MgO Nanoparticles Obtained from an Innovative and Sustainable Route and Their Applications in Cancer Therapy
Abstract
:1. Introduction
2. Materials
2.1. Synthesis of MgO NPs Obtained Starting from Mg(OH)2 Precursor
2.2. Characterization of the Produced MgO NPs
2.3. In Vitro Tests to Evaluate the MgO NPs’ Efficacy as Toxicological Agents against Cancer Cells
3. Results
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vinardell, M.P.; Mitjans, M. Antitumor Activities of Metal Oxide Nanoparticles. Nanomaterials 2015, 5, 1004–1021. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, K.; Moon, J.Y.; Hyun, H.B.; Cho, S.K.; Kim, S.-J. Mechanistic investigation on the toxicity of MgO nanoparticles toward cancer cells. J. Mater. Chem. 2012, 22, 24610–24617. [Google Scholar] [CrossRef]
- Fahmy, H.M.; El-Hakim, M.H.; Nady, D.S.; Elkaramany, Y.; Mohamed, F.A.; Yasien, A.M.; Moustafa, M.A.; Elmsery, B.E.; Yousef, H.A. Review on MgO nanoparticles multifunctional role in the biomedical field: Properties and applications. Nanomed. J. 2022, 9, 1–14. [Google Scholar]
- Caputo, F.; De Nicola, M.; Ghibelli, L. Pharmacological potential of bioactive engineered nanomaterials. Biochem. Pharmacol. 2014, 92, 112–130. [Google Scholar] [CrossRef]
- Kumaran, R.S.; Choi, Y.-K.; Singh, V.; Song, H.-J.; Song, K.-G.; Kim, K.J.; Kim, H.J. In Vitro Cytotoxic Evaluation of MgO Nanoparticles and Their Effect on the Expression of ROS Genes. Int. J. Mol. Sci. 2015, 16, 7551–7564. [Google Scholar] [CrossRef] [PubMed]
- Manke, A.; Wang, L.; Rojanasakul, Y. Mechanisms of Nanoparticle-Induced Oxidative Stress and Toxicity. BioMed Res. Int. 2013, 2013, 942916. [Google Scholar] [CrossRef] [PubMed]
- Park, E.-J.; Choi, J.; Park, Y.-K.; Park, K. Oxidative stress induced by cerium oxide nanoparticles in cultured BEAS-2B cells. Toxicology 2008, 245, 90–100. [Google Scholar] [CrossRef]
- Huang, Y.-W.; Wu, C.-H.; Aronstam, R.S. Toxicity of Transition Metal Oxide Nanoparticles: Recent Insights from in vitro Studies. Materials 2010, 3, 4842–4859. [Google Scholar] [CrossRef]
- Stone, V.; Shaw, J.; Brown, D.; MacNee, W.; Faux, S.; Donaldson, K. The role of oxidative stress in the prolonged inhibitory effect of ultrafine carbon black on epithelial cell function. Toxicol. Vitr. 1998, 12, 649–659. [Google Scholar] [CrossRef]
- Pugazhendhi, A.; Prabhu, R.; Muruganantham, K.; Shanmuganathan, R.; Natarajan, S. Anticancer, antimicrobial and photocatalytic activities of green synthesized magnesium oxide nanoparticles (MgONPs) using aqueous extract of Sargassum wightii. J. Photochem. Photobiol. B: Biol. 2018, 190, 86–97. [Google Scholar] [CrossRef]
- Majeed, S.; Danish, M.; Muhadi, N.F.B.B. Genotoxicity and apoptotic activity of biologically synthesized magnesium oxide nanoparticles against human lung cancer A-549 cell line. Adv. Nat. Sci. Nanosci. Nanotechnol. 2018, 9, 025011. [Google Scholar] [CrossRef]
