General Strategies for Preparing Hybrid Polymer/Quantum Dot Nanocomposites for Color Conversion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Surface Modification of QDs and Assembly of QD/MPS in SiTiOx Beads
2.3. Preparation of QD/Polymer Color-Enhanced Films
2.4. Characterization
3. Results and Discussion
3.1. QDs in a Transparent Matrix
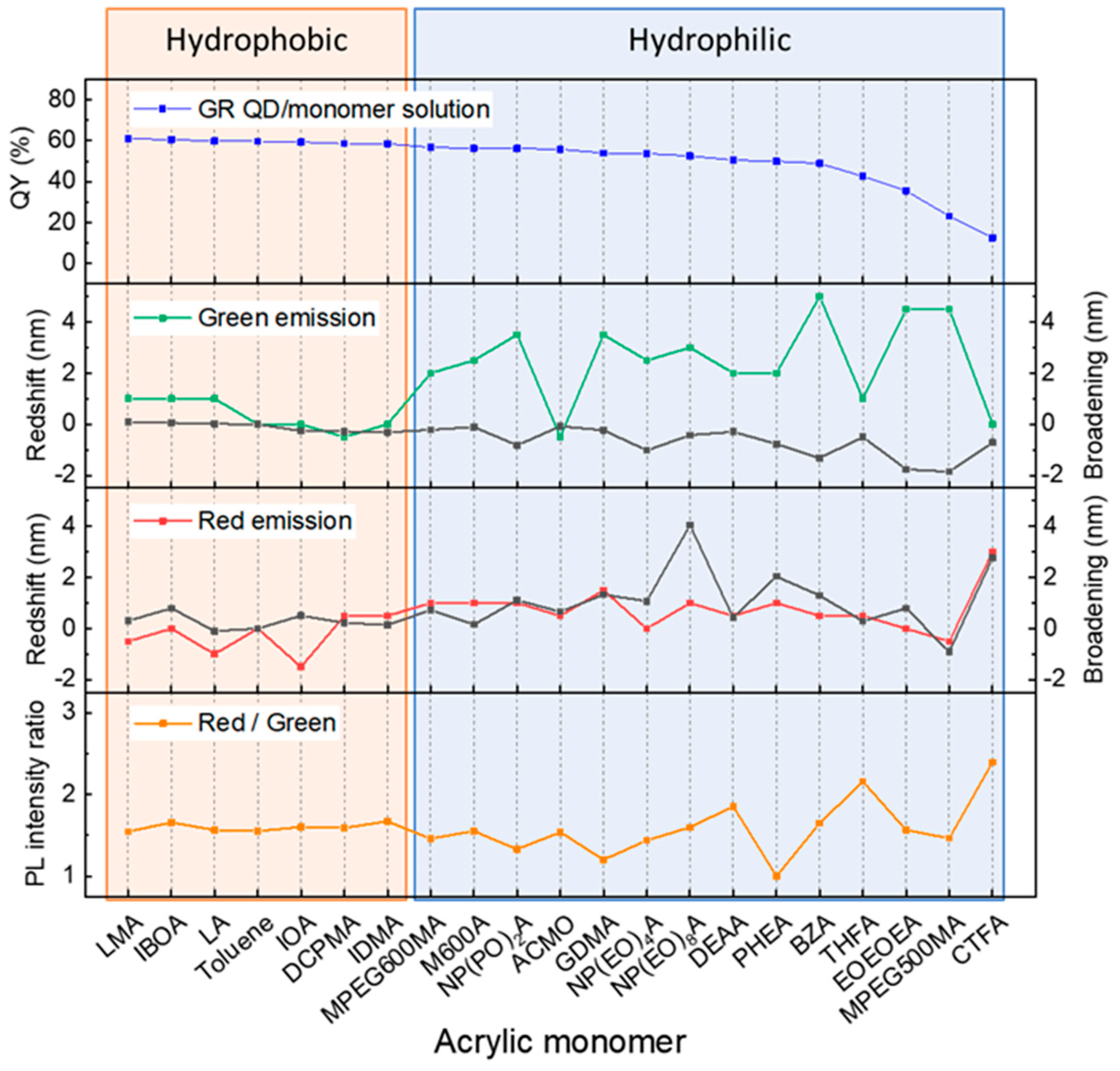
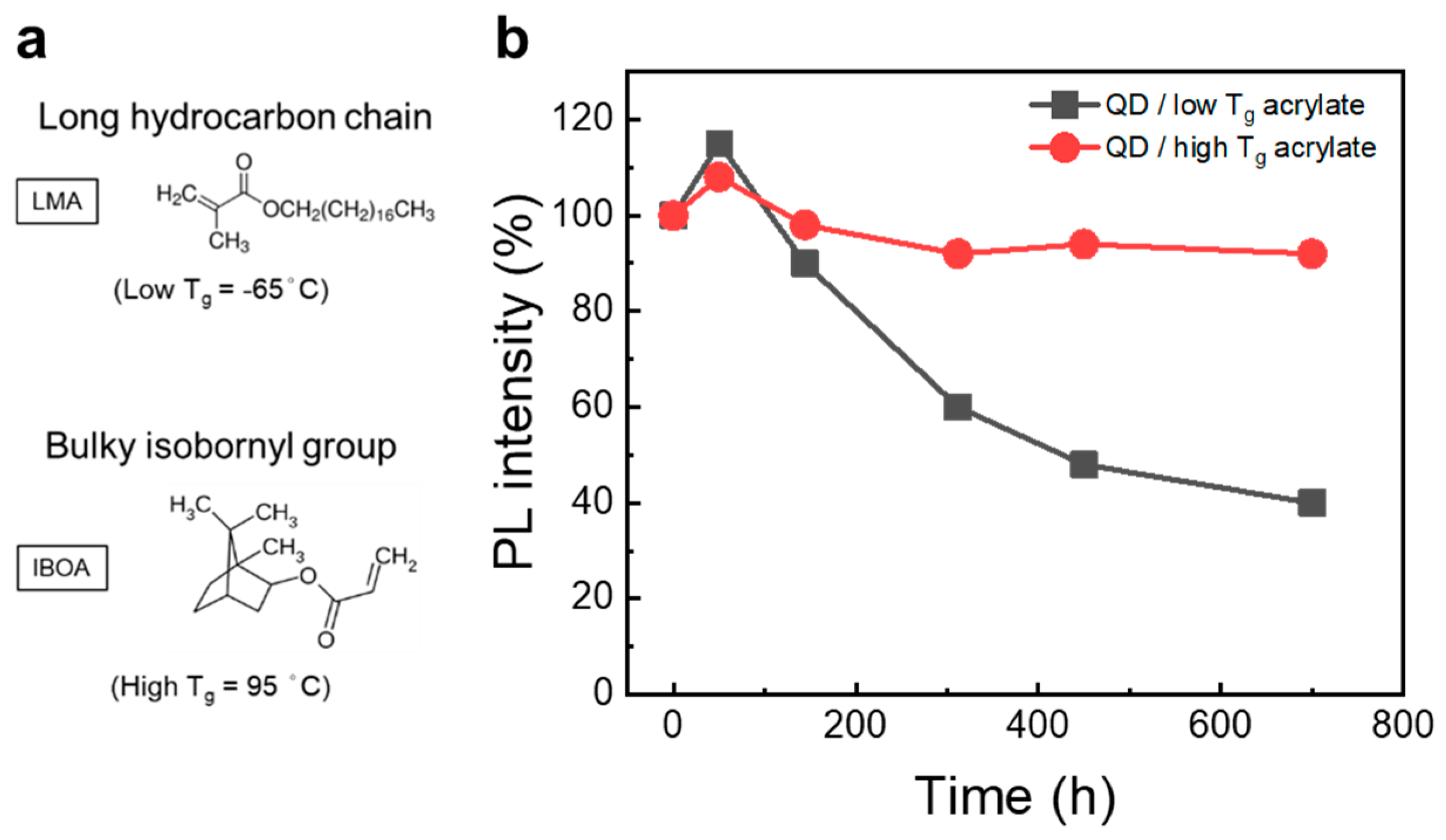
3.1.1. QDs in Hydrophobic/Hydrophilic Polymer Matrix
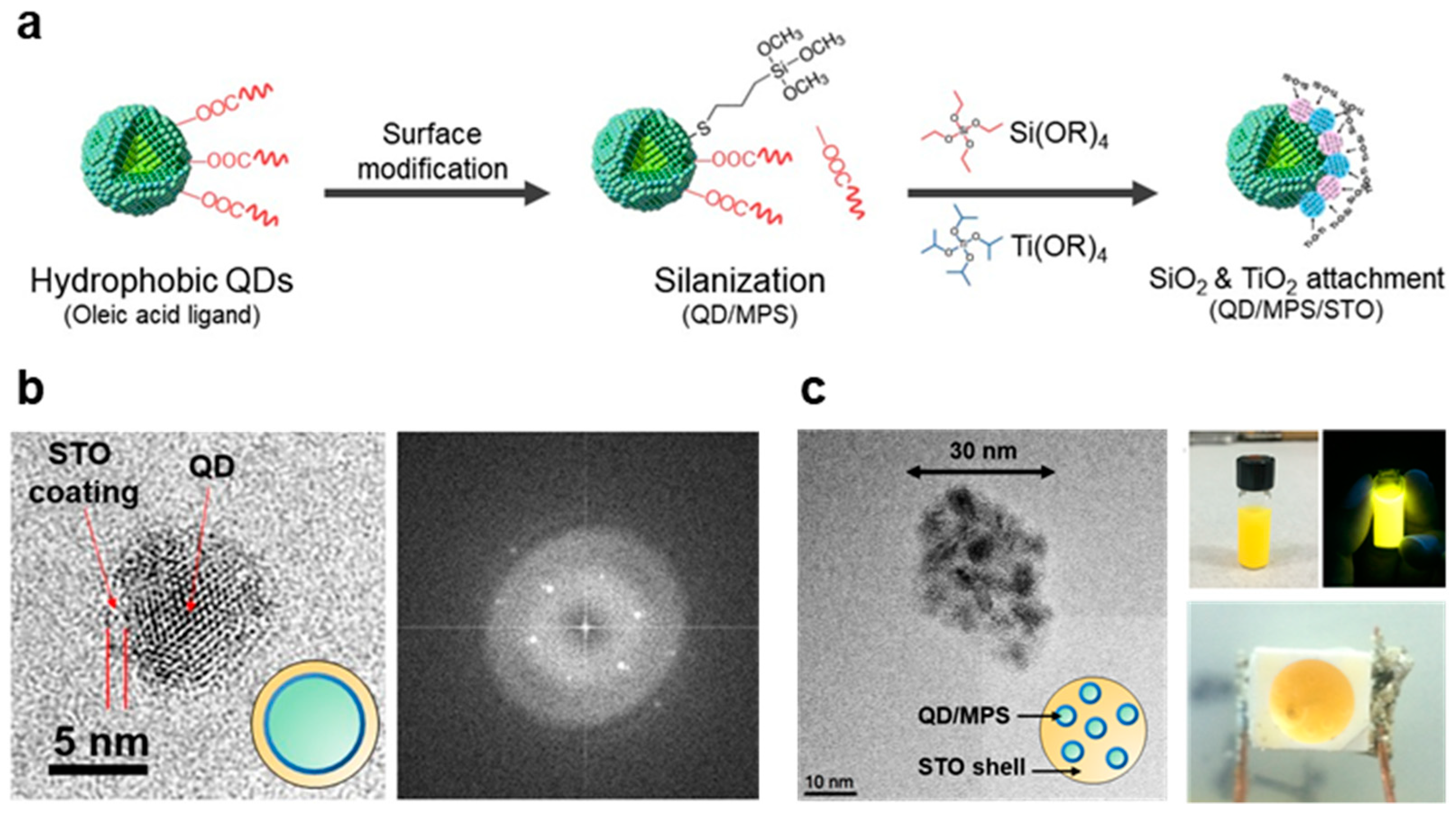
3.1.2. Chemical Modification of QDs in Hydrophilic Silica–Titania Glass
3.2. Preparation of QDCC for Lighting and Displays
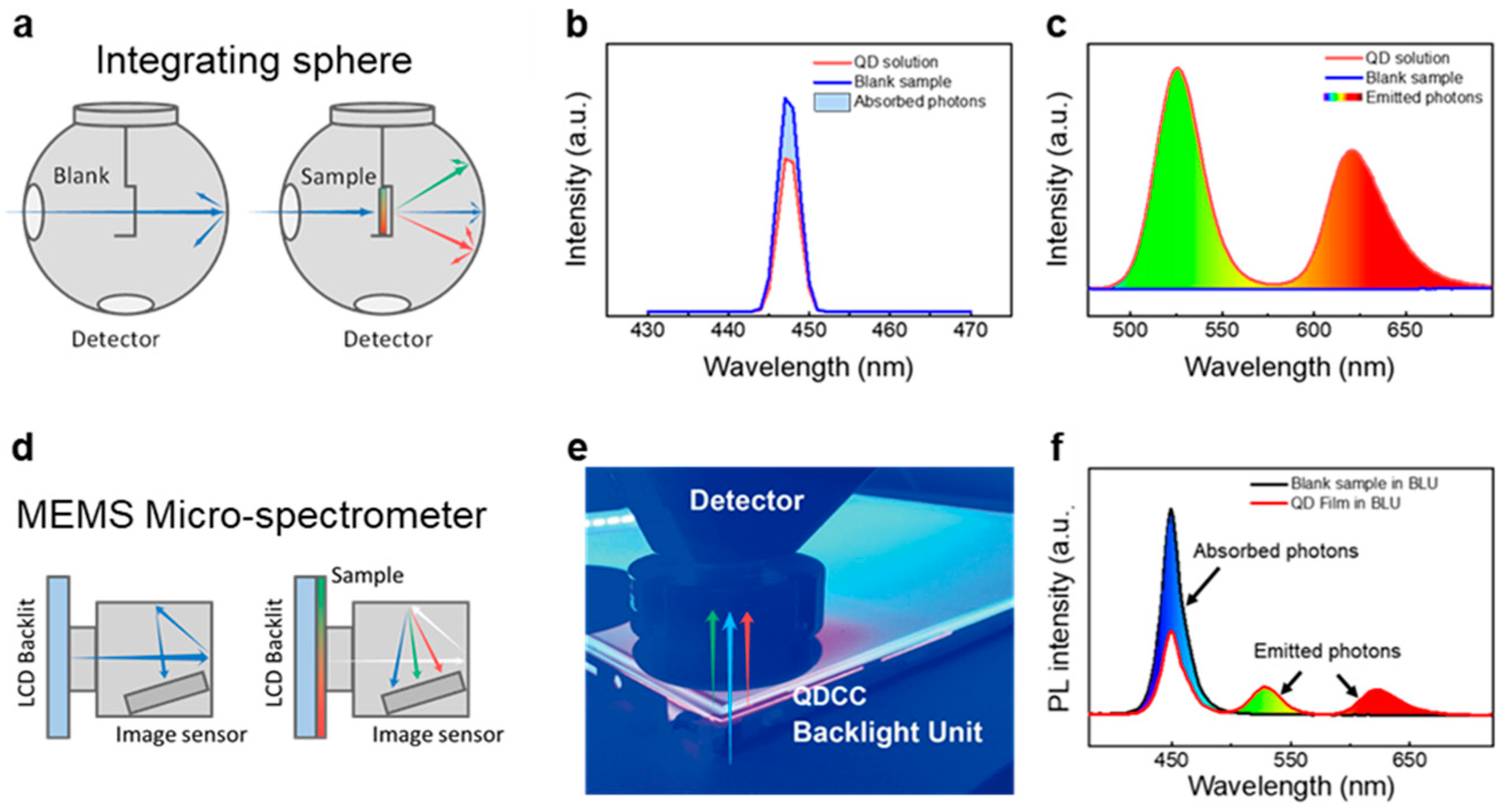
3.2.1. Calculations of Luminescence Efficiency of QDCCs
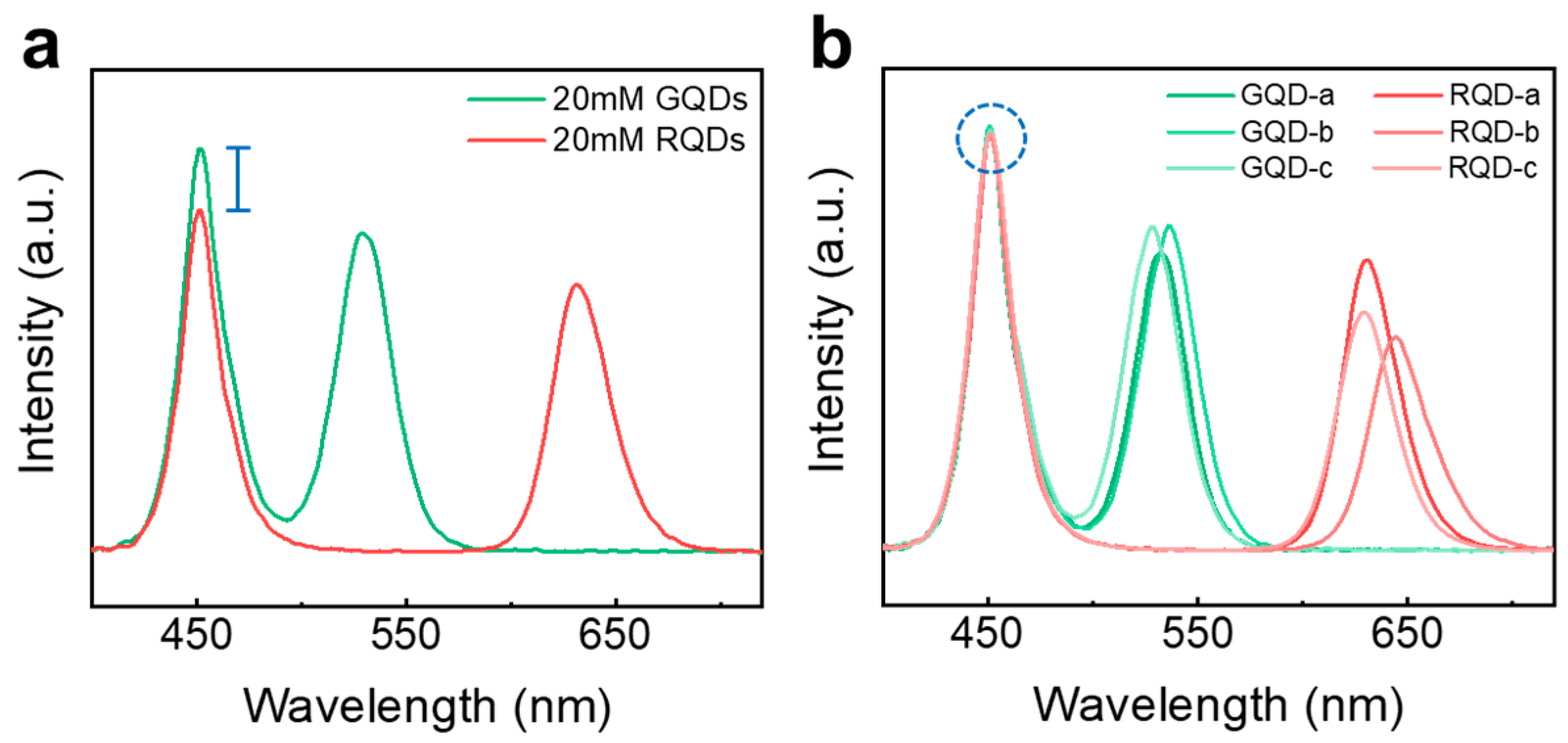
3.2.2. Quantification of QD via Gravimetric and Optical Methods
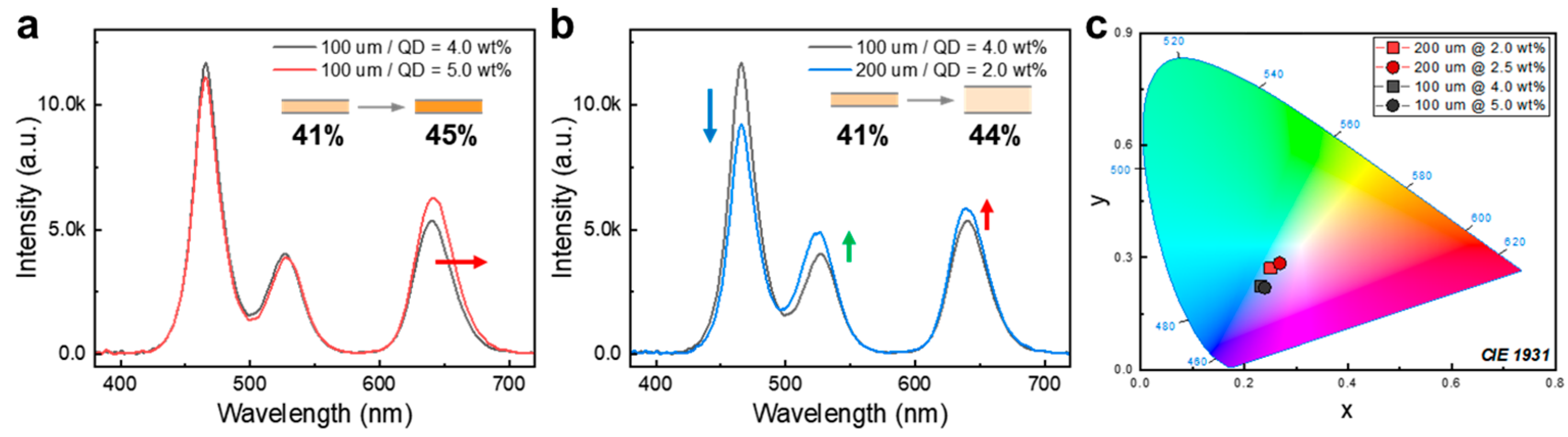
3.2.3. Effect of QD Concentration and Film Thickness in QDCCs’ Optical Performance
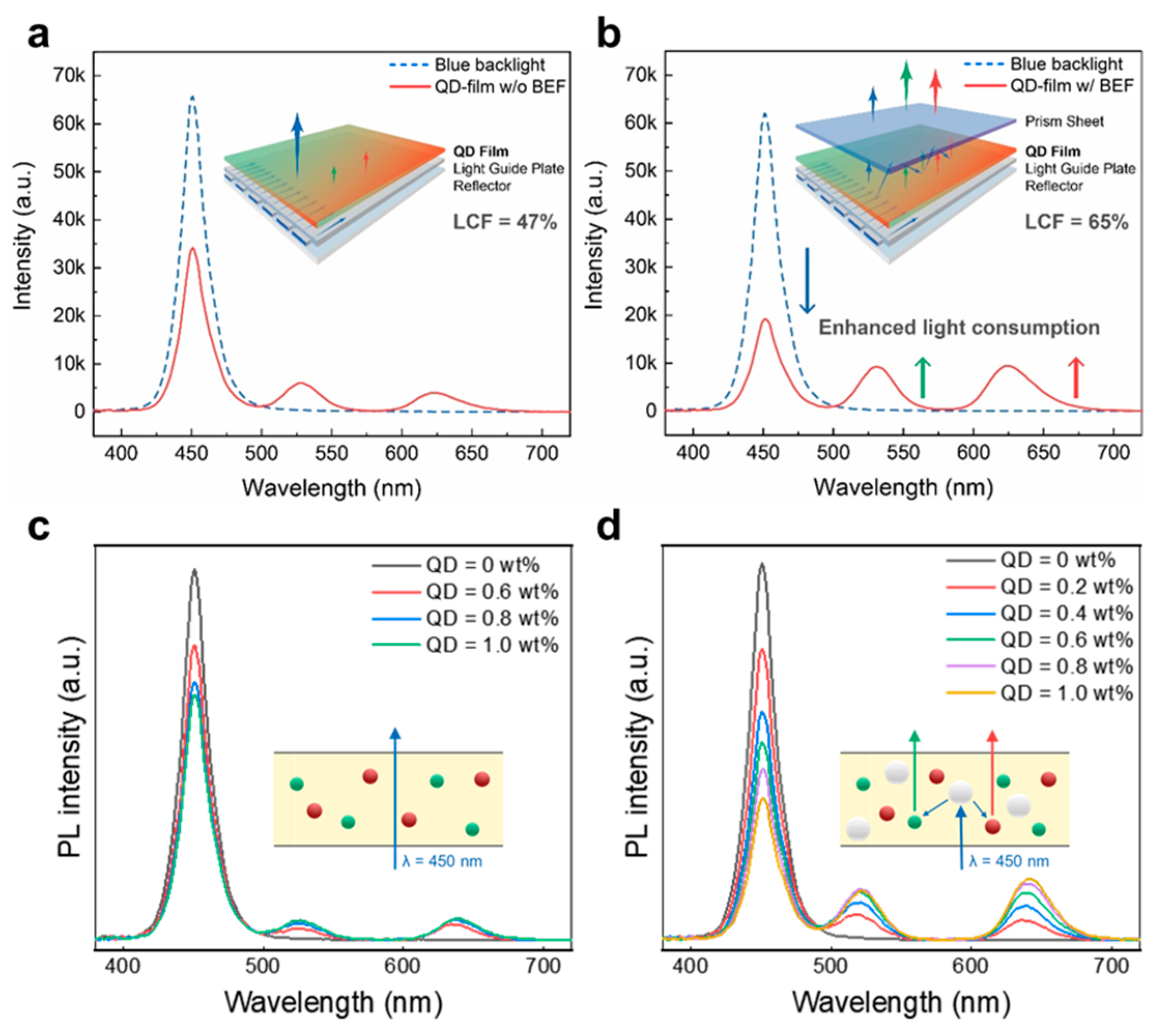
3.2.4. Light Extraction of QD/Polymer Nanocomposites
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kamtekar, K.T.; Monkman, A.P.; Bryce, M.R. Recent Advances in White Organic Light-Emitting Materials and Devices (WOLEDs). Adv. Mater. 2010, 22, 572–582. [Google Scholar] [CrossRef] [PubMed]
- Crawford, M.H. LEDs for Solid-State Lighting: Performance Challenges and Recent Advances. IEEE J. Sel. Top. Quantum Electron. 2009, 15, 1028–1040. [Google Scholar] [CrossRef]
- Ryu, J.; Park, S.; Park, Y.; Ryu, S.; Hwang, K.; Jang, H.W. Technological Breakthroughs in Chip Fabrication, Transfer, and Color Conversion for High-Performance Micro-LED Displays. Adv. Mater. 2022, 35, e2204947. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Chen, J.; Chen, Z.; Liang, L.; Hu, J.; Shen, W.; Li, Z.; Zeng, H. Progress in Color Conversion Technology for Micro-LED. Adv. Mater. Technol. 2023, 8, 2200632. [Google Scholar] [CrossRef]
