Ti3C2-MXene/NiO Nanocomposites-Decorated CsPbI3 Perovskite Active Materials under UV-Light Irradiation for the Enhancement of Crystal-Violet Dye Photodegradation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ti3C2-MXene Nanosheets
2.3. Preparation of Ti3C2-MXene/NiO Composite
2.4. Preparation of CsPbI3
2.5. Preparation of Ti3C2-MXene/NiO/CsPbI3 Composites
2.6. Characterizations
2.7. Photocatalytic Degradation of Dye
3. Results and Discussion
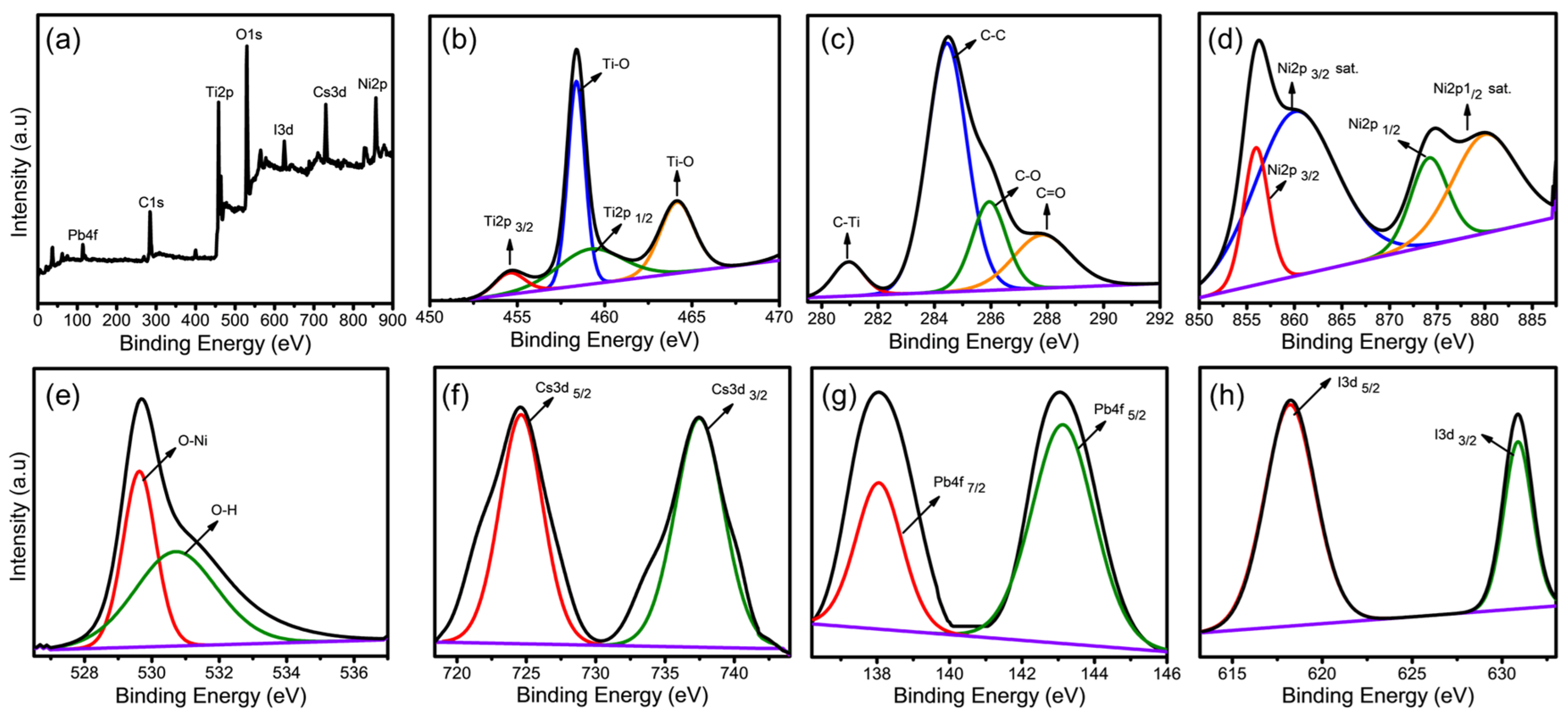
3.1. Surface Analysis of Ti3C2-MXene/NiO/CsPbI3 Composites
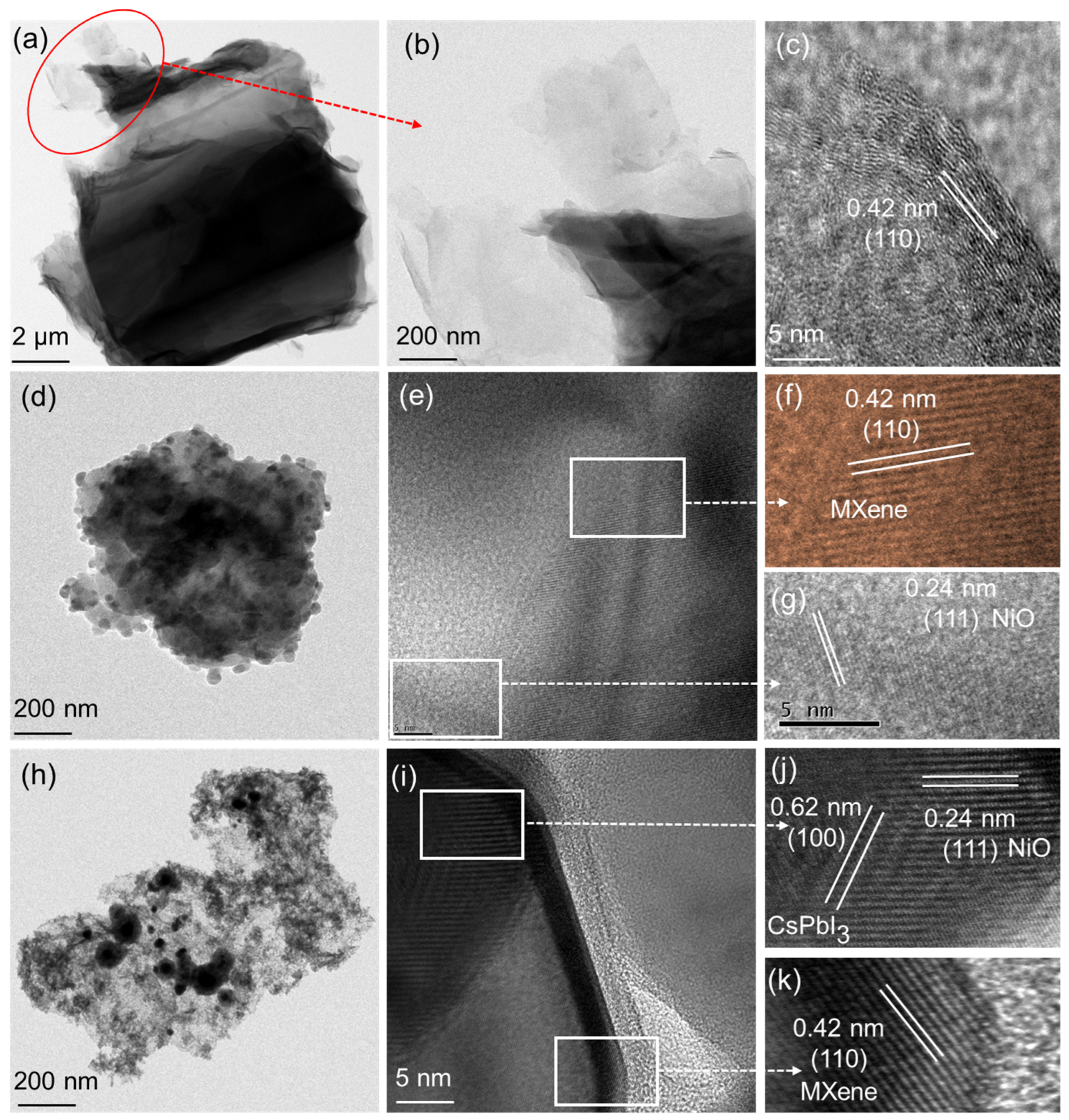
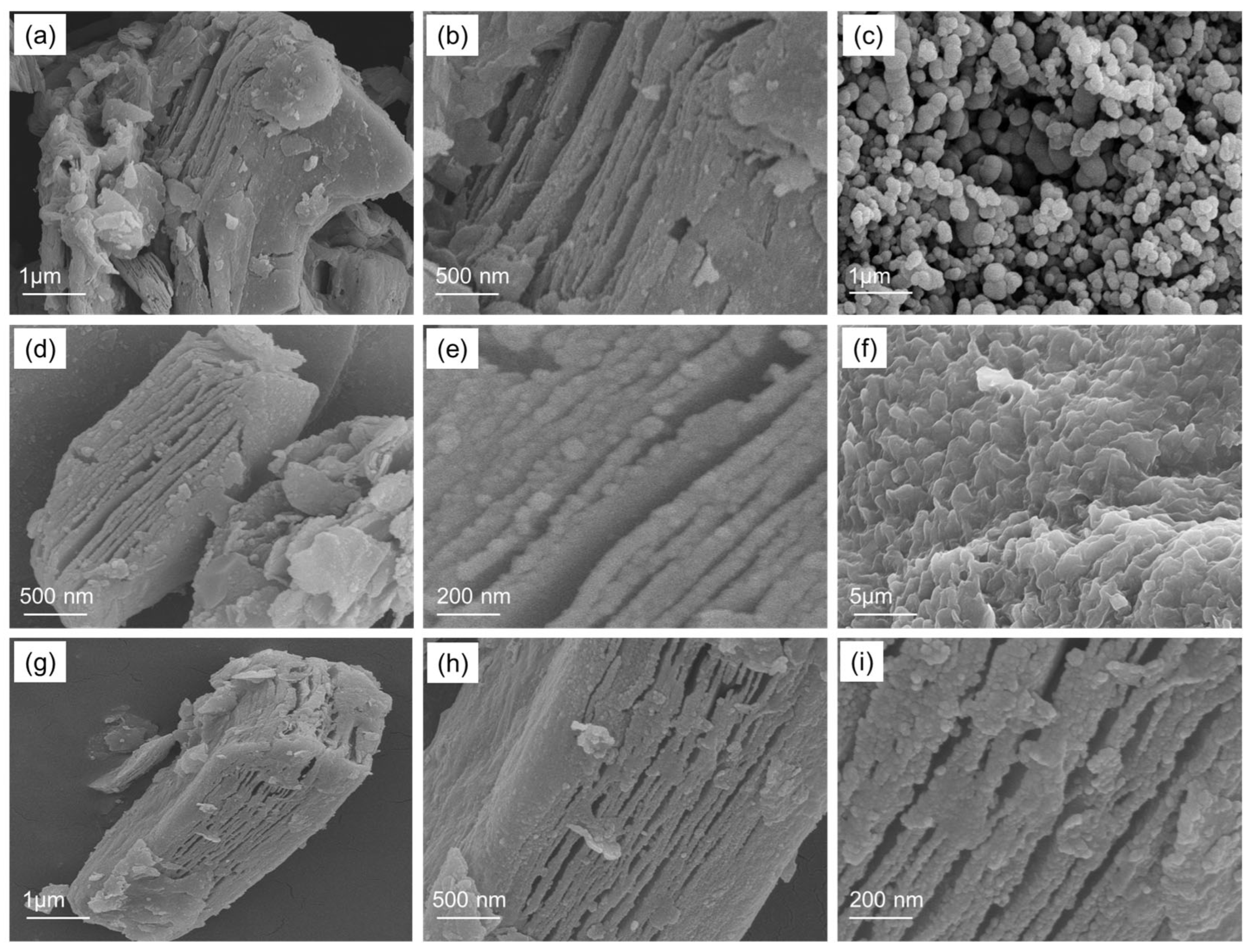
3.2. Morphological Properties of Ti3C2-MXene/NiO/CsPbI3 Composites
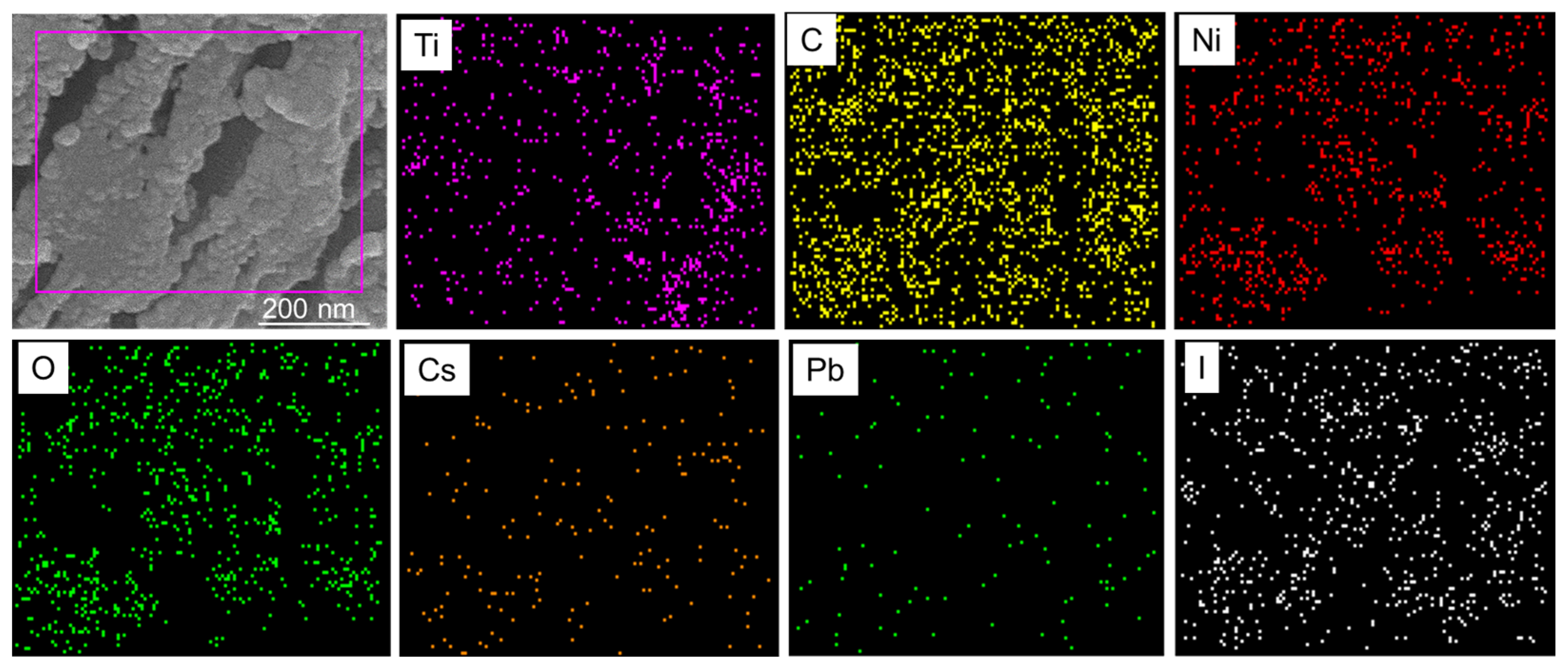
3.3. Elemental Composition of Ti3C2-MXene/NiO/CsPbI3 Composites
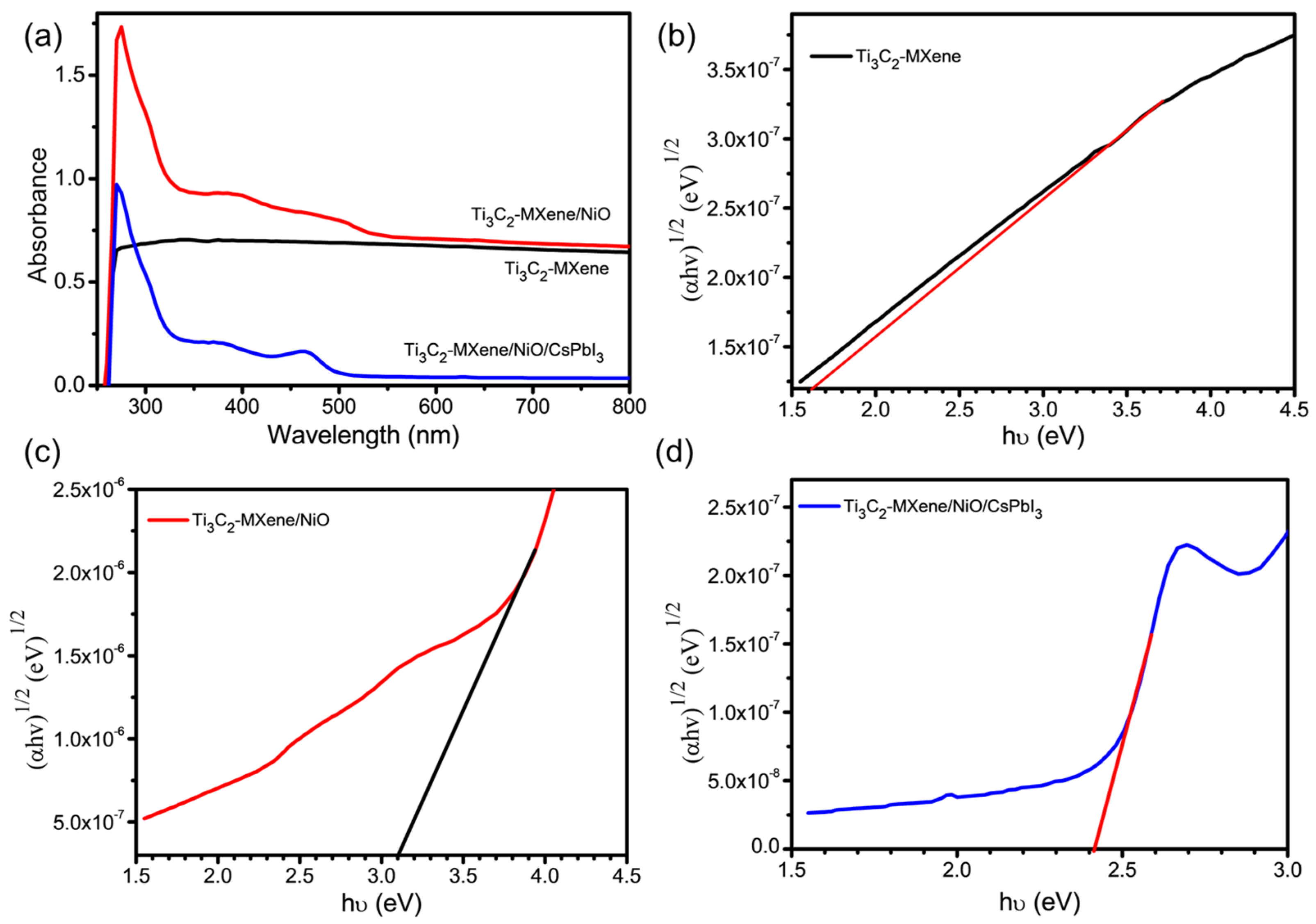
3.4. Optical Properties of Ti3C2-MXene/NiO/CsPbI3 Composites
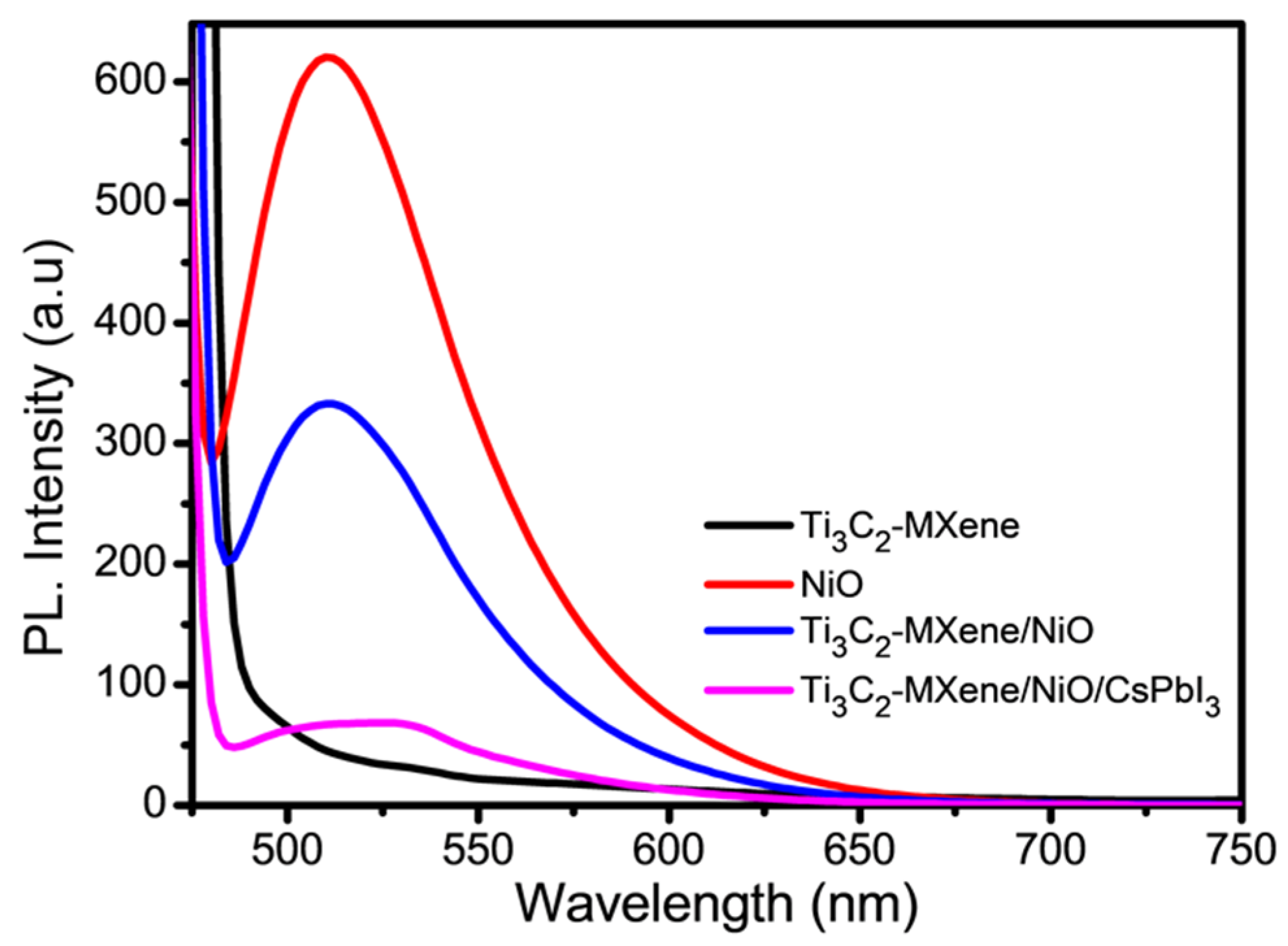
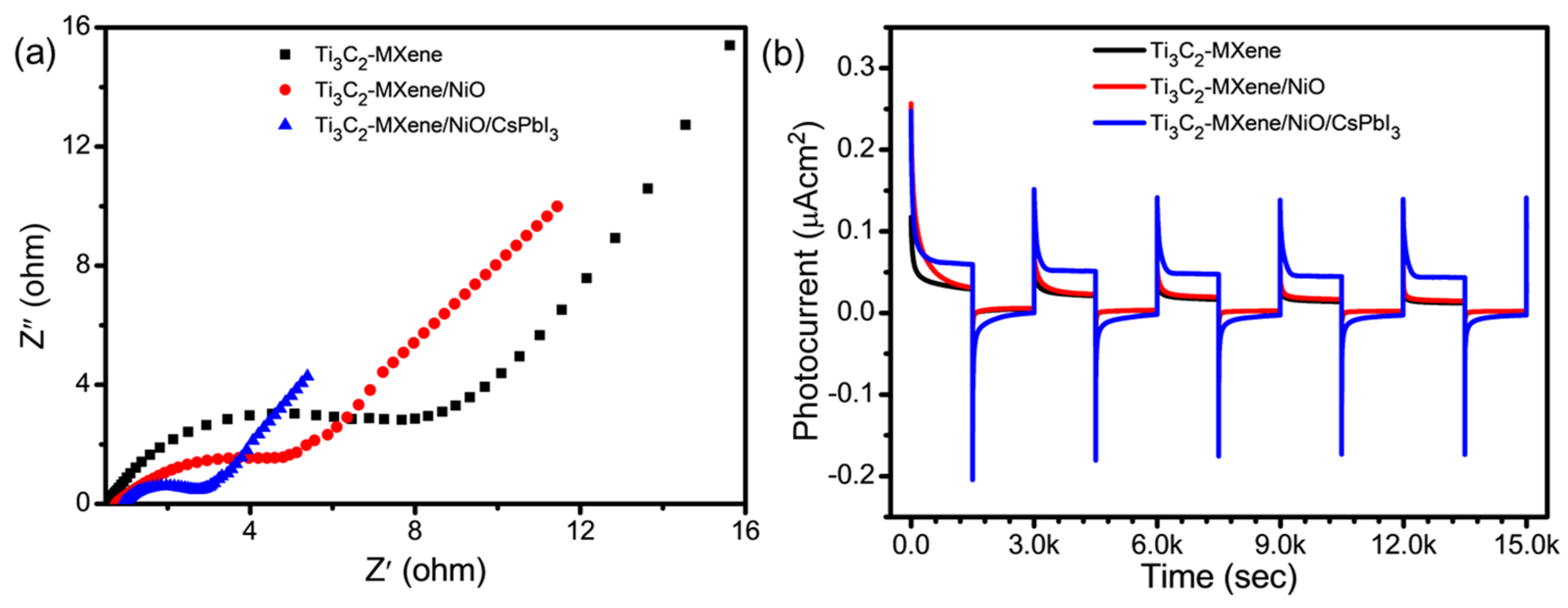
3.5. Charge-Transfer Behavior of Ti3C2-MXene/NiO/CsPbI3 Composites
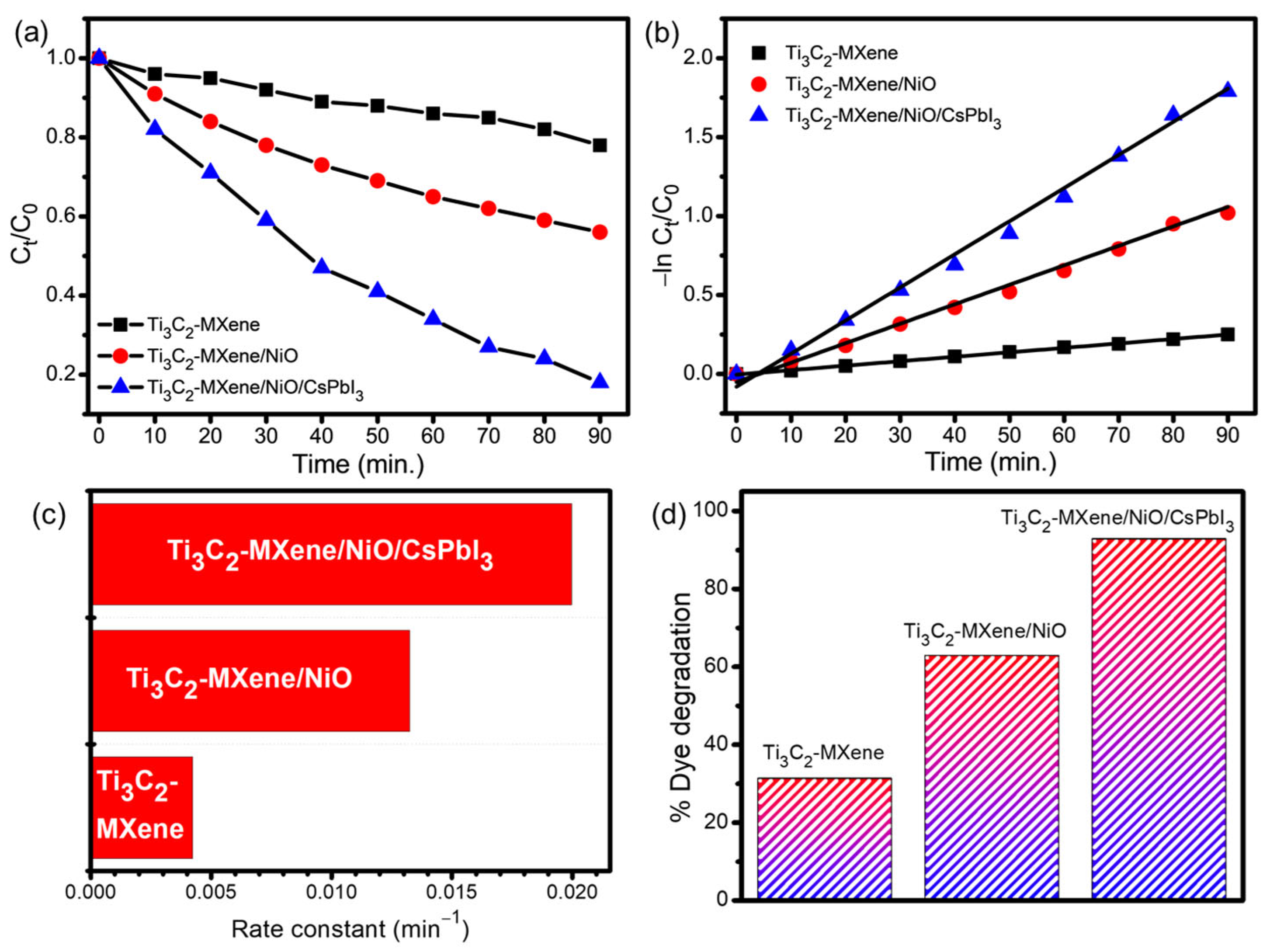
3.6. Photocatalytic Degradation of Dye
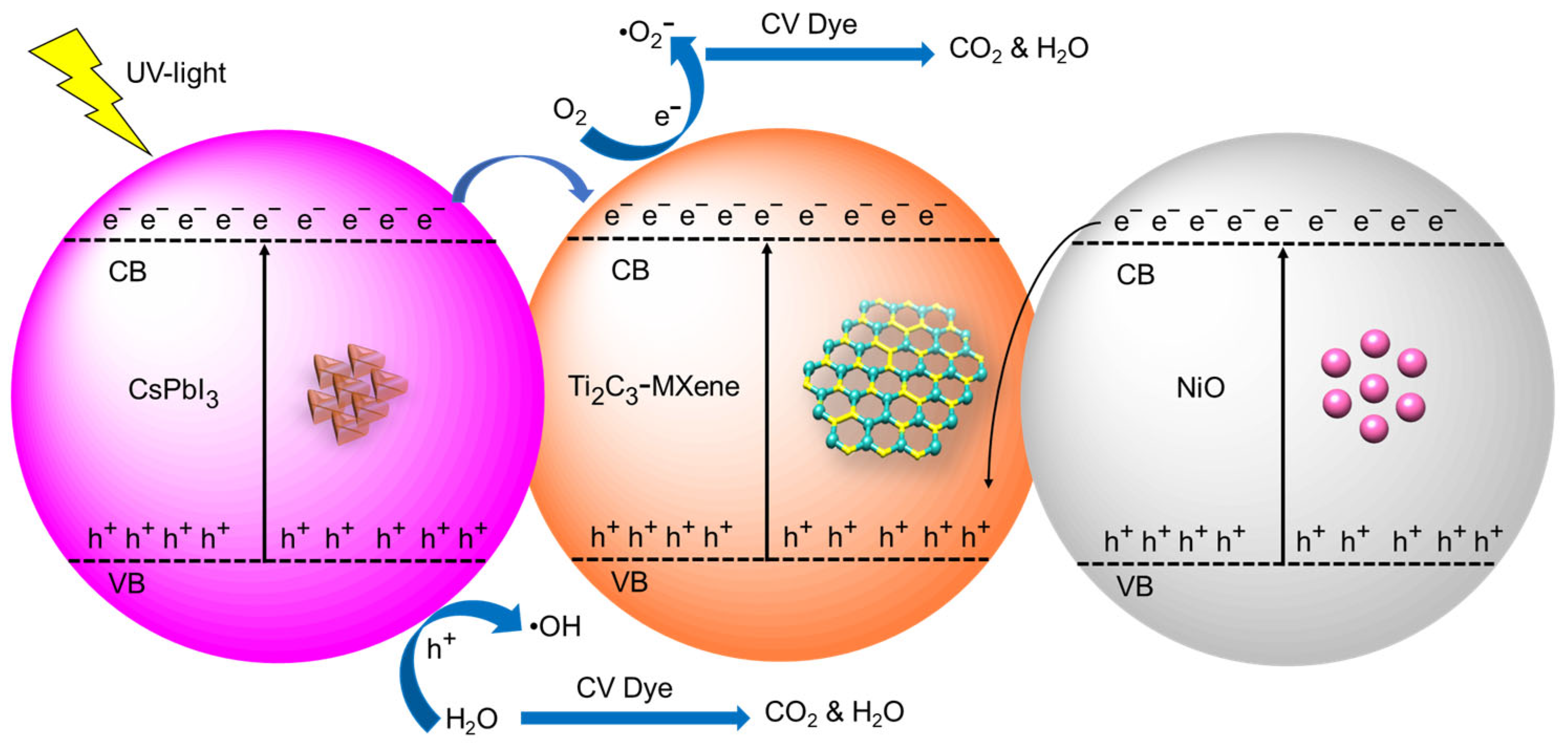
3.7. Proposed Photocatalytic Mechanism
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Chu, E.W.; Karr, J.R. Environmental Impact: Concept, Consequences, Measurement. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–22. [Google Scholar] [CrossRef]
- Akinnawo, S.O. Eutrophication: Causes, Consequences, Physical, Chemical and Biological Techniques for Mitigation Strategies. Environ. Chall. 2023, 12, 100733. [Google Scholar] [CrossRef]
- Afrin, S.; Shuvo, H.R.; Sultana, B.; Islam, F.; Rus’d, A.A.; Begum, S.; Hossain, M.N. The Degradation of Textile Industry Dyes Using the Effective Bacterial Consortium. Heliyon 2021, 7, e08102. [Google Scholar] [CrossRef] [PubMed]
- Benkhaya, S.; M’rabet, S.; El Harfi, A. Classifications, Properties, Recent Synthesis and Applications of Azo Dyes. Heliyon 2020, 6, E03271. [Google Scholar] [CrossRef] [PubMed]
- Shabir, M.; Yasin, M.; Hussain, M.; Shafiq, I.; Akhter, P.; Nizami, A.S.; Jeon, B.H.; Park, Y.K. A Review on Recent Advances in the Treatment of Dye-Polluted Wastewater. J. Ind. Eng. Chem. 2022, 112, 1–19. [Google Scholar] [CrossRef]
- Gómez-Gómez, A.L.; Martínez-Ayala, A.L.; Moguel-Concha, D.d.R.; Borges-Martínez, J.E.; Perea-Flores, M.d.J.; Dávila-Ortiz, G. Relationship of Nanomaterials’ Structure Based on Their Application in the Food Industry: Physicochemical and Techno-Functional Characteristics. Appl. Sci. 2023, 13, 7167. [Google Scholar] [CrossRef]
