Hierarchically Porous Carbon Networks Derived from Chitosan for High-Performance Electrochemical Double-Layer Capacitors
Abstract
:1. Introduction
2. Materials and Methods
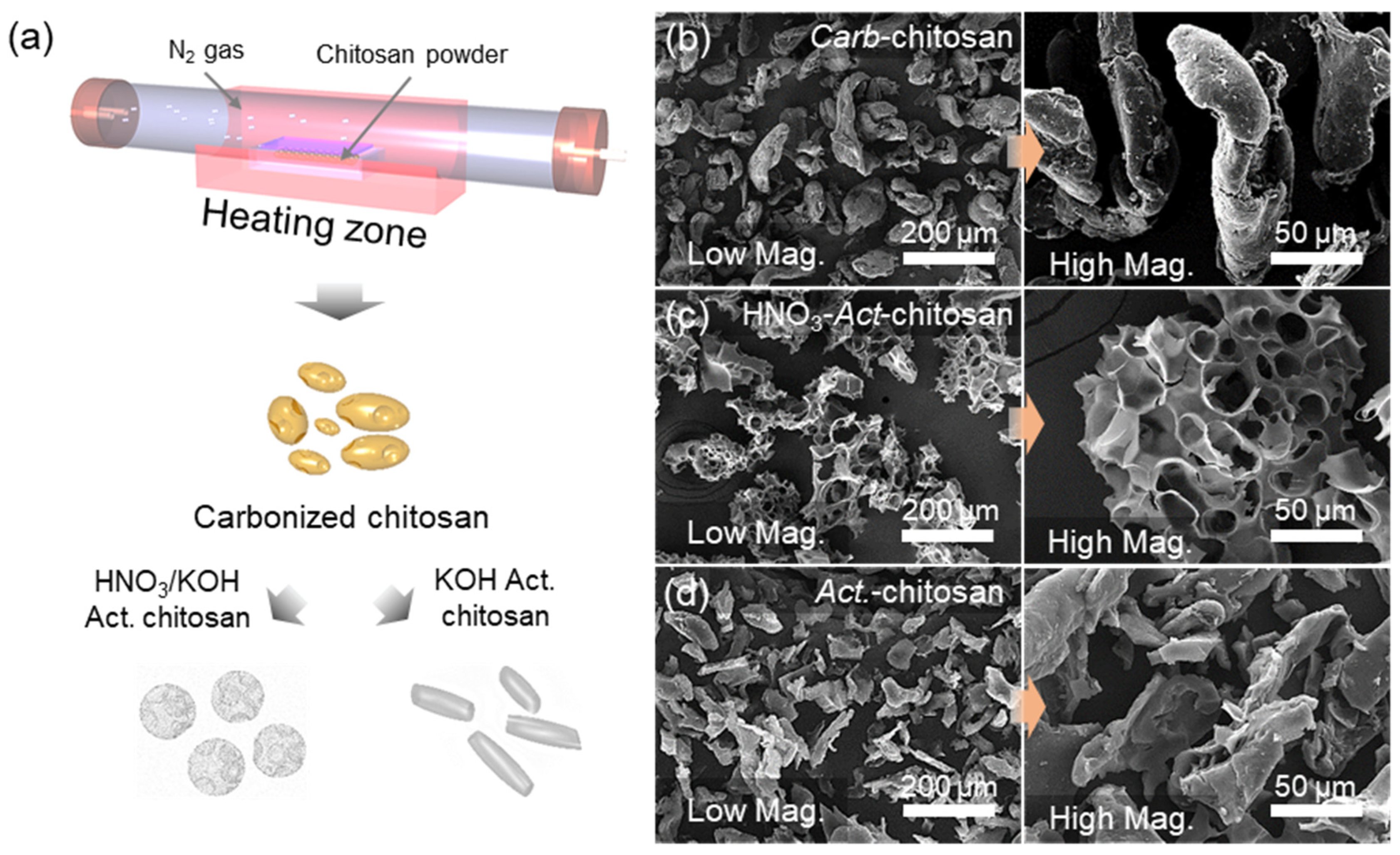
2.1. Fabrication of Porous Activated Carbons from Chitosan Precursor
2.2. Characterizations
2.3. Electrochemical Characterization
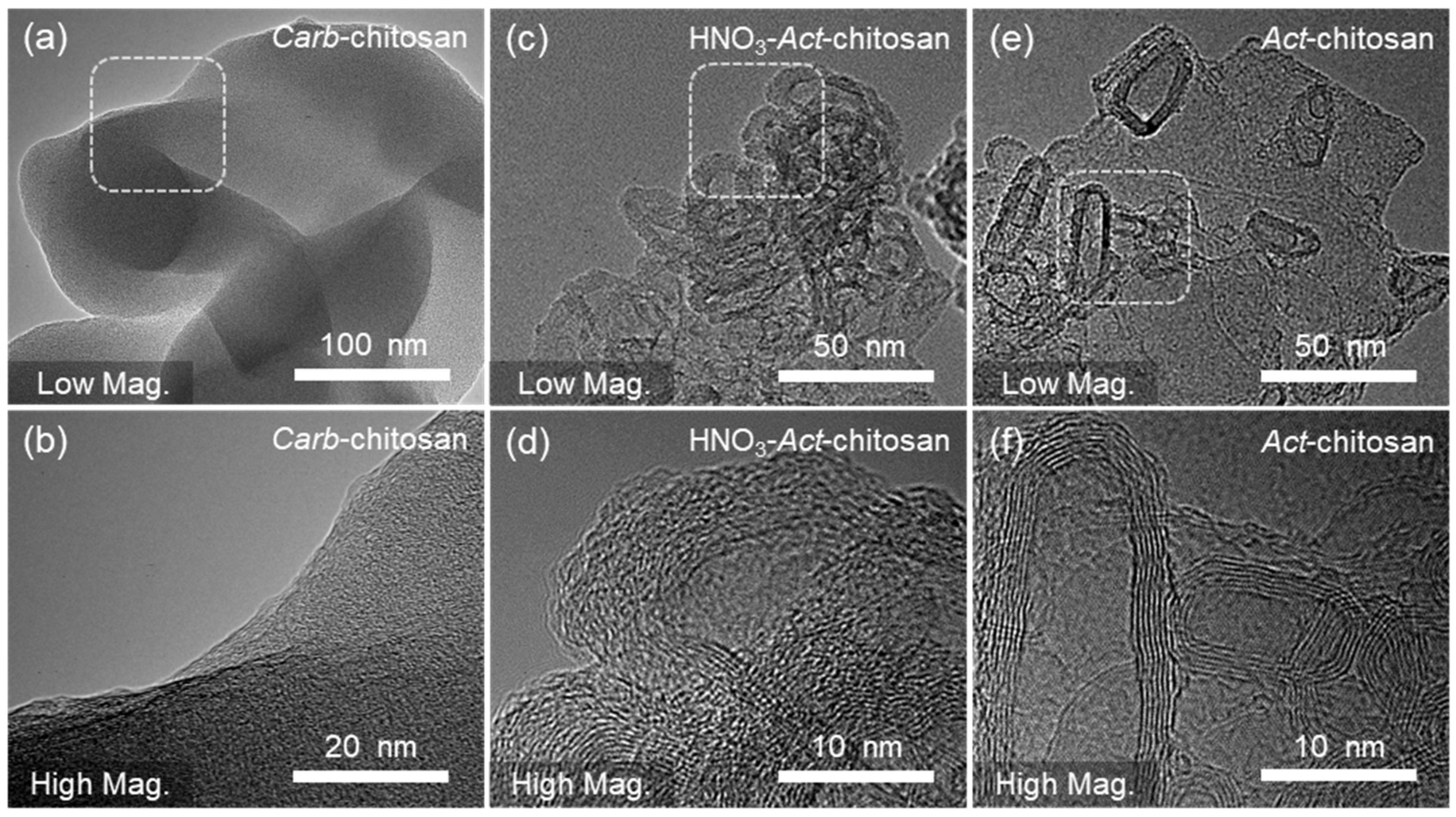
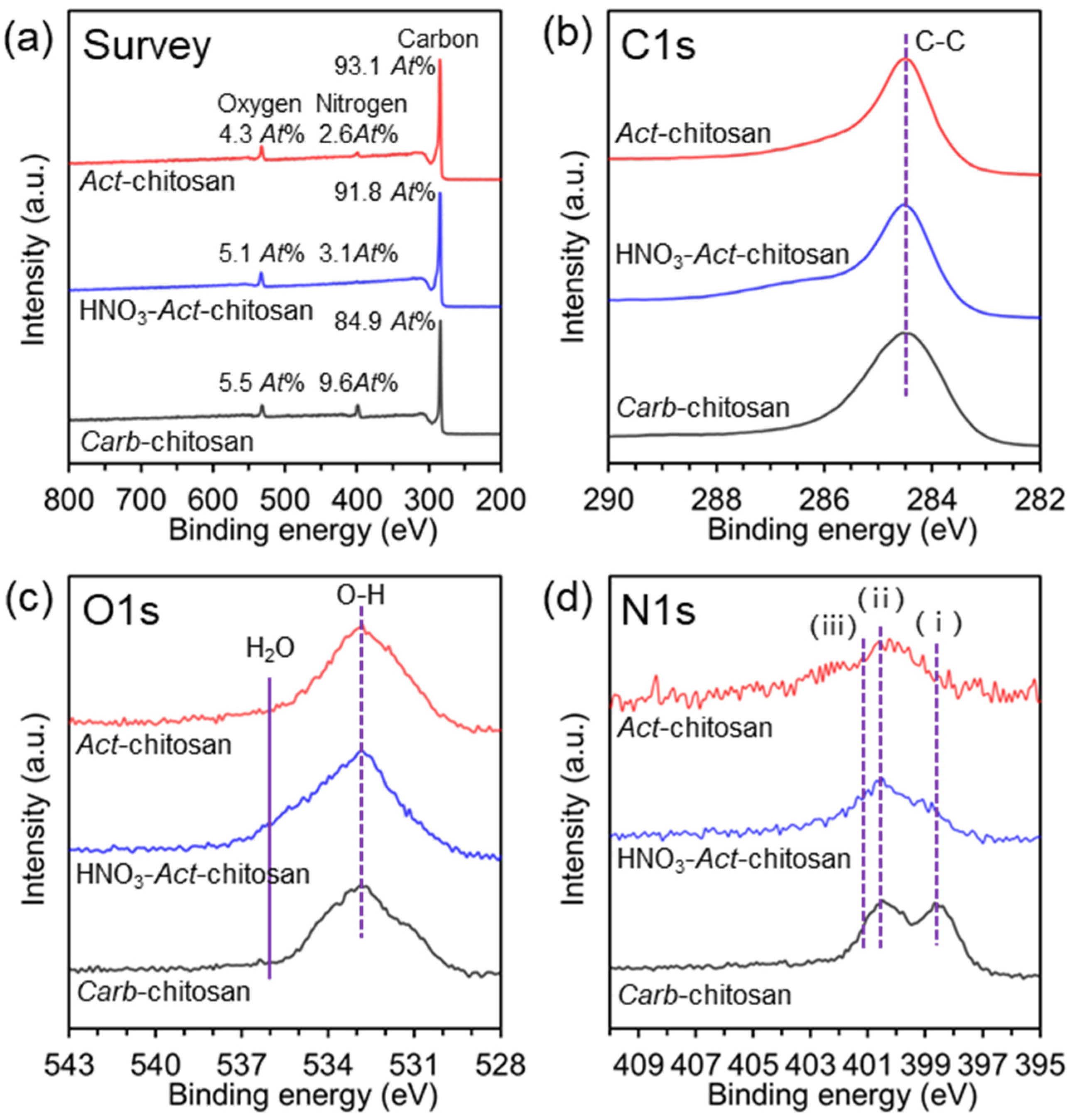
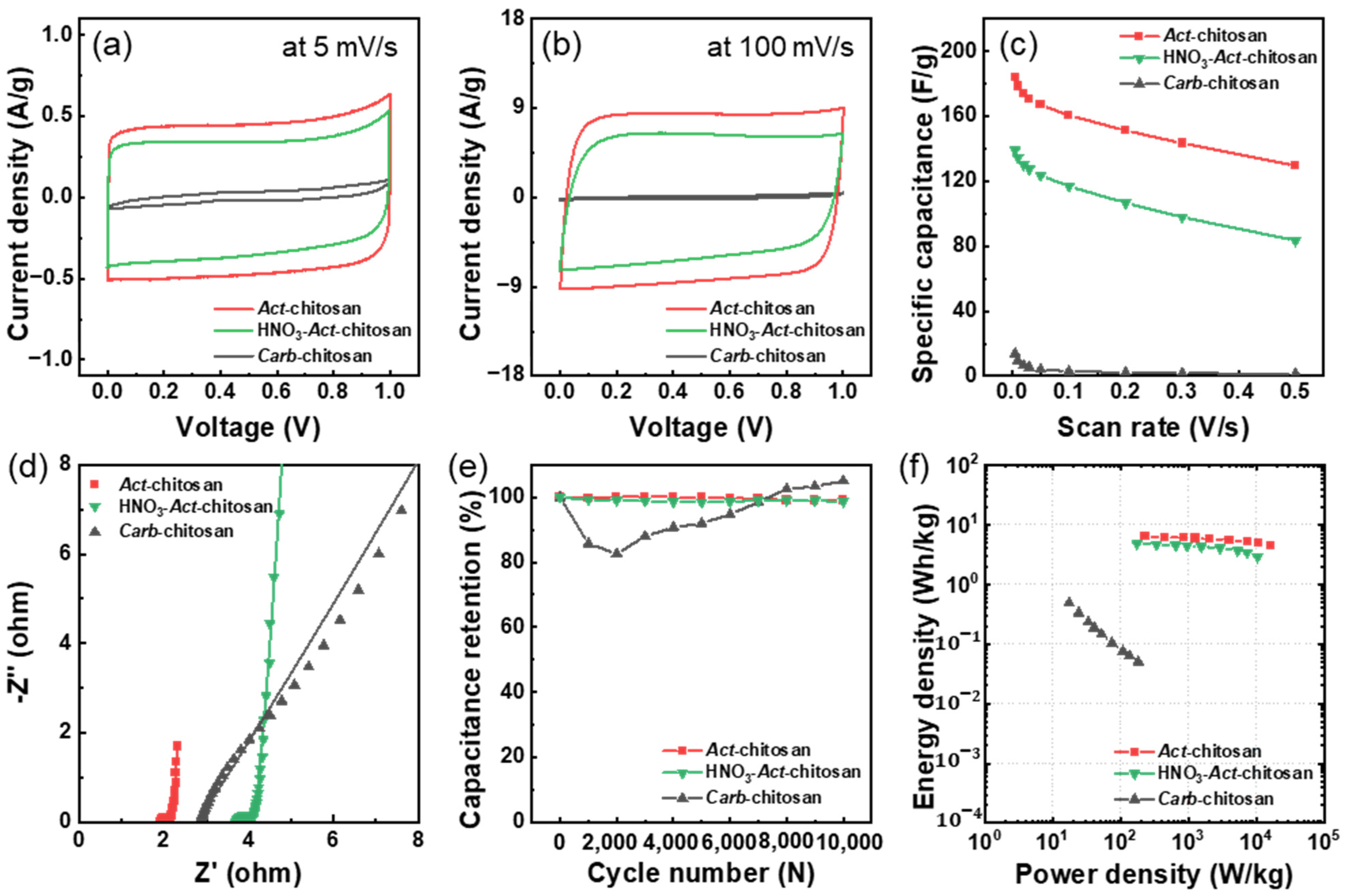
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Sevilla, M.; Mokaya, R. Energy storage applications of activated carbons: Supercapacitors and hydrogen storage. Energy Environ. Sci. 2014, 7, 1250–1280. [Google Scholar] [CrossRef]
- Dong, S.; He, X.; Zhang, H.; Xie, X.; Yu, M.; Yu, C.; Xiao, N.; Qiu, J. Surface modification of biomass-derived hard carbon by grafting porous carbon nanosheets for high-performance supercapacitors. J. Mater. Chem. A 2018, 6, 15954–15960. [Google Scholar] [CrossRef]
- Wang, Q.; Yan, J.; Fan, Z. Carbon materials for high volumetric performance supercapacitors: Design, progress, challenges and opportunities. Energy Environ. Sci. 2016, 9, 729–762. [Google Scholar] [CrossRef]
- Stein, A.; Wang, Z.; Fierke, M.A. Functionalization of Porous Carbon Materials with Designed Pore Architecture. Adv. Mater. 2009, 21, 265–293. [Google Scholar] [CrossRef]
- Shi, Y.; Zhou, X.; Yu, G. Material and Structural Design of Novel Binder Systems for High-Energy, High-Power Lithium-Ion Batteries. Acc. Chem. Res. 2017, 50, 2642–2652. [Google Scholar] [CrossRef]
- Jiang, J.; Zhang, L.; Wang, X.; Holm, N.; Rajagopalan, K.; Chen, F.; Ma, S. Highly ordered macroporous woody biochar with ultra-high carbon content as supercapacitor electrodes. Electrochim. Acta 2013, 113, 481–489. [Google Scholar] [CrossRef]
