Fractional Factorial Design to Evaluate the Synthesis and Electrochemical Transfer Parameters of h-BN Coatings
Abstract
:1. Introduction
2. Material and Methods
2.1. Synthesis of h-BN
2.2. Electrochemical Transfer Process
2.3. Characterization
2.3.1. Optical Microscopy
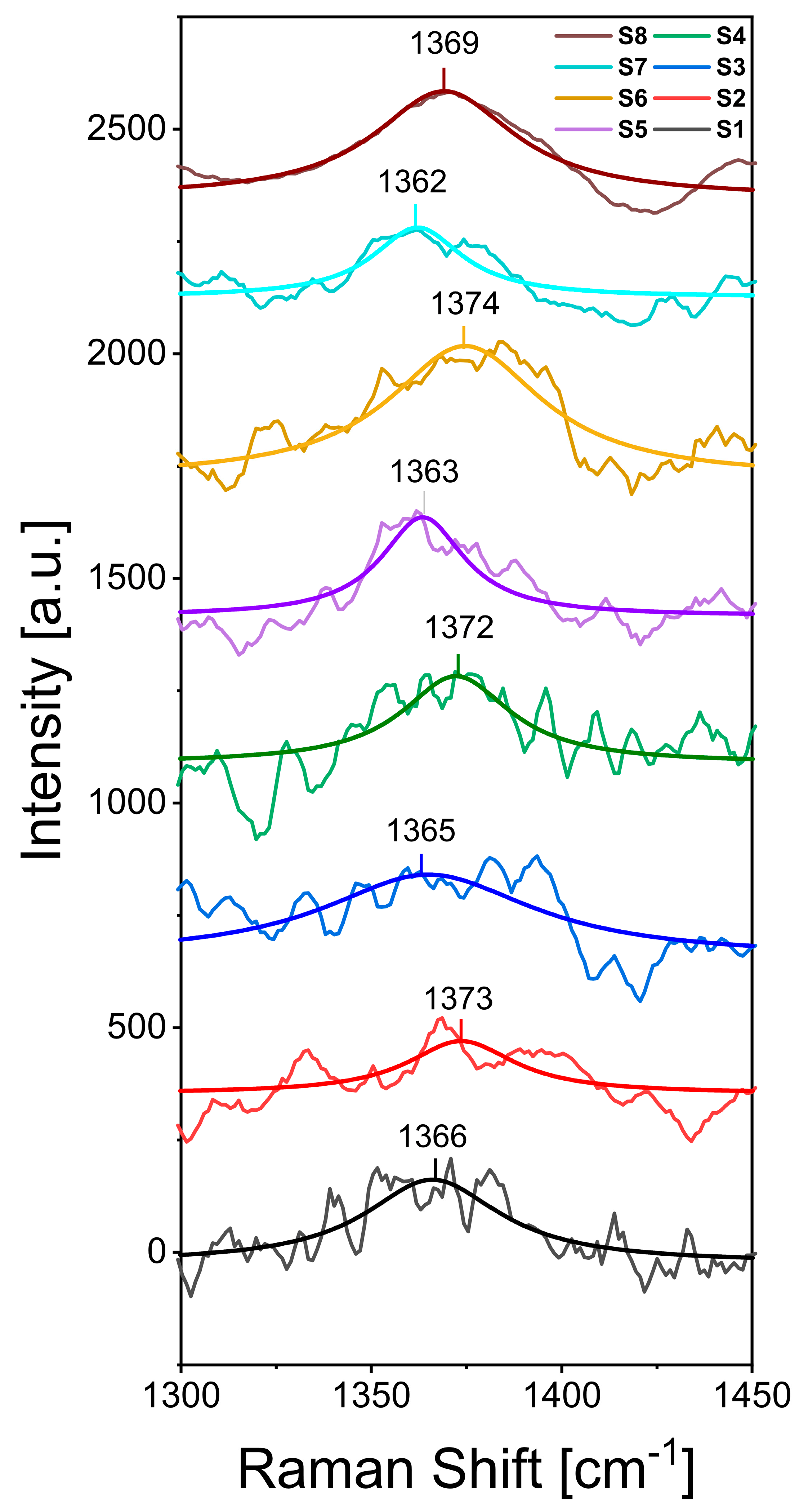
2.3.2. RAMAN Spectroscopy
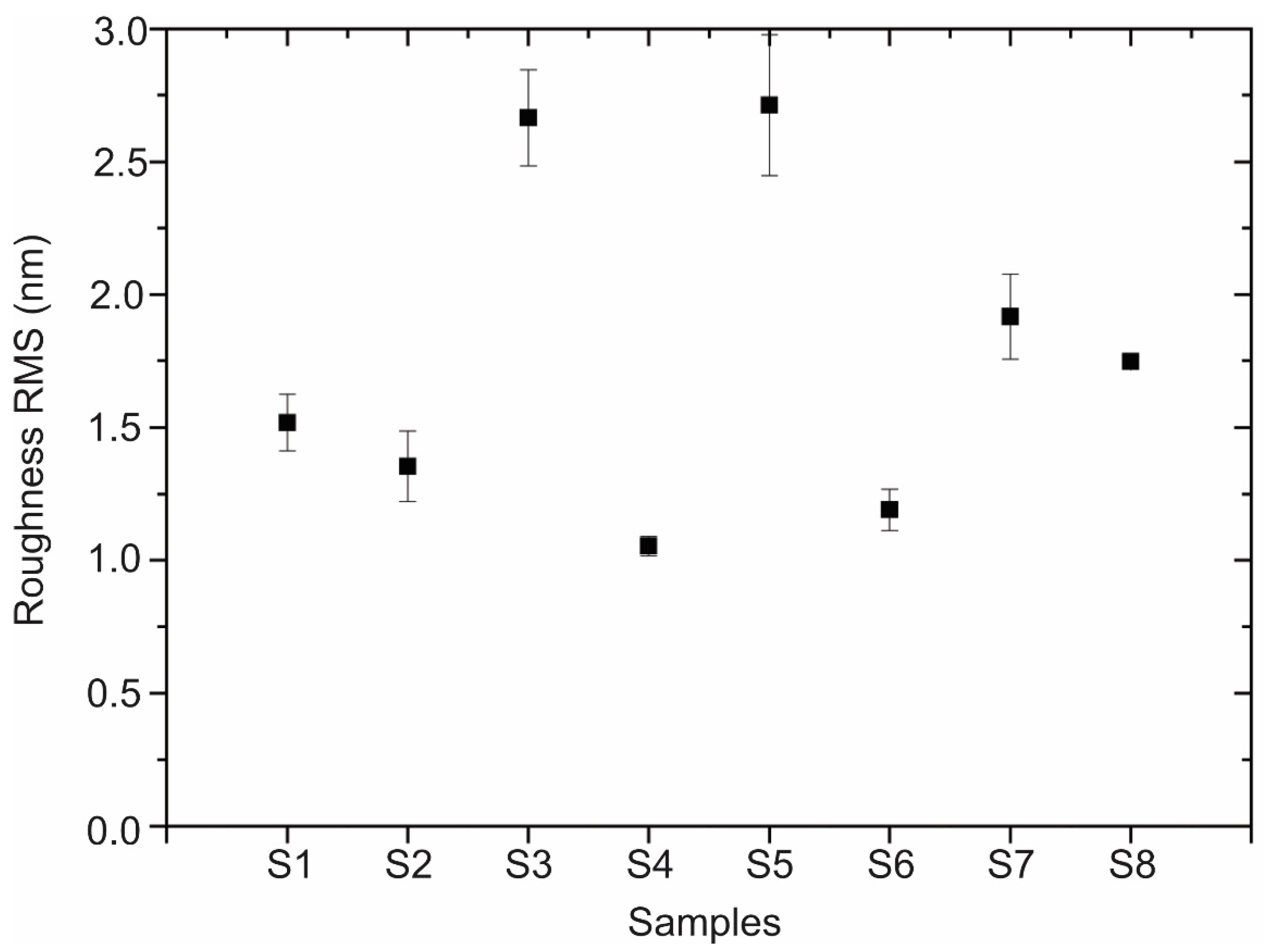