- Li, X.; Bai, Y.; Shi, X.; Su, N.; Nie, G.; Zhang, R.; Nie, H.; Ye, L. Applications of MXene (Ti3C2T: X) in Photocatalysis: A Review. Mater. Adv. 2021, 2, 1570–1594. [Google Scholar] [CrossRef]
- Yu, H.; Jiang, H.; Zhang, S.; Feng, X.; Yin, S.; Zhao, W. Review of Two-Dimensional MXenes (Ti3C2Tx) Materials in Photocatalytic Applications. Processes 2023, 11, 1413. [Google Scholar] [CrossRef]
- Amrillah, T.; Supandi, A.R.; Puspasari, V.; Hermawan, A.; Seh, Z.W. MXene-Based Photocatalysts and Electrocatalysts for CO2 Conversion to Chemicals. Trans. Tianjin Univ. 2022, 28, 307–322. [Google Scholar] [CrossRef]
- Raizada, P.; Soni, V.; Kumar, A.; Singh, P.; Parwaz Khan, A.A.; Asiri, A.M.; Thakur, V.K.; Nguyen, V.H. Surface Defect Engineering of Metal Oxides Photocatalyst for Energy Application and Water Treatment. J. Mater. 2021, 7, 388–418. [Google Scholar] [CrossRef]
- Park, H.; Son, N.; Park, B.H.; Joo, S.W.; Kang, M. Visible Light-Induced Stable HER Performance Using Duality of Ultrafine Pt NPs in a Z-Scheme p-n Junction Fe2O3@Pt@FeS Catalyst. Appl. Surf. Sci. 2021, 541, 148347. [Google Scholar] [CrossRef]
- Taeño, M.; Maestre, D.; Cremades, A. An Approach to Emerging Optical and Optoelectronic Applications Based on NiO Micro- and Nanostructures. Nanophotonics 2021, 10, 1785–1799. [Google Scholar] [CrossRef]
- Kumari, H.; Sonia; Suman; Ranga, R.; Chahal, S.; Devi, S.; Sharma, S.; Kumar, S.; Kumar, P.; Kumar, S.; et al. A Review on Photocatalysis Used for Wastewater Treatment: Dye Degradation. Water Air Soil Pollut. 2023, 234, 349. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Pradhan, B.; Hofkens, J.; Roeffaers, M.B.J.; Steele, J.A. Solar-Driven Metal Halide Perovskite Photocatalysis: Design, Stability, and Performance. ACS Energy Lett. 2020, 5, 1107–1123. [Google Scholar] [CrossRef]
- Fu, H.; Liu, X.; Fu, J.; Wu, Y.; Zhang, Q.; Wang, Z.; Liu, Y.; Zheng, Z.; Cheng, H.; Dai, Y.; et al. 2D/Quasi-2D Ruddlesden—Popper Perovskite: A High-Performance Photocatalyst for Hydrogen Evolution. ACS Catal. 2023, 13, 14716–14724. [Google Scholar] [CrossRef]
- Drozdowski, D.; Gągor, A.; Stefańska, D.; Zarȩba, J.K.; Fedoruk, K.; Mączka, M.; Sieradzki, A. Three-Dimensional Methylhydrazinium Lead Halide Perovskites: Structural Changes and Effects on Dielectric, Linear, and Nonlinear Optical Properties Entailed by the Halide Tuning. J. Phys. Chem. C 2022, 126, 1600–1610. [Google Scholar] [CrossRef]