- Wu, F.-C.; Tseng, R.-L.; Hu, C.-C.; Wang, C.-C. Effects of pore structure and electrolyte on the capacitive characteristics of steam- and KOH-activated carbons for supercapacitors. J. Power Sources 2005, 144, 302–309. [Google Scholar] [CrossRef]
- Wang, Z.; Tammela, P.; Strømme, M.; Nyholm, L. Cellulose-based Supercapacitors: Material and Performance Considerations. Adv. Energy Mater. 2017, 7, 1700130. [Google Scholar] [CrossRef]
- Jin, Z.; Yan, X.; Yu, Y.; Zhao, G. Sustainable activated carbon fibers from liquefied wood with controllable porosity for high-performance supercapacitors. J. Mater. Chem. A 2014, 2, 11706–11715. [Google Scholar] [CrossRef]
- Huang, Y.; Ma, E.; Zhao, G. Preparation of liquefied wood-based activated carbon fibers by different activation methods for methylene blue adsorption. RSC Adv. 2015, 5, 70287–70296. [Google Scholar] [CrossRef]
- Tsubouchi, N.; Nishio, M.; Shinohara, Y.; Bud, J.; Mochizuki, Y. Production of activated carbon from peat by with natural soda ash and effect of nitrogen addition on the development of surface area. Fuel Process. Technol. 2018, 176, 76–84. [Google Scholar] [CrossRef]
- Yang, K.; Peng, J.; Srinivasakannan, C.; Zhang, L.; Xia, H.; Duan, X. Preparation of high surface area activated carbon from coconut shells using microwave heating. Bioresour. Technol. 2010, 101, 6163–6169. [Google Scholar] [CrossRef]
- Rodríguez Correa, C.; Stollovsky, M.; Hehr, T.; Rauscher, Y.; Rolli, B.; Kruse, A. Influence of the Carbonization Process on Activated Carbon Properties from Lignin and Lignin-Rich Biomasses. ACS Sustain. Chem. Eng. 2017, 5, 8222–8233. [Google Scholar] [CrossRef]
