Precise Confinement and Position Distribution of Atomic Cu and Zn in ZSM-5 for CO2 Hydrogenation to Methanol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Catalyst Synthesis
2.3. Characterization Techniques
2.4. Catalytic Tests
3. Results
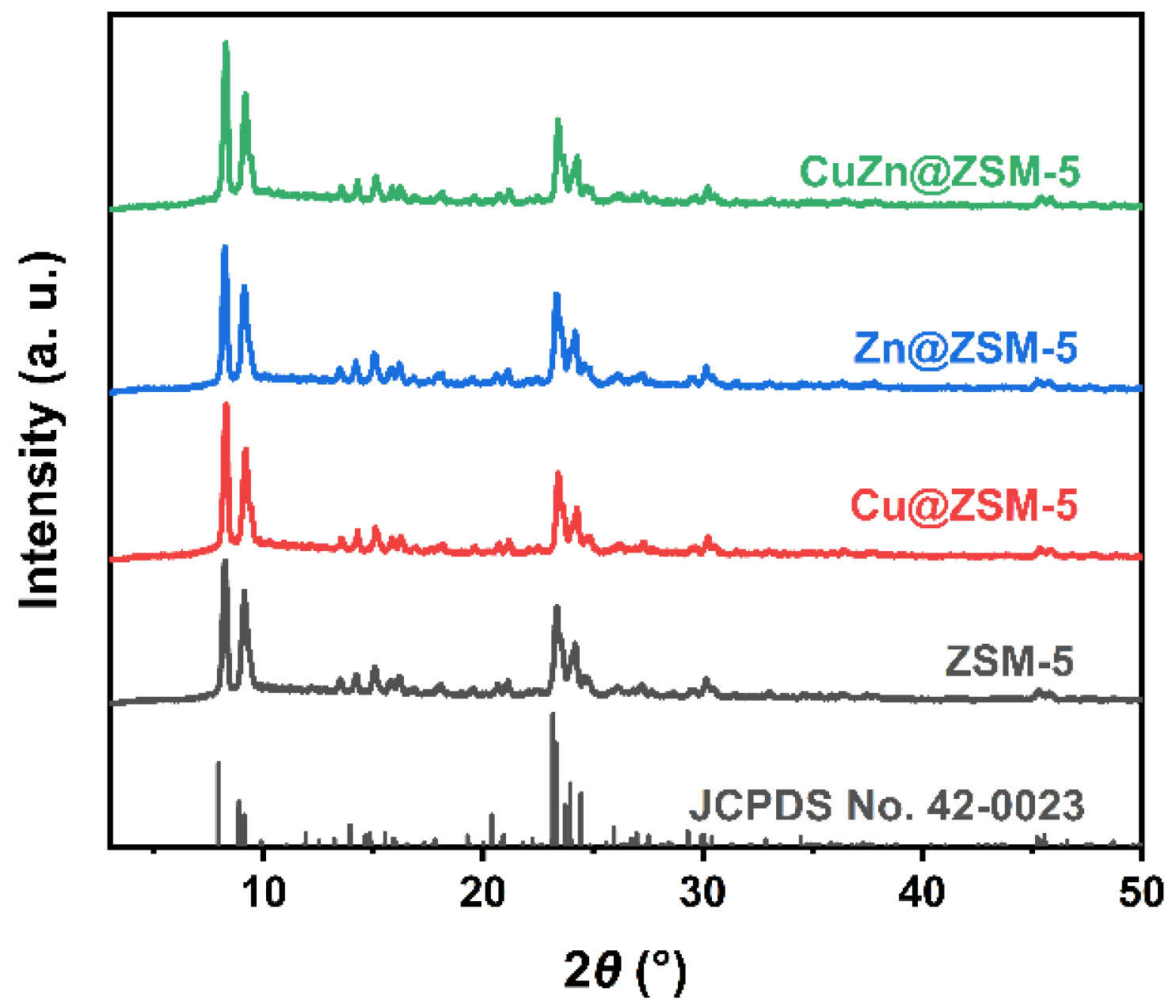
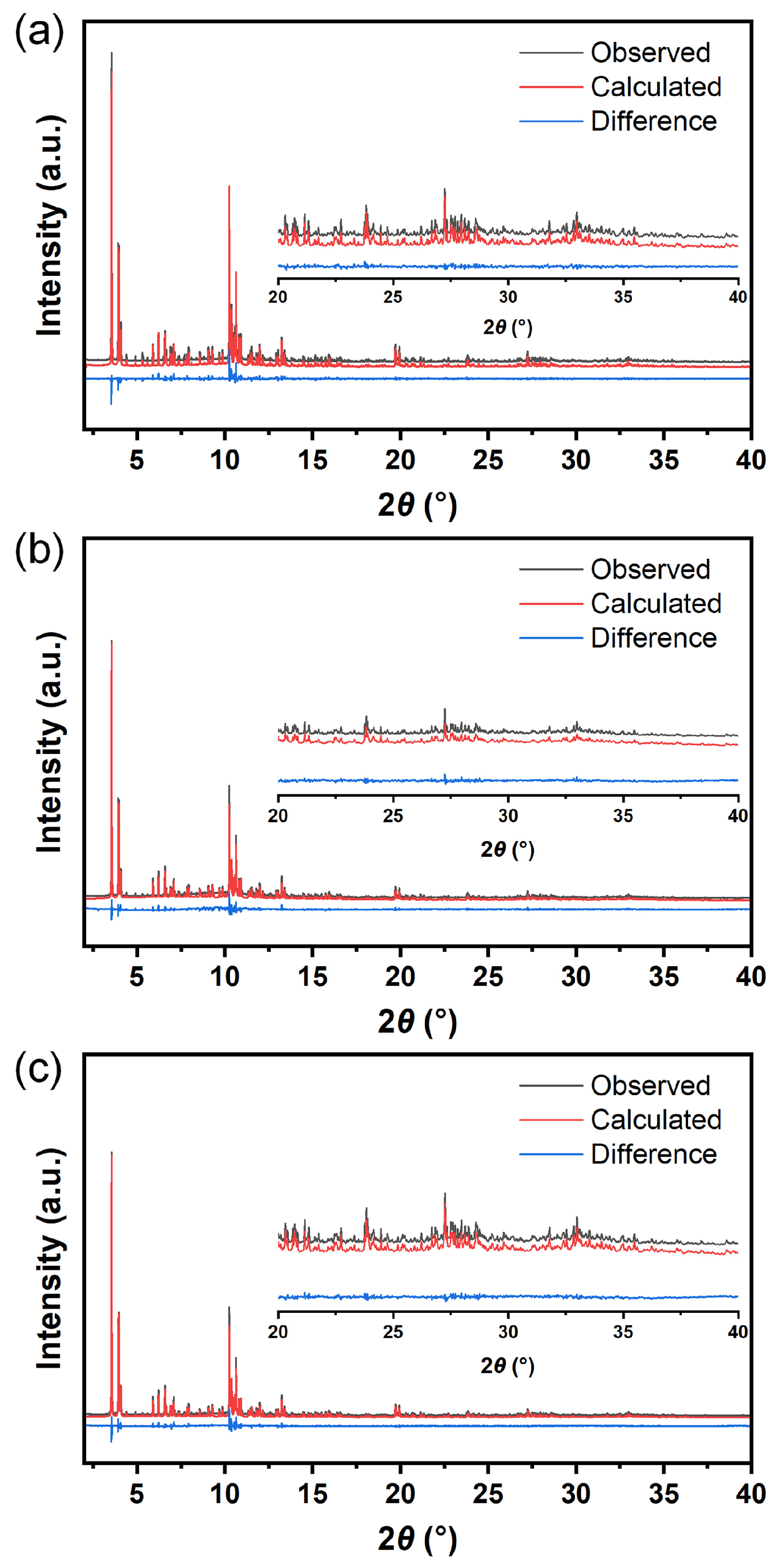
3.1. Structural Characterization
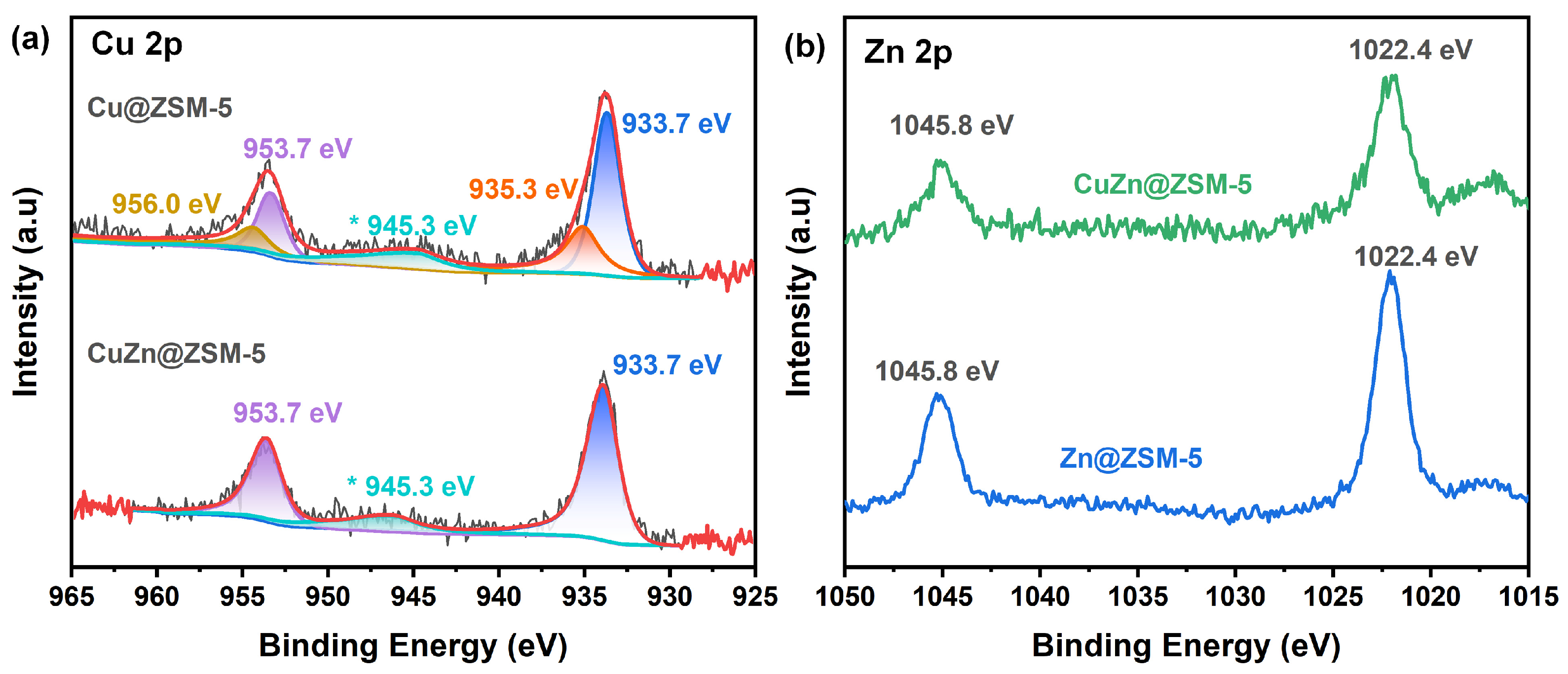
3.2. Insight into the Confined Position of the Metal in ZSM-5
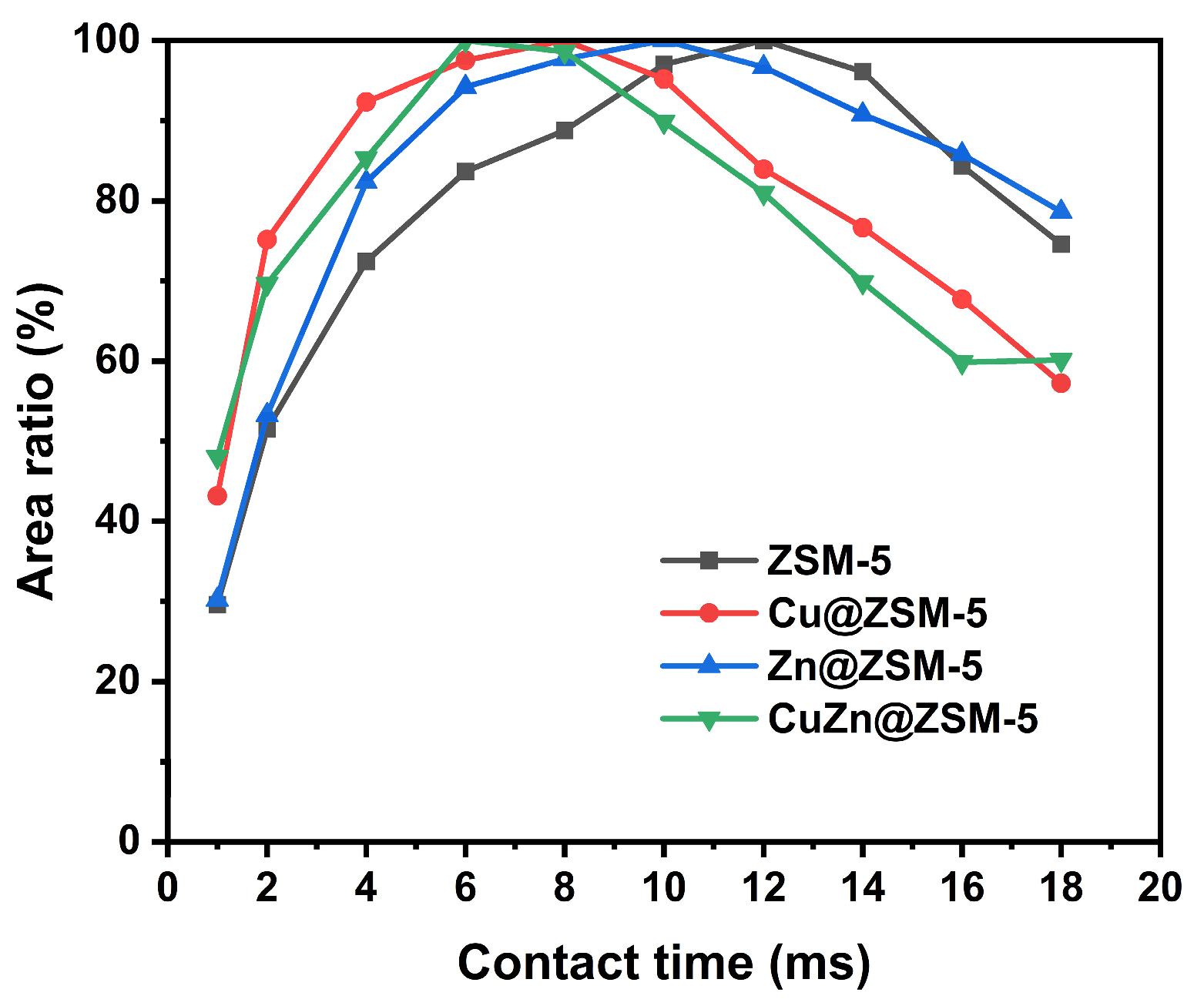
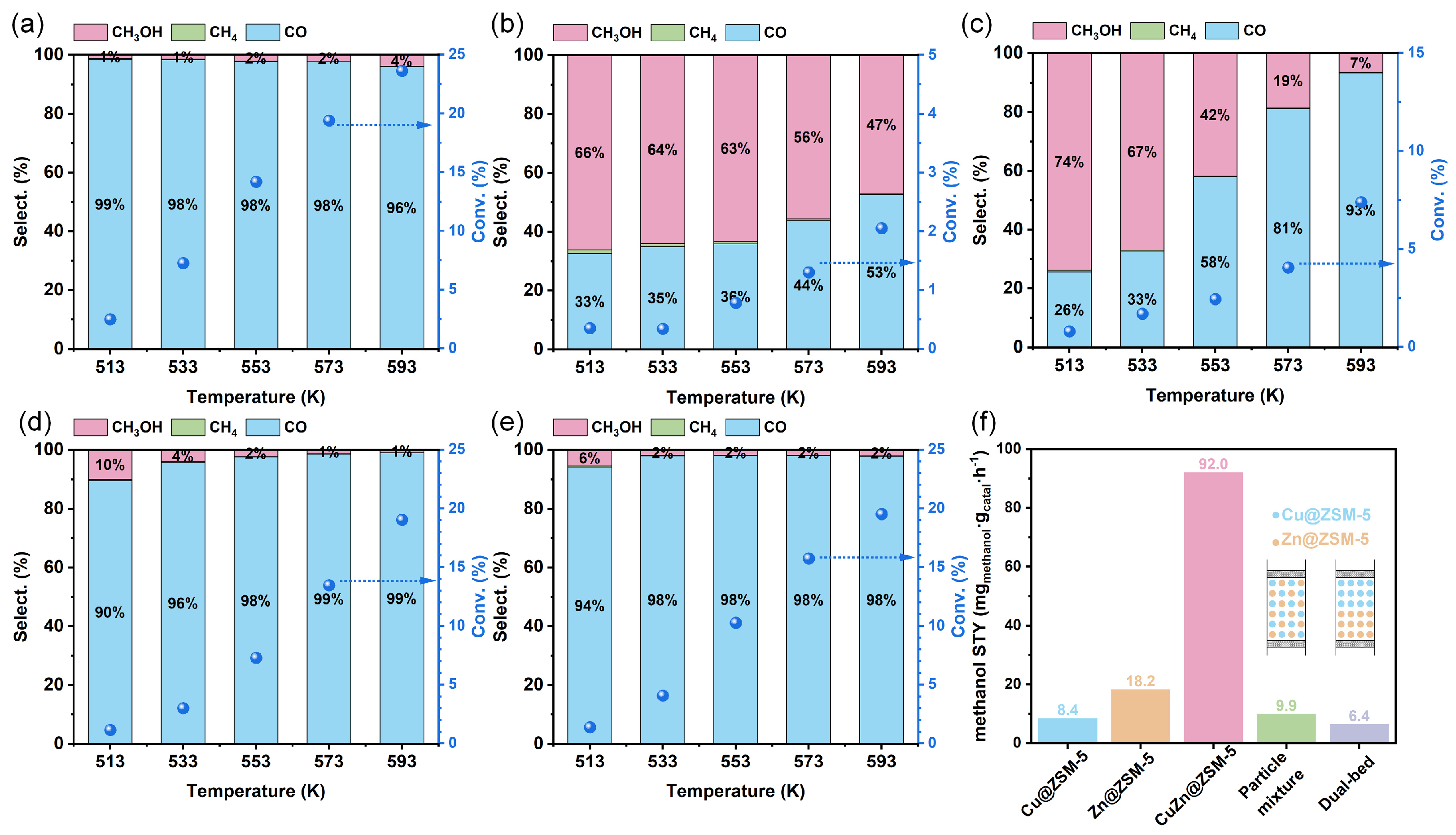
3.3. Catalytic Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Q.; Yu, J.H.; Corma, A. Applications of zeolites to C1 chemistry: Recent advances, challenges, and opportunities. Adv. Mater. 2020, 32, 2002927. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.J.; Huang, R.; Deng, D.H. Catalytic conversion of C1 molecules under mild conditions. EnergyChem 2021, 3, 100050. [Google Scholar] [CrossRef]
- Zhou, W.; Cheng, K.; Kang, J.C.; Zhou, C.; Subramanian, V.; Zhang, Q.H.; Wang, Y. New horizon in C1 chemistry: Breaking the selectivity limitation in transformation of syngas and hydrogenation of CO2 into hydrocarbon chemicals and fuels. Chem. Soc. Rev. 2019, 48, 3193–3228. [Google Scholar] [CrossRef]
- Bhattacharyya, S.S.; Leite, F.F.G.D.; Adeyemi, M.A.; Sarker, A.J.; Cambareri, G.S.; Faverin, C.; Tieri, M.P.; Castillo-Zacarías, C.; Melchor-Martínez, E.M.; Iqbal, H.M.N.; et al. A paradigm shift to CO2 sequestration to manage global warming—With the emphasis on developing countries. Sci. Total Environ. 2021, 790, 148169. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.T.; Deng, D.H.; Bao, X.H. Catalysis for Selected C1 Chemistry. Chem 2020, 6, 2497–2514. [Google Scholar] [CrossRef]
- Wang, W.; Wang, S.P.; Ma, X.B.; Gong, J.L. Recent advances in catalytic hydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727. [Google Scholar] [CrossRef]
