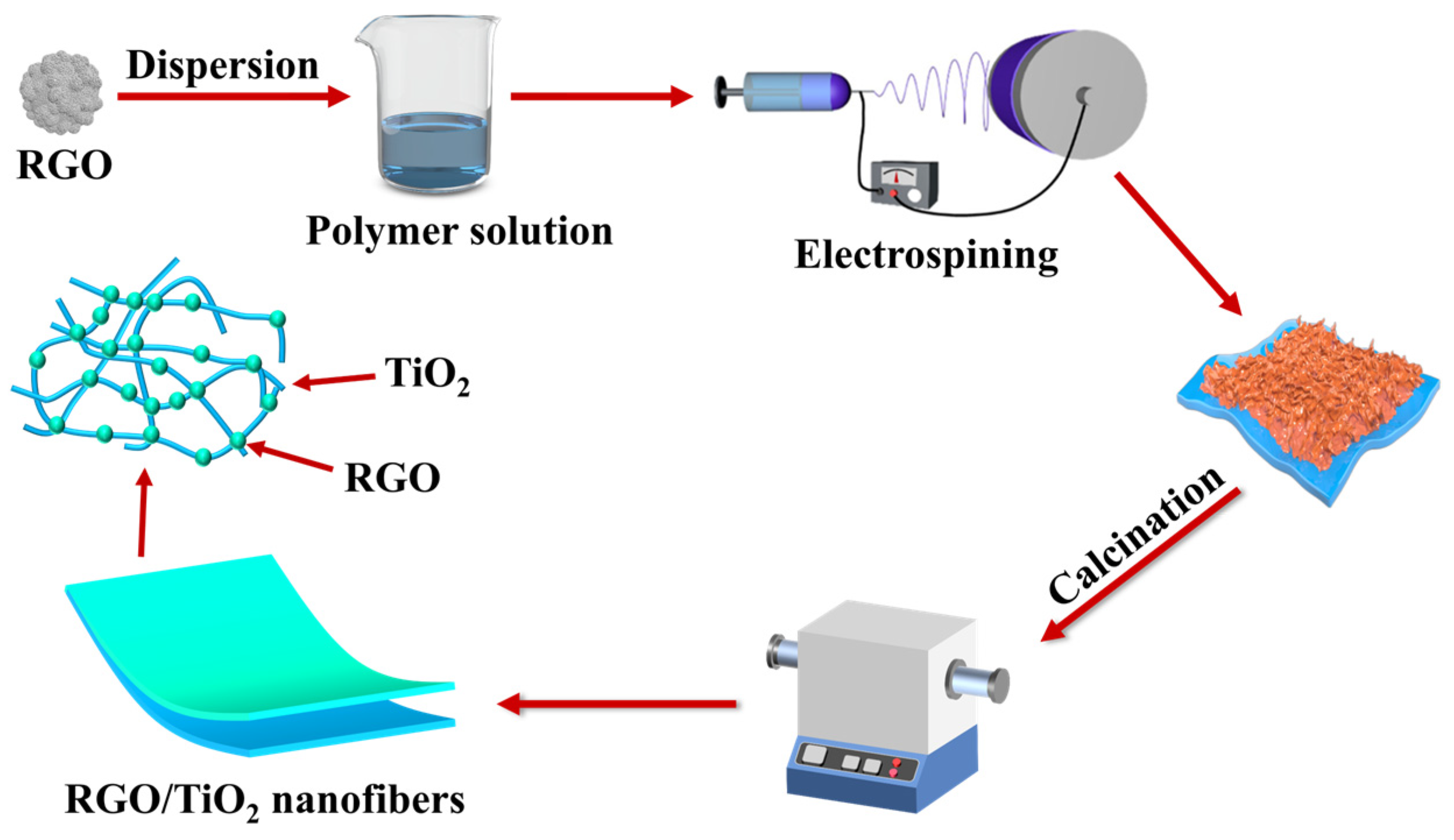
2.2. Fabrication of RGO/TiO2 Nanofibrous Membranes
2.3. Characterization
2.4. Antibacterial Experiments
3. Results and Discussion
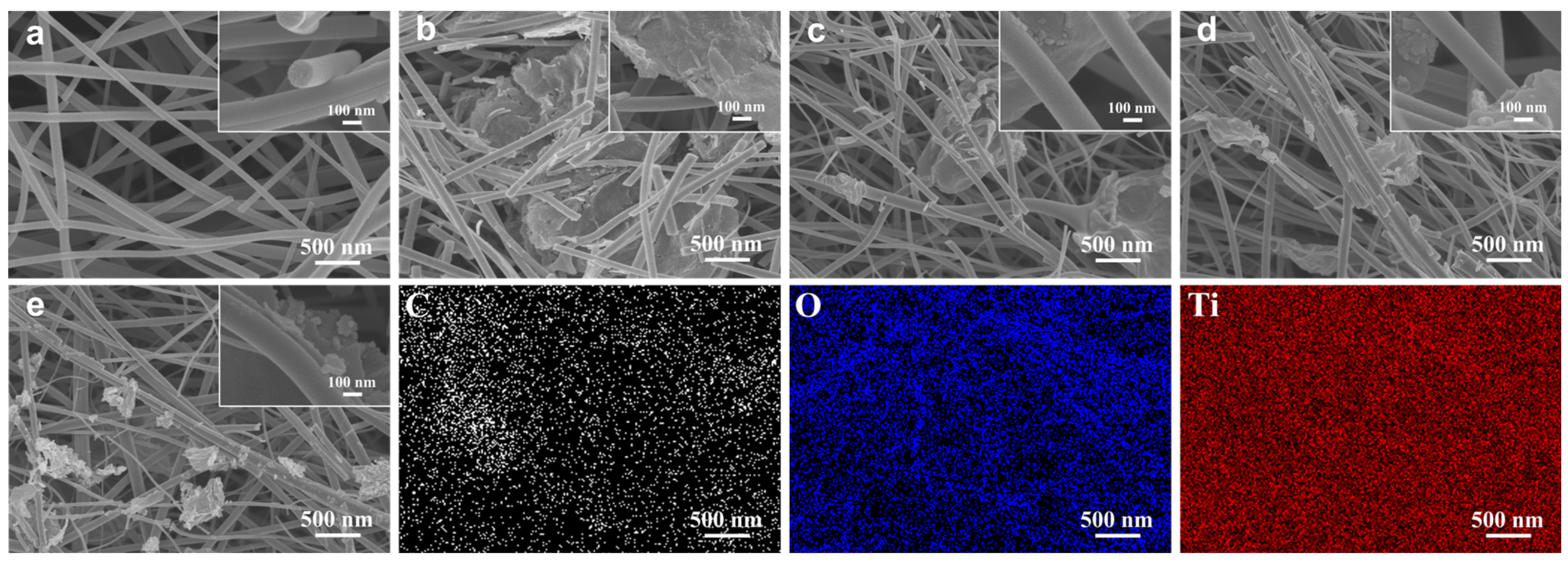
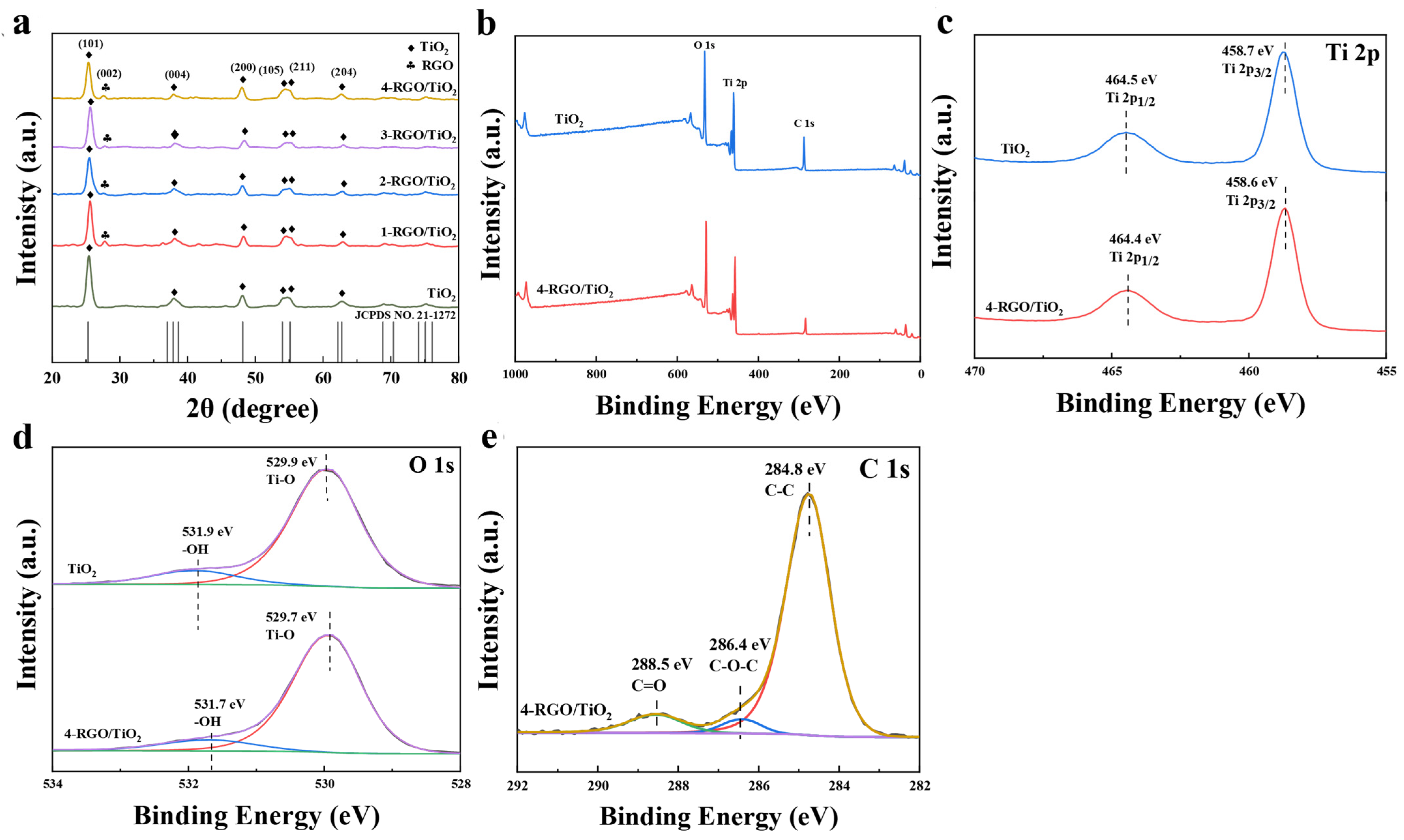
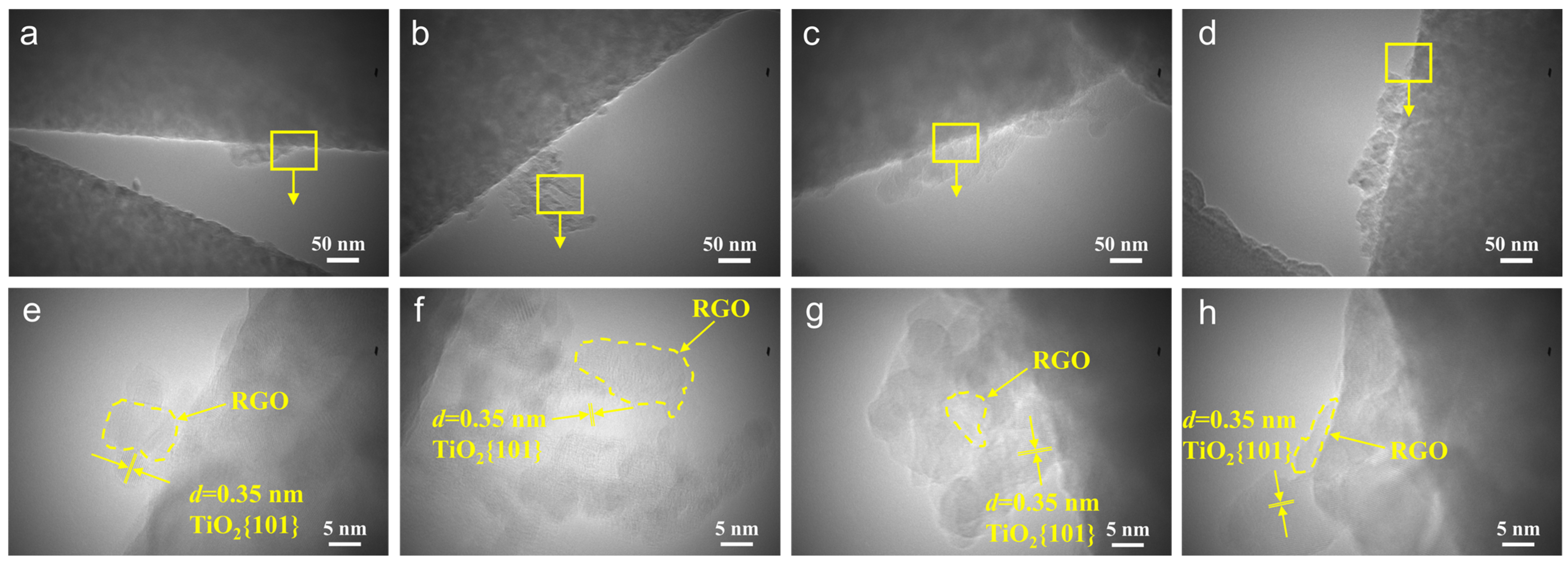
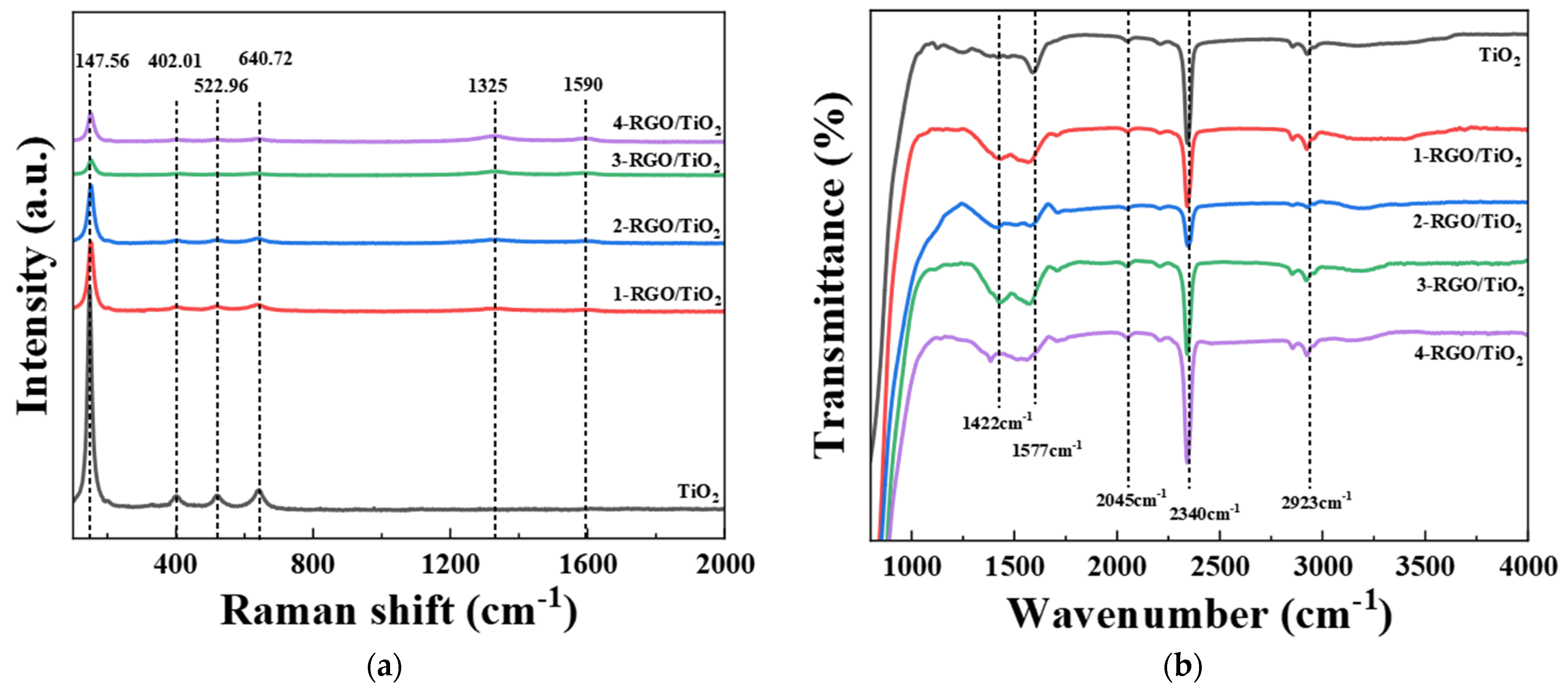
3.1. Morphology and Structures of RGO/TiO2 Nanofibrous Membranes
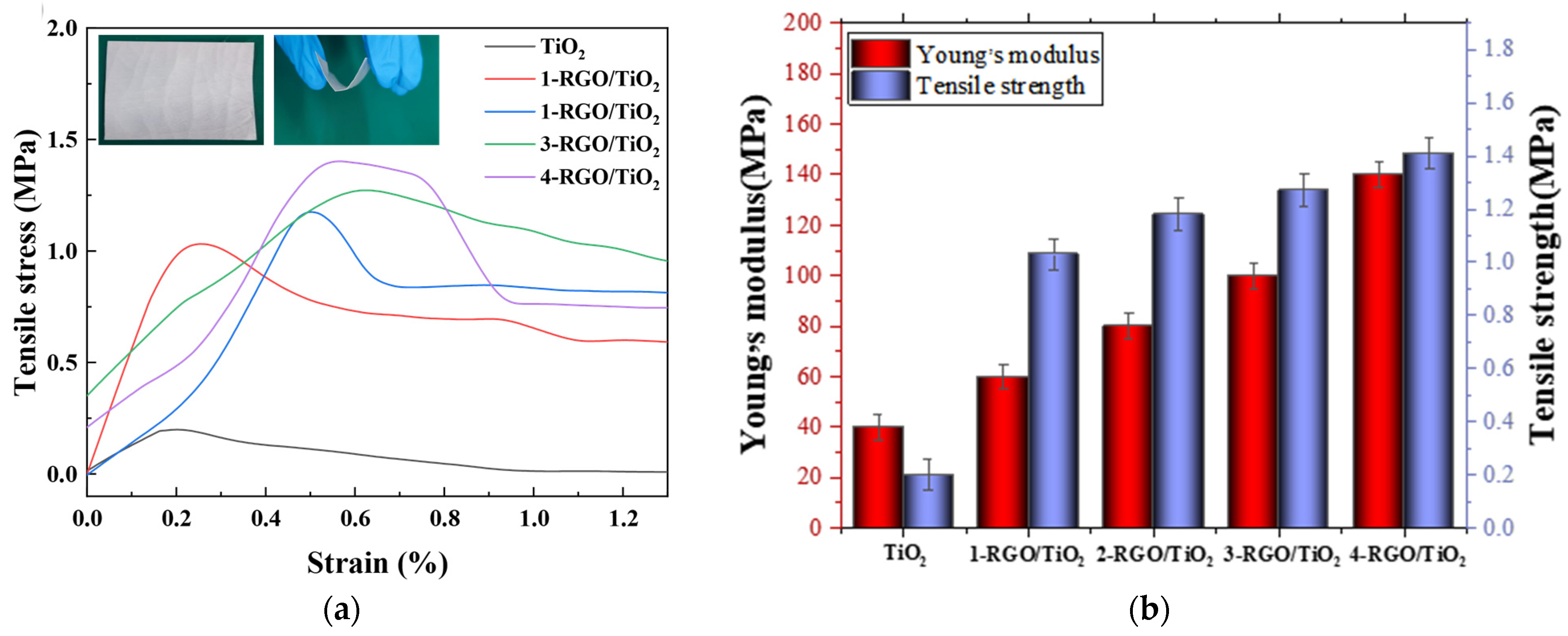
3.2. Mechanical Properties of RGO/TiO2 Nanofibrous Membrane
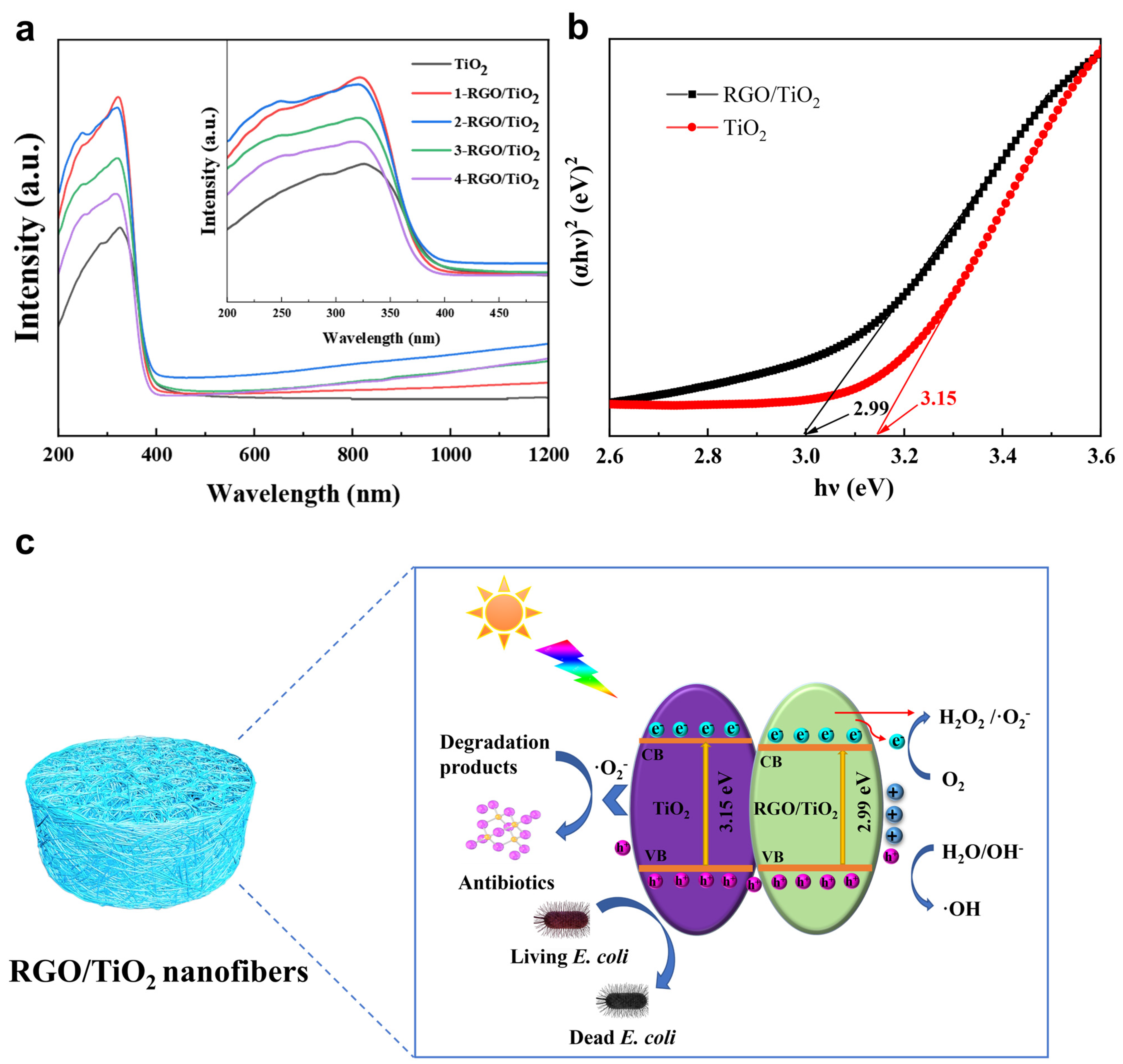
3.3. Optical Properties of RGO/TiO2 Nanofibrous Membrane
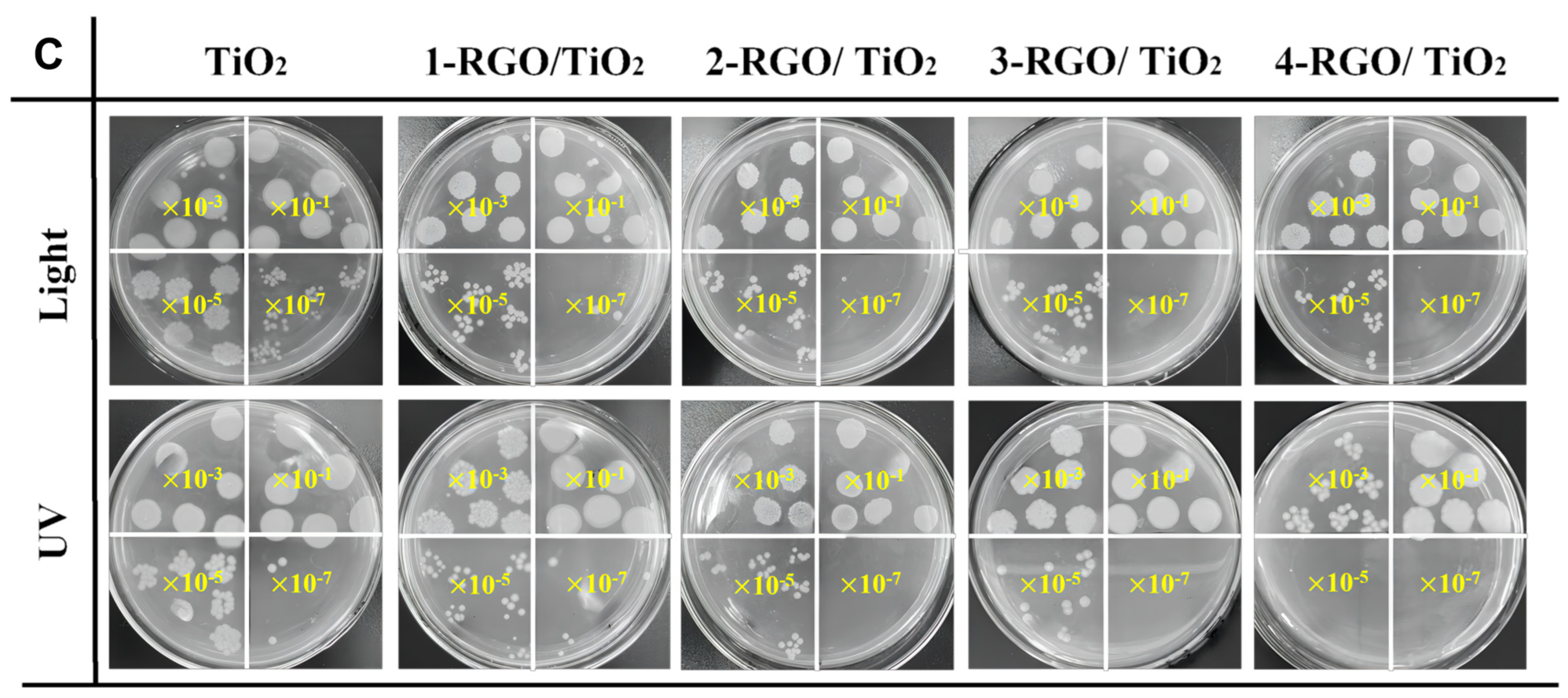
3.4. Photocatalytic Antibacterial Performance of RGO/TiO2 Nanofibrous Membrane
3.5. Photocatalytic Mechanism Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raeke, J.; Lechtenfeld, O.J.; Seiwert, B.; Meier, T.; Riemenschneider, C.; Reemtsma, T. Photochemically Induced Bound Residue Formation of Carbamazepine with Dissolved Organic Matter. Environ. Sci. Technol. 2017, 51, 5523–5530. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Kong, X.; Zhang, W.; Zhu, W.; Huang, L.; Wang, J.; Zhang, X.; Liu, X.; Hu, N.; Suo, Y.; et al. Mechanism insight into rapid photocatalytic disinfection of Salmonella based on vanadate QDs-interspersed g-C3N4 heterostructures. Appl. Catal. B Environ. 2018, 225, 228–237. [Google Scholar] [CrossRef]
- Si, Y.; Li, J.; Zhao, C.; Deng, Y.; Ma, Y.; Wang, D.; Sun, G. Biocidal and Rechargeable N-Halamine Nanofibrous Membranes for Highly Efficient Water Disinfection. ACS Biomater. Sci. Eng. 2017, 3, 854–862. [Google Scholar] [CrossRef] [PubMed]
