A Pyridine Diketopyrrolopyrrole-Grafted Graphene Oxide Nanocomposite for the Sensitive Detection of Chloramphenicol by a Direct Electrochemical Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instruments
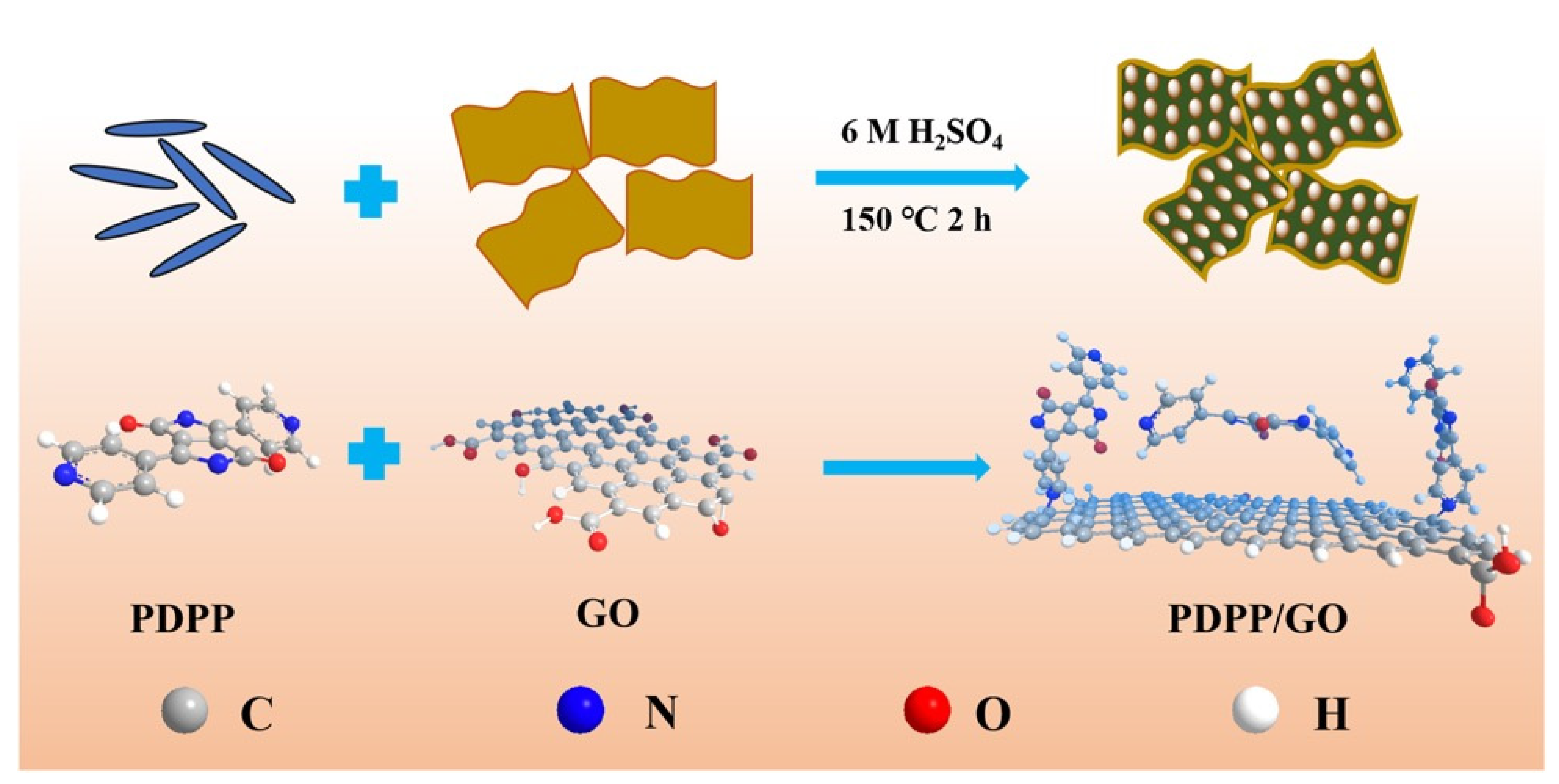
2.2. Preparation of the PDPP/GO Composite
2.3. Construction of the PDPP/GO/GCE-Modified Electrode
3. Results and Discussion
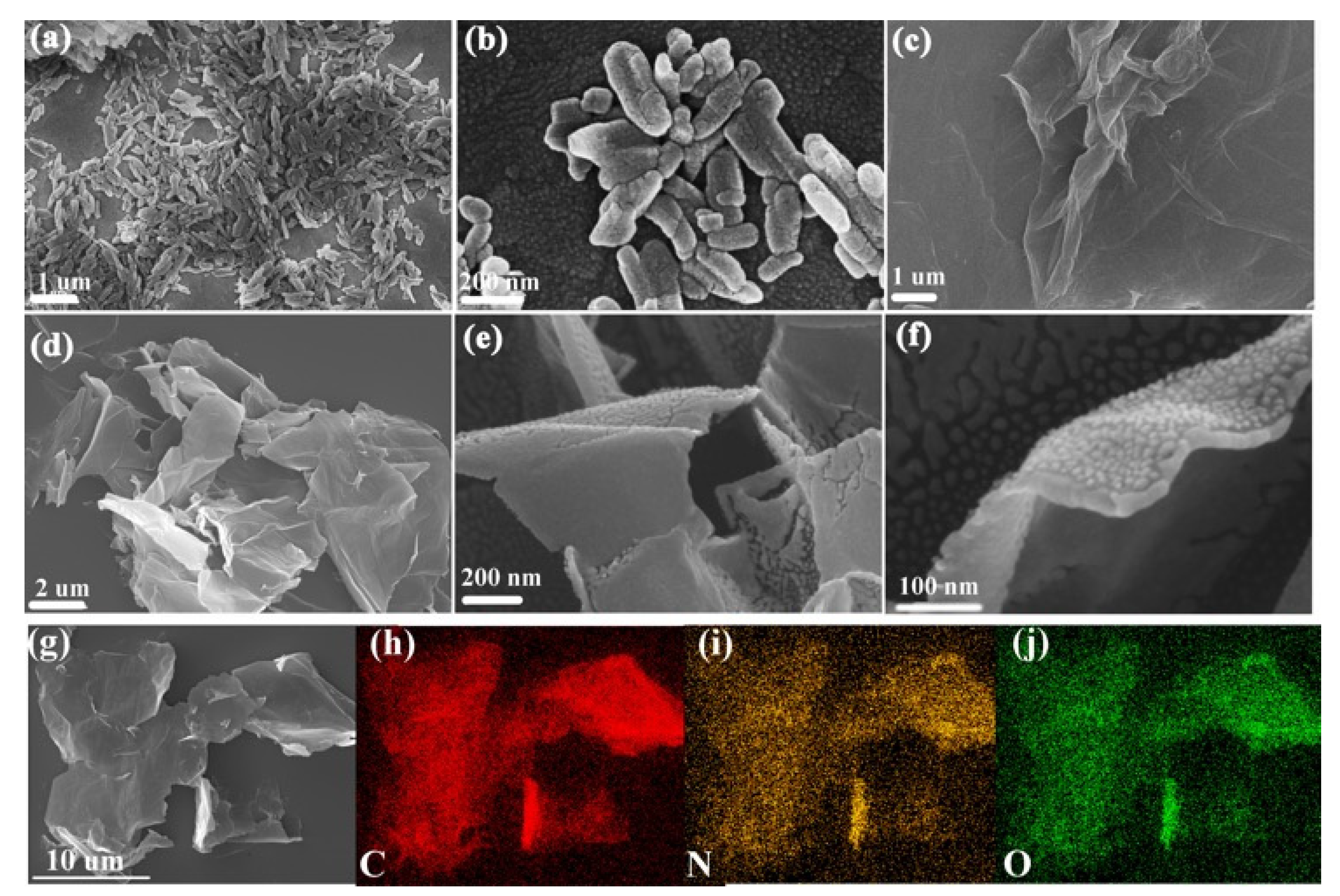
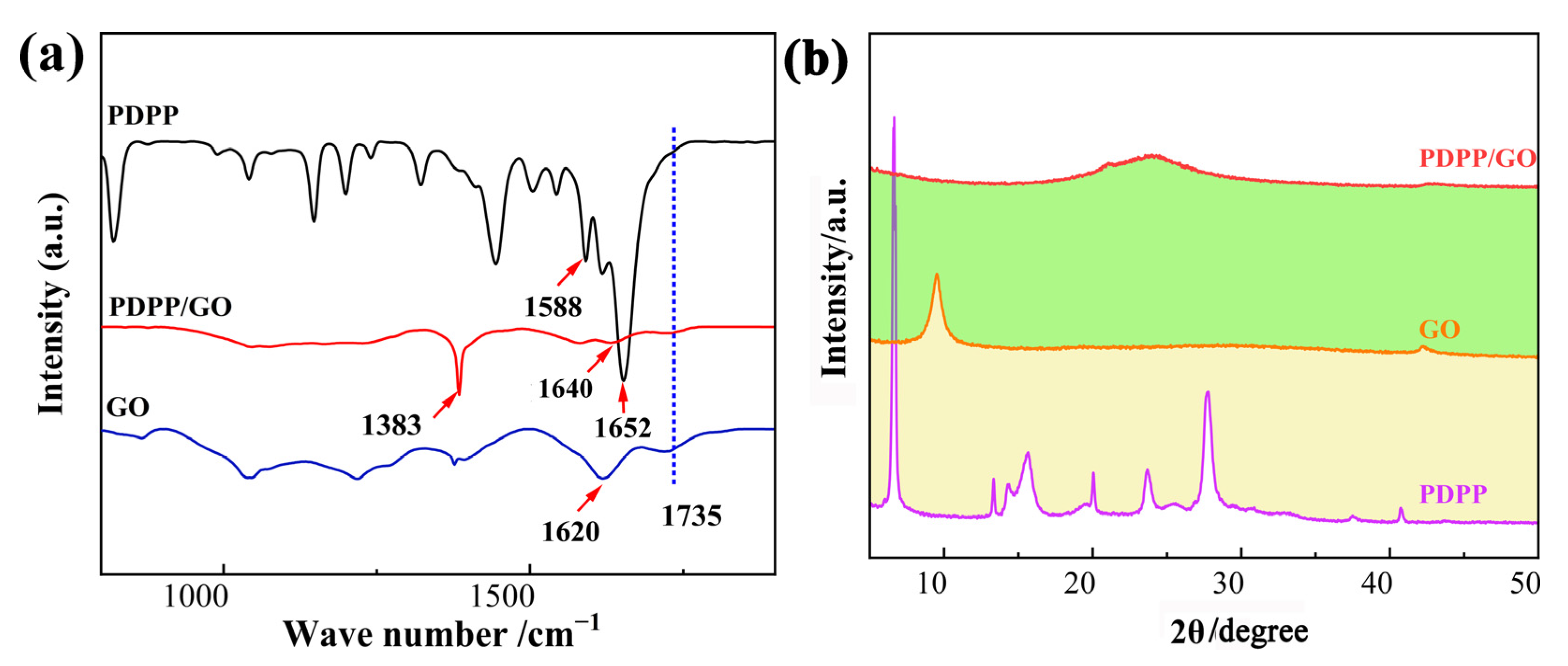
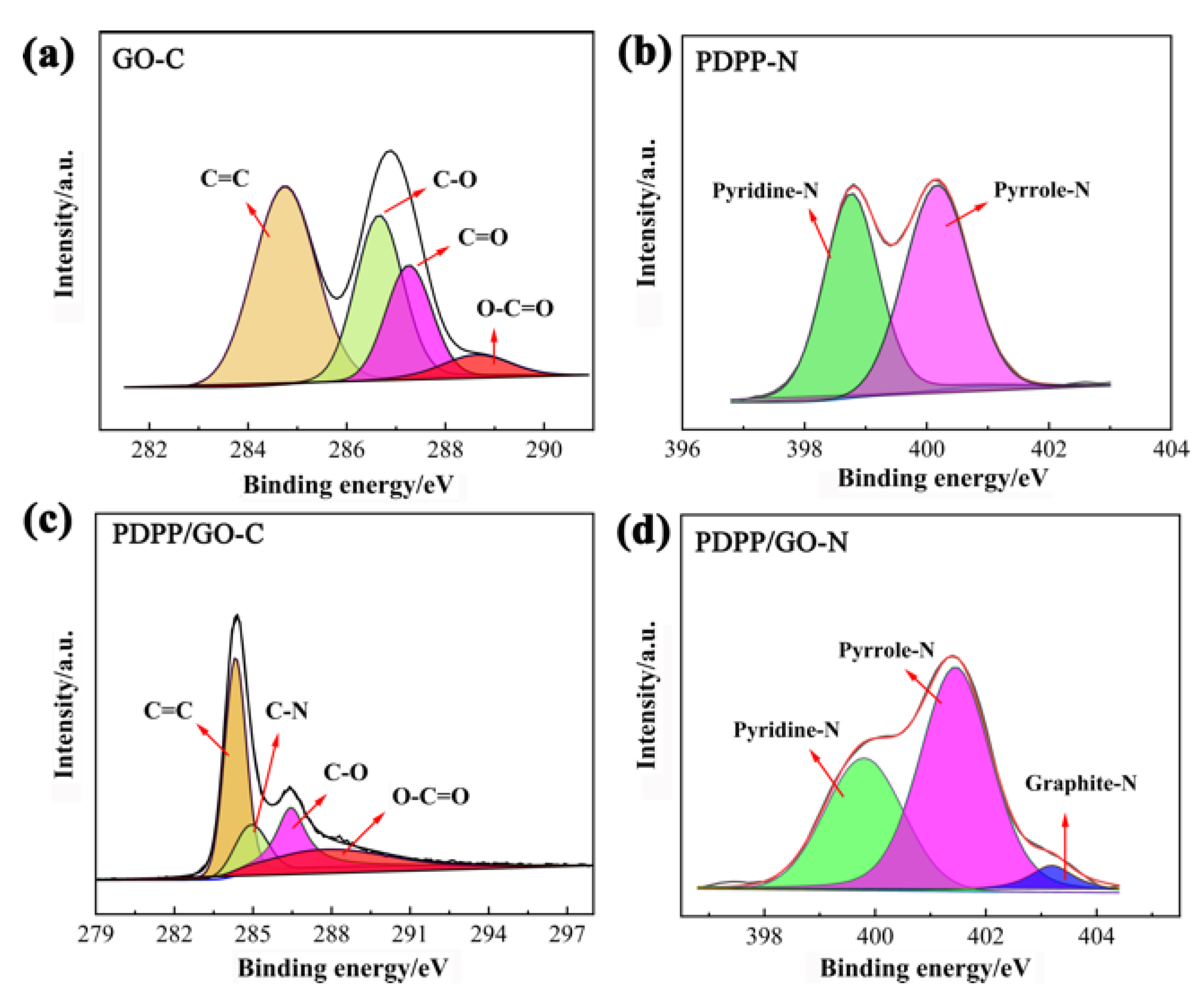
3.1. Morphology and Structure Characterization
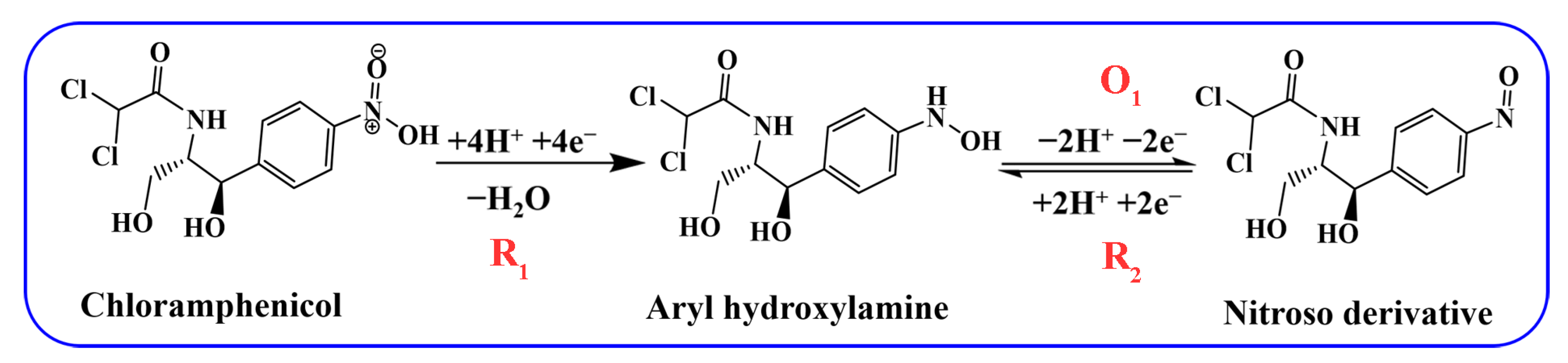
3.2. Electrochemical Test for Different Electrodes Regarding CAP
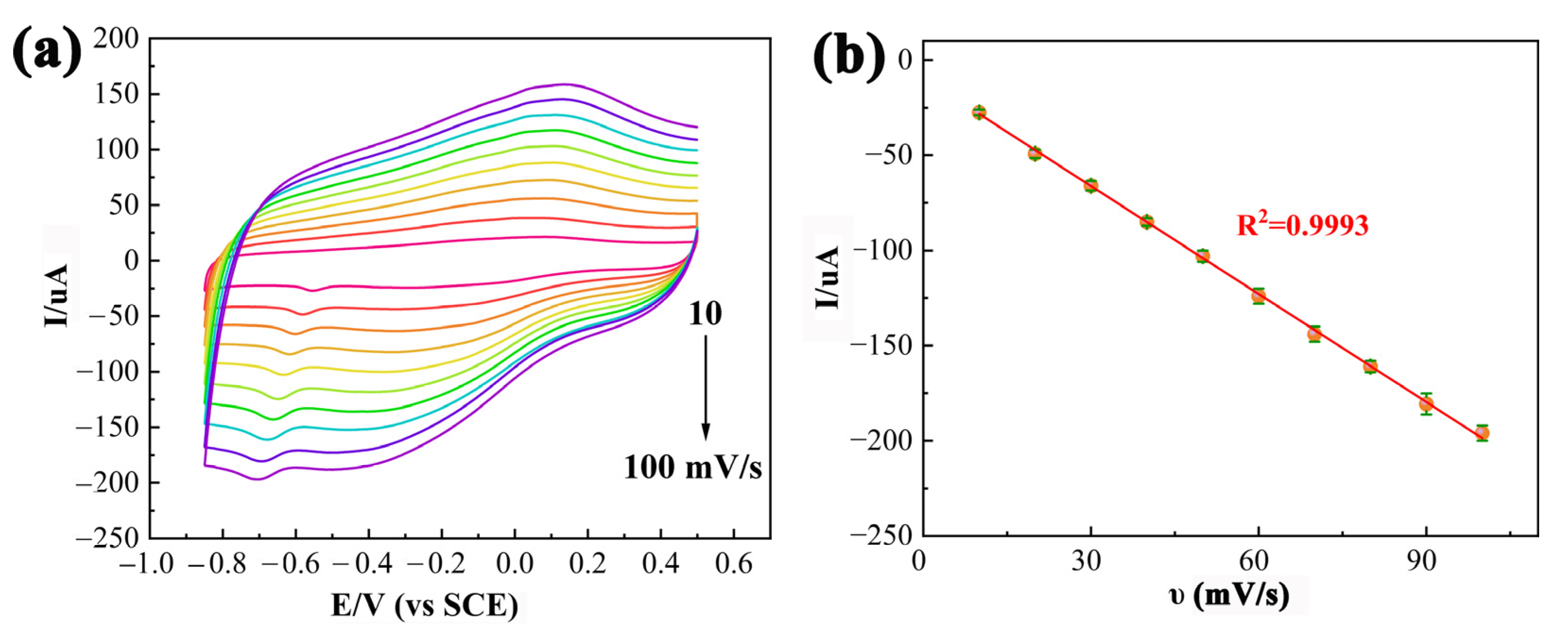
3.3. Effect of the pH and Scan Rate
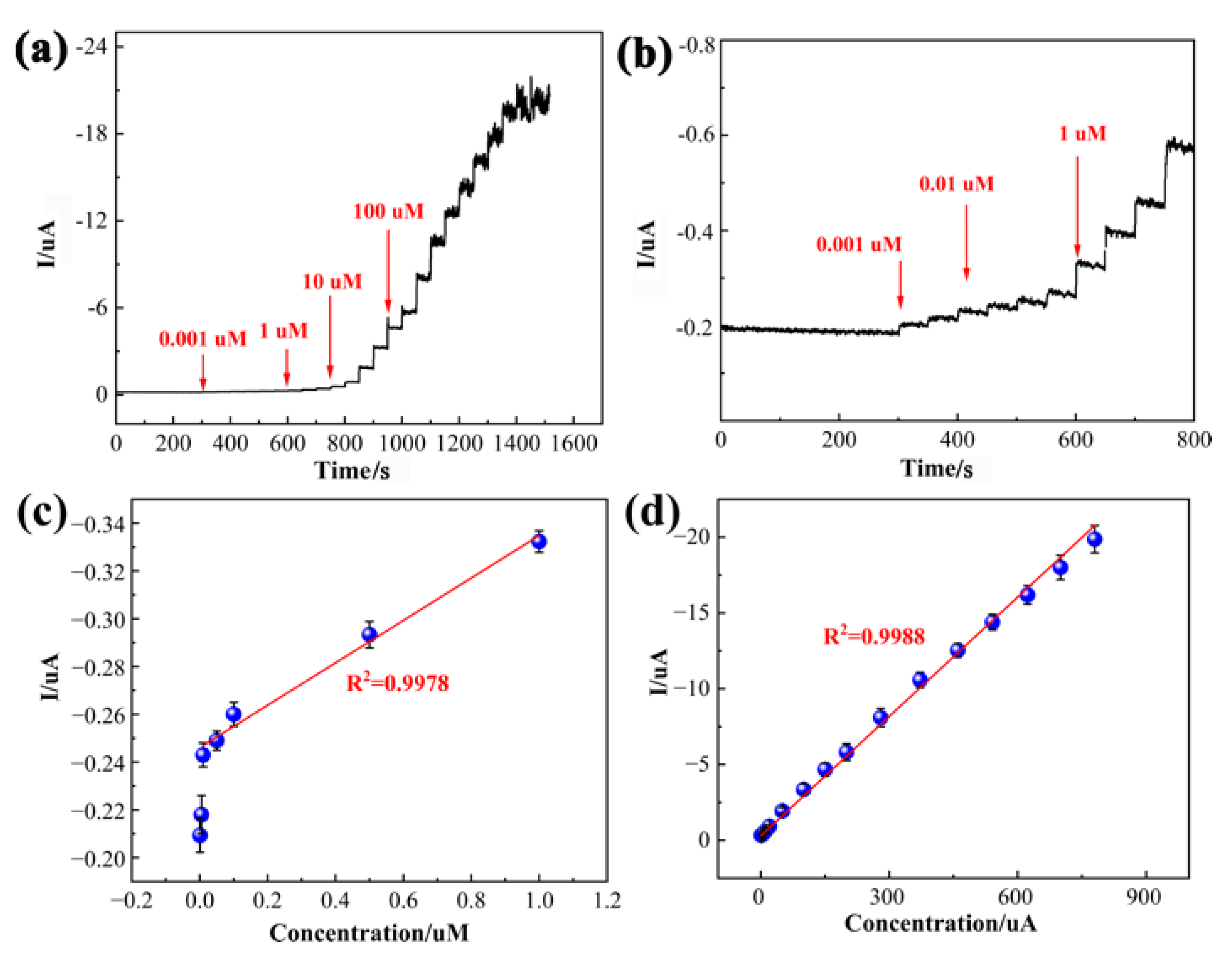
3.4. Determination of CAP
3.5. Selectivity, Reproducibility, and Stability
3.6. Real Sample Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Feder, H.M.J.; Osier, C.; Maderazo, E.G. Chloramphenicol: A Review of Its Use in Clinical Practice. Rev. Infect. Dis. 1981, 3, 479–491. [Google Scholar] [CrossRef] [PubMed]
- White, D.G.; Hudson, C.; Maurer, J.J.; Ayers, S.; Zhao, S.H.; Lee, M.D.; Bolton, L.; Foley, T.; Sherwood, J. Characterization of Chloramphenicol and Florfenicol Resistance in Escherichia coli Associated with Bovine Diarrhea. J. Clin. Microbiol. 2000, 38, 4593–4598. [Google Scholar] [CrossRef] [Green Version]
- Dinos, G.P.; Athanassopoulos, C.M.; Missiri, D.A.; Giannopoulou, P.C.; Vlachogiannis, I.A.; Papadopoulos, G.E.; Papaioannou, D.; Kalpaxis, D.L. Chloramphenicol Derivatives as Antibacterial and Anticancer Agents: Historic Problems and Current Solutions. Antibiotics 2016, 5, 20. [Google Scholar] [CrossRef] [PubMed]
- Umapathi, R.; Ghoreishian, S.M.; Sonwal, S.; Rani, G.M.; Huh, Y.S. Portable electrochemical sensing methodologies for on-site detection of pesticide residues in fruits and vegetables. Coord. Chem. Rev. 2022, 453, 214305. [Google Scholar] [CrossRef]
