Functionalization of MWCNTs for Bioelectrocatalysis by Bacterial Two-Domain Laccase from Catenuloplanes japonicus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Enzyme Isolation
2.2. Multiwall Carbon Nanotubes Processing and Measurements
2.3. Oxidation of MWCNTs
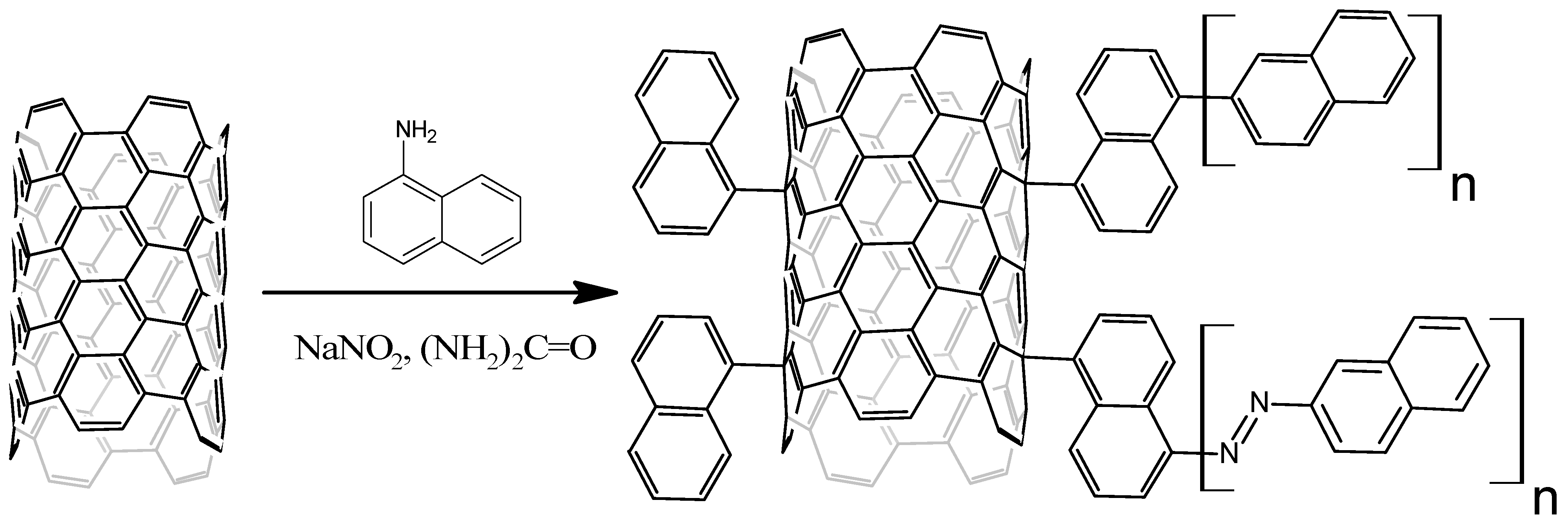
2.4. Naphthylation of MWCNTs
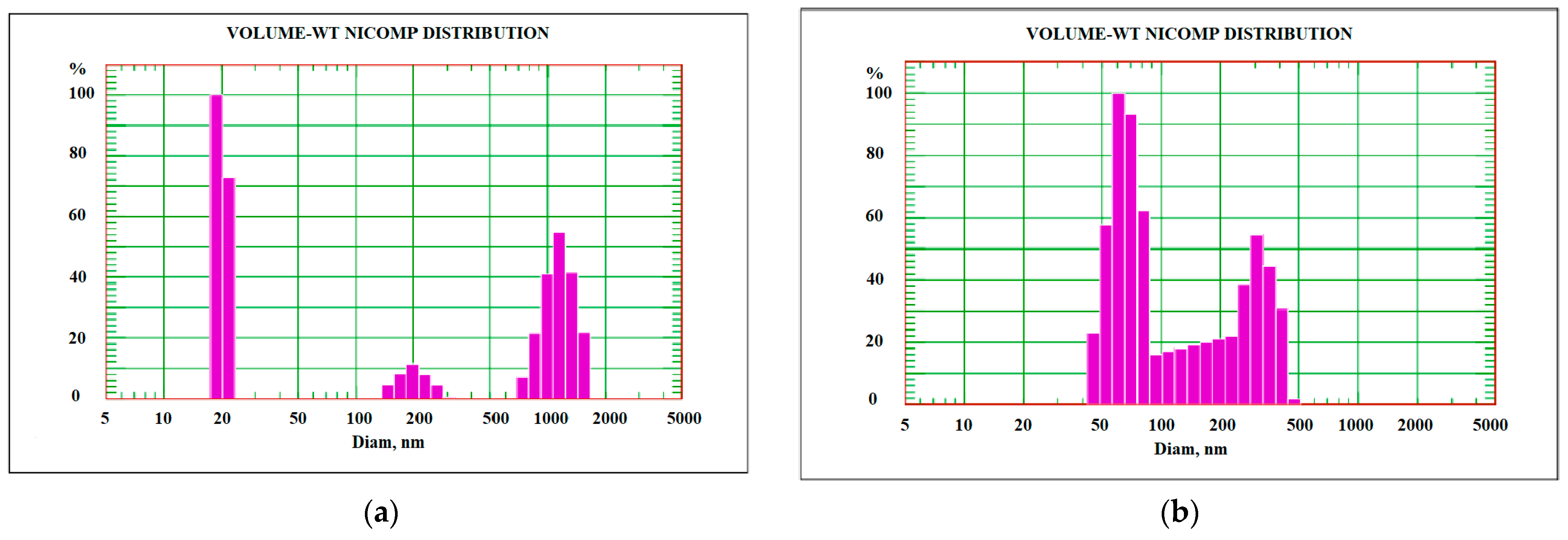
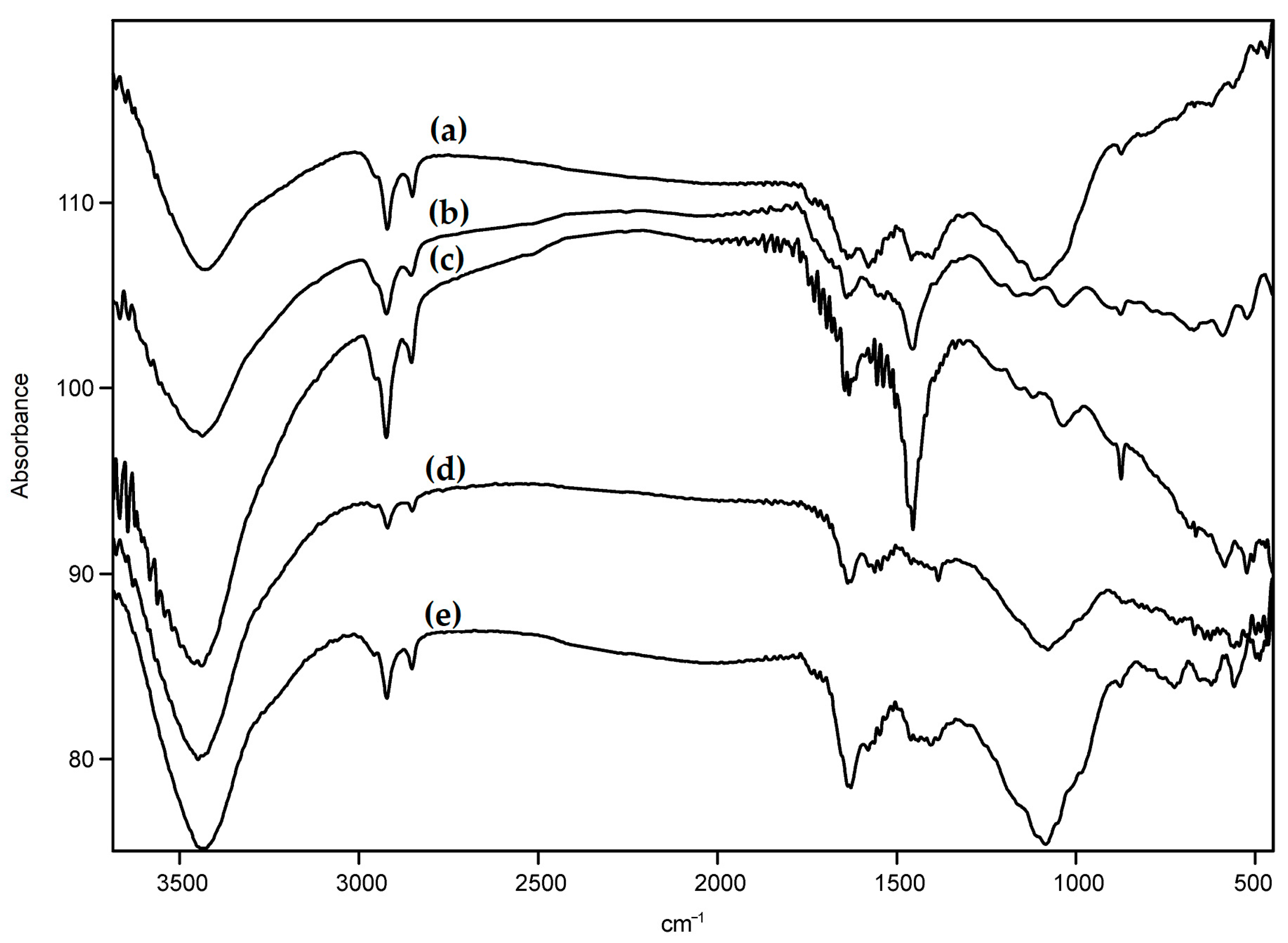
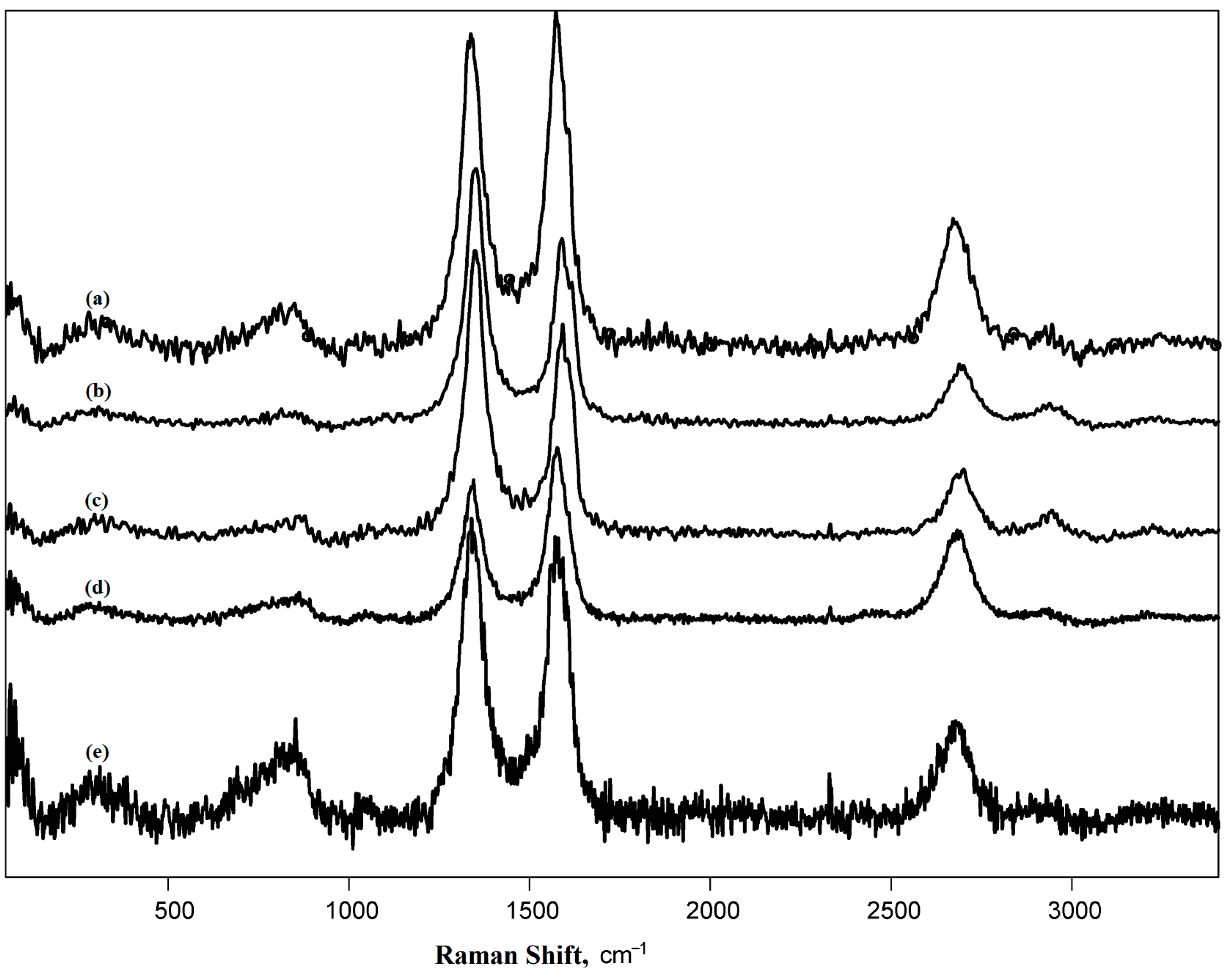
2.5. Characteristics of MWCNTs
2.6. Modification of Graphite Rod Electrodes (GRE)
2.7. Modification of Carbon Paste Electrode (CPE)
2.8. Determination of Electrochemically Active Surface (EAS)
2.9. Laccase Immobilization onto the Electrode Surface
2.10. Amperometric Measurements
2.11. Spectral Graphite Rod Electrodes and Biofuel Cell Model with Gluconobacter oxydans Natural Enzyme Cascades
2.12. Molecular Docking Studies
3. Results
3.1. Characteristics of the Pristine and Modified Carbon Nanotubes
3.2. Determination of Redox Potential of Ac-875 Laccase in Bioelectocatalytical Systems Based on Graphite Paste Electrodes
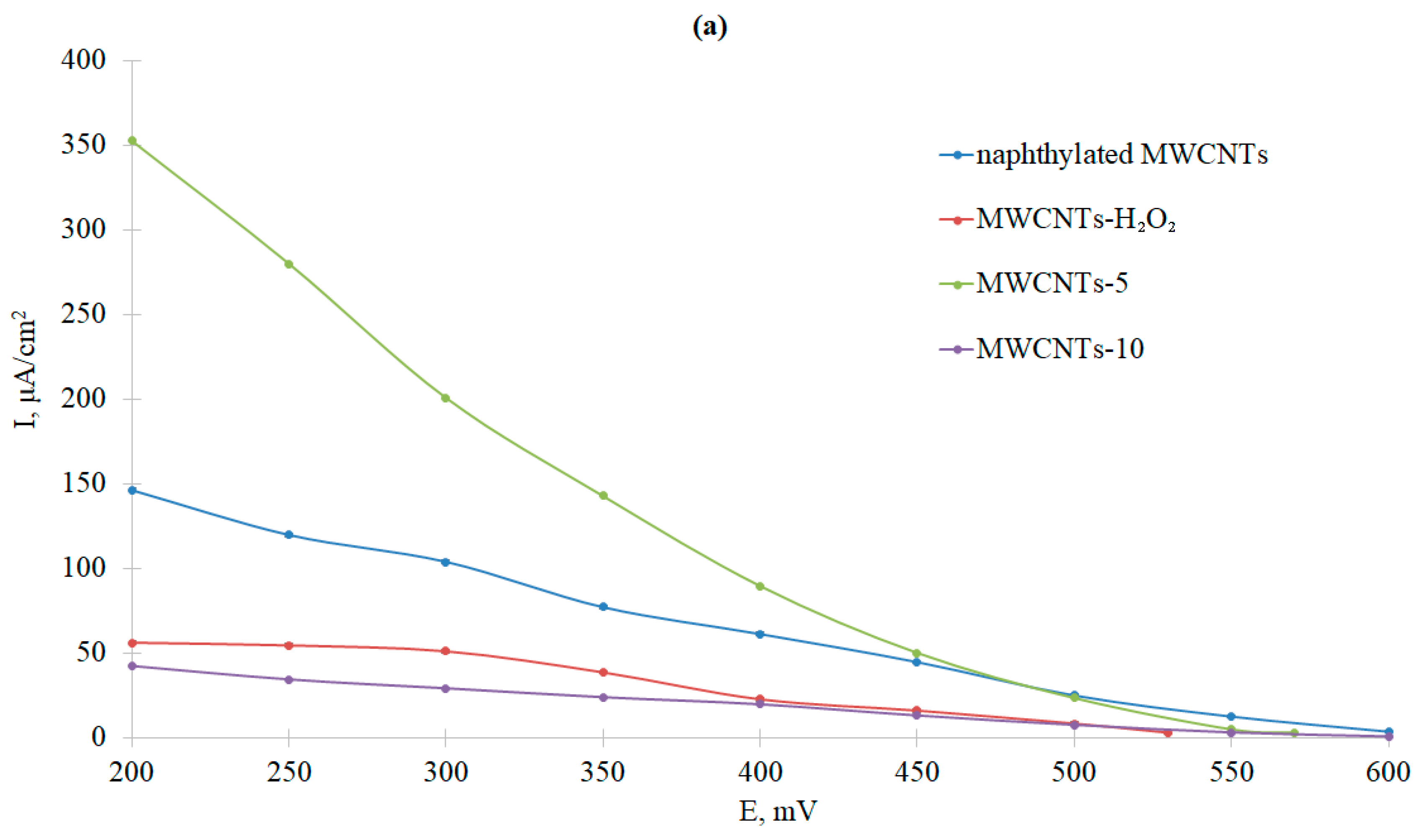
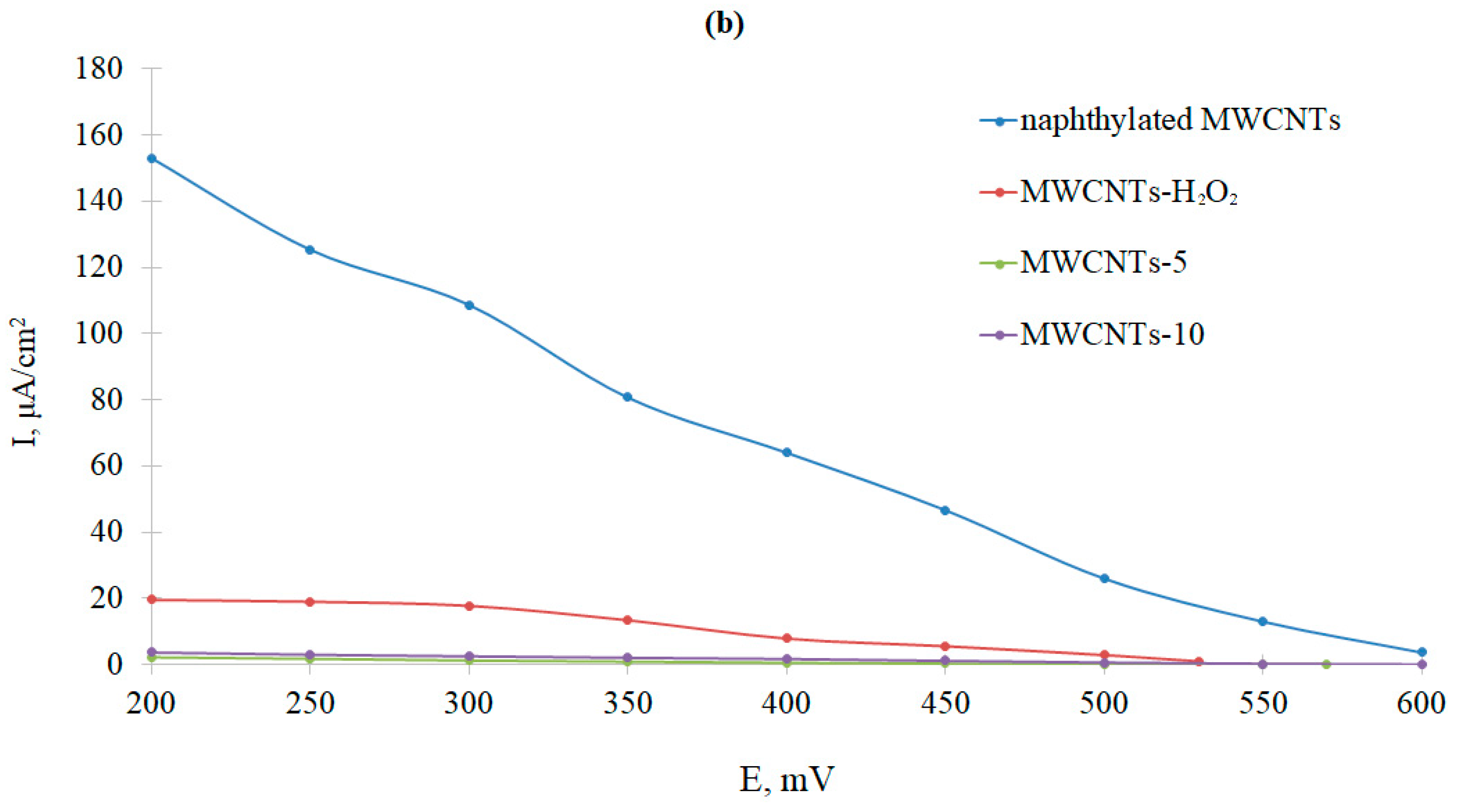
3.3. Comparative Analysis of Bioelectrocatalytic Oxygen Reduction on Graphite Pencil Rods Modified with Ac-875 Laccase Immobilized on Functionalized Carbon Nanotubes
3.4. Biofuel Cell with Anode Biocatalyst of Natural Enzyme Cascades of Bacteria Gluconobacter oxydans
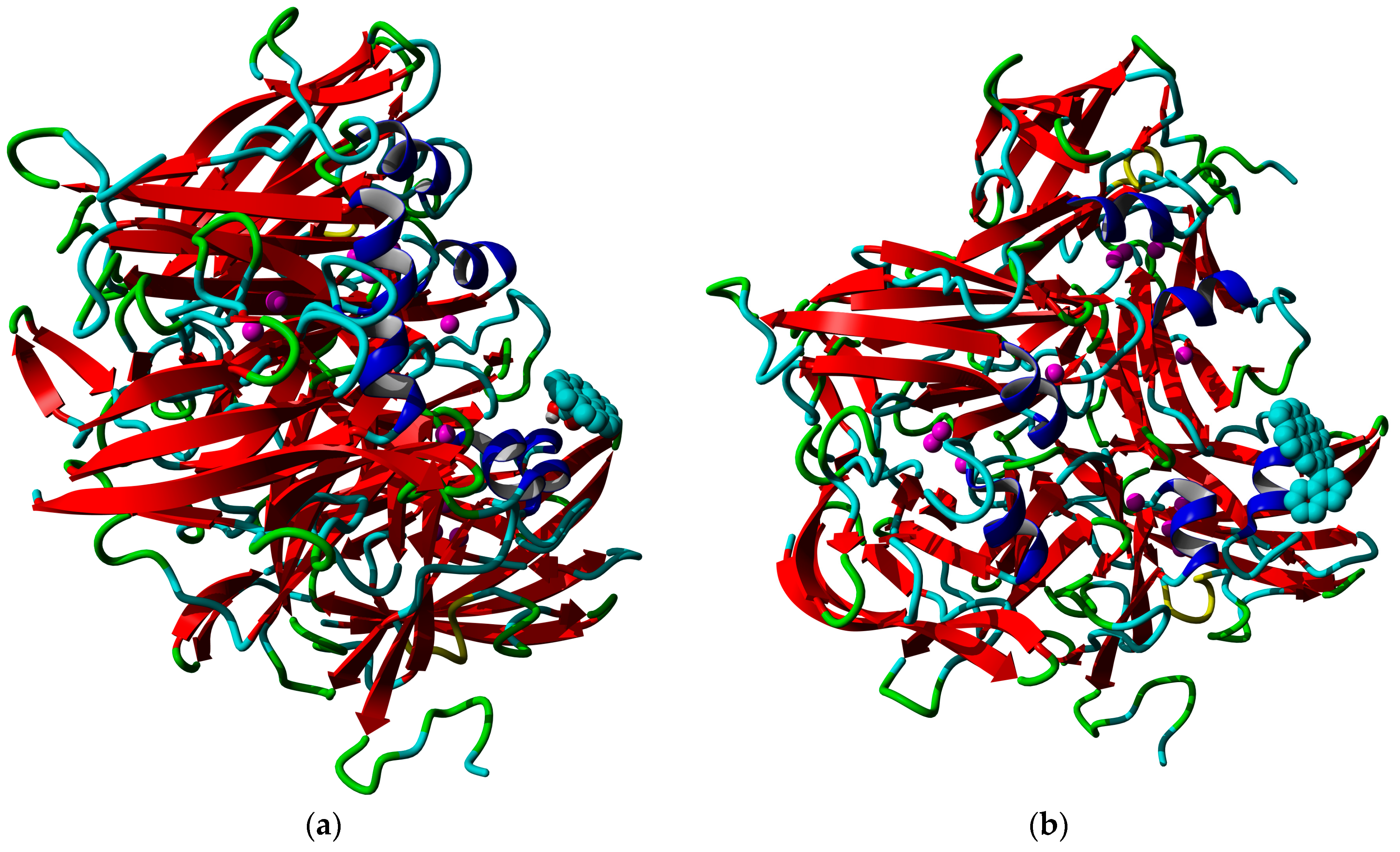
3.5. Molecular Docking Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morozan, A.; Jousselme, B.; Palacin, S. Low-Platinum and Platinum-Free Catalysts for the Oxygen Reduction Reaction at Fuel Cell Cathodes. Energy Environ. Sci. 2011, 4, 1238–1254. [Google Scholar] [CrossRef]
- Kodama, K.; Nagai, T.; Kuwaki, A.; Jinnouchi, R.; Morimoto, Y. Challenges in Applying Highly Active Pt-Based Nanostructured Catalysts for Oxygen Reduction Reactions to Fuel Cell Vehicles. Nat. Nanotechnol. 2021, 16, 140–147. [Google Scholar] [CrossRef]
- Jaouen, F.; Proietti, E.; Lefèvre, M.; Chenitz, R.; Dodelet, J.-P.; Wu, G.; Taek Chung, H.; Marie Johnston, C.; Zelenay, P. Recent Advances in Non-Precious Metal Catalysis for Oxygen—Reduction Reaction in Polymer Electrolyte Fuel Cells. Energy Environ. Sci. 2011, 4, 114–130. [Google Scholar] [CrossRef]
- Jiao, P.; Ye, D.; Zhu, C.; Wu, S.; Qin, C.; An, C.; Hu, N.; Deng, Q. Non-Precious Transition Metal Single-Atom Catalysts for the Oxygen Reduction Reaction: Progress and Prospects. Nanoscale 2022, 14, 14322–14340. [Google Scholar] [CrossRef]
- de Poulpiquet, A.; Ciaccafava, A.; Gadiou, R.; Gounel, S.; Giudici-Orticoni, M.T.; Mano, N.; Lojou, E. Design of a H2/O2 Biofuel Cell Based on Thermostable Enzymes. Electrochem. Commun. 2014, 42, 72–74. [Google Scholar] [CrossRef]
- Yoo, E.; Nagashima, Y.; Yamazaki, T.; Matsumoto, T.; Nakamura, J. Reduction of Pt Usage in Fuel Cell Electrocatalysts Using Carbon Nanotubes and non-Pt Metals. Polym. Adv. Technol. 2006, 17, 540–543. [Google Scholar] [CrossRef]
- Zhou, Z.; Orcutt, E.K.; Anderson, H.C.; Stowers, K.J. Hydrogen Surface Modification of a Carbon Nanotube Catalyst for the Improvement of Ethane Oxidative Dehydrogenation. Carbon 2019, 152, 924–931. [Google Scholar] [CrossRef]
- Liang, Y.; Cai, R.; Hickey, D.P.; Kitt, J.P.; Harris, J.M.; Minteer, S.D.; Korzeniewski, C. Infrared Microscopy as a Probe of Composition within a Model Biofuel Cell Electrode Prepared from Trametes versicolor Laccase. ChemElectroChem 2019, 6, 818–826. [Google Scholar] [CrossRef]