- Shamsi, J.; Urban, A.S.; Imran, M.; De Trizio, L.; Manna, L. Metal Halide Perovskite Nanocrystals: Synthesis, Post-Synthesis Modifications, and Their Optical Properties. Chem. Rev. 2019, 119, 3296–3348. [Google Scholar] [CrossRef]
- Temerov, F.; Baghdadi, Y.; Rattner, E.; Eslava, S. A Review on Halide Perovskite-Based Photocatalysts: Key Factors and Challenges. ACS Appl. Energy Mater. 2022, 5, 14605–14637. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Zhang, W.; Yang, H.; Fan, Q.; Xiong, F.; Liu, S.; Li, D.S.; Liu, B. Halide Perovskite Composites for Photocatalysis: A Mini Review. EcoMat 2021, 3, e12079. [Google Scholar] [CrossRef]
- Dandia, A.; Saini, P.; Sharma, R.; Parewa, V. Visible Light Driven Perovskite-Based Photocatalysts: A New Candidate for Green Organic Synthesis by Photochemical Protocol. Curr. Res. Green Sustain. Chem. 2020, 3, 100031. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, A.; Cao, Y.; Xie, J.; Jia, W.; Jia, D. Interstitial N-Doped SrSnO3 Perovskite: Structural Design, Modification and Photocatalytic Degradation of Dyes. New J. Chem. 2019, 43, 10965–10972. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, J.; Geng, J.; Bao, R.; Wang, Z.; Xia, J.; Li, H. Perovskite LaNiO3/TiO2 Step-Scheme Heterojunction with Enhanced Photocatalytic Activity. Appl. Surf. Sci. 2020, 503, 144287. [Google Scholar] [CrossRef]
- Kitchamsetti, N.; Didwal, P.N.; Mulani, S.R.; Patil, M.S.; Devan, R.S. Photocatalytic Activity of MnTiO3 Perovskite Nanodiscs for the Removal of Organic Pollutants. Heliyon 2021, 7, e07297. [Google Scholar] [CrossRef] [PubMed]
- Lele, N.; Bambo, M.F.; Mmutlane, E.M.; Dlamini, L.N. Construction of a Multifunctional MXene@β-Cyclodextrin Nanocomposite with Photocatalytic Properties. Emergent Mater. 2023, 6, 605–626. [Google Scholar] [CrossRef]
- Tan, L.; Lv, J.; Xu, X.; Zhao, H.; He, C.; Wang, H.; Zheng, W. Construction of MXene/NiO Composites through in-Situ Precipitation Strategy for Dispersibility Improvement of NiO Nanoparticles. Ceram. Int. 2019, 45, 6597–6600. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, X.; Zhou, Y.; Jiang, Q.; Ye, Q.; Chu, Z.; Li, X.; Yang, X.; Yin, Z.; You, J. Solvent-Controlled Growth of Inorganic Perovskite Films in Dry Environment for Efficient and Stable Solar Cells. Nat. Commun. 2018, 9, 2225. [Google Scholar] [CrossRef]
- Karthikeyan, P.; Elanchezhiyan, S.S.; Preethi, J.; Talukdar, K.; Meenakshi, S.; Park, C.M. Two-Dimensional (2D) Ti3C2Tx MXene Nanosheets with Superior Adsorption Behavior for Phosphate and Nitrate Ions from the Aqueous Environment. Ceram. Int. 2021, 47, 732–739. [Google Scholar] [CrossRef]