- Ranganathan, R.; Madanmohan, S.; Kesavan, A.; Baskar, G.; Krishnamoorthy, Y.R.; Santosham, R.; Ponraju, D.; Rayala, S.K.; Venkatraman, G. Nanomedicine: Towards development of patient-friendly drug-delivery systems for oncological applications. Int. J. Nanomed. 2012, 7, 1043–1060. [Google Scholar] [CrossRef]
- Beeler, E.; Gabani, P.; Singh, O.V. Implementation of nanoparticles in therapeutic radiation oncology. J. Nanoparticle Res. 2017, 19, 179. [Google Scholar] [CrossRef]
- Ciccarese, F.; Raimondi, V.; Sharova, E.; Silic-Benussi, M.; Ciminale, V. Nanoparticles as Tools to Target Redox Homeostasis in Cancer Cells. Antioxidants 2020, 9, 211. [Google Scholar] [CrossRef]
- Jin, T.; He, Y. Antibacterial activities of magnesium oxide (MgO) nanoparticles against foodborne pathogens. J. Nanoparticle Res. 2011, 13, 6877–6885. [Google Scholar] [CrossRef]
- Tang, Z.-X.; Lv, B.-F. MgO nanoparticles as antibacterial agent: Preparation and activity. Braz. J. Chem. Eng. 2014, 31, 591–601. [Google Scholar] [CrossRef]
- Bertinetti, L.; Drouet, C.; Combes, C.; Rey, C.; Tampieri, A.; Coluccia, S.; Martra, G. Surface Characteristics of Nanocrystalline Apatites: Effect of Mg Surface Enrichment on Morphology, Surface Hydration Species, and Cationic Environments. Langmuir 2009, 25, 5647–5654. [Google Scholar] [CrossRef]
- Leung, Y.H.; Ng, A.M.C.; Xu, X.; Shen, Z.; Gethings, L.A.; Wong, M.T.; Chan, C.M.N.; Guo, M.Y.; Ng, Y.H.; Djurišić, A.B.; et al. Mechanisms of Antibacterial Activity of MgO: Non-ROS Mediated Toxicity of MgO Nanoparticles Towards Escherichia coli. Small 2014, 10, 1171–1183. [Google Scholar] [CrossRef]
- Mirhosseini, M. Evaluation of antibacterial effect of magnesium oxide nanoparticles with nisin and heat in milk. Mashhad Univ. Med. Sci. 2016, 3, 135–142. [Google Scholar]
- Di, D.-R.; He, Z.-Z.; Sun, Z.-Q.; Liu, J. A new nano-cryosurgical modality for tumor treatment using biodegradable MgO nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 1233–1241. [Google Scholar] [CrossRef]
- Karthik, K.; Dhanuskodi, S.; Kumar, S.P.; Gobinath, C.; Sivaramakrishnan, S. Microwave assisted green synthesis of MgO nanorods and their antibacterial and anti-breast cancer activities. Mater. Lett. 2017, 206, 217–220. [Google Scholar] [CrossRef]
- Camtakan, Z.; Erenturk, S.; Yusan, S. Magnesium oxide nanoparticles: Preparation, characterization, and uranium sorption properties. Environ. Prog. Sustain. Energy 2011, 31, 536–543. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, Y.J.; Jun, K.W.; Baeg, J.O.; Yim, D.J. Chemical synthesis and characterization of highly oil dispersed MgO nanoparticles. J. Ind. Eng. Chem. 2006, 12, 882–887. [Google Scholar]
- Yu, J.C.; Xu, A.; Zhang, L.; Song, R.; Wu, L. Synthesis and Characterization of Porous Magnesium Hydroxide and Oxide Nanoplates. J. Phys. Chem. B 2004, 108, 64–70. [Google Scholar] [CrossRef]
- Wang, L.; Wang, J.; Engelsen, D.D.; Wu, J.; Du, Y.; Li, H.; Jia, X. Controlled Synthesis of Magnesium Oxide Nanoparticles for Dye Adsorption. J. Nanoelectron. Optoelectron. 2017, 12, 512–517. [Google Scholar] [CrossRef]
