Unraveling the Structural and Compositional Peculiarities in CTAB-Templated CeO2-ZrO2-MnOx Catalysts for Soot and CO Oxidation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of CeO2-ZrO2-MnOx Oxide Systems
2.2. Materials Characterization
2.3. Catalytic Tests in Oxidation CO and Soot
3. Results
3.1. Chemical Composition
3.2. Low-Temperature Nitrogen Adsorption
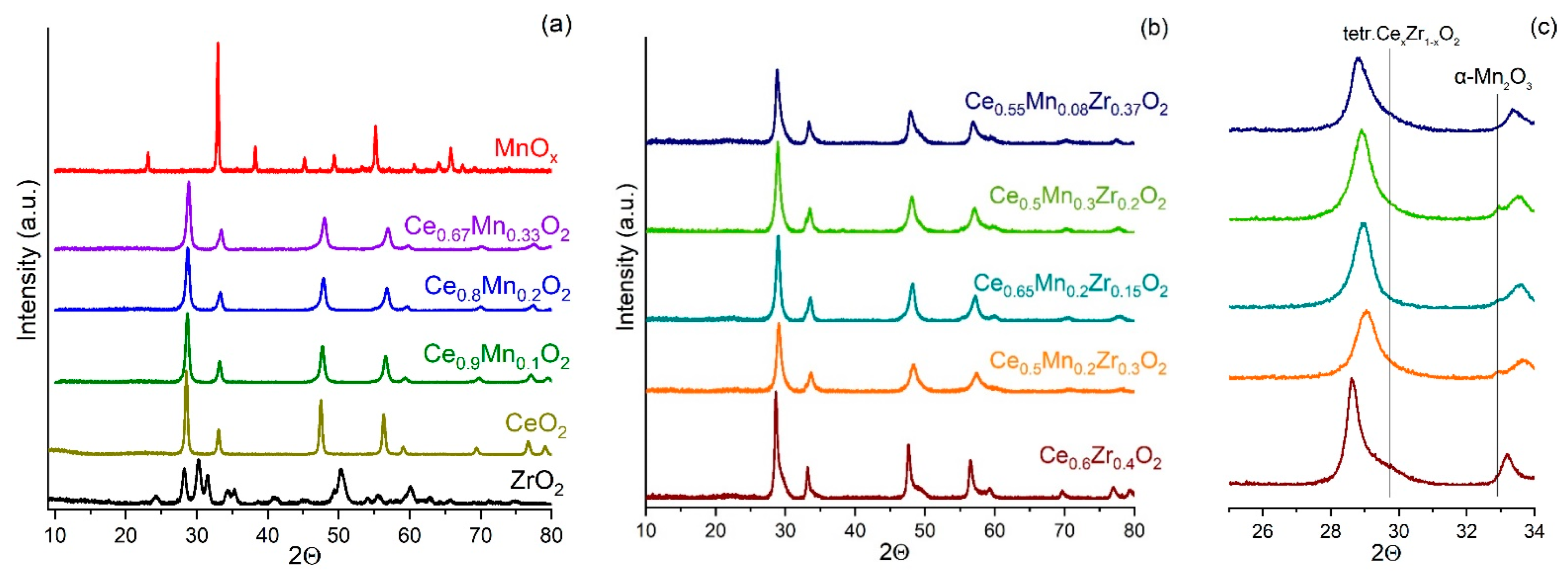
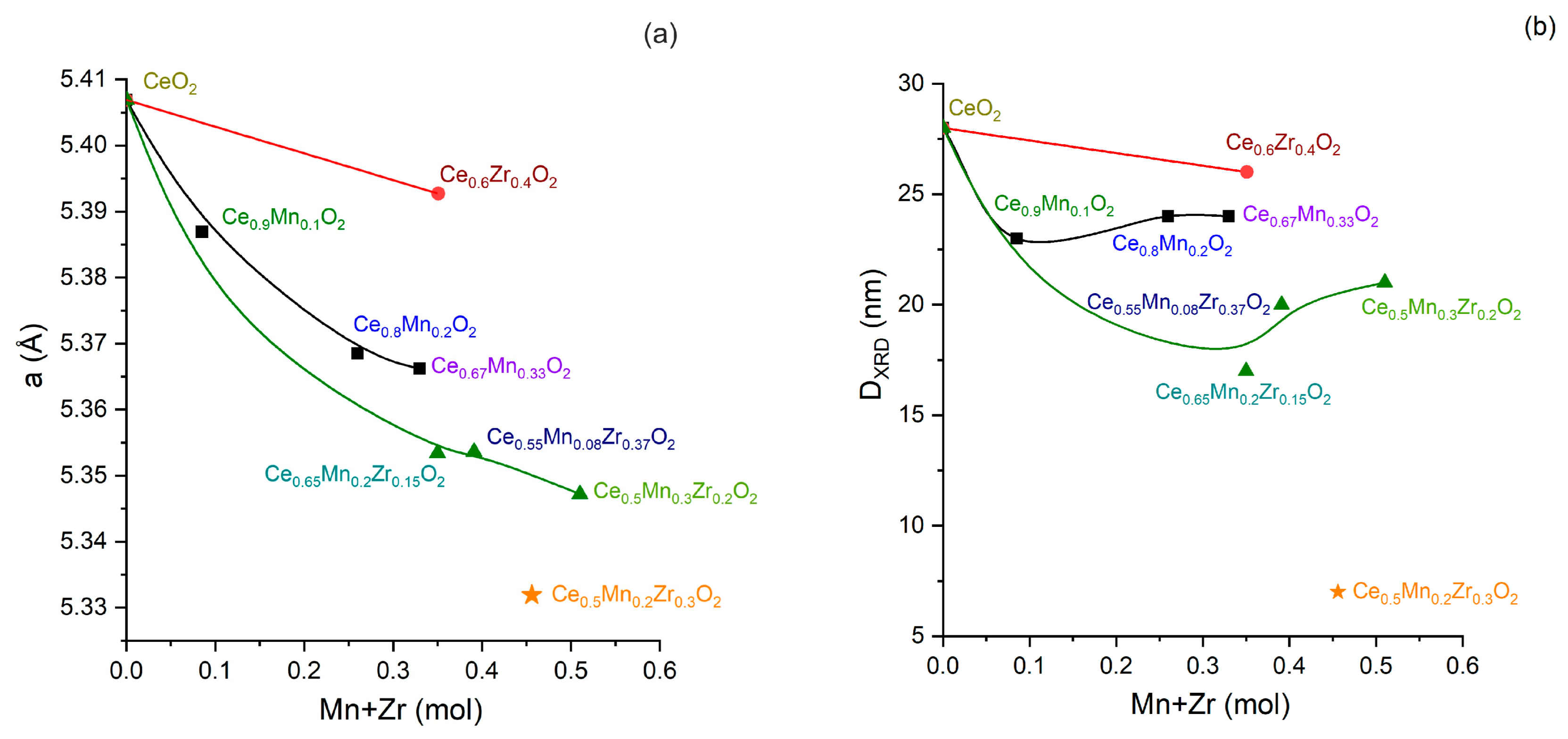
3.3. X-ray Phase Analysis (XRD)
3.4. Raman Spectroscopy
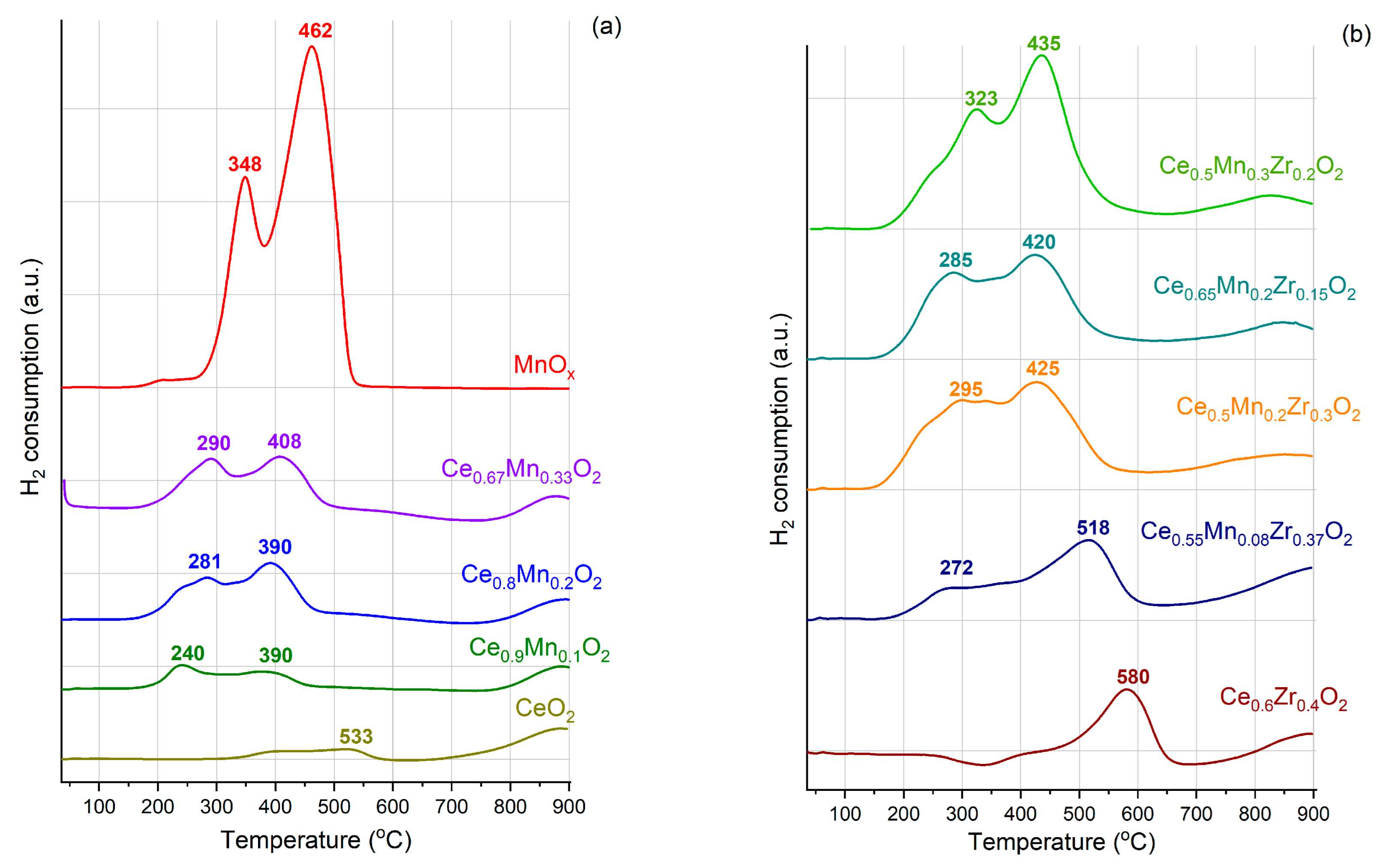
3.5. Temperature-Programmed Reduction in Hydrogen (TPR-H2)
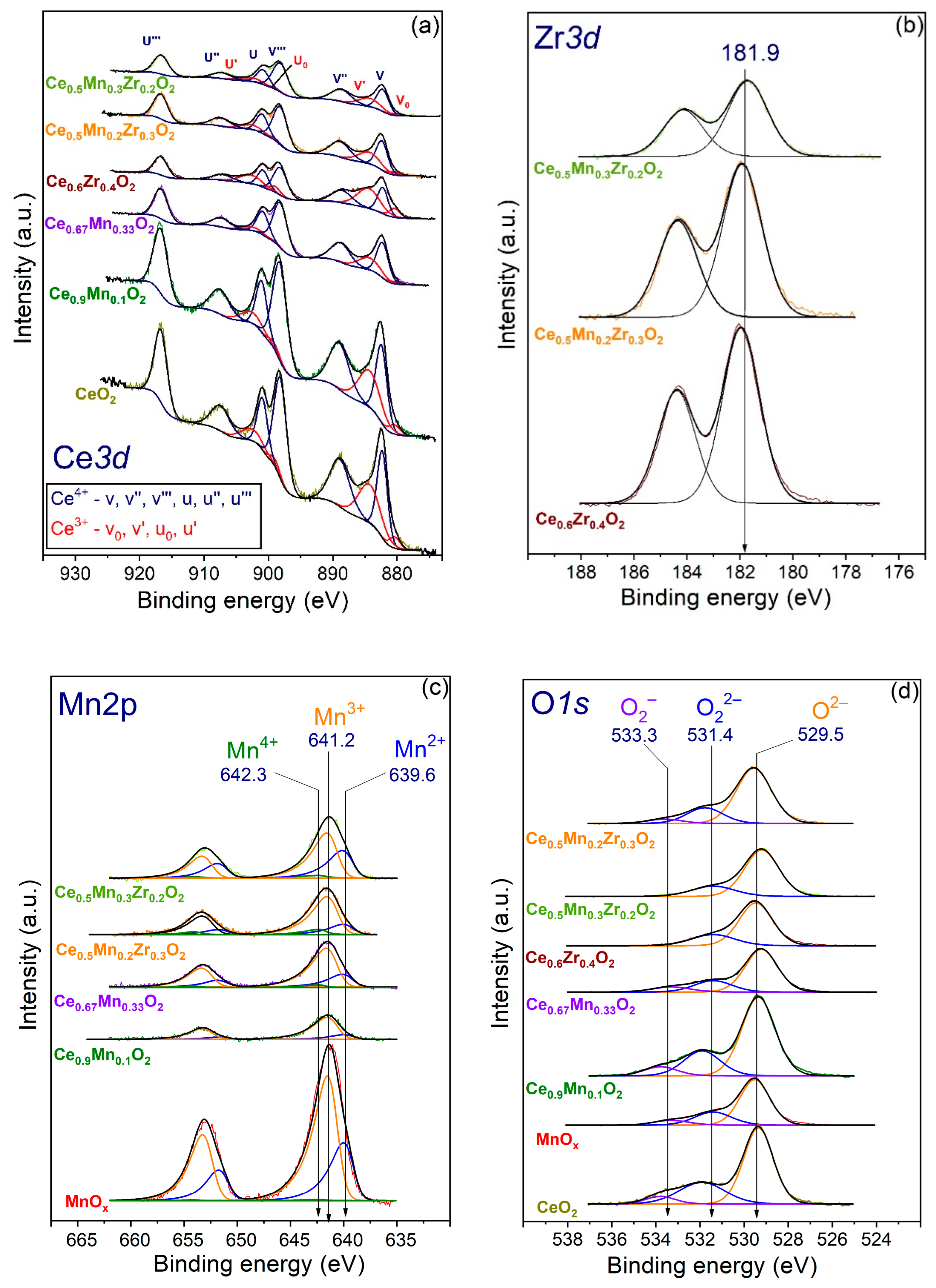
3.6. XPS
3.7. TEM
3.8. Catalytic Activity in CO and Soot Oxidation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yashnik, S.A. Catalytic Diesel Exhaust Systems: Modern Problems and Technological Solutions for Modernization of the Oxidation Catalyst. Catal. Ind. 2022, 14, 283–297. [Google Scholar] [CrossRef]
- Salaev, M.A.; Salaeva, A.A.; Kharlamova, T.S.; Mamontov, G.V. Pt–CeO2-based composites in environmental catalysis: A review. Appl. Catal. B Environ. 2021, 295, 120286. [Google Scholar] [CrossRef]
- Stadnichenko, A.I.; Simanenko, A.A.; Slavinskaya, E.M.; Fedorova, E.A.; Stonkus, O.A.; Romanenko, A.V.; Boronin, A.I. Study of Pt/Ce-Mn-Ox catalysts for the low-temperature co oxidation reaction. J. Struct. Chem. 2022, 63, 1199–1214. [Google Scholar] [CrossRef]
- Stadnichenko, A.I.; Slavinskaya, E.M.; Fedorova, E.A.; Goncharova, D.A.; Zaikovskii, V.I.; Kardash, T.Y.; Svetlichnyi, V.A.; Boronin, A.I. Activation of Au–CeO2 composites prepared by pulsed laser ablation in the reaction of low-temperature co oxidation. J. Struct. Chem. 2021, 62, 1918–1934. [Google Scholar] [CrossRef]
- Shilov, V.A.; Rogozhnikov, V.N.; Potemkin, D.I.; Snytnikov, P.V. Regeneration of Rh/Ce0.75Zr0.25O2–δ/θ-Al2O3/FeCrAl Catalyst after Autothermal Reforming of Diesel Fuel. Kinet. Catal. 2023, 64, 215–220. [Google Scholar] [CrossRef]
- Yashnik, S.A.; Ismagilov, Z.R. Diesel Oxidation Catalyst Pt–Pd/MnOx–Al2O3 for Soot Emission Control: Effect of NO and Water Vapor on Soot Oxidation. Top. Catal. 2023, 66, 860–874. [Google Scholar] [CrossRef]
- Topsøe, H. Developments in operando studies and in situ characterization of heterogeneous catalysts. J. Catal. 2003, 216, 155–164. [Google Scholar] [CrossRef]
- Bulavchenko, O.A.; Konovalova, V.P.; Saraev, A.A.; Kremneva, A.M.; Rogov, V.A.; Gerasimov, E.Y.; Afonasenko, T.N. The Catalytic Performance of CO Oxidation over MnOx-ZrO2 Catalysts: The Role of Synthetic Routes. Catalysts 2023, 13, 57. [Google Scholar] [CrossRef]
- Gu, Z.; Sang, X.; Wang, H.; Li, K. Structure and catalytic property of CeO2-ZrO2-Fe2O3 mixed oxide catalysts for diesel soot combustion: Effect of preparation method. J. Rare Earths. 2014, 32, 817–823. [Google Scholar] [CrossRef]