- Gorton, L.; Bollella, P.; Kano, K.; Hibino, Y.; Antiochia, R.; Rojas-Carrillo, O. (Invited) Fructose Biosensors Based on Direct Electron Transfer between Fructose Dehydrogenase and Electrodes. Meet. Abstr. 2019, MA2019-04, 384. [Google Scholar] [CrossRef]
- Bollella, P.; Hibino, Y.; Kano, K.; Gorton, L.; Antiochia, R. Enhanced Direct Electron Transfer of Fructose Dehydrogenase Rationally Immobilized on a 2-Aminoanthracene Diazonium Cation Grafted Single-Walled Carbon Nanotube Based Electrode. ACS Catal. 2018, 8, 10279–10289. [Google Scholar] [CrossRef]
- Bensghaïer, A.; Mousli, F.; Lamouri, A.; Postnikov, P.S.; Chehimi, M.M. The Molecular and Macromolecular Level of Carbon Nanotube Modification Via Diazonium Chemistry: Emphasis on the 2010s Years. Chem. Afr. 2020, 3, 535–569. [Google Scholar] [CrossRef]
- Jang, C.; Ji, J.; Yu, J. Applicability of CNT as Support Candidate for Thiophene Hydrodesulfurization and 1-Octene Hydrogenation Catalyst. Inorg. Chem. Commun. 2021, 129, 108615. [Google Scholar] [CrossRef]
- Dey, B.; Dutta, T. Laccases: Thriving the Domain of Bio-Electrocatalysis. Bioelectrochemistry 2022, 146, 108144. [Google Scholar] [CrossRef]
- Matsumoto, T.; Eguchi, S.; Nakai, H.; Hibino, T.; Yoon, K.-S.; Ogo, S. [NiFe]Hydrogenase from Citrobacter sp. S-77 Surpasses Platinum as an Electrode for H2 Oxidation Reaction. Angew. Chem. 2014, 126, 9041–9044. [Google Scholar] [CrossRef]
- Den Boer, D.; De Heer, H.C.; Buda, F.; Hetterscheid, D.G.H. Challenges in Elucidating the Free Energy Scheme of the Laccase Catalyzed Reduction of Oxygen. ChemCatChem 2023, 15, e202200878. [Google Scholar] [CrossRef]
- Thorum, M.S.; Anderson, C.A.; Hatch, J.J.; Campbell, A.S.; Marshall, N.M.; Zimmerman, S.C.; Lu, Y.; Gewirth, A.A. Direct, Electrocatalytic Oxygen Reduction by Laccase on Anthracene-2-Methanethiol-Modified Gold. J. Phys. Chem. Lett. 2010, 1, 2251–2254. [Google Scholar] [CrossRef]
- Szczesny, J.; Birrell, J.A.; Conzuelo, F.; Lubitz, W.; Ruff, A.; Schuhmann, W. Redox-Polymer-Based High-Current-Density Gas-Diffusion H2-Oxidation Bioanode Using [FeFe] Hydrogenase from Desulfovibrio desulfuricans in a Membrane-free Biofuel Cell. Angew. Chem. Int. Ed. 2020, 59, 16506–16510. [Google Scholar] [CrossRef]
- Xia, H.; So, K.; Kitazumi, Y.; Shirai, O.; Nishikawa, K.; Higuchi, Y.; Kano, K. Dual Gas-Diffusion Membrane- and Mediatorless Dihydrogen/Air-Breathing Biofuel Cell Operating at Room Temperature. J. Power Sources 2016, 335, 105–112. [Google Scholar] [CrossRef]
- Tsygankov, A.A.; Zorin, N.A.; Starodubov, A.S.; Khasimov, M.K.; Melnikova, M.S.; Doronin, I.A.; Vasilov, R.G. A Simple Method for Oriented Immobilization of HydSL Hydrogenase of Thiocapsa Bogorovii on Carbon Electrodes. Int. J. Hydrogen Energy 2023, 48, 39989–39999. [Google Scholar] [CrossRef]
- Patra, S.; Verma, J.; Mishra, Y.K.; Kurinec, S.; Wang, Q.; Syväjärvi, M.; Tiwari, A. The Positioning of Biofuel Cells-Based Biobatteries for Net-Zero Energy Future. J. Energy Storage 2023, 72, 107919. [Google Scholar] [CrossRef]
- Dutta, S.; Patil, R.; Dey, T. Electron Transfer-Driven Single and Multi-Enzyme Biofuel Cells for Self-Powering and Energy Bioscience. Nano Energy 2022, 96, 107074. [Google Scholar] [CrossRef]