- Chae, S.; Lee, H.; Pikhitsa, P.V.; Kim, C.; Shin, S.; Kim, D.H.; Choi, M. Synthesis of terraced and spherical MgO nanoparticles using flame metal combustion. Powder Technol. 2017, 305, 132–140. [Google Scholar] [CrossRef]
- Balamurugan, S.; Ashna, L.; Parthiban, P. Synthesis of Nanocrystalline MgO Particles by Combustion Followed by Annealing Method Using Hexamine as a Fuel. J. Nanotechnol. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Abinaya, S.; Kavitha, H.P.; Prakash, M.; Muthukrishnaraj, A. Green synthesis of magnesium oxide nanoparticles and its applications: A review. Sustain. Chem. Pharm. 2021, 19, 100368. [Google Scholar] [CrossRef]
- Khan, M.I.; Akhtar, M.N.; Ashraf, N.; Najeeb, J.; Munir, H.; Awan, T.I.; Tahir, M.B.; Kabli, M.R. Green synthesis of magnesium oxide nanoparticles using Dalbergia sissoo extract for photocatalytic activity and antibacterial efficacy. Appl. Nanosci. 2020, 10, 2351–2364. [Google Scholar] [CrossRef]
- Sugirtha, P.; Divya, R.; Yedhukrishnan, R.; Suganthi, K.; Anusha, N.; Ponnusami, V.; Rajan, K. Green Synthesis of Magnesium Oxide Nanoparticles Using Brassica oleracea and Punica granatum Peels and their Anticancer and Photocatalytic Activity. Asian J. Chem. 2015, 27, 2513–2517. [Google Scholar] [CrossRef]
- McKelvy, M.J.; Sharma, R.; Chizmeshya, A.V.G.; Carpenter, R.W.; Streib, K. Magnesium Hydroxide Dehydroxylation: In Situ Nanoscale Observations of Lamellar Nucleation and Growth. Chem. Mater. 2001, 13, 921–926. [Google Scholar] [CrossRef]
- Taglieri, G.; Daniele, V.; Mondelli, C. MgO nanoparticles synthesized starting from an innovative one-step process. J. Am. Ceram. Soc. 2017, 101, 1780–1789. [Google Scholar] [CrossRef]
- Taglieri, G.; Felice, B.; Daniele, V.; Ferrante, F. Mg(OH)2 nanoparticles produced at room temperature by an innovative, facile, and scalable synthesis route. J. Nanoparticle Res. 2015, 17, 1–13. [Google Scholar] [CrossRef]
- Volpe, R.; Taglieri, G.; Daniele, V.; Del Re, G. A Process for the Synthesis of Ca(OH)2 Nanoparticles by Means of Ionic Exchange Resins. European Patent EP2880101B1, 21 December 2016. [Google Scholar]
- Taglieri, G.; Macera, L.; Daniele, V. Procedimento per la Sintesi di Nanoparticelle di Ferridrite o di Magnetite Mediante Resine a Scambio Ionico. Italian Patent 102019000017981, 4 October 2019. [Google Scholar]
- Fiala, J. D. L. Bish, J. E. Post (eds). Modern powder diffraction. Mineralogical society of America, Washington, 1989, XI + 369 p, 167 figures, $20.00, ISBN 0-939950-24-3. Cryst. Res. Technol. 1990, 25, 1358. [Google Scholar] [CrossRef]
- Tolnai, S. A method for viable cell count. Tissue Cult. Assoc. Man. 1975, 1, 37–38. [Google Scholar] [CrossRef]
- Clark, N.A.; Hafner, M.; Kouril, M.; Williams, E.H.; Muhlich, J.L.; Pilarczyk, M.; Niepel, M.; Sorger, P.K.; Medvedovic, M. GRcalculator: An online tool for calculating and mining dose–response data. BMC Cancer 2017, 17, 698. [Google Scholar] [CrossRef] [PubMed]
- GR Metrics. Available online: http://www.grcalculator.org/grcalculator/ (accessed on 8 November 2023).