- Efome, J.E.; Rana, D.; Matsuura, T.; Lan, C.Q. Metal–organic frameworks supported on nanofibers to remove heavy metals. J. Mater. Chem. A 2018, 6, 4550–4555. [Google Scholar] [CrossRef]
- Dong, L.; Hou, L.A.; Wang, Z.; Gu, P.; Chen, G.; Jiang, R. A new function of spent activated carbon in BAC process: Removing heavy metals by ion exchange mechanism. J. Hazard. Mater. 2018, 359, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, X. Titanium Dioxide Nanomaterials: Self-Structural Modifications. Chem. Rev. 2014, 114, 9890–9918. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Tian, J.; Sang, Y.; Cabot, A.; Liu, H. Structure, Synthesis, and Applications of TiO2 Nanobelts. Adv. Mater. 2015, 27, 2557–2582. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Zhu, L.; Ong, W.L.; Wang, J.; Ho, G.W. Structural design of TiO2-based photocatalyst for H2 production and degradation applications. Catal. Sci. Technol. 2015, 5, 4703–4726. [Google Scholar] [CrossRef]
- Tan, Y.; Shu, Z.; Zhou, J.; Li, T.; Wang, W.; Zhao, Z. One-step synthesis of nanostructured g-C3N4/TiO2 composite for highly enhanced visible-light photocatalytic H2 evolution. Appl. Catal. B Environ. 2018, 230, 260–268. [Google Scholar] [CrossRef]
- Pan, J.; Dong, Z.; Wang, B.; Jiang, Z.; Zhao, C.; Wang, J.; Song, C.; Zheng, Y.; Li, C. The enhancement of photocatalytic hydrogen production via Ti3+ self-doping black TiO2/g-C3N4 hollow core-shell nano-heterojunction. Appl. Catal. B Environ. 2019, 242, 92–99. [Google Scholar] [CrossRef]