- Norhasyima, R.S.; Mahlia, T.M.I. Advances in CO₂ utilization technology: A patent landscape review. J.CO2 Util. 2018, 26, 323–335. [Google Scholar] [CrossRef]
- Jiang, X.; Nie, X.W.; Guo, X.W.; Song, C.S.; Chen, J.G. Recent Advances in Carbon Dioxide Hydrogenation to Methanol via Heterogeneous Catalysis. Chem. Rev. 2020, 120, 7984–8034. [Google Scholar] [CrossRef]
- Qi, S.C.; Liu, X.Y.; Zhu, R.R.; Xue, D.M.; Liu, X.Q.; Sun, L.B. Causation of catalytic activity of Cu-ZnO for CO2 hydrogenation to methanol. Chem. Eng. J. 2022, 430, 132784. [Google Scholar] [CrossRef]
- Kuld, S.; Conradsen, C.; Moses, P.G.; Chorkendorff, I.; Sehested, J. Quantification of zinc atoms in a surface alloy on copper in an industrial-type methanol synthesis catalyst. Angew. Chem. Int. Ed. 2014, 53, 5941–5945. [Google Scholar] [CrossRef]
- Kattel, S.; Ramírez, P.J.; Chen, J.G.; Rodriguez, J.A.; Liu, P. Active sites for CO2 hydrogenation to methanol on Cu/ZnO catalysts. Science 2017, 355, 1296–1299. [Google Scholar] [CrossRef] [PubMed]
- Van Deelen, T.W.; Hernández Mejía, C.; de Jong, K.P. Control of metal-support interactions in heterogeneous catalysts to enhance activity and selectivity. Nat. Catal. 2019, 2, 955–970. [Google Scholar] [CrossRef]
- Hartley, U.W.; Murugesan, P.K. Chapter 12: Dual Functional Nano Zeolites for CO2 Capture and Conversion; Elsevier: Amsterdam, The Netherlands, 2023; pp. 309–332. [Google Scholar]
- Wang, H.; Wang, L.; Xiao, F.S. Metal@zeolite hybrid materials for catalysis. ACS Cent. Sci. 2020, 6, 1685–1697. [Google Scholar] [CrossRef]
- Zhang, F.Y.; Zhou, W.; Xiong, X.W.; Wang, Y.H.; Cheng, K.; Kang, J.C.; Zhang, Q.H.; Wang, Y. Selective hydrogenation of CO2 to ethanol over sodium-modified Rhodium nanoparticles embedded in zeolite Silicalite-1. J. Phys. Chem. C 2021, 125, 24429–24439. [Google Scholar] [CrossRef]
- Leung, K.C.; Hong, S.; Li, G.C.; Xing, Y.D.; Ng, B.K.Y.; Ho, P.L.; Ye, D.P.; Zhao, P.; Tan, E.; Safonova, O.; et al. Confined Ru sites in a 13X zeolite for ultrahigh H2 production from NH3 decomposition. J. Am. Chem. Soc. 2023, 145, 14548–14561. [Google Scholar] [CrossRef]
