Achieving Order in Disorder: Stabilizing Red Light-Emitting α-Phase Formamidinium Lead Iodide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Synthesis of δ-FAPbI3
2.3. Synthesis of α-FAPbI3
2.4. Characterization Methods
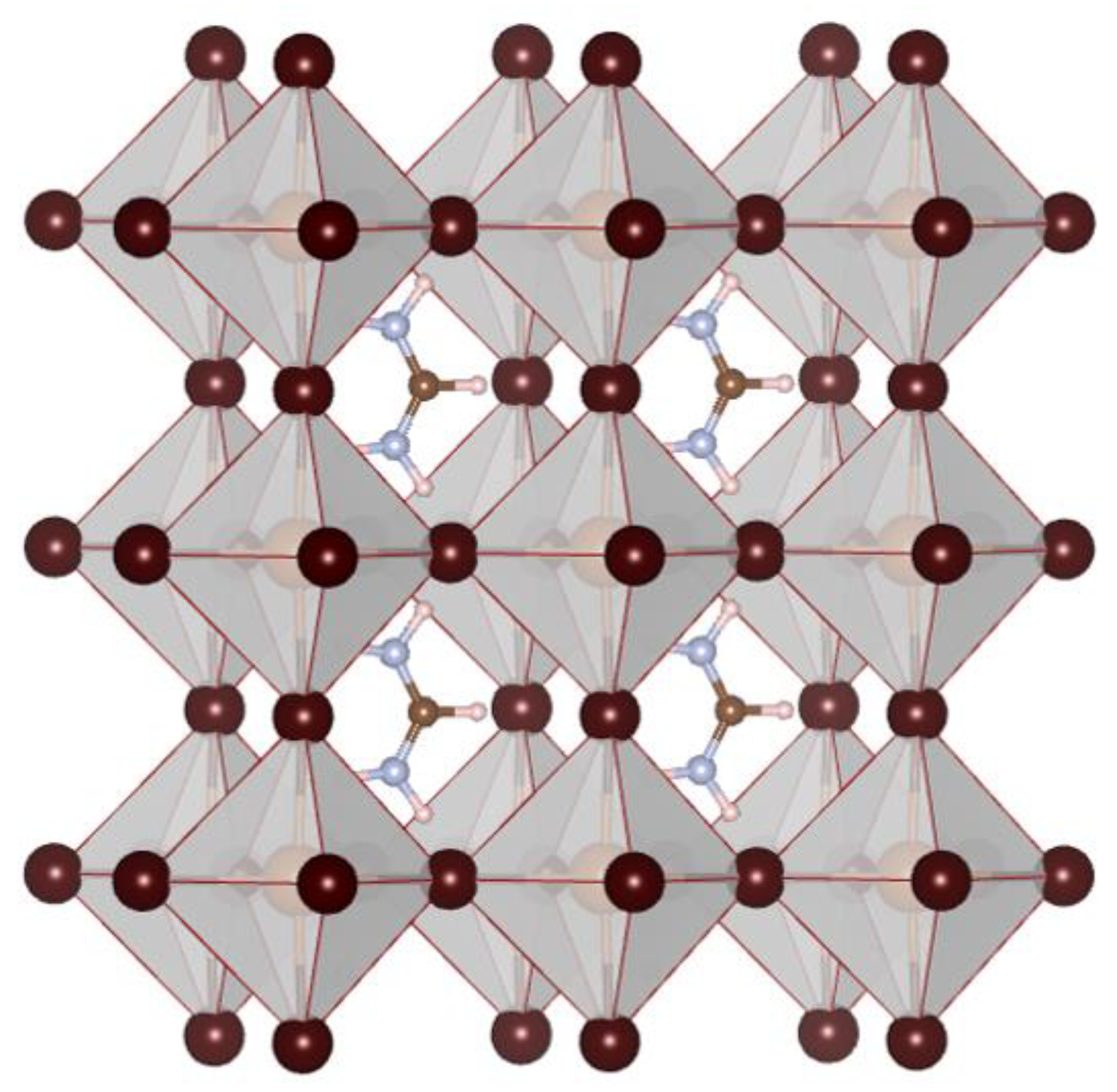
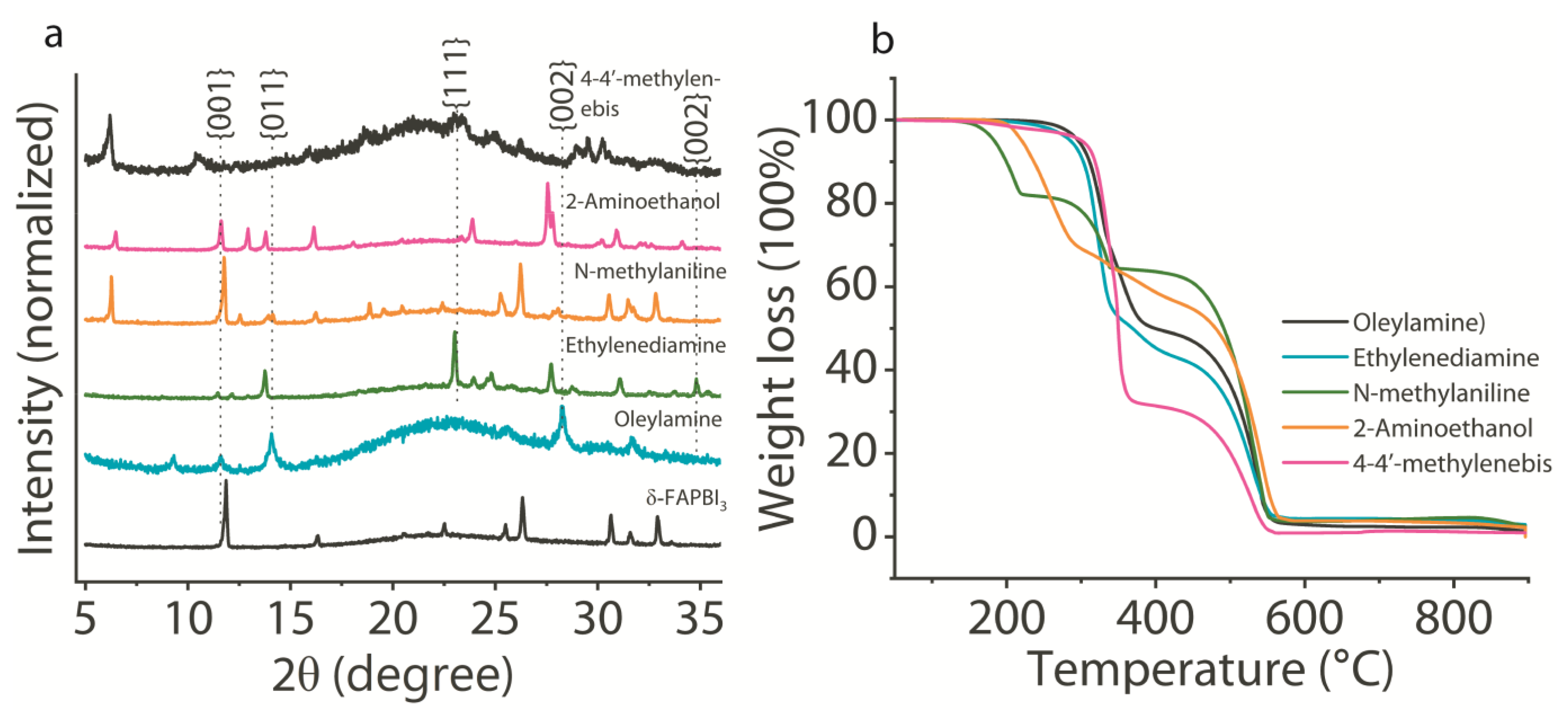
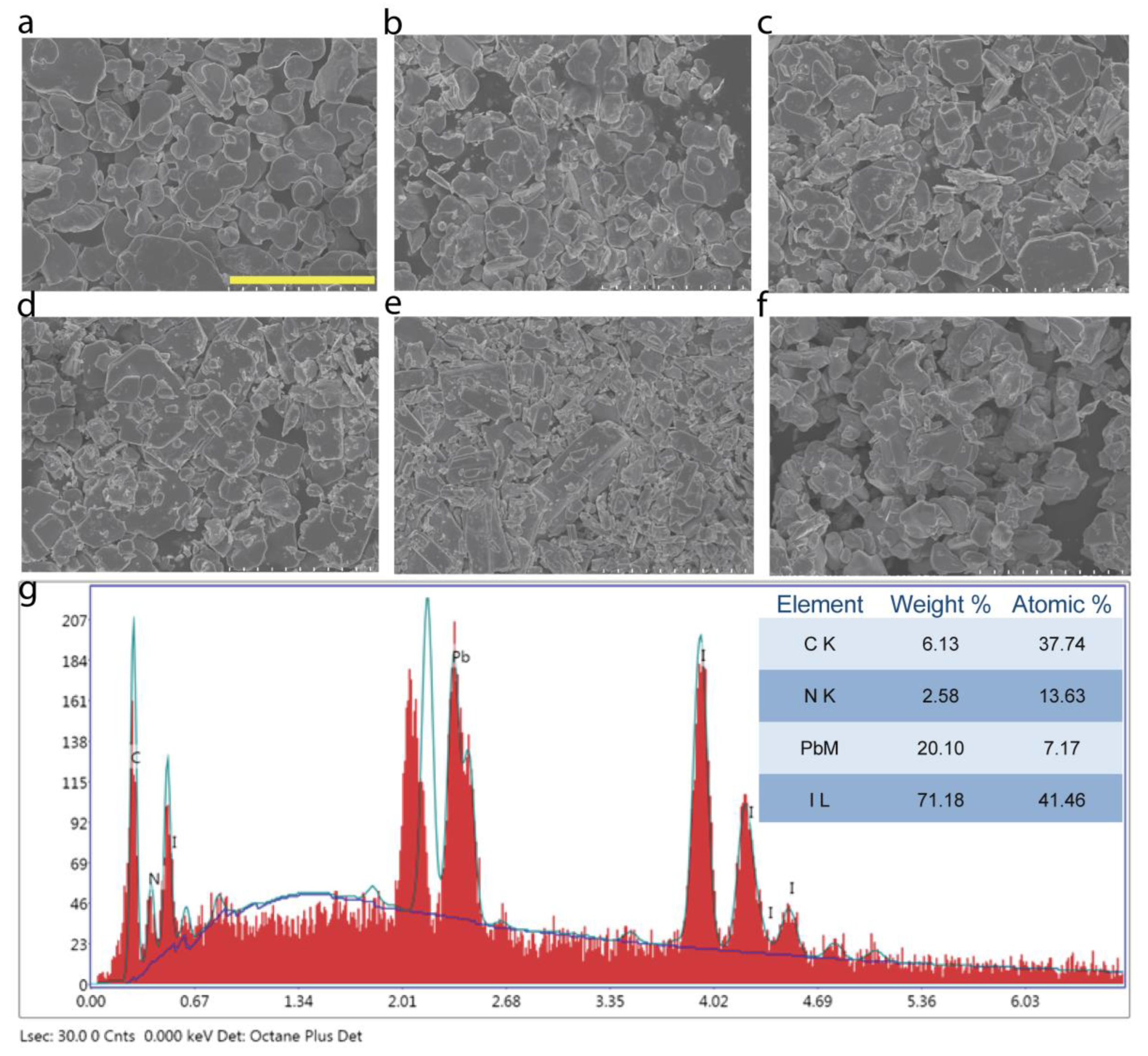
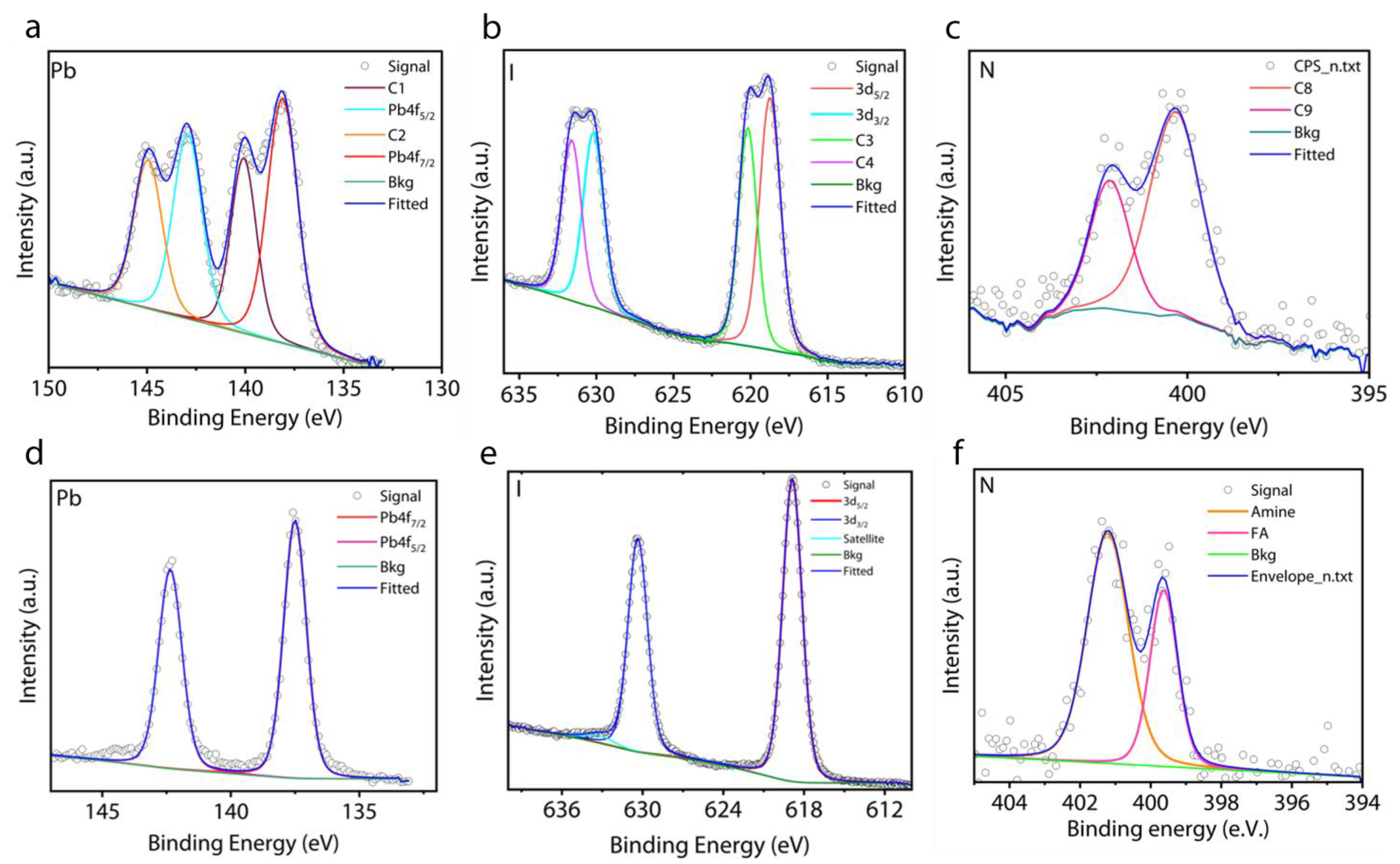
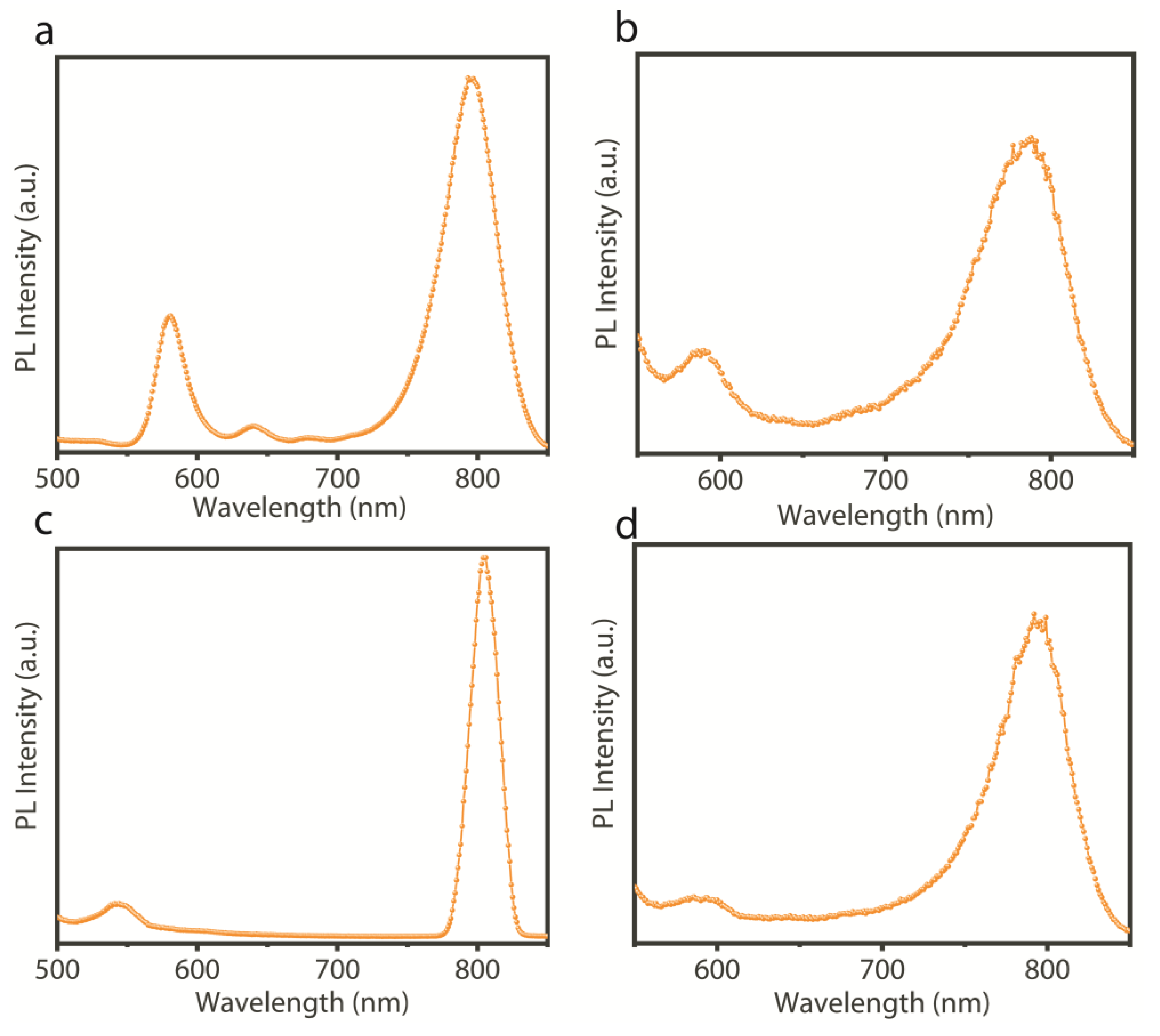
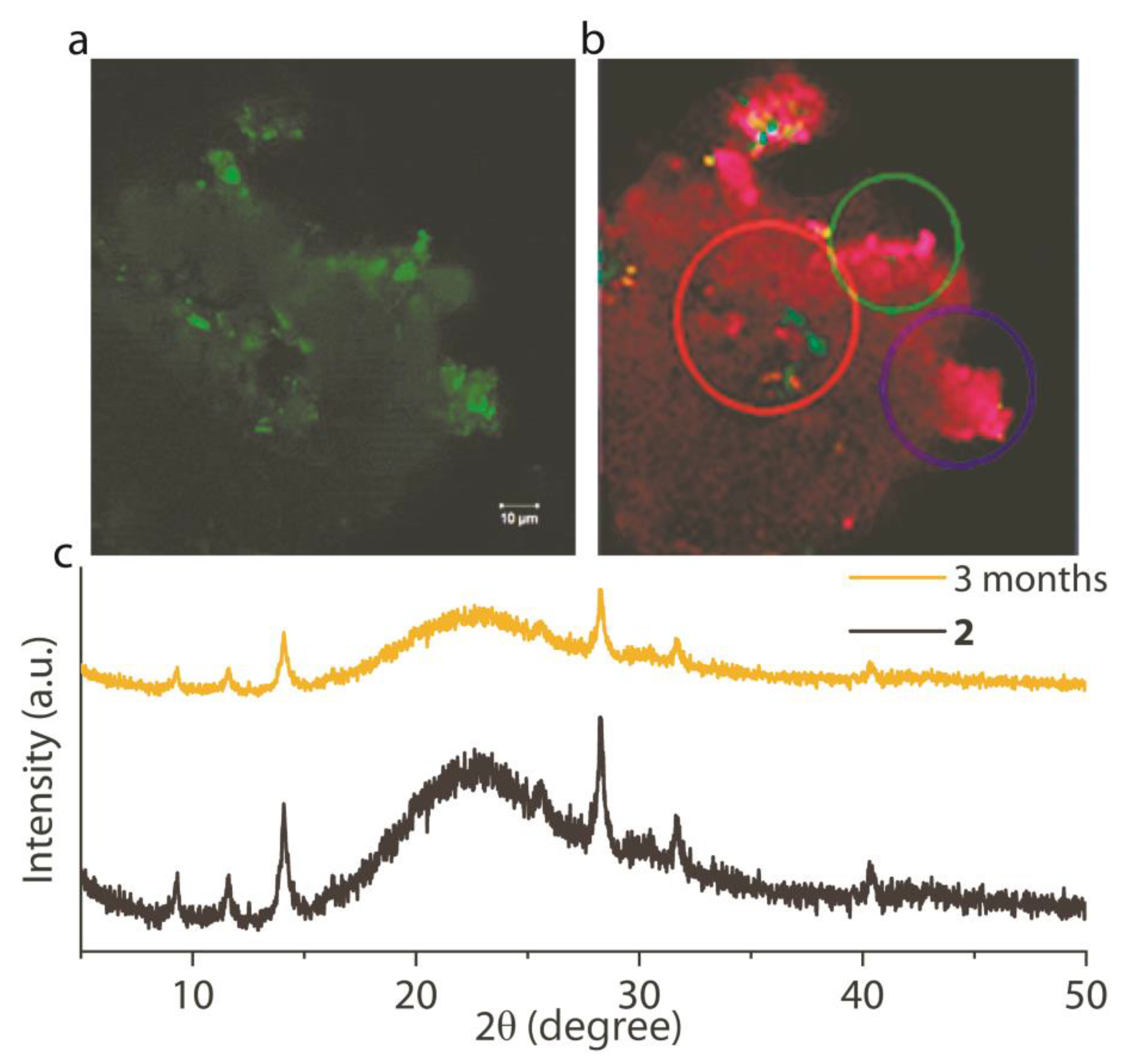
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wagner, L.; Mastroianni, S.; Hinsch, A. Reverse Manufacturing Enables Perovskite Photovoltaics to Reach the Carbon Footprint Limit of a Glass Substrate. Joule 2020, 4, 882–901. [Google Scholar] [CrossRef]
- Kurtz, S.; Haegel, N.; Sinton, R.; Margolis, R. A new era for solar. Nat. Photon. 2017, 11, 3–5. [Google Scholar] [CrossRef]
- Snaith, H.J. Perovskites: The Emergence of a New Era for Low-Cost, High-Efficiency Solar Cells. J. Phys. Chem. Lett. 2013, 4, 3623–3630. [Google Scholar] [CrossRef]
- Singh, A.N.; Kim, M.-H.; Meena, A.; Wi, T.-U.; Lee, H.-W.; Kim, K.S. Na/Al Codoped Layered Cathode with Defects as Bifunctional Electrocatalyst for High-Performance Li-Ion Battery and Oxygen Evolution Reaction. Small 2021, 17, 2005605. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.N.; Islam, M.; Meena, A.; Faizan, M.; Han, D.; Bathula, C.; Hajibabaei, A.; Anand, R.; Nam, K.-W. Unleashing the Potential of Sodium-Ion Batteries: Current State and Future Directions for Sustainable Energy Storage. Adv. Funct. Mater. 2023, 33, 2304617. [Google Scholar] [CrossRef]
- Steele, B.C.H.; Heinzel, A. Materials for fuel-cell technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Cullen, D.A.; Neyerlin, K.C.; Ahluwalia, R.K.; Mukundan, R.; More, K.L.; Borup, R.L.; Weber, A.Z.; Myers, D.J.; Kusoglu, A. New roads and challenges for fuel cells in heavy-duty transportation. Nat. Energy 2021, 6, 462–474. [Google Scholar] [CrossRef]
