Facile Fabrication of Nickel Supported on Reduced Graphene Oxide Composite for Oxygen Reduction Reaction
Abstract
:1. Introduction
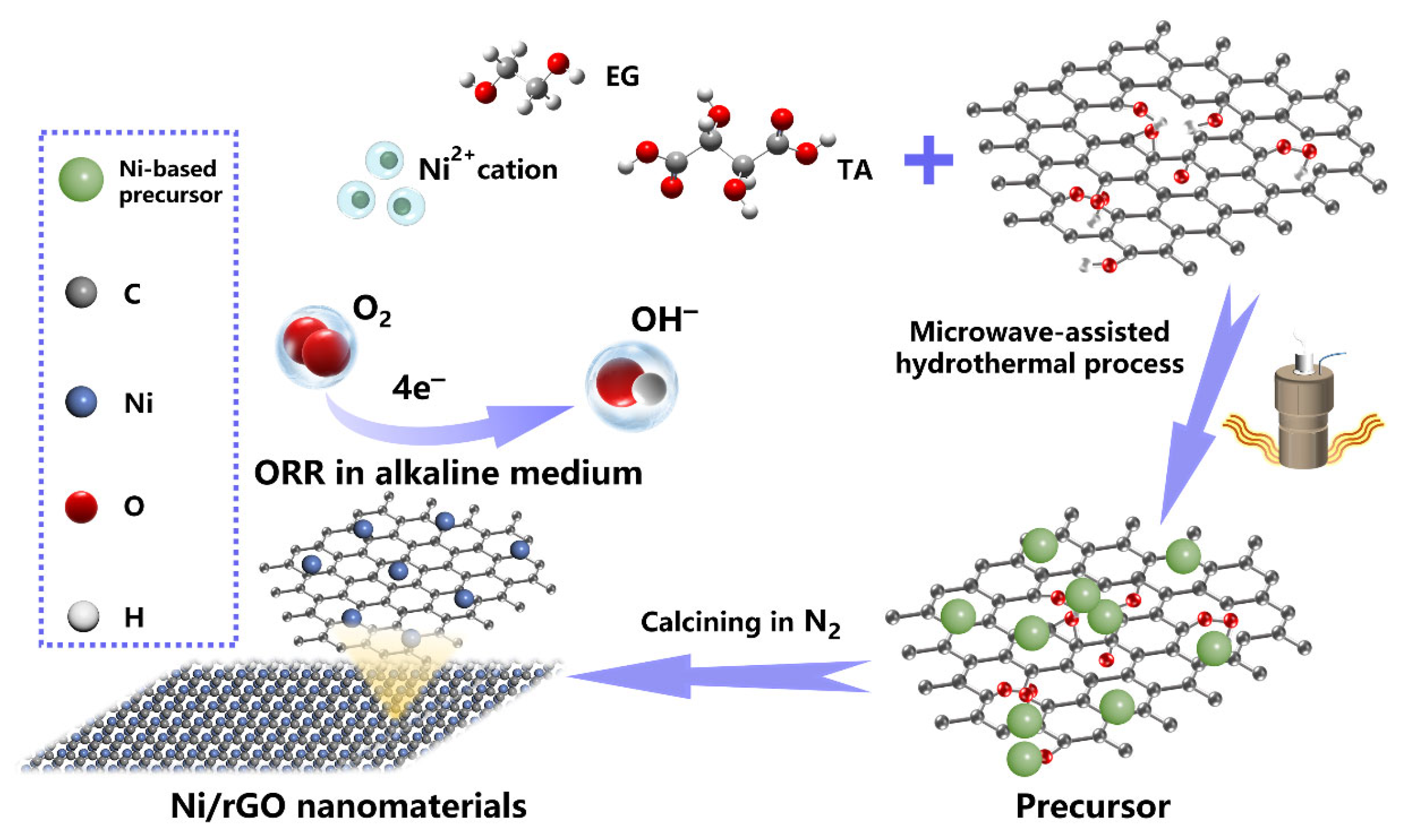
2. Fabrication Process of Ni/rGO Composite
3. Results and Discussion
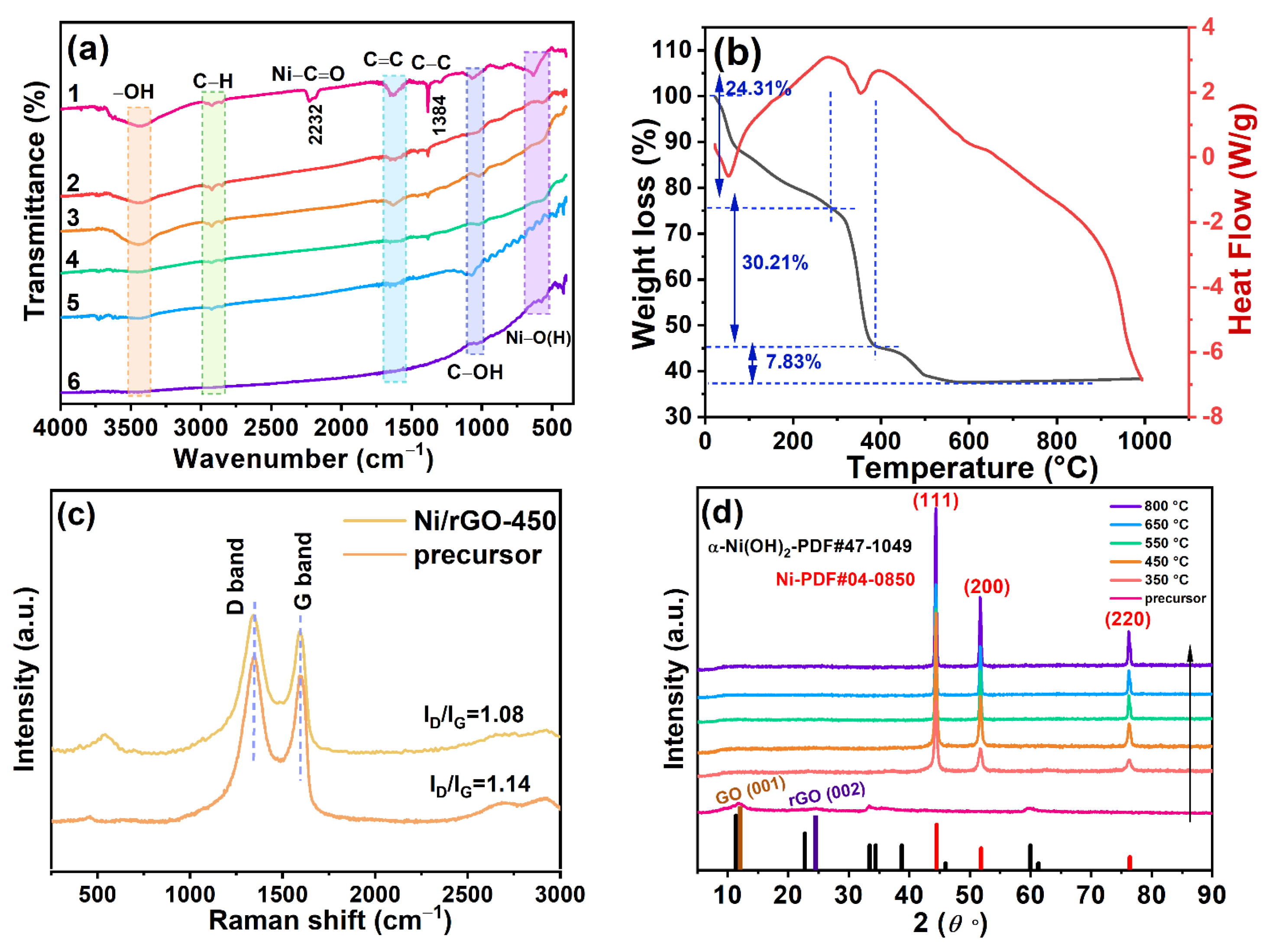
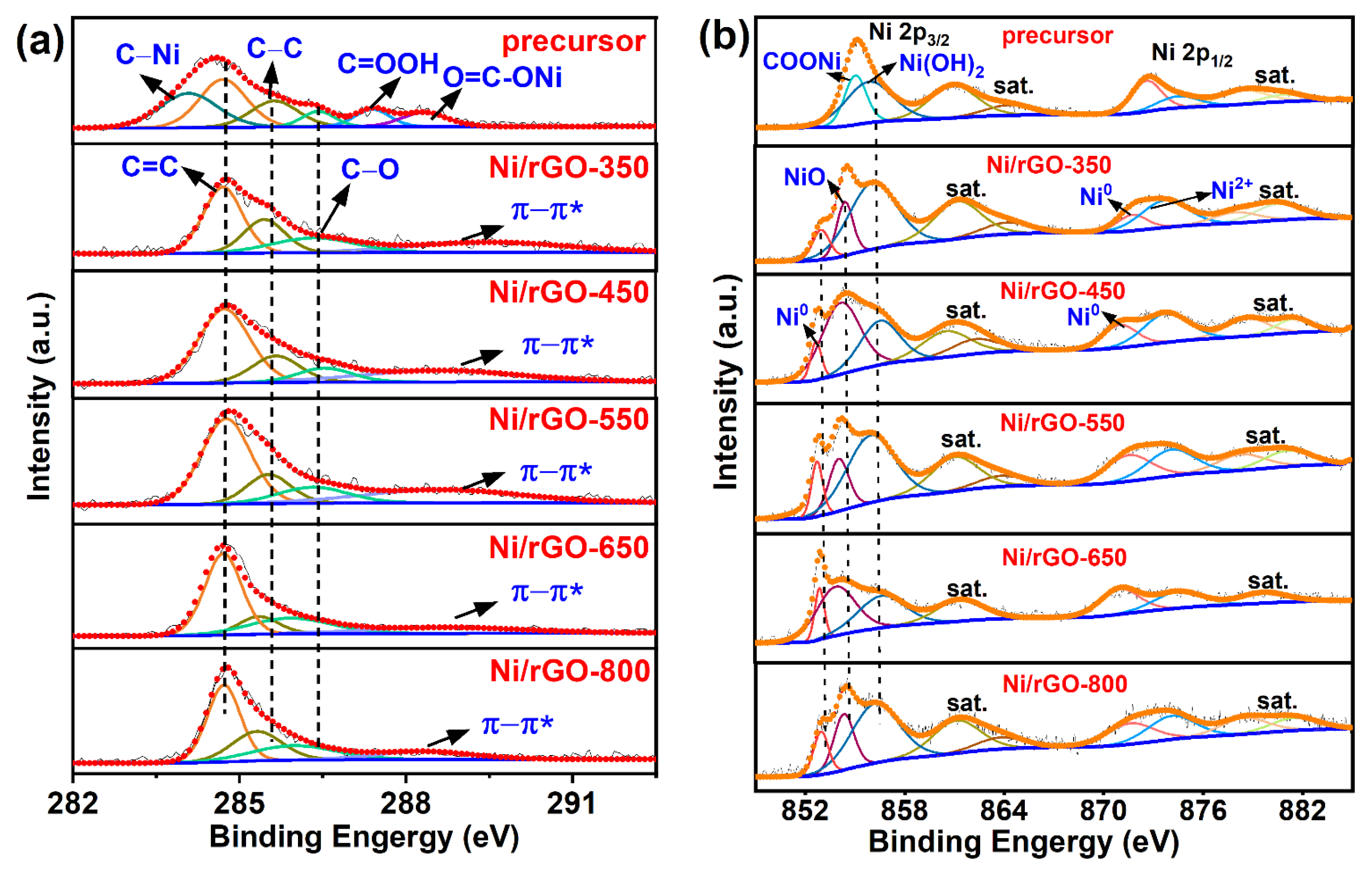
3.1. Morphology, Phase, and Element Composition Analysis
3.2. ORR Performance Analysis
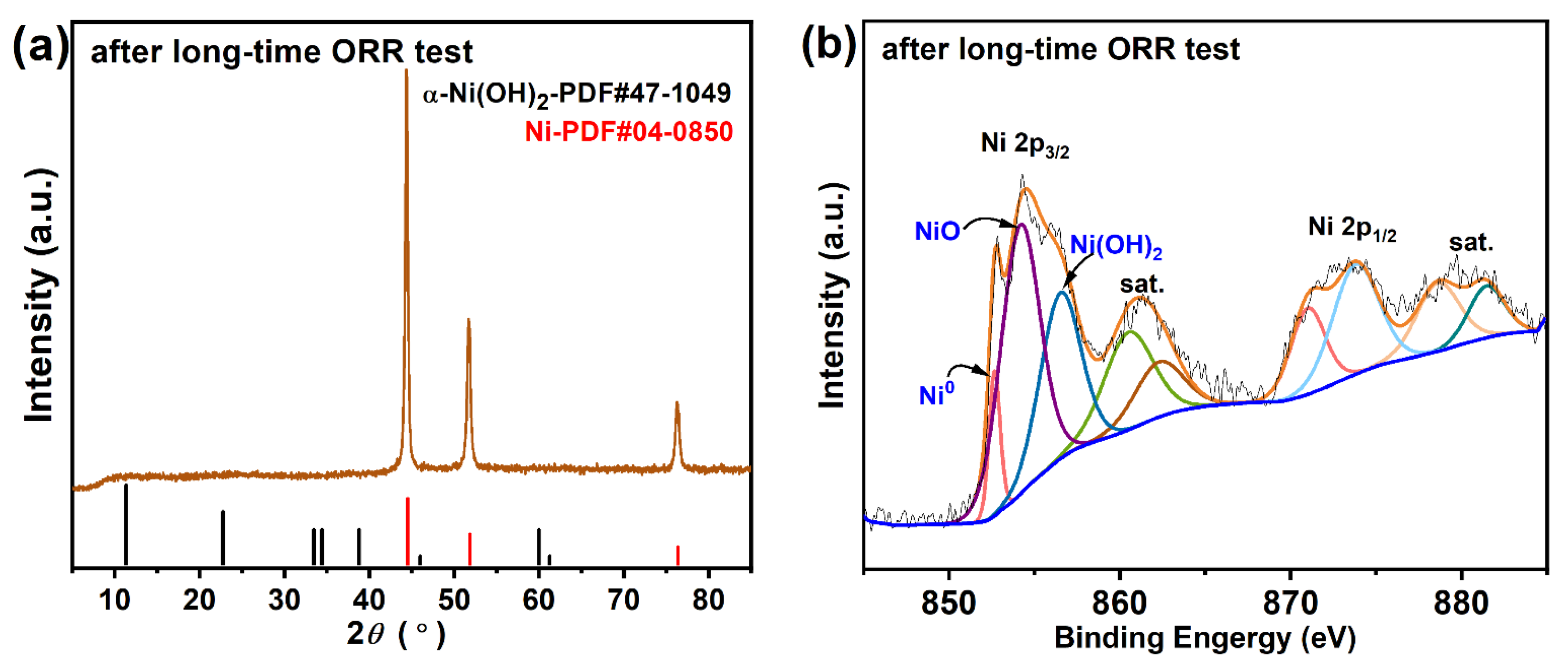
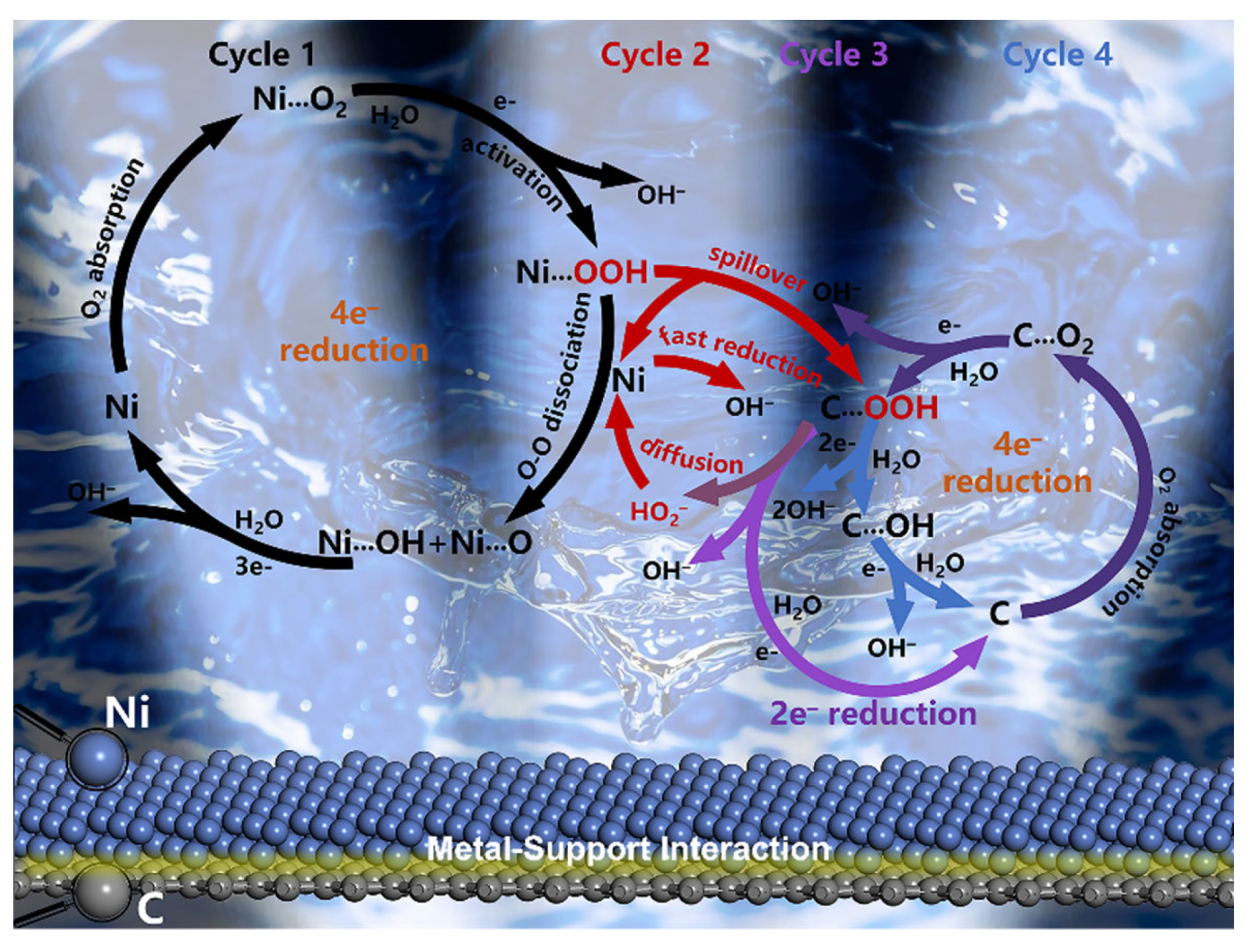
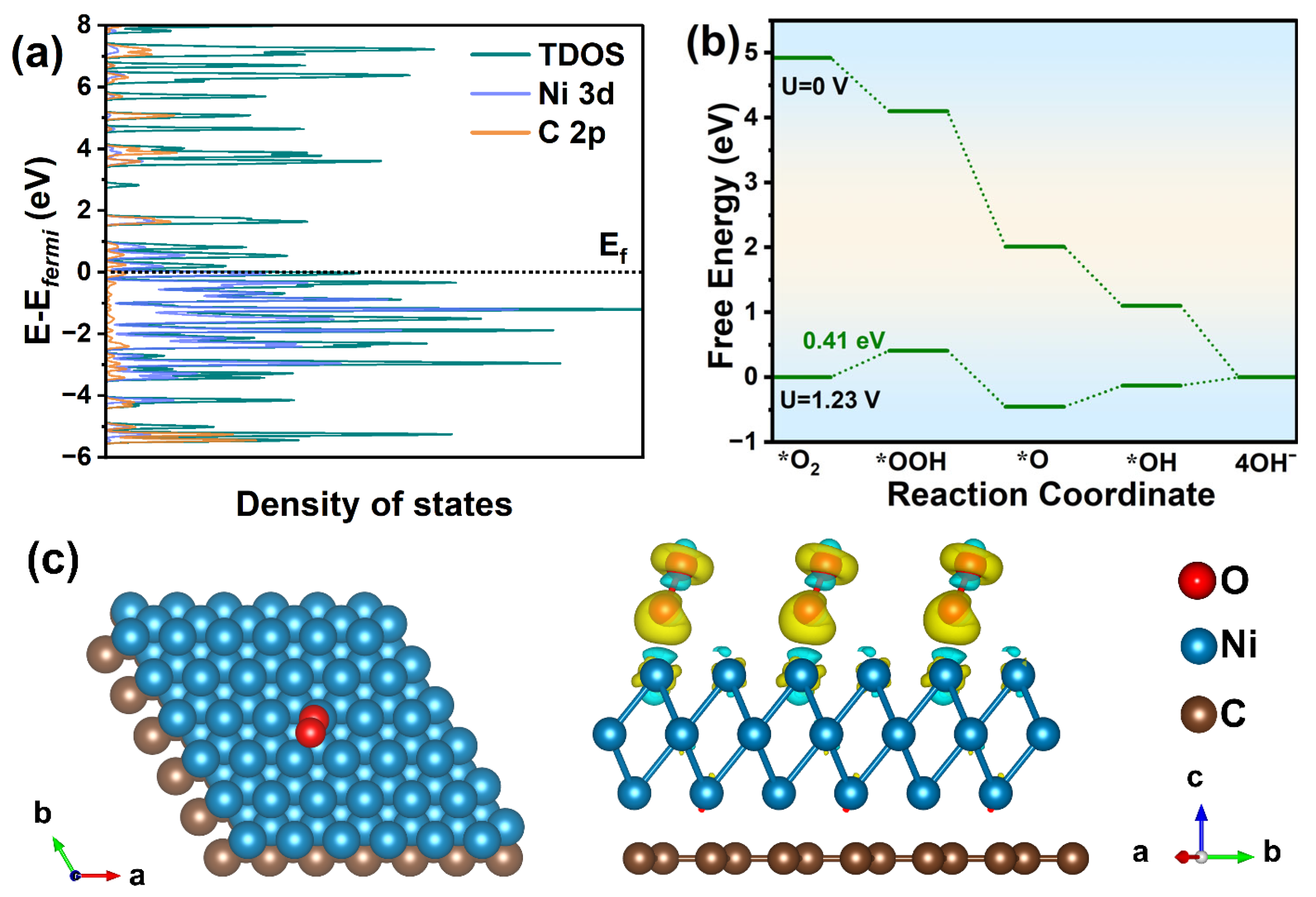
3.3. Mechanism towards Oxygen Reduction Reaction for Ni/rGO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nanadegani, F.S.; Sunden, B. Review of exergy and energy analysis of fuel cells. Int. J. Hydrogen Energy 2023, 48, 32875–32942. [Google Scholar] [CrossRef]
- Zhang, Y.; Song, Y.; Zhao, J.; Li, S.; Li, Y. Ultrahigh electrocatalytic activity and durability of bimetallic Au@Ni core-shell nanoparticles supported on rGO for methanol oxidation reaction in alkaline electrolyte. J. Alloys Compd. 2020, 822, 153322. [Google Scholar] [CrossRef]
- Han, X.-W.; Guo, S.; Li, T.; Peng, J.; Pan, H. Construction of Ag/3D-reduced graphene oxide nanocomposite with advanced catalytic capacity for 4-nitrophenol and methylene blue. Colloid Surf. A 2022, 650, 128688. [Google Scholar] [CrossRef]
- Habib, B.; Chen, S.; Nichols, F.; Talib, S.H.; Arshad, N.; Zafar, A.; Mahmood, A.; Zaman, S.; Janjua, N.K. Cu/Fe embedded N-doped carbon as a highly durable oxygen reduction electrocatalyst. Mater. Adv. 2023, 4, 5353–5360. [Google Scholar] [CrossRef]
- Zheng, Y.; Khan, N.A.; Ni, X.; Zhang, K.A.I.; Shen, Y.; Huang, N.; Kong, X.Y.; Ye, L. Emerging covalent triazine framework-based nanomaterials for electrochemical energy storage and conversion. Chem. Commun. 2023, 59, 6314–6334. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Mei, S.; Zheng, Y.; Wang, L.; Ye, L. High-entropy oxides as photocatalysts for organic conversion. Chem. Commun. 2023, 59, 13478–13481. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Guan, Q.; Wu, H.; Liu, W.; Lin, Y.; Sun, Z.; Ye, X.; Zheng, X.; Pan, H.; Zhu, J.; et al. Highly active and stable metal single-atom catalysts achieved by strong electronic metal–support interactions. J. Am. Chem. Soc. 2019, 141, 14515–14519. [Google Scholar] [CrossRef]