- Romanova, E.V.; Nam, A.V.; Kharlamova, T.S. Peculiarities of the Active Component Formation in Alumina-Supported Copper Molybdate Catalysts for Soot Combustion. Kinet. Catal. 2021, 62, 675–687. [Google Scholar] [CrossRef]
- Liu, H.; Dai, X.; Wang, K.; Yan, Z.; Qian, L. Highly efficient catalysts of Mn1−xAgxCo2O4 spinel oxide for soot combustion. Catal. Commun. 2017, 101, 134–137. [Google Scholar] [CrossRef]
- Prasad, R.; Singh, S.V. Catalytic abatement of CO, HCs and soot emissions over spinel-based catalysts from diesel engines: An overview. J. Environ. Chem. Eng. 2020, 8, 103627. [Google Scholar]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and catalytic applications of CeO2-based materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef]
- Afonasenko, T.N.; Glyzdova, D.V.; Yurpalov, V.L.; Konovalova, V.P.; Rogov, V.A.; Gerasimov, E.Y.; Bulavchenko, O.A. The Study of Thermal Stability of Mn-Zr-Ce, Mn-Ce and Mn-Zr Oxide Catalysts for CO Oxidation. Materials 2022, 15, 7553. [Google Scholar] [CrossRef]
- Kaplin, I.Y.; Lokteva, E.S.; Tikhonov, A.V.; Zhilyaev, K.A.; Golubina, E.V.; Maslakov, K.I.; Kamaev, A.O.; Isaikina, O.Y. Templated Synthesis of Copper Modified Tin-Doped Ceria for Catalytic CO Oxidation. Top. Catal. 2020, 63, 86–98. [Google Scholar] [CrossRef]
- Liao, Y.; Liu, P.; Zhang, J.; Wang, C.; Chen, L.; Yan, D.; Ren, Q.; Liang, X.; Fu, M.; Steven, L.S.; et al. Electrospun Ce–Mn oxide as an efficient catalyst for soot combustion: Ce–Mn synergy, soot-catalyst contact, and catalytic oxidation mechanism. Chemosphere 2023, 334, 138995. [Google Scholar] [CrossRef]
- Ye, Z.; Liu, Y.; Nikiforov, A.; Ji, J.; Zhao, B.; Wang, J. The research on CO oxidation over Ce–Mn oxides: The preparation method effects and oxidation mechanism. Chemosphere 2023, 336, 139130. [Google Scholar] [CrossRef]
- Greca, E.L.; Kharlamova, T.S.; Grabchenko, M.V.; Svetlichnyi, V.A.; Pantaleo, G.; Consentino, L.; Stonkus, O.A.; Vodyankina, O.V.; Liotta, L.F. Influence of Y Doping on Catalytic Activity of CeO2, MnOx, and CeMnOx Catalysts for Selective Catalytic Reduction of NO by NH3. Catalysts 2023, 13, 901. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Y.; Yu, Y.; Shan, W.; He, H. Surface oxygen species essential for the catalytic activity of Ce-M-Sn (M = Mn or Fe) in soot oxidation. Catal. Sci. Technol. 2021, 11, 895–903. [Google Scholar] [CrossRef]
- Martín-Martín, J.A.; González-Marcos, M.P.; Aranzabal, A.; González-Velasco, J.R. Effect of interaction degree between Mn and Ce of MnOX-CeO2 formulation on NO reduction and o-DCB oxidation performed simultaneously. J. Environ. Chem. Eng. 2023, 11, 110200. [Google Scholar] [CrossRef]
- Chupradit, S.; Kavitha, M.; Suksatan, W.; Ansari, M.J.; Al Mashhadani, Z.I.; Kadhim, M.M.; Mustafa, Y.F.; Shafik, S.S.; Kianfar, E. Morphological Control: Properties and Applications of Metal Nanostructures. Adv. Mater. Sci. Eng. 2022, 2022, 1971891. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, C.; Liang, C.; Li, S.; Liu, X.; Du, X.; Yang, K.; Zhao, J.; Yu, Q.; Zhai, Y.; et al. Regulating CeO2 morphologies on the catalytic oxidation of toluene at lower temperature: A study of the structure–activity relationship. J. Catal. 2023, 418, 151–162. [Google Scholar] [CrossRef]