- Pan, Y.; Xu, X.; Zhong, Y.; Ge, L.; Chen, Y.; Veder, J.-P.M.; Guan, D.; O’Hayre, R.; Li, M.; Wang, G.; et al. Direct evidence of boosted oxygen evolution over perovskite by enhanced lattice oxygen participation. Nat. Commun. 2020, 11, 2002. [Google Scholar] [CrossRef]
- Egger, D.A.; Rappe, A.M.; Kronik, L. Hybrid Organic–Inorganic Perovskites on the Move. Acc. Chem. Res. 2016, 49, 573–581. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhou, R.; Zhang, Z.; Dong, Z.; Xu, J. Boosted charge transfer and CO2 photoreduction by construction of S-scheme heterojunctions between Cs2AgBiBr6 nanosheets and two-dimensional metal–organic frameworks. J. Mater. Chem. C 2023, 11, 2540–2551. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, D.; Chu, Y.; Chang, L.; Xu, J. Space-Confined Growth of Cs2CuBr4 Perovskite Nanodots in Mesoporous CeO2 for Photocatalytic CO2 Reduction: Structure Regulation and Built-in Electric Field Construction. J. Phys. Chem. Lett. 2023, 14, 5249–5259. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, D.; Dong, Z.; Jiang, Y.; Li, X.; Chu, Y.; Xu, J. Lead-Free Cs2AgBiBr6 Nanocrystals Confined in MCM-48 Mesoporous Molecular Sieve for Efficient Photocatalytic CO2 Reduction. Sol. RRL 2023, 7, 2300038. [Google Scholar] [CrossRef]
- Jeon, N.J.; Noh, J.H.; Kim, Y.C.; Yang, W.S.; Ryu, S.; Seok, S.I. Solvent engineering for high-performance inorganic–organic hybrid perovskite solar cells. Nat. Mater. 2014, 13, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Jana, A.; Ba, Q.; Nissimagoudar, A.S.; Kim, K.S. Formation of a photoactive quasi-2D formamidinium lead iodide perovskite in water. J. Mater. Chem. A 2019, 7, 25785–25790. [Google Scholar] [CrossRef]
- Kong, X.; Li, Z.; Jiang, Y.; Xu, Z.; Feng, S.-P.; Zhou, G.; Liu, J.-M.; Gao, J. Improving stability and efficiency of perovskite solar cells via a cerotic acid interfacial layer. Surf. Interfaces 2021, 25, 101163. [Google Scholar] [CrossRef]