- Guo, N.; Zeng, Y.; Li, H.; Xu, X.; Yu, H.; Han, X. Novel mesoporous TiO2@g-C3N4 hollow core@shell heterojunction with enhanced photocatalytic activity for water treatment and H2 production under simulated sunlight. J. Hazard. Mater. 2018, 353, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Liang, X.; Zhou, K.; Li, S.; Lu, H.; Zhang, M.; Wang, H.; Xu, Z.; Zhang, Y. Biomimetic Mechanically Enhanced Carbon Nanotube Fibers by Silk Fibroin Infiltration. Small 2021, 17, 2100066. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.A.; Mohamed, A.A. Recent progress in semiconductor/graphene photocatalysts: Synthesis, photocatalytic applications, and challenges. RSC Adv. 2023, 13, 421–439. [Google Scholar] [CrossRef]
- Chua, C.K.; Pumera, M. Chemical reduction of graphene oxide: A synthetic chemistry viewpoint. Chem. Soc. Rev. 2014, 43, 291–312. [Google Scholar] [CrossRef]
- Ghosh, R.; Midya, A.; Santra, S.; Ray, S.K.; Guha, P.K. Chemically Reduced Graphene Oxide for Ammonia Detection at Room Temperature. ACS Appl. Mater. Interfaces 2013, 5, 7599–7603. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, Y.; Zhou, K.; Zhu, H.; Gu, D.; Ge, W.; Gan, Y.; Hao, J. Constructing BiOBr/g-C3N4/Bi2O2CO3 Z-Scheme Photocatalyst with Enhanced Photocatalytic Activity; Research Square Platform LLC: Durham, NC, USA, 2021. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, J.; Xiong, X.; Lin, J.; Yang, S.; Xi, J.; Kong, Z. A novel Z-type multidimensional FeSe2/CuSe heterojunction photocatalyst with high photocatalytic and photoelectrochemical performance. Int. J. Hydrog. Energy 2022, 47, 28879–28893. [Google Scholar] [CrossRef]