- Chavan, R.A.; Kamble, G.P.; Dhavale, S.B.; Rasal, A.S.; Kolekar, S.S.; Chang, J.Y.; Ghule, A.V. NiO@MXene Nanocomposite as an Anode with Enhanced Energy Density for Asymmetric Supercapacitors. Energy Fuels 2023, 37, 4658–4670. [Google Scholar] [CrossRef]
- Shu, M.; Li, R.; Zhang, Z. CsPbI3 Quantum Dots/Polypyrrole Microrod 0D/1D Heterostructure: Synthesis, Formation Mechanism and Enhanced Charge Transport Property. Mater. Chem. Phys. 2021, 274, 125193. [Google Scholar] [CrossRef]
- Mahmood, M.; Rasheed, A.; Ayman, I.; Rasheed, T.; Munir, S.; Ajmal, S.; Agboola, P.O.; Warsi, M.F.; Shahid, M. Synthesis of Ultrathin MnO2nanowire-Intercalated 2D-MXenes for High-Performance Hybrid Supercapacitors. Energy Fuels 2021, 35, 3469–3478. [Google Scholar] [CrossRef]
- Zhang, K.; Ying, G.; Liu, L.; Ma, F.; Su, L.; Zhang, C.; Wu, D.; Wang, X.; Zhou, Y. Three-Dimensional Porous Ti3C2 Tx-NiO Composite Electrodes with Enhanced Electrochemical Performance for Supercapacitors. Materials 2019, 12, 188. [Google Scholar] [CrossRef]
- Shen, Q.; Ripolles, T.S.; Even, J.; Ogomi, Y.; Nishinaka, K.; Izuishi, T.; Nakazawa, N.; Zhang, Y.; Ding, C.; Liu, F.; et al. Slow Hot Carrier Cooling in Cesium Lead Iodide Perovskites. Appl. Phys. Lett. 2017, 111, 153903. [Google Scholar] [CrossRef]
- Kiran, N.U.; Deore, A.B.; More, M.A.; Late, D.J.; Rout, C.S.; Mane, P.; Chakraborty, B.; Besra, L.; Chatterjee, S. Comparative Study of Cold Electron Emission from 2D Ti3C2TXMXene Nanosheets with Respect to Its Precursor Ti3SiC2MAX Phase. ACS Appl. Electron. Mater. 2022, 4, 2656–2666. [Google Scholar] [CrossRef]
- Huang, B.; Tong, X.; Zhang, X.; Feng, Q.; Rumyantseva, M.N.; Prakash, J.; Li, X. MXene/NiO Composites for Chemiresistive-Type Room Temperature Formaldehyde Sensor. Chemosensors 2023, 11, 258. [Google Scholar] [CrossRef]
- Zhang, Q.; Tai, M.; Zhou, Y.; Zhou, Y.; Wei, Y.; Tan, C.; Wu, Z.; Li, J.; Lin, H. Enhanced Photocatalytic Property of γ-CsPbI3 Perovskite Nanocrystals with WS2. ACS Sustain. Chem. Eng. 2020, 8, 1219–1229. [Google Scholar] [CrossRef]
- Aleksanyan, E.; Aprahamian, A.; Mukasyan, A.S.; Harutyunyan, V.; Manukyan, K.V. Mechanisms of Mechanochemical Synthesis of Cesium Lead Halides: Pathways toward Stabilization of α-CsPbI3. J. Mater. Sci. 2020, 55, 8665–8678. [Google Scholar] [CrossRef]