- Chen, H.; Simoska, O.; Lim, K.; Grattieri, M.; Yuan, M.; Dong, F.; Lee, Y.S.; Beaver, K.; Weliwatte, S.; Gaffney, E.M.; et al. Fundamentals, Applications, and Future Directions of Bioelectrocatalysis. Chem. Rev. 2020, 120, 12903–12993. [Google Scholar] [CrossRef]
- Berezin, I.V.; Bogdanovskaya, V.A.; Varfolomeev, S.D.; Tarasevich, M.R.; Yaropolov, A.I. The Oxygen Equilibrium Bioelectrocatalysis. Potential in the Presence of Laccase. Dokl. Proc. Acad. Sci. USSR Phys. Chem. 1978, 455, 240–241. [Google Scholar]
- Piontek, K.; Antorini, M.; Choinowski, T. Crystal Structure of a Laccase from the FungusTrametes Versicolor at 1.90-Å Resolution Containing a Full Complement of Coppers. J. Biol. Chem. 2002, 277, 37663–37669. [Google Scholar] [CrossRef]
- Xie, T.; Liu, Z.; Wang, G. Structural Basis for Monolignol Oxidation by a Maize Laccase. Nat. Plants 2020, 6, 231–237. [Google Scholar] [CrossRef]
- Miranda-Blancas, R.; Avelar, M.; Rodriguez-Arteaga, A.; Sinicropi, A.; Rudiño-Piñera, E. The β-Hairpin from the Thermus thermophilus HB27 Laccase Works as a pH-Dependent Switch to Regulate Laccase Activity. J. Struct. Biol. 2021, 213, 107740. [Google Scholar] [CrossRef]
- Minteer, S.D. Oxidative Bioelectrocatalysis: From Natural Metabolic Pathways to Synthetic Metabolons and Minimal Enzyme Cascades. Biochim. Biophys. Acta (BBA)-Bioenerg. 2016, 1857, 621–624. [Google Scholar] [CrossRef]
- Milton, R.D.; Wang, T.; Knoche, K.L.; Minteer, S.D. Tailoring Biointerfaces for Electrocatalysis. Langmuir 2016, 32, 2291–2301. [Google Scholar] [CrossRef]
- Hoegger, P.J.; Kilaru, S.; James, T.Y.; Thacker, J.R.; Kues, U. Phylogenetic Comparison and Classification of Laccase and Related Multicopper Oxidase Protein Sequences. FEBS J. 2006, 273, 2308–2326. [Google Scholar] [CrossRef]
- Bento, I.; Peixoto, C.; Zaitsev, V.N.; Lindley, P.F. Ceruloplasmin Revisited: Structural and Functional Roles of Various Metal Cation-Binding Sites. Acta Crystallogr. Sect. D Biol. Crystallogr. 2007, 63, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Aleksejeva, O.; Sokolov, A.V.; Marquez, I.; Gustafsson, A.; Bushnev, S.; Eriksson, H.; Ljunggren, L.; Shleev, S. Autotolerant Ceruloplasmin Based Biocathodes for Implanted Biological Power Sources. Bioelectrochemistry 2021, 140, 107794. [Google Scholar] [CrossRef]
- Solomon, E.I.; Sundaram, U.M.; Machonkin, T.E. Multicopper Oxidases and Oxygenases. Chem. Rev. 1996, 96, 2563–2606. [Google Scholar] [CrossRef]
- Loi, M.; Glazunova, O.; Fedorova, T.; Logrieco, A.F.; Mulè, G. Fungal Laccases: The Forefront of Enzymes for Sustainability. J. Fungi 2021, 7, 1048. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Ali, S.; Liu, S.; Zhou, J.; Tang, Y. Characterization of Plant Laccase Genes and Their Functions. Gene 2023, 852, 147060. [Google Scholar] [CrossRef]
- Abdel-Banat, B.M.A.; El-Shafie, H.A.F. Expression Profiling, Phylogenetic, and Structural Analyses of a Laccase Gene from the Red Palm Weevil, Rhynchophorus ferrugineus. Afr. J. Biotechnol. 2019, 18, 978–990. [Google Scholar] [CrossRef]
- Skálová, T.; Dušková, J.; Hašek, J.; Štěpánková, A.; Kovaľ, T.; Østergaard, L.H.; Dohnálek, J. Structure of Laccase from Streptomyces coelicolor after Soaking with Potassium Hexacyanoferrate and at an Improved Resolution of 2.3 Å. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2011, 67, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Lukk, T.; Solbiati, J.O.; Bauer, S.; Nair, S.K.; Cronan, J.E.; Gerlt, J.A. Roles of Small Laccases from Streptomyces in Lignin Degradation. Biochemistry 2014, 53, 4047–4058. [Google Scholar] [CrossRef] [PubMed]
