Constructing the Sulfur-Doped CdO@In2O3 Nanofibers Ternary Heterojunction for Efficient Photocatalytic Hydrogen Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
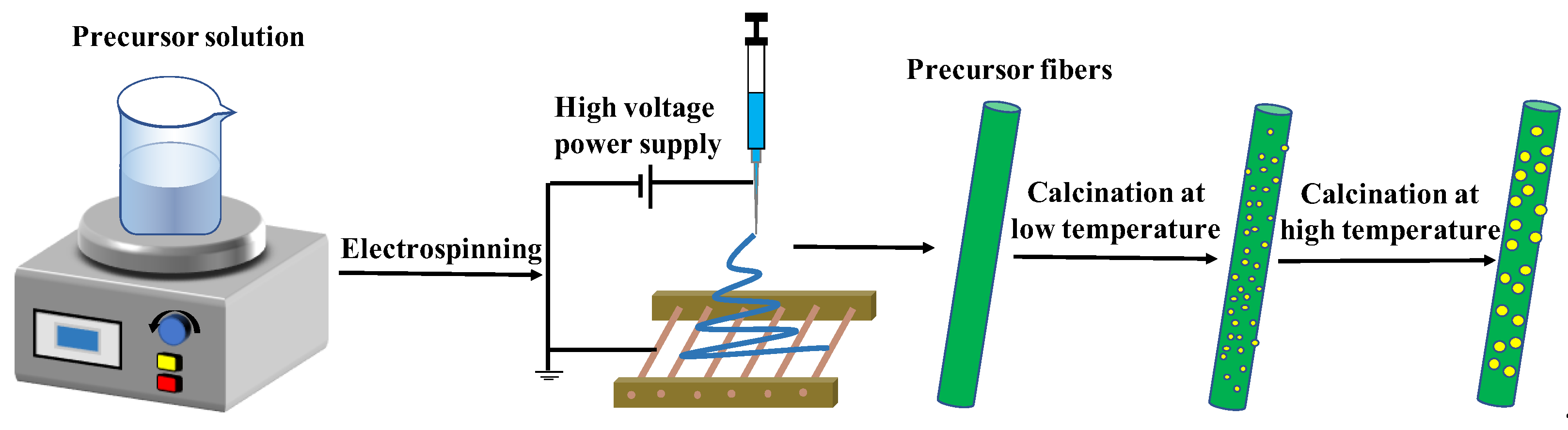
2.2. Preparation of Samples
2.3. Characterization of the Materials
2.4. The Preparation of Catalyst Films and Photoelectrochemical Measurements
2.5. Photocatalytic Performance Measurement
2.6. Theoretical Calculation
3. Results and Discussion
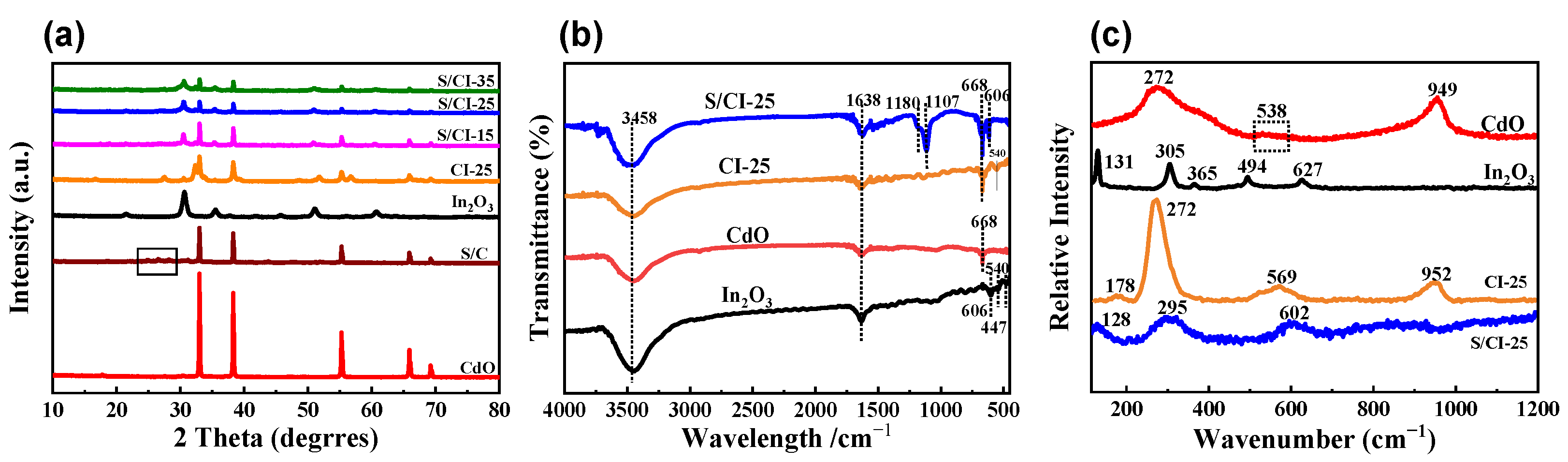
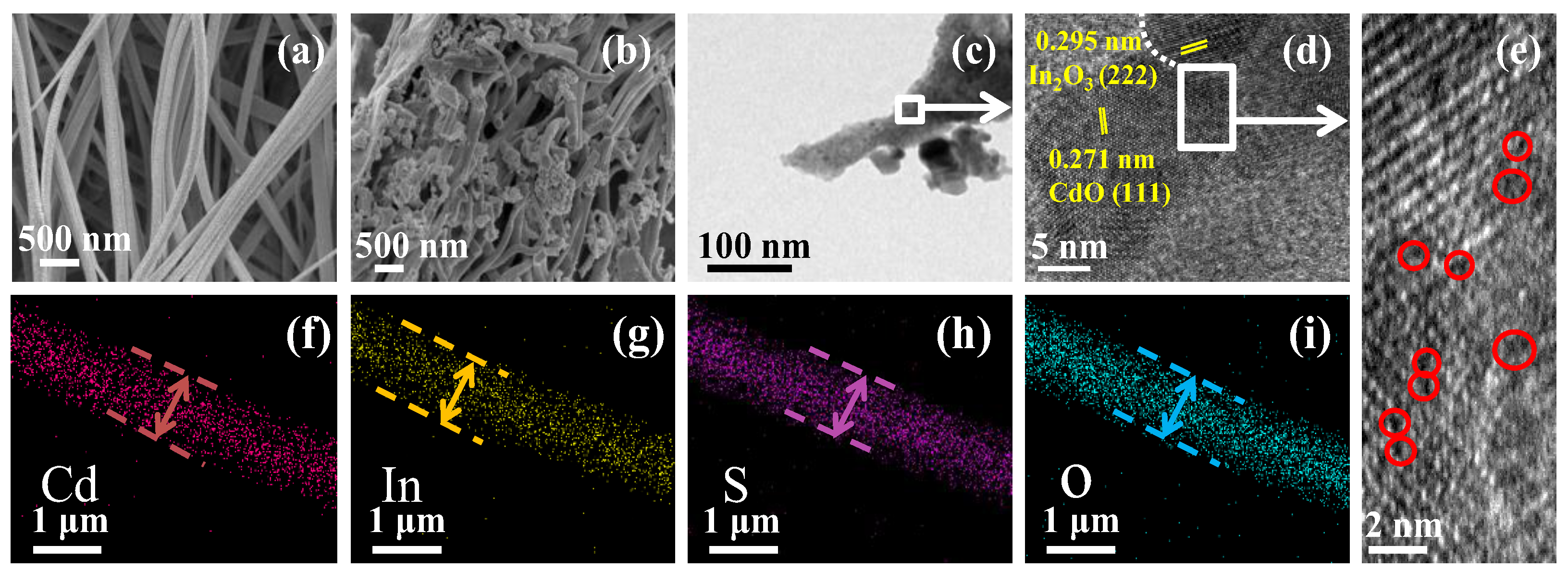
3.1. Structures and Morphologies Characterization of S/CdO@In2O3 Nanofibers
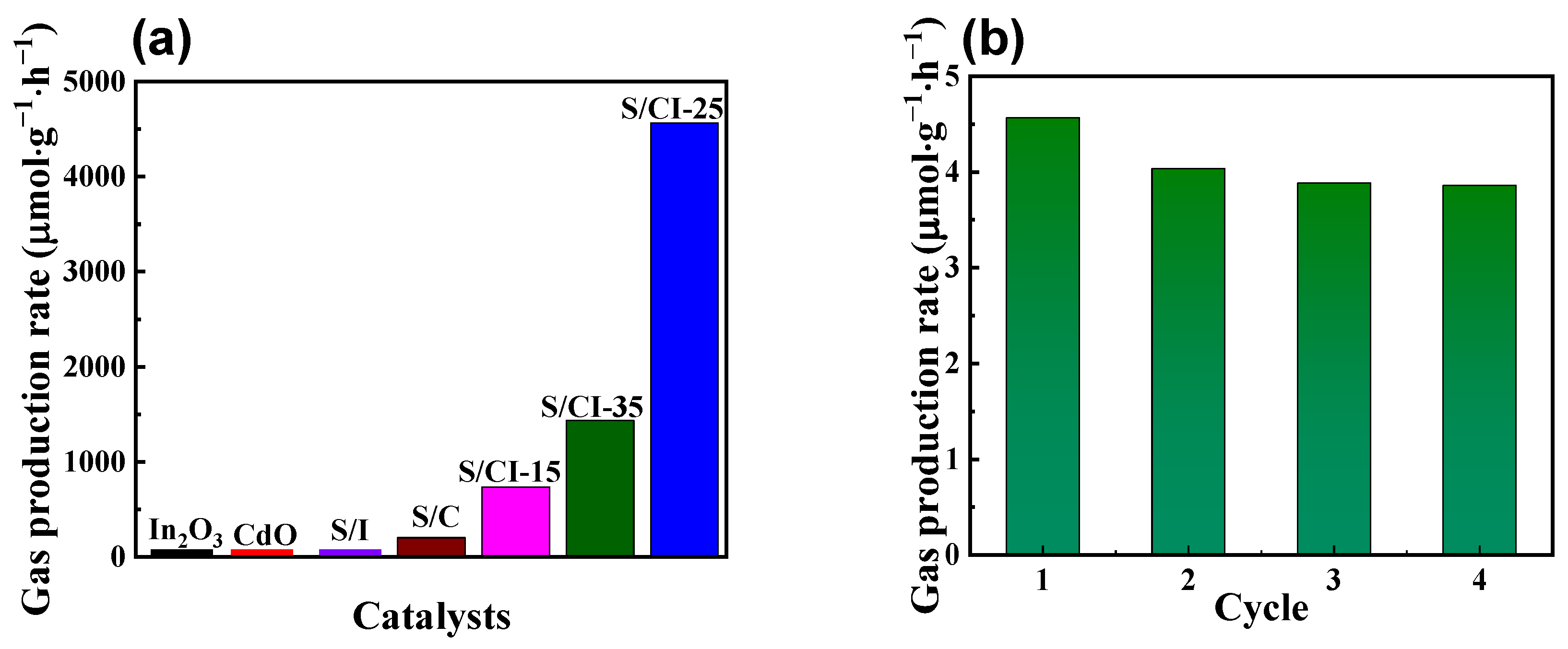
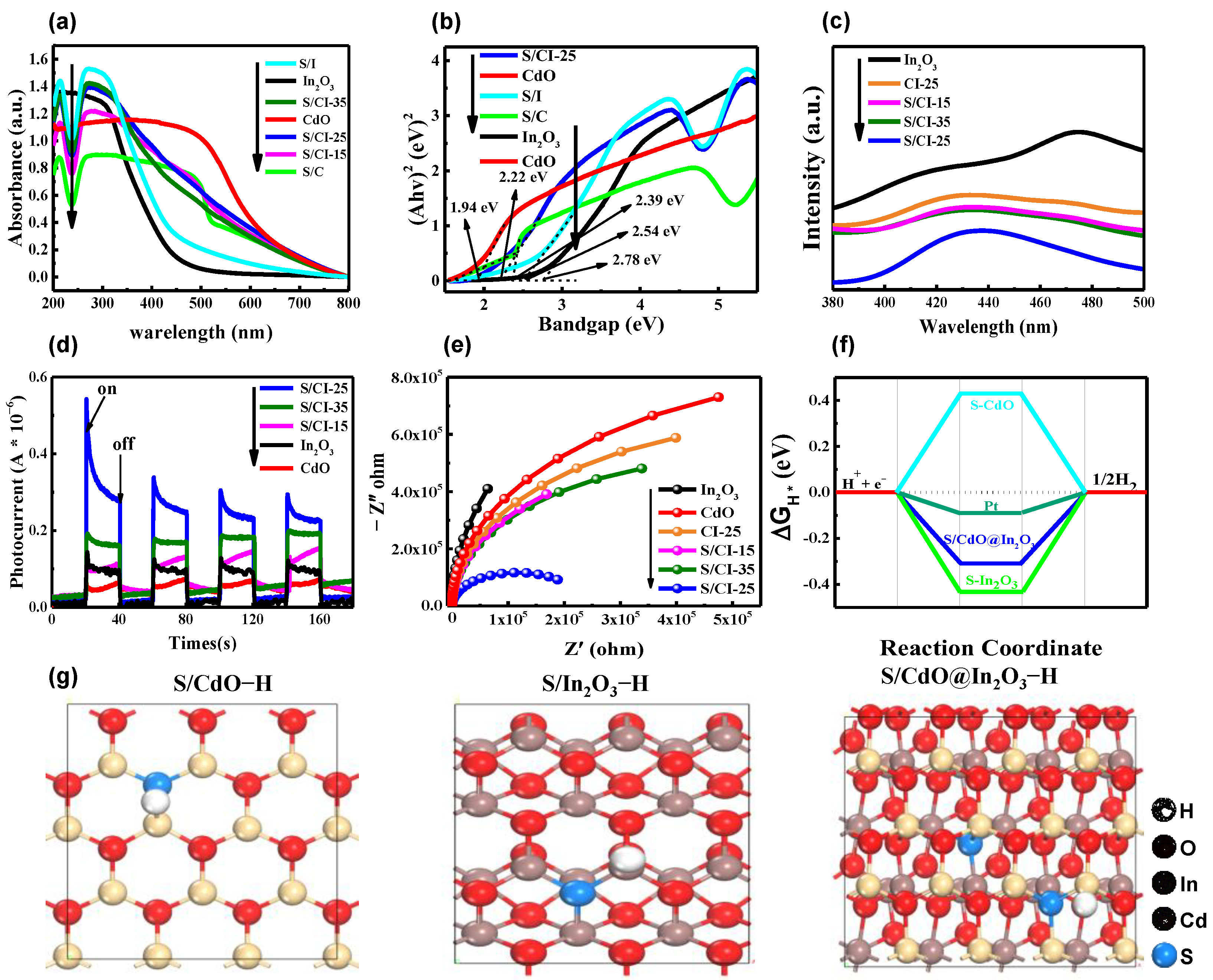
3.2. Photocatalytic Performance and Mechanism of Hydrogen Evolution Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, R.T.; Ren, Z.F.; Liang, Y.; Zhang, G.H.; Dittrich, T.; Liu, R.Z.; Liu, Y.; Zhao, Y.; Pang, S.; An, H.Y.; et al. Spatiotemporal imaging of charge transfer in photocatalyst particles. Nature 2022, 610, 296–301. [Google Scholar] [CrossRef]
- Di, J.; Xiong, J.; Li, H.; Liu, Z. Ultrathin 2D Photocatalysts: Electronic-Structure Tailoring, Hybridization, and Applications. Adv. Mater. 2018, 30, 1704548. [Google Scholar] [CrossRef]
- Cai, C.; Teng, Y.; Wu, J.-H.; Li, J.-Y.; Chen, H.-Y.; Chen, J.-H.; Kuang, D.-B. In Situ Photosynthesis of an MAPbI3/CoP Hybrid Heterojunction for Efficient Photocatalytic Hydrogen Evolution. Adv. Funct. Mater. 2020, 30, 2001478. [Google Scholar] [CrossRef]
- Teng, Y.; Gao, J.-X.; Li, J.-Y.; Chen, H.-Y.; Wang, X.-D.; Kuang, D.-B. Engineering multinary heterointerfaces in two-dimensional cobalt molybdenum phosphide hybrid nanosheets for efficient electrocatalytic water splitting. Sustain. Energy Fuels 2021, 5, 3458–3466. [Google Scholar] [CrossRef]
- Ganguly, P.; Harb, M.; Cao, Z.; Cavallo, L.G.; Breen, A.; Dervin, S.; Dionysiou, D.D.; Pillai, S.C. 2D Nanomaterials for Photocatalytic Hydrogen Production. ACS Energy Lett. 2019, 4, 1687–1709. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Liao, J.-F.; Chen, H.-Y.; Zhang, H.-H.; Li, J.-Y.; Wang, X.-D.; Kuang, D.-B. All-Solid-State Z-Scheme α-Fe2O3/Amine-RGO/CsPbBr3 Hybrids for Visible-Light-Driven Photocatalytic CO2 Reduction. Chem 2020, 6, 766–780. [Google Scholar] [CrossRef]