- Kaplin, I.Y.; Lokteva, E.S.; Golubina, E.V.; Lunin, V.V. Template synthesis of porous ceria-based catalysts for environmental application. Molecules 2020, 25, 4244. [Google Scholar] [CrossRef]
- Mehdizadeh, P.; Jamdar, M.; Mahdi, M.A.; Abdulsahib, W.K.; Jasim, L.S.; Raheleh Yousefi, S.; Salavati-Niasari, M. Rapid microwave fabrication of new nanocomposites based on Tb-Co-O nanostructures and their application as photocatalysts under UV/Visible light for removal of organic pollutants in water. Arab. J. Chem. 2023, 16, 104579. [Google Scholar] [CrossRef]
- Zhu, M.; Zhao, C.; Liu, X.; Wang, X.; Zhou, F.; Wang, J.; Hu, Y.; Zhao, Y.; Yao, T.; Yang, L.-M.; et al. Single Atomic Cerium Sites with a High Coordination Number for Efficient Oxygen Reduction in Proton-Exchange Membrane Fuel Cells. ACS Catal. 2021, 11, 3923–3929. [Google Scholar] [CrossRef]
- Yousefi, S.R.; Alshamsi, H.A.; Amiri, O.; Salavati-Niasari, M. Synthesis, characterization and application of Co/Co3O4 nanocomposites as an effective photocatalyst for discoloration of organic dye contaminants in wastewater and antibacterial properties. J. Mol. Liq. 2021, 337, 116405. [Google Scholar] [CrossRef]
- Li, H.; Pan, Q.; Liu, J.; Liu, W.; Li, Q.; Wang, L.; Wang, Z. Synthesis and catalytic properties of praseodymium oxide (Pr6O11) nanorods for diesel soot oxidation. J. Environ. Chem. Eng. 2023, 11, 109152. [Google Scholar] [CrossRef]
- Zhu, H.; Xu, J.; Yichuan, Y.; Wang, Z.; Gao, Y.; Liu, W.; Yin, H. Catalytic oxidation of soot on mesoporous ceria-based mixed oxides with cetyltrimethyl ammonium bromide (CTAB)-assisted synthesis. J. Colloid Interface Sci. 2017, 508, 1–13. [Google Scholar] [CrossRef]
- Kaplin, I.Y.; Lokteva, E.S.; Bataeva, S.V.; Maslakov, K.I.; Fionov, A.V.; Shumyantsev, A.V.; Isaikina, O.Y.; Kamaev, A.O.; Golubina, E.V. Effect of MnOx modification and template type on the catalytic performance of ceria-zirconia in CO and soot oxidation. Pure Appl. Chem. 2021, 93, 447–462. [Google Scholar] [CrossRef]
- Kaplin, I.Y.; Lokteva, E.S.; Golubina, E.V.; Shishova, V.V.; Maslakov, K.I.; Fionov, A.V.; Isaikina, O.Y.; Lunin, V.V. Efficiency of manganese modified CTAB-templated ceria-zirconia catalysts in total CO oxidation. Appl. Surf. Sci. 2019, 485, 432–440. [Google Scholar] [CrossRef]
- Pan, C.; Zhang, D.; Shi, L. CTAB assisted hydrothermal synthesis, controlled conversion and CO oxidation properties of CeO2 nanoplates, nanotubes, and nanorods. J. Solid-State Chem. 2008, 181, 1298–1306. [Google Scholar] [CrossRef]
- Gao, Y.; Wu, X.; Liu, S.; Weng, D.; Ran, R. MnOx–CeO2 mixed oxides for diesel soot oxidation: A review. Catal. Surv. Asia 2018, 22, 230–240. [Google Scholar] [CrossRef]