- Dong, X.; Yan, X.; Li, M.; Liu, H.; Li, J.; Wang, L.; Wang, K.; Wang, X.L.; Lu, X.; Wang, S.Y.; et al. Ultrasensitive detection of chloramphenicol using electrochemical aptamer sensor: A mini review. Electrochem. Commun. 2020, 120, 106835. [Google Scholar] [CrossRef]
- Ghosh, S.; Sachdeva, B.; Sachdeva, P.; Chaudhary, V.; Rani, G.M.; Sinha, J.K. Graphene quantum dots as a potential diagnostic and therapeutic tool for the management of Alzheimer’s disease. Carbon Lett. 2022, 32, 1–14. [Google Scholar] [CrossRef]
- Yadav, S.K.; Agrawal, B.; Chandra, P.; Goyal, R.N. In vitro chloramphenicol detection in a Haemophilus influenza model using an aptamer-polymer based electrochemical biosensor. Biosens. Bioelectron. 2014, 55, 337–342. [Google Scholar] [CrossRef]
- Khoshbin, Z.; Verdian, A.; Housaindokht, M.R.; Izadyar, M.; Rouhbakhsh, Z. Aptasensors as the future of antibiotics test kits-a case study of the aptamer application in the chloramphenicol detection. Biosens. Bioelectron. 2018, 122, 263–283. [Google Scholar] [CrossRef]
- Ma, P.; Sun, Y.; Khan, I.M.; Gu, Q.; Yue, L.; Wang, Z. Structure-switching fluorescence aptasensor for sensitive detection of chloramphenicol. Mikrochim. Acta 2020, 187, 505. [Google Scholar] [CrossRef]
- Gantverg, A.; Shishani, I.; Hoffman, M. Determination of chloramphenicol in animal tissues and urine. Ana. Chim. Acta 2003, 483, 125–135. [Google Scholar] [CrossRef]
- Yao, T.; Yao, S. Magnetic ionic liquid aqueous two-phase system coupled with high performance liquid chromatography: A rapid approach for determination of chloramphenicol in water environment. J. Chromatogr. A 2017, 1481, 12–22. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Fei, D.; Liu, P.; Guo, X.; Peng, L.; Wang, Y.; Xu, N.; Wei, X. Surface-Enhanced Raman Scattering–Based Lateral Flow Immunoassay for the Detection of Chloramphenicol Antibiotics Using Au@Ag Nanoparticles. Food Anal. Method. 2021, 14, 2642–2650. [Google Scholar] [CrossRef]