- Qin, Y.; Li, H.; Lu, J.; Ding, Y.; Ma, C.; Liu, X.; Meng, M.; Yan, Y. Fabrication of Bi2WO6/In2O3 photocatalysts with efficient photocatalytic performance for the degradation of organic pollutants: Insight into the role of oxygen vacancy and heterojunction. Adv. Powder Technol. 2020, 31, 2890–2900. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Q.; Xu, M.; Yuan, H.; Chen, Y.; Zhang, J.; Luo, K.; Zhang, J.; You, B. Novel and efficient synthesis of Ag-ZnO nanoparticles for the sunlight-induced photocatalytic degradation. Appl. Surf. Sci. 2019, 476, 632–640. [Google Scholar] [CrossRef]
- Meng, A.Y.; Zhang, L.Y.; Cheng, B.; Yu, J.G. Dual Cocatalysts in TiO2 Photocatalysis. Adv. Mater. 2019, 31, 1807660. [Google Scholar] [CrossRef]
- Munawar, T.; Yasmeen, S.; Hussain, F.; Mahmood, K.; Hussain, A.; Asghar, M.; Iqbal, F. Synthesis of novel heterostructured ZnO-CdO-CuO nanocomposite: Characterization and enhanced sunlight driven photocatalytic activity. Mater. Chem. Phys. 2020, 249, 122983. [Google Scholar] [CrossRef]
- Kipkorir, A.; DuBose, J.; Cho, J.; Kamat, P.V. CsPbBr3-CdS heterostructure: Stabilizing perovskite nanocrystals for photocatalysis. Chem. Sci. 2021, 12, 14815–14825. [Google Scholar] [CrossRef]
- Nagakawa, H.; Nagata, M. Photoreforming of Organic Waste into Hydrogen Using a Thermally Radiative CdOx/CdS/SiC Photocatalyst. ACS Appl. Mater. Interfaces 2021, 13, 47511–47519. [Google Scholar] [CrossRef]
- Hattali, O.A.A.; Marzouqi, F.A.; Mamari, S.A.; Kuvarega, A.T.; Selvaraj, R. CdO nanoplates for photocatalytic degradation of Levofloxacin and Nizatidine under natural solar light irradiation. Inorg. Chem. Comm. 2022, 146, 110071. [Google Scholar] [CrossRef]
- Upadhyay, G.K.; Kumar, V.; Purohit, L.P. Optimized CdO:TiO2 nanocomposites for heterojunction solar cell applications. J. Alloy. Compd. 2021, 856, 157453. [Google Scholar] [CrossRef]
- Mohanraj, K.; Balasubramanian, D.; Chandrasekaran, J. Synthesis and characterization of ruthenium-doped CdO nanoparticle and its n-RuCdO/p-Si junction diode application. J. Alloy. Compd. 2019, 779, 762–775. [Google Scholar] [CrossRef]
- Zhou, G.S.; Cheng, Y.; Yu, Z.H.; Liu, X.L.; Chen, D.H.; Wang, J.Q.; Hang, Y.; Xu, Y.R.; Li, C.X.; Lu, Z.Y. Regulation of coordination and doping environment via target molecular transformation for boosting selective photocatalytic ability. Chem. Commun. 2022, 58, 10036–10039. [Google Scholar] [CrossRef]
