Corrosion Behavior of Ni/NiCr/NiCrAlSi Composite Coating on Copper for Application as a Heat Exchanger in Sea Water
Abstract
:1. Introduction
2. Experimental
2.1. Preparation of the Composite Coatings
2.2. Characterization
2.3. Electrochemical Measurements
3. Results and Discussion
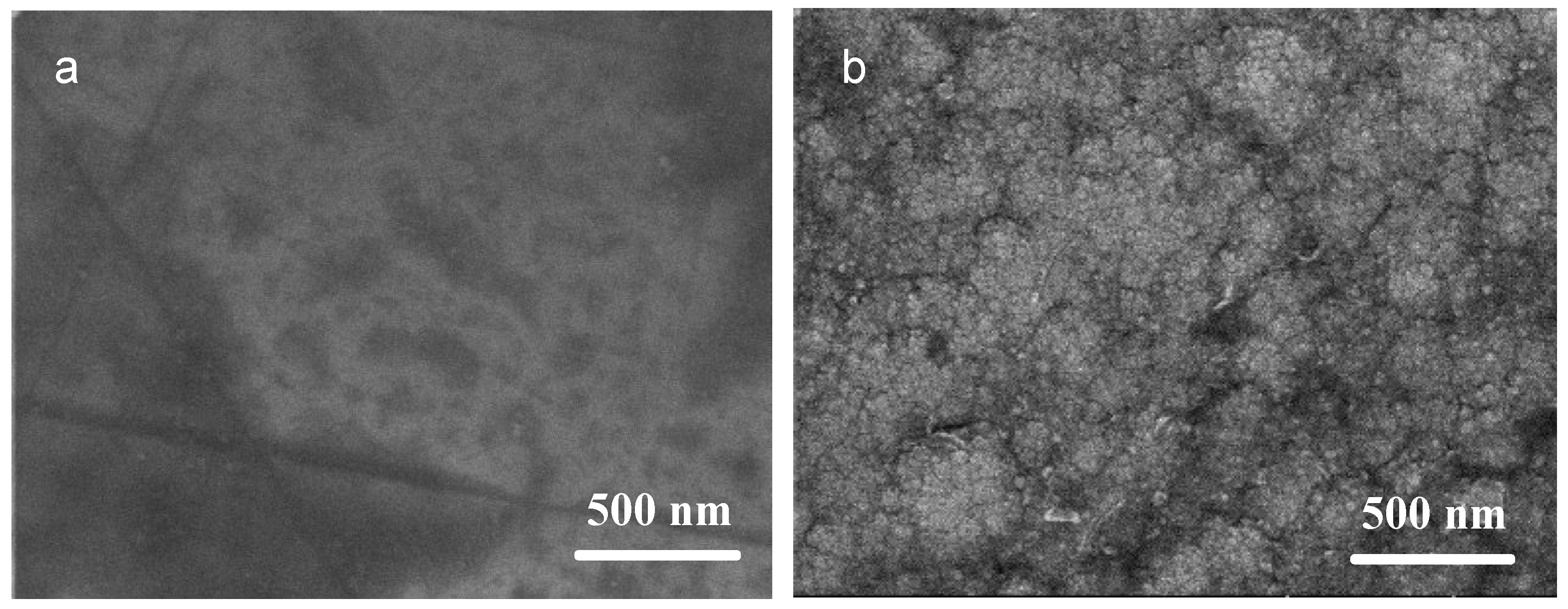
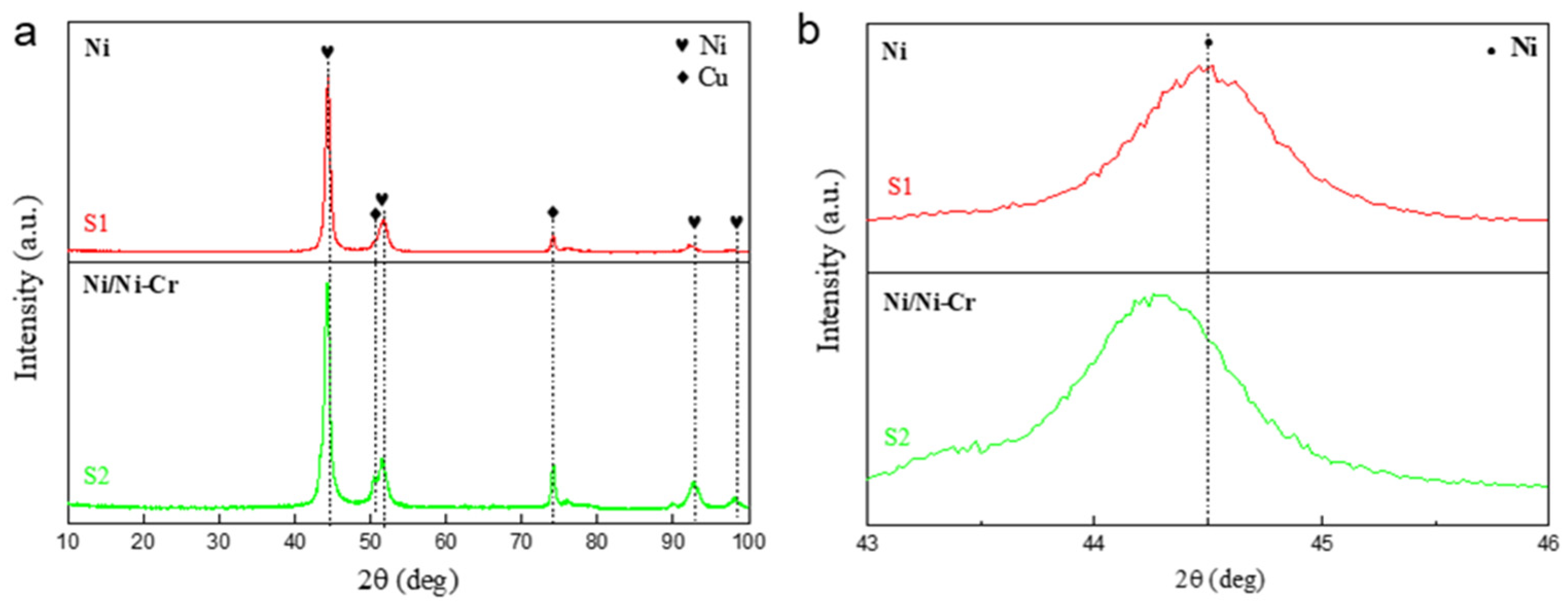
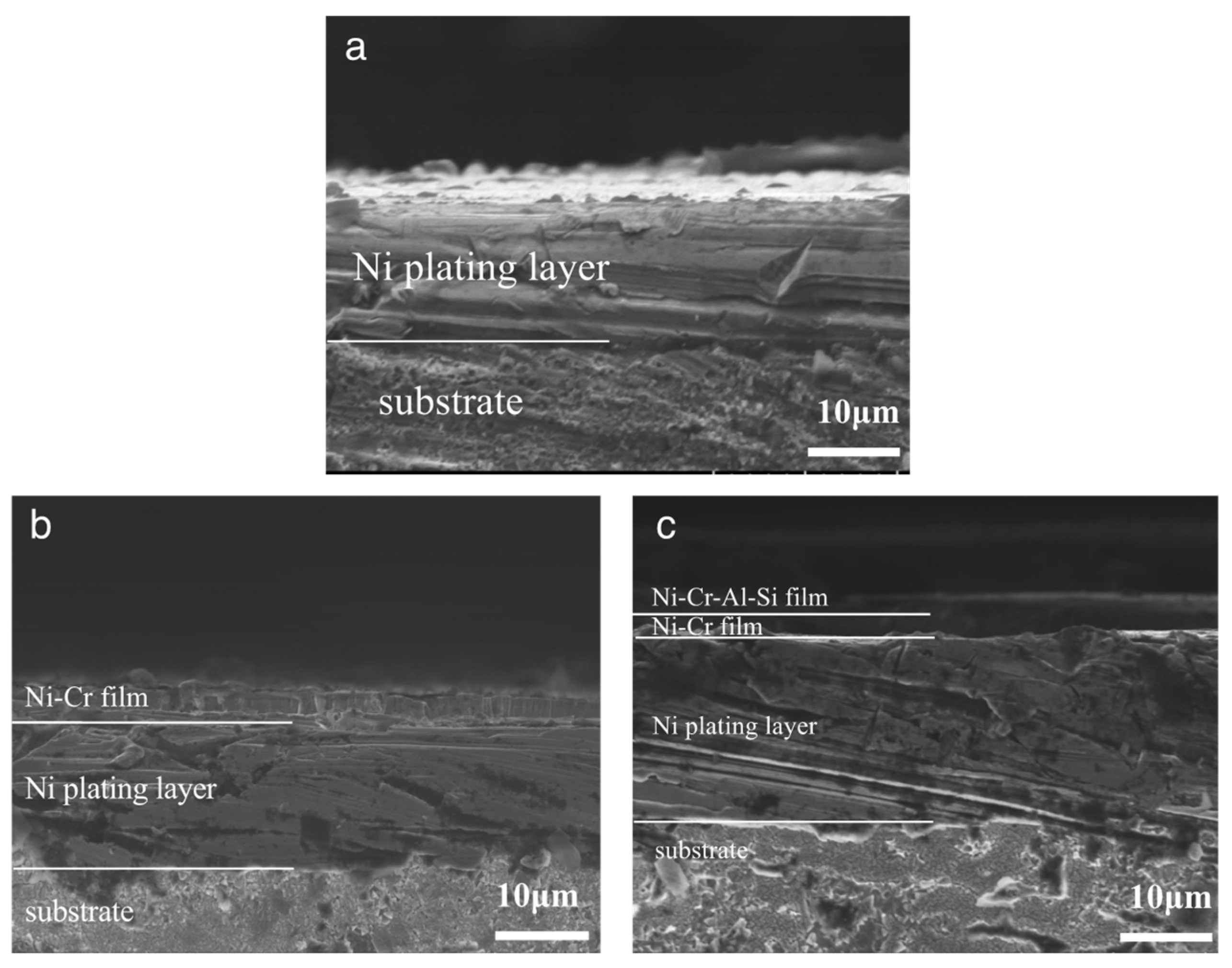
3.1. Composition and Structure of the Ni/NiCr Composite Coating
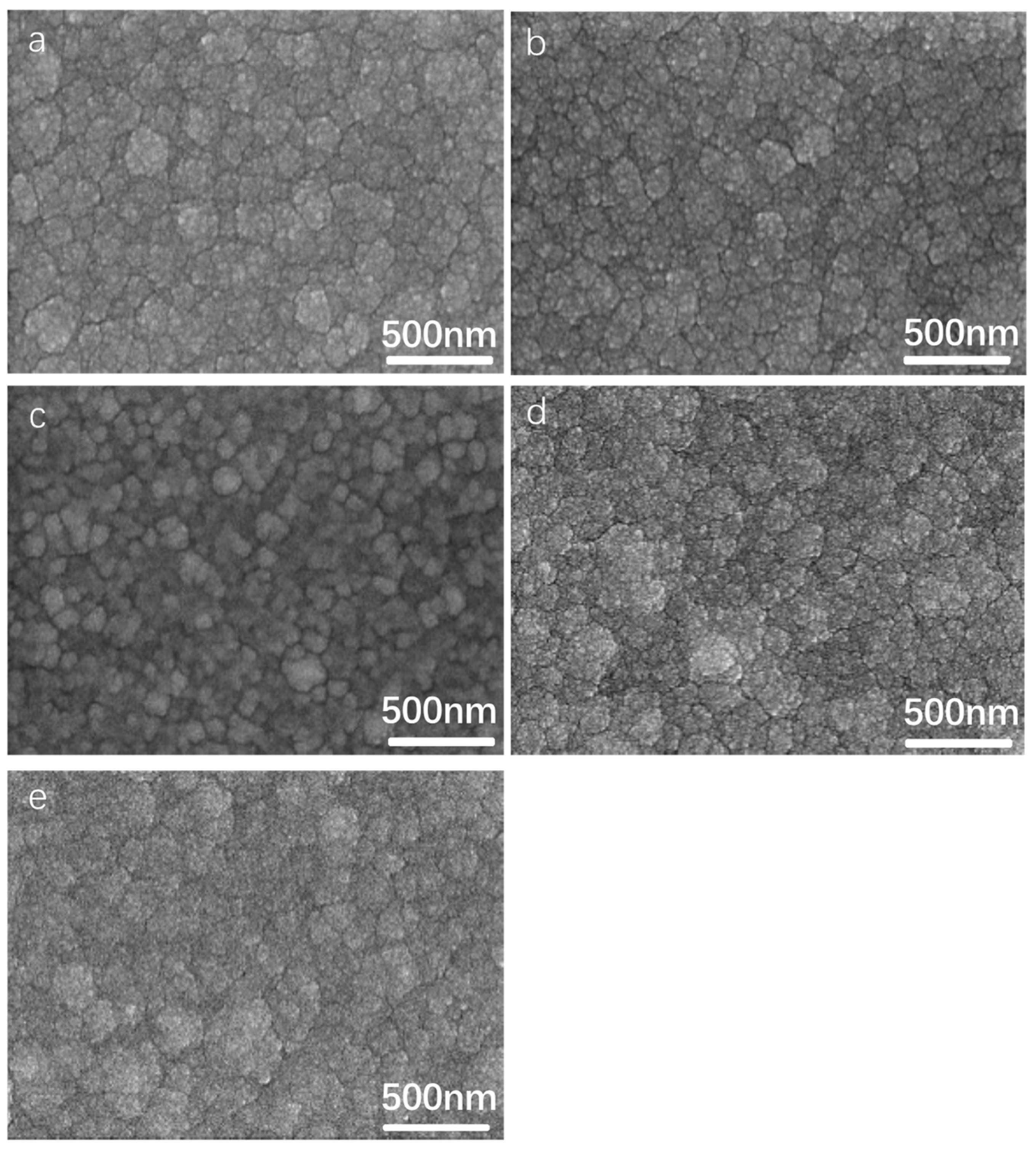
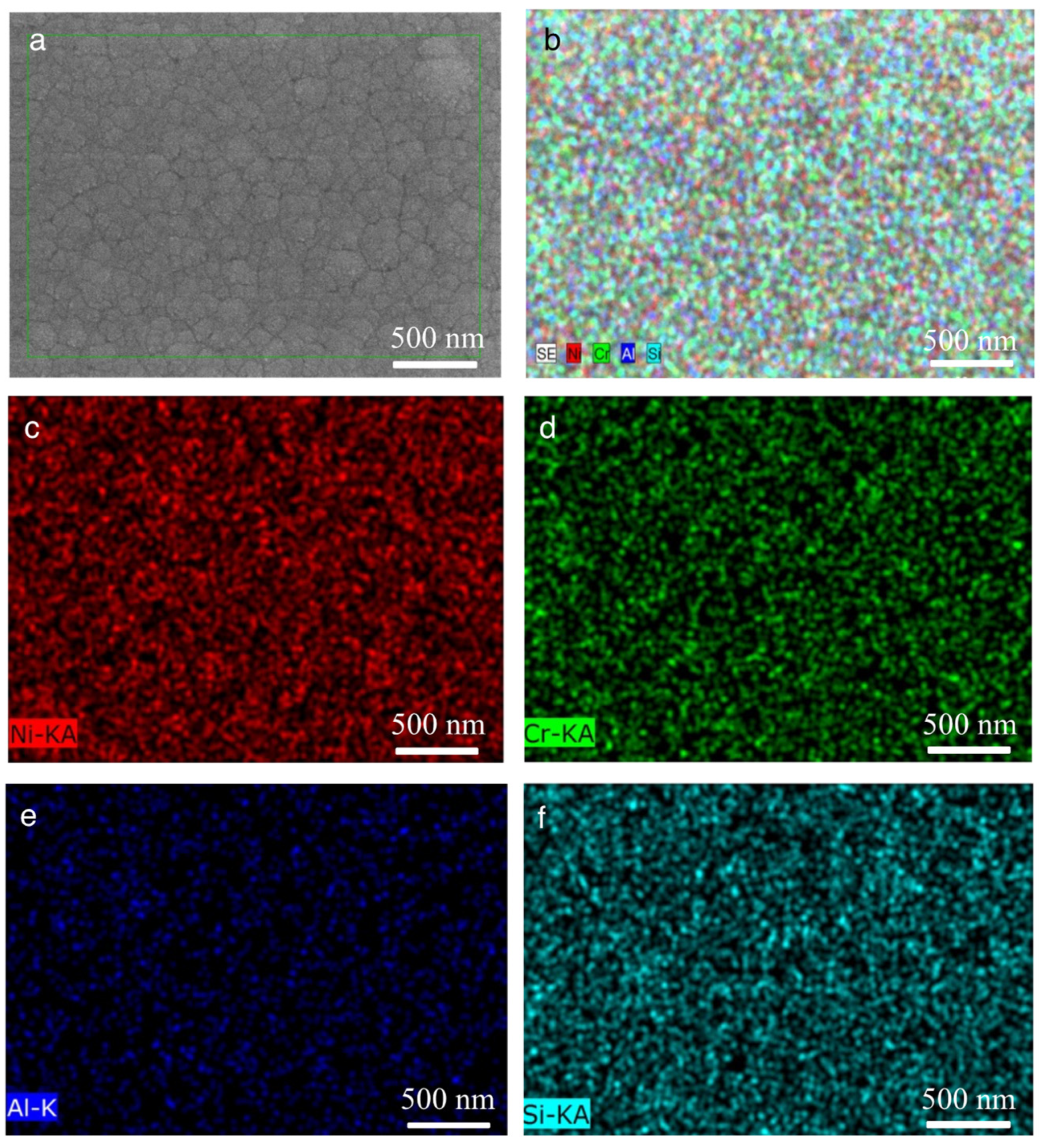
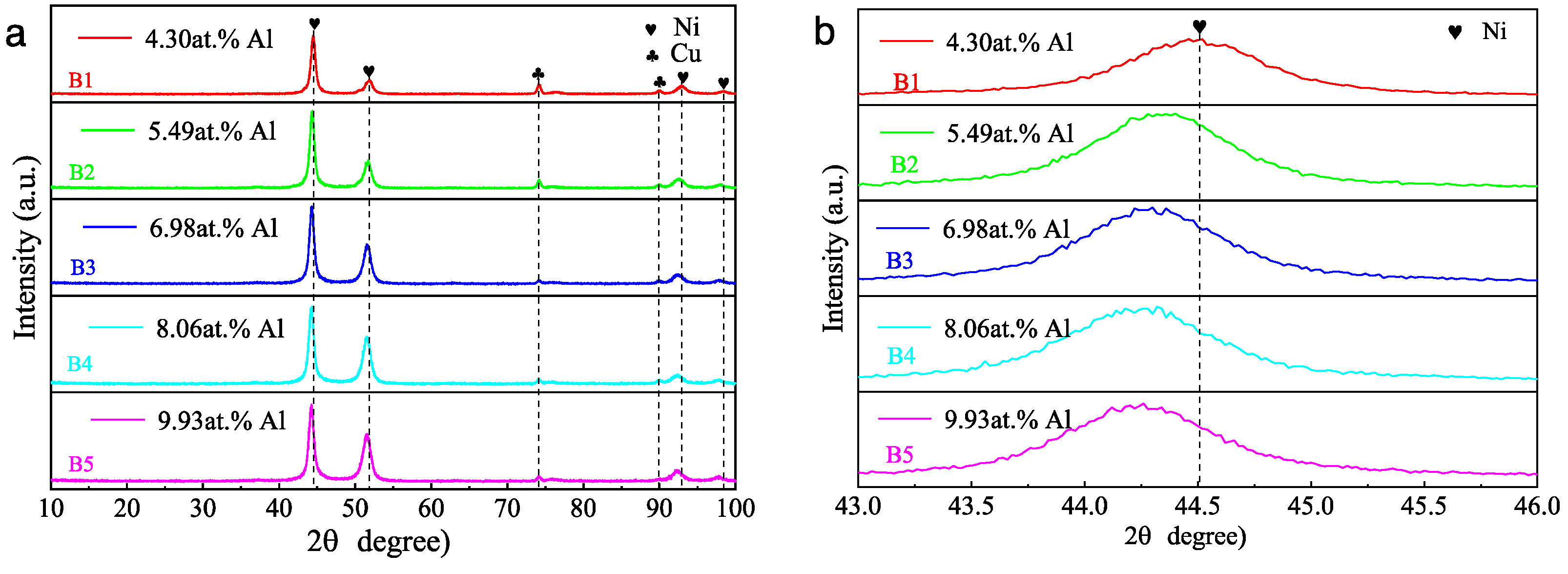
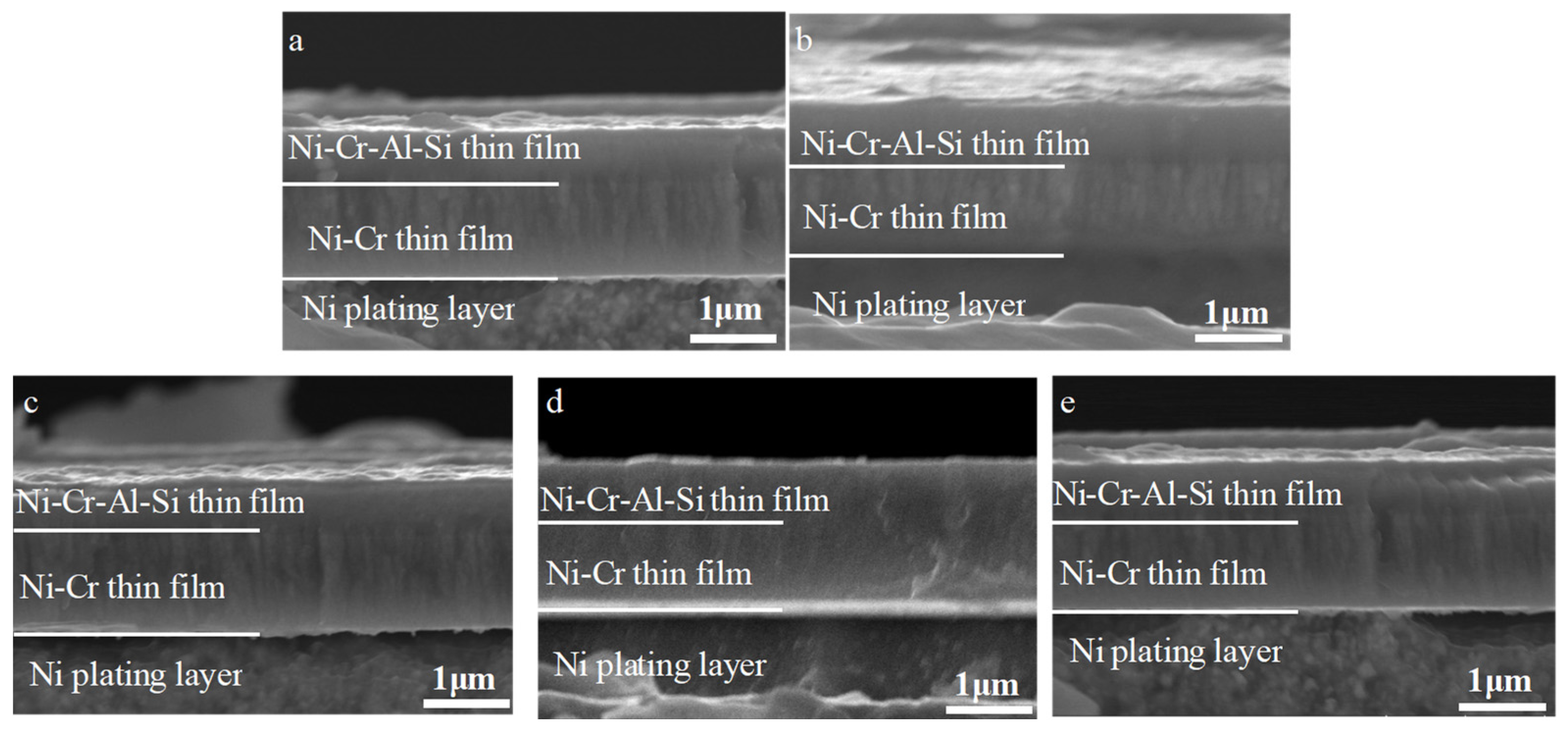
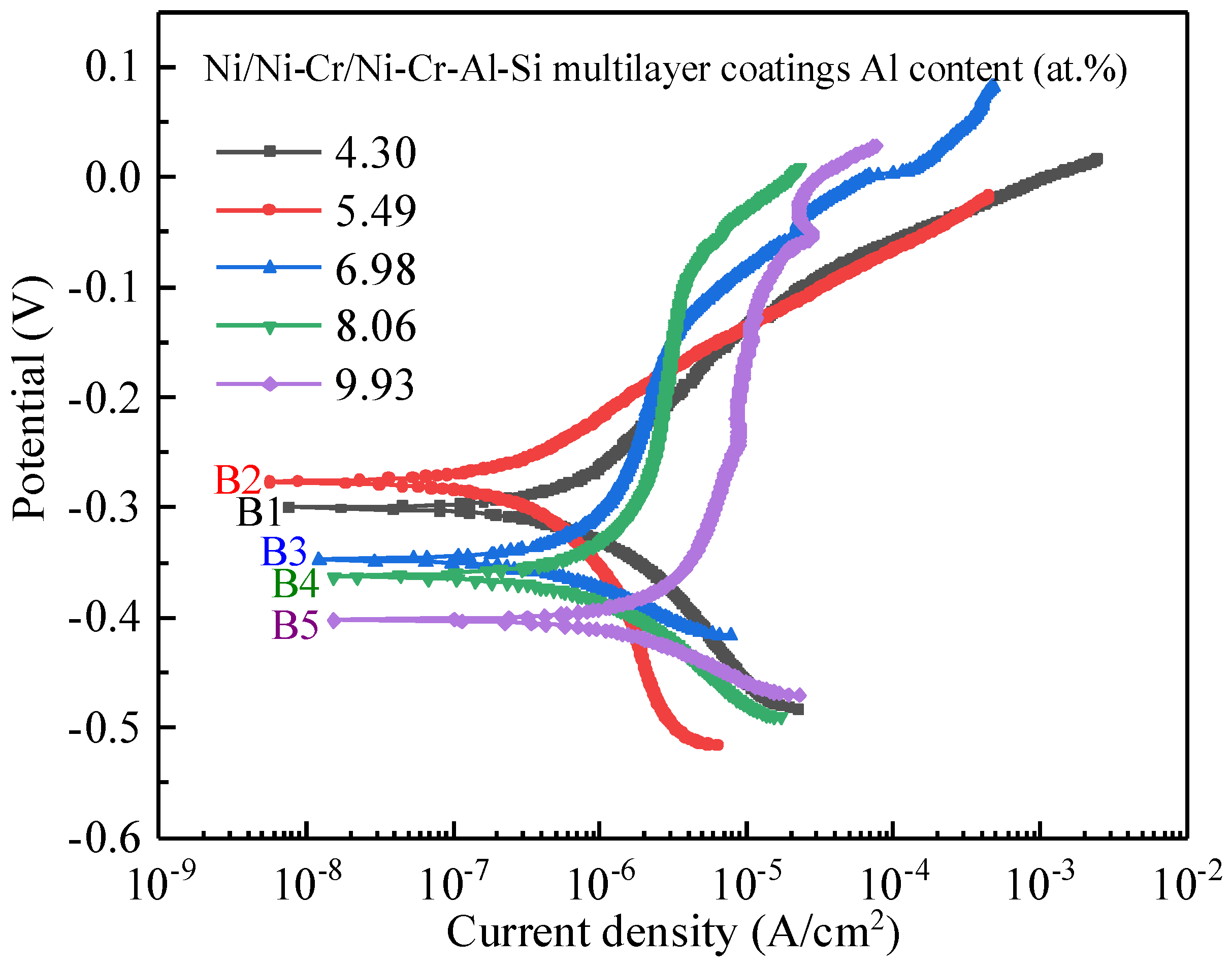
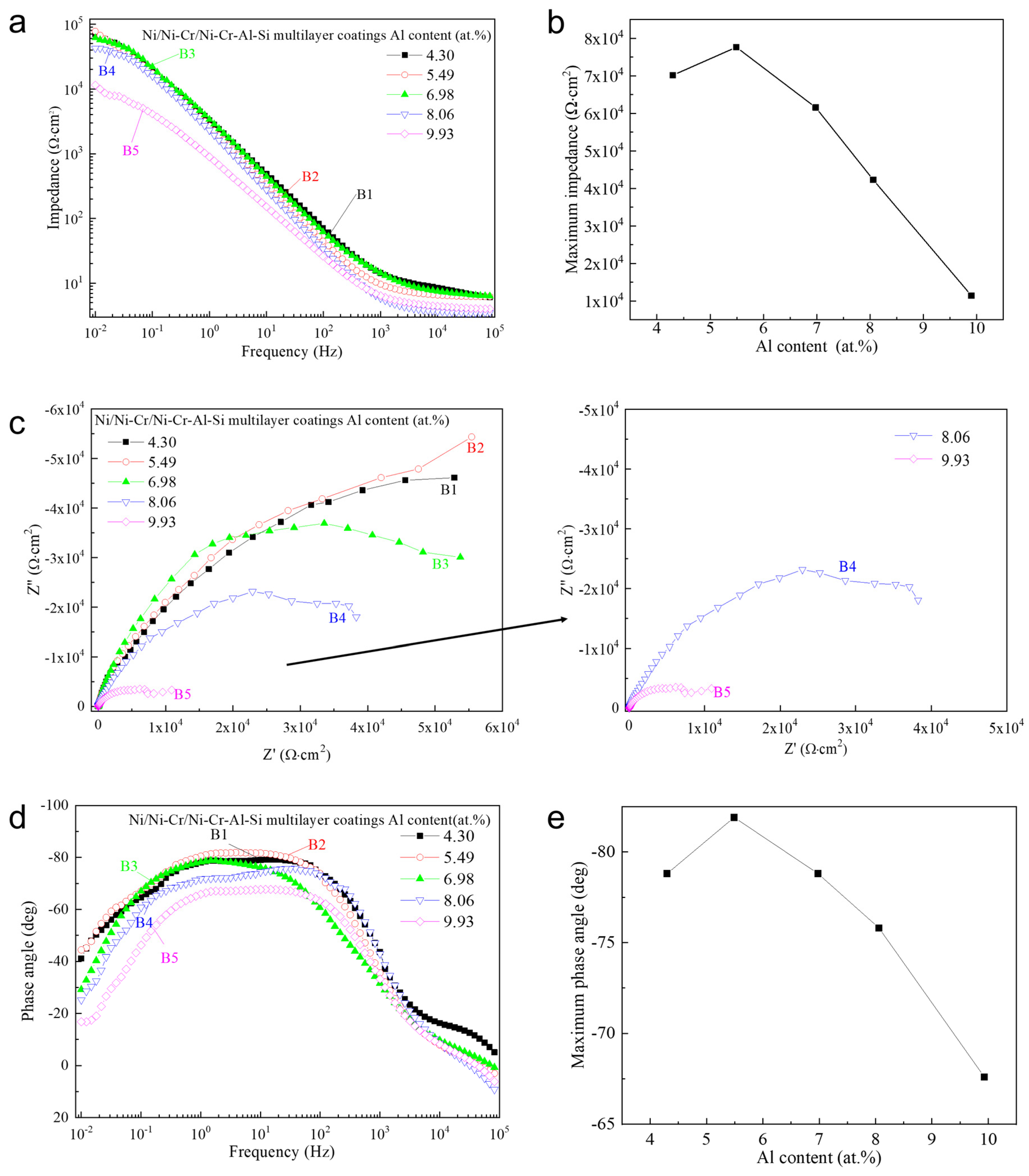
3.2. Effect of Aluminum Content in NiCrAlSi Layer on the Structure and Properties
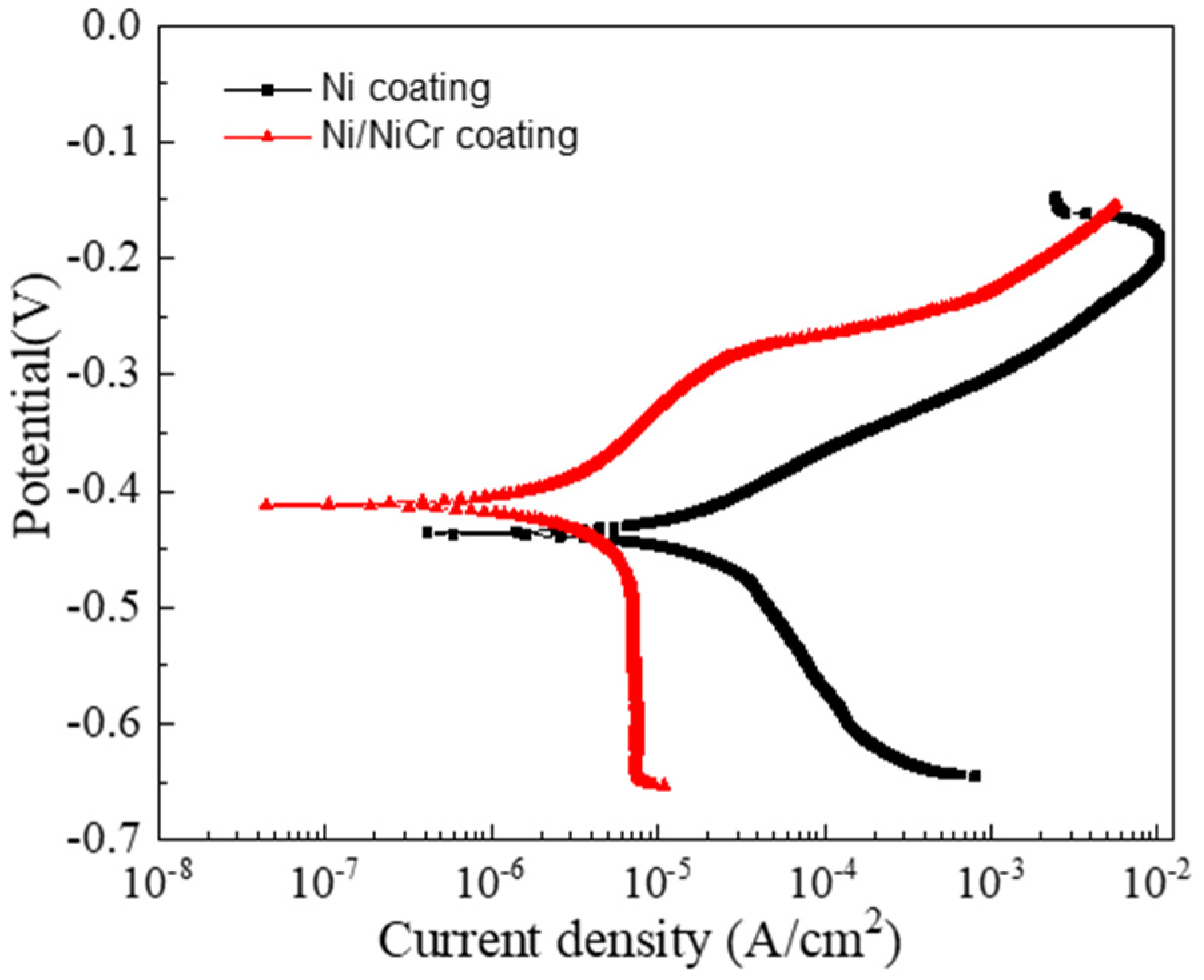
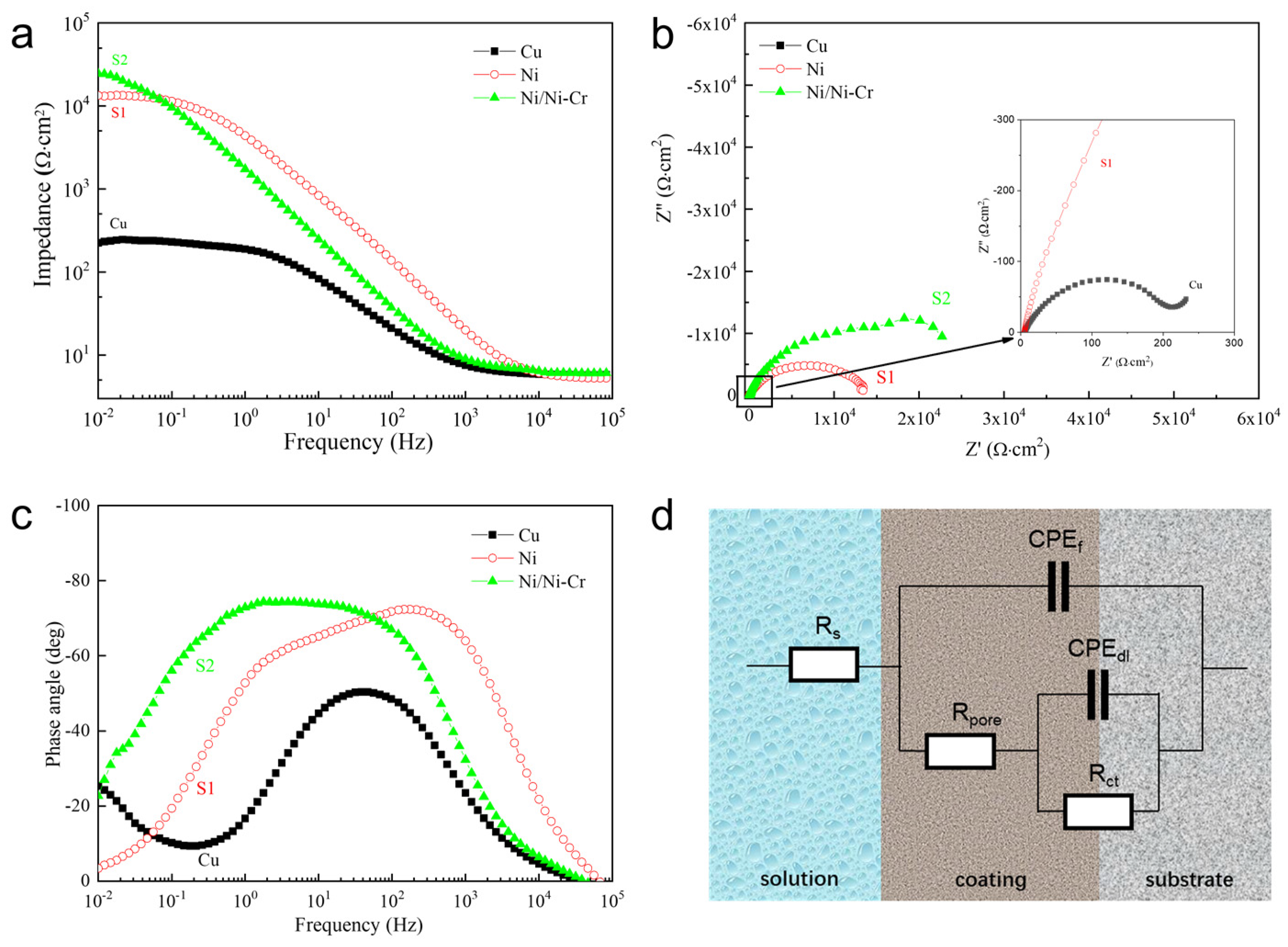
3.3. Corrosion Behavior of the Ni/NiCr Coating
3.3.1. Potentiodynamic Polarization Measurements
3.3.2. AC Impedance Measurements
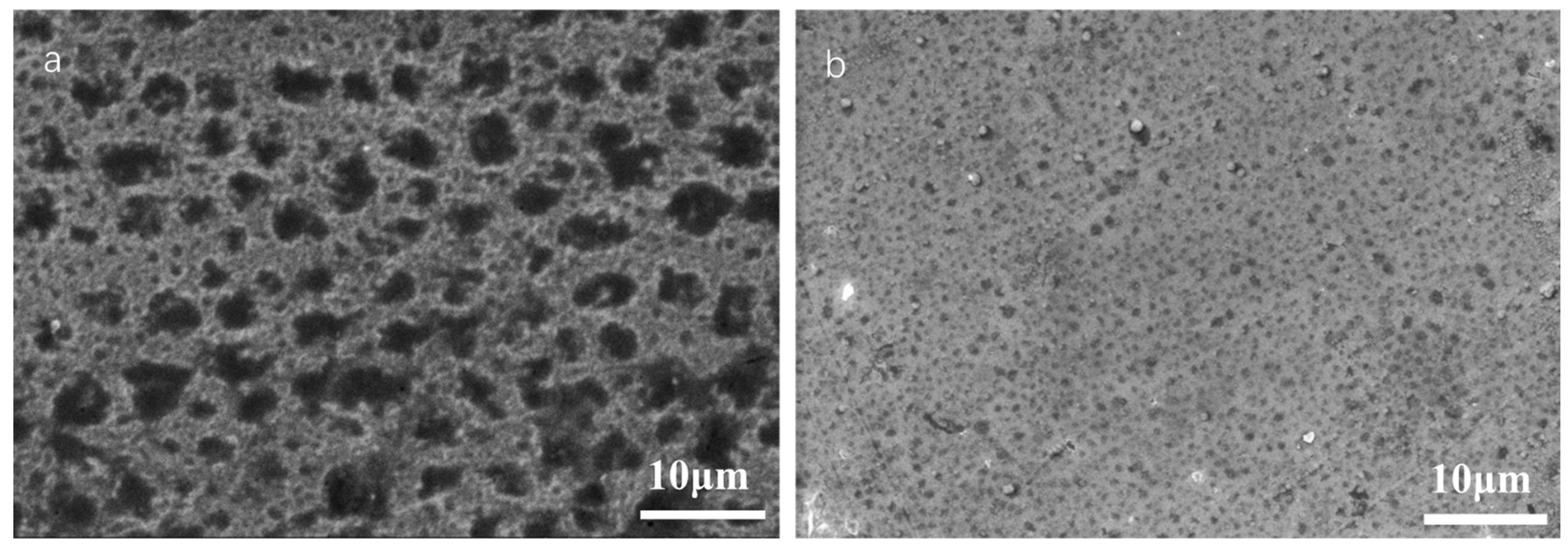
3.3.3. SEM Observation on Sample Surface after Potentiodynamic Polarization Measurement
3.4. Corrosion Behavior of the Ni/NiCr/NiCrAlSi Coating
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dahmani, K.; Ouakki, M.G.M.; Cherkaoui, M.; Touir, R.; Erkan, S.; El Ibrahimi, B. Quantum chemical and molecular dynamic simulation studies for the identification of the extracted cinnamon essential oil constituent responsible for copper corrosion inhibition in acidified 3.0 wt% NaCl medium. Inorg. Chem. Commun. 2021, 124, 108409. [Google Scholar] [CrossRef]