- Choi, M.; Wu, Z.J.; Iglesia, E. Mercaptosilane-assisted synthesis of metal clusters within zeolites and catalytic consequences of encapsulation. J. Am. Chem. Soc. 2010, 132, 9129–9137. [Google Scholar] [CrossRef]
- Goel, S.; Wu, Z.J.; Zones, S.I.; Iglesia, E. Synthesis and catalytic properties of metal clusters encapsulated within small-pore (SOD, GIS, ANA) zeolites. J. Am. Chem. Soc. 2012, 134, 17688–17695. [Google Scholar] [CrossRef]
- Goel, S.; Zones, S.I.; Iglesia, E. Encapsulation of metal clusters within MFI via interzeolite transformations and direct hydrothermal syntheses and catalytic consequences of their confinement. J. Am. Chem. Soc. 2014, 136, 15280–15290. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Sun, Q.M.; Bai, R.S.; Li, X.; Guo, G.Q.; Yu, J.H. In situ confinement of ultrasmall Pd clusters within nanosized Silicalite-1 zeolite for highly efficient catalysis of hydrogen generation. J. Am. Chem. Soc. 2016, 138, 7484–7487. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Shao, Y.; Wang, Y.Q.; Gates, B.C.; Xiao, F.S. A Pd@zeolite catalyst for nitroarene hydrogenation with high product selectivity by sterically controlled adsorption in the zeolite micropores. Angew. Chem. Int. Ed. 2017, 56, 9747–9751. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.; Zhang, B.S.; Zhao, H.S.; Kolb, U.; Zhu, Y.H.; Liu, L.M.; Han, Y.; Wang, G.X.; Wang, C.T.; et al. Sinter-resistant metal nanoparticle catalysts achieved by immobilization within zeolite crystals via seed-directed growth. Nat. Catal. 2018, 1, 540–546. [Google Scholar] [CrossRef]
- Cui, W.G.; Zhang, Q.; Zhou, L.; Wei, Z.C.; Yu, L.; Dai, J.J.; Zhang, H.B.; Hu, T.L. Hybrid MOF template-directed construction of hollow-structured In2O3@ZrO2 heterostructure for enhancing hydrogenation of CO2 to methanol. Small 2022, 19, 2204914. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Gao, Y.X.; Zhang, M.; Gao, H.; Chen, J.; Jia, H.P. Efficient infrared-light-driven photothermal CO2 reduction over MOF-derived defective Ni/TiO2. Appl. Catal. B 2022, 303, 120905. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.H.; Deng, D.H.; Bao, X.H. Two-dimensional materials confining single atoms for catalysis. Chin. J. Catal. 2017, 38, 1443–1453. [Google Scholar] [CrossRef]
- Tang, X.; Ye, J.J.; Guo, L.S.; Pu, T.C.; Cheng, L.; Cao, X.M.; Guo, Y.L.; Wang, L.; Guo, Y.; Zhan, W.C.; et al. Atomic Insights into the Cu Species Supported on Zeolite for Direct Oxidation of Methane to Methanol via Low-Damage HAADF-STEM. Adv. Mater. 2023, 35, 2208504. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Song, S.J.; Liu, C.C.; Liu, L.m.; Zhang, L.K.; Zhao, Y.H.; Wang, X.; Xu, H.; Guan, Y.J.; Jiang, J.A.; et al. Germanium-enriched double-four-membered-ring units inducing zeolite-confined subnanometric Pt clusters for efficient propane dehydrogenation. Nat. Catal. 2023, 6, 506–518. [Google Scholar] [CrossRef]
- Chai, Y.C.; Qin, B.; Li, B.N.; Dai, W.L.; Wu, G.J.; Guan, N.J.; Li, L.D. Zeolite-encaged mononuclear copper centers catalyze CO2 selective hydrogenation to methanol. Natl. Sci. Rev. 2023, 10, nwad043. [Google Scholar] [CrossRef]
- Shamzhy, M.; Opanasenko, M.; Concepción, P.; Martínez, A. New trends in tailoring active sites in zeolite-based catalysts. Chem. Soc. Rev. 2019, 48, 1095–1149. [Google Scholar] [CrossRef]
- Kokotailo, G.T.; Lawton, S.L.; Olson, D.H.; Meier, W.M. Structure of synthetic zeolite ZSM-5. Nature 1978, 272, 437–438. [Google Scholar] [CrossRef]
- Olson, D.H.; Kokotailo, G.T.; Lawton, S.L.; Meier, W.M. Crystal structure and structure-related properties of ZSM-5. J. Phys. Chem. 1981, 85, 2238–2243. [Google Scholar] [CrossRef]
- Van Koningsveld, H.; Jansen, J.C.; van Bekkum, H. The monoclinic framework structure of zeolite H-ZSM-5. Comparison with the orthorhombic framework of as-synthesized ZSM-5. Zeolites 1990, 10, 235–242. [Google Scholar] [CrossRef]