- Tong, T.T.V.; Cao, T.T.; Tran, N.H.; Le, T.K.V.; Le, D.C. Green, Cost-effective simultaneous assay of chloramphenicol, methyl paraben, and propylparaben in eye-drops by capillary zone electrophoresis. J. Anal. Methods Chem. 2021, 2021, 5575701. [Google Scholar] [CrossRef]
- Jia, B.J.; He, X.; Cui, P.L.; Liu, J.X.; Wang, J.P. Detection of chloramphenicol in meat with a chemiluminescence resonance energy transfer platform based on molecularly imprinted graphene. Anal. Chim. Acta 2019, 1063, 136–143. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Jian, D.; Zhang, Y.; Shan, Y.; Wang, S.; Liu, F. Paper-based antibiotic sensor (PAS) relying on colorimetric indirect competitive enzyme-linked immunosorbent assay for quantitative tetracycline and chloramphenicol detection. Sensor. Actuat. B-Chem. 2021, 329, 129173. [Google Scholar] [CrossRef]
- Cardoso, A.R.; Tavares, A.P.M.; Sales, M.G.F. In-situ generated molecularly imprinted material for chloramphenicol electrochemical sensing in waters down to the nanomolar level. Sensor. Actuat. B-Chem. 2018, 256, 420–428. [Google Scholar] [CrossRef]
- Kokulnathan, T.; Sharma, T.S.K.; Chen, S.-M.; Chen, T.-W.; Dinesh, B. Ex-situ decoration of graphene oxide with palladium nanoparticles for the highly sensitive and selective electrochemical determination of chloramphenicol in food and biological samples. J. Taiwan Inst. Chem. E. 2018, 89, 26–38. [Google Scholar] [CrossRef]
- Song, J.; Yang, J.; Zeng, J.; Tan, J.; Zhang, L. Graphite oxide film-modified electrode as an electrochemical sensor for acetaminophen. Sensor. Actuat. B-Chem. 2011, 155, 220–225. [Google Scholar] [CrossRef]
- Santos, J.S.; Pontes, M.S.; Santiago, E.F.; Fiorucci, A.R.; Arruda, G.J. An efficient and simple method using a graphite oxide electrochemical sensor for the determination of glyphosate in environmental samples. Sci. Total. Environ. 2020, 749, 142385. [Google Scholar] [CrossRef]
