Mössbauer Study on the Conversion of Different Iron-Based Catalysts Used in Carbon Nanotube Synthesis
Abstract
:1. Introduction
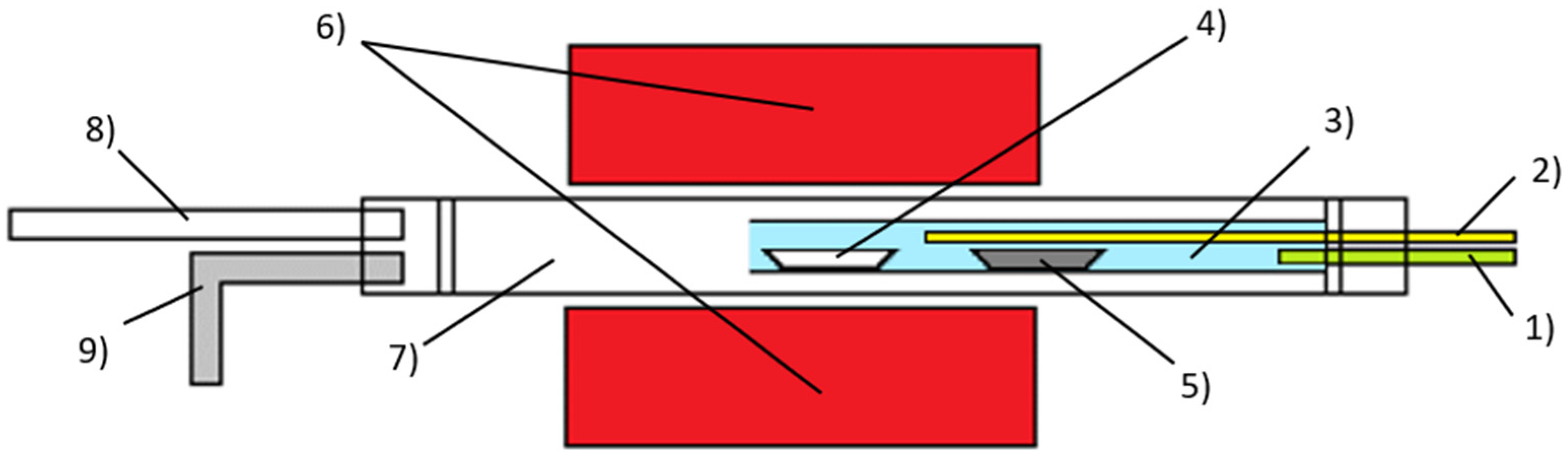
2. Materials and Methods
3. Results and Discussion
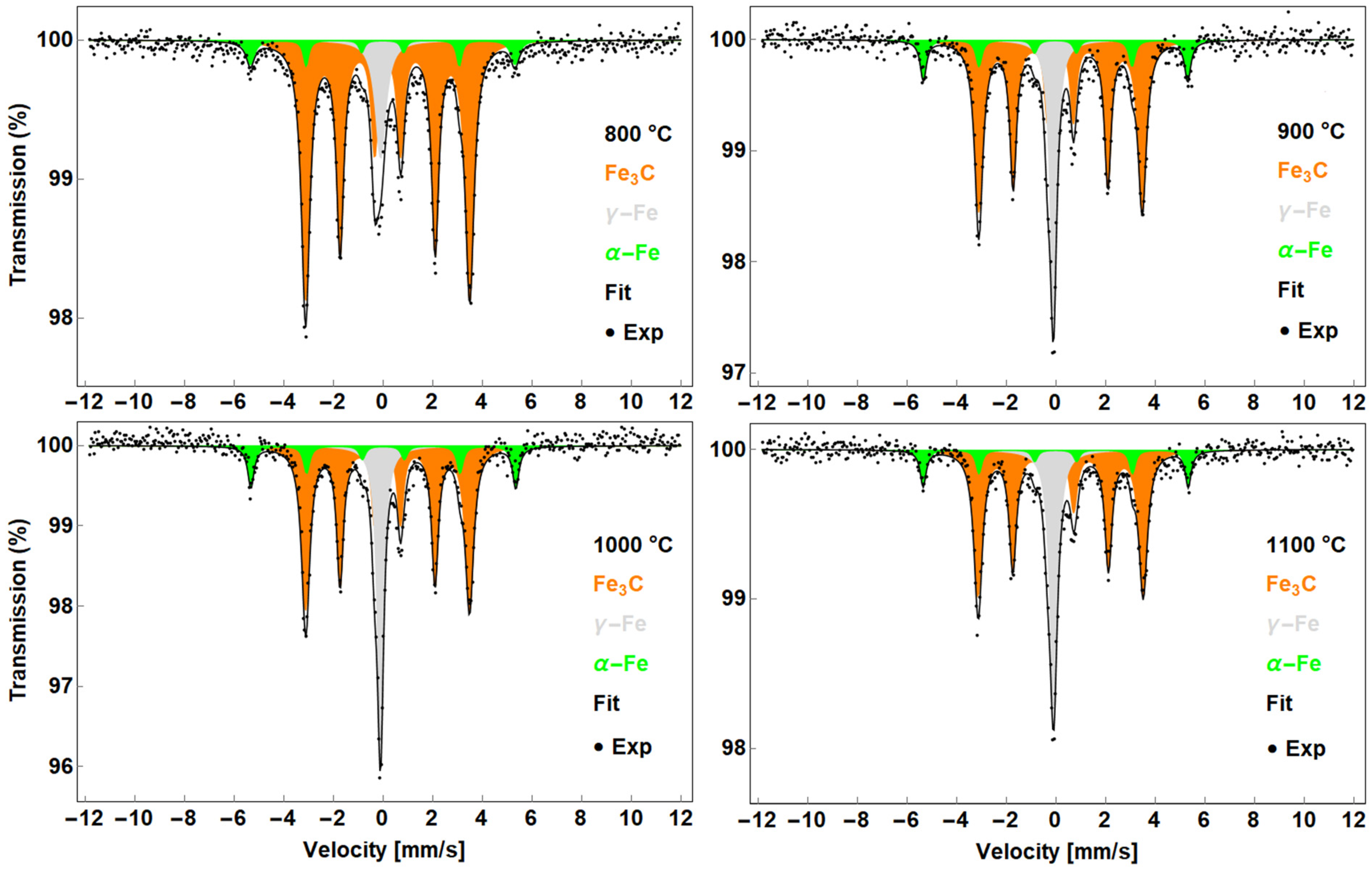
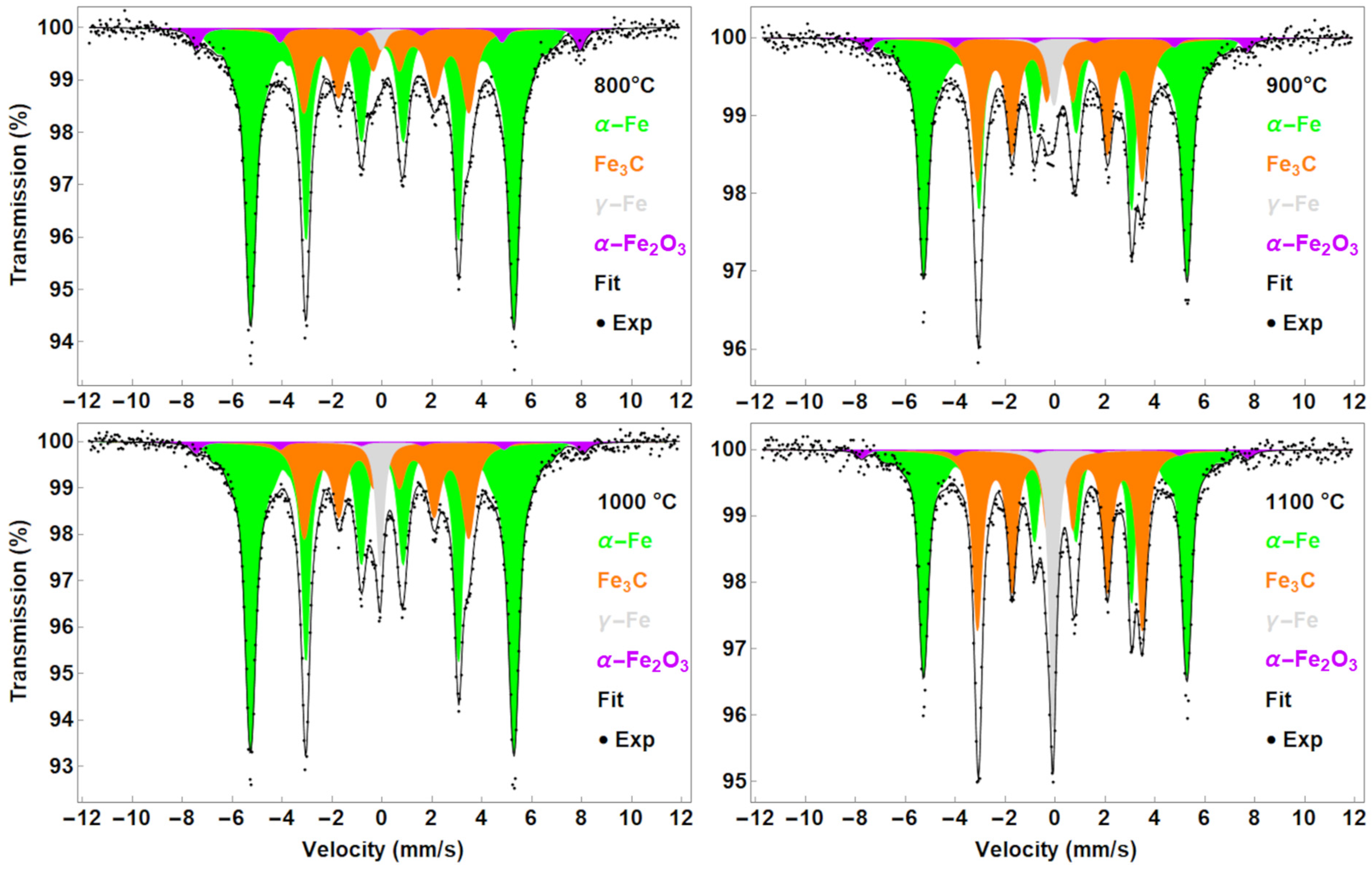
3.1. Mössbauer Spectroscopy
3.1.1. CNTs Synthesised from F + T Solution
3.1.2. CNTs Synthesised from Solid Ferrocene
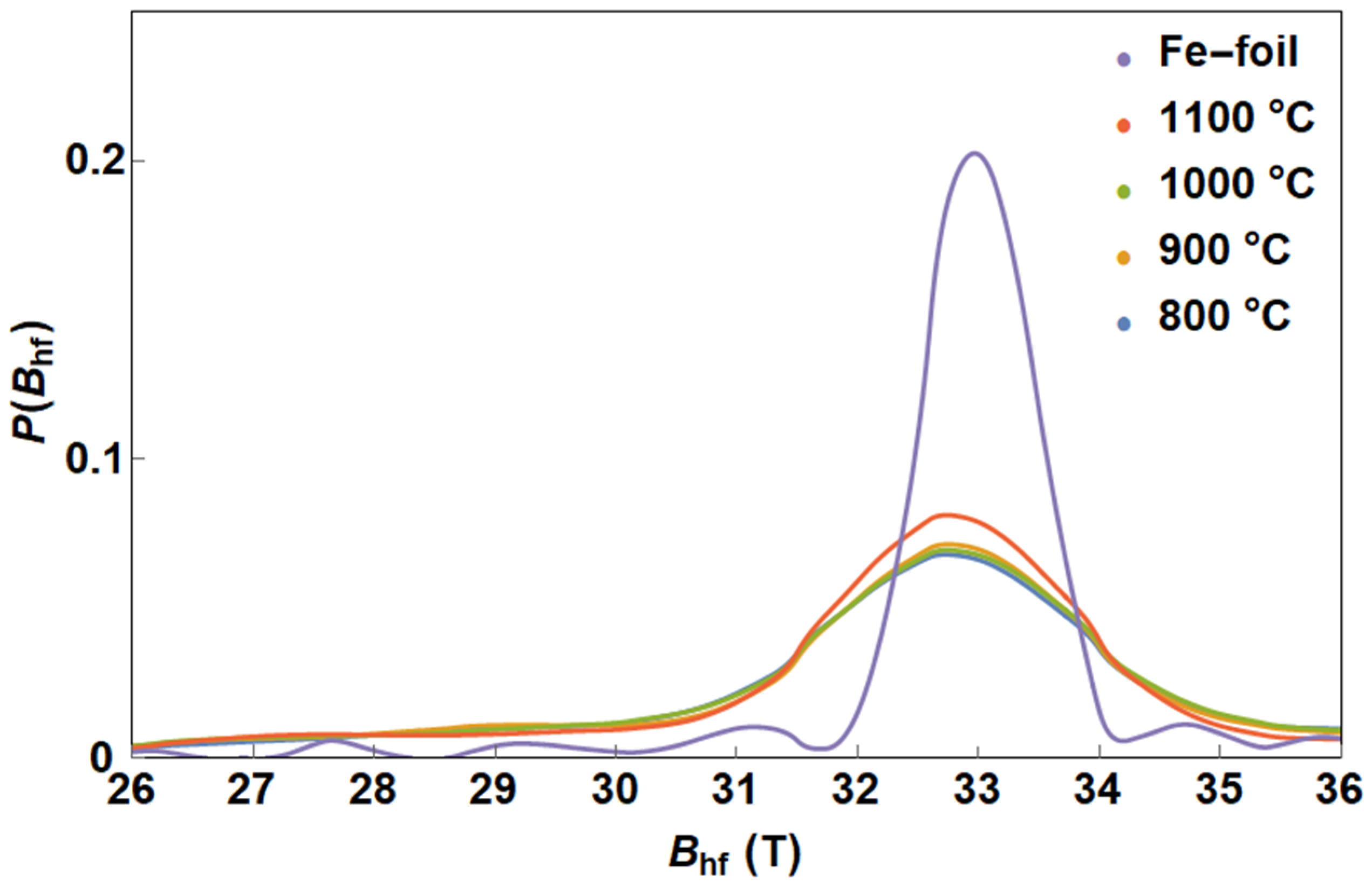
3.1.3. CNTs Synthesised with ZVI Nanoparticle Catalyst
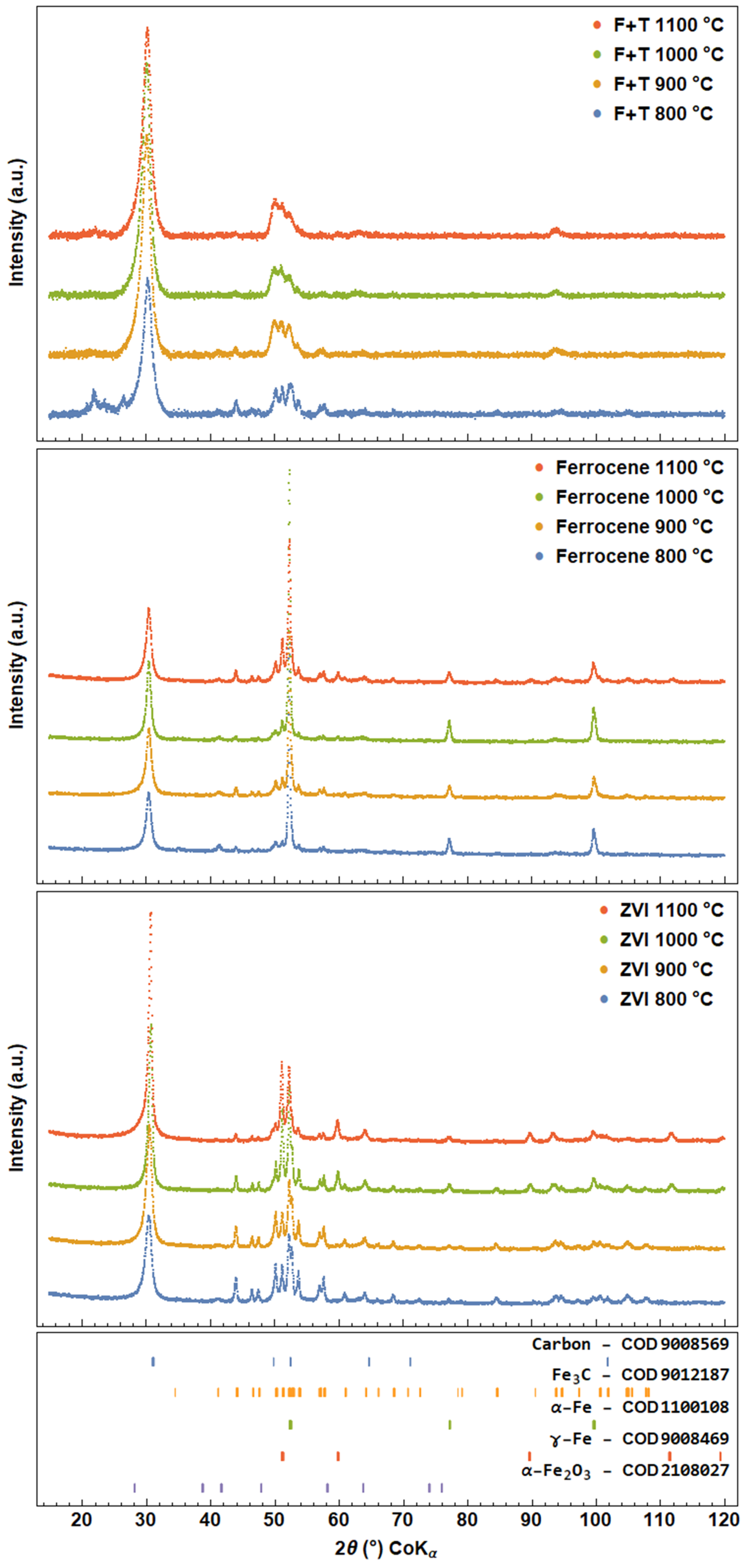
3.2. X-ray Powder Diffraction
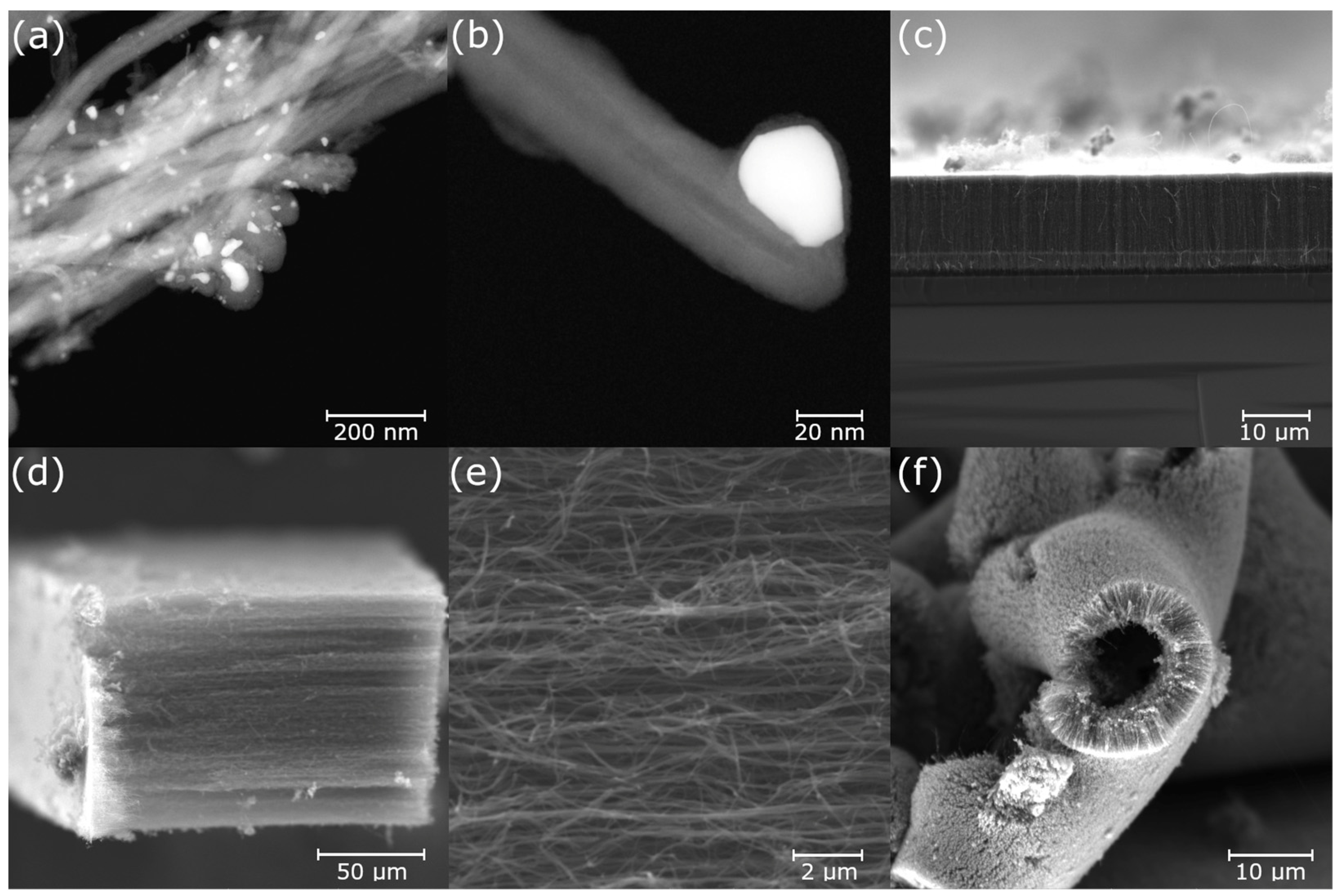
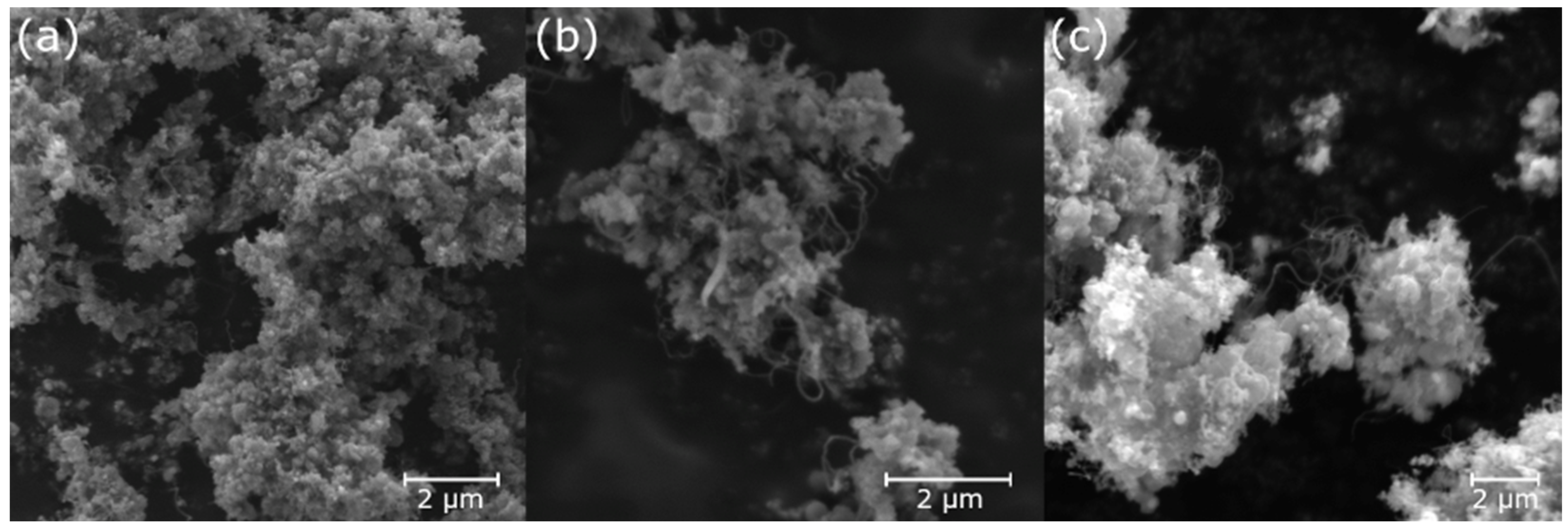
3.3. Electron Microscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harris, P.J.F. Carbon Nanotube Science: Synthesis, Properties and Applications, 3rd ed.; Cambridge University Press: Cambridge, UK, 2009; ISBN 9780511609701. [Google Scholar]
- Pei, B.; Wang, W.; Dunne, N.; Li, X. Applications of Carbon Nanotubes in Bone Tissue Regeneration and Engineering: Superiority, Concerns, Current Advancements, and Prospects. Nanomaterials 2019, 9, 1501. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.; Flahaut, E.; Golzio, M. Overview of Carbon Nanotubes for Biomedical Applications. Materials 2019, 12, 624. [Google Scholar] [CrossRef] [PubMed]
- Norizan, M.N.; Moklis, M.H.; Zulaikha, S.; Demon, N.; Halim, N.A.; Samsuri, A.; Mohamad, I.S.; Knight, V.F.; Abdullah, N. Carbon Nanotubes: Functionalisation and Their Application in Chemical Sensors. RSC Adv. 2020, 10, 43704–43732. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.-M.; Zhang, Z.; Wang, S. Carbon Nanotube Electronics: Recent Advances. Mater. Today 2014, 17, 433–442. [Google Scholar] [CrossRef]