- Fan, X.; Zhang, L.; Zhang, G.; Shu, Z.; Shi, J. Chitosan derived nitrogen-doped microporous carbons for high performance CO2 capture. Carbon 2013, 61, 423–430. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Y.; Li, K.; Wang, Z.; Tian, P.; Liu, D.; Yang, T.; Wang, J. Activated carbon derived from chitosan as air cathode catalyst for high performance in microbial fuel cells. J. Power Sources 2018, 378, 1–9. [Google Scholar] [CrossRef]
- Hao, P.; Zhao, Z.; Leng, Y.; Tian, J.; Sang, Y.; Boughton, R.I.; Wong, C.P.; Liu, H.; Yang, B. Graphene-based nitrogen self-doped hierarchical porous carbon aerogels derived from chitosan for high performance supercapacitors. Nano Energy 2015, 15, 9–23. [Google Scholar] [CrossRef]
- Huang, J.; Liang, Y.; Hu, H.; Liu, S.; Cai, Y.; Dong, H.; Zheng, M.; Xiao, Y.; Liu, Y. Ultrahigh-surface-area hierarchical porous carbon from chitosan: Acetic acid mediated efficient synthesis and its application in superior supercapacitors. J. Mater. Chem. A 2017, 5, 24775–24781. [Google Scholar] [CrossRef]
- Cheng, J.; Xu, Q.; Wang, X.; Li, Z.; Wu, F.; Shao, J.; Xie, H. Ultrahigh-surface-area nitrogen-doped hierarchically porous carbon materials derived from chitosan and betaine hydrochloride sustainable precursors for high-performance supercapacitors. Sustain. Energy Fuels 2019, 3, 1215–1224. [Google Scholar] [CrossRef]
- Kucinska, A.; Cyganiuk, A.; Lukaszewicz, J.P. A microporous and high surface area active carbon obtained by the heat-treatment of chitosan. Carbon 2012, 50, 3098–3101. [Google Scholar] [CrossRef]
- Chang, B.; Guo, Y.; Li, Y.; Yin, H.; Zhang, S.; Yang, B.; Dong, X. Graphitized hierarchical porous carbon nanospheres: Simultaneous activation/graphitization and superior supercapacitance performance. J. Mater. Chem. A 2015, 3, 9565–9577. [Google Scholar] [CrossRef]