- Dong, J.; Wang, S.; Xi, P.; Zhang, X.; Zhu, X.; Wang, H.; Huang, T. Reduced Graphene Oxide-Supported Iron-Cobalt Alloys as High-Performance Catalysts for Oxygen Reduction Reaction. Nanomaterials 2023, 13, 2735. [Google Scholar] [CrossRef]
- Zaman, S.; Huang, L.; Douka, A.I.; Yang, H.; You, B.; Xia, B.Y. Oxygen Reduction Electrocatalysts toward Practical Fuel Cells: Progress and Perspectives. Angew. Chem. Int. Ed. 2021, 60, 17832–17852. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Yin, P.; Liang, H. Engineering the electronic interaction between metals and carbon supports for oxygen/hydrogen electrocatalysis. ACS Mater. Lett. 2021, 3, 1197–1212. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, S.; Yu, X.; Li, K.; Ni, X.; Ye, L. Nitrogen-doped carbon spheres with precisely-constructed pyridinic-N active sites for efficient oxygen reduction. Appl. Surf. Sci. 2022, 598, 153786. [Google Scholar] [CrossRef]
- Artchuea, T.; Srikhaow, A.; Sriprachuabwong, C.; Tuantranont, A.; Tang, I.-M.; Pon-On, W. Copper zinc sulfide (CuZnS) quantum dot-decorated (NiCo)-S/conductive carbon matrix as the cathode for Li-S batteries. Nanomaterials 2022, 12, 2403. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M.G.; Beltrán, A.M.; Fernández, A.; Cadús, L.E.; Morales, M.R. Pd supported on defective TiO2 polymorphic mixtures: Effect of metal-support interactions upon glycerol selective oxidation. Results Eng. 2022, 16, 100737. [Google Scholar] [CrossRef]
- Arif, M.; Bilal, S.; Shah, A.u.H.A. Fabrication and Integration of Functionalized N-rGO-Ni/Ag and N-rGO-Ni/Co Nanocomposites as Synergistic Oxygen Electrocatalysts in Fuel Cells. Nanomaterials 2022, 12, 585. [Google Scholar] [CrossRef] [PubMed]
- Ramirez-Barria, C.S.; Fernandes, D.M.; Freire, C.; Villaro-Abalos, E.; Guerrero-Ruiz, A.; Rodríguez-Ramos, I. Upgrading the Properties of Reduced Graphene Oxide and Nitrogen-Doped Reduced Graphene Oxide Produced by Thermal Reduction toward Efficient ORR Electrocatalysts. Nanomaterials 2019, 9, 1761. [Google Scholar] [CrossRef]
- Zaman, S.; Wang, M.; Liu, H.; Sun, F.; Yu, Y.; Shui, J.; Chen, M.; Wang, H. Carbon-based catalyst supports for oxygen reduction in proton-exchange membrane fuel cells. Trends Chem. 2022, 4, 886–906. [Google Scholar] [CrossRef]