- Sehrawat, P.; Julien, C.; Islam, S.S. Carbon Nanotubes in Li-Ion Batteries: A Review. Mater. Sci. Eng. B 2016, 213, 12–40. [Google Scholar] [CrossRef]
- Ullah Rather, S. Preparation, Characterization and Hydrogen Storage Studies of Carbon Nanotubes and Their Composites: A Review. Int. J. Hydrog. Energy 2020, 45, 4653–4672. [Google Scholar] [CrossRef]
- Nieto, A.; Budan, J.; Gupta, R.K.; Ansell, T.Y. 3D Printed Carbon Nanotube Reinforced Stainless Steel via Selective Laser Melting. MRS Commun. 2022, 12, 578–584. [Google Scholar] [CrossRef]
- Lu, D.; Shi, X.; Zhong, J. Understanding the Role of Unzipped Carbon Nanotubes in Cement Pastes. Cem. Concr. Compos. 2022, 126, 104366. [Google Scholar] [CrossRef]
- Kim, Y.A.; Hayashi, T.; Endo, M.; Dresselhaus, M.S. Carbon Nanofibers. In Springer Handbook of Nanomaterials; Vajtai, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 233–262. ISBN 9783642205958. [Google Scholar]
- Ahmad, M.; Silva, S.R.P. Low Temperature Growth of Carbon Nanotubes—A Review. Carbon 2019, 158, 24–44. [Google Scholar] [CrossRef]
- Hou, G.; Chauhan, D.; Ng, V.; Xu, C.; Yin, Z.; Paine, M.; Su, R.; Shanov, V.; Mast, D.; Schulz, M.; et al. Gas Phase Pyrolysis Synthesis of Carbon Nanotubes at High Temperature. Mater. Des. 2017, 132, 112–118. [Google Scholar] [CrossRef]
- Hata, K.; Futaba, D.N.; Mizuno, K.; Namai, T.; Yumura, M.; Iijima, S. Water-Assisted Highly Efficient Synthesis of Impurity-Free Single-Walled Carbon Nanotubes. Science 2004, 306, 1362–1364. [Google Scholar] [CrossRef] [PubMed]
- Mayne, M.; Grobert, N.; Terrones, M.; Kamalakaran, R.; Rühle, M.; Kroto, H.W.; Walton, D.R.M. Pyrolytic Production of Aligned Carbon Nanotubes from Homogeneously Dispersed Benzene-Based Aerosols. Chem. Phys. Lett. 2001, 338, 101–107. [Google Scholar] [CrossRef]