- Ren, L.M.; Zhu, L.F.; Yang, C.G.; Chen, Y.M.; Sun, Q.; Zhang, H.Y.; Li, C.J.; Nawaz, F.; Meng, X.J.; Xiao, F.S. Designed copper–amine complex as an efficient template for one-pot synthesis of Cu-SSZ-13 zeolite with excellent activity for selective catalytic reduction of NOx by NH3. Chem. Commun. 2011, 47, 9789–9791. [Google Scholar] [CrossRef]
- Wang, Y.Z.; Han, J.F.; Chen, M.Y.; Lv, W.T.; Meng, P.T.; Gao, W.; Meng, X.J.; Fan, W.B.; Xu, J.; Yan, W.F.; et al. Low-silica Cu-CHA zeolite enriched with Al Pairs transcribed from silicoaluminophosphate seed: Synthesis and ammonia selective catalytic reduction performance. Angew. Chem. Int. Ed. 2023, 62, e202306174. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.L.; Zeng, S.; Zhang, X.L.; Deng, Q.Z.; Li, X.J.; Yu, W.G.; Zhu, K.K.; Xu, S.T.; Liu, J.C.; Han, L. Generating assembled MFI nanocrystals with reduced b-axis through structure-directing agent exchange induced recrystallization. Angew. Chem. Int. Ed. 2021, 60, 13959–13968. [Google Scholar] [CrossRef]
- Kawaguchi, S.; Takemoto, M.; Osaka, K.; Nishibori, E.; Moriyoshi, C.; Kubota, Y.; Kuroiwa, Y.; Sugimoto, K. High-throughput powder diffraction measurement system consisting of multiple MYTHEN detectors at beamline BL02B2 of SPring-8. Rev. Sci. Instrum. 2017, 88, 085111. [Google Scholar] [CrossRef]
- Wang, L.L.; Jiang, Y.L.; Zhou, Y.; Shi, R.K.; Hosokawa, F.; Terasaki, O.; Zhang, Q. Improving Data Quality in Traditional Low-Dose Scanning Transmission Electron Microscopy Imaging. Part. Part. Syst. Charact. 2023, 40, 2200122. [Google Scholar] [CrossRef]
- Choudhary, M.K.; Kumar, M.; Rimer, J.D. Regulating nonclassical pathways of Silicalite-1 crystallization through controlled evolution of amorphous precursors. Angew. Chem. Int. Ed. 2019, 58, 15712–15716. [Google Scholar] [CrossRef]
- Choi, M.; Na, K.; Kim, J.; Sakamoto, Y.; Terasaki, O.; Ryoo, R. Stable single-unit-cell nanosheets of zeolite MFI as active and long-lived catalysts. Nature 2009, 461, 246–249. [Google Scholar] [CrossRef]