- Roushani, M.; Rahmati, Z.; Farokhi, S.; Hoseini, S.J.; Fath, R.H. The development of an electrochemical nanoaptasensor to sensing chloramphenicol using a nanocomposite consisting of graphene oxide functionalized with (3-Aminopropyl) triethoxysilane and silver nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110388. [Google Scholar] [CrossRef]
- Karthik, R.; Govindasamy, M.; Chen, S.M.; Mani, V.; Lou, B.S.; Devasenathipathy, R.; Hou, Y.S.; Elangovan, A. Green synthesized gold nanoparticles decorated graphene oxide for sensitive determination of chloramphenicol in milk, powdered milk, honey and eye drops. J. Colloid. Interface Sci. 2016, 475, 46–56. [Google Scholar] [CrossRef]
- Yi, W.; Li, Z.; Dong, C.; Li, H.-W.; Li, J. Electrochemical detection of chloramphenicol using palladium nanoparticles decorated reduced graphene oxide. Microchem. J. 2019, 148, 774–783. [Google Scholar] [CrossRef]
- Rajaji, U.; Manavalan, S.; Chen, S.-M.; Govindasamy, M.; Chen, T.-W.; Maiyalagan, T. Microwave-assisted synthesis of europium(III) oxide decorated reduced graphene oxide nanocomposite for detection of chloramphenicol in food samples. Compos. Part B-Eng. 2019, 161, 29–36. [Google Scholar] [CrossRef]
- Yadav, M.; Ganesan, V.; Gupta, R.; Yadav, D.K.; Sonkar, P.K. Cobalt oxide nanocrystals anchored on graphene sheets for electrochemical determination of chloramphenicol. Microchem. J. 2019, 146, 881–887. [Google Scholar] [CrossRef]
- Yang, L.; Huang, Y.; Peng, Y.; Liu, F.; Zhang, Q.; He, H.; Wang, J.; Jiang, L.; Zhou, Y. Pyridine-Diketopyrrolopyrrole-Based Novel Metal-Free Visible-Light Organophotoredox Catalyst for Atom-Transfer Radical Polymerization. J. Phys. Chem. A 2020, 124, 1068–1075. [Google Scholar] [CrossRef]
- Kanimozhi, C.; Yaacobi-Gross, N.; Chou, K.W.; Amassian, A.; Anthopoulos, T.D.; Patil, S. Diketopyrrolopyrrole-diketopyrrolopyrrole-based conjugated copolymer for high-mobility organic field-effect transistors. J. Am. Chem. Soc. 2012, 134, 16532–16535. [Google Scholar] [CrossRef]