- Xiong, J.; Wei, Y.; Zhang, Y.; Mei, X.; Wu, Q.; Zhao, Z.; Liu, J.; Wu, D.; Li, J. Facile synthesis of 3D ordered macro-mesoporous Ce1-xZrxO2 catalysts with enhanced catalytic activity for soot oxidation. Catal. Today 2020, 355, 587–595. [Google Scholar] [CrossRef]
- Afonasenko, T.N.; Glyzdova, D.V.; Konovalov, V.P.; Saraev, A.A.; Aydakov, E.E.; Bulavchenko, O.A. Effect of Calcination Temperature on the Properties of Mn–Zr–Ce Catalysts in the Oxidation of Carbon Monoxide. Kinet. Catal. 2022, 63, 431–439. [Google Scholar] [CrossRef]
- Cychosz, K.A.; Guillet-Nicolas, R.; García-Martínez, J.; Thommes, M. Recent advances in the textural characterization of hierarchically structured nanoporous materials. Chem. Soc. Rev. 2017, 46, 389–414. [Google Scholar] [CrossRef]
- Williamson, G.K.; Hall, W.H. X-ray line broadening from filed aluminium and wolfram. Acta Metall. 1953, 1, 22–31. [Google Scholar] [CrossRef]
- Martínez, L.M.; Araque, M.; Centeno, M.A.; Roger, A.C. Role of ruthenium on the catalytic properties of CeZr and CeZrCo mixed oxides for glycerol steam reforming reaction toward H2 production. Catal. Today 2015, 242, 80–90. [Google Scholar] [CrossRef]
- Agarwal, S.; Zhu, X.; Hensen, E.J.M.; Leferts, L.; Mojet, B.L. Defect chemistry of ceria nanorods. J. Phys. Chem. C 2014, 118, 4131–4142. [Google Scholar] [CrossRef]
- Radinger, H.; Connor, P.; Stark, R.; Jaegermann, W.; Kaiser, B. Manganese Oxide as an Inorganic Catalyst for the Oxygen Evolution Reaction Studied by X-ray Photoelectron and Operando Raman Spectroscopy. ChemCatChem 2021, 13, 1175–1185. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhu, W.; Lu, J.; Liao, W.; Qi, N.; Luo, Y.; Dionysiou, D.D. Induced synthesis of CeO2 with abundant crystal boundary for promoting catalytic oxidation of gaseous styrene. Appl. Catal. B. Environ. 2024, 342, 123461. [Google Scholar] [CrossRef]
- Krishna, K.; Bueno-López, A.; Makkee, M.; Moulijn, J.A. Potential rare earth modified CeO2 catalysts for soot oxidation: I. Characterisation and catalytic activity with O2. Appl. Catal. B Environ. 2007, 75, 189–200. [Google Scholar] [CrossRef]
- Lin, X.; Li, S.; He, H.; Wu, Z.; Wu, J.; Chen, L.; Fu, M. Evolution of oxygen vacancies in MnOx-CeO2 mixed oxides for soot oxidation. Appl. Catal. B Environ. 2018, 223, 91–102. [Google Scholar] [CrossRef]
- Reddy, B.M.; Reddy, G.K.; Katta, L. Structural characterization and dehydration activity of CeO2–SiO2 and CeO2–ZrO2 mixed oxides prepared by a rapid microwave-assisted combustion synthesis method. J. Mol. Catal. A Chem. 2010, 319, 52–57. [Google Scholar] [CrossRef]
- Derevyannikova, E.A.; Kardash, T.Y.; Stadnichenko, A.I.; Stonkus, O.A.; Slavinskaya, E.M.; Svetlichnyi, V.A.; Boronin, A.I. Structural Insight into Strong Pt–CeO2 Interaction: From Single Pt Atoms to PtOx Clusters. J. Phys. Chem. C 2019, 123, 1320–1334. [Google Scholar] [CrossRef]