- Singh, A.N.; Kajal, S.; Kim, J.; Jana, A.; Kim, J.Y.; Kim, K.S. Interface Engineering Driven Stabilization of Halide Perovskites against Moisture, Heat, and Light for Optoelectronic Applications. Adv. Energy Mater. 2020, 10, 2000768. [Google Scholar] [CrossRef]
- Carpenella, V.; Ripanti, F.; Stellino, E.; Fasolato, C.; Nucara, A.; Petrillo, C.; Malavasi, L.; Postorino, P. High-Pressure Behavior of δ-Phase of Formamidinium Lead Iodide by Optical Spectroscopies. J. Phys. Chem. C 2023, 127, 2440–2447. [Google Scholar] [CrossRef]
- Yang, F.; Dong, L.; Jang, D.; Tam, K.C.; Zhang, K.; Li, N.; Guo, F.; Li, C.; Arrive, C.; Bertrand, M.; et al. Fully Solution Processed Pure α-Phase Formamidinium Lead Iodide Perovskite Solar Cells for Scalable Production in Ambient Condition. Adv. Energy Mater. 2020, 10, 2001869. [Google Scholar] [CrossRef]
- Bouich, A.; Torres, J.C.; Khattak, Y.H.; Baig, F.; Marí-Guaita, J.; Soucase, B.M.; Mendez-Blas, A.; Palacios, P. Bright future by controlling α/δ phase junction of formamidinium lead iodide doped by imidazolium for solar cells: Insight from experimental, DFT calculations and SCAPS simulation. Surf. Interfaces 2023, 40, 103159. [Google Scholar] [CrossRef]
- Ma, F.; Li, J.; Li, W.; Lin, N.; Wang, L.; Qiao, J. Stable α/δ phase junction of formamidinium lead iodide perovskites for enhanced near-infrared emission. Chem. Sci. 2017, 8, 800–805. [Google Scholar] [CrossRef]
- Kim, B.; Kim, J.; Park, N. First-principles identification of the charge-shifting mechanism and ferroelectricity in hybrid halide perovskites. Sci. Rep. 2020, 10, 19635. [Google Scholar] [CrossRef] [PubMed]
- Weller, M.T.; Weber, O.J.; Frost, J.M.; Walsh, A. Cubic Perovskite Structure of Black Formamidinium Lead Iodide, α-[HC(NH2)2]PbI3, at 298 K. J. Phys. Chem. Lett. 2015, 6, 3209–3212. [Google Scholar] [CrossRef]