- Xu, J.; Bi, S.; Tang, W.; Kang, Q.; Niu, D.; Hu, S.; Zhao, S.; Wang, L.; Xin, Z.; Zhang, X. Duplex trapping and charge transfer with polysulfides by a diketopyrrolopyrrole-based organic framework for high-performance lithium–sulfur batteries. J. Mater. Chem. A 2019, 7, 18100–18108. [Google Scholar] [CrossRef]
- Yang, L.; Yu, Y.; Feng, J.; Wu, J.; Jiang, L.; Dan, Y.; Qiu, Y. Charge transfer induced unexpected red-shift absorption of Zn and Cu porous coordination polymers based on electron-withdrawing ligand. J. Photochem. Photobio. A 2018, 350, 103–110. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Jafari-Asl, M.; Rezaei, B. Pyridine-functionalized graphene oxide, an efficient metal free electrocatalyst for oxygen reduction reaction. Electrochim. Acta 2016, 194, 95–103. [Google Scholar] [CrossRef]
- Lin, H.; Xu, Z.; Zhang, L.; Yang, X.; Ju, Q.; Xue, L.; Zhou, J.; Zhuo, S.; Wu, Y. Diketopyrrolopyrrole derivative functionalized graphene for high performance visible-light photodetectors. New J. Chem. 2017, 41, 4302–4307. [Google Scholar] [CrossRef]
- Bronstein, H.; Chen, Z.; Ashraf, R.S.; Zhang, W.; Du, J.; Durrant, J.R.; Tuladhar, P.S.; Song, K.; Watkins, S.E.; Geerts, Y.; et al. Thieno[3,2-b]thiophene-diketopyrrolopyrrole containing polymers for high-performance organic field-effect transistors and organic photovoltaic devices. J. Am. Chem. Soc. 2011, 133, 3272–3275. [Google Scholar] [CrossRef]
- Chen, D.; Feng, H.; Li, J. Graphene oxide: Preparation, functionalization, and electrochemical applications. Chem. Rev. 2012, 112, 6027–6053. [Google Scholar] [CrossRef]
- Song, J.; Wang, X.; Chang, C.T. Preparation and characterization of graphene oxide. J. Nanomater. 2014, 2014, 1–6. [Google Scholar] [CrossRef]
- Jahan, M.; Bao, Q.; Loh, K.P. Electrocatalytically active graphene-porphyrin MOF composite for oxygen reduction reaction. J. Am. Chem. Soc. 2012, 134, 6707–6713. [Google Scholar] [CrossRef]