- Kolyadenko, I.; Scherbakova, A.; Kovalev, K.; Gabdulkhakov, A.; Tishchenko, S. Engineering the Catalytic Properties of Two-Domain Laccase from Streptomyces griseoflavus Ac-993. Int. J. Mol. Sci. 2021, 23, 65. [Google Scholar] [CrossRef]
- Trubitsina, L.I.; Tishchenko, S.V.; Gabdulkhakov, A.G.; Lisov, A.V.; Zakharova, M.V.; Leontievsky, A.A. Structural and Functional Characterization of Two-Domain Laccase from Streptomyces viridochromogenes. Biochimie 2015, 112, 151–159. [Google Scholar] [CrossRef]
- Trubitsina, L.I.; Abdullatypov, A.V.; Larionova, A.P.; Trubitsin, I.V.; Alferov, S.V.; Ponamoreva, O.N.; Leontievsky, A.A. Expression of Thermophilic Two-Domain Laccase from Catenuloplanes japonicus in Escherichia coli and Its Activity against Triarylmethane and Azo Dyes. PeerJ 2021, 9, e11646. [Google Scholar] [CrossRef]
- Fedina, V.; Lavrova, D.; Dyachkova, T.; Pasko, A.; Zvonarev, A.; Panfilov, V.; Ponamoreva, O.; Alferov, S. Polymer-Based Conductive Nanocomposites for the Development of Bioanodes Using Membrane-Bound Enzyme Systems of Bacteria Gluconobacter Oxydans in Biofuel Cells. Polymers 2023, 15, 1296. [Google Scholar] [CrossRef]
- Dyachkova, T.P.; Khan, Y.A.; Orlova, N.V.; Kondrashov, S.V. Oxidation of Multiwalled Carbon Nanotubes by Hydrogene Peroxide Vapor: Laws and Effects. Vestnik 2016, 22, 323–333. [Google Scholar] [CrossRef]
- Dyachkova, T.P.; Tkachev, A.G. Methods for Functionalization and Modification of Carbon Nanotubes; M Publishing House: London, UK, 2013; ISBN 978-5-4442-0050-6. (In Russian) [Google Scholar]
- Boehm, H.P. Surface Oxides on Carbon and Their Analysis: A Critical Assessment. Carbon 2002, 40, 145–149. [Google Scholar] [CrossRef]
- Zhu, P.; Zhao, Y. Cyclic Voltammetry Measurements of Electroactive Surface Area of Porous Nickel: Peak Current and Peak Charge Methods and Diffusion Layer Effect. Mater. Chem. Phys. 2019, 233, 60–67. [Google Scholar] [CrossRef]
- Randviir, E.P. A Cross Examination of Electron Transfer Rate Constants for Carbon Screen-Printed Electrodes Using Electrochemical Impedance Spectroscopy and Cyclic Voltammetry. Electrochim. Acta 2018, 286, 179–186. [Google Scholar] [CrossRef]
- Nefedkin, S.I.; Guterman, V.E.; Alekseenko, A.A.; Belenov, S.V.; Ivanenko, A.V.; Klimova, M.A.; Pavlov, V.I.; Panov, S.V.; Paperzh, K.O.; Shubenkov, S.V. Russian Technologies and Nanostructural Materials in High Specific Power Systems Based on Hydrogen–Air Fuel Cells with an Open Cathode. Nanotechnologies Russ. 2020, 15, 370–378. [Google Scholar] [CrossRef]
- Krieger, E.; Dunbrack, R.L.; Hooft, R.W.W.; Krieger, B. Assignment of Protonation States in Proteins and Ligands: Combining pKa Prediction with Hydrogen Bonding Network Optimization. Methods Mol. Biol. 2012, 819, 405–421. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Oskin, P.; Demkina, I.; Dmitrieva, E.; Alferov, S. Functionalization of Carbon Nanotubes Surface by Aryl Groups: A Review. Nanomaterials 2023, 13, 1630. [Google Scholar] [CrossRef] [PubMed]
- Żelechowska, K.; Stolarczyk, K.; Łyp, D.; Rogalski, J.; Roberts, K.P.; Bilewicz, R.; Biernat, J.F. Aryl and N-Arylamide Carbon Nanotubes for Electrical Coupling of Laccase to Electrodes in Biofuel Cells and Biobatteries. Biocybern. Biomed. Eng. 2013, 33, 235–245. [Google Scholar] [CrossRef]