- Yoon, H.-I.; Lee, D.-K.; Bae, H.B.; Jo, G.-Y.; Chung, H.-S.; Kim, J.-G.; Kang, S.-J.L.; Chung, S.-Y. Probing dopant segregation in distinct cation sites at perovskite oxide polycrystal interfaces. Nat. Commun. 2017, 8, 1417. [Google Scholar] [CrossRef]
- Pei, Z.; Zhang, H.; Wu, Z.-P.; Lu, X.F.; Luan, D.; Lou, X.W. Atomically dispersed Ni activates adjacent Ce sites for enhanced electrocatalytic oxygen evolution activity. Sci. Adv. 2023, 9, eadh1320. [Google Scholar] [CrossRef]
- Yao, P.; Jiang, Y.; Liu, Y.; Wu, C.; Chou, K.-C.; Lyu, T.; Li, Q. Catalytic effect of Ni@rGO on the hydrogen storage properties of MgH2. J. Magnes. Alloys 2020, 8, 461–471. [Google Scholar] [CrossRef]
- Trisunaryanti, W.; Wijaya, K.; Triyono, T.; Adriani, A.R.; Larasati, S. Green synthesis of hierarchical porous carbon prepared from coconut lumber sawdust as Ni-based catalyst support for hydrotreating Callophyllum inophyllum oil. Results Eng. 2021, 11, 100258. [Google Scholar] [CrossRef]
- Zhang, B.; Su, D.S. Probing the metal–support interaction in carbon-supported catalysts by using electron microscopy. ChemCatChem 2015, 7, 3639–3645. [Google Scholar] [CrossRef]
- Xue, C.; Cai, W.; Weng, X.; Owens, G.; Chen, Z. A one step synthesis of hybrid Fe/Ni-rGO using green tea extract for the removal of mixed contaminants. Chemosphere 2021, 284, 131369. [Google Scholar] [CrossRef]
- Ouyang, J.; Duan, L.; Tieke, B. Effects of carboxylic acids on the crystal growth of calcium oxalate nanoparticles in lecithin−water liposome systems. Langmuir 2003, 19, 8980–8985. [Google Scholar] [CrossRef]
- Alinejadian, N.; Nasirpouri, F.; Yus, J.; Ferrari, B. Reduction-based engineering of three-dimensional morphology of Ni-rGO nanocomposite. Mat. Sci. Eng. B 2021, 271, 115259. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, C.; Li, J.; Zhao, Z.; Xu, J.; Liu, L.; Qian, J. Fabrication of interface-engineered Ni/NiO/rGO nanobush for highly efficient and durable oxygen reduction. Mat. Sci. Semicon. Proc. 2023, 156, 107259. [Google Scholar] [CrossRef]
- Elessawy, N.A.; Abdel Rafea, M.; Roushdy, N.; Youssef, M.E.; Gouda, M.H. Development and evaluation of cost-effective and green Bi-functional nickel oxide decorated graphene electrocatalysts for alkaline fuel cells. Results Eng. 2023, 17, 100871. [Google Scholar] [CrossRef]