- Claramunt, S.; Varea, A.; López-Díaz, D.; Velázquez, M.M.; Cornet, A.; Cirera, A. The importance of interbands on the interpretation of the raman spectrum of graphene oxide. J. Phys. Chem. C 2015, 119, 10123–10129. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, D.; Yang, M.; Xia, X.; Chen, H.; Chen, Y.; Liu, H. Pyridine-enriched graphene sheets for high volumetric performance supercapacitors. J. Solid State Electrochem. 2018, 22, 1921–1931. [Google Scholar] [CrossRef]
- Si, W.; Lei, W.; Hao, Q.; Xia, X.; Zhang, H.; Li, J.; Li, Q.; Cong, R. Facile synthesis of nitrogen-doped graphene derived from graphene oxide and vitamin B3 as high-performance sensor for imidacloprid determination. Electrochim. Acta 2016, 212, 784–790. [Google Scholar] [CrossRef]
- Lim, J.; Yeo, H.; Kim, S.G.; Park, O.K.; Yu, J.; Hwang, J.Y.; Goh, M.; Ku, B.C.; Lee, H.S.; You, N.H. Pyridine-functionalized graphene/polyimide nanocomposites; mechanical, gas barrier, and catalytic effects. Compos. Part B-Eng. 2017, 114, 280–288. [Google Scholar] [CrossRef]
- Vinothkumar, V.; Abinaya, M.; Chen, S.M. Ultrasonic assisted preparation of CoMoO4 nanoparticles modified electrochemical sensor for chloramphenicol determination. J. Solid State Chem. 2021, 302, 122392. [Google Scholar] [CrossRef]
- Jakubec, P.; Urbanova, V.; Medrikova, Z.; Zboril, R. Advanced Sensing of Antibiotics with Magnetic Gold Nanocomposite: Electrochemical Detection of Chloramphenicol. Chemistry 2016, 22, 14279–14284. [Google Scholar] [CrossRef]
- Hu, Q.; Li, G.; Liu, X.; Zhu, B.; Li, G.; Fan, L.; He, C. Coupling pentlandite nanoparticles and dual-doped carbon networks to yield efficient and stable electrocatalysts for acid water oxidation. J. Mater. Chem. A 2019, 7, 461–468. [Google Scholar] [CrossRef]
- Yang, B.; Shao, M.; Xu, Y.; Du, Y.; Yang, H.; Bin, D.; Liu, B.; Lu, H. Core-shell ZIF-8@ZIF-67-derived cobalt nanoparticles in situ grown on N-doped carbon nanotube polyhedra for ultrasensitive electrochemical detection of chloramphenicol. ChemElectroChem 2022, 9, 202200438. [Google Scholar] [CrossRef]