- Paul, S.; Samdarshi, S.K. A Green Precursor for Carbon Nanotube Synthesis. New Carbon Mater. 2011, 26, 85–88. [Google Scholar] [CrossRef]
- Hiramatsu, M.; Hori, M. Aligned Growth of Single-Walled and Double-Walled Carbon Nanotube Films by Control of Catalyst Preparation. In Carbon Nanotubes—Synthesis, Characterization, Applications; Yellampalli, S., Ed.; Intech: London, UK, 2011; ISBN 9789533074979. [Google Scholar]
- Moisala, A.; Nasibulin, A.G.; Kauppinen, E.I. The Role of Metal Nanoparticles in the Catalytic Production of Single-Walled Carbon—A Review. J. Phys. Condens. Matter 2003, 15, 3011–3035. [Google Scholar] [CrossRef]
- Kumar, M. Carbon Nanotube Synthesis and Growth Mechanism. In Carbon Nanotubes—Synthesis, Characterization, Applications; Yellampalli, S., Ed.; InTech: London, UK, 2011; ISBN 9789533074979. [Google Scholar]
- Yuan, D.; Ding, L.; Chu, H.; Feng, Y.; McNicholas, T.P.; Liu, J. Horizontally Aligned Single-Walled Carbon Nanotube on Quartz from a Large Variety of Metal Catalysts. Nano Lett. 2008, 8, 2576–2579. [Google Scholar] [CrossRef]
- de Jong, K.P.; Geus, J.W. Carbon Nanofibers: Catalytic Synthesis and Applications. Catal. Rev. 2000, 42, 481–510. [Google Scholar] [CrossRef]
- Deck, C.P.; Vecchio, K. Prediction of Carbon Nanotube Growth Success by the Analysis of Carbon–Catalyst Binary Phase Diagrams. Carbon 2006, 44, 267–275. [Google Scholar] [CrossRef]
- Tian, T.; Cheng, Y.; Sun, Z.; Huang, K.; Lei, M.; Tang, H. Carbon Nanotubes Supported Oxygen Reduction Reaction Catalysts: Role of Inner Tubes. Adv. Compos. Hybrid Mater. 2022, 6, 7. [Google Scholar] [CrossRef]
- Gupta, V.K.; Saleh, T.A. Sorption of Pollutants by Porous Carbon, Carbon Nanotubes and Fullerene—An Overview. Environ. Sci. Pollut. Res. 2013, 20, 2828–2843. [Google Scholar] [CrossRef]