- Gull, S.; Batool, S.; Li, G.; Idrees, M. Synthesis of Cesium Lead Halide Perovskite/Zinc Oxide (CsPbX3/ZnO, X = Br, I) as Heterostructure Photocatalyst with Improved Activity for Heavy Metal Degradation. Front. Chem. 2022, 10, 1020484. [Google Scholar] [CrossRef]
- Ding, C.; Chen, X.; Zhang, T.; Zhou, C.; Liu, X.; Wang, J.; Lin, J.; Chen, X. Electrochemical Synthesis of Annealing-Free and Highly Stable Black-Phase CsPbI3perovskite. Chem. Commun. 2021, 57, 8981–8984. [Google Scholar] [CrossRef]
- Luo, Q.; Chai, B.; Xu, M.; Cai, Q. Preparation and Photocatalytic Activity of TiO2-Loaded Ti3C2 with Small Interlayer Spacing. Appl. Phys. A Mater. Sci. Process. 2018, 124, 495. [Google Scholar] [CrossRef]
- El-Kemary, M.; Nagy, N.; El-Mehasseb, I. Nickel Oxide Nanoparticles: Synthesis and Spectral Studies of Interactions with Glucose. Mater. Sci. Semicond. Process. 2013, 16, 1747–1752. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Tariq, A.; Zaheer, A.; Gul, S.; Ali, S.I.; Iqbal, M.Z.; Akinwande, D.; Rizwan, S. Ti3C2-MXene/Bismuth Ferrite Nanohybrids for Efficient Degradation of Organic Dyes and Colorless Pollutants. ACS Omega 2019, 4, 20530–20539. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Ali, S.I.; Amin, F.; Tariq, A.; Iqbal, M.Z.; Rizwan, S. La-and Mn-Codoped Bismuth Ferrite/Ti3C2 MXene Composites for Efficient Photocatalytic Degradation of Congo Red Dye. ACS Omega 2019, 4, 8661–8668. [Google Scholar] [CrossRef] [PubMed]
- Khan, J.; Ahmad, R.T.M.; Yu, Q.; Liu, H.; Khan, U.; Liu, B. A La2O3/MXene Composite Electrode for Supercapacitors with Improved Capacitance and Cycling Performance. Sci. Technol. Adv. Mater. 2023, 24, 2242262. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wen, C.; Yuan, M.; Sun, Z.; Wei, Y.; Ma, L.; Li, H.; Sun, G. Nickel Oxide Nanoparticles Decorated Highly Conductive Ti3C2 MXene as Cathode Catalyst for Rechargeable Li–O2 Battery. J. Alloys Compd. 2020, 824, 153803. [Google Scholar] [CrossRef]
- Neampet, S.; Ruecha, N.; Qin, J.; Wonsawat, W.; Chailapakul, O.; Rodthongkum, N. A Nanocomposite Prepared from Platinum Particles, Polyaniline and a Ti3C2 MXene for Amperometric Sensing of Hydrogen Peroxide and Lactate. Microchim. Acta 2019, 186, 752. [Google Scholar] [CrossRef]
- Sekar, S.; Bathula, C.; Rabani, I.; Lee, J.W.; Lee, S.H.; Seo, Y.S.; Lee, S. Enhanced Photocatalytic Crystal-Violet Degradation Performances of Sonochemically-Synthesized AC-CeO2 Nanocomposites. Ultrason. Sonochem. 2022, 90, 10677. [Google Scholar] [CrossRef]