- Li, L.; Chen, F.; Lu, J.-Q.; Luo, M.-F. Study of defect sites in Ce1−xMxO2−δ (x = 0.2) solid solutions using Raman spectroscopy. J. Phys. Chem. A 2011, 115, 7972–7977. [Google Scholar] [CrossRef] [PubMed]
- Vinodkumar, T.; Rao, B.G.; Reddy, B.M. Infuence of isovalent and aliovalent dopants on the reactivity of cerium oxide for catalytic applications. Catal. Today 2015, 253, 57–64. [Google Scholar] [CrossRef]
- Wu, Z.; Li, M.; Howe, J.; Meyer, H.M.; Overbury, S.H. Probing defect sites on CeO2 nanocrystals with well-defned surface planes by Raman spectroscopy and O2 adsorption. Langmuir 2010, 26, 16595–16606. [Google Scholar] [CrossRef]
- Sartoretti, E.; Novara, C.; Giorgis, F.; Piumetti, M.; Bensaid, S.; Russo, N.; Fino, D. In situ Raman analyses of the soot oxidation reaction over nanostructured ceria-based catalysts. Sci. Rep. 2019, 9, 3875. [Google Scholar] [CrossRef]
- Jiang, Y.; Gao, J.; Zhang, Q.; Liu, Z.; Fu, M.; Wu, J.; Hu, Y.; Ye, D. Enhanced oxygen vacancies to improve ethyl acetate oxidation over MnOx-CeO2 catalyst derived from MOF template. Chem. Eng. J. 2019, 371, 78–87. [Google Scholar] [CrossRef]
- Wu, L.; Wang, J.; Yang, C.; Gao, X.; Fang, Y.; Wang, X.; Duo Wu, W.; Wu, Z. Understanding the catalytic ozonation process on ceria nanorods: Efficacy, Frenkel-type oxygen vacancy as a key descriptor and mechanism insight. Appl. Catal. B Environ. 2023, 323, 122152. [Google Scholar] [CrossRef]
- Julien, C.M.; Massot, M.; Poinsignon, C. Lattice vibrations of manganese oxides: Part I. Periodic structures. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2004, 60, 689–700. [Google Scholar] [CrossRef] [PubMed]
- Naeem, R.; Ali Ehsan, M.; Yahya, R.; Sohail, M.; Khaledi, H.; Mazhar, M. Fabrication of Pristine Mn2O3 and Ag–Mn2O3 Composite Thin Films by AACVD for Photoelectrochemical Water Splitting. Dalton Trans. 2016, 45, 14928. [Google Scholar] [CrossRef]
- Bernardini, S.; Bellatreccia, F.; Casanova Municchia, A.; Della Ventura, G.; Sodo, A. Raman spectra of natural manganese oxides. J. Raman Spectrosc. 2019, 50, 873–888. [Google Scholar] [CrossRef]
- Nakajima, A.; Yoshihara, A.; Ishigame, M. Defect-induced Raman spectra in doped CeO2. Phys. Rev. B 1994, 50, 13297. [Google Scholar] [CrossRef]
- Cao, L.; Pan, L.; Ni, C.; Yuan, Z.; Wang, S. Autothermal reforming of methane over Rh/Ce0.5Zr0.5O2 catalyst: Effects of the crystal structure of the supports. Fuel Process. Technol. 2010, 91, 306–312. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, H.; Wu, D. Performance of an Aliovalent-Doping MnCeOx/Cordierite Monolithic Catalyst Derived from MOFs for o-Xylene Oxidation. Catal. Lett. 2023. [Google Scholar] [CrossRef]
- Feng, Y.; Wu, J.; Chi, Q.; Li, W.; Yu, Y.; Fei, W. Defects and Aliovalent Doping Engineering in Electroceramics. Chem. Rev. 2020, 120, 1710–1787. [Google Scholar] [CrossRef]
- Andreoli, S.; Deorsola, F.A.; Galletti, C.; Pirone, R. Nanostructured MnOx catalysts for low-temperature NOx SCR. Chem. Eng. J. 2015, 278, 174–182. [Google Scholar] [CrossRef]