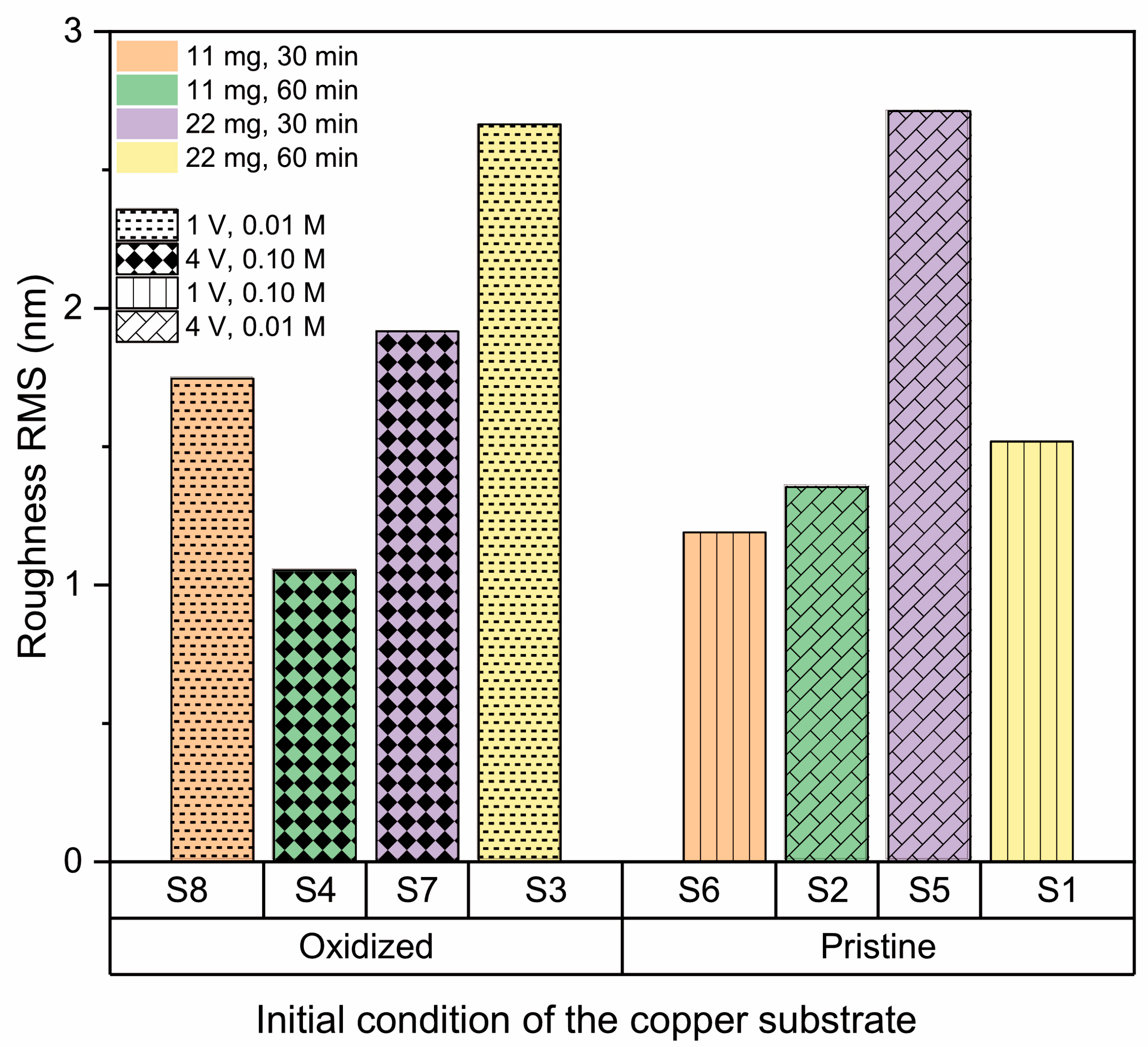
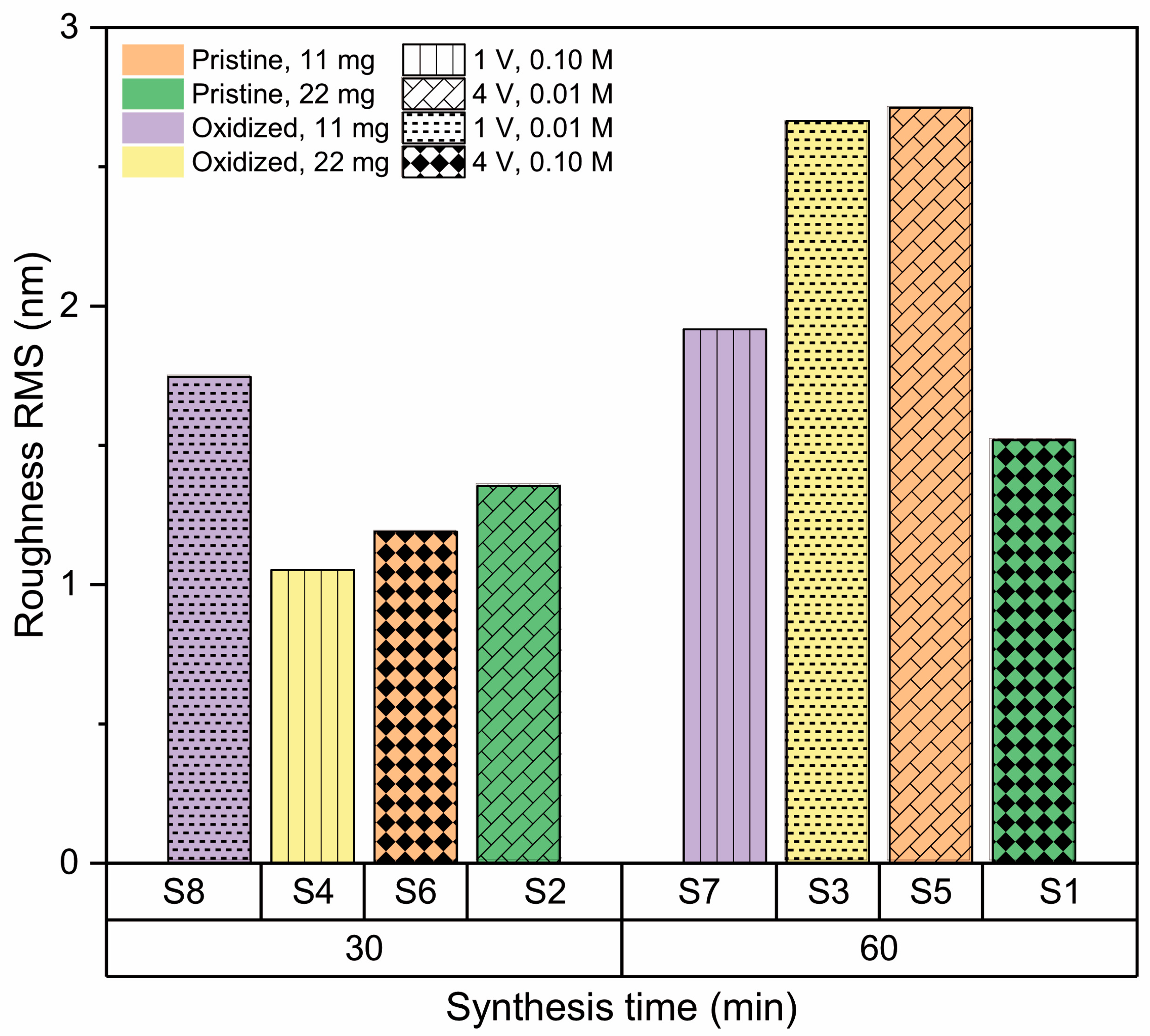
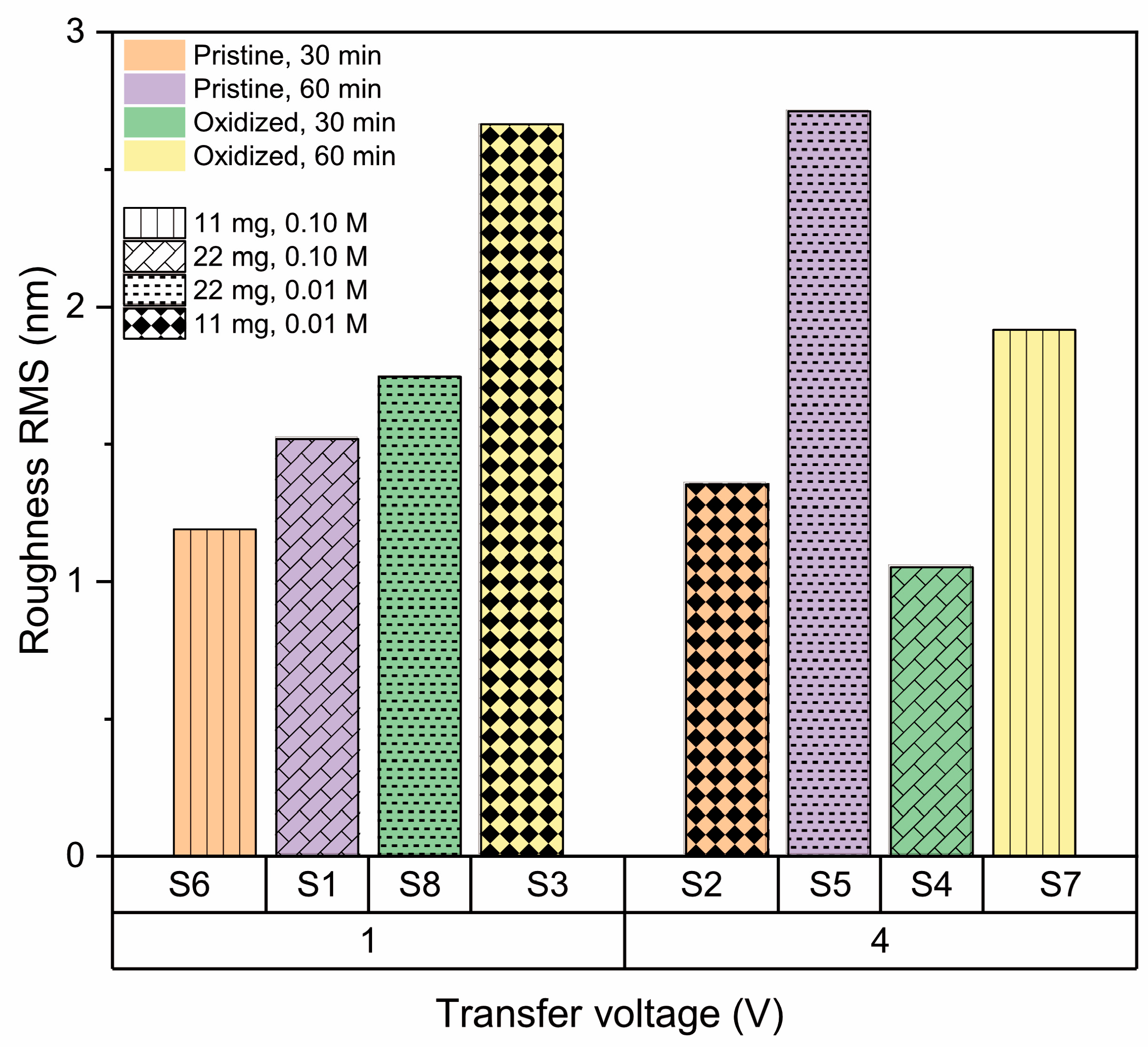
2.3.3. Surface Roughness
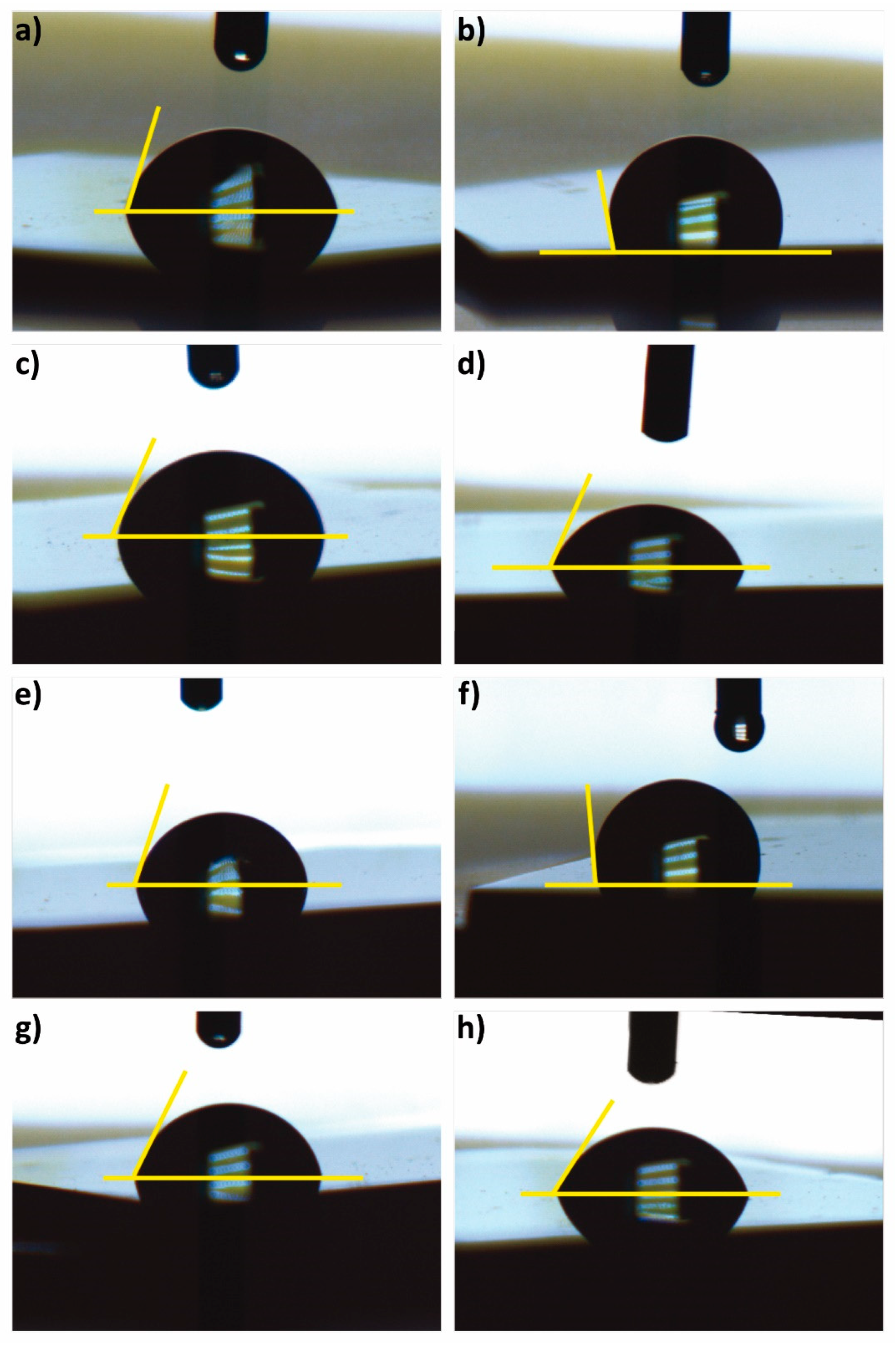
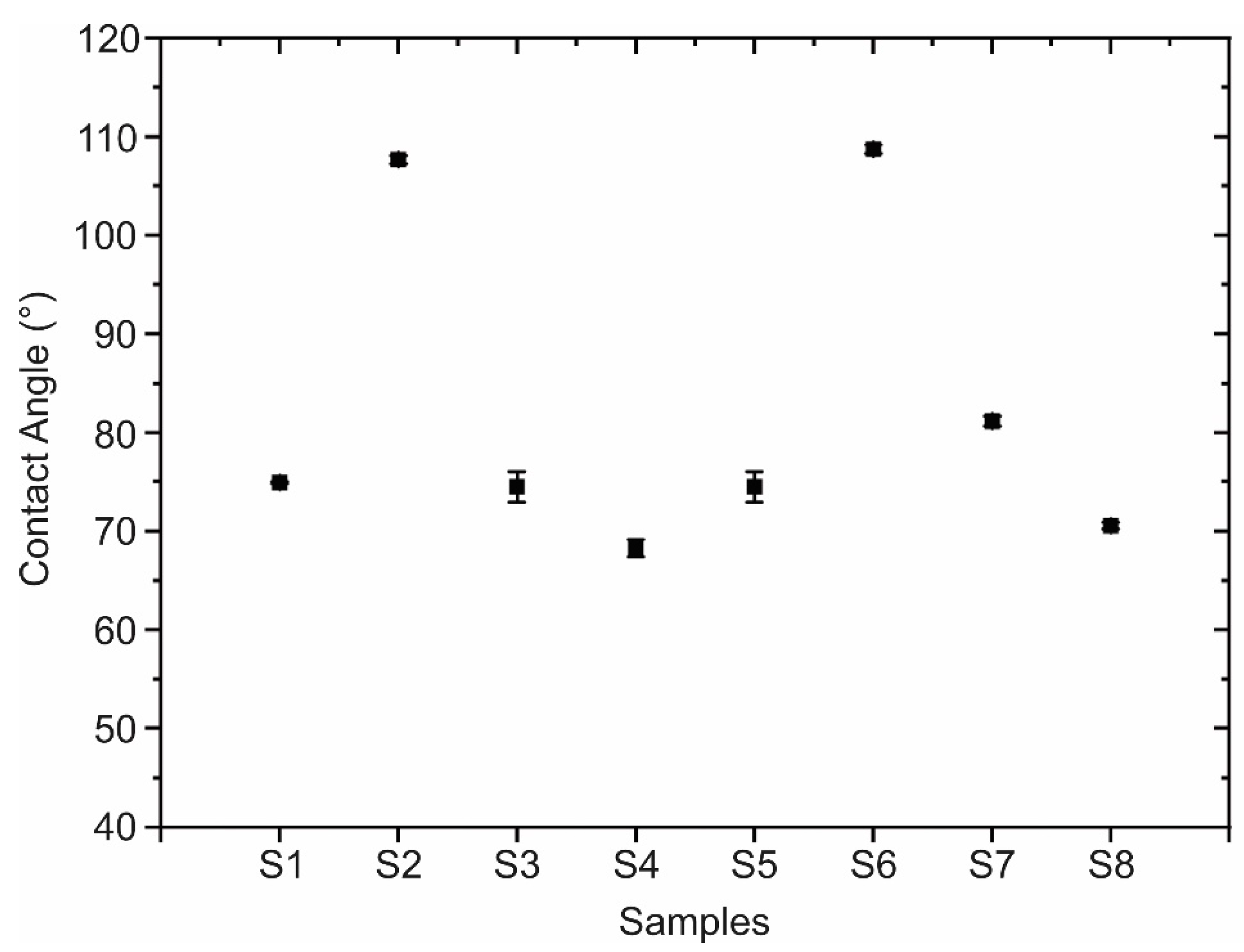
2.3.4. Contact Angle
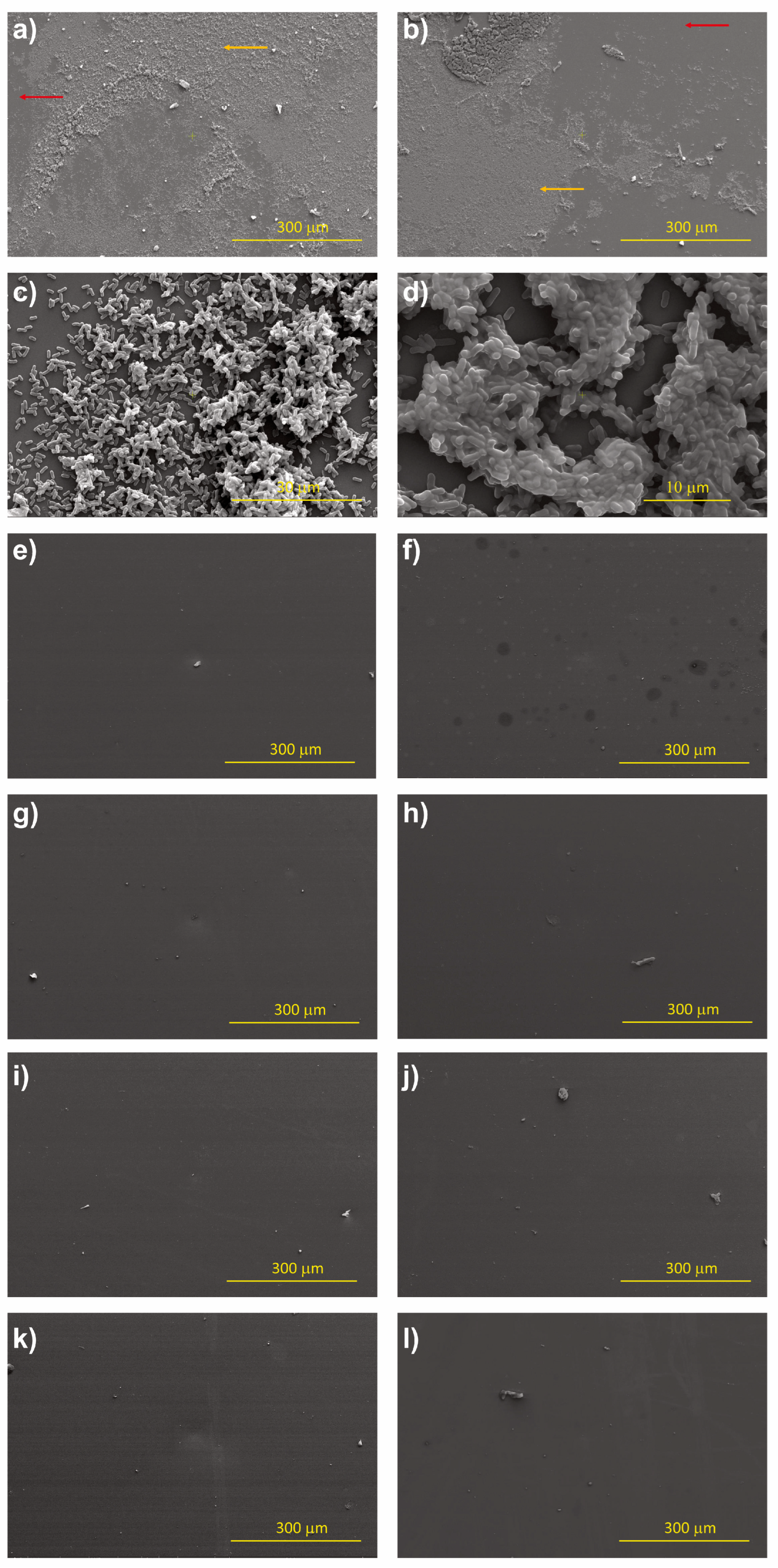
2.3.5. Morphology
2.4. Preparation of Bacterial Cultures
2.5. Fractional Factorial Experimental Design for Quality of h-BN Transferred Layer
3. Results and Discussion
3.1. Characterization
3.2. Experimental Design
3.2.1. Empirical Model of Roughness Equation
3.2.2. Empirical Model for Contact Angle Equation
3.2.3. Effect of Amount of Precursor
- Precursor decomposition: during this phase, precursors like ammonia borane and borazine undergo breakdown into boron- and nitrogen-based compounds at elevated temperatures.