- Adhamash, E.; Pathak, R.; Chen, K.; Rahman, M.T.; El-Magrous, A.; Gu, Z.; Lu, S.; Qiao, Q.; Zhou, Y. High-energy plasma activation of renewable carbon for enhanced capacitive performance of supercapacitor electrode. Electrochim. Acta 2020, 362, 137148. [Google Scholar] [CrossRef]
- Ruiz, V.; Blanco, C.; Raymundo-Piñero, E.; Khomenko, V.; Béguin, F.; Santamaría, R. Effects of thermal treatment of activated carbon on the electrochemical behaviour in supercapacitors. Electrochim. Acta 2007, 52, 4969–4973. [Google Scholar] [CrossRef]
- Ling, Z.; Wang, Z.; Zhang, M.; Yu, C.; Wang, G.; Dong, Y.; Liu, S.; Wang, Y.; Qiu, J. Sustainable Synthesis and Assembly of Biomass-Derived B/N Co-Doped Carbon Nanosheets with Ultrahigh Aspect Ratio for High-Performance Supercapacitors. Adv. Funct. Mater. 2015, 26, 111–119. [Google Scholar] [CrossRef]
- Li, M.; Han, X.; Chang, X.; Yin, W.; Ma, J. Nitrogen/Sulfur-Codoped Carbon Materials from Chitosan for Supercapacitors. J. Electron. Mater. 2016, 45, 4331–4337. [Google Scholar] [CrossRef]
- Ling, Z.; Wang, G.; Zhang, M.; Fan, X.; Yu, C.; Yang, J.; Xiao, N.; Qiu, J. Boric acid-mediated B,N-codoped chitosan-derived porous carbons with a high surface area and greatly improved supercapacitor performance. Nanoscale 2015, 7, 5120–5125. [Google Scholar] [CrossRef]
- Hwang, K.-J.; Balathanigaimani, M.S.; Choi, Y.H.; Yoon, S.-D.; Shim, W.-G. Influence of ultra-micropore volume of activated carbons prepared from noble mung bean on the adsorption properties of CO2, CH4, and N2. New J. Chem. 2022, 46, 17577–17584. [Google Scholar] [CrossRef]
- Lee, S.G.; Park, K.H.; Shim, W.G.; Balathanigaimani, M.S.; Moon, H. Performance of electrochemical double layer capacitors using highly porous activated carbons prepared from beer lees. J. Ind. Eng. Chem. 2011, 17, 450–454. [Google Scholar] [CrossRef]