- Kumar, R.; Sahoo, B. One-Step Pyrolytic Synthesis and Growth Mechanism of Core-Shell Type Fe/Fe 3 C-Graphite Nanoparticles-Embedded Carbon Globules. Nano-Struct. Nano-Objects 2018, 16, 77–85. [Google Scholar] [CrossRef]
- Ruskov, T.; Spirov, I.; Ritschel, M.; Müller, C.; Leonhardt, A.; Ruskov, R. Mössbauer Morphological Analysis of Fe-Filled Multiwalled Carbon Nanotube Samples. J. Appl. Phys. 2006, 100, 84326. [Google Scholar] [CrossRef]
- Oshima, H.; Shimazu, T.; Siry, M.; Mibu, K. Analysis of Fe Catalyst during Carbon Nanotube Synthesis by Mössbauer Spectroscopy. J. Phys. Chem. C 2009, 113, 18523–18526. [Google Scholar] [CrossRef]
- Mendonça, F.G.; Ardisson, J.D.; Rosmaninho, M.G.; Lago, R.M.; Tristão, J.C. Mössbauer Study of Carbon Coated Iron Magnetic Nanoparticles Produced by Simultaneous Reduction/Pyrolysis. Hyperfine Interact. 2011, 202, 123–129. [Google Scholar] [CrossRef]
- Stejskal, A.; Procházka, V.; Novák, P.; Dudka, M. Mössbauer Spectrometer Designed for Measurements of Fast Processes. Nucl. Instrum. Methods Phys. Res. A 2020, 984, 164597. [Google Scholar] [CrossRef]
- Klencsar, Z.; Kuzmann, E.; Vertes, A. User-Friendly Software for Mössbauer Spectrum Analysis. J. Radioanal. Nucl. Chem. 1996, 210, 105–118. [Google Scholar] [CrossRef]
- Sajitha, E.P.; Prasad, V.; Subramanyam, S.V.; Kumar Mishra, A.; Sarkar, S.; Bansal, C. Structural, Magnetic and Mössbauer Studies of Iron Inclusions in a Carbon Matrix. J. Magn. Magn. Mater. 2007, 313, 329–336. [Google Scholar] [CrossRef]
- Cook, D.C. Strain Induced Martensite Formation in Stainless Steel. Metall. Trans. A 1987, 18, 201–210. [Google Scholar] [CrossRef]
- Lyubutin, I.S.; Anosova, O.A.; Frolov, K.V.; Sulyanov, S.N.; Okotrub, A.V.; Kudashov, A.G.; Bulusheva, L.G. Iron Nanoparticles in Aligned Arrays of Pure and Nitrogen-Doped Carbon Nanotubes. Carbon 2012, 50, 2628–2634. [Google Scholar] [CrossRef]