- Abadikhah, H.; Naderi Kalali, E.; Khodi, S.; Xu, X.; Agathopoulos, S. Multifunctional Thin-Film Nanofiltration Membrane Incorporated with Reduced Graphene Oxide@TiO2@Ag Nanocomposites for High Desalination Performance, Dye Retention, and Antibacterial Properties. ACS Appl. Mater. Interfaces 2019, 11, 23535–23545. [Google Scholar] [CrossRef]
- Zhang, Q.; Han, F.; Yan, Y.; Dai, Q.; Proctor, G.; Cheah, P.; Avijit, P.; Chandra, R.P.; Kang, N.; Hu, M.; et al. Preparation and properties of visible light responsive RGO/In2TiO5 nanobelts for photocatalytic degradation of organic pollutants. Appl. Surf. Sci. 2019, 485, 547–553. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Z.; Wang, W.; Wang, Y.; Hu, X.; Liu, J.; Gong, X.; Miao, W.; Ding, L.; Li, X.; et al. Synthesis, modification and application of titanium dioxide nanoparticles: A review. Nanoscale 2022, 14, 6709–6734. [Google Scholar] [CrossRef]
- Meng, A.; Cheng, B.; Tan, H.; Fan, J.; Su, C.; Yu, J. TiO2/polydopamine S-scheme heterojunction photocatalyst with enhanced CO2-reduction selectivity. Appl. Catal. B Environ. 2021, 289, 120039. [Google Scholar] [CrossRef]
- Song, J.; Sun, G.; Yu, J.; Si, Y.; Ding, B. Construction of ternary Ag@ZnO/TiO2 fibrous membranes with hierarchical nanostructures and mechanical flexibility for water purification. Ceram. Int. 2020, 46, 468–475. [Google Scholar] [CrossRef]
- Xu, C.; Kou, X.; Cao, B.; Fang, H.-T. Hierarchical graphene@TiO2 sponges for sodium-ion storage with high areal capacity and robust stability. Electrochim. Acta 2020, 355, 136782. [Google Scholar] [CrossRef]