- Deposition and nucleation: The resulting boron- and nitrogen-based compounds are deposited onto metal surfaces, subsequently forming clusters.
- Continuous growth: These clusters then expand, forming larger h-BN islands, which eventually merge to create a seamless film.
3.2.4. Effect of the Copper Substrate Condition
3.2.5. Effect of Synthesis Time
3.2.6. Effect of Transference Parameters
3.3. Bacterial Adhesion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, Y.; Xiao, P.; Wang, Y.; Hao, Y. Mechanisms and Control Measures of Mature Biofilm Resistance to Antimicrobial Agents in the Clinical Context. ACS Omega 2020, 5, 22684–22690. [Google Scholar] [CrossRef] [PubMed]
- Ciofu, O.; Moser, C.; Jensen, P.Ø.; Høiby, N. Tolerance and resistance of microbial biofilms. Nat. Rev. Microbiol. 2022, 20, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.-E.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Front. Bioeng. Biotechnol. 2021, 9, 643722. [Google Scholar]
- Li, X.; Wu, B.; Chen, H.; Nan, K.; Jin, Y.; Sun, L.; Wang, B. Recent developments in smart antibacterial surfaces to inhibit biofilm formation and bacterial infections. J. Mater. Chem. B 2018, 6, 4274–4292. [Google Scholar] [CrossRef] [PubMed]
- DeFlorio, W.; Liu, S.; Arcot, Y.; Ulugun, B.; Wang, X.; Min, Y.; Cisneros-Zevallos, L.; Akbulut, M. Durable superhydrophobic coatings for stainless-steel: An effective defense against Escherichia coli and Listeria fouling in the post-harvest environment. Food Res. Int. 2023, 173, 113227. [Google Scholar] [CrossRef]
- Yuan, Y.; Lee, T.R. Contact Angle and Wetting Properties. In Surface Science Techniques; Bracco, G., Holst, B., Eds.; Springer Series in Surface Sciences; Springer: Berlin/Heidelberg, Germany, 2013; pp. 3–34. [Google Scholar] [CrossRef]
- Lu, A.; Gao, Y.; Jin, T.; Luo, X.; Zeng, Q.; Shang, Z. Effects of surface roughness and texture on the bacterial adhesion on the bearing surface of bio-ceramic joint implants: An in vitro study. Ceram. Int. 2020, 46, 6550–6559. [Google Scholar] [CrossRef]
- Cheng, Y.; Feng, G.; Moraru, C.I. Micro- and Nanotopography Sensitive Bacterial Attachment Mechanisms: A Review. Front. Microbiol. 2019, 10, 191. [Google Scholar]
- Kolewe, K.W.; Zhu, J.; Mako, N.R.; Nonnenmann, S.S.; Schiffman, J.D. Bacterial Adhesion Is Affected by the Thickness and Stiffness of Poly(ethylene glycol) Hydrogels. ACS Appl. Mater. Interfaces 2018, 10, 2275–2281. [Google Scholar] [CrossRef]
- Chandra, J.; Patel, J.D.; Li, J.; Zhou, G.; Mukherjee, P.K.; McCormick, T.S.; Anderson, J.M.; Ghannoum, M.A. Modification of Surface Properties of Biomaterials Influences the Ability of Candida albicans To Form Biofilms. Appl. Environ. Microbiol. 2005, 71, 8795–8801. [Google Scholar] [CrossRef]
- Arias, S.L.; Devorkin, J.; Civantos, A.; Allain, J.P. Escherichia coli Adhesion and Biofilm Formation on Polydimethylsiloxane are Independent of Substrate Stiffness. Langmuir 2021, 37, 16–25. [Google Scholar] [CrossRef]
- Yang, K.; Shi, J.; Wang, L.; Chen, Y.; Liang, C.; Yang, L.; Wang, L.-N. Bacterial anti-adhesion surface design: Surface patterning, roughness and wettability: A review. J. Mater. Sci. Technol. 2022, 99, 82–100. [Google Scholar] [CrossRef]
- Mu, M.; Liu, S.; DeFlorio, W.; Hao, L.; Wang, X.; Salazar, K.S.; Taylor, M.; Castillo, A.; Cisneros-Zevallos, L.; Oh, J.K.; et al. Influence of Surface Roughness, Nanostructure, and Wetting on Bacterial Adhesion. Langmuir 2023, 39, 5426–5439. [Google Scholar] [CrossRef]
- Sahli, C.; Moya, S.E.; Lomas, J.S.; Gravier-Pelletier, C.; Briandet, R.; Hémadi, M. Recent advances in nanotechnology for eradicating bacterial biofilm. Theranostics 2022, 12, 2383–2405. [Google Scholar] [CrossRef]
- Luo, X.; Yao, S.; Zhang, H.; Cai, M.; Liu, W.; Pan, R.; Chen, C.; Wang, X.; Wang, L.; Zhong, M. Biocompatible nano-ripples structured surfaces induced by femtosecond laser to rebel bacterial colonization and biofilm formation. Opt. Laser Technol. 2020, 124, 105973. [Google Scholar] [CrossRef]
- Kumari, N.; Kumar, S.; Karmacharya, M.; Dubbu, S.; Kwon, T.; Singh, V.; Chae, K.H.; Kumar, A.; Cho, Y.-K.; Lee, I.S. Surface-Textured Mixed-Metal-Oxide Nanocrystals as Efficient Catalysts for ROS Production and Biofilm Eradication. Nano Lett. 2021, 21, 279–287. [Google Scholar] [CrossRef]
- Meyer, F.; Enax, J.; Epple, M.; Amaechi, B.T.; Simader, B. Cariogenic Biofilms: Development, Properties, and Biomimetic Preventive Agents. Dent. J. 2021, 9, 88. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, M.; Moriya, H.; Miyauchi, A. Biomimetic Design Inspired Sharkskin Denticles for Growth Suppression of Biofilm. J. Photopolym. Sci. Technol. 2019, 32, 295–301. [Google Scholar] [CrossRef]
- Sullivan, T.; O’Callaghan, I. Recent Developments in Biomimetic Antifouling Materials: A Review. Biomimetics 2020, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhou, L.; Chiao, M.; Yang, W. Antibacterial hydrogel coating: Strategies in surface chemistry. Adv. Colloid Interface Sci. 2020, 285, 102280. [Google Scholar] [CrossRef]
- Ren, L.; Lin, X.; Tan, L.; Yang, K. Effect of surface coating on antibacterial behavior of magnesium based metals. Mater. Lett. 2011, 65, 3509–3511. [Google Scholar] [CrossRef]
- Gokce, C.; Gurcan, C.; Besbinar, O.; Altay Unal, M.; Yilmazer, A. Emerging 2D materials for antimicrobial applications in the pre- and post-pandemic era. Nanoscale 2022, 14, 239–249. [Google Scholar] [CrossRef]
- Mazinani, A.; Rastin, H.; Nine, M.J.; Lee, J.; Tikhomirova, A.; Tung, T.T.; Ghomashchi, R.; Kidd, S.; Vreugde, S.; Losic, D. Comparative antibacterial activity of 2D materials coated on porous-titania. J. Mater. Chem. B 2021, 9, 6412–6424. [Google Scholar] [CrossRef] [PubMed]
- Mba, I.E.; Nweze, E.I. Nanoparticles as therapeutic options for treating multidrug-resistant bacteria: Research progress, challenges, and prospects. World J. Microbiol. Biotechnol. 2021, 37, 108. [Google Scholar] [CrossRef]