- Noriega, O.A.G.; Porcayo-Calderon, J.; Martinez, H.; Lopez-Sesenes, R.; Gonzalez-Rodriguez, J.G. Effect of plasma treatment of copper on its corrosion behaviour in 3.5% NaCl solution. Int. J. Electrochem. Sci. 2023, 18, 100049. [Google Scholar] [CrossRef]
- Schindelholz, E.J.; Cong, H.; Jove-Colon, C.F. Electrochemical aspects of copper atmospheric corrosion in the presence of sodium chloride. Electrochim. Acta 2018, 276, 194–206. [Google Scholar] [CrossRef]
- Liao, X.; Cao, F.; Zheng, L.; Liu, W.; Chen, A.; Zhang, J.; Cao, C. Corrosion behaviour of copper under chloride-containing thin electrolyte layer. Corros. Sci. 2011, 53, 3289–3298. [Google Scholar] [CrossRef]
- Chen, S.; Zhang, D. Study of corrosion behavior of copper in 3.5 wt.% NaCl solution containing extracellular polymeric substances of an aerotolerant sulphate-reducing bacteria. Corros. Sci. 2018, 136, 275–284. [Google Scholar] [CrossRef]
- Lu, X.; Liu, Y.; Zhao, H.; Pan, C.; Wang, Z. Corrosion behavior of copper in extremely harsh marine atmosphere in Nansha Islands. China Trans. Nonferrous Met. Soc. China 2021, 31, 703–714. [Google Scholar] [CrossRef]
- Wan, Y.; Wang, X.; Sun, H.; Li, Y.; Zhang, K.; Wu, Y. Corrosion Behavior of Copper at Elevated Temperature. Int. J. Electrochem. Sci. 2012, 7, 7902–7914. [Google Scholar] [CrossRef]