- Gaurav, A.; Tsai, C.-Y.; Wang, G.-W.; Tsai, H.-Y.; Ye, Z.T.; Lin, C.-F. Ultrahigh-resolution full-color micro-LED array with enhanced efficiency based on a color conversion technique. Photon Res. 2023, 11, 925–935. [Google Scholar] [CrossRef]
- Du, J.; Liu, S.; Song, Z.; Liu, Q. All-Inorganic Green Synthesis of Small-Sized and Efficient K2SiF6:Mn4+ Phosphor for Mini-LED Displays. ACS Appl. Mater. Interfaces 2023, 15, 53738–53745. [Google Scholar] [CrossRef]
- Huang, L.; Lou, S.; Cao, L.; Liu, B.; Zhu, Y.; Liu, Y.; Wang, C.; Wang, J. Surfactant- and HF-Free Cation Exchange Synthesis of Highly-Efficient Nanoscale K2SiF6:Mn4+ and Potential Usage for High-Resolution Micro-LED Display. ACS Mater. Lett. 2022, 4, 1716–1720. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, Y.A.; Li, L.S.; Wang, D.; Zhu, T.; Xu, J.; Yang, C.; Li, Y. Bright, multicoloured light-emitting diodes based on quantum dots. Nat. Photon. 2007, 1, 717–722. [Google Scholar] [CrossRef]
- Shields, A.J. Semiconductor quantum light sources. Nat. Photon. 2007, 1, 215–223. [Google Scholar] [CrossRef]
- Shirasaki, Y.; Supran, G.J.; Bawendi, M.G.; Bulović, V. Emergence of colloidal quantum-dot light-emitting technologies. Nat. Photon. 2012, 7, 13–23. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, C.-H.; Hyun, B.-R.; Sher, C.-W.; Lv, Z.; Luo, B.; Jiang, F.; Wu, T.; Ho, C.-H.; Kuo, H.-C.; et al. Micro-light-emitting diodes with quantum dots in display technology. Light. Sci. Appl. 2020, 9, 83. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; He, J.; Wu, S.-T. Recent Advances on Quantum-Dot-Enhanced Liquid-Crystal Displays. IEEE J. Sel. Top. Quantum Electron. 2017, 23, 1–11. [Google Scholar] [CrossRef]
- Mahshid, S.; Askari, M.; Sasani Ghamsari, M.; Afshar, N.; Lahuti, S. Mixed-phase TiO2 nanoparticles preparation using sol–gel method. J. Alloys Compd. 2009, 478, 586–589. [Google Scholar] [CrossRef]