- Oskin, P.; Tula State University, Tula, Russia; Dyachkova, T.; Tambov State Technical University, Tambov, Russia. Personal communication, 2023.
- Pandolfo, A.G.; Hollenkamp, A.F. Carbon Properties and Their Role in Supercapacitors. J. Power Sources 2006, 157, 11–27. [Google Scholar] [CrossRef]
- Li, Z.; Deng, L.; Kinloch, I.A.; Young, R.J. Raman Spectroscopy of Carbon Materials and Their Composites: Graphene, Nanotubes and Fibres. Prog. Mater. Sci. 2023, 135, 101089. [Google Scholar] [CrossRef]
- Bonpua, J.; Yagües, Y.; Aleshin, A.; Dasappa, S.; Camacho, J. Flame Temperature Effect on Sp2 Bonds on Nascent Carbon Nanoparticles Formed in Premixed Flames (T > 2100 K): A Raman Spectroscopy and Particle Mobility Sizing Study. Proc. Combust. Inst. 2019, 37, 943–951. [Google Scholar] [CrossRef]
- Cançado, L.G.; Jorio, A.; Ferreira, E.H.M.; Stavale, F.; Achete, C.A.; Capaz, R.B.; Moutinho, M.V.O.; Lombardo, A.; Kulmala, T.S.; Ferrari, A.C. Quantifying Defects in Graphene via Raman Spectroscopy at Different Excitation Energies. Nano Lett. 2011, 11, 3190–3196. [Google Scholar] [CrossRef]
- Ribeiro-Soares, J.; Oliveros, M.E.; Garin, C.; David, M.V.; Martins, L.G.P.; Almeida, C.A.; Martins-Ferreira, E.H.; Takai, K.; Enoki, T.; Magalhães-Paniago, R.; et al. Structural Analysis of Polycrystalline Graphene Systems by Raman Spectroscopy. Carbon 2015, 95, 646–652. [Google Scholar] [CrossRef]
- Ghosh, S.; Ganesan, K.; Polaki, S.R.; Ravindran, T.R.; Krishna, N.G.; Kamruddin, M.; Tyagi, A.K. Evolution and Defect Analysis of Vertical Graphene Nanosheets: Defect Analysis of Vertical Graphene Nanosheets. J. Raman Spectrosc. 2014, 45, 642–649. [Google Scholar] [CrossRef]
- Chernyak, S.A.; Ivanov, A.S.; Stolbov, D.N.; Egorova, T.B.; Maslakov, K.I.; Shen, Z.; Lunin, V.V.; Savilov, S.V. N-Doping and Oxidation of Carbon Nanotubes and Jellyfish-like Graphene Nanoflakes through the Prism of Raman Spectroscopy. Appl. Surf. Sci. 2019, 488, 51–60. [Google Scholar] [CrossRef]
- Eckmann, A.; Felten, A.; Mishchenko, A.; Britnell, L.; Krupke, R.; Novoselov, K.S.; Casiraghi, C. Probing the Nature of Defects in Graphene by Raman Spectroscopy. Nano Lett. 2012, 12, 3925–3930. [Google Scholar] [CrossRef] [PubMed]
- Venezuela, P.; Lazzeri, M.; Mauri, F. Theory of Double-Resonant Raman Spectra in Graphene: Intensity and Line Shape of Defect-Induced and Two-Phonon Bands. Phys. Rev. B 2011, 84, 035433. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Basko, D.M. Raman Spectroscopy as a Versatile Tool for Studying the Properties of Graphene. Nat. Nanotechnol. 2013, 8, 235–246. [Google Scholar] [CrossRef]
- Metwally, N.H.; Saad, G.R.; Abd El-Wahab, E.A. Grafting of Multiwalled Carbon Nanotubes with Pyrazole Derivatives: Characterization, Antimicrobial Activity and Molecular Docking Study. Int. J. Nanomed. 2019, 14, 6645–6659. [Google Scholar] [CrossRef]
- Singh, D.K.; Iyer, P.K.; Giri, P.K. Diameter Dependence of Interwall Separation and Strain in Multiwalled Carbon Nanotubes Probed by X-ray Diffraction and Raman Scattering Studies. Diam. Relat. Mater. 2010, 19, 1281–1288. [Google Scholar] [CrossRef]
- Mathur, A.; Wadhwa, S.; Tweedie, M.; Hazra, K.S.; Dickinson, C.; Roy, S.S.; Mitra, S.K.; Misra, D.S.; McLaughlin, J.A. A Comparative Study of the Growth, Microstructural and Electrical Properties of Multiwall CNTs Grown by Thermal and Microwave Plasma Enhanced CVD Methods. Phys. E Low-Dimens. Syst. Nanostructures 2011, 44, 29–36. [Google Scholar] [CrossRef]