- Chen, L.; Liu, G.; Feng, N.; Yu, J.; Meng, J.; Fang, F.; Guan, G. Effect of calcination temperature on structural properties and catalytic soot combustion activity of MnOx/wire-mesh monoliths. Appl. Surf. Sci. 2019, 467, 1088–1103. [Google Scholar] [CrossRef]
- Trovarelli, A. Catalytic properties of ceria and CeO2-containing materials. Catal. Rev. 1996, 38, 439–520. [Google Scholar] [CrossRef]
- Watanabe, S.; Ma, X.; Song, C. Characterization of structural and surface properties of nanocrystalline TiO2−CeO2 mixed oxides by XRD, XPS, TPR, and TPD. J. Phys. Chem. C 2009, 113, 14249–14257. [Google Scholar] [CrossRef]
- Atribak, I.; Bueno-López, A.; García-García, A. Combined removal of diesel soot particulates and NOx over CeO2–ZrO2 mixed oxides. J. Catal. 2008, 259, 123–132. [Google Scholar] [CrossRef]
- Wangcheng, Z.H.A.N.; ZHANG, X.; Yanglong, G.U.O.; Li, W.A.N.G.; Yun, G.U.O.; Guanzhong, L.U. Synthesis of mesoporous CeO2-MnOx binary oxides and their catalytic performances for CO oxidation. J. Rare Earths 2014, 32, 146–152. [Google Scholar] [CrossRef]
- Zou, Z.Q.; Meng, M.; Zha, Y.Q. Surfactant-assisted synthesis, characterizations, and catalytic oxidation mechanisms of the mesoporous MnOx−CeO2 and Pd/MnOx−CeO2 catalysts used for CO and C3H8 oxidation. J. Phys. Chem. C 2010, 114, 468–477. [Google Scholar] [CrossRef]
- Afonasenko, T.N.; Yurpalova, D.V.; Vinokurov, Z.S.; Saraev, A.A.; Aidakov, E.E.; Konovalova, V.P.; Rogov, V.A.; Bulavchenko, O.A. The Formation of Mn-Ce-Zr Oxide Catalysts for CO and Propane Oxidation: The Role of Element Content Ratio. Catalysts 2023, 13, 211. [Google Scholar] [CrossRef]
- Tholkappiyan, R.; Nirmalesh Naveen, A.; Vishista, K.; Fathalla, H. Investigation on the electrochemical performance of hausmannite Mn3O4 nanoparticles by ultrasonic irradiation assisted co-precipitation method for supercapacitor electrodes. J. Taibah Uni. Sci. 2018, 12, 669–677. [Google Scholar] [CrossRef]
- Bulavchenko, O.; Vinokurov, Z.; Afonasenko, T.; Tsyrul’Nikov, P.; Tsybulya, S.; Saraev, A.; Kaichev, V. Reduction of mixed Mn–Zr oxides: In situ XPS and XRD studies. Dalton Trans. 2015, 44, 15499–15507. [Google Scholar] [CrossRef]
- Liberman, E.Y.; Kleusov, B.S.; Naumkin, A.V.; Zagaynov, I.V.; Konkova, T.V.; Simakina, E.A.; Izotova, A.O. Thermal Stability and Catalytic Activity of the MnOx–CeO2 and the MnOx–ZrO2–CeO2 Highly Dispersed Materials in the Carbon Monoxide Oxidation Reaction. Inorg. Mater. Appl. Res. 2021, 2, 468–476. [Google Scholar] [CrossRef]
- Grabchenko, M.V.; Mamontov, G.V.; Chernykh, M.V.; Vodyankina, O.V.; Salaev, M.A. Synergistic effect in ternary CeO2-ZrO2-MnOx catalysts for CO oxidation and soot combustion. Chem. Eng. Sci. 2023, 9593. [Google Scholar] [CrossRef]
- Sacco, N.A.; Bortolozzi, J.P.; Milt, V.G.; Miró, E.E.; Banús, E.D. One step citric acid-assisted synthesis of Mn-Ce mixed oxides and their application to diesel soot combustion. Fuel 2022, 332, 124201. [Google Scholar] [CrossRef]