- Xin, S.H.; Wang, Q.; Xu, J.; Chu, Y.Y.; Wang, P.F.; Feng, N.D.; Qi, G.D.; Trébosc, J.; Lafon, O.; Fan, W.B.; et al. The acidic nature of “NMR-invisible” tri-coordinated framework aluminum species in zeolites. Chem. Sci. 2019, 10, 10159–10169. [Google Scholar] [CrossRef]
- Fyfe, C.A.; Brouwer, D.H. Effect of molecular oxygen on the variable-temperature 29Si MAS NMR spectra of zeolite−sorbate complexes. J. Am. Chem. Soc. 2004, 126, 1306–1307. [Google Scholar] [CrossRef]
- Christiansen, S.C.; Zhao, D.; Janicke, M.T.; Landry, C.C.; Stucky, G.D.; Chmelka, B.F. Molecularly ordered inorganic frameworks in layered silicate surfactant mesophases. J. Am. Chem. Soc. 2001, 123, 4519–4529. [Google Scholar] [CrossRef] [PubMed]
- Hedin, N.; Graf, R.; Christiansen, S.C.; Gervais, C.; Hayward, R.C.; Eckert, J.; Chmelka, B.F. Structure of a surfactant-templated silicate framework in the absence of 3D crystallinity. J. Am. Chem. Soc. 2004, 126, 9425–9432. [Google Scholar] [CrossRef] [PubMed]
- Pines, A.; Gibby, M.G.; Waugh, J.S. Proton-enhanced NMR of dilute spins in solids. J. Chem. Phys. 1973, 59, 569–590. [Google Scholar] [CrossRef]
- Wu, X.L.; Zilm, K.W. Complete spectral editing in CPMAS NMR. J. Magn. Reson. A 1993, 102, 205–213. [Google Scholar] [CrossRef]
- Palmas, P.; Tekely, P.; Canet, D. Local-field measurements on powder samples from polarization inversion of the rare-spin magnetization. J. Magn. Reson. A 1993, 104, 26–36. [Google Scholar] [CrossRef]
- Brouwer, D.H.; Cadars, S.; Eckert, J.; Liu, Z.; Terasaki, O.; Chmelka, B.F. A general protocol for determining the structures of molecularly ordered but noncrystalline silicate frameworks. J. Am. Chem. Soc. 2013, 135, 5641–5655. [Google Scholar] [CrossRef] [PubMed]
- Biesinger, M.C.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Sc, Ti, V, Cu and Zn. Appl. Surf. Sci. 2010, 257, 887–898. [Google Scholar] [CrossRef]