- Yang, P.; Ando, M.; Murase, N. Highly Luminescent CdSe/CdxZn1–xS Quantum Dots Coated with Thickness-Controlled SiO2 Shell through Silanization. Langmuir 2011, 27, 9535–9540. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Yoon, D.-E.; Jang, J.; Lee, D.; Choi, G.-M.; Chang, J.H.; Lee, J.Y.; Lee, D.C.; Bae, B.-S. Quantum Dot/Siloxane Composite Film Exceptionally Stable against Oxidation under Heat and Moisture. J. Am. Chem. Soc. 2016, 138, 16478–16485. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.-H.; Yang, S.-H.; Yeh, C.-W.; Ho, S.-J.; Liu, M.-C.; Chen, H.-S. Polycarbonate light guide plates with embedded quantum dots fabricated by large-scale injection moulding for wide colour gamut displays. Mater. Des. 2021, 201, 109504. [Google Scholar] [CrossRef]
- Sharma, V.D.; Kansay, V.; Bhatia, A.; Bera, M. Influence of intentional phosphorus doping on Brassica juncea-derived unintentionally N, Ca-doped carbon quantum dots@bioplastic composites for applications in LED and UV-tube down-conversion. Mater. Chem. Phys. 2023, 303, 127834. [Google Scholar] [CrossRef]
- Das, P.; Ganguly, S.; Rosenkranz, A.; Wang, B.; Yu, J.; Srinivasan, S.; Rajabzadeh, A.R. MXene/0D nanocomposite architectures: Design, properties and emerging applications. Mater. Today Nano 2023, 24, 100428. [Google Scholar] [CrossRef]
- Zeng, B.; Palui, G.; Zhang, C.; Zhan, N.; Wang, W.; Ji, X.; Chen, B.; Mattoussi, H. Characterization of the Ligand Capping of Hydrophobic CdSe–ZnS Quantum Dots Using NMR Spectroscopy. Chem. Mater. 2018, 30, 225–238. [Google Scholar] [CrossRef]
- Krivenkov, V.; Samokhvalov, P.; Zvaigzne, M.; Martynov, I.L.; Chistyakov, A.A.; Nabiev, I. Ligand-Mediated Photobrightening and Photodarkening of CdSe/ZnS Quantum Dot Ensembles. J. Phys. Chem. C 2018, 122, 15761–15771. [Google Scholar] [CrossRef]