- Qazi, S.J.S.; Rennie, A.R.; Cockcroft, J.K.; Vickers, M. Use of wide-angle X-ray diffraction to measure shape and size of dispersed colloidal particles. J. Colloid Interface Sci. 2009, 338, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Qiu, L.; Qu, B. Controlled synthesis of magnesium hydroxide nanoparticles with different morphological structures and related properties in flame retardant ethylene–vinyl acetate blends. Nanotechnology 2004, 15, 1576–1581. [Google Scholar] [CrossRef]
- Henrist, C.; Mathieu, J.-P.; Vogels, C.; Rulmont, A.; Cloots, R. Morphological study of magnesium hydroxide nanoparticles precipitated in dilute aqueous solution. J. Cryst. Growth 2003, 249, 321–330. [Google Scholar] [CrossRef]
- Lv, J.; Qiu, L.; Qu, B. Controlled growth of three morphological structures of magnesium hydroxide nanoparticles by wet precipitation method. J. Cryst. Growth 2004, 267, 676–684. [Google Scholar] [CrossRef]
- Alothman, Z.A. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Condon, J.B. Surface Area and Porosity Determinations by Physisorption: Measurement, Classical Theories and Quantum Theory; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Chang, S.-S.; Clair, B.; Ruelle, J.; Beauchêne, J.; Di Renzo, F.; Quignard, F.; Zhao, G.-J.; Yamamoto, H.; Gril, J. Mesoporosity as a new parameter for understanding tension stress generation in trees. J. Exp. Bot. 2009, 60, 3023–3030. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, I.M.; Saleh, I.A.; Abdelhami, M.R. Synthesis of MgO Nanoparticles from Different Organic Precursors: Catalytic Decontamination of Organic Pollutants and Antitumor Activity. J. Mater. Sci. Eng. 2017, 6, 1000359. [Google Scholar] [CrossRef]

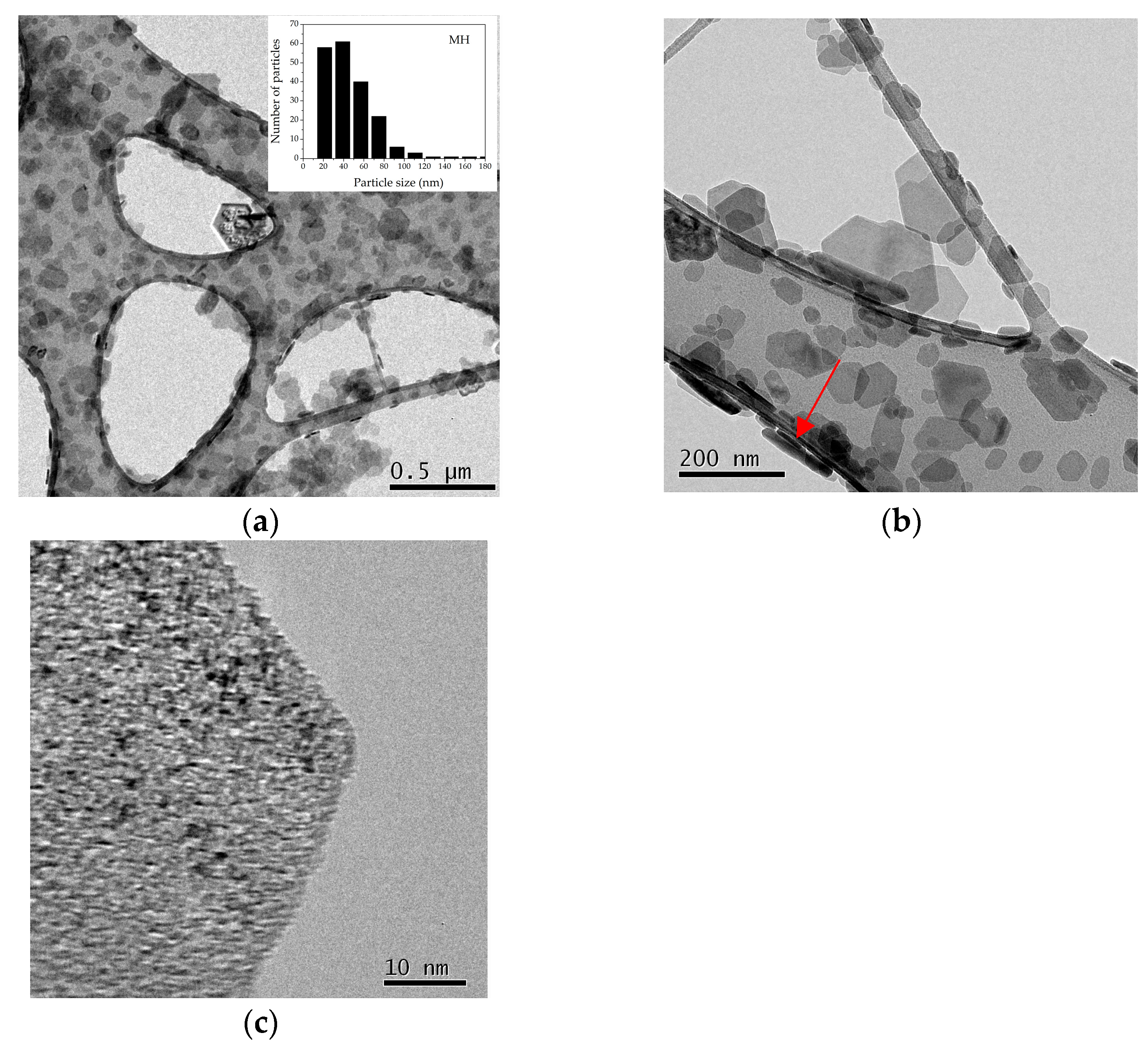

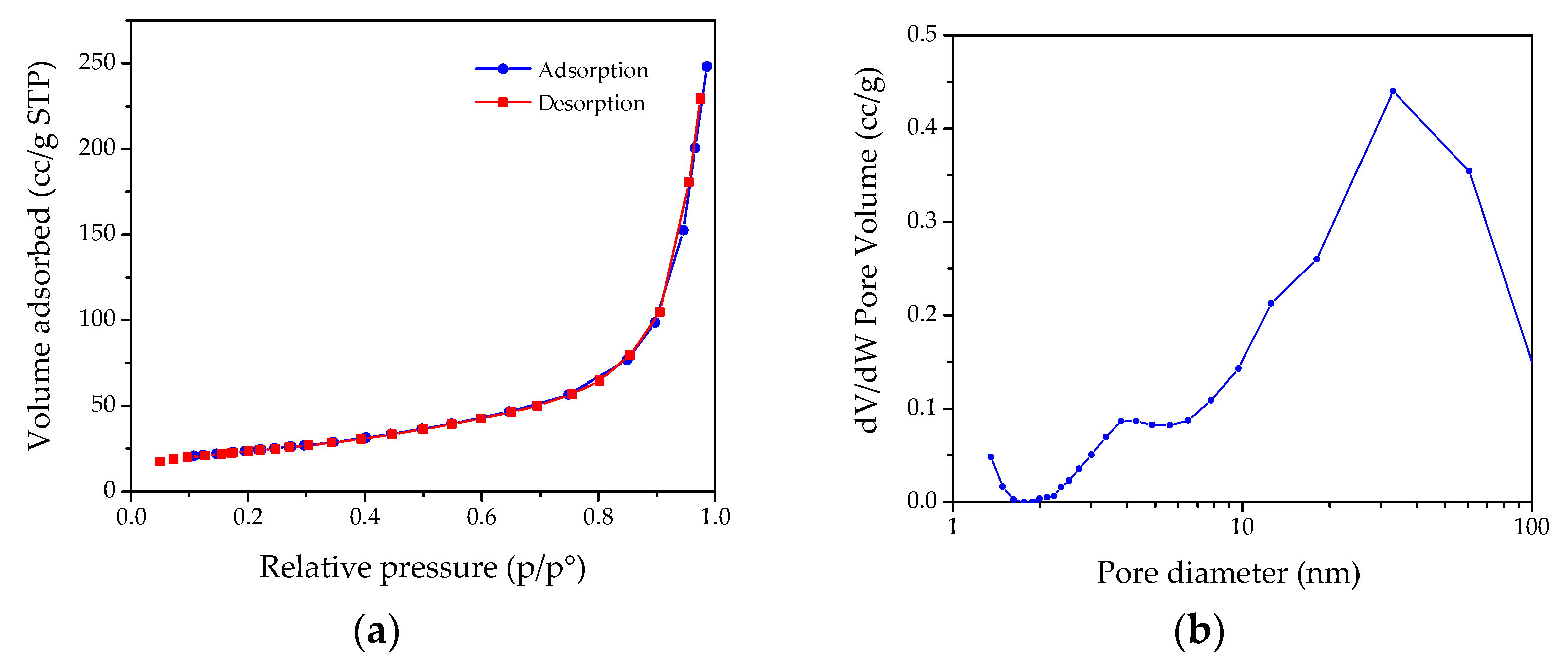
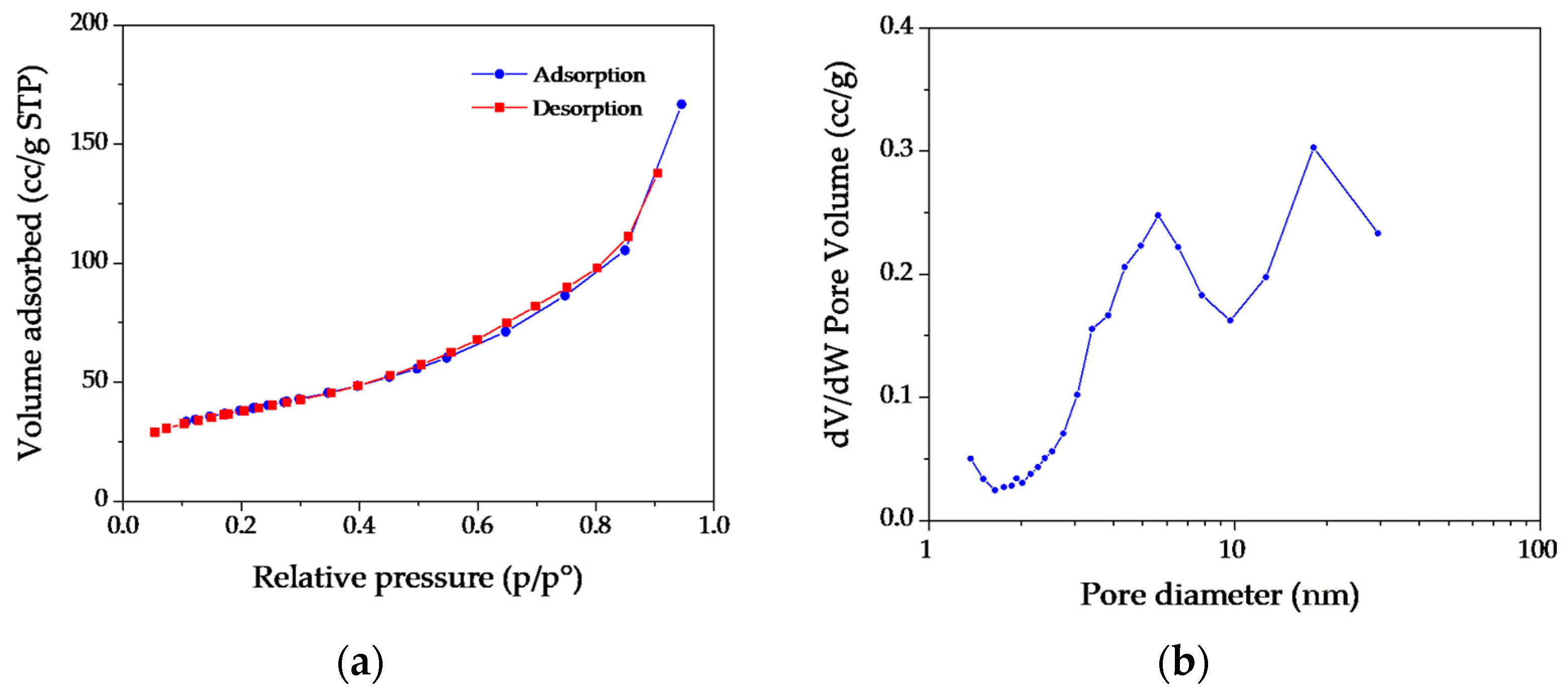
| ICSD 98-008-9823 | MH NPs | ICSD 98-017-0905 | MgO NPs | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| a = 3.1430 Å c = 4.7670 Å | a = 3.1478 Å c = 4.7819 Å | a = 4.2270 Å c = 4.2270 Å | a = 4.2227 Å c = 4.2227 Å | ||||||||