- Yan, T.J.; Li, N.; Wang, L.L.; Ran, W.G.; Duchesne, P.N.; Wan, L.L.; Nguyen, N.T.; Wang, L.; Xia, M.K.; Ozin, G.A. Bismuth atom tailoring of indium oxide surface frustrated Lewis pairs boosts heterogeneous CO2 photocatalytic hydrogenation. Nat. Commun. 2020, 11, 6095. [Google Scholar] [CrossRef]
- Meng, M.; Wang, L.W.; Li, C.Y.; Xu, K.; Chen, Y.Y.; Li, J.T.; Gan, Z.X.; Yuan, H.L.; Liu, L.Z.; Li, J. Boosting charge separation on epitaxial In2O3 octahedron-nanowire crystal facet-based homojunctions for robust photoelectrochemical water splitting. Appl. Catal. B 2023, 321, 122071. [Google Scholar] [CrossRef]
- Reshak, A.H. Quantum dots in photocatalytic applications: Efficiently enhancing visible light photocatalytic activity by integrating CdO quantum dots as sensitizers. Phys. Chem. Chem. Phys. 2017, 19, 24915–24927. [Google Scholar] [CrossRef]
- Wang, S.; Guan, B.Y.; Lu, Y.; Lou, X.W.D. Formation of Hierarchical In2S3-CdIn2S3 Heterostructured Nanotubes for Efficient and Stable Visible Light CO2 Reduction. J. Am. Chem. Soc. 2017, 139, 17305–17308. [Google Scholar] [CrossRef]
- Xiao, Z.H.; Xiao, J.; Sun, Q.; Wang, Y.F.; Pan, L.; Shi, C.X.; Zhang, X.W.; Zou, J.-J. Interface Engineering of Conjugated Polymer-Based Composites for Photocatalysis. Chem. Eur. J. 2022, 28, e202202593. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-D.; Huang, Y.-H.; Liao, J.-F.; Jiang, Y.; Zhou, L.; Zhang, X.-Y.; Chen, H.-Y.; Kuang, D.-B. In Situ Construction of a Cs2SnI6 Perovskite Nanocrystal/SnS2 Nanosheet Heterojunction with Boosted Interfacial Charge Transfer. J. Am. Chem. Soc. 2019, 141, 13434–13441. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Li, K.; Wu, X.; Wang, N.; Li, X.; Li, Q.; Li, L.; Lv, K. Dramatic promotion of visible-light photoreactivity of TiO2 hollow microspheres towards NO oxidation by introduction of oxygen vacancy. Appl. Catal. B 2019, 256, 117860. [Google Scholar] [CrossRef]