- Dake, L.S.; Baer, D.R.; Zachara, J.M. Auger parameter measurements of zinc compounds relevant to zinc transport in the environment. Surf. Interface Anal. 1989, 14, 71–75. [Google Scholar] [CrossRef]
- Zhao, H.B.; Yu, R.F.; Ma, S.C.; Xu, K.Z.; Chen, Y.; Jiang, K.; Fang, Y.; Zhu, C.X.; Liu, X.C.; Tang, Y.; et al. The role of Cu1–O3 species in single-atom Cu/ZrO2 catalyst for CO2 hydrogenation. Nat. Catal. 2022, 5, 818–831. [Google Scholar] [CrossRef]
- Behrens, M.; Studt, F.; Kasatkin, I.; Kühl, S.; Hävecker, M.; Abild-Pedersen, F.; Zander, S.; Girgsdies, F.; Kurr, P.; Kniep, B.-L.; et al. The active site of methanol synthesis over Cu/ZnO/Al2O3 industrial catalysts. Science 2012, 336, 893–897. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Yin, H.R.; Yu, G.D.; He, S.; Kang, J.C.; Liu, Z.M.; Cheng, K.; Zhang, Q.H.; Wang, Y. Selective hydrogenation of CO2 and CO into olefins over sodium and zinc promoted iron carbide catalysts. J. Catal. 2021, 395, 350–361. [Google Scholar] [CrossRef]
- Peter, A.; Bernhard, K.; David, D.; Norbert, K.; Thomas, G.; Patrick, L.; Christoph, R.; Kevin, P.; Djuro, B.; Hsin-Yi, W.; et al. The state of zinc in methanol synthesis over a Zn/ZnO/Cu(211) model catalyst. Science 2022, 376, 603–608. [Google Scholar]
- Jo, D.Y.; Lee, M.W.; Ham, H.C.; Lee, K.Y. Role of the Zn atomic arrangements in enhancing the activity and stability of the kinked Cu(211) site in CH3OH production by CO2 hydrogenation and dissociation: First-principles microkinetic modeling study. J. Catal. 2019, 373, 336–350. [Google Scholar] [CrossRef]




| Sample | Theoretical Value a | Experimental Value b | ||
|---|---|---|---|---|
| Cu (wt.%) | Zn (wt.%) | Cu (wt.%) | Zn (wt.%) | |
| Cu@ZSM-5 | 2.07 | - | 1.82 | - |
| Zn@ZSM-5 | - | 1.06 | - | 0.91 |
| CuZn@ZSM-5 | 2.05 | 1.04 | 2.23 | 0.89 |
| Sample | SBET a (m2·g−1) | Smicro (m2·g−1) | Vmicro b (cm3·g−1) | Vtotal (cm3·g−1) | Pore Diameter c (nm) |
|---|---|---|---|---|---|
| ZSM-5 | 437 | 337 | 0.13 | 0.22 | 0.52 |
| Cu@ZSM-5 | 410 | 259 | 0.10 | 0.21 | 0.52 |