- Dyachkova, T.P.; Rukhov, A.V.; Tkachev, A.G.; Tugolukov, E.N. Functionalization of Carbon Nanotubes: Methods, Mechanisms and Technological Realization. Adv. Mater. Technol. 2018, 2, 18–41. [Google Scholar] [CrossRef]
- Rehman, A.; Abbas, S.M.; Ammad, H.M.; Badshah, A.; Ali, Z.; Anjum, D.H. A Facile and Novel Approach towards Carboxylic Acid Functionalization of Multiwalled Carbon Nanotubes and Efficient Water Dispersion. Mater. Lett. 2013, 108, 253–256. [Google Scholar] [CrossRef]
- Velický, M.; Toth, P.S.; Woods, C.R.; Novoselov, K.S.; Dryfe, R.A.W. Electrochemistry of the Basal Plane versus Edge Plane of Graphite Revisited. J. Phys. Chem. C 2019, 123, 11677–11685. [Google Scholar] [CrossRef]
- Iamprasertkun, P.; Hirunpinyopas, W.; Keerthi, A.; Wang, B.; Radha, B.; Bissett, M.A.; Dryfe, R.A.W. Capacitance of Basal Plane and Edge-Oriented Highly Ordered Pyrolytic Graphite: Specific Ion Effects. J. Phys. Chem. Lett. 2019, 10, 617–623. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.; Wang, H.; Zhang, L.; Wilkinson, D.P.; Zhang, J. Recent Progresses in Oxygen Reduction Reaction Electrocatalysts for Electrochemical Energy Applications. Electrochem. Energy Rev. 2019, 2, 518–538. [Google Scholar] [CrossRef]
- Torrinha, Á.; Amorim, C.G.; Montenegro, M.C.B.S.M.; Araújo, A.N. Biosensing Based on Pencil Graphite Electrodes. Talanta 2018, 190, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Nathani, A.; Vishnu, N.; Sharma, C.S. Review—Pencil Graphite Electrodes as Platform for Enzyme and Enzyme-Like Protein Immobilization for Electrochemical Detection. J. Electrochem. Soc. 2020, 167, 037520. [Google Scholar] [CrossRef]
- Torrinha, Á.; Jiyane, N.; Sabela, M.; Bisetty, K.; Montenegro, M.C.B.S.M.; Araújo, A.N. Nanostructured Pencil Graphite Electrodes for Application as High Power Biocathodes in Miniaturized Biofuel Cells and Bio-Batteries. Sci. Rep. 2020, 10, 16535. [Google Scholar] [CrossRef]
- Miyama, A.; Okada, T.; Takahashi, F.; Jin, J.; Tatsumi, H. Electrochemical Measurements with a Periodically Renewable Pencil Electrode. J. Electroanal. Chem. 2022, 908, 116094. [Google Scholar] [CrossRef]
- Seinberg, J.-M.; Kullapere, M.; Mäeorg, U.; Maschion, F.C.; Maia, G.; Schiffrin, D.J.; Tammeveski, K. Spontaneous Modification of Glassy Carbon Surface with Anthraquinone from the Solutions of Its Diazonium Derivative: An Oxygen Reduction Study. J. Electroanal. Chem. 2008, 624, 151–160. [Google Scholar] [CrossRef]
- Ejuh, G.W.; Tchangnwa Nya, F.; Djongyang, N.; Ndjaka, J.M.B. Theoretical Study on the Electronic, Optoelectronic, Linear and Non Linear Optical Properties and UV–Vis Spectrum of Coronene and Coronene Substituted with Chlorine. SN Appl. Sci. 2020, 2, 1247. [Google Scholar] [CrossRef]
- Rani, M.; Sehrawat, M.; Sharma, S.; Singh, B.P. Recent Advancement and Challenges in Multifunctional Carbon Nanotube Buckypaper and Its Composites for Energy Storage and Conversion Applications. J. Energy Storage 2023, 73, 109063. [Google Scholar] [CrossRef]
- Buzzetti, P.H.M.; Berezovska, A.; Nedellec, Y.; Cosnier, S. Hollow Bioelectrodes Based on Buckypaper Assembly. Application to the Electroenzymatic Reduction of O2. Nanomaterials 2022, 12, 2399. [Google Scholar] [CrossRef] [PubMed]