- Xu, Y.-F.; Yang, M.-Z.; Chen, B.-X.; Wang, X.-D.; Chen, H.-Y.; Kuang, D.-B.; Su, C.-Y. A CsPbBr3 Perovskite Quantum Dot/Graphene Oxide Composite for Photocatalytic CO2 Reduction. J. Am. Chem. Soc. 2017, 139, 5660–5663. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Huan, J.; Hao, N.; Qian, J.; Mao, H.; Wang, K. Engineering of Heterojunction-Mediated Biointerface for Photoelectrochemical Aptasensing: Case of Direct Z-Scheme CdTe-Bi2S3 Heterojunction with Improved Visible-Light-Driven Photoelectrical Conversion Efficiency. ACS Appl. Mater. Inter. 2017, 9, 18369–18376. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.; Yang, L.; Zhang, L. Z-Scheme 2D/3D hierarchical MoS2@CoMoS4 flower-shaped arrays with enhanced full spectrum light photoelectrocatalytic activity for H2O2/p-aminophenol production and contaminant degradation. J. Mater. Chem. A 2020, 8, 25890–25903. [Google Scholar] [CrossRef]
- Yang, L.; Hu, Y.; Zhang, L. Architecting Z-scheme Bi2S3@CoO with 3D chrysanthemums-like architecture for both photoeletro-oxidization and-reduction performance under visible light. Chem. Eng. J. 2019, 378, 122092. [Google Scholar] [CrossRef]
- Su, Y.; Yu, X.; Fu, X.; Zhu, Q.; Liu, L.; Zhu, Y.; Zhang, Y. Embedding Ag nanoparticles to construct BiOI/Ag/PANI with enhanced photoelectrocatalytic activity: A demonstration of the switch from type-II to Z-scheme. Electrochim. Acta 2020, 344, 136144. [Google Scholar] [CrossRef]
- He, S.; Yan, C.; Chen, X.Z.; Wang, Z.; Ouyang, T.; Guo, M.-L.; Liu, Z.Q. Construction of core-shell heterojunction regulating α-Fe2O3 layer on CeO2 nanotube arrays enables highly efficient Z-scheme photoelectrocatalysis. Appl. Catal. B 2020, 276, 119138. [Google Scholar] [CrossRef]
- He, S.; Xiao, K.; Chen, X.Z.; Li, T.; Ouyang, T.; Wang, Z.; Guo, M.L.; Liu, Z.Q. Enhanced photoelectrocatalytic activity of direct Z-scheme porous amorphous carbon nitride/manganese dioxide nanorod arrays. J. Colloid Interf. Sci. 2019, 557, 644–654. [Google Scholar] [CrossRef]
- She, X.; Wu, J.; Xu, H.; Zhong, J.; Wang, Y.; Song, Y.; Nie, K.; Liu, Y.; Yang, Y.; Rodrigues, M.-T.F.; et al. High Efficiency Photocatalytic Water Splitting Using 2D α-Fe2O3/g-C3N4 Z-Scheme Catalysts. Adv. Energy Mater. 2017, 7, 1700025. [Google Scholar] [CrossRef]
- Yuan, L.; Lu, K.-Q.; Zhang, F.; Fu, X.; Xu, Y.-J. Unveiling the interplay between light-driven CO2 photocatalytic reduction and carbonaceous residues decomposition: A case study of Bi2WO6-TiO2 binanosheets. Appl. Catal. B 2018, 237, 424–431. [Google Scholar] [CrossRef]
