Emerging and Promising Multifunctional Nanomaterial for Textile Application Based on Graphitic Carbon Nitride Heterostructure Nanocomposites
Abstract
:1. Introduction
2. Ag/g-C3N4 Nanocomposites
2.1. Preparation and Photocatalytic Mechanism of Ag/g-C3N4 Nanocomposites
2.2. Ag/g-C3N4 Nanocomposites for Textile Application
3. TiO2/g-C3N4 Heterojunctions
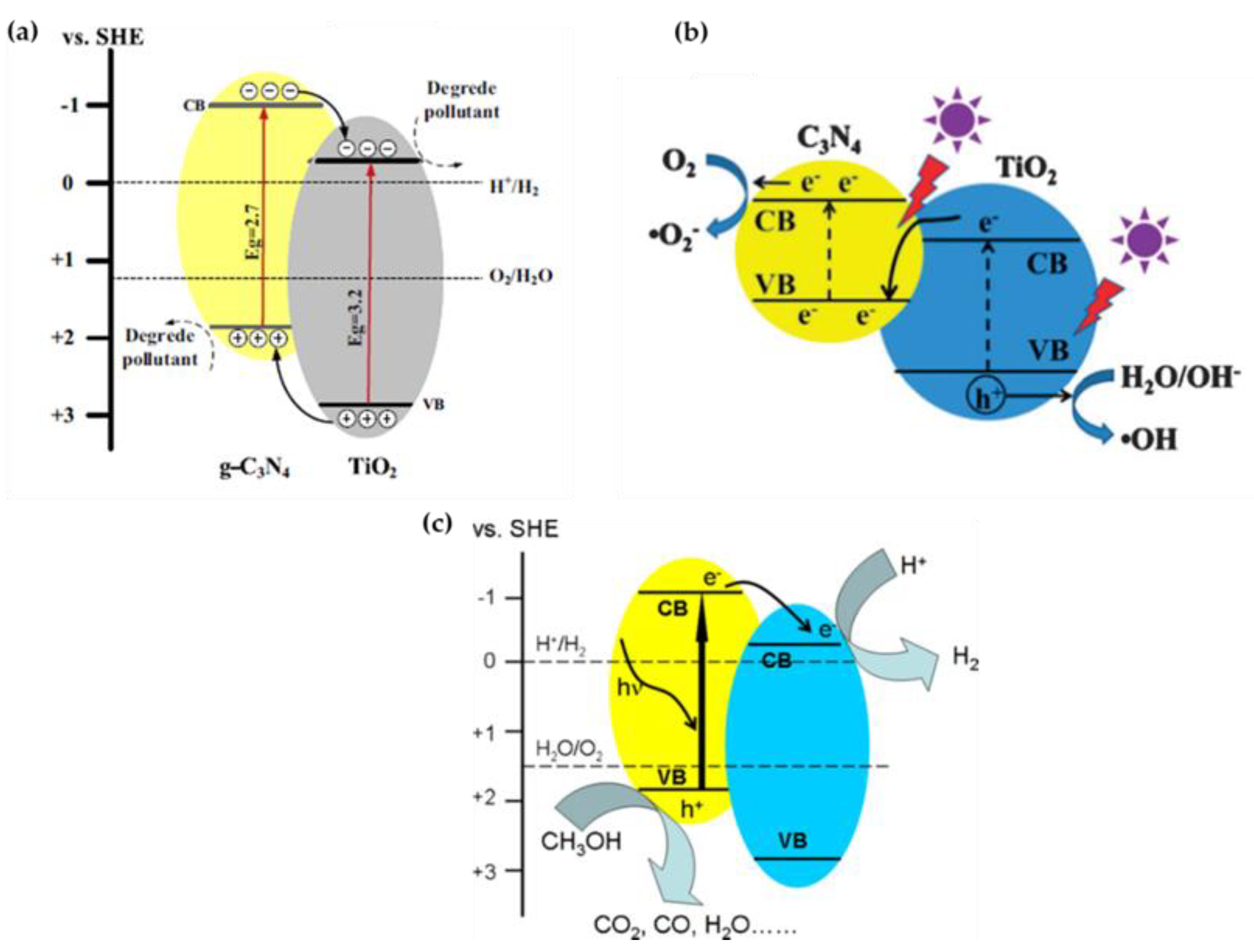
3.1. Preparation and Photocatalytic Mechanism of TiO2/g-C3N4 Nanocomposites
3.2. TiO2/g-C3N4 Nanocomposites for Textile Application
4. Ag/TiO2/g-C3N4 Heterostructure
Preparation and Photocatalytic Mechanism of Ag/TiO2/g-C3N4 Nanocomposites
5. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mamba, G.; Mishra, A.K. Graphitic carbon nitride (g-C3N4) nanocomposites: A new and exciting generation of visible light driven photocatalysts for environmental pollution remediation. Appl. Catal. B Environ. 2016, 198, 347–377. [Google Scholar] [CrossRef]
- Sudhaik, A.; Raizada, P.; Shandilya, P.; Jeong, D.Y.; Lim, J.H.; Singh, P. Review on fabrication of graphitic carbon nitride based efficient nanocomposites for photodegradation of aqueous phase organic pollutants. J. Ind. Eng. Chem. 2018, 67, 28–51. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S. A critical review on graphitic carbon nitride (g-C3N4)-based materials: Preparation, modification and environmental application. Coord. Chem. Rev. 2022, 453, 214338. [Google Scholar] [CrossRef]
- Hao, Q.; Jia, G.; Wei, W.; Vinu, A.; Wang, Y.; Arandiyan, H.; Ni, B.J. Graphitic carbon nitride with different dimensionalities for energy and environmental applications. Nano Res. 2020, 13, 18–37. [Google Scholar] [CrossRef] [Green Version]
- Ashritha, M.G.; Hareesh, K. A review on Graphitic Carbon Nitride based binary nanocomposites as supercapacitors. J. Energy Storage 2020, 32, 101840. [Google Scholar] [CrossRef]
- Ratshiedana, R.; Kuvarega, A.T.; Mishra, A.K. Titanium dioxide and graphitic carbon nitride–based nanocomposites and nanofibres for the degradation of organic pollutants in water: A review. Environ. Sci. Pollut. Res. 2021, 28, 10357–10374. [Google Scholar] [CrossRef]