- Hwang, H.; Yang, S.; Yuk, S.; Lee, K.-S.; Byun, S.; Lee, D. Ti3C2Tx MXene as a growth template for amorphous RuOx in carbon nanofiber-based flexible electrodes for enhanced pseudocapacitive energy storage. NPG Asia Mater. 2023, 15, 29. [Google Scholar] [CrossRef]
- Hall, P.J.; Mirzaeian, M.; Fletcher, S.I.; Sillars, F.B.; Rennie, A.J.R.; Shitta-Bey, G.O.; Wilson, G.; Cruden, A.; Carter, R. Energy storage in electrochemical capacitors: Designing functional materials to improve performance. Energy Environ. Sci. 2010, 3, 1238–1251. [Google Scholar] [CrossRef]
- Byun, S.; Shin, B. Densely packed hybrid films comprising SnO2 and reduced graphite oxide for high-density electrochemical capacitors. J. Mater. Chem. A 2016, 4, 16175–16183. [Google Scholar] [CrossRef]
- Byun, S.; Kim, J.H.; Song, S.H.; Lee, M.; Park, J.-J.; Lee, G.; Hong, S.H.; Lee, D. Ordered, Scalable Heterostructure Comprising Boron Nitride and Graphene for High-Performance Flexible Supercapacitors. Chem. Mater. 2016, 28, 7750–7756. [Google Scholar] [CrossRef]
- Stoller, M.D.; Ruoff, R.S. Best practice methods for determining an electrode material’s performance for ultracapacitors. Energy Environ. Sci. 2010, 3, 1294–1301. [Google Scholar] [CrossRef]
- Yu, H.; Son, Y.R.; Yoo, H.; Cha, H.G.; Lee, H.; Kim, H.S. Chitosan-Derived Porous Activated Carbon for the Removal of the Chemical Warfare Agent Simulant Dimethyl Methylphosphonate. Nanomaterials 2019, 9, 1703. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Lee, D.; Kim, J.; Song, J.; Lee, Y.M.; Kim, H.T.; Park, J.K. Defect-free, size-tunable graphene for high-performance lithium ion battery. Nano Lett. 2014, 14, 4306–4313. [Google Scholar] [CrossRef]
- Becerril, H.A.; Mao, J.; Liu, Z.; Stoltenberg, R.M.; Bao, Z.; Chen, Y. Evaluation of Solution-Processed Reduced Graphene Oxide Films as Transparent Conductors. ACS Nano 2008, 2, 463–470. [Google Scholar] [CrossRef]