- Shaik, M.A.; Golla, B.R.; Khaple, S. Effect of powder processing and alloying additions (Al/ZrB2) on the microstructure, mechanical and electrical properties of Cu. Adv. Powder Technol. 2021, 32, 3314–3323. [Google Scholar] [CrossRef]
- Sheng, L.Y.; Yang, F.; Xi, T.F.; Lai, C.; Ye, H.Q. Influence of heat treatment on interface of Cu/Al bimetal composite fabricated by cold rolling. Compos. Part B-Eng. 2011, 42, 1468–1473. [Google Scholar] [CrossRef]
- Kim, I.-K.; Hong, S.I. Mechanochemical joining in cold roll-cladding of tri-layered Cu/Al/Cu composite and the interface cracking behavior. Mater. Des. 2014, 57, 625–631. [Google Scholar] [CrossRef]
- Liang, F.; Tan, H.F.; Zhang, B.; Zhang, G.P. Maximizing necking-delayed fracture of sandwich-structured Ni/Cu/Ni composites. Scr. Mater. 2017, 134, 28–32. [Google Scholar] [CrossRef]
- Tang, H.; Sun, J.; Pan, J. Electrochemical and localized corrosion behavior of 316LSS/copper/316LSS sandwich composite in chloride-containing environment. Corros. Sci. 2022, 209, 110719. [Google Scholar] [CrossRef]
- Yi, P.; Dong, C.F.; Xiao, K.; Li, X.G. Surface failure analysis of a field-exposed copper-clad plate in a marine environment with industrial pollution. Appl. Surf. Sci. 2017, 399, 608–616. [Google Scholar] [CrossRef]
- Zhang, X.; Li, S.; Sun, W.; Wang, L.; Wang, J.; Liu, G. Study on the corrosion behavior of copper coupled with TiO2 with different crystal structures. Corros. Sci. 2021, 183, 109352. [Google Scholar] [CrossRef]