- Baker, R.T.K. Catalytic Growth of Carbon Filaments. Carbon 1989, 27, 315–323. [Google Scholar] [CrossRef]
- Zboril, R.; Mashlan, M.; Petridis, D. Iron(III) oxides from thermal processes-synthesis, structural and magnetic properties, Mössbauer spectroscopy characterization, and applications. Chem. Mater. 2002, 14, 969–982. [Google Scholar] [CrossRef]
- Futaba, D.N.; Yamada, T.; Kobashi, K.; Yumura, M.; Hata, K. Macroscopic Wall Number Analysis of Single-Walled, Double-Walled, and Few-Walled Carbon Nanotubes by X-ray Diffraction. J. Am. Chem. Soc. 2011, 133, 5716–5719. [Google Scholar] [CrossRef]
- Abdel-Ghani, N.T.; El-Chaghaby, G.A.; Helal, F.S. Individual and Competitive Adsorption of Phenol and Nickel onto Multiwalled Carbon Nanotubes. J. Adv. Res. 2015, 6, 405–415. [Google Scholar] [CrossRef]
- Ban, F.Y.; Majid, S.R.; Huang, N.M.; Lim, H.N. Graphene Oxide and Its Electrochemical Performance. Int. J. Electrochem. Sci. 2012, 7, 4345–4351. [Google Scholar] [CrossRef]
- Venkatesan, S.; Visvalingam, B.; Mannathusamy, G.; Viswanathan, V.; Rao, A.G. Effect of Chemical Vapor Deposition Parameters on the Diameter of Multi-Walled Carbon Nanotubes. Int. Nano Lett. 2018, 8, 297–308. [Google Scholar] [CrossRef]
- Pérez-Cabero, M.; Taboada, J.B.; Guerrero-Ruiz, A.; Overweg, A.R.; Rodríguez-Ramos, I. The role of alpha-iron and cementite phases in the growing mechanism of carbon nanotubes: A 57Fe Mössbauer spectroscopy study. Phys. Chem. Chem. Phys. 2006, 8, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Venkataraman, A.; Amadi, V.; Chen, Y.; Papadopoulos, C. Carbon Nanotube Assembly and Integration for Applications. Nanoscale Res. Lett. 2019, 14, 220. [Google Scholar] [CrossRef] [PubMed]