- Ashfaq, M.Y.; Al-Ghouti, M.A.; Zouari, N. Investigating the effect of polymer-modified graphene oxide coating on RO membrane fouling. J. Water Process Eng. 2022, 49, 103164. [Google Scholar] [CrossRef]
- Swidan, N.S.; Hashem, Y.A.; Elkhatib, W.F.; Yassien, M.A. Antibiofilm activity of green synthesized silver nanoparticles against biofilm associated enterococcal urinary pathogens. Sci. Rep. 2022, 12, 3869. [Google Scholar] [CrossRef]
- Mei, L.; Zhu, S.; Yin, W.; Chen, C.; Nie, G.; Gu, Z.; Zhao, Y. Two-dimensional nanomaterials beyond graphene for antibacterial applications: Current progress and future perspectives. Theranostics 2020, 10, 757–781. [Google Scholar] [CrossRef] [PubMed]
- Dey, N.; Vickram, S.; Thanigaivel, S.; Kamatchi, C.; Subbaiya, R.; Karmegam, N.; Govarthanan, M. Graphene materials: Armor against nosocomial infections and biofilm formation—A review. Environ. Res. 2022, 214, 113867. [Google Scholar] [CrossRef]
- Parra, C.; Montero-Silva, F.; Gentil, D.; Del Campo, V.; Henrique Rodrigues da Cunha, T.; Henríquez, R.; Häberle, P.; Garín, C.; Ramírez, C.; Fuentes, R.; et al. The Many Faces of Graphene as Protection Barrier. Performance under Microbial Corrosion and Ni Allergy Conditions. Materials 2017, 10, 1406. [Google Scholar] [CrossRef]
- Parra, C.; Dorta, F.; Jimenez, E.; Henríquez, R.; Ramírez, C.; Rojas, R.; Villalobos, P. A nanomolecular approach to decrease adhesion of biofouling-producing bacteria to graphene-coated material. J. Nanobiotechnol. 2015, 13, 82. [Google Scholar] [CrossRef] [PubMed]
- Parra, C.; Montero-Silva, F.; Henríquez, R.; Flores, M.; Garín, C.; Ramírez, C.; Moreno, M.; Correa, J.; Seeger, M.; Häberle, P. Suppressing Bacterial Interaction with Copper Surfaces through Graphene and Hexagonal-Boron Nitride Coatings. ACS Appl. Mater. Interfaces 2015, 7, 6430–6437. [Google Scholar] [CrossRef]
- Parra, C.; Aristizabal, J.; Arce, B.; Montero-Silva, F.; Lascano, S.; Henriquez, R.; Lazcano, P.; Giraldo-Gallo, P.; Ramírez, C.; Henrique Rodrigues da Cunha, T.; et al. Graphene Coating as an Effective Barrier to Prevent Bacteria-Mediated Dissolution of Gold. Metals 2021, 11, 147. [Google Scholar] [CrossRef]
- Qureshi, N.; Patil, R.; Shinde, M.; Umarji, G.; Causin, V.; Gade, W.; Mulik, U.; Bhalerao, A.; Amalnerkar, D.P. Innovative biofilm inhibition and anti-microbial behavior of molybdenum sulfide nanostructures generated by microwave-assisted solvothermal route. Appl. Nanosci. 2015, 5, 331–341. [Google Scholar] [CrossRef]
- Santos, J.; Moschetta, M.; Rodrigues, J.; Alpuim, P.; Capasso, A. Interactions Between 2D Materials and Living Matter: A Review on Graphene and Hexagonal Boron Nitride Coatings. Front. Bioeng. Biotechnol. 2021, 9, 612669. [Google Scholar]
- Sahoo, J.; Sarkhel, S.; Mukherjee, N.; Jaiswal, A. Nanomaterial-Based Antimicrobial Coating for Biomedical Implants: New Age Solution for Biofilm-Associated Infections. ACS Omega 2022, 7, 45962–45980. [Google Scholar] [CrossRef]
- Hayat, A.; Sohail, M.; Jery, A.E.; Al-Zaydi, K.M.; Raza, S.; Ali, H.; Ajmal, Z.; Zada, A.; Taha, T.A.; Din, I.U.; et al. Recent advances, properties, fabrication and opportunities in two-dimensional materials for their potential sustainable applications. Energy Storage Mater. 2023, 59, 102780. [Google Scholar] [CrossRef]
- Ren, G.; Wan, K.; Kong, H.; Guo, L.; Wang, Y.; Liu, X.; Wei, G. Recent advance in biomass membranes: Fabrication, functional regulation, and antimicrobial applications. Carbohydr. Polym. 2023, 305, 120537. [Google Scholar] [CrossRef]
- Kıvanç, M.; Barutca, B.; Koparal, A.T.; Göncü, Y.; Bostancı, S.H.; Ay, N. Effects of hexagonal boron nitride nanoparticles on antimicrobial and antibiofilm activities, cell viability. Mater. Sci. Eng. C 2018, 91, 115–124. [Google Scholar] [CrossRef]
- Merlo, A.; Mokkapati, V.R.S.S.; Pandit, S.; Mijakovic, I. Boron nitride nanomaterials: Biocompatibility and bio-applications. Biomater. Sci. 2018, 6, 2298–2311. [Google Scholar] [CrossRef]
- Smith McWilliams, A.D.; Martínez-Jiménez, C.; Matatyaho Ya’akobi, A.; Ginestra, C.J.; Talmon, Y.; Pasquali, M.; Martí, A.A. Understanding the Exfoliation and Dispersion of Hexagonal Boron Nitride Nanosheets by Surfactants: Implications for Antibacterial and Thermally Resistant Coatings. ACS Appl. Nano Mater. 2021, 4, 142–151. [Google Scholar] [CrossRef]
- Mukheem, A.; Shahabuddin, S.; Akbar, N.; Miskon, A.; Muhamad Sarih, N.; Sudesh, K.; Ahmed Khan, N.; Saidur, R.; Sridewi, N. Boron Nitride Doped Polyhydroxyalkanoate/Chitosan Nanocomposite for Antibacterial and Biological Applications. Nanomaterials 2019, 9, 645. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Neupane, N.; Dahal, P.R.; Rahimi, S.; Cao, Z.; Pandit, S.; Mijakovic, I. Antibiotic-Loaded Boron Nitride Nanoconjugate with Strong Performance against Planktonic Bacteria and Biofilms. ACS Appl. Bio Mater. 2023, 6, 3131–3142. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Hao, L.; Chen, L.; Gao, F.; Qiu, S.; Zhou, H.; Chen, H.; Zhou, X. Facile Mechanical-Induced Functionalization of Hexagonal Boron Nitride and Its Application as Vehicles for Antibacterial Essential Oil. ACS Sustain. Chem. Eng. 2020, 8, 15120–15133. [Google Scholar] [CrossRef]
- Nithya, J.S.M.; Pandurangan, A. Aqueous dispersion of polymer coated boron nitride nanotubes and their antibacterial and cytotoxicity studies. RSC Adv. 2014, 4, 32031–32046. [Google Scholar] [CrossRef]
- Zurob, E.; Dennett, G.; Gentil, D.; Montero-Silva, F.; Gerber, U.; Naulín, P.; Gómez, A.; Fuentes, R.; Lascano, S.; Rodrigues da Cunha, T.H.; et al. Inhibition of Wild Enterobacter cloacae Biofilm Formation by Nanostructured Graphene- and Hexagonal Boron Nitride-Coated Surfaces. Nanomaterials 2019, 9, 49. [Google Scholar] [CrossRef]