| Zn@ZSM-5 | 408 | 296 | 0.12 | 0.20 | 0.53 |
| CuZn@ZSM-5 | 285 | 193 | 0.07 | 0.23 | 0.53 |
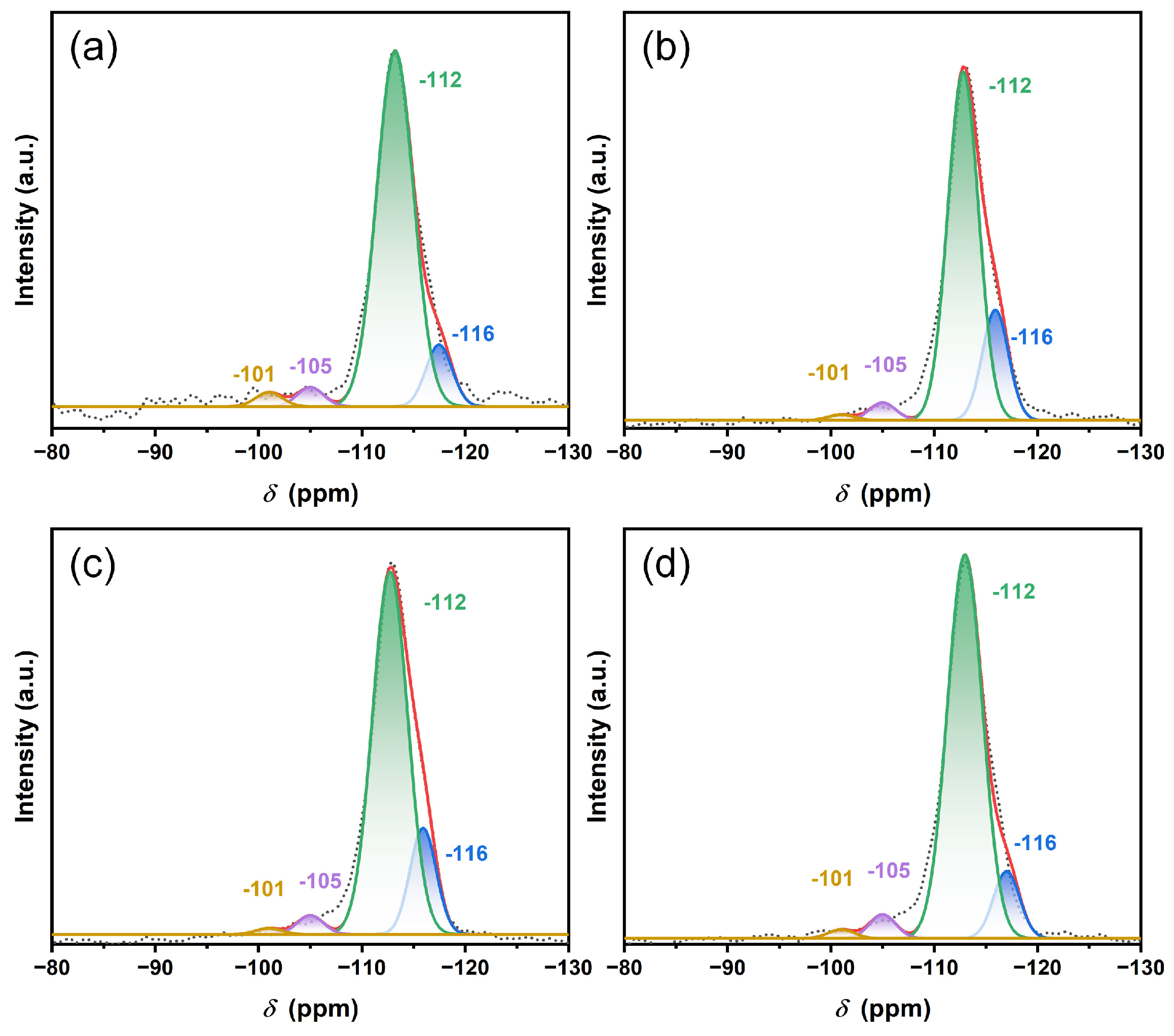
| Sample | Chemical Environment (%) | Si/Al * (NMR) | Si/Al (ICP-AES) | |||
|---|---|---|---|---|---|---|
| Si(OSi)3(OAl)1 | Si(OSi)4 | |||||
| −101 ppm | −105 ppm | −112 ppm | −116 ppm | |||
| ZSM-5 | 2.4 | 3.2 | 83.9 | 10.5 | 71 | 86 |
| Cu@ZSM-5 | 1.6 | 3.3 | 75.2 | 19.9 | 82 | 79 |
| Zn@ZSM-5 | 1.5 | 3.1 | 78.1 | 17.3 | 87 | 82 |
| CuZn@ZSM-5 | 1.5 | 3.3 | 83.6 | 11.6 | 83 | 75 |
| Sample | Chemical Environment (%) | Area Ratio of Cu-2:Cu-1 | |||
|---|---|---|---|---|---|
| Cu 2p1/2 | Cu 2p3/2 | ||||
| Cu-1 (956.0 eV) | Cu-2 (953.7 eV) | Cu-1 (935.3 eV) | Cu-2 (933.7 eV) | ||
| Cu@ZSM-5 | 12.5% | 15.5% | 32.0% | 40.0% | 1.25 |
| CuZn@ZSM-5 | - | 34.1% | - | 65.9% | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, H.; Zhang, J.; Feng, W.; Yao, Q.; Zhang, L.; Ren, Y.; Ye, L.; Yue, B.; He, H. Precise Confinement and Position Distribution of Atomic Cu and Zn in ZSM-5 for CO2 Hydrogenation to Methanol. Nanomaterials 2023, 13, 3053. https://doi.org/10.3390/nano13233053
Ding H, Zhang J, Feng W, Yao Q, Zhang L, Ren Y, Ye L, Yue B, He H. Precise Confinement and Position Distribution of Atomic Cu and Zn in ZSM-5 for CO2 Hydrogenation to Methanol. Nanomaterials. 2023; 13(23):3053. https://doi.org/10.3390/nano13233053
Chicago/Turabian StyleDing, Hongxin, Jinwen Zhang, Wenhua Feng, Qingying Yao, Li Zhang, Yuanhang Ren, Lin Ye, Bin Yue, and Heyong He. 2023. "Precise Confinement and Position Distribution of Atomic Cu and Zn in ZSM-5 for CO2 Hydrogenation to Methanol" Nanomaterials 13, no. 23: 3053. https://doi.org/10.3390/nano13233053
APA StyleDing, H., Zhang, J., Feng, W., Yao, Q., Zhang, L., Ren, Y., Ye, L., Yue, B., & He, H. (2023). Precise Confinement and Position Distribution of Atomic Cu and Zn in ZSM-5 for CO2 Hydrogenation to Methanol. Nanomaterials, 13(23), 3053. https://doi.org/10.3390/nano13233053