- Lima, M.D.; Bonadiman, R.; De Andrade, M.J.; Toniolo, J.; Bergmann, C.P. Synthesis of Multi-Walled Carbon Nanotubes by Catalytic Chemical Vapor Deposition Using Cr2−xFexO3 as Catalyst. Diam. Relat. Mater. 2006, 15, 1708–1713. [Google Scholar] [CrossRef]
- Nessim, G.D.; Hart, A.J.; Kim, J.S.; Acquaviva, D.; Oh, J.; Morgan, C.D.; Seita, M.; Leib, J.S.; Thompson, C.V. Tuning of Vertically-Aligned Carbon Nanotube Diameter and Areal Density through Catalyst Pre-Treatment. Nano Lett. 2008, 8, 3587–3593. [Google Scholar] [CrossRef]

| Temperature | Phase | IS (mm/s) | FWHM (mm/s) | B (T) | A (%) |
|---|---|---|---|---|---|
| 800 °C | Fe3C | 0.19 ± 0.01 | (3, 4) 0.34 ± 0.01 | 20.5 ± 0.5 | 82 ± 2 |
| (2, 5) 0.35 ± 0.01 | |||||
| (1, 6) 0.43 ± 0.01 | |||||
| α-Fe | 0.01 ± 0.01 | (3, 4) 0.26 ± 0.07 | 33.1 ± 0.5 | 7 ± 2 | |
| (2, 5) 0.26 ± 0.04 | |||||
| (1, 6) 0.41 ± 0.06 | |||||
| γ-Fe | −0.10 ± 0.01 | 0.51 ± 0.02 | - | 11 ± 2 | |
| 900 °C | Fe3C | 0.18 ± 0.01 | (3, 4) 0.29 ± 0.01 | 20.5 ± 0.5 | 67 ± 2 |
| (2, 5) 0.32 ± 0.01 | |||||
| (1, 6) 0.41 ± 0.01 | |||||
| α-Fe | 0.02 ± 0.01 | 0.30 ± 0.03 | 33.1 ± 0.5 | 12 ± 2 | |
| γ-Fe | −0.10 ± 0.01 | 0.32 ± 0.01 | - | 21 ± 2 | |
| 1000 °C | Fe3C | 0.18 ± 0.01 | (3, 4) 0.29 ± 0.01 | 20.5 ± 0.5 | 65 ± 2 |
| (2, 5) 0.32 ± 0.01 | |||||
| (1, 6) 0.38 ± 0.01 | |||||
| α-Fe | 0.01 ± 0.01 | 0.28 * | 33.3 ± 0.5 | 12 ± 2 | |
| γ-Fe | −0.10 ± 0.01 | 0.38 ± 0.02 | - | 23 ± 2 | |
| 1100 °C | Fe3C | 0.19 ± 0.01 | (3, 4) 0.34 ± 0.02 | 20.7 ± 0.5 | 64 ± 2 |
| (2, 5) 0.35 ± 0.01 | |||||
| (1, 6) 0.42 ± 0.01 | |||||
| α-Fe | 0.01 ± 0.01 | 0.29 ± 0.03 | 33.2 ± 0.5 | 11 ± 2 | |
| γ-Fe | −0.09 ± 0.01 | 0.37 ± 0.01 | - | 25 ± 2 |
| Temperature | Phase | IS (mm/s) | QS/ε (mm/s) | FWHM (mm/s) | B (T) | A (%) |
|---|---|---|---|---|---|---|
| 800 °C | Fe3C | 0.16 ± 0.01 | - | (3, 4) 0.50 ± 0.04 | 20.5 ± 0.5 | 25 ± 2 |