- Lignos, I.; Morad, V.; Shynkarenko, Y.; Bernasconi, C.; Maceiczyk, R.M.; Protesescu, L.; Bertolotti, F.; Kumar, S.; Ochsenbein, S.T.; Masciocchi, N. Exploration of near-infrared-emissive colloidal multinary lead halide perovskite nanocrystals using an automated microfluidic platform. ACS Nano 2018, 12, 5504–5517. [Google Scholar] [CrossRef]
- Niu, T.; Chao, L.; Dong, X.; Fu, L.; Chen, Y. Phase-Pure α-FAPbI3 for Perovskite Solar Cells. J. Phys. Chem. Lett. 2022, 13, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-Y.; Wang, C.-P.; Raja, R.; Wang, L.; Tsao, C.-S.; Su, W.-F. High-efficiency bulk heterojunction perovskite solar cell fabricated by one-step solution process using single solvent: Synthesis and characterization of material and film formation mechanism. J. Mater. Chem. A 2018, 6, 4179–4188. [Google Scholar] [CrossRef]
- Huang, H.; Li, Y.; Tong, Y.; Yao, E.P.; Feil, M.W.; Richter, A.F.; Döblinger, M.; Rogach, A.L.; Feldmann, J.; Polavarapu, L. Spontaneous Crystallization of Perovskite Nanocrystals in Nonpolar Organic Solvents: A Versatile Approach for their Shape-Controlled Synthesis. Angew. Chem. Int. Ed. 2019, 58, 16558–16562. [Google Scholar] [CrossRef]
- Bathula, C.; Jana, A.; Opoku, H.; Youi, H.-K.; Ghfar, A.A.; Ahmed, A.T.A.; Kim, H.-S. Interfacial engineering of quasi-2-D formamidinium lead iodide nanosheets for perovskite solar cell by mechanochemical approach. Surf. Interfaces 2021, 27, 101551. [Google Scholar] [CrossRef]
- Zheng, X.; Wu, C.; Jha, S.K.; Li, Z.; Zhu, K.; Priya, S. Improved phase stability of formamidinium lead triiodide perovskite by strain relaxation. ACS Energy Lett. 2016, 1, 1014–1020. [Google Scholar] [CrossRef]
- Fu, Y.; Wu, T.; Wang, J.; Zhai, J.; Shearer, M.J.; Zhao, Y.; Hamers, R.J.; Kan, E.; Deng, K.; Zhu, X.Y.; et al. Stabilization of the Metastable Lead Iodide Perovskite Phase via Surface Functionalization. Nano Lett. 2017, 17, 4405–4414. [Google Scholar] [CrossRef]