- Wang, Y.-Y.; Chen, Y.-X.; Barakat, T.; Zeng, Y.-J.; Liu, J.; Siffert, S.; Su, B.-L. Recent advances in non-metal doped titania for solar-driven photocatalytic/photoelectrochemical water-splitting. J. Energy Chem. 2022, 66, 529–559. [Google Scholar] [CrossRef]
- Wang, W.; Zeng, Z.; Zeng, G.; Zhang, C.; Xiao, R.; Zhou, C.; Xiong, W.; Yang, Y.; Lei, L.; Liu, Y.; et al. Sulfur doped carbon quantum dots loaded hollow tubular g-C3N4 as novel photocatalyst for destruction of Escherichia coli and tetracycline degradation under visible light. Chem. Eng. J. 2019, 378, 122132. [Google Scholar] [CrossRef]
- Shen, Q.Y.; Xu, T.Y.; Zhuang, G.-l.; Zhuang, Y.; Sun, L.M.; Han, X.G.; Wang, X.J.; Zhan., W.W. Spatially Separated Photoinduced Charge Carriers for the Enhanced Photocatalysis Over the One-Dimensional Yolk-Shell In2Se3@N-C Nanoreactor. ACS Catal. 2021, 11, 12931–12939. [Google Scholar] [CrossRef]
- Odhiambo, V.O.; Ongarbayeva, A.; Kéri, O.; Simon, L.; Szilágyi, I.M. Synthesis of TiO2/WO3 Composite Nanofibers by a Water-Based Electrospinning Process and Their Application in Photocatalysis. Nanomaterials 2020, 10, 882. [Google Scholar] [CrossRef]
- Shepa, I.; Mudra, E.; Dusza, J. Electrospinning through the prism of time. Mater. Today Chem. 2021, 21, 100543. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, S.; Ren, M.; Song, L.; Zhao, G. Preparation and characterization of hollow In2O3/Co3O4 heterostructured microribbons by electrospinning process. J. Sol-Gel Sci. Technol. 2011, 61, 169–174. [Google Scholar] [CrossRef]
- Zhao, F.; Lu, Q.; Liu, S. Preparation and characterization of In2O3/ZnO heterostructured microbelts by sol-gel combined with electrospinning method. J. Sol-Gel Sci. Technol. 2013, 69, 357–363. [Google Scholar] [CrossRef]
- Maafa, I.M.; Al-Enizi, A.M.; Abutaleb, A.; Zouli, N.I.; Ubaidullah, M.; Shaikh, S.F.; Al-Abdrabalnabi, M.A.; Yousef, A. One-pot preparation of CdO/ZnO core/shell nanofibers: An efficient photocatalyst. Alex. Eng. J. 2021, 60, 1819–1826. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kresse, G.; Joubert, D. From Ultrasoft Pseudopotentials to the Projector Augmented-Wave Method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector Augmented-Wave Method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Yang, L.; Hong, Y.; Liu, E.; Zhang, X.; Wang, L.; Lin, X.; Shi, J. Significant enhancement of photocatalytic H2 production simultaneous with dye degradation over Ni2P modified In2O3 nanocomposites. Sep. Purif. Technol. 2021, 263, 118366. [Google Scholar] [CrossRef]