| (2, 5) 0.68 ± 0.03 | ||||||
| (1, 6) 0.65 ± 0.03 | ||||||
| α-Fe | 0.00 ± 0.01 | - | (3, 4) 0.47 ± 0.01 | 33.1 * | 70 ± 2 | |
| (2, 5) 0.18 ± 0.03 | ||||||
| (1, 6) 0.17 ± 0.05 | ||||||
| γ-Fe | −0.05 ± 0.01 | - | 0.44 ± 0.11 | - | 1 ± 2 | |
| α-Fe2O3 | 0.30 ± 0.01 | −0.10 ± 0.01 | 0.47 * | 47.7 ± 0.5 | 4 ± 2 | |
| 900 °C | Fe3C | 0.18 ± 0.01 | - | (3, 4) 0.45 ± 0.03 | 20.5 ± 0.5 | 36 ± 2 |
| (2, 5) 0.51 ± 0.01 | ||||||
| (1, 6) 0.54 ± 0.02 | ||||||
| α-Fe | 0.00 ± 0.01 | - | (3, 4) 0.44 ± 0.02 | 33.0 * | 57 ± 2 | |
| (2, 5) 0.17 ± 0.04 | ||||||
| (1, 6) 0.17 ± 0.07 | ||||||
| γ-Fe | −0.06 ± 0.01 | - | 0.51 ± 0.04 | - | 4 ± 2 | |
| α-Fe2O3 | 0.23 ± 0.01 | −0.29 ± 0.01 | 0.47 * | 47.2 ± 0.5 | 3 ± 2 | |
| 1000 °C | Fe3C | 0.17 ± 0.01 | - | (3, 4) 0.50 ± 0.03 | 20.5 ± 0.5 | 25 ± 2 |
| (2, 5) 0.60 ± 0.03 | ||||||
| (1, 6) 0.63 ± 0.02 | ||||||
| α-Fe | 0.00 ± 0.01 | - | (3, 4) 0.45 ± 0.01 | 33.0 * | 68 ± 2 | |
| (2, 5) 0.17 ± 0.03 | ||||||
| (1, 6) 0.17 ± 0.05 | ||||||
| γ-Fe | −0.09 ± 0.01 | - | 0.35 ± 0.01 | - | 5 ± 2 | |
| α-Fe2O3 | 0.35 ± 0.01 | −0.10 ± 0.01 | 0.47 * | 48.1 ± 0.5 | 2 ± 2 | |
| 1100 °C | Fe3C | 0.18 ± 0.01 | - | (3, 4) 0.37 ± 0.02 | 20.5 ± 0.5 | 39 ± 2 |
| (2, 5) 0.43 ± 0.01 | ||||||
| (1, 6) 0.46 ± 0.01 | ||||||
| α-Fe | 0.00 ± 0.01 | - | (3, 4) 0.37 ± 0.02 | 32.9 * | 48 ± 2 | |
| (2, 5) 0.15 ± 0.04 | ||||||
| (1, 6) 0.16 ± 0.07 | ||||||
| γ-Fe | −0.10 ± 0.01 | - | 0.34 ± 0.04 | - | 11 ± 2 | |
| α-Fe2O3 | 0.24 ± 0.01 | −0.52 ± 0.01 | 0.47 * | 48.1 ± 0.5 | 2 ± 2 |
| Temperature | Phase | IS (mm/s) | FWHM (mm/s) | B (T) | A (%) |
|---|---|---|---|---|---|
| 800 °C | α-Fe | −0.01 ± 0.01 | (3, 4) 0.33 ± 0.02 | 32.7 ± 0.5 | 5 ± 2 |
| (2, 5) 0.22 ± 0.02 | |||||
| (1, 6) 0.27 ± 0.02 | |||||
| Fe3C | 0.19 ± 0.01 | (3, 4) 0.27 ± 0.01 | 20.3 ± 0.5 | 34 ± 2 | |