- Hung, C.-C.; Ho, S.-J.; Yeh, C.-W.; Chen, G.-H.; Huang, J.-H.; Chen, H.-S. Highly Luminescent Dual-Color-Emitting Alloyed [ZnxCd1–xSeyS1–y] Quantum Dots: Investigation of Bimodal Growth and Application to Lighting. J. Phys. Chem. C 2017, 121, 28373–28384. [Google Scholar] [CrossRef]
- Qin, Z.; Su, Q.; Chen, S. Unraveling the Energy Transfer Mechanisms in Bi-Color and Tri-Color Quantum Dots: Toward Efficient White Quantum Dot Light-Emitting Diodes. Adv. Opt. Mater. 2023, 11, 2202451. [Google Scholar] [CrossRef]
- Chen, H.-S.; Huang, S.-H.; Perng, T.-P. Preparation and Characterization of Molecularly Homogeneous Silica–Titania Film by Sol–Gel Process with Different Synthetic Strategies. ACS Appl. Mater. Interfaces 2012, 4, 5188–5195. [Google Scholar] [CrossRef]
- Chen, G.-H.; Chen, H.-S. Nanometer-Thick Sol–Gel Silica–Titania Film Infused with Superparamagnetic Fe3O4 Nanoparticles for Electromagnetic Interference Shielding. ACS Appl. Nano Mater. 2020, 3, 8858–8865. [Google Scholar] [CrossRef]
- Yeh, C.-W.; Chen, G.-H.; Ho, S.-J.; Chen, H.-S. Inhibiting the Surface Oxidation of Low-Cadmim-Content ZnS:(Cd,Se) Quantum Dots for Enhancing Application Reliability. ACS Appl. Nano Mater. 2019, 2, 5290–5301. [Google Scholar] [CrossRef]
- Jung, N.-T.; Chen, P.-R.; Ho, S.-J.; Tung, C.-C.; Chen, P.-Y.; Chen, H.-S. 3D quantum dot-lens fabricated by stereolithographic printing with in-situ UV curing for lighting and displays. Compos. Part B Eng. 2021, 226, 109350. [Google Scholar] [CrossRef]
- Yang, K.P.; Son, S.-R.; Baek, S.H.; Lee, J.H.; Lee, K. Fabrication of light emitting diodes using photo-patternable quantum dot-acrylate resins. J. Mater. Chem. C 2021, 9, 17084–17088. [Google Scholar] [CrossRef]
- Mendes-Felipe, C.; Oliveira, J.; Etxebarria, I.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. State-of-the-Art and Future Challenges of UV Curable Polymer-Based Smart Materials for Printing Technologies. Adv. Mater. Technol. 2019, 4, 1800618. [Google Scholar] [CrossRef]