- Velusamy, P.; Babu, R.R.; Ramamurthi, K.; Elangovan, E.; Viegas, J.; Dahlem, M.S.; Arivanandhan, M. Characterization of spray pyrolytically deposited high mobility praseodymium doped CdO thin films. Ceram. Int. 2016, 42, 12675–12685. [Google Scholar] [CrossRef]
- Shen, Q.; Sun, L.; Zhuang, Y.; Zhan, W.; Wang, X.; Han, X. Hollow Dodecahedral Structure of In2O3-In2S3 Heterojunction Encapsulated by N-Doped C as an Excellent Visible-Light-Active Photocatalyst for Organic Transformation. Inorg. Chem. 2020, 59, 17650–17658. [Google Scholar] [CrossRef]
- Sreekanth, T.V.M.; Dillip, G.R.; Lee, Y.R. Picrasma quassioides mediated cerium oxide nanostructures and their post-annealing treatment on the microstructural, morphological and enhanced catalytic performance. Ceram. Int. 2016, 42, 6610–6618. [Google Scholar] [CrossRef]
- Balamurugan, S.; Balu, A.R.; Narasimman, V.; Selvan, G.; Usharani, K.; Srivind, J.; Suganya, M.; Manjula, N.; Rajashree, C.; Nagarethinam, V.S. Multi metal oxide CdO-Al2O3-NiO nanocomposite-synthesis, photocatalytic and magnetic properties. Mater. Res. Express 2018, 6, 015022. [Google Scholar] [CrossRef]
- Akashi, R.; Naya, S.-I.; Negishi, R.; Tada, H. Two-Step Excitation-Driven Au-TiO2-CuO Three-Component Plasmonic Photocatalyst: Selective Aerobic Oxidation of Cyclohexylamine to Cyclohexanone. J. Phys. Chem. C 2016, 120, 27989–27995. [Google Scholar] [CrossRef]
- Kuang, P.Y.; Zheng, X.J.; Lin, J.; Huang, X.B.; Li, N.; Li, X.; Liu, Z.Q. Facile Construction of Dual p-n Junctions in CdS/Cu2O/ZnO Photoanode with Enhanced Charge Carrier Separation and Transfer Ability. ACS Omega 2017, 2, 852–863. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.G.; Ng, K.H.; Zhu, Y.C.; Zhang, Y.Z.; Li, Z.X.; Xu, S.; Huang, J.Y.; Hu, J.; Chen, Z.; Cai, W.L.; et al. Mo-activated VC as effective cocatalyst for an enhanced photocatalytic hydrogen evolution activity of CdS. Chem. Eng. J. 2023, 452, 139325. [Google Scholar] [CrossRef]