- Le, V.T.; Doan, V.D.; Le, T.T.N.; Dao, M.U.; Vo, T.T.T.; Do, H.H.; Viet, D.Q.; Tran, V.A. Efficient Photocatalytic Degradation of Crystal Violet under Natural Sunlight Using Fe3O4/ZnO Nanoparticles Embedded Carboxylate-Rich Carbon. Mater. Lett. 2021, 283, 128749. [Google Scholar] [CrossRef]
- Alsafari, I.A.; Munir, S.; Zulfiqar, S.; Saif, M.S.; Warsi, M.F.; Shahid, M. Synthesis, Characterization, Photocatalytic and Antibacterial Properties of Copper Ferrite/MXene (CuFe2O4/Ti3C2) Nanohybrids. Ceram. Int. 2021, 47, 28874–28883. [Google Scholar] [CrossRef]
- Sun, B.; Tao, F.; Huang, Z.; Yan, W.; Zhang, Y.; Dong, X.; Wu, Y.; Zhou, G. Ti3C2 MXene-Bridged Ag/Ag3PO4 Hybrids toward Enhanced Visible-Light-Driven Photocatalytic Activity. Appl. Surf. Sci. 2021, 535, 147354. [Google Scholar] [CrossRef]
- Chaiwichian, S.; Wetchakun, K.; Kangwansupamonkon, W.; Wetchakun, N. Novel Visible-Light-Driven BiFeO3-Bi2WO6 Nanocomposites toward Degradation of Dyes. J. Photochem. Photobiol. A Chem. 2017, 349, 183–192. [Google Scholar] [CrossRef]
- Qamar, S.; Fatima, K.; Ullah, N.; Akhter, Z.; Waseem, A.; Sultan, M. Recent Progress in Use of MXene in Perovskite Solar Cells: For Interfacial Modification, Work-Function Tuning and Additive Engineering. Nanoscale 2022, 14, 13018–13039. [Google Scholar] [CrossRef]
- Song, T.; Hou, L.; Long, B.; Ali, A.; Deng, G.J. Ultrathin MXene “Bridge” to Accelerate Charge Transfer in Ultrathin Metal-Free 0D/2D Black Phosphorus/g-C3N4 Heterojunction toward Photocatalytic Hydrogen Production. J. Colloid Interface Sci. 2021, 584, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Fu, X.; Zhou, Z. 3D/2D Heterojunction of CeO2/Ultrathin MXene Nanosheets for Photocatalytic Hydrogen Production. ACS Omega 2022, 7, 21684–21693. [Google Scholar] [CrossRef] [PubMed]
- Sanakousar, M.F.; Vidyasagar, C.C.; Jiménez-Pérez, V.M.; Jayanna, B.K.; Mounesh; Shridhar, A.H.; Prakash, K. Efficient Photocatalytic Degradation of Crystal Violet Dye and Electrochemical Performance of Modified MWCNTs/Cd-ZnO Nanoparticles with Quantum Chemical Calculations. J. Hazard. Mater. Adv. 2021, 2, 100004. [Google Scholar] [CrossRef]
- Filipe, O.M.S.; Santos, E.B.H.; Otero, M.; Gonçalves, E.A.C.; Neves, M.G.P.M.S. Photodegradation of Metoprolol in the Presence of Aquatic Fulvic Acids. Kinetic Studies, Degradation Pathways and Role of Singlet Oxygen, OH Radicals and Fulvic Acids Triplet States. J. Hazard. Mater. 2020, 385, 121523. [Google Scholar] [CrossRef] [PubMed]