- Huang, S.-C.; Yeh, C.-W.; Chen, G.-H.; Liu, M.-C.; Chen, H.-S. Investigation of Luminescence Enhancement and Decay of QD-LEDs: Interface Reactions between QDs and Atmospheres. ACS Appl. Mater. Interfaces 2019, 11, 2516–2525. [Google Scholar] [CrossRef]
- Emara, M.M.; Van Patten, P.G. Effect of oxygen and polymer matrix on photo-induced changes in CdSe quantum dots. Mater. Chem. Phys. 2020, 256, 123652. [Google Scholar] [CrossRef]
- Chen, G.-H.; Chen, P.-H.; Lin, C.-T.; Jang, T.-W.; Yang, P.; Chen, H.-S. Enhanced Photostability of Core/Shell Quantum Dots under Intense Blue Light Irradiation through Positive Photoaging Mechanism. ACS Appl. Mater. Interfaces 2023. [Google Scholar] [CrossRef]
- Jang, E.-P.; Jo, J.-H.; Kim, M.-S.; Yoon, S.-Y.; Lim, S.-W.; Kim, J.; Yang, H. Near-complete photoluminescence retention and improved stability of InP quantum dots after silica embedding for their application to on-chip-packaged light-emitting diodes. RSC Adv. 2018, 8, 10057–10063. [Google Scholar] [CrossRef]
- Quesnel, E.; Poncet, S.; Altazin, S.; Lin, Y.P.; D’amico, M. Lifetime prediction of encapsulated CdSexS1−x quantum platelets for color conversion in high luminance LED microdisplays. Opt. Express 2023, 31, 10955–10964. [Google Scholar] [CrossRef]
- Yang, P.; Zhang, A.; Ando, M.; Murase, N. Multiple hydrophobic QDs assembled in SiO2 particles using silane coupling agent. Colloids Surf. A Physicochem. Eng. Asp. 2012, 397, 92–98. [Google Scholar] [CrossRef]
- Coto-García, A.M.; Fernández-Argüelles, M.T.; Costa-Fernández, J.M.; Sanz-Medel, A. Entrapment of quantum dots in sol–gel matrices to develop sensing material based on fluorescence resonance energy transfer. Chem. Commun. 2009, 36, 5454–5456. [Google Scholar] [CrossRef] [PubMed]
- Schlamp, M.C.; Peng, X.; Alivisatos, A.P. Improved efficiencies in light emitting diodes made with CdSe(CdS) core/shell type nanocrystals and a semiconducting polymer. J. Appl. Phys. 1997, 82, 5837–5842. [Google Scholar] [CrossRef]