- Wang, P.-Q.; Bai, Y.; Luo, P.-Y.; Liu, J.-Y. Graphene–WO3 nanobelt composite: Elevated conduction band toward photocatalytic reduction of CO2 into hydrocarbon fuels. Catal. Commun. 2013, 38, 82–85. [Google Scholar] [CrossRef]
- Zhu, Z.; Liu, Z.; Zhong, L.; Song, C.; Shi, W.; Cui, F.; Wang, W. Breathable and asymmetrically superwettable Janus membrane with robust oil-fouling resistance for durable membrane distillation. J. Membr. Sci. 2018, 563, 602–609. [Google Scholar] [CrossRef]
- Adhikari, S.P.; Awasthi, G.P.; Kim, H.J.; Park, C.H.; Kim, C.S. Electrospinning Directly Synthesized Porous TiO2 Nanofibers Modified by Graphitic Carbon Nitride Sheets for Enhanced Photocatalytic Degradation Activity under Solar Light Irradiation. Langmuir 2016, 32, 6163–6175. [Google Scholar] [CrossRef]
- Si, Y.; Yu, J.; Tang, X.; Ge, J.; Ding, B. Ultralight nanofibre-assembled cellular aerogels with superelasticity and multifunctionality. Nat. Commun. 2014, 5, 5802. [Google Scholar] [CrossRef]
- Si, Y.; Wang, X.; Dou, L.; Yu, J.; Ding, B. Ultralight and fire-resistant ceramic nanofibrous aerogels with temperature-invariant superelasticity. Sci. Adv. 2018, 4, eaas8925. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Zhao, Y.; Chen, Y.; Yu, J.; Wang, J. The influence of oxygen functional groups on gas-sensing properties of reduced graphene oxide (rGO) at room temperature. RSC Adv. 2016, 6, 52339–52346. [Google Scholar] [CrossRef]