- Liu, Y.; Li, S.; Zhang, J.; Liu, J.; Han, Z.; Ren, L. Corrosion inhibition of biomimetic super-hydrophobic electrodeposition coatings on copper substrate. Corros. Sci. 2015, 94, 190–196. [Google Scholar] [CrossRef]
- Huang, Y.; Sarkar, D.K.; Gallant, D.; Chen, X.G. Corrosion resistance properties of superhydrophobic copper surfaces fabricated by one-step electrochemical modification process. Appl. Surf. Sci. 2013, 282, 689–694. [Google Scholar] [CrossRef]
- Grisales, M.A.; Giraldo, F.; Echavarría, A.M.; Bolivar, F.J.; Bejarano, G.G. A novel ReN/TiAlN multilayer coating on M2 steel by magnetron sputtering: Development and electrochemical behavior. Surf. Coat. Technol. 2022, 448, 128883. [Google Scholar] [CrossRef]
- Liu, Y.R.; Du, H.; Zuo, X.; Guo, P.; Liu, L.; Lee, K.R.; Wang, A.Y.; Ke, P.L. Cr/GLC multilayered coating in simulated deep-sea environment: Corrosion behavior and growth defect evolution. Corros. Sci. 2021, 188, 109528. [Google Scholar] [CrossRef]
- Liu, Y.R.; Li, S.Y.; Li, H.; Ma, G.S.; Sun, L.L.; Guo, P.; Ke, P.L.; Lee, K.R.; Wang, A.Y. Controllable defect engineering to enhance the corrosion resistance of Cr/GLC multilayered coating for deep-sea applications. Corros. Sci. 2022, 199, 110175. [Google Scholar] [CrossRef]
- Ahn, S.H.; Lee, J.H.; Kim, J.G.; Han, J.G. Localized corrosion mechanisms of the multilayered coatings related to growth defects. Surf. Coat. Technol. 2004, 177–178, 638–644. [Google Scholar] [CrossRef]