- Shimizu, K.T.; Böhmer, M.; Estrada, D.; Gangwal, S.; Grabowski, S.; Bechtel, H.; Kang, E.; Vampola, K.J.; Chamberlin, D.; Shchekin, O.B.; et al. Toward commercial realization of quantum dot based white light-emitting diodes for general illumination. Photon. Res. 2017, 5, A1–A6. [Google Scholar] [CrossRef]
- Li, J.-S.; Tang, Y.; Li, Z.-T.; Ding, X.-R.; Rao, L.-S.; Yu, B.-H. Effect of Quantum Dot Scattering and Absorption on the Optical Performance of White Light-Emitting Diodes. IEEE Trans. Electron Devices 2018, 65, 2877–2884. [Google Scholar] [CrossRef]
- Yang, D.; Xie, Y.; Geng, C.; Shen, C.; Liu, J.G.; Sun, M.; Li, S.; Bi, W.; Xu, S. Thermal Analysis and Performance Optimization of Quantum Dots in LEDs by Microsphere Model. IEEE Trans. Electron Devices 2019, 66, 3896–3902. [Google Scholar] [CrossRef]
- Shakeri, B.; Meulenberg, R.W. A Closer Look into the Traditional Purification Process of CdSe Semiconductor Quantum Dots. Langmuir 2015, 31, 13433–13440. [Google Scholar] [CrossRef]
- Kango, S.; Kalia, S.; Celli, A.; Njuguna, J.; Habibi, Y.; Kumar, R. Surface modification of inorganic nanoparticles for development of organic–inorganic nanocomposites—A review. Prog. Polym. Sci. 2013, 38, 1232–1261. [Google Scholar] [CrossRef]
- Knauf, R.R.; Lennox, J.C.; Dempsey, J.L. Quantifying Ligand Exchange Reactions at CdSe Nanocrystal Surfaces. Chem. Mater. 2016, 28, 4762–4770. [Google Scholar] [CrossRef]
- Boles, M.A.; Ling, D.; Hyeon, T.; Talapin, D.V. The surface science of nanocrystals. Nat. Mater. 2016, 15, 141–153. [Google Scholar] [CrossRef] [PubMed]
- Masui, H.; Nakamura, S.; DenBaars, S.P. Effects of Phosphor Application Geometry on White Light-Emitting Diodes. Jpn. J. Appl. Phys. 2006, 45, L910–L912. [Google Scholar] [CrossRef]