- Gu, X.Y.; Chen, E.Z.; Ma, M.Y.; Yang, Z.Y.; Bai, J.L.; Chen, L.L.; Sun, G.Z.; Zhang, Z.X.; Pan, X.J.; Zhou, J.Y.; et al. Effect of TiO2-rGO heterojunction on electron collection efficiency and mechanical properties of fiber-shaped dye-sensitized solar cells. J. Phys. D Appl. Phys. 2019, 52, 095502. [Google Scholar] [CrossRef]
- Rout, C.S.; Joshi, P.D.; Kashid, R.V.; Joag, D.S.; More, M.A.; Simbeck, A.J.; Washington, M.; Nayak, S.K.; Late, D.J. Superior Field Emission Properties of Layered WS2-RGO Nanocomposites. Sci. Rep. 2013, 3, 3282. [Google Scholar] [CrossRef]
- Wang, W.; Fang, J.; Shao, S.; Lai, M.; Lu, C. Compact and uniform TiO2@g-C3N4 core-shell quantum heterojunction for photocatalytic degradation of tetracycline antibiotics. Appl. Catal. B Environ. 2017, 217, 57–64. [Google Scholar] [CrossRef]
- Chen, N.; Li, X.; Wang, X.; Yu, J.; Wang, J.; Tang, Z.; Akbar, S.A. Enhanced room temperature sensing of Co3O4-intercalated reduced graphene oxide based gas sensors. Sens. Actuators B Chem. 2013, 188, 902–908. [Google Scholar] [CrossRef]
- Wu, Z.X.; Zhang, Y.W.; Jhon, M.H.; Srolovitz, D.J. Anatomy of nanomaterial deformation: Grain boundary sliding, plasticity and cavitation in nanocrystalline Ni. Acta Mater. 2013, 61, 5807–5820. [Google Scholar] [CrossRef]
- Meyers, M.A.; Mishra, A.; Benson, D.J. Mechanical properties of nanocrystalline materials. Prog. Mater. Sci. 2006, 51, 427–556. [Google Scholar] [CrossRef]
- Huang, S.; Wu, H.; Zhou, M.; Zhao, C.; Yu, Z.; Ruan, Z.; Pan, W. A flexible and transparent ceramic nanobelt network for soft electronics. NPG Asia Mater. 2014, 6, e86. [Google Scholar] [CrossRef]