- Kumar Verma, A.; Govind Rajan, A. Surface Roughness Explains the Observed Water Contact Angle and Slip Length on 2D Hexagonal Boron Nitride. Langmuir 2022, 38, 9210–9220. [Google Scholar] [CrossRef]
- Cai, Y.; Yu, C.; Zhong, S.; Chen, G.; Liu, L. Roughness-controlled cell-surface interactions mediate early biofilm development in drinking water systems. J. Environ. Chem. Eng. 2023, 11, 110101. [Google Scholar] [CrossRef]
- Uzoma, P.C.; Etim, I.-I.N.; Okonkwo, B.O.; Olanrele, O.S.; Njoku, D.I.; Kolawole, S.K.; Emori, W.; Ikeuba, A.I.; Njoku, C.N.; Ekerenam, O.O.; et al. Recent design approaches, adhesion mechanisms, and applications of antibacterial surfaces. Chem. Eng. J. Adv. 2023, 16, 100563. [Google Scholar] [CrossRef]
- Muhammad, M.H.; Idris, A.L.; Fan, X.; Guo, Y.; Yu, Y.; Jin, X.; Qiu, J.; Guan, X.; Huang, T. Beyond Risk: Bacterial Biofilms and Their Regulating Approaches. Front. Microbiol. 2020, 11, 928. [Google Scholar] [CrossRef]
- Krasowska, A.; Sigler, K. How microorganisms use hydrophobicity and what does this mean for human needs? Front. Cell. Infect. Microbiol. 2014, 4, 112. [Google Scholar] [CrossRef]
- Yuan, Y.; Hays, M.P.; Hardwidge, P.R.; Kim, J. Surface characteristics influencing bacterial adhesion to polymeric substrates. RSC Adv. 2017, 7, 14254–14261. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Zhang, R.; Han, Z.; Dong, H.; Yu, G.; Geng, D.; Yang, H.Y. A minireview on chemical vapor deposition growth of wafer-scale monolayer h-BN single crystals. Nanoscale 2021, 13, 17310–17317. [Google Scholar] [CrossRef]
- Li, M.; Zhou, S.; Wang, R.; Yu, Y.; Wong, H.; Luo, Z.; Li, H.; Gan, L.; Zhai, T. In situ formed nanoparticle-assisted growth of large-size single crystalline h-BN on copper. Nanoscale 2018, 10, 17865–17872. [Google Scholar] [CrossRef] [PubMed]
- Stehle, Y.; Meyer, H.M.I.; Unocic, R.R.; Kidder, M.; Polizos, G.; Datskos, P.G.; Jackson, R.; Smirnov, S.N.; Vlassiouk, I.V. Synthesis of Hexagonal Boron Nitride Monolayer: Control of Nucleation and Crystal Morphology. Chem. Mater. 2015, 27, 8041–8047. [Google Scholar] [CrossRef]
- Yasnó, J.P.; Kiminami, R.H.G.A. Short time reaction synthesis of nano-hexagonal boron nitride. Adv. Powder Technol. 2020, 31, 4436–4443. [Google Scholar] [CrossRef]
- Kim, K.K.; Hsu, A.; Jia, X.; Kim, S.M.; Shi, Y.; Hofmann, M.; Nezich, D.; Rodriguez-Nieva, J.F.; Dresselhaus, M.; Palacios, T.; et al. Synthesis of Monolayer Hexagonal Boron Nitride on Cu Foil Using Chemical Vapor Deposition. Nano Lett. 2012, 12, 161–166. [Google Scholar] [CrossRef]
- Lopes, J.M.J. Synthesis of hexagonal boron nitride: From bulk crystals to atomically thin films. Prog. Cryst. Growth Charact. Mater. 2021, 67, 100522. [Google Scholar] [CrossRef]
- Ismach, A.; Chou, H.; Ferrer, D.A.; Wu, Y.; McDonnell, S.; Floresca, H.C.; Covacevich, A.; Pope, C.; Piner, R.; Kim, M.J.; et al. Toward the Controlled Synthesis of Hexagonal Boron Nitride Films. ACS Nano 2012, 6, 6378–6385. [Google Scholar] [CrossRef]
- Singhal, R.; Echeverria, E.; McIlroy, D.N.; Singh, R.N. Synthesis of hexagonal boron nitride films on silicon and sapphire substrates by low-pressure chemical vapor deposition. Thin Solid Films 2021, 733, 138812. [Google Scholar] [CrossRef]
- Echeverría, J.C.; Moriones, P.; Garrido, J.J.; Ugarte, M.D.; Cervera, L.; Garaio, E.; Gómez-Polo, C.; Pérez-Landazábal, J.I. Steering the synthesis of Fe3O4 nanoparticles under sonication by using a fractional factorial design. Mater. Chem. Phys. 2021, 270, 124760. [Google Scholar] [CrossRef]
- Mosquera-Romero, S.; Anaya-Garzon, J.; Garcia-Timermans, C.; Van Dorpe, J.; Hoorens, A.; Commenges-Bernole, N.; Verbeken, K.; Rabaey, K.; Varia, J. Combined Gold Recovery and Nanoparticle Synthesis in Microbial Systems Using Fractional Factorial Design. Nanomaterials 2023, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Rocha, L.S.; Sousa, É.M.L.; Gil, M.V.; Oliveira, J.A.B.P.; Otero, M.; Esteves, V.I.; Calisto, V. Producing Magnetic Nanocomposites from Paper Sludge for the Adsorptive Removal of Pharmaceuticals from Water—A Fractional Factorial Design. Nanomaterials 2021, 11, 287. [Google Scholar] [CrossRef]
- Chinatangkul, N.; Pengon, S.; Limmatvapirat, C.; Limmatvapirat, S. Design of Experiment Approach for Fabrication Process of Electrospun Shellac Nanofibers Using Factorial Designs. Key Eng. Mater. 2017, 757, 120–124. [Google Scholar] [CrossRef]
- Pinto, A.H. The Importance of Factorial Design of Experiments in Functional Nanomaterials Preparation and Performance. In Functional Properties of Advanced Engineering Materials and Biomolecules; La Porta, F.A., Taft, C.A., Eds.; Engineering Materials; Springer International Publishing: Cham, Switzerland, 2021; pp. 387–438. ISBN 978-3-030-62226-8. [Google Scholar]
- Arciola, C.R.; Campoccia, D.; Montanaro, L. Implant infections: Adhesion, biofilm formation and immune evasion. Nat. Rev. Microbiol. 2018, 16, 397–409. [Google Scholar] [CrossRef]
- Zhao, S.; Zhou, F.; Li, Z.; Liu, H. Effect of precursor purity and flow rate on the CVD growth of hexagonal boron nitride. J. Alloys Compd. 2016, 688, 1006–1012. [Google Scholar] [CrossRef]
- Liu, H.; You, C.Y.; Li, J.; Galligan, P.R.; You, J.; Liu, Z.; Cai, Y.; Luo, Z. Synthesis of hexagonal boron nitrides by chemical vapor deposition and their use as single photon emitters. Nano Mater. Sci. 2021, 3, 291–312. [Google Scholar] [CrossRef]
- Babenko, V.; Lane, G.; Koos, A.A.; Murdock, A.T.; So, K.; Britton, J.; Meysami, S.S.; Moffat, J.; Grobert, N. Time-dependent decomposition of ammonia borane for the controlled production of 2D hexagonal boron nitride. Sci. Rep. 2017, 7, 14297. [Google Scholar] [CrossRef]
- Wang, X.; Hooper, T.N.; Kumar, A.; Priest, I.K.; Sheng, Y.; Samuels, T.O.M.; Wang, S.; Robertson, A.W.; Pacios, M.; Bhaskaran, H.; et al. Oligomeric aminoborane precursors for the chemical vapour deposition growth of few-layer hexagonal boron nitride. CrystEngComm 2017, 19, 285–294. [Google Scholar] [CrossRef]