- Yao, P.; He, J.; Jiang, X.; Jiao, Y.; Wang, J.; Chen, Y. Factors determining gasoline soot abatement over CeO2–ZrO2-MnOx catalysts under low oxygen concentration condition. J. Energy Inst. 2020, 93, 774–783. [Google Scholar] [CrossRef]
- Kibis, L.S. Interface Interactions And CO Oxidation Activity of Ag/CeO2 Catalysts: A New Approach Using Model Catalytic Systems. Appl. Catal. A Gen. 2019, 570, 51–61. [Google Scholar] [CrossRef]


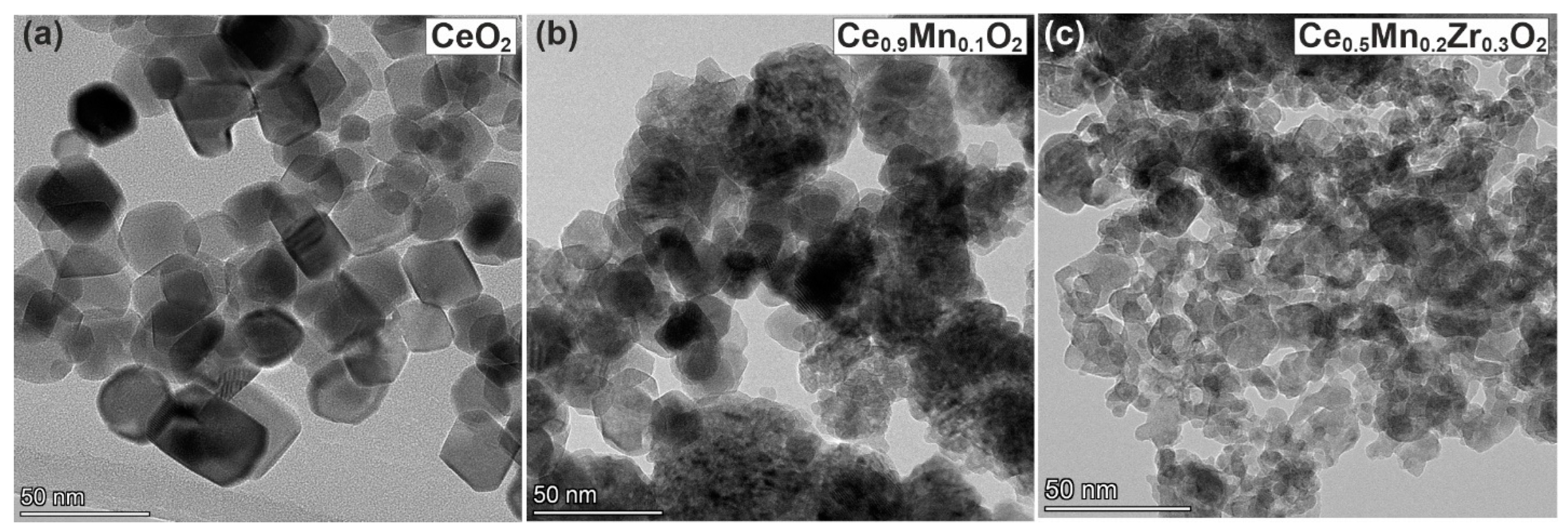
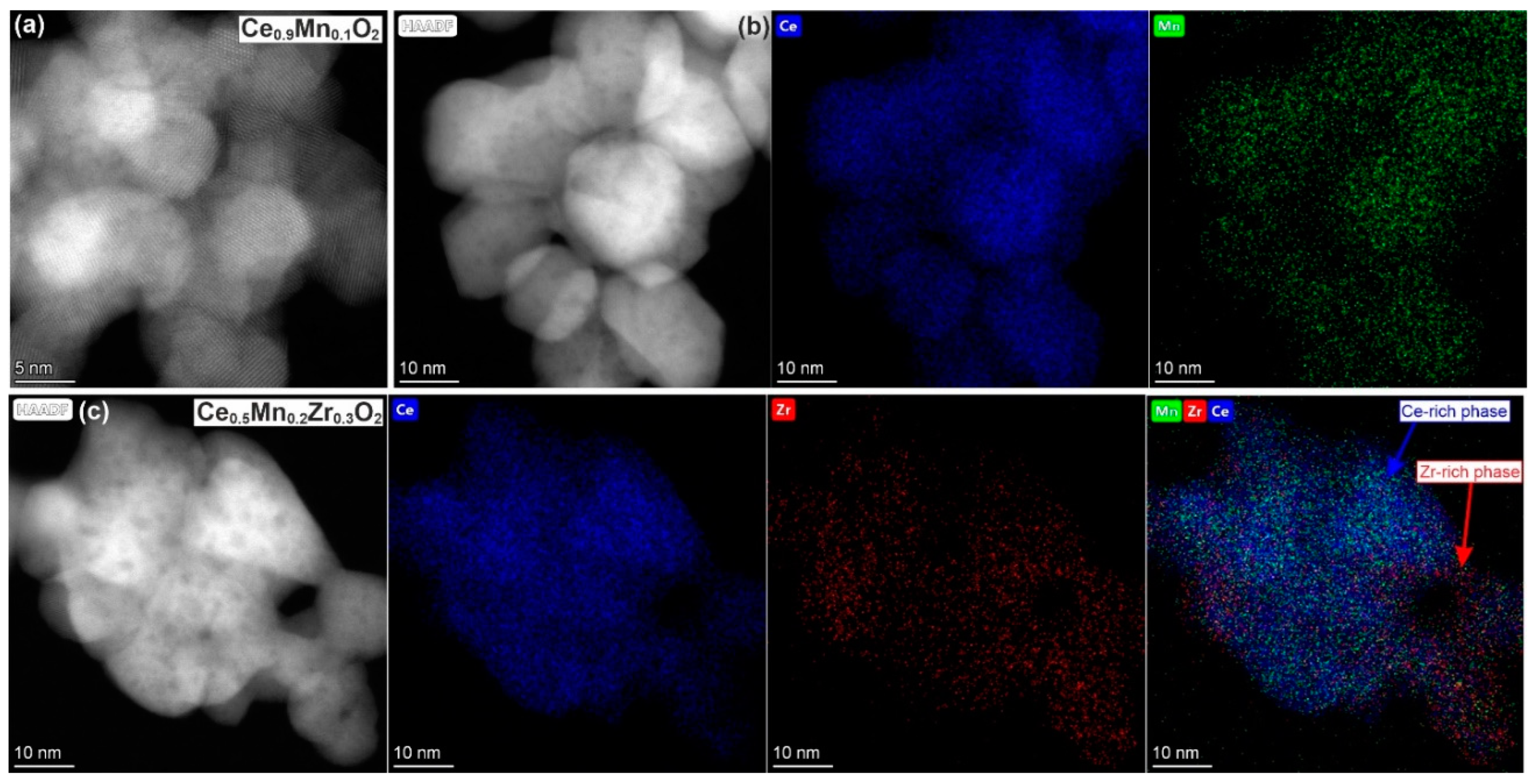
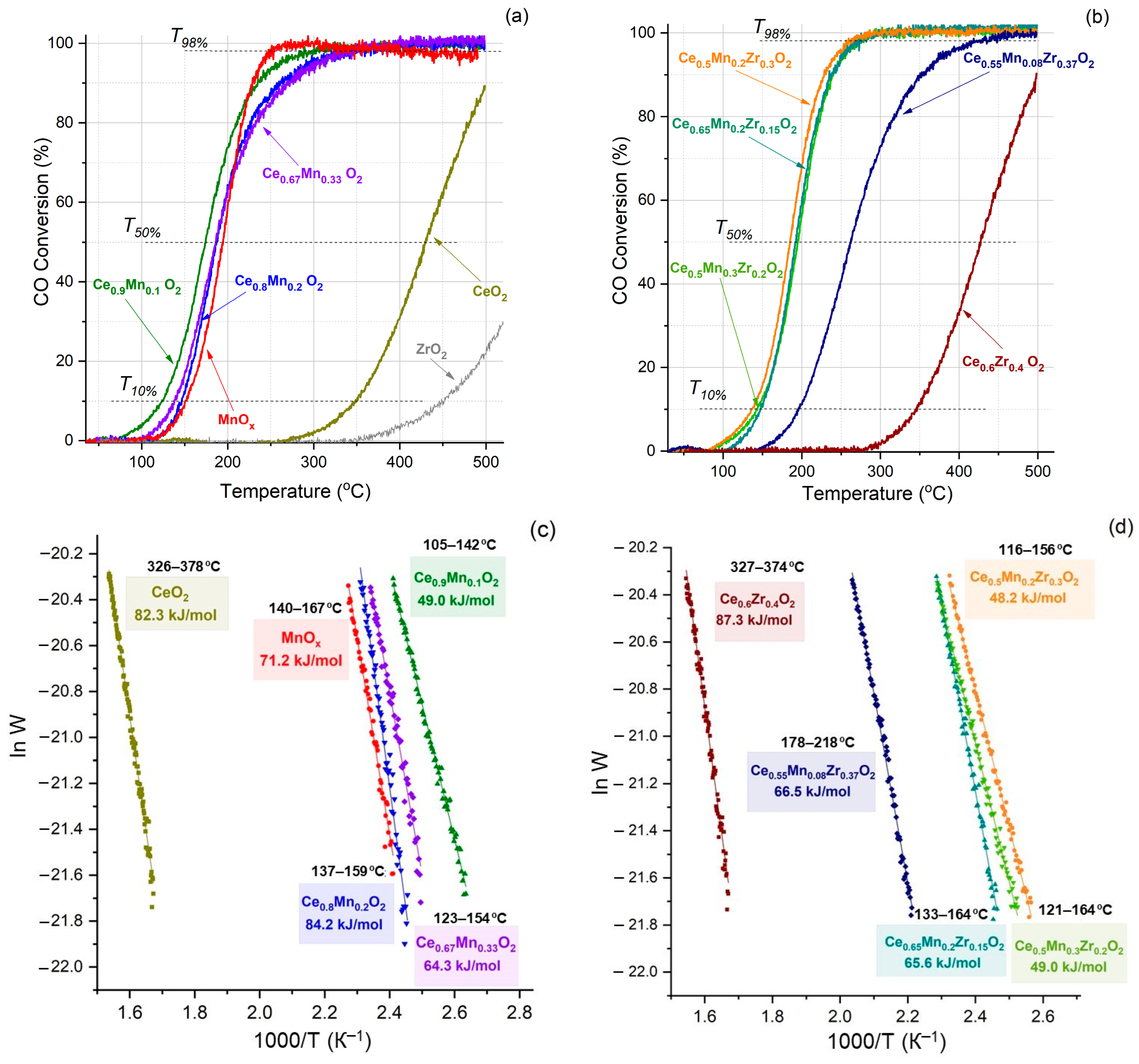

| Sample | n(Ce)/ n(Sum) | n(Mn)/ n(Sum) | n(Zr)/ n(Sum) | Ce/Zr | Ce/Mn | Zr/Mn | |||
|---|---|---|---|---|---|---|---|---|---|
| XRF | XPS | XRF | XPS | XRF | XPS | ||||
| Ce0.9Mn0.1O2 | 0.915 | 0.085 | – | – | – | 10.76 | 9.20 | – | – |
| Ce0.8Mn0.2O2 | 0.738 | 0.262 | – | – | – | 2.82 | – | – | – |
| Ce0.67Mn0.33O2 | 0.686 | 0.314 | – | – | – | 2.18 | 2.41 | – | – |
| Ce0.6Zr0.4O2 | 0.648 | – | 0.351 | 2.06 | 1.43 | – | – | – | – |
| Ce0.5Mn0.2Zr0.3O2 | 0.544 | 0.184 | 0.272 | 2.00 | 0.93 | 3.09 | 1.59 | 1.48 | 1.71 |
| Ce0.65Mn0.2Zr0.15O2 | 0.692 | 0.176 | 0.132 | 5.24 | n.d. | 3.93 | n.d. | 0.75 | n.d. |
| Ce0.5Mn0.3Zr0.2O2 | 0.459 | 0.382 | 0.158 | 2.90 | 2.98 | 1.20 | 1.37 | 0.41 | 0.46 |
| Ce0.55Mn0.08Zr0.37O2 | 0.609 | 0.045 | 0.346 | 1.76 | n.d. | 13.53 | n.d. | 7.69 | n.d. |
| Sample | SSA, m2/g | V, cm3/g | Pore Size, nm |
|---|---|---|---|
| MnOx | 6 | 0.03 | 37 |
| ZrO2 | 39 | 0.11 | 8 |
| CeO2 | 37 | 0.18 | 19 |
| Ce0.9Mn0.1O2 | 40 | 0.18 | 19 |
| Ce0.8Mn0.2O2 | 45 | 0.29 | 24 |
| Ce0.67Mn0.33O2 | 38 | 0.26 | 27 |
| Ce0.6Zr0.4O2 | 46 | 0.11 | 8 |
| Ce0.5Mn0.2Zr0.3O2 | 52 | 0.16 | 10 |
| Ce0.5Mn0.3Zr0.2O2 | 46 | 0.18 | 14 |
| Ce0.55Mn0.08Zr0.37O2 | 50 | 0.13 | 8 |
| Ce0.65Mn0.2Zr0.15O2 | 52 | 0.24 | 17 |
| Sample | Phase | Structural Parameters | ||||
|---|---|---|---|---|---|---|
| a, Å | b, Å | c, Å | ∆d/d × 10−3 | DXRD, nm | ||
| CeO2 | cub. CeO2 | 5.407 | - | - | 0.99 | 28 |
| MnOx | cub. Mn2O3 | 9.404 | - | - | 0.91 | 43 |
| ZrO2 | mon. ZrO2 | 5.147 | 5.2032 | 5.313 | 3.69 | 15 |
| tetr. ZrO2 | 3.597 | - | 5.177 | 1.92 | 15 | |
| Ce0.9Mn0.1O2 | cub. Ce1−xMnxO2−δ | 5.387 | - | - | 2.16 | 23 |
| Ce0.8Mn0.2O2 | cub. Ce1−xMnxO2−δ | 5.369 | - | - | 3.76 | 24 |
| Ce0.67Mn0.33O2 | cub. Ce1−xMnxO2−δ | 5.366 | - | - | 5.31 | 24 |
| Ce0.6Zr0.4O2 | cub. Ce1−xZrxO2−δ | 5.393 | - | - | 7.32 | 26 |
| cub. CexZr1−xO2−δ/ tetr. CexZr1−xO2−δ | 5.229/ 3.624 | - | -/ 5.277 | 1.36 | 8 | |
| Ce0.5Mn0.2Zr0.3O2 | cub. Ce1−xZrxO2−δ | 5.331 | - | - | 5.90 | 7 |
| tetr. CexZr1−xO2−δ | 3.701 | - | 5.339 | 9.2 | 5 | |
| cub. Mn2O3 | n.a. | - | - | n.a. | n.a. | |
| Ce0.65Mn0.2Zr0.15O2 | cub. Ce1−xZrxO2−δ | 5.343 | - | - | 4.35 | 17 |
| cub. Mn2O3 | n.a. | - | - | n.a. | n.a. | |
| Ce0.5Mn0.3Zr0.2O2 | cub. Ce1−xZrxO2−δ | 5.347 | - | - | 9.38 | 21 |
| tetr. CexZr1−xO2−δ | 3.696 | - | 5.277 | 9.4 | 4 | |