- Manjula, N.; Chen, S.-M. Synthesis of highly electroactive nanoflowers like manganese in oxide and electroanalytical application for chloramphenicol determination in milk and honey samples. J. Electroanal. Chem. 2021, 880, 114914. [Google Scholar] [CrossRef]
- Umesh, N.; Sathiyan, A.; Wang, S.F.; Elanthamilan, E.; Merlin, J.P.; Jesila, J.A. A simple chemical approach for synthesis of Sr2Co2O5 nanoparticles and its application in the detection of chloramphenicol and in energy storage systems. J. Electroanal. Chem. 2021, 880, 114911. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.C.; Zhang, J.W. A highly selective electrochemical sensor for chloramphenicol based on three-dimensional reduced graphene oxide architectures. Talanta 2016, 161, 567–573. [Google Scholar] [CrossRef]
- Niu, X.; Bo, X.; Guo, L. MOF-derived hollow NiCo2O4/C composite for simultaneous electrochemical determination of furazolidone and chloramphenicol in milk and honey. Food Chem. 2021, 364, 130368. [Google Scholar] [CrossRef]
- Jaysiva, G.; Manavalan, S.; Chen, S.M.; Veerakumar, P.; Keerthi, M.; Tu, H.S. MoN nanorod/sulfur-doped graphitic carbon nitride for electrochemical determination of chloramphenicol. ACS Sustainable Chem. Eng. 2020, 8, 11088–11098. [Google Scholar] [CrossRef]
- Sakthivel, M.; Sukanya, R.; Chen, S.M.; Ho, K.C. Synthesis and characterization of samarium-substituted molybdenum diselenide and its graphene oxide nanohybrid for enhancing the selective sensing of chloramphenicol in a milk sample. ACS Appl. Mater. Interfaces 2018, 10, 29712–29723. [Google Scholar] [CrossRef]
- Vilian, A.T.E.; Oh, S.Y.; Rethinasabapathy, M.; Umapathi, R.; Hwang, S.K.; Oh, C.W.; Park, B.; Huh, Y.S.; Han, Y.K. Improved conductivity of flower-like MnWO4 on defect engineered graphitic carbon nitride as an efficient electrocatalyst for ultrasensitive sensing of chloramphenicol. J. Hazard. Mater. 2020, 399, 122868. [Google Scholar] [CrossRef]



| Modified Electrode | Method | Linear range (uM) | LOD (uM) | Ref. |