- Hasanat, A.U.; Khoja, A.H.; Naeem, N.; Al-Anazi, A.; Liaquat, R.; Khan, B.A.; Din, I.U. Thermocatalytic partial oxidation of methane to syngas (H2, CO) production using Ni/La2O3 modified biomass fly ash supported catalyst. Results Eng. 2023, 19, 101333. [Google Scholar] [CrossRef]
- Chandel, M.; Makkar, P.; Ghosh, B.K.; Moitra, D.; Ghosh, N.N. A facile synthesis methodology for preparation of Ag–Ni-reduced graphene oxide: A magnetically separable versatile nanocatalyst for multiple organic reactions and density functional study of its electronic structures. RSC Adv. 2018, 8, 37774–37788. [Google Scholar] [CrossRef] [PubMed]
- Zeenat; Maryum Javed, S.; Ahmad, Z.; Ahmed, S.; Iqbal, S.; Naqvi, I.J.; Usman, M.; Ashiq, M.N.; Elnaggar, A.Y.; El-Bahy, Z.M. Highly dispersed active sites of Ni nanoparticles onto hierarchical reduced graphene oxide architecture towards efficient water oxidation. Fuel 2022, 312, 122926. [Google Scholar] [CrossRef]
- Li, W.; Liu, Y.; Guo, F.; Du, Y.; Chen, Y. Self-assembly sandwich-like Fe, Co, or Ni nanoparticles/reduced graphene oxide composites with excellent microwave absorption performance. Appl. Surf. Sci. 2021, 562, 150212. [Google Scholar] [CrossRef]
- Niu, L.; Wang, J.; Hong, W.; Sun, J.; Fan, Z.; Ye, X.; Wang, H.; Yang, S. Solvothermal synthesis of Ni/reduced graphene oxide composites as electrode material for supercapacitors. Electrochim. Acta 2014, 123, 560–568. [Google Scholar] [CrossRef]
- Gavrilov, N.; Momčilović, M.; Dobrota, A.S.; Stanković, D.M.; Jokić, B.; Babić, B.; Skorodumova, N.V.; Mentus, S.V.; Pašti, I.A. A study of ordered mesoporous carbon doped with Co and Ni as a catalyst of oxygen reduction reaction in both alkaline and acidic media. Surf. Coat. Technol. 2018, 349, 511–521. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, D.; Karunanithi, P.; Kumar, S.; Goswami, M.; Singh, A.; Singh, N.; Alam, F.; Sathish, N.; Choudhary, D.; et al. Self-assembled nickel anchored reduced graphene oxide hybrids: Synergistic performance of electro-catalyst for oxygen reduction reaction in non-aqueous medium. Mater. Res. Express 2019, 6, 125520. [Google Scholar] [CrossRef]