- Santos, A.M.M.D.; Batista, R.J.C.; Martins, L.A.M.; Ilha, M.; Vieira, M.Q.; Miquita, D.R.; Guma, F.C.R.; Muller, I.L.; Manhabosco, T.M. Corrosion and cell viability studies of graphite-like hydrogenated amorphous carbon films deposited on bare and nitrided titanium alloy. Corros. Sci. 2014, 82, 297–303. [Google Scholar] [CrossRef]
- William Grips, V.K.; Barshilia Harish, C.; Ezhil Selvi, V.; Kalavati Rajam, K.S. Electrochemical behavior of single layer CrN, TiN, TiAlN coatings and nanolayered TiAlN/CrN multilayer coatings prepared by reactive direct current magnetron sputtering. Thin Solid Film. 2006, 514, 204–211. [Google Scholar] [CrossRef]
- Wong, K.P.; Chan, K.C.; Yue, T.M. Influence of spike current in different shaped waveforms on the surface finish of nickel electroforms. Surf. Coat. Technol. 2001, 140, 284–292. [Google Scholar] [CrossRef]
- Gaona-Tiburcio, C.; Montoya-Rangel, M.; Cabral-Miramontes, J.A.; Lopez, F.E.; Zambrano-Robledo, P.; Cruz, R.O.; Chacon-Nava, J.G.; Baltazar-Zamora, M.A.; Almeraya-Calderon, F. Corrosion resistance of multilayer coatings deposited by PVD on Inconel 718 using electrochemical impedance spectroscopy technique. Coatings 2020, 10, 521. [Google Scholar] [CrossRef]
- Song, B.; Hua, Y.; Zhou, C.; Li, Y.; Yang, L.; Song, Z. Fabrication and anticorrosion behavior of a bi-phase TaNbHfZr/CoCrNi multilayer coating through magnetron sputtering. Corros. Sci. 2022, 196, 110020. [Google Scholar] [CrossRef]