| hkl | I (%) | 2 Theta (°) | I (%) | Dhkl (nm) | <Dhkl> average value (nm) | hkl | I (%) | 2 Theta (°) | I (%) | Dhkl (nm) | <Dhkl> average value (nm) |
| 001 | 96.30 | 18.60 | 96.20 | 12.61 | 14.27 | 111 | 11.40 | 36.80 | 11.47 | 7.53 | 8.37 |
| 010 | 2.60 | 32.88 | 5.15 | 12.95 | 002 | 100 | 42.75 | 100 | 7.67 | ||
| 011 | 100 | 38.04 | 100 | 13.13 | 022 | 45.00 | 62.05 | 44.95 | 8.35 | ||
| 012 | 37.80 | 50.88 | 37.86 | 13.76 | 113 | 4.90 | 74.37 | 4.94 | 9.01 | ||
| 110 | 27.80 | 58.70 | 27.75 | 14.28 | 222 | 10.80 | 78.29 | 10.83 | 9.27 | ||
| 111 | 15.70 | 62.14 | 15.72 | 14.55 | |||||||
| 103 | 15.30 | 68.30 | 15.39 | 15.10 | |||||||
| 201 | 9.70 | 72.12 | 9.68 | 15.50 | |||||||
| 202 | 6.50 | 81.35 | 6.45 | 16.60 | |||||||
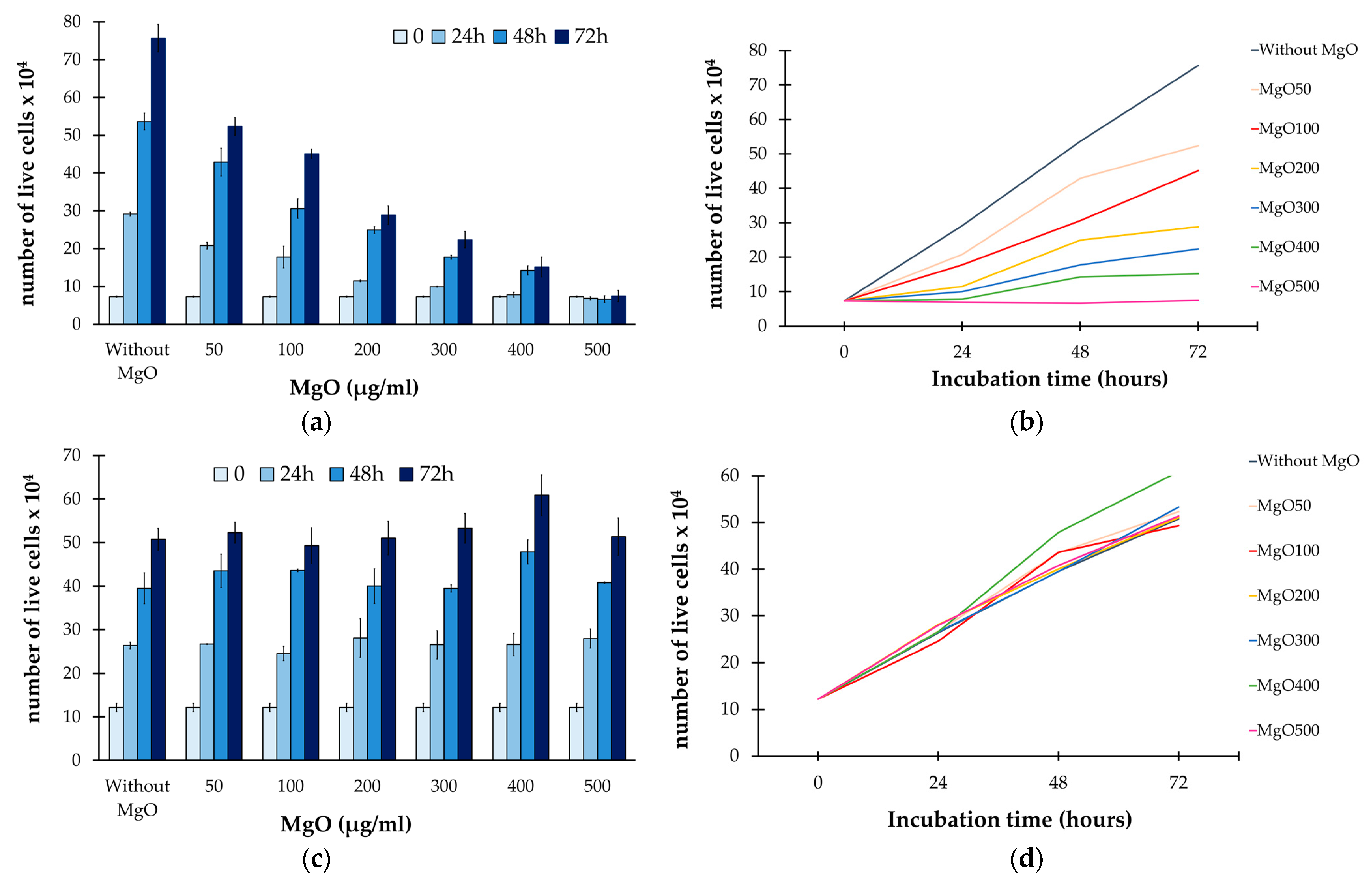
| Number of Live Melanoma Cells | ||||||||
|---|---|---|---|---|---|---|---|---|
| Time (hours) | Without MgO | MgO50 | MgO100 | MgO200 | MgO300 | MgO400 | MgO500 | |
| 0 | Σ | 7.3 × 104 | ||||||
| σ | 0.14 | |||||||
| 24 | Σ | 29.16 × 104 | 20.79 × 104 | 17.78 × 104 | 11.50 × 104 | 9.98 × 104 | 7.81 × 104 | 6.86 × 104 |