- Nosaka, Y.; Nosaka, A. Understanding Hydroxyl Radical (•OH) Generation Processes in Photocatalysis. ACS Energy Lett. 2016, 1, 356–359. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhang, L.; Zuo, P.; Wang, Y.; Yu, Z. Enhanced Photocatalytic Performance of Spherical BiOI/MnO2 Composite and Mechanism Investigation. RSC Adv. 2018, 8, 36161–36166. [Google Scholar] [CrossRef]
- Yamashita, H.; Ichihashi, Y.; Zhang, S.G.; Matsumura, Y.; Souma, Y.; Tatsumi, T.; Anpo, M. Photocatalytic Decomposition of NO at 275 K on Titanium Oxide Catalysts Anchored within Zeolite Cavities and Framework. Appl. Surf. Sci. 1997, 121–122, 305–309. [Google Scholar] [CrossRef]
- Li, C.; Kan, C.; Meng, X.; Liu, M.; Shang, Q.; Yang, Y.; Wang, Y.; Cui, X. Self-Assembly 2D Ti3C2/g-C3N4 MXene Heterojunction for Highly Efficient Photocatalytic Degradation of Tetracycline in Visible Wavelength Range. Nanomaterials 2022, 12, 4015. [Google Scholar] [CrossRef]
- Murali, G.; Reddy Modigunta, J.K.; Park, Y.H.; Lee, J.H.; Rawal, J.; Lee, S.Y.; In, I.; Park, S.J. A Review on MXene Synthesis, Stability, and Photocatalytic Applications. ACS Nano 2022, 16, 13370–13429. [Google Scholar] [CrossRef]
- Liu, C.; Xiao, W.; Liu, X.; Wang, Q.; Hu, J.; Zhang, S.; Xu, J.; Zhang, Q.; Zou, Z. Rationally Designed Ti3C2 MXene/CaIn2S4 Schottky Heterojunction for Enhanced Photocatalytic Cr(VI) Reduction: Performance, Influence Factors and Mechanism. J. Mater. Sci. Technol. 2023, 161, 123–135. [Google Scholar] [CrossRef]
- Tahir, M.; Khan, A.A.; Tasleem, S.; Mansoor, R.; Sherryna, A.; Tahir, B. Recent Advances in Titanium Carbide MXene-Based Nanotextures with Influential Effect of Synthesis Parameters for Solar CO2 Reduction and H2 Production: A Critical Review. J. Energy Chem. 2023, 76, 295–331. [Google Scholar] [CrossRef]
- Liu, C.; Xiao, W.; Yu, G.; Wang, Q.; Hu, J.; Xu, C.; Du, X.; Xu, J.; Zhang, Q.; Zou, Z. Interfacial Engineering of Ti3C2 MXene/CdIn2S4 Schottky Heterojunctions for Boosting Visible-Light H2 Evolution and Cr(VI) Reduction. J. Colloid Interface Sci. 2023, 640, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Chinnasamy, C.; Perumal, N.; Choubey, A.; Rajendran, S. Recent Advancements in MXene-Based Nanocomposites as Photocatalysts for Hazardous Pollutant Degradation—A Review. Environ. Res. 2023, 233, 116459. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Navid, I.A.; Ma, Y.; Xiao, Y.; Wang, P.; Ye, Z.; Zhou, B.; Sun, K.; Mi, Z. Solar-to-Hydrogen Efficiency of More than 9% in Photocatalytic Water Splitting. Nature 2023, 613, 66–70. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Q.; Zou, Z. Recent Advances in Designing ZnIn2S4-Based Heterostructured Photocatalysts for Hydrogen Evolution. J. Mater. Sci. Technol. 2023, 139, 167–188. [Google Scholar] [CrossRef]