- LaMarche, C.Q.; Leadley, S.; Liu, P.; Kellogg, K.M.; Hrenya, C.M. Method of quantifying surface roughness for accurate adhesive force predictions. Chem. Eng. Sci. 2017, 158, 140–153. [Google Scholar] [CrossRef]
- Horcas, I.; Fernández, R.; Gómez-Rodríguez, J.M.; Colchero, J.; Gómez-Herrero, J.; Baro, A.M. WSXM: A software for scanning probe microscopy and a tool for nanotechnology. Rev. Sci. Instrum. 2007, 78, 013705. [Google Scholar] [CrossRef]
- Bresnehan, M.S.; Hollander, M.J.; Wetherington, M.; LaBella, M.; Trumbull, K.A.; Cavalero, R.; Snyder, D.W.; Robinson, J.A. Integration of Hexagonal Boron Nitride with Quasi-freestanding Epitaxial Graphene: Toward Wafer-Scale, High-Performance Devices. ACS Nano 2012, 6, 5234–5241. [Google Scholar] [CrossRef]
- Bhimanapati, G.R.; Glavin, N.R.; Robinson, J.A. Chapter Three-2D Boron Nitride: Synthesis and Applications. In Semiconductors and Semimetals; Iacopi, F., Boeckl, J.J., Jagadish, C., Eds.; 2D Materials; Elsevier: Amsterdam, The Netherlands, 2016; Volume 95, pp. 101–147. [Google Scholar]
- Khan, M.H.; Liu, H.K.; Sun, X.; Yamauchi, Y.; Bando, Y.; Golberg, D.; Huang, Z. Few-atomic-layered hexagonal boron nitride: CVD growth, characterization, and applications. Mater. Today 2017, 20, 611–628. [Google Scholar] [CrossRef]
- Song, X.; Gao, J.; Nie, Y.; Gao, T.; Sun, J.; Ma, D.; Li, Q.; Chen, Y.; Jin, C.; Bachmatiuk, A.; et al. Chemical vapor deposition growth of large-scale hexagonal boron nitride with controllable orientation. Nano Res. 2015, 8, 3164–3176. [Google Scholar] [CrossRef]
- Watson, A.J.; Lu, W.; Guimarães, M.H.D.; Stöhr, M. Transfer of large-scale two-dimensional semiconductors: Challenges and developments. 2D Mater. 2021, 8, 032001. [Google Scholar] [CrossRef]
- Chen, T.-A.; Chuu, C.-P.; Tseng, C.-C.; Wen, C.-K.; Wong, H.-S.P.; Pan, S.; Li, R.; Chao, T.-A.; Chueh, W.-C.; Zhang, Y.; et al. Wafer-scale single-crystal hexagonal boron nitride monolayers on Cu (111). Nature 2020, 579, 219–223. [Google Scholar] [CrossRef]
- Tay, R.Y. Chemical Vapor Deposition Growth and Characterization of Two-Dimensional Hexagonal Boron Nitride; Springer Theses; Springer: Singapore, 2018; ISBN 978-981-10-8808-7. [Google Scholar]
- Gorbachev, R.V.; Riaz, I.; Nair, R.R.; Jalil, R.; Britnell, L.; Belle, B.D.; Hill, E.W.; Novoselov, K.S.; Watanabe, K.; Taniguchi, T.; et al. Hunting for Monolayer Boron Nitride: Optical and Raman Signatures. Small 2011, 7, 465–468. [Google Scholar] [CrossRef]
- Chandni, U.; Watanabe, K.; Taniguchi, T.; Eisenstein, J.P. Evidence for Defect-Mediated Tunneling in Hexagonal Boron Nitride-Based Junctions. Nano Lett. 2015, 15, 7329–7333. [Google Scholar] [CrossRef]
- Chen, V.; Shin, Y.C.; Mikheev, E.; Lin, Q.; Martis, J.; Zhang, Z.; Chatterjee, S.; Majumdar, A.; Wong, H.-S.P.; Goldhaber-Gordon, D.; et al. Application-driven synthesis and characterization of hexagonal boron nitride deposited on metals and carbon nanotubes. 2D Mater. 2021, 8, 045024. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.; Liu, J.; Zhang, Y.; Xie, Y. Large-Scale Synthesis h-BN Films on Copper-Nickel Alloy by Atmospheric Pressure Chemical Vapor Deposition. Crystals 2022, 12, 985. [Google Scholar] [CrossRef]
- Huhtamäki, T.; Tian, X.; Korhonen, J.T.; Ras, R.H.A. Surface-wetting characterization using contact-angle measurements. Nat. Protoc. 2018, 13, 1521–1538. [Google Scholar] [CrossRef]
- Song, K.; Lee, J.; Choi, S.-O.; Kim, J. Interaction of Surface Energy Components between Solid and Liquid on Wettability, and Its Application to Textile Anti-Wetting Finish. Polymers 2019, 11, 498. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Altenried, S.; Zogg, A.; Zuber, F.; Maniura-Weber, K.; Ren, Q. Role of the Surface Nanoscale Roughness of Stainless Steel on Bacterial Adhesion and Microcolony Formation. ACS Omega 2018, 3, 6456–6464. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.H.; Huang, Z.; Xiao, F.; Casillas, G.; Chen, Z.; Molino, P.J.; Liu, H.K. Synthesis of Large and Few Atomic Layers of Hexagonal Boron Nitride on Melted Copper. Sci. Rep. 2015, 5, 7743. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Ma, H.; Hossain, M.; Zhong, M.; Xia, Q.; Li, B.; Duan, X. Substrates in the Synthesis of Two-Dimensional Materials via Chemical Vapor Deposition. Chem. Mater. 2020, 32, 10321–10347. [Google Scholar] [CrossRef]
- Ji, Y.; Calderon, B.; Han, Y.; Cueva, P.; Jungwirth, N.R.; Alsalman, H.A.; Hwang, J.; Fuchs, G.D.; Muller, D.A.; Spencer, M.G. Chemical Vapor Deposition Growth of Large Single-Crystal Mono-, Bi-, Tri-Layer Hexagonal Boron Nitride and Their Interlayer Stacking. ACS Nano 2017, 11, 12057–12066. [Google Scholar] [CrossRef]
- Golberg, D.; Bando, Y.; Huang, Y.; Terao, T.; Mitome, M.; Tang, C.; Zhi, C. Boron Nitride Nanotubes and Nanosheets. ACS Nano 2010, 4, 2979–2993. [Google Scholar] [CrossRef] [PubMed]
- Auwärter, W. Hexagonal boron nitride monolayers on metal supports: Versatile templates for atoms, molecules and nanostructures. Surf. Sci. Rep. 2019, 74, 1–95. [Google Scholar] [CrossRef]
- Tay, R.Y.; Griep, M.H.; Mallick, G.; Tsang, S.H.; Singh, R.S.; Tumlin, T.; Teo, E.H.T.; Karna, S.P. Growth of Large Single-Crystalline Two-Dimensional Boron Nitride Hexagons on Electropolished Copper. Nano Lett. 2014, 14, 839–846. [Google Scholar] [CrossRef]
- Oh, H.; Jo, J.; Tchoe, Y.; Yoon, H.; Hwi Lee, H.; Kim, S.-S.; Kim, M.; Sohn, B.-H.; Yi, G.-C. Centimeter-sized epitaxial h-BN films. NPG Asia Mater. 2016, 8, e330. [Google Scholar] [CrossRef]
- Cherian, C.T.; Giustiniano, F.; Martin-Fernandez, I.; Andersen, H.; Balakrishnan, J.; Özyilmaz, B. ‘Bubble-Free’ Electrochemical Delamination of CVD Graphene Films. Small 2015, 11, 189–194. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Xu, X.; Dubuisson, E.; Bao, Q.; Lu, J.; Loh, K.P. Electrochemical Delamination of CVD-Grown Graphene Film: Toward the Recyclable Use of Copper Catalyst. ACS Nano 2011, 5, 9927–9933. [Google Scholar] [CrossRef]