| cub. Mn2O3 | n.a. | - | - | n.a. | n.a. | |
| Ce0.55Mn0.08Zr0.37O2 | cub. Ce1−xZrxO2−δ | 5.354 | - | - | 7.56 | 20 |
| tetr. CexZr1−xO2−δ | 3.696 | - | 5.277 | 4.2 | 7 | |
| Sample | H2 Consumption (mmol/mol Catalyst) | |||
| 150–350 °C | 350–650 °C | 600–900 °C | Σ | |
| CeO2 | - | 0.024 (surface CeO2) | 0.057 (bulk CeO2) | 0.081 |
| MnOx | 0.215 (dispersed MnOx) | 0.472 (reduction of MnOx) | - | 0.687 |
| Ce0.6Zr0.4O2 | - | 0.031 | 0.016 | 0.047 |
| Catalysts | 150–500 °C (Dispersed MnOx and Surface CeO2) | 500–650 °C (Surface CeO2) | 650–900 °C (Bulk CeO2) | Σ |
| Ce0.9Mn0.1O2 | 0.060 | 0.002 | 0.038 | 0.669 |
| Ce0.8Mn0.2O2 | 0.130 | 0.001 | 0.037 | 1.296 |
| Ce0.67Mn0.33O2 | 0.146 | 0.001 | 0.032 | 1.426 |
| Ce0.5Mn0.2Zr0.3O2 | 0.120 | 0.005 | 0.027 | 1.233 |
| Ce0.65Mn0.2Zr0.15O2 | 0.110 | 0.003 | 0.015 | 1.112 |
| Ce0.5Mn0.3Zr0.2O2 | 0.173 | 0.015 | 0.033 | 1.702 |
| Ce0.55Mn0.08Zr0.37O2 | 0.065 | 0.020 | 0.025 | 0.675 |
| Sample | Ce4+, % | Mn2+, % | Mn3+, % | Mn4+, % | (O22− + O2−)/Ot 1 | O2−, % | O22−, % | O2−, % |
|---|---|---|---|---|---|---|---|---|
| MnOx | – | 31 | 69 | 1 | 0.32 | 68 | 24 | 8 |
| Ce0.67Mn0.33O2 | 85 | 24 | 76 | 4 | 0.31 | 69 | 21 | 10 |
| Ce0.5Mn0.3Zr0.2O2 | 84 | 38 | 62 | 5 | 0.22 | 78 | 22 | 0 |
| Ce0.5Mn0.2Zr0.3O2 | 82 | 19 | 73 | 9 | 0.27 | 73 | 21 | 6 |
| Ce0.9Mn0.1O2 | 81 | 17 | 83 | n.d. | 0.32 | 68 | 24 | 8 |
| Ce0.6Zr0.4O2 | 65 | – | – | – | 0.25 | 75 | 25 | 0 |
| CeO2 | 80 | – | – | – | 0.35 | 65 | 29 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabchenko, M.V.; Mikheeva, N.N.; Mamontov, G.V.; Cortés Corberán, V.; Litvintseva, K.A.; Svetlichnyi, V.A.; Vodyankina, O.V.; Salaev, M.A. Unraveling the Structural and Compositional Peculiarities in CTAB-Templated CeO2-ZrO2-MnOx Catalysts for Soot and CO Oxidation. Nanomaterials 2023, 13, 3108. https://doi.org/10.3390/nano13243108
Grabchenko MV, Mikheeva NN, Mamontov GV, Cortés Corberán V, Litvintseva KA, Svetlichnyi VA, Vodyankina OV, Salaev MA. Unraveling the Structural and Compositional Peculiarities in CTAB-Templated CeO2-ZrO2-MnOx Catalysts for Soot and CO Oxidation. Nanomaterials. 2023; 13(24):3108. https://doi.org/10.3390/nano13243108
Chicago/Turabian StyleGrabchenko, Maria V., Natalia N. Mikheeva, Grigory V. Mamontov, Vicente Cortés Corberán, Kseniya A. Litvintseva, Valery A. Svetlichnyi, Olga V. Vodyankina, and Mikhail A. Salaev. 2023. "Unraveling the Structural and Compositional Peculiarities in CTAB-Templated CeO2-ZrO2-MnOx Catalysts for Soot and CO Oxidation" Nanomaterials 13, no. 24: 3108. https://doi.org/10.3390/nano13243108
APA StyleGrabchenko, M. V., Mikheeva, N. N., Mamontov, G. V., Cortés Corberán, V., Litvintseva, K. A., Svetlichnyi, V. A., Vodyankina, O. V., & Salaev, M. A. (2023). Unraveling the Structural and Compositional Peculiarities in CTAB-Templated CeO2-ZrO2-MnOx Catalysts for Soot and CO Oxidation. Nanomaterials, 13(24), 3108. https://doi.org/10.3390/nano13243108