| (2, 5) 0.34 ± 0.01 | |||||
| (1, 6) 0.39 ± 0.01 | |||||
| Bcg peak | 0.20 ± 0.01 | 11.99 ± 0.54 | - | 61 ± 2 | |
| 900 °C | α-Fe | −0.01 ± 0.01 | (3, 4) 0.27 ± 0.03 | 32.7 ± 0.5 | 14 ± 2 |
| (2, 5) 0.27 ± 0.02 | |||||
| (1, 6) 0.29 ± 0.01 | |||||
| Fe3C | 0.19 ± 0.01 | (3, 4) 0.31 ± 0.01 | 20.4 ± 0.5 | 84 ± 2 | |
| (2, 5) 0.37 ± 0.01 | |||||
| (1, 6) 0.44 ± 0.01 | |||||
| γ-Fe | −0.05 ± 0.01 | 0.30 * | - | 2 ± 2 | |
| 1000 °C | α-Fe | −0.00 ± 0.01 | (3, 4) 0.23 ± 0.02 | 32.8 ± 0.5 | 27 ± 2 |
| (2, 5) 0.24 ± 0.01 | |||||
| (1, 6) 0.25 ± 0.01 | |||||
| Fe3C | 0.18 ± 0.01 | (3, 4) 0.28 ± 0.01 | 20.5 ± 0.5 | 50 ± 2 | |
| (2, 5) 0.30 ± 0.01 | |||||
| (1, 6) 0.35 ± 0.01 | |||||
| γ-Fe | −0.09 ± 0.01 | 0.30 ± 0.01 | - | 23 ± 2 | |
| 1100 °C | α-Fe | 0.00 ± 0.01 | (3, 4) 0.25 ± 0.02 | 32.7 ± 0.5 | 35 ± 2 |
| (2, 5) 0.27 ± 0.01 | |||||
| (1, 6) 0.28 ± 0.01 | |||||
| Fe3C | 0.19 ± 0.01 | (3, 4) 0.28 ± 0.03 | 20.4 ± 0.5 | 26 ± 2 | |
| (2, 5) 0.30 ± 0.01 | |||||
| (1, 6) 0.33 ± 0.02 | |||||
| γ-Fe | −0.09 ± 0.01 | 0.30 ± 0.01 | - | 39 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kořenek, M.; Ivanova, T.; Svačinová, V.; Mašláň, M. Mössbauer Study on the Conversion of Different Iron-Based Catalysts Used in Carbon Nanotube Synthesis. Nanomaterials 2023, 13, 3010. https://doi.org/10.3390/nano13233010
Kořenek M, Ivanova T, Svačinová V, Mašláň M. Mössbauer Study on the Conversion of Different Iron-Based Catalysts Used in Carbon Nanotube Synthesis. Nanomaterials. 2023; 13(23):3010. https://doi.org/10.3390/nano13233010
Chicago/Turabian StyleKořenek, Michal, Tatiana Ivanova, Veronika Svačinová, and Miroslav Mašláň. 2023. "Mössbauer Study on the Conversion of Different Iron-Based Catalysts Used in Carbon Nanotube Synthesis" Nanomaterials 13, no. 23: 3010. https://doi.org/10.3390/nano13233010