- Sun, J.; Fan, X.; Guo, W.; Liu, L.; Liu, X.; Deng, J.; Xu, C. Mechanism of Electrochemical Delamination of Two-Dimensional Materials from Their Native Substrates by Bubbling. Sensors 2015, 15, 31811–31820. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, X.; Zhan, Z.; Guo, W.; Xu, C.; Deng, J.; Chakarov, D.; Hyldgaard, P.; Schröder, E.; Yurgens, A.; et al. A Mechanism for Highly Efficient Electrochemical Bubbling Delamination of CVD-Grown Graphene from Metal Substrates. Adv. Mater. Interfaces 2016, 3, 1500492. [Google Scholar] [CrossRef]
- Magnuson, C.W.; Kong, X.; Ji, H.; Tan, C.; Li, H.; Piner, R.; Ventrice, C.A.; Ruoff, R.S. Copper oxide as a “self-cleaning” substrate for graphene growth. J. Mater. Res. 2014, 29, 403–409. [Google Scholar] [CrossRef]
- Çelik, Y.; Escoffier, W.; Yang, M.; Flahaut, E.; Suvacı, E. Relationship between heating atmosphere and copper foil impurities during graphene growth via low pressure chemical vapor deposition. Carbon 2016, 109, 529–541. [Google Scholar] [CrossRef]
- Mazhar, H.; Adamson, D.H.; Al-Harthi, M.A. Differently oxidized portions of functionalized hexagonal boron nitride. Mater. Chem. Phys. 2023, 308, 128243. [Google Scholar] [CrossRef]
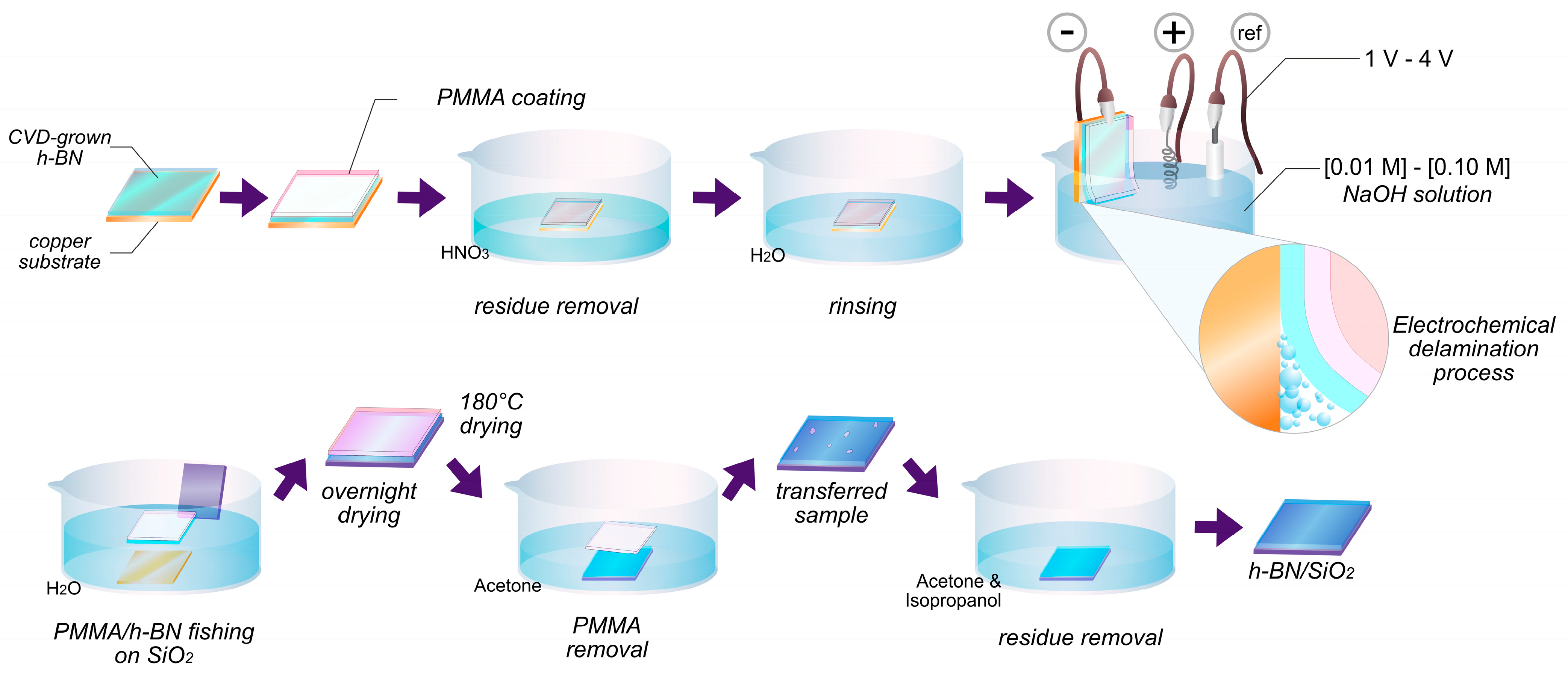


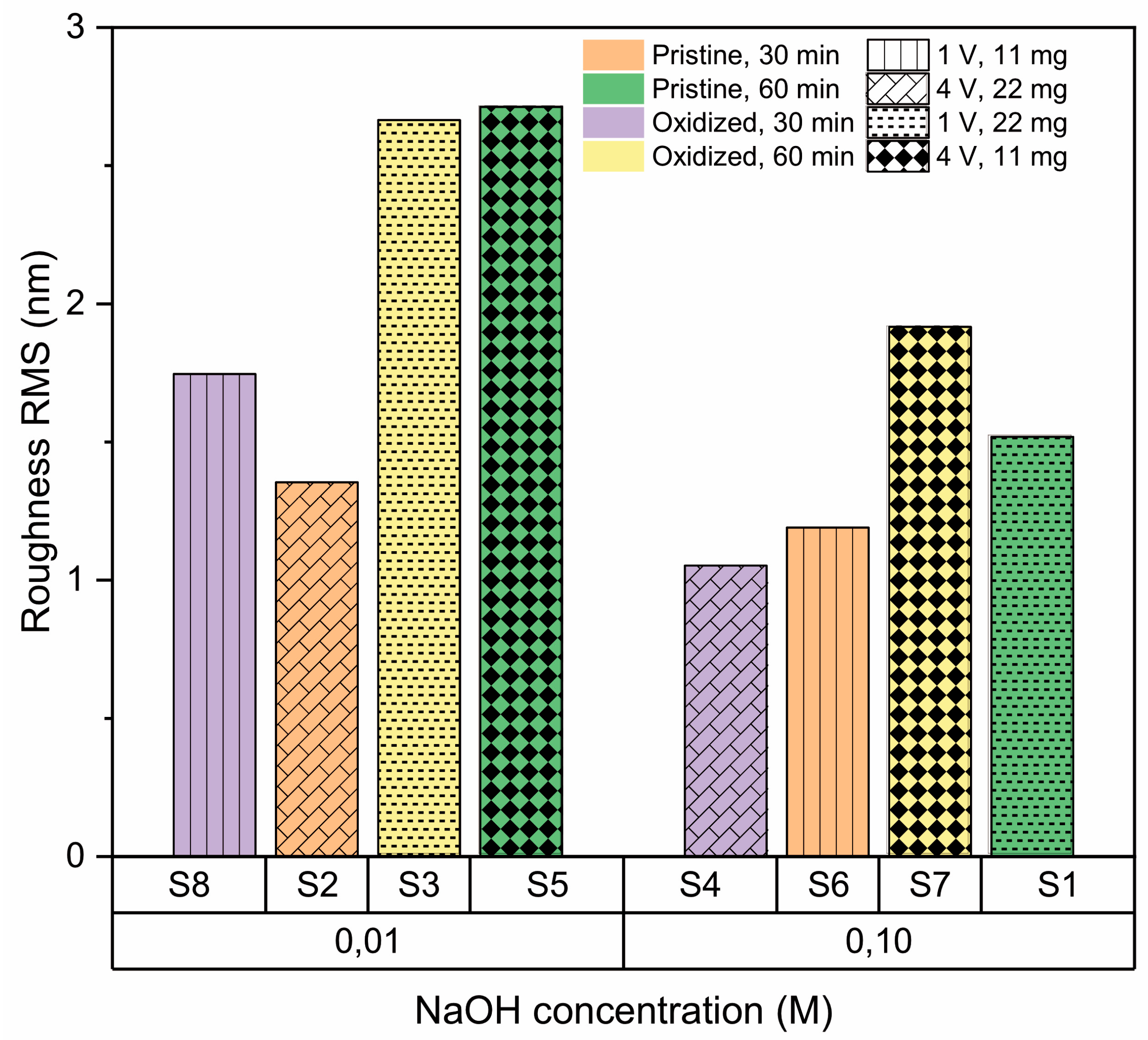
| Sample | A—Amount of Precursor (mg) | B—Condition of Substrate | C—Synthesis Time (min) | D—Transfer Voltage (V) | E—NaOH Concentration (M) |
|---|---|---|---|---|---|
| S1 | 22 | Pristine | 60 | 1 | 0.10 |
| S2 | 22 | Pristine | 30 | 4 | 0.01 |
| S3 | 22 | Oxidized | 60 | 1 | 0.01 |
| S4 | 22 | Oxidized | 30 | 4 | 0.10 |
| S5 | 11 | Pristine | 60 | 4 | 0.01 |
| S6 | 11 | Pristine | 30 | 1 | 0.10 |
| S7 | 11 | Oxidized | 60 | 4 | 0.10 |
| S8 | 11 | Oxidized | 30 | 1 | 0.01 |
| Parameters in Equation (1) | Effects Considered |
|---|---|
| I − ACD − BDE | |
| A − CD + BCE | |
| B − DE + ACE | |
| C − AD + ABE | |
| D − AC − BE | |
| E − BD + ABC | |
| AB + CE − ADE − BCD | |
| AE + BC − ABD − CDE |
| Sample | A (mg) | B | C (min) | D (V) | E (M) | Roughness RMS (nm) | Contact Angle (°) | RAMAN (cm−1) |
|---|---|---|---|---|---|---|---|---|
| S1 | 22 | Pristine | 60 | 1 | 0.10 | 1.52 ± 0.21 | 75.0 ± 0.1 | 1366 |
| S2 | 22 | Pristine | 30 | 4 | 0.01 | 1.35 ± 0.26 | 107.7 ± 1.1 | 1373 |
| S3 | 22 | Oxidized | 60 | 1 | 0.01 | 2.66 ± 0.36 | 74.5 ± 1.9 | 1365 |
| S4 | 22 | Oxidized | 30 | 4 | 0.10 | 1.05 ± 0.07 | 68.3 ± 4.4 | 1372 |
| S5 | 11 | Pristine | 60 | 4 | 0.01 | 2.71 ± 0.53 | 74.5 ± 1.9 | 1363 |
| S6 | 11 | Pristine | 30 | 1 | 0.10 | 1.19 ± 0.15 | 108.8 ± 0.5 | 1374 |
| S7 | 11 | Oxidized | 60 | 4 | 0.10 | 1.92 ± 0.31 | 81.2 ± 0.6 | 1362 |
| S8 | 11 | Oxidized | 30 | 1 | 0.01 | 1.75 ± 0.05 | 70.6 ± 0.4 | 1369 |
| Source | DF | Contribution | SC Ajust. | MC Ajust. | F-Value |
|---|---|---|---|---|---|
| Model | 7 | 83.83% | 16.78 | 2.40 | 29.61 |
| Lineal | 5 | 79.38% | 15.89 | 3.18 | 39.26 |
| A | 1 | 3.56% | 0.71 | 0.71 | 8.80 |
| B | 1 | 1.37% | 0.27 | 0.28 | 3.40 |
| C | 1 | 45.08% | 9.03 | 9.03 | 111.48 |
| D | 1 | 0.03% | 0.01 | 0.01 | 0.06 |
| E | 1 | 29.34% | 5.88 | 5.88 | 72.56 |
| Two-term interactions | 2 | 4.44% | 0.89 | 0.44 | 5.50 |
| A×B | 1 | 4.41% | 0.88 | 0.88 | 10.91 |
| A×E | 1 | 0.03% | 0.01 | 0.01 | 0.08 |
| Error | 40 | 16.17% | 3.24 | 0.08 | |
| Total | 47 | 100.00% |
| Case | A (mg) | B | C (min) | D (M) | Roughness RMS (nm) |
|---|---|---|---|---|---|
| Minimum | 22 | Pristine | 30 | 0.10 | 0.63 |
| Maximum | 11 | Pristine | 60 | 0.01 | 2.73 |
| Source | DF | Contribution | SC Ajust. | MC Ajust. | F-Value |
|---|---|---|---|---|---|
| Model | 7 | 98.96% | 5559.13 | 794.16 | 217.25 |
| Lineal | 5 | 51.65% | 2901.51 | 580.30 | 158.74 |
| A | 1 | 0.62% | 34.67 | 34.67 | 9.48 |
| B | 1 | 34.00% | 1909.99 | 1909.99 | 522.48 |
| C | 1 | 16.75% | 940.98 | 940.98 | 257.41 |
| D | 1 | 0.05% | 2.93 | 2.93 | 0.80 |
| E | 1 | 0.23% | 12.95 | 12.95 | 3.54 |
| Two-term interactions | 2 | 47.31% | 2657.63 | 1328.81 | 363.50 |
| A*B | 1 | 0.46% | 25.72 | 25.72 | 7.04 |
| A*E | 1 | 46.85% | 2631.91 | 2631.91 | 719.97 |
| Error | 16 | 1.04% | 58.49 | 3.66 | |
| Total | 23 | 100.00% |
| Samples | A (mg) | B | C (min) | D (M) | Contact Angle (°) |
|---|---|---|---|---|---|
| Minimum | 22 | Oxidized | 60 | 0.10 | 55.1 |
| Maximum | 11 | Pristine | 30 | 0.10 | 109.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueroa, H.; Aristizabal, J.; Reinoso-Guerra, E.; Arce, B.; Vargas-Straube, M.J.; Gentil, D.; Ramírez, C.; Cordero, J.; Barrera, N.P.; Parra, C. Fractional Factorial Design to Evaluate the Synthesis and Electrochemical Transfer Parameters of h-BN Coatings. Nanomaterials 2023, 13, 2992. https://doi.org/10.3390/nano13232992
Figueroa H, Aristizabal J, Reinoso-Guerra E, Arce B, Vargas-Straube MJ, Gentil D, Ramírez C, Cordero J, Barrera NP, Parra C. Fractional Factorial Design to Evaluate the Synthesis and Electrochemical Transfer Parameters of h-BN Coatings. Nanomaterials. 2023; 13(23):2992. https://doi.org/10.3390/nano13232992
Chicago/Turabian StyleFigueroa, Helen, Juliet Aristizabal, Elías Reinoso-Guerra, Bárbara Arce, María José Vargas-Straube, Dana Gentil, Cristian Ramírez, José Cordero, Nelson P. Barrera, and Carolina Parra. 2023. "Fractional Factorial Design to Evaluate the Synthesis and Electrochemical Transfer Parameters of h-BN Coatings" Nanomaterials 13, no. 23: 2992. https://doi.org/10.3390/nano13232992
APA StyleFigueroa, H., Aristizabal, J., Reinoso-Guerra, E., Arce, B., Vargas-Straube, M. J., Gentil, D., Ramírez, C., Cordero, J., Barrera, N. P., & Parra, C. (2023). Fractional Factorial Design to Evaluate the Synthesis and Electrochemical Transfer Parameters of h-BN Coatings. Nanomaterials, 13(23), 2992. https://doi.org/10.3390/nano13232992