- Mazurenko, I.; Hitaishi, V.P.; Lojou, E. Recent Advances in Surface Chemistry of Electrodes to Promote Direct Enzymatic Bioelectrocatalysis. Curr. Opin. Electrochem. 2020, 19, 113–121. [Google Scholar] [CrossRef]
- Cosnier, S.; Gross, A.J.; Le Goff, A.; Holzinger, M. Recent Advances on Enzymatic Glucose/Oxygen and Hydrogen/Oxygen Biofuel Cells: Achievements and Limitations. J. Power Sources 2016, 325, 252–263. [Google Scholar] [CrossRef]
- Gentil, S.; Rousselot-Pailley, P.; Sancho, F.; Robert, V.; Mekmouche, Y.; Guallar, V.; Tron, T.; Le Goff, A. Efficiency of Site-Specific Clicked Laccase–Carbon Nanotubes Biocathodes towards O2 Reduction. Chem. A Eur. J 2020, 26, 4798–4804. [Google Scholar] [CrossRef]
- Xu, Z.; Yang, S.; Xie, Y.; Yu, H.; Zhou, J. Modulating the Adsorption Orientation of Methionine-Rich Laccase by Tailoring the Surface Chemistry of Single-Walled Carbon Nanotubes. Colloids Surf. B Biointerfaces 2022, 217, 112660. [Google Scholar] [CrossRef]
- Mani, P.; Fidal, V.T.; Keshavarz, T.; Chandra, T.S.; Kyazze, G. Laccase Immobilization Strategies for Application as a Cathode Catalyst in Microbial Fuel Cells for Azo Dye Decolourization. Front. Microbiol. 2021, 11, 620075. [Google Scholar] [CrossRef] [PubMed]
- Blanford, C.F.; Heath, R.S.; Armstrong, F.A. A Stable Electrode for High-Potential, Electrocatalytic O2 Reduction Based on Rational Attachment of a Blue Copper Oxidase to a Graphite Surface. Chem. Commun. 2007, 17, 1710–1712. [Google Scholar] [CrossRef] [PubMed]
- Meredith, M.T.; Minson, M.; Hickey, D.; Artyushkova, K.; Glatzhofer, D.T.; Minteer, S.D. Anthracene-Modified Multi-Walled Carbon Nanotubes as Direct Electron Transfer Scaffolds for Enzymatic Oxygen Reduction. ACS Catal. 2011, 1, 1683–1690. [Google Scholar] [CrossRef]
- Stolarczyk, K.; Sepelowska, M.; Lyp, D.; Żelechowska, K.; Biernat, J.F.; Rogalski, J.; Farmer, K.D.; Roberts, K.N.; Bilewicz, R. Hybrid Biobattery Based on Arylated Carbon Nanotubes and Laccase. Bioelectrochemistry 2012, 87, 154–163. [Google Scholar] [CrossRef]
- Giroud, F.; Sawada, K.; Taya, M.; Cosnier, S. 5,5-Dithiobis(2-Nitrobenzoic Acid) Pyrene Derivative-Carbon Nanotube Electrodes for NADH Electrooxidation and Oriented Immobilization of Multicopper Oxidases for the Development of Glucose/O2 Biofuel Cells. Biosens. Bioelectron. 2017, 87, 957–963. [Google Scholar] [CrossRef]
- Amaral, T.K.M.; Dos Santos, G.O.; De Oliveira, P.S.C.; Pires, N.J.; De Castro, V.G.; Silva, G.G.; Denadai, Â.M.L.; Alves, M.O.; Trigueiro, J.P.C.; Lavall, R.L.; et al. The Effect of Debris on the Adsorption and Electron-Transfer Capacity at the Interface of Oxidized Carbon Nanotubes. Chem. Eng. J. 2020, 388, 124379. [Google Scholar] [CrossRef]
- Gellett, W.; Schumacher, J.; Kesmez, M.; Le, D.; Minteer, S.D. High Current Density Air-Breathing Laccase Biocathode. J. Electrochem. Soc. 2010, 157, B557. [Google Scholar] [CrossRef]

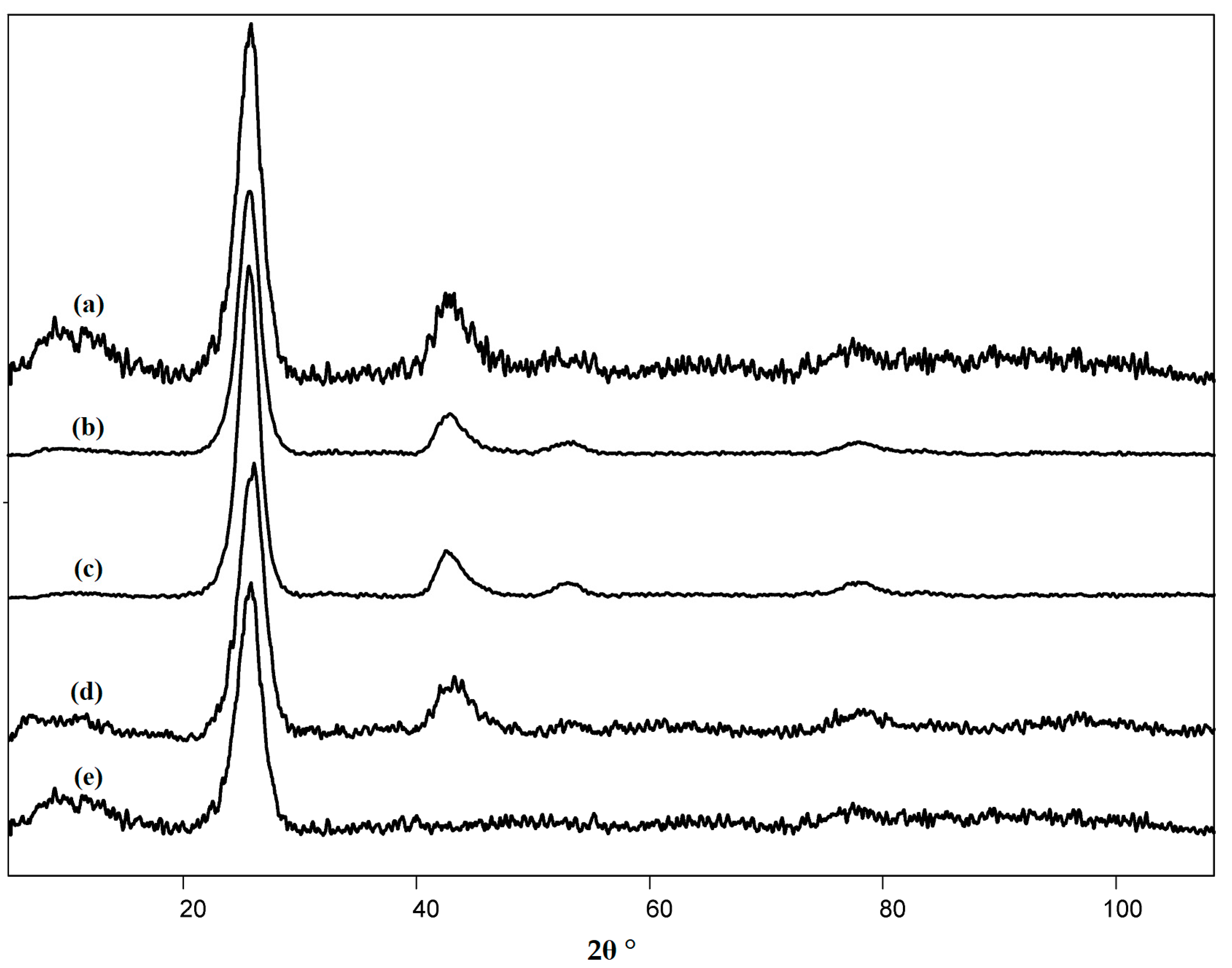
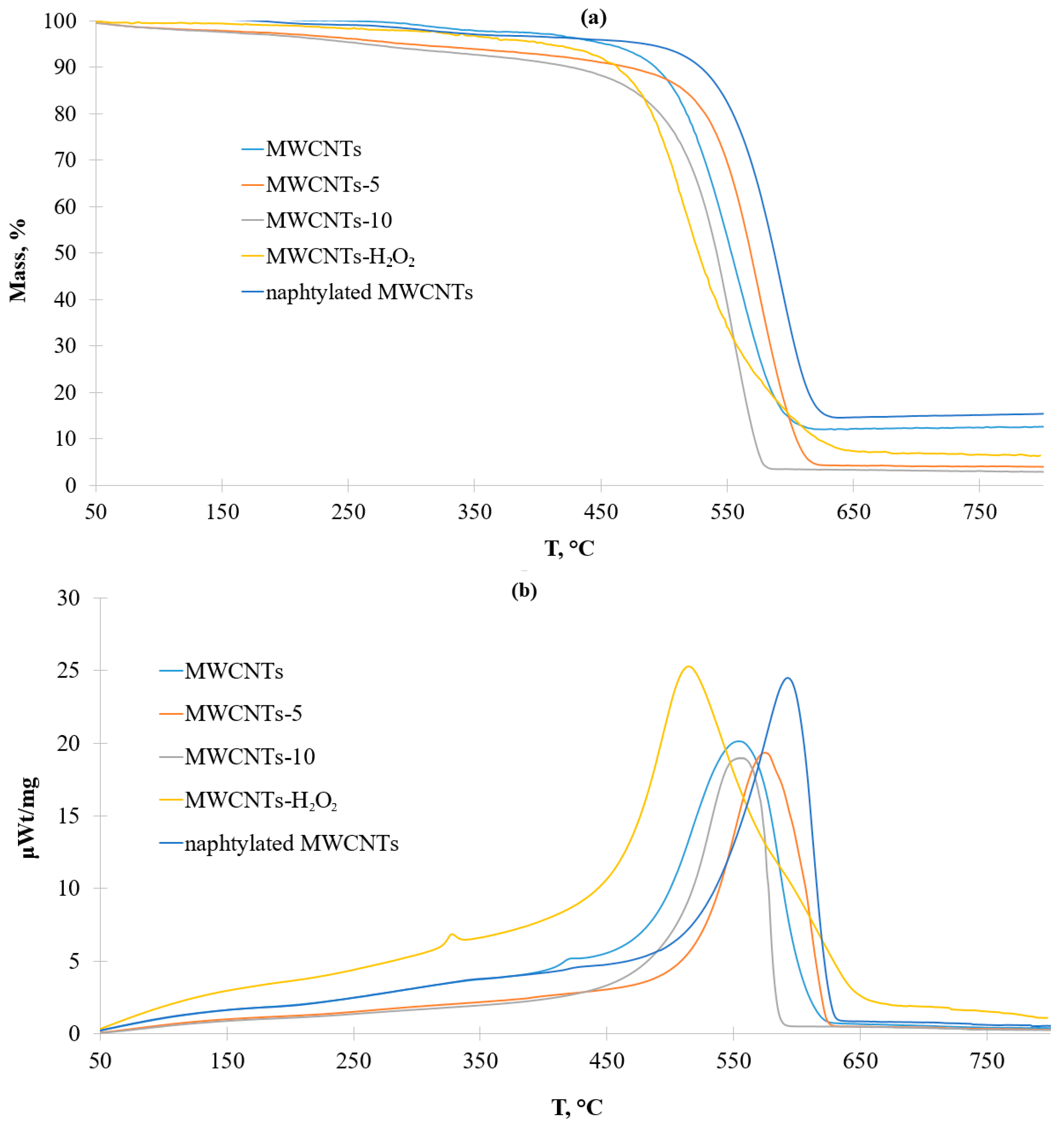
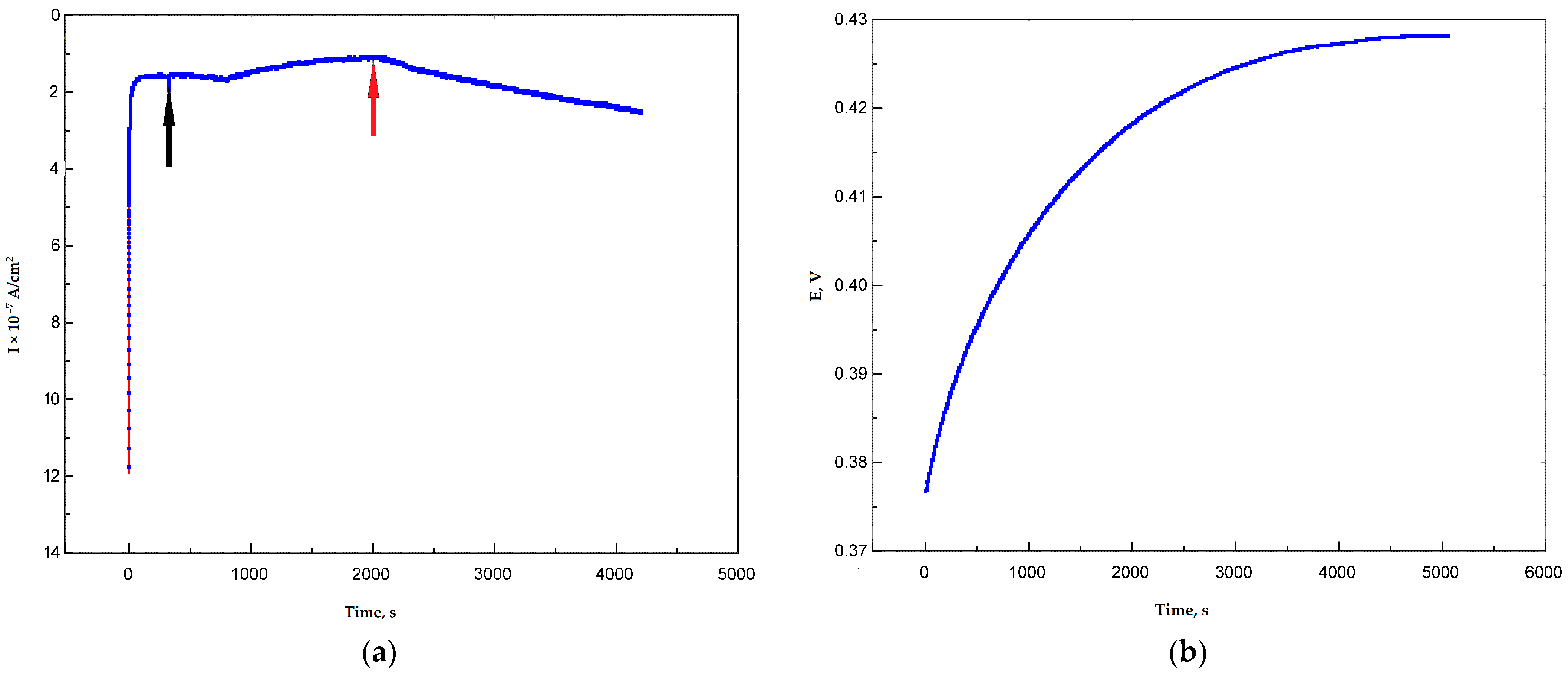
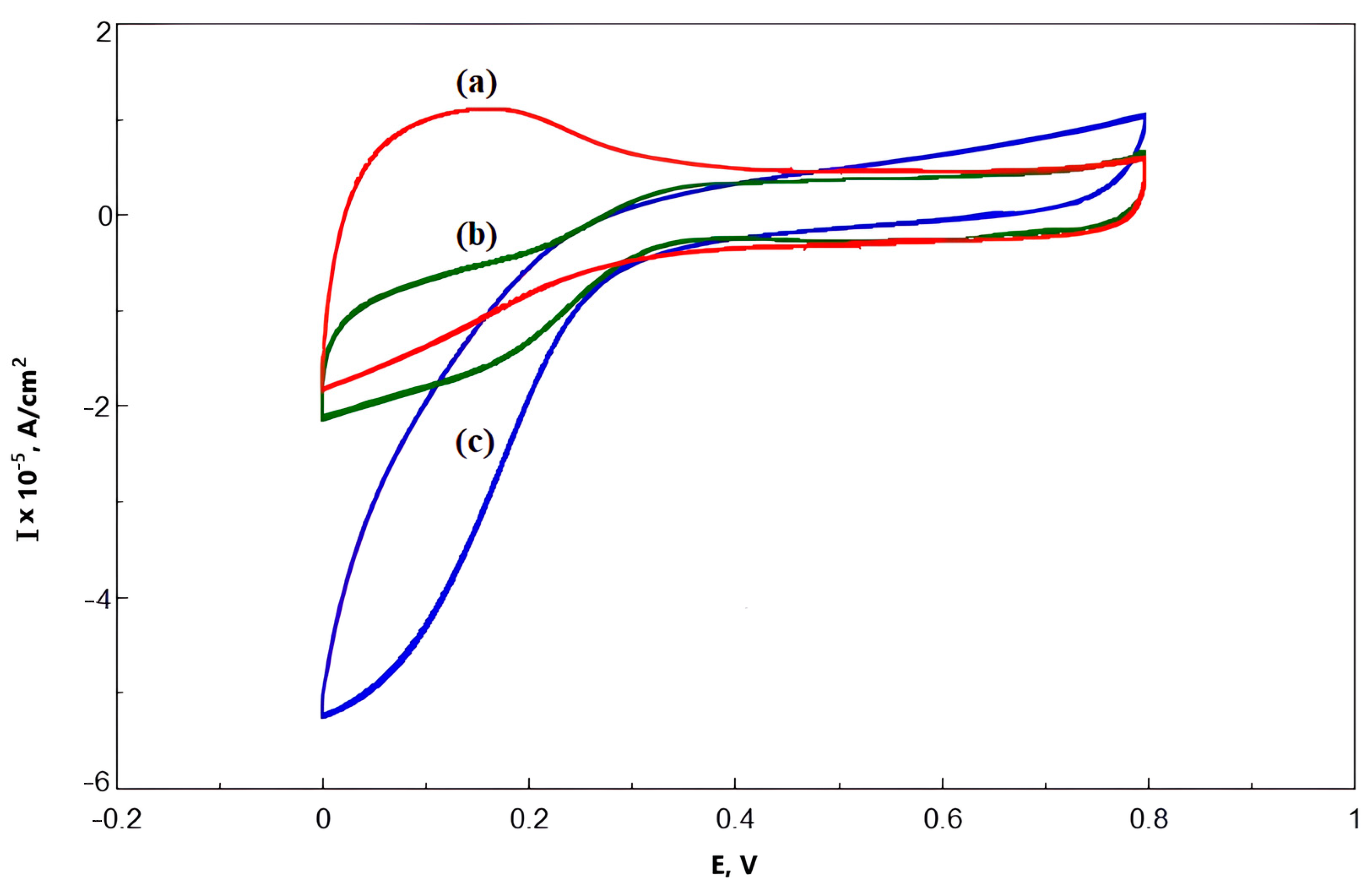
| Sample | Degree of Functionalization by the Groups (mmol/g) | |||
|---|---|---|---|---|
| -OH (Phenolic) | Lactone | -COOH (Carboxylic) | Total-Content | |
| MWCNT-5 | 0.8 | 0.5 | 0.4 | 1.7 |
| MWCNT-10 | 0.7 | 0.6 | 0.4 | 1.7 |
| CNT Type | ID*/IG | ID/IG | ID”/IG | ID′/IG | ID/ID′ | I2D/IG | LD, nm | nD × 10−9, cm−2 | La, nm |
|---|---|---|---|---|---|---|---|---|---|