| σ | 0.51 | 0.89 | 2.86 | 0.23 | 0.11 | 0.62 | 0.39 | |
| 48 | Σ | 53.64 × 104 | 42.90 × 104 | 30.60 × 104 | 24.95 × 104 | 17.75 × 104 | 14.25 × 104 | 6.63 × 104 |
| σ | 2.21 | 3.68 | 2.54 | 0.93 | 0.49 | 1.20 | 0.92 | |
| 72 | Σ | 75.65 × 104 | 52.36 × 104 | 45.09 × 104 | 28.85 × 104 | 22.40 × 104 | 15.15 × 104 | 7.46 × 104 |
| σ | 3.61 | 2.33 | 1.23 | 2.47 | 2.18 | 2.62 | 1.40 | |
| Number of live skin fibroblasts | ||||||||
| Time (hours) | Without MgO | MgO50 | MgO100 | MgO200 | MgO300 | MgO400 | MgO500 | |
| 0 | Σ | 12.2 × 104 | ||||||
| σ | 0.91 | |||||||
| 24 | Σ | 26.36 × 104 | 26.67 × 104 | 24.54 × 104 | 28.11 × 104 | 26.53 × 104 | 26.57 × 104 | 27.97 × 104 |
| σ | 0.74 | 0.08 | 1.58 | 4.40 | 3.21 | 2.54 | 2.18 | |
| 48 | Σ | 39.50 × 104 | 43.50 × 104 | 43.60 × 104 | 40.00 × 104 | 39.47 × 104 | 47.86 × 104 | 40.77 × 104 |
| σ | 3.53 | 3.82 | 0.28 | 3.96 | 0.81 | 2.74 | 0.16 | |
| 72 | Σ | 50.75 × 104 | 52.30 × 104 | 49.30 × 104 | 51.03 × 104 | 53.28 × 104 | 60.90 × 104 | 51.36 × 104 |