- Arunpandiyan, S.; Raja, A.; Bharathi, S.; Arivarasan, A. Fabrication of ZnO/NiO:rGO coated Ni foam binder-free electrode via hydrothermal method for supercapacitor application. J. Alloys Compd. 2021, 883, 160791. [Google Scholar] [CrossRef]
- Tao, Y.; Jinfei, C.; Tingting, Y.; Zaijun, L. Template-free synthesis of α-Ni(OH)2 hollow microspheres with flower-like morphology for high-performance supercapacitors. Mater. Res. Bull. 2014, 60, 612–620. [Google Scholar] [CrossRef]
- Xu, W.; Li, S.; Zhang, W.; Hu, S.; Yu, W.; Zhou, Y. Core@shell Ti3C2Tx@Ni particles with enhanced microwave absorption properties and prolonged stability. Mater. Res. Bull. 2023, 164, 112250. [Google Scholar] [CrossRef]
- Panigrahi, B.B. Sintering and grain growth kinetics of ball milled nanocrystalline nickel powder. Mat. Sci. Eng. A Struct. 2007, 460–461, 7–13. [Google Scholar] [CrossRef]
- Wei, L.; Ye, S.; Tian, Y.; Xie, Y.; Chen, Y. Effects of ammonium citrate additive on crystal morphology of aluminum phosphate ammonium taranakite. J. Cryst. Growth 2009, 311, 3359–3363. [Google Scholar] [CrossRef]
- Sahu, R.S.; Li, D.-L.; Doong, R.-a. Unveiling the hydrodechlorination of trichloroethylene by reduced graphene oxide supported bimetallic Fe/Ni nanoparticles. Chem. Eng. J. 2018, 334, 30–40. [Google Scholar] [CrossRef]
- Jena, G.; Thinaharan, C.; George, R.P.; Philip, J. Robust nickel-reduced graphene oxide-myristic acid superhydrophobic coating on carbon steel using electrochemical codeposition and its corrosion resistance. Surf. Coat. Technol. 2020, 397, 125942. [Google Scholar] [CrossRef]
- Xue, T.; Liao, S.-J.; Yang, Y.; Yan, X.-H.; Zou, Z.-L.; Luo, M. Nickel induced in situ growth of nickel hydroxide nanoflakes on reduced graphite oxide with high energy and power density. J. Colloid Interface Sci. 2019, 537, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Darabdhara, G.; Das, M.R.; Amin, M.A.; Mersal, G.A.M.; Mostafa, N.Y.; Abd El-Rehim, S.S.; Szunerits, S.; Boukherroub, R. AuNi alloy nanoparticles supported on reduced graphene oxide as highly efficient electrocatalysts for hydrogen evolution and oxygen reduction reactions. Int. J. Hydrogen Energy 2018, 43, 1424–1438. [Google Scholar] [CrossRef]
- Zhang, W.; Li, Y.; Peng, S. Template-free synthesis of hollow Ni/reduced graphene oxide composite for efficient H2 evolution. J. Mater. Chem. A 2017, 5, 13072–13078. [Google Scholar] [CrossRef]