- Green, B.; Kovalev, S.; Asgekar, V.; Geloni, G.; Lehnert, U.; Golz, T.; Kuntzsch, M.; Bauer, C.; Hauser, J.; Voigtlaender, J.; et al. High-Field High-Repetition-Rate Sources for the Coherent THz Control of Matter. Sci. Rep. 2016, 6, 22256. [Google Scholar] [CrossRef]
- Shao, H.; Wu, Y.C.; Lin, Z.; Taberna, P.L.; Simon, P. Nanoporous carbon for electrochemical capacitive energy storage. Chem. Soc. Rev. 2020, 49, 3005–3039. [Google Scholar] [CrossRef]
- Zhu, L.; Shen, F.; Smith, R.L.; Qi, X. High-Performance Supercapacitor Electrode Materials from Chitosan via Hydrothermal Carbonization and Potassium Hydroxide Activation. Energy Technol. 2016, 5, 452–460. [Google Scholar] [CrossRef]
- Kim, J.; Kim, S.; Yuk, S.; Hwang, H.; Song, S.H.; Byun, S.; Lee, D. Densely Packed Fiber Electrodes Composed of Liquid Crystalline MXenes for High-Areal-Density Supercapacitors. Energy Technol. 2023, 11, 2201349. [Google Scholar] [CrossRef]
- Yang, N.; Yu, S.; Zhang, W.; Cheng, H.M.; Simon, P.; Jiang, X. Electrochemical Capacitors with Confined Redox Electrolytes and Porous Electrodes. Adv. Mater. 2022, 34, e2202380. [Google Scholar] [CrossRef]
- Jäckel, N.; Simon, P.; Gogotsi, Y.; Presser, V. Increase in Capacitance by Subnanometer Pores in Carbon. ACS Energy Lett. 2016, 1, 1262–1265. [Google Scholar] [CrossRef]
- Largeot, C.; Portet, C.; Chmiola, J.; Taberna, P.-L.; Gogotsi, Y.; Simon, P. Relation between the Ion Size and Pore Size for an Electric Double-Layer Capacitor. J. Am. Chem. Soc. 2008, 130, 2730–2731. [Google Scholar] [CrossRef]