- Chen, H.; Ye, Y.W.; Wang, C.T.; Ma, X.L.; Wang, H.X.; Liu, W. Understanding the corrosion and tribological behaviors of CrSiN coatings with various Si contents in HCl solution. Tribol. Int. 2019, 131, 530–540. [Google Scholar] [CrossRef]
- Drnovšek, A.; Vo, H.T.; de Figueiredo, M.R.; Kolozsvári, S.; Hosemann, P.; Franz, R. High temperature fracture toughness of single-layer CrAlN and CrAlSiN hard coatings. Surf. Coat. Technol. 2021, 409, 126909. [Google Scholar] [CrossRef]
- Ziat, Y.; Hammi, M.; Zarhri, Z.; Laghlimi, C. Epoxy coating modified with graphene: A promising composite against corrosion behavior of copper surface in marine media. J. Alloys Compd. 2020, 820, 153380. [Google Scholar] [CrossRef]
- Cai, F.; Zhou, Q.; Chen, J.; Zhang, S. Effect of inserting the Zr layers on the tribo-corrosion behavior of Zr/ZrN multilayer coatings on titanium alloys. Corros. Sci. 2023, 213, 111002. [Google Scholar] [CrossRef]
- Blundy, R.G.; Pryor, M.J. The potential dependence of ration product composition on copper-nickel alloys. Corros. Sci. 1972, 12, 65–75. [Google Scholar] [CrossRef]
- Xi, K.; Wu, H.; Zhou, C.; Qi, Z.; Yang, K.; Fu, R.K.; Xiao, S.; Wu, G.; Ding, K.; Chen, G.; et al. Improved corrosion and wear resistance of micro-arc oxidation coatings on the 2024 aluminum alloy by incorporation of quasi-two-dimensional sericite microplates. Appl. Surf. Sci. 2022, 585, 152693. [Google Scholar] [CrossRef]


| Component | Concentration (g/L) |
|---|---|