- Hara, Y.; Nicol, M. Raman spectra and the structure of rutile at high pressures. Phys. Status Solidi (B) 1979, 94, 317–322. [Google Scholar] [CrossRef]
- Sharma, N.; Sharma, V.; Sharma, S.K.; Sachdev, K. Gas sensing behaviour of green synthesized reduced graphene oxide (rGO) for H2 and NO. Mater. Lett. 2019, 236, 444–447. [Google Scholar] [CrossRef]
- Pfaffeneder-Kmen, M.; Casas, I.F.; Naghilou, A.; Trettenhahn, G.; Kautek, W. A Multivariate Curve Resolution evaluation of an in-situ ATR-FTIR spectroscopy investigation of the electrochemical reduction of graphene oxide. Electrochim. Acta 2017, 255, 160–167. [Google Scholar] [CrossRef]
- Gui, Y.; Zhao, J.; Wang, W.; Tian, J.; Zhao, M. Synthesis of hemispherical WO3/graphene nanocomposite by a microwave-assisted hydrothermal method and the gas-sensing properties to triethylamine. Mater. Lett. 2015, 155, 4–7. [Google Scholar] [CrossRef]
- Song, J.; Wu, X.; Zhang, M.; Liu, C.; Yu, J.; Sun, G.; Si, Y.; Ding, B. Highly flexible, core-shell heterostructured, and visible-light-driven titania-based nanofibrous membranes for antibiotic removal and E. coil inactivation. Chem. Eng. J. 2020, 379, 122269. [Google Scholar] [CrossRef]
- Nasr, M.; Balme, S.; Eid, C.; Habchi, R.; Miele, P.; Bechelany, M. Enhanced Visible-Light Photocatalytic Performance of Electrospun rGO/TiO2 Composite Nanofibers. J. Phys. Chem. C 2016, 121, 261–269. [Google Scholar] [CrossRef]
- Zhang, Y.; Park, S.-J. Stabilization of dispersed CuPd bimetallic alloy nanoparticles on ZIF-8 for photoreduction of Cr(VI) in aqueous solution. Chem. Eng. J. 2019, 369, 353–362. [Google Scholar] [CrossRef]