- Calabrese, J.; Jones, N.L.; Harlow, R.L.; Herron, N.; Thorn, D.L.; Wang, Y. Preparation and characterization of layered lead halide compounds. J. Am. Chem. Soc. 1991, 113, 2328–2330. [Google Scholar] [CrossRef]
- Mitzi, D.B.; Feild, C.A.; Harrison, W.T.A.; Guloy, A.M. Conducting tin halides with a layered organic-based perovskite structure. Nature 1994, 369, 467–469. [Google Scholar] [CrossRef]
- Jayanthi, K.; Spanopoulos, I.; Zibouche, N.; Voskanyan, A.A.; Vasileiadou, E.S.; Islam, M.S.; Navrotsky, A.; Kanatzidis, M.G. Entropy Stabilization Effects and Ion Migration in 3D “Hollow” Halide Perovskites. J. Am. Chem. Soc. 2022, 144, 8223–8230. [Google Scholar] [CrossRef]
- Zhu, Y.; Jia, S.; Zheng, J.; Lin, Y.; Wu, Y.; Wang, J. Facile synthesis of nitrogen-doped graphene frameworks for enhanced performance of hole transport material-free perovskite solar cells. J. Mater. Chem. C 2018, 6, 3097–3103. [Google Scholar] [CrossRef]
- Stergiou, A.; Sideri, I.K.; Kafetzi, M.; Ioannou, A.; Arenal, R.; Mousdis, G.; Pispas, S.; Tagmatarchis, N. Methylammonium lead bromide perovskite nano-crystals grown in a poly [styrene-co-(2-(dimethylamino) ethyl methacrylate)] matrix immobilized on exfoliated graphene nano-sheets. Nanomaterials 2022, 12, 1275. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Tang, S.; Yuan, G.; Zhu, W.; Huang, Y.; Li, S.; Lin, M. Tailored graphene quantum dots to passivate defects and accelerate charge extraction for all-inorganic CsPbIBr2 perovskite solar cells. J. Alloys Compd. 2022, 895, 162529. [Google Scholar] [CrossRef]
- Fu, F.; Pisoni, S.; Jeangros, Q.; Sastre-Pellicer, J.; Kawecki, M.; Paracchino, A.; Moser, T.; Werner, J.; Andres, C.; Duchêne, L. I 2 vapor-induced degradation of formamidinium lead iodide based perovskite solar cells under heat–light soaking conditions. Energy Environ. Sci. 2019, 12, 3074–3088. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhou, Y.; Wei, Y.; Tai, M.; Nan, H.; Gu, Y.; Han, J.; Yin, X.; Li, J.; Lin, H. Improved phase stability of γ-CsPbI3 perovskite nanocrystals using the interface effect using iodine modified graphene oxide. J. Mater. Chem. C 2020, 8, 2569–2578. [Google Scholar] [CrossRef]
- Noel, N.K.; Abate, A.; Stranks, S.D.; Parrott, E.S.; Burlakov, V.M.; Goriely, A.; Snaith, H.J. Enhanced Photoluminescence and Solar Cell Performance via Lewis Base Passivation of Organic–Inorganic Lead Halide Perovskites. ACS Nano 2014, 8, 9815–9821. [Google Scholar] [CrossRef]
- Jana, A.; Bathula, C.; Park, Y.; Kadam, A.; Sree, V.G.; Ansar, S.; Kim, H.-S.; Im, H. Facile synthesis and optical study of organic-inorganic lead bromide perovskite-clay (kaolinite, montmorillonite, and halloysite) composites. Surf. Interfaces 2022, 29, 101785. [Google Scholar] [CrossRef]
- Protesescu, L.; Yakunin, S.; Kumar, S.; Bär, J.; Bertolotti, F.; Masciocchi, N.; Guagliardi, A.; Grotevent, M.; Shorubalko, I.; Bodnarchuk, M.I.; et al. Dismantling the “Red Wall” of Colloidal Perovskites: Highly Luminescent Formamidinium and Formamidinium–Cesium Lead Iodide Nanocrystals. ACS Nano 2017, 11, 3119–3134. [Google Scholar] [CrossRef]
- Mishra, A.; Kubicki, D.J.; Boziki, A.; Chavan, R.D.; Dankl, M.; Mladenović, M.; Prochowicz, D.; Grey, C.P.; Rothlisberger, U.; Emsley, L. Interplay of Kinetic and Thermodynamic Reaction Control Explains Incorporation of Dimethylammonium Iodide into CsPbI3. ACS Energy Lett. 2022, 7, 2745–2752. [Google Scholar] [CrossRef] [PubMed]
- Swarnkar, A.; Marshall, A.R.; Sanehira, E.M.; Chernomordik, B.D.; Moore, D.T.; Christians, J.A.; Chakrabarti, T.; Luther, J.M. Quantum dot–induced phase stabilization of α-CsPbI3 perovskite for high-efficiency photovoltaics. Science 2016, 354, 92–95. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Lee, J.-W.; Dai, Z.; Wang, R.; Nuryyeva, S.; Liao, M.E.; Chang, S.-Y.; Meng, L.; Meng, D.; Sun, P.; et al. Surface Ligand Management for Stable FAPbI3 Perovskite Quantum Dot Solar Cells. Joule 2018, 2, 1866–1878. [Google Scholar] [CrossRef]