| Pristine MWCNT | - | 1.27 | 0.11 | 0.38 | 3.34 | 0.35 | 10.7 | 273 | 13.0 |
| Naphthylated MWCNT | 0.02 | 1.53 | 0.12 | 0.74 | 2.06 | 0.45 | 9.8 | 330 | 10.8 |
| MWCNT-H2O2 | - | 1.11 | - | 0.36 | 3.08 | 0.52 | 11.5 | 239 | 12.8 |
| MWCNT-5 | - | 1.40 | - | 0.44 | 3.18 | 0.31 | 10.2 | 301 | 11.5 |
| MWCNT-10 | - | 1.42 | 0.21 | 0.84 | 1.69 | 0.31 | 10.1 | 306 | 11.7 |
| Type of Electrode | EAS, cm2 | ks × 104, cm·c−1 | fe, % |
|---|---|---|---|
| Blank pencil core electrode | 0.011 ± 0.002 | 4 ± 1 | 0.4 ± 0.1 |
| Electrode with MWCNTs-5 | 5 ± 1 | 150 ± 20 | 15 ± 2 |
| Electrode with MWCNTs-10 | 1.2 ± 0.3 | 800 ± 300 | 80 ± 30 |
| Electrode with MWCNTs-H2O2 | 0.09 ± 0.4 | 250 ± 80 | 25 ± 8 |
| Electrode with naphthylated MWCNTs | 0.03 ± 0.01 | 20 ± 0.4 | 2.00 ± 0.04 |
| Modification of the Anode, the Cathode Was Modified by MWCNTs-5 | ||||
| Type of Anode | OCP, mV | CCP, mV | P, μW | R, kΩ |
| Graphite rod + Chitosan + MWCNTs-5 | 215 ± 9 | 115 ± 5 | 1.64 ± 0.02 | 9.5 |
| Modified graphite rod + Chitosan + MWCNTs-5 | 125 ± 6 | 125 ± 3 | 1.4 ± 0.2 | 13 |
| Modified graphite rod + BSA + MWCNTs-5 | 100 ± 7 | 120 ± 4 | 1.3 ± 0.1 | 13 |
| Modification of the laccase cathode, the anode was formed according to paragraph 2.11 | ||||
| CNT type | OCP, mV | CCP, mV | P, μW | R, kΩ |
| Naphthylated MWCNTs | 120 ± 5 | 160 ± 3 | 5.3 ± 0.8 | 5 |
| MWCNTs-H2O2 | 130 ± 4 | 145 ± 5 | 5.4 ± 0.9 | 4 |
| MWCNTs-5 | 330 ± 17 | 135 ± 12 | 2.0 ± 0.1 | 10 |
| Derivative | Binding Affinity, kcal/mol | Distance to Copper, Å |
|---|---|---|
| Coronene, unmodified | −7.9 | 11.464 |
| Coronene, naphthylated (center) | −8.6 | 11.526 |
| Coronene, naphthylated (edge) | −9.0 | 11.973 |
| Coronene, phenolic (center) | −7.7 | 11.703 |
| Coronene, phenolic (edge) | −8.4 | 12.577 |
| Coronene, carboxylated (center) | −7.8 | 11.443 |
| Coronene, carboxylated (edge) | −8.3 | 11.332 |
| Coronene, lactone (center) | −8.6 | 12.274 |
| Coronene, lactone (edge) | −8.8 | 11.600 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullatypov, A.; Oskin, P.; Fedina, V.; Trubitsina, L.; Yakimovich, S.; Shuvalova, E.; Verma, P.; Dyachkova, T.; Ponamoreva, O.; Alferov, S. Functionalization of MWCNTs for Bioelectrocatalysis by Bacterial Two-Domain Laccase from Catenuloplanes japonicus. Nanomaterials 2023, 13, 3019. https://doi.org/10.3390/nano13233019
Abdullatypov A, Oskin P, Fedina V, Trubitsina L, Yakimovich S, Shuvalova E, Verma P, Dyachkova T, Ponamoreva O, Alferov S. Functionalization of MWCNTs for Bioelectrocatalysis by Bacterial Two-Domain Laccase from Catenuloplanes japonicus. Nanomaterials. 2023; 13(23):3019. https://doi.org/10.3390/nano13233019
Chicago/Turabian StyleAbdullatypov, Azat, Pavel Oskin, Veronika Fedina, Liubov Trubitsina, Sofiya Yakimovich, Ekaterina Shuvalova, Pradeep Verma, Tatyana Dyachkova, Olga Ponamoreva, and Sergey Alferov. 2023. "Functionalization of MWCNTs for Bioelectrocatalysis by Bacterial Two-Domain Laccase from Catenuloplanes japonicus" Nanomaterials 13, no. 23: 3019. https://doi.org/10.3390/nano13233019
APA StyleAbdullatypov, A., Oskin, P., Fedina, V., Trubitsina, L., Yakimovich, S., Shuvalova, E., Verma, P., Dyachkova, T., Ponamoreva, O., & Alferov, S. (2023). Functionalization of MWCNTs for Bioelectrocatalysis by Bacterial Two-Domain Laccase from Catenuloplanes japonicus. Nanomaterials, 13(23), 3019. https://doi.org/10.3390/nano13233019