- Augustyn, V.; Come, J.; Lowe, M.A.; Kim, J.W.; Taberna, P.-L.; Tolbert, S.H.; Abruña, H.D.; Simon, P.; Dunn, B. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 2013, 12, 518–522. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liu, J.; Yu, W.; Gao, J.; Cui, X.; Jiang, L. Steering Pt surface via N-doped carbon layer to highly active CO-tolerant hydrogen oxidation reaction catalyst in alkaline media. Appl. Surf. Sci. 2023, 614, 156131. [Google Scholar] [CrossRef]
- Hwang, H.; Byun, S.; Yuk, S.; Kim, S.; Song, S.H.; Lee, D. High-rate electrospun Ti3C2Tx MXene/Carbon Nanofiber Electrodes for Flexible Supercapacitors. Appl. Surf. Sci. 2021, 556, 149710. [Google Scholar] [CrossRef]
- Luo, M.; Wang, X.; Meng, T.; Yang, P.; Zhu, Z.; Min, H.; Chen, M.; Chen, W.; Zhou, X. Rapid One-Step Preparation of Hierarchical Porous Carbon from Chitosan-Based Hydrogel for High-Rate Supercapacitors: The effect of gelling agent concentration. Int. J. Biol. Macromol. 2020, 146, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Platek, A.; Nita, C.; Ghimbeu, C.M.; Frąckowiak, E.; Fic, K. Electrochemical Capacitors Operating in Aqueous Electrolyte with Volumetric Characteristics Improved by Sustainable Templating of Electrode Materials. Electrochim. Acta 2020, 338, 135788. [Google Scholar] [CrossRef]
- Li, X.; Wang, H.; Robinson, J.T.; Sanchez, H.; Diankov, G.; Dai, H. Simultaneous Nitrogen Doping and Reduction of Graphene Oxide. J. Am. Chem. Soc. 2009, 131, 15939–15944. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Dong, L.; Lai, G.; Wei, M.; Jiang, X.; Bai, L. Nitrogen-Doped Hierarchically Porous Carbons Derived from Polybenzoxazine for Enhanced Supercapacitor Performance. Nanomaterials 2019, 9, 131. [Google Scholar] [CrossRef]
- Park, K.H.; Park, T.; Yuk, S.; Ko, B.; Ahn, J.; Hong, S.J.; Lee, D.; Song, S.H. Engineering Nanopores in Graphene-Based Nanoplatelets Derived from Cellulose-Based Biomass for High-Performance Capacitors. ACS Mater. Lett. 2023, 5, 3263–3272. [Google Scholar] [CrossRef]