- Sepehrmansourie, H.; Alamgholiloo, H.; Noroozi Pesyan, N.; Zolfigol, M.A. A MOF-on-MOF Strategy to Construct Double Z-Scheme Heterojunction for High-Performance Photocatalytic Degradation. Appl. Catal. B Environ. 2023, 321, 122082. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Y.; Huang, H.; Wang, J.; He, G.; Feng, J.; Yu, T.; Li, Z.; Zou, Z. Spatial Decoupling of Redox Chemistry for Efficient and Highly Selective Amine Photoconversion to Imines. J. Am. Chem. Soc. 2023, 145, 7181–7189. [Google Scholar] [CrossRef]
- Xia, J.; Sohail, M.; Nazeeruddin, M.K. Efficient and Stable Perovskite Solar Cells by Tailoring of Interfaces. Adv. Mater. 2023, 35, 2211324. [Google Scholar] [CrossRef]
- Saranin, D.; Pescetelli, S.; Pazniak, A.; Rossi, D.; Liedl, A.; Yakusheva, A.; Luchnikov, L.; Podgorny, D.; Gostischev, P.; Didenko, S.; et al. Transition Metal Carbides (MXenes) for Efficient NiO-Based Inverted Perovskite Solar Cells. Nano Energy 2021, 82, 105771. [Google Scholar] [CrossRef]
- Vijayakumar, E.; Govinda Raj, M.; Narendran, M.G.; Preetha, R.; Mohankumar, R.; Neppolian, B.; John Bosco, A. Promoting Spatial Charge Transfer of ZrO2Nanoparticles: Embedded on Layered MoS2/g-C3N4Nanocomposites for Visible-Light-Induced Photocatalytic Removal of Tetracycline. ACS Omega 2022, 7, 5079–5095. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alothman, A.A.; Khan, M.R.; Albaqami, M.D.; Mohandoss, S.; Alothman, Z.A.; Ahmad, N.; Alqahtani, K.N. Ti3C2-MXene/NiO Nanocomposites-Decorated CsPbI3 Perovskite Active Materials under UV-Light Irradiation for the Enhancement of Crystal-Violet Dye Photodegradation. Nanomaterials 2023, 13, 3026. https://doi.org/10.3390/nano13233026
Alothman AA, Khan MR, Albaqami MD, Mohandoss S, Alothman ZA, Ahmad N, Alqahtani KN. Ti3C2-MXene/NiO Nanocomposites-Decorated CsPbI3 Perovskite Active Materials under UV-Light Irradiation for the Enhancement of Crystal-Violet Dye Photodegradation. Nanomaterials. 2023; 13(23):3026. https://doi.org/10.3390/nano13233026
Chicago/Turabian StyleAlothman, Asma A., Mohammad Rizwan Khan, Munirah D. Albaqami, Sonaimuthu Mohandoss, Zeid A. Alothman, Naushad Ahmad, and Khadraa N. Alqahtani. 2023. "Ti3C2-MXene/NiO Nanocomposites-Decorated CsPbI3 Perovskite Active Materials under UV-Light Irradiation for the Enhancement of Crystal-Violet Dye Photodegradation" Nanomaterials 13, no. 23: 3026. https://doi.org/10.3390/nano13233026