- Stoumpos, C.C.; Malliakas, C.D.; Kanatzidis, M.G. Semiconducting tin and lead iodide perovskites with organic cations: Phase transitions, high mobilities, and near-infrared photoluminescent properties. Inorg. Chem. 2013, 52, 9019–9038. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Chen, Q.; Li, G.; Luo, S.; Song, T.-b.; Duan, H.-S.; Hong, Z.; You, J.; Liu, Y.; Yang, Y. Interface engineering of highly efficient perovskite solar cells. Science 2014, 345, 542–546. [Google Scholar] [CrossRef] [PubMed]
- Persson, I.; Lyczko, K.; Lundberg, D.; Eriksson, L.; Płaczek, A. Coordination chemistry study of hydrated and solvated lead (II) ions in solution and solid state. Inorg. Chem. 2011, 50, 1058–1072. [Google Scholar] [CrossRef] [PubMed]
- Sandeep, K.; Gopika, K.Y.; Revathi, M.R. Role of Capped Oleyl Amine in the Moisture-Induced Structural Transformation of CsPbBr3 Perovskite Nanocrystals. Phys. Status Solidi (RRL)–Rapid Res. Lett. 2019, 13, 1900387. [Google Scholar] [CrossRef]
- Acik, M.; Park, I.K.; Koritala, R.E.; Lee, G.; Rosenberg, R.A. Oxygen-induced defects at the lead halide perovskite/graphene oxide interfaces. J. Mater. Chem. A 2018, 6, 1423–1442. [Google Scholar] [CrossRef]
- Huang, S.; Li, Z.; Wang, B.; Zhu, N.; Zhang, C.; Kong, L.; Zhang, Q.; Shan, A.; Li, L. Morphology Evolution and Degradation of CsPbBr3 Nanocrystals under Blue Light-Emitting Diode Illumination. ACS Appl. Mater. Interfaces 2017, 9, 7249–7258. [Google Scholar] [CrossRef]
- Prathapani, S.; Choudhary, D.; Mallick, S.; Bhargava, P.; Yella, A. Experimental evaluation of room temperature crystallization and phase evolution of hybrid perovskite materials. CrystEngComm 2017, 19, 3834–3843. [Google Scholar] [CrossRef]