- Hao, J.; Li, C.; Wu, C.; Wu, K. In-situ synthesis of carbon-encapsulated Ni nanoparticles decorated graphene nanosheets with high reactivity toward glucose oxidation and sensing. Carbon 2019, 148, 44–51. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, J.; Xing, J.; Xu, J.; Wang, X.; Yu, X.; Liu, L. Preparation of TiO2/Sb–SnO2 composite by a polymer pyrolysis method for conducting fillers. Mat. Sci. Semicon. Proc. 2021, 133, 105922. [Google Scholar] [CrossRef]
- Tang, H.; Bian, Z.; Peng, Y.; Li, S.; Wang, H. Stepwise dechlorination of chlorinated alkenes on an Fe-Ni/rGO/Ni foam cathode: Product control by one-electron-transfer reactions. J. Hazard. Mater. 2022, 433, 128744. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, Y.; Wang, H.; Dai, H. Strongly coupled inorganic/nanocarbon hybrid materials for advanced electrocatalysis. J. Am. Chem. Soc. 2013, 135, 2013–2036. [Google Scholar] [CrossRef]
- Tyagi, A.; Kar, K.K.; Yokoi, H. Atomically dispersed Ni/NixSy anchored on doped mesoporous networked carbon framework: Boosting the ORR performance in alkaline and acidic media. J. Colloid Interface Sci. 2020, 571, 285–296. [Google Scholar] [CrossRef]
- Feng, L.; Sun, X.; Yao, S.; Liu, C.; Xing, W.; Zhang, J. 3-Electrocatalysts and catalyst layers for oxygen reduction reaction. In Rotating Electrode Methods and Oxygen Reduction Electrocatalysts; Xing, W., Yin, G., Zhang, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 67–132. [Google Scholar]
- Wan, Z.; Bai, X.; Mo, H.; Yang, J.; Wang, Z.; Zhou, L. Multi-porous NiAg-doped Pd alloy nanoparticles immobilized on reduced graphene oxide/CoMoO4 composites as a highly active electrocatalyst for direct alcohol fuel cell. Colloid Surf. A 2021, 614, 126048. [Google Scholar] [CrossRef]
- Feng, Z.; Li, D.; Wang, L.; Sun, Q.; Lu, P.; Xing, P.; An, M. A 3D porous Ni-Zn/RGO catalyst with superaerophobic surface for high-performance hydrazine electrooxidation. J. Alloys Compd. 2019, 788, 1240–1245. [Google Scholar] [CrossRef]
- He, X.; Chang, L.; Han, P.; Li, K.; Wu, H.; Tang, Y.; Gao, F.; Zhang, Y.; Zhou, A. High-performance Co-N-C catalyst derived from PS@ZIF-8 @ZIF-67 for improved oxygen reduction reaction. Colloid Surf. A 2023, 663, 130988. [Google Scholar] [CrossRef]