- Boonprakob, N.; Wetchakun, N.; Phanichphant, S.; Waxler, D.; Sherrell, P.; Nattestad, A.; Chen, J.; Inceesungvorn, B. Enhanced visible-light photocatalytic activity of g-C3N4/TiO2 films. J. Colloid Interface Sci. 2014, 417, 402–409. [Google Scholar] [CrossRef]
- Phan, D.-T.; Chung, G.-S. P–n junction characteristics of graphene oxide and reduced graphene oxide on n-type Si(111). J. Phys. Chem. Solids 2013, 74, 1509–1514. [Google Scholar] [CrossRef]
- Krishna Kumar, A.S.; Tseng, W.-B.; Arputharaj, E.; Huang, P.-J.; Tseng, W.-L.; Bajda, T. Covalent Organic Framework Nanosheets as an Enhancer for Light-Responsive Oxidase-like Nanozymes: Multifunctional Applications in Colorimetric Sensing, Antibiotic Degradation, and Antibacterial Agents. ACS Sustain. Chem. Eng. 2023, 11, 6956–6969. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, S.; Chong, Z.; Zuo, X.; Qi, W. Construction of Binary RGO/TiO2 Fibrous Membranes with Enhanced Mechanical Properties for E. coli Inactivation. Nanomaterials 2023, 13, 2954. https://doi.org/10.3390/nano13222954
Zhao S, Chong Z, Zuo X, Qi W. Construction of Binary RGO/TiO2 Fibrous Membranes with Enhanced Mechanical Properties for E. coli Inactivation. Nanomaterials. 2023; 13(22):2954. https://doi.org/10.3390/nano13222954
Chicago/Turabian StyleZhao, Suyi, Zhenzeng Chong, Xiaogang Zuo, and Wenjun Qi. 2023. "Construction of Binary RGO/TiO2 Fibrous Membranes with Enhanced Mechanical Properties for E. coli Inactivation" Nanomaterials 13, no. 22: 2954. https://doi.org/10.3390/nano13222954