| σ | 2.47 | 2.40 | 4.10 | 3.86 | 3.39 | 4.67 | 4.30 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daniele, V.; Volpe, A.R.; Cesare, P.; Taglieri, G. MgO Nanoparticles Obtained from an Innovative and Sustainable Route and Their Applications in Cancer Therapy. Nanomaterials 2023, 13, 2975. https://doi.org/10.3390/nano13222975
Daniele V, Volpe AR, Cesare P, Taglieri G. MgO Nanoparticles Obtained from an Innovative and Sustainable Route and Their Applications in Cancer Therapy. Nanomaterials. 2023; 13(22):2975. https://doi.org/10.3390/nano13222975
Chicago/Turabian StyleDaniele, Valeria, Anna Rita Volpe, Patrizia Cesare, and Giuliana Taglieri. 2023. "MgO Nanoparticles Obtained from an Innovative and Sustainable Route and Their Applications in Cancer Therapy" Nanomaterials 13, no. 22: 2975. https://doi.org/10.3390/nano13222975
APA StyleDaniele, V., Volpe, A. R., Cesare, P., & Taglieri, G. (2023). MgO Nanoparticles Obtained from an Innovative and Sustainable Route and Their Applications in Cancer Therapy. Nanomaterials, 13(22), 2975. https://doi.org/10.3390/nano13222975