- Camardello, S.J.; Butts, M.D.; Cassidy, R.; Murphy, J.E.; Parthasarathy, G.; Setlur, A.A.; Siclovan, O.P.; Welch, J.; Yakimov, A. 62-11: Development of New Green Phosphors for Liquid Crystal Display Backlights. SID Symp. Dig. Tech. Pap. 2021, 52, 917–919. [Google Scholar] [CrossRef]
- Murphy, J.; Camardello, S.; Doherty, M.; Liu, J.; Smigelski, P.; Setlur, A. 11.1: Invited Paper: Narrow-Band Phosphors for Next Generation MiniLED and MicroLED Displays. SID Symp. Dig. Tech. Pap. 2021, 52, 165–168. [Google Scholar] [CrossRef]
- You, J.P.; Tran, N.T.; Lin, Y.-C.; He, Y.; Shi, F.G. Phosphor-Concentration-Dependent Characteristics of White LEDs in Different Current Regulation Modes. J. Electron. Mater. 2009, 38, 761–766. [Google Scholar] [CrossRef]
- Chen, J.; Hardev, V.; Hartlove, J.; Hofler, J.; Lee, E. 66.1: Distinguised Paper: A High-Efficiency Wide-Color-Gamut Solid-State Backlight System for LCDs Using Quantum Dot Enhancement Film. SID Symp. Dig. Tech. Pap. 2012, 43, 895–896. [Google Scholar] [CrossRef]
- Myeong, S.; Chon, B.; Kumar, S.; Son, H.-J.; Kang, S.O.; Seo, S. Quantum dot photolithography using a quantum dot photoresist composed of an organic–inorganic hybrid coating layer. Nanoscale Adv. 2022, 4, 1080–1087. [Google Scholar] [CrossRef]
- Ho, S.-J.; Hsu, H.-C.; Yeh, C.-W.; Chen, H.-S. Inkjet-Printed Salt-Encapsulated Quantum Dot Film for UV-Based RGB Color-Converted Micro-Light Emitting Diode Displays. ACS Appl. Mater. Interfaces 2020, 12, 33346–33351. [Google Scholar] [CrossRef]
- Li, J.; Li, Z.; Qiu, J.; Li, J. Photothermal Optimization of Quantum Dot Converters for High-Power Solid-State Light Sources. Adv. Opt. Mater. 2022, 10, 2102201. [Google Scholar] [CrossRef]
- Ho, S.-J.; Chen, H.-S. Inverse μ-photonic crystals enhanced the features of mini-sized quantum dot LEDs. J. Mater. Chem. C 2020, 8, 4309–4313. [Google Scholar] [CrossRef]