- Alipour Moghadam Esfahani, R.; Black-Araujo, K.; Melino, P.D.; Sullivan, M.T.; Easton, E.B. Silicon-doped niobium suboxide (NbOS): A fuel cell electrocatalyst support with enhanced conductivity and corrosion-resistance. Results Eng. 2022, 16, 100767. [Google Scholar] [CrossRef]
- Wang, W.; Chen, J.; Tao, Y.; Zhu, S.; Zhang, Y.; Wu, X. Flowerlike Ag-supported Ce-doped Mn3O4 nanosheet heterostructure for a highly efficient oxygen reduction reaction: Roles of metal oxides in Ag surface states. ACS Catal. 2019, 9, 3498–3510. [Google Scholar] [CrossRef]
- Beall, C.E.; Fabbri, E.; Schmidt, T.J. Perovskite oxide based electrodes for the oxygen reduction and evolution reactions: The underlying mechanism. ACS Catal. 2021, 11, 3094–3114. [Google Scholar] [CrossRef]
- Mefford, J.T.; Kurilovich, A.A.; Saunders, J.; Hardin, W.G.; Abakumov, A.M.; Forslund, R.P.; Bonnefont, A.; Dai, S.; Johnston, K.P.; Stevenson, K.J. Decoupling the roles of carbon and metal oxides on the electrocatalytic reduction of oxygen on La1−xSrxCoO3−δ perovskite composite electrodes. Phys. Chem. Chem. Phys. 2019, 21, 3327–3338. [Google Scholar] [CrossRef] [PubMed]
- Dong, Q.; Ji, S.; Wang, H.; Linkov, V.; Wang, R. Oxygen spillover effect at Cu/Fe2O3 heterointerfaces to enhance oxygen electrocatalytic reactions for rechargeable Zn–Air batteries. ACS Appl. Mater. Interfaces 2022, 14, 51222–51233. [Google Scholar] [CrossRef] [PubMed]
- Nørskov, J.K.; Rossmeisl, J.; Logadottir, A.; Lindqvist, L.; Kitchin, J.R.; Bligaard, T.; Jónsson, H. Origin of the overpotential for oxygen reduction at a fuel-cell cathode. J. Phys. Chem. B 2004, 108, 17886–17892. [Google Scholar] [CrossRef]
- Tripković, V.; Skúlason, E.; Siahrostami, S.; Nørskov, J.K.; Rossmeisl, J. The oxygen reduction reaction mechanism on Pt(111) from density functional theory calculations. Electrochim. Acta 2010, 55, 7975–7981. [Google Scholar] [CrossRef]
- Ge, F.; Qiao, Q.; Chen, X.; Wu, Y. Probing the catalytic activity of M-N4−xOx embedded graphene for the oxygen reduction reaction by density functional theory. Front. Chem. Sci. Eng. 2021, 15, 1206–1216. [Google Scholar] [CrossRef]