- Chmiola, J.; Largeot, C.; Taberna, P.-L.; Simon, P.; Gogotsi, Y. Monolithic Carbide-Derived Carbon Films for Micro-Supercapacitors. Science 2010, 328, 480–483. [Google Scholar] [CrossRef] [PubMed]
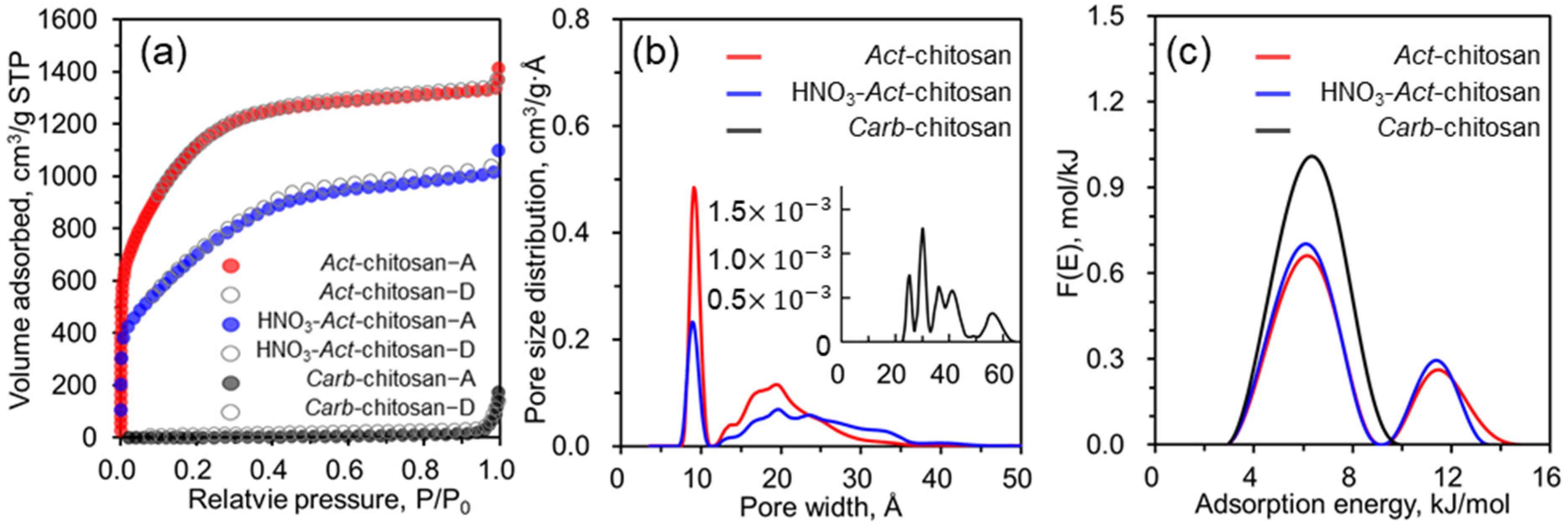
| Sample | Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Width (nm) | ||
|---|---|---|---|---|---|
| SBET | S2D-NLDFT | V2D-NLDFT, <2 nm | V2D-NLDFT, >2 nm | W2D-NLDFT | |
| Carb-chitosan | 13 | 8 | 0 | 0.013 | 17 |
| HNO3-Act. chitosan | 2584 | 1765 | 0.701 | 0.718 | 0.89/1.98 |
| Act-chitosan | 4128 | 2731 | 1.338 | 0.539 | 0.91/1.93 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, K.H.; Byun, S.; Ko, B.; Hong, W.-G.; Kim, J.; Lee, D.; Shim, W.G.; Song, S.H. Hierarchically Porous Carbon Networks Derived from Chitosan for High-Performance Electrochemical Double-Layer Capacitors. Nanomaterials 2023, 13, 2961. https://doi.org/10.3390/nano13222961
Park KH, Byun S, Ko B, Hong W-G, Kim J, Lee D, Shim WG, Song SH. Hierarchically Porous Carbon Networks Derived from Chitosan for High-Performance Electrochemical Double-Layer Capacitors. Nanomaterials. 2023; 13(22):2961. https://doi.org/10.3390/nano13222961
Chicago/Turabian StylePark, Kwang Hyun, Segi Byun, Boemjin Ko, Woong-Gil Hong, Jungmo Kim, Dongju Lee, Wang Geun Shim, and Sung Ho Song. 2023. "Hierarchically Porous Carbon Networks Derived from Chitosan for High-Performance Electrochemical Double-Layer Capacitors" Nanomaterials 13, no. 22: 2961. https://doi.org/10.3390/nano13222961
APA StylePark, K. H., Byun, S., Ko, B., Hong, W.-G., Kim, J., Lee, D., Shim, W. G., & Song, S. H. (2023). Hierarchically Porous Carbon Networks Derived from Chitosan for High-Performance Electrochemical Double-Layer Capacitors. Nanomaterials, 13(22), 2961. https://doi.org/10.3390/nano13222961