- Lee, T.-Y.; Miao, W.-C.; Hung, Y.-Y.; Bai, Y.-H.; Chen, P.-T.; Huang, W.-T.; Chen, K.-A.; Lin, C.-C.; Chen, F.-C.; Hong, Y.-H.; et al. Ameliorating Uniformity and Color Conversion Efficiency in Quantum Dot-Based Micro-LED Displays through Blue–UV Hybrid Structures. Nanomaterials 2023, 13, 2099. [Google Scholar] [CrossRef] [PubMed]
- Han, H.-V.; Lin, H.-Y.; Lin, C.-C.; Chong, W.-C.; Li, J.-R.; Chen, K.-J.; Yu, P.; Chen, T.-M.; Chen, H.-M.; Lau, K.-M.; et al. Resonant-enhanced full-color emission of quantum-dot-based micro LED display technology. Opt. Express 2015, 23, 32504–32515. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-T.; Li, J.-X.; Li, J.-S.; Deng, Z.-H.; Deng, Y.-H.; Tang, Y. Scattering Effect on Optical Performance of Quantum Dot White Light-Emitting Diodes Incorporating SiO2 Nanoparticles. IEEE J. Quantum Electron. 2020, 56, 1–9. [Google Scholar] [CrossRef]
- Gao, H.; Xie, Y.; Geng, C.; Xu, S.; Bi, W. Efficiency Enhancement of Quantum-Dot-Converted LEDs by 0D–2D Hybrid Scatterers. ACS Photon. 2020, 7, 3430–3439. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, G.-H.; Lin, C.-T.; Chen, P.-H.; Jang, T.-W.; Chen, H.-S. General Strategies for Preparing Hybrid Polymer/Quantum Dot Nanocomposites for Color Conversion. Nanomaterials 2023, 13, 3072. https://doi.org/10.3390/nano13233072
Chen G-H, Lin C-T, Chen P-H, Jang T-W, Chen H-S. General Strategies for Preparing Hybrid Polymer/Quantum Dot Nanocomposites for Color Conversion. Nanomaterials. 2023; 13(23):3072. https://doi.org/10.3390/nano13233072
Chicago/Turabian StyleChen, Guan-Hong, Chen-Te Lin, Po-Hsun Chen, Tyng-Woei Jang, and Hsueh-Shih Chen. 2023. "General Strategies for Preparing Hybrid Polymer/Quantum Dot Nanocomposites for Color Conversion" Nanomaterials 13, no. 23: 3072. https://doi.org/10.3390/nano13233072
APA StyleChen, G.-H., Lin, C.-T., Chen, P.-H., Jang, T.-W., & Chen, H.-S. (2023). General Strategies for Preparing Hybrid Polymer/Quantum Dot Nanocomposites for Color Conversion. Nanomaterials, 13(23), 3072. https://doi.org/10.3390/nano13233072