| NiSO4·6H2O | 200 |
| NiCl2 | 15 |
| H3BO3 | 41 |
| Additive (Coumarin) | 3 |
| Sample | Ni (at.%) | Cr (at.%) | Al (at.%) | Si (at.%) |
|---|---|---|---|---|
| S1 | 100 | 0 | 0 | 0 |
| S2 | 81.43 | 18.57 | 0 | 0 |
| Target for NiCr deposition | 80 | 20 | - | - |
| Sample | Ni (at.%) | Cr (at.%) | Al (at.%) | Si (at.%) | [Si]/[Ni + Cr] |
|---|---|---|---|---|---|
| B1 | 63.51 | 12.50 | 4.30 | 19.69 | 0.259 |
| B2 | 61.80 | 12.36 | 5.49 | 20.35 | 0.274 |
| B3 | 62.57 | 12.57 | 6.98 | 17.88 | 0.237 |
| B4 | 62.07 | 12.54 | 8.06 | 17.33 | 0.232 |
| B5 | 61.26 | 12.33 | 9.93 | 16.49 | 0.224 |
| Target | 60 | 13 | 5 | 20 | 0.274 |
| Sample | Rs/Ω·cm−2 | CPEf/μF·cm−2 | nc | Rpore/Ω·cm−2 | CPEdl/μF·cm−2 | nt | Rct/Ω·cm−2 |
|---|---|---|---|---|---|---|---|
| S1 | 5.279 | 2.121 × 10−5 | 0.8972 | 982.9 | 3.014 × 10−5 | 0.6964 | 1.299 × 104 |
| S2 | 6.212 | 1.216 × 10−5 | 0.8459 | 2.029 × 104 | 2.283 × 10−5 | 0.5033 | 2.55 × 104 |
| Sample | Ecorr (V Vs. SCE) | icorr (A·cm−2) | βc (V/Decade) |
|---|---|---|---|
| B1 | −0.301 ± 0.013 | (5.461 ± 0.195) × 10−7 | −0.127 ± 0.006 |
| B2 | −0.277 ± 0.022 | (2.666 ± 0.171) × 10−7 | −0.132 ± 0.009 |
| B3 | −0.348 ± 0.011 | (7.299 ± 0.32) × 10−7 | −0.080 ± 0.008 |
| B4 | −0.363 ± 0.012 | (1.046 ± 0.043) × 10−6 | −0.108 ± 0.011 |
| B5 | −0.403 ± 0.023 | (1.567 ± 0.093) × 10−6 | −0.070 ± 0.007 |
| Sample | Rs/Ω cm−2 | CPEf/μF·cm−2 | nc | Rpore/Ω cm−2 | CPEdl/μF·cm−2 | nt | Rct/Ω cm−2 |
|---|---|---|---|---|---|---|---|