- Ren, J.-T.; Yuan, K.; Wu, K.; Zhou, L.; Zhang, Y.-W. A robust CdS/In2O3 hierarchical heterostructure derived from a metal-organic framework for efficient visible-light photocatalytic hydrogen production. Inorg. Chem. Front. 2019, 6, 366–375. [Google Scholar] [CrossRef]
- Margan, P.; Haghighi, M. Sono-coprecipitation synthesis and physicochemical characterization of CdO-ZnO nanophotocatalyst for removal of acid orange 7 from wastewater. Ultrason. Sonochem. 2018, 40, 323–332. [Google Scholar] [CrossRef]
- Du, Q.; Ma, J.; Shao, X.; Wang, W.; Tian, G. Core-shell structured TiO2@In2O3 for highly active visible-light photocatalysis. Chem. Phys. Lett. 2019, 714, 208–212. [Google Scholar] [CrossRef]
- McManamon, C.; O’Connell, J.; Delaney, P.; Rasappa, S.; Holmes, J.D.; Morris, M.A. A facile route to synthesis of S-doped TiO2 nanoparticles for photocatalytic activity. J. Mol. Catal. A Chem. 2015, 406, 51–57. [Google Scholar] [CrossRef]
- Ahamad, T.; Naushad, M.; Alshehri, S.M. Analysis of degradation pathways and intermediates products for ciprofloxacin using a highly porous photocatalyst. Chem. Eng. J. 2021, 417, 127969. [Google Scholar] [CrossRef]
- Ma, D.; Shi, J.W.; Zou, Y.; Fan, Z.; Shi, J.; Cheng, L.; Sun, D.; Wang, Z.; Niu, C. Multiple carrier-transfer pathways in a flower-like In2S3/CdIn2S4/In2O3 ternary heterostructure for enhanced photocatalytic hydrogen production. Nanoscale 2018, 10, 7860–7870. [Google Scholar] [CrossRef]
- Navarro, R.; Delvalle, F.; Fierro, J. Photocatalytic hydrogen evolution from CdS-ZnO-CdO systems under visible light irradiation: Effect of thermal treatment and presence of Pt and Ru cocatalysts. Int. J. Hydrog. Energy 2008, 33, 4265–4273. [Google Scholar] [CrossRef]
- Ma, D.; Shi, J.-W.; Sun, D.; Zou, Y.; Cheng, L.; He, C.; Wang, Z.; Niu, C. Au Nanoparticle and CdS Quantum Dot Codecoration of In2O3 Nanosheets for Improved H2 Evolution Resulting from Efficient Light Harvesting and Charge Transfer. ACS Sustain.Chem. Eng. 2018, 7, 547–557. [Google Scholar] [CrossRef]
- Munusamy, T.D.; Chin, S.Y.; Tarek, M.; Khan, M.M.R. Sustainable hydrogen production by CdO/exfoliated g-C3N4 via photoreforming of formaldehyde containing wastewater. Int. J. Hydrog. Energy 2021, 46, 30988–30999. [Google Scholar] [CrossRef]