- Durante, C. Metal–carbon interaction in metal nanoparticles and implication in the electrocatalysis of oxygen reduction. Curr. Opin. Electrochem. 2022, 36, 101119. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for liquid metals. Phys. Rev. B 1993, 47, 558–561. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Yu, J.; Jiang, Z.; Wang, J.; Fang, H.; Huang, T.; Sun, S. Synthesis of high performing Cu0.31Ni0.69O/rGO hybrid for oxygen reduction reaction in alkaline medium. Int. J. Hydrogen Energy 2019, 44, 13345–13353. [Google Scholar] [CrossRef]
- Nassr, A.B.A.A.; Kottakkat, T.; Bron, M. A simple microwave process for the preparation of cobalt oxide nanoparticles supported on carbon nanotubes for electrocatalytic applications. J. Solid State Electrochem. 2020, 24, 131–136. [Google Scholar] [CrossRef]
- Zhang, L.; Lu, J.; Wei, J.; Wang, Y. Novel flower-like CuO/N-rGO as enhanced electrocatalyst for oxygen reduction reaction. Nano 2019, 14, 1950132. [Google Scholar] [CrossRef]
- Wu, Z.-Y.; Chen, P.; Wu, Q.-S.; Yang, L.-F.; Pan, Z.; Wang, Q. Co/Co3O4/C–N, a novel nanostructure and excellent catalytic system for the oxygen reduction reaction. Nano Energy 2014, 8, 118–125. [Google Scholar] [CrossRef]



| Samples | Specific Surface Area (m2 g−1) | Ni NPs Size (nm) | lnD | 1/T (K−1) |
|---|---|---|---|---|
| Precursor | 97.182 | ─ | ─ | ─ |
| Ni/rGO-350 | 63.913 | 16.90318 | 2.8275 | 0.0016 |
| Ni/rGO-450 | 74.545 | 21.60161 | 3.07277 | 0.00138 |
| Ni/rGO-550 | 34.024 | 26.30211 | 3.26965 | 0.00121 |
| Ni/rGO-650 | 26.307 | 29.52183 | 3.38513 | 0.00108 |
| Ni/rGO-800 | 11.500 | 32.90283 | 3.49356 | 0.00093 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Qian, J.; Li, J.; Xing, J.; Liu, L. Facile Fabrication of Nickel Supported on Reduced Graphene Oxide Composite for Oxygen Reduction Reaction. Nanomaterials 2023, 13, 3087. https://doi.org/10.3390/nano13243087
Wang Y, Qian J, Li J, Xing J, Liu L. Facile Fabrication of Nickel Supported on Reduced Graphene Oxide Composite for Oxygen Reduction Reaction. Nanomaterials. 2023; 13(24):3087. https://doi.org/10.3390/nano13243087
Chicago/Turabian StyleWang, Yanan, Jianhua Qian, Junhua Li, Jinjuan Xing, and Lin Liu. 2023. "Facile Fabrication of Nickel Supported on Reduced Graphene Oxide Composite for Oxygen Reduction Reaction" Nanomaterials 13, no. 24: 3087. https://doi.org/10.3390/nano13243087