|---|---|---|---|---|
| 3DRGO/GCE | DPV | 1–113 | 0.1500 | [45] |
| NiCo2O4@C/GCE | DPV | 0.5–320 | 0.0352 | [46] |
| MoN@S-GCN/GCE | DPV | 0.5–2450 | 0.0069 | [47] |
| GO/SmMoSe2/GCE | LSV | 0.01–244 | 0.0050 | [48] |
| g-C3N4/MnWO4/GCE | DPV | 0.004–0.071 | 0.0013 | [49] |
| Co3O4@rGO/GCE | i-t | 0.1–1500.0 | 0.1000 | [24] |
| PDPP/GO/GCE | i-t | 0.01–780 | 0.0016 | This work |
| Samples | Detected (μM) | Added (μM) | Found (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| Tap water | 0 | 0.1 | 0.0938 ± 0.002 | 93.8 | 1.9 |
| 0 | 10 | 10.55 ± 0.33 | 105.5 | 3.1 | |
| 0 | 100 | 99.3 ± 1.79 | 99.3 | 1.8 | |
| Milk | 0 | 0.1 | 0.0965 ± 0.004 | 96.5 | 4.1 |
| 0 | 10 | 10.83 ± 0.49 | 108.3 | 4.6 | |
| 0 | 100 | 105.5 ± 4.75 | 105.5 | 4.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, L.; Hao, J.; Yang, L.; Wang, J.; Huang, L.; Liu, K. A Pyridine Diketopyrrolopyrrole-Grafted Graphene Oxide Nanocomposite for the Sensitive Detection of Chloramphenicol by a Direct Electrochemical Method. Nanomaterials 2023, 13, 392. https://doi.org/10.3390/nano13030392
Jia L, Hao J, Yang L, Wang J, Huang L, Liu K. A Pyridine Diketopyrrolopyrrole-Grafted Graphene Oxide Nanocomposite for the Sensitive Detection of Chloramphenicol by a Direct Electrochemical Method. Nanomaterials. 2023; 13(3):392. https://doi.org/10.3390/nano13030392
Chicago/Turabian StyleJia, Lingpu, Juan Hao, Long Yang, Jun Wang, Lijuan Huang, and Kunping Liu. 2023. "A Pyridine Diketopyrrolopyrrole-Grafted Graphene Oxide Nanocomposite for the Sensitive Detection of Chloramphenicol by a Direct Electrochemical Method" Nanomaterials 13, no. 3: 392. https://doi.org/10.3390/nano13030392
APA StyleJia, L., Hao, J., Yang, L., Wang, J., Huang, L., & Liu, K. (2023). A Pyridine Diketopyrrolopyrrole-Grafted Graphene Oxide Nanocomposite for the Sensitive Detection of Chloramphenicol by a Direct Electrochemical Method. Nanomaterials, 13(3), 392. https://doi.org/10.3390/nano13030392