- Han, L.; Jing, F.; Zhang, J.; Luo, X.-Z.; Zhong, Y.-L.; Wang, K.; Zang, S.-H.; Teng, D.-H.; Liu, Y.; Chen, J.; et al. Environment friendly and remarkably efficient photocatalytic hydrogen evolution based on metal organic framework derived hexagonal/cubic In2O3 phase-junction. Appl. Catal. B-Environ. 2021, 282, 119602. [Google Scholar] [CrossRef]
- Cao, S.-W.; Liu, X.-F.; Yuan, Y.-P.; Zhang, Z.-Y.; Liao, Y.-S.; Fang, J.; Loo, S.C.J.; Sum, T.C.; Xue, C. Solar-to-fuels conversion over In2O3/g-C3N4 hybrid photocatalysts. Appl. Catal. B-Environ. 2014, 147, 940–946. [Google Scholar] [CrossRef]
- Yang, J.; Wu, X.; Mei, Z.; Zhou, S.; Su, Y.; Wang, G. CVD Assisted Synthesis of Macro/Mesoporous TiO2/g-C3N4 S-Scheme Heterojunction for Enhanced Photocatalytic Hydrogen. Evol. Adv. Sustain. Syst. 2022, 6, 220005. [Google Scholar]
- Zhu, H.; Yang, X.; Zhang, M.; Li, Q.; Yang, J. Construction of 2D/2D TiO2/g-C3N4 nanosheet heterostructures with improved photocatalytic activity. Mater. Res. Bull. 2020, 125, 110765. [Google Scholar] [CrossRef]
- Gong, R.; Yang, L.; Qiu, S.; Chen, W.-T.; Wang, Q.; Xie, J.; Waterhouse, G.I.N.; Xu, J. A Nitrogen-Rich Covalent Triazine Framework as a Photocatalyst for Hydrogen Production. Adv. Polym. Tech. 2020, 2020, 7819049. [Google Scholar] [CrossRef]
- Xiao, M.; Jiao, Y.; Luo, B.; Wang, S.; Chen, P.; Lyu, M.; Du, A.; Wang, L. Understanding the roles of carbon in carbon/g-C3N4 based photocatalysts for H2 evolution. Nano Res. 2021. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhu, Z.; Yang, M.; Li, Y.; Lin, X.; Li, M.; Tang, S.; Teng, Y.; Kuang, D.-B. Constructing the Sulfur-Doped CdO@In2O3 Nanofibers Ternary Heterojunction for Efficient Photocatalytic Hydrogen Production. Nanomaterials 2023, 13, 401. https://doi.org/10.3390/nano13030401
Zhang H, Zhu Z, Yang M, Li Y, Lin X, Li M, Tang S, Teng Y, Kuang D-B. Constructing the Sulfur-Doped CdO@In2O3 Nanofibers Ternary Heterojunction for Efficient Photocatalytic Hydrogen Production. Nanomaterials. 2023; 13(3):401. https://doi.org/10.3390/nano13030401
Chicago/Turabian StyleZhang, Haiyan, Zi Zhu, Min Yang, Youji Li, Xiao Lin, Ming Li, Senpei Tang, Yuan Teng, and Dai-Bin Kuang. 2023. "Constructing the Sulfur-Doped CdO@In2O3 Nanofibers Ternary Heterojunction for Efficient Photocatalytic Hydrogen Production" Nanomaterials 13, no. 3: 401. https://doi.org/10.3390/nano13030401