| B1 | 5.577 | 5.502 × 10−5 | 0.7369 | 7.445 × 104 | 1.69 × 10−5 | 0.9614 | 1.445 × 105 |
| B2 | 6.469 | 7.357 × 10−5 | 0.8906 | 5.101 × 104 | 5.97 × 10−5 | 0.6616 | 1.502 × 105 |
| B3 | 7.006 | 5.456 × 10−5 | 0.8329 | 4.206 × 104 | 1.015 × 10−5 | 0.9889 | 8.792 × 104 |
| B4 | 3.602 | 8.083 × 10−5 | 0.9128 | 1.148 × 104 | 2.173 × 10−5 | 0.4612 | 4.977 × 104 |
| B5 | 4.146 | 2.069 × 10−5 | 1 | 1686 | 2.489 × 10−5 | 0.7436 | 1.108 × 104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, H.; Wen, J.; Song, G.; Wu, H.; Yin, Y. Corrosion Behavior of Ni/NiCr/NiCrAlSi Composite Coating on Copper for Application as a Heat Exchanger in Sea Water. Nanomaterials 2023, 13, 3129. https://doi.org/10.3390/nano13243129
Du H, Wen J, Song G, Wu H, Yin Y. Corrosion Behavior of Ni/NiCr/NiCrAlSi Composite Coating on Copper for Application as a Heat Exchanger in Sea Water. Nanomaterials. 2023; 13(24):3129. https://doi.org/10.3390/nano13243129
Chicago/Turabian StyleDu, Hao, Jiayuan Wen, Guihong Song, Hao Wu, and Yansheng Yin. 2023. "Corrosion Behavior of Ni/NiCr/NiCrAlSi Composite Coating on Copper for Application as a Heat Exchanger in Sea Water" Nanomaterials 13, no. 24: 3129. https://doi.org/10.3390/nano13243129
APA StyleDu, H., Wen, J., Song, G., Wu, H., & Yin, Y. (2023). Corrosion Behavior of Ni/NiCr/NiCrAlSi Composite Coating on Copper for Application as a Heat Exchanger in Sea Water. Nanomaterials, 13(24), 3129. https://doi.org/10.3390/nano13243129