- Hidalgo, J.; Atourki, L.; Li, R.; Castro-Méndez, A.-F.; Kim, S.; Sherman, E.A.; Bieber, A.S.; Sher, M.-J.; Nienhaus, L.; Perini, C.A.R.; et al. Bulky cation hinders undesired secondary phases in FAPbI3 perovskite solar cells. Mater. Today 2023, 68, 13–21. [Google Scholar] [CrossRef]
- Feng, W.; Tan, Y.; Yang, M.; Jiang, Y.; Lei, B.-X.; Wang, L.; Wu, W.-Q. Small amines bring big benefits to perovskite-based solar cells and light-emitting diodes. Chem 2022, 8, 351–383. [Google Scholar] [CrossRef]
| Sr. No | Chemicals | Mw. (g) | Density (g/mol) | mmol | Vol. Used (µL) |
|---|---|---|---|---|---|
| 1. | Oleylamine | 267.49 | 0.813 | 3 | 987 |
| 2. | Ethylenediamine | 60.10 | 0.899 | 3 | 200 |
| 3. | N-methylaniline | 107.16 | 0.989 | 3 | 325 |
| 4. | 2-Aminoethanol | 61.08 | 1.01 | 3 | 182 |
| 5. | 4-4′-methylenebis (cyclohexylamine) | 210.37 | 0.95 | 6 | 1328 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.N.; Jana, A.; Selvaraj, M.; Assiri, M.A.; Yun, S.; Nam, K.-W. Achieving Order in Disorder: Stabilizing Red Light-Emitting α-Phase Formamidinium Lead Iodide. Nanomaterials 2023, 13, 3049. https://doi.org/10.3390/nano13233049
Singh AN, Jana A, Selvaraj M, Assiri MA, Yun S, Nam K-W. Achieving Order in Disorder: Stabilizing Red Light-Emitting α-Phase Formamidinium Lead Iodide. Nanomaterials. 2023; 13(23):3049. https://doi.org/10.3390/nano13233049
Chicago/Turabian StyleSingh, Aditya Narayan, Atanu Jana, Manickam Selvaraj, Mohammed A. Assiri, Sua Yun, and Kyung-Wan Nam. 2023. "Achieving Order in Disorder: Stabilizing Red Light-Emitting α-Phase Formamidinium Lead Iodide" Nanomaterials 13, no. 23: 3049. https://doi.org/10.3390/nano13233049
APA StyleSingh, A. N., Jana, A., Selvaraj, M., Assiri, M. A., Yun, S., & Nam, K.-W. (2023). Achieving Order in Disorder: Stabilizing Red Light-Emitting α-Phase Formamidinium Lead Iodide. Nanomaterials, 13(23), 3049. https://doi.org/10.3390/nano13233049