- Chouhan, R.S.; Jerman, I.; Heath, D.; Bohm, S.; Gandhi, S.; Sadhu, V.; Baker, S.; Horvat, M. Emerging tri-s-triazine-based graphitic carbon nitride: A potential signal-transducing nanostructured material for sensor applications. Nano Sel. 2021, 2, 712–743. [Google Scholar] [CrossRef]
- Liang, Q.; Shao, B.; Tong, S.; Liu, Z.; Tang, L.; Liu, Y.; Cheng, M.; He, Q.; Wu, T.; Pan, Y.; et al. Recent advances of melamine self-assembled graphitic carbon nitride-based materials: Design, synthesis and application in energy and environment. Chem. Eng. J. 2021, 405, 126951. [Google Scholar] [CrossRef]
- Shen, Y.; Dos Santos-Garcia, A.J.; de Vidales, M.J.M. Graphitic carbon nitride-based composite in advanced oxidation processes for aqueous organic pollutants removal: A review. Processes 2021, 9, 66. [Google Scholar] [CrossRef]
- Tan, J.; Li, Z.; Li, J.; Wu, J.; Yao, X.; Zhang, T. Graphitic carbon nitride-based materials in activating persulfate for aqueous organic pollutants degradation: A review on materials design and mechanisms. Chemosphere 2021, 262, 127675. [Google Scholar] [CrossRef]
- Guo, R.-t.; Wang, J.; Bi, Z.-x.; Chen, X.; Hu, X.; Pan, W.-g. Recent advances and perspectives of g–C3N4–based materials for photocatalytic dyes degradation. Chemosphere 2022, 295, 133834. [Google Scholar] [CrossRef]
- Cao, S.W.; Yuan, Y.P.; Barber, J.; Loo, S.C.J.; Xue, C. Noble-metal-free g-C3N4/Ni(dmgH)2 composite for efficient photocatalytic hydrogen evolution under visible light irradiation. Appl. Surf. Sci. 2014, 319, 344–349. [Google Scholar] [CrossRef]
- Kang, Y.; Yang, Y.; Yin, L.C.; Kang, X.; Liu, G.; Cheng, H.M. An Amorphous Carbon Nitride Photocatalyst with Greatly Extended Visible-Light-Responsive Range for Photocatalytic Hydrogen Generation. Adv. Mater. 2015, 27, 4572–4577. [Google Scholar] [CrossRef]
- Zhang, G.; Lan, Z.A.; Wang, X. Surface engineering of graphitic carbon nitride polymers with cocatalysts for photocatalytic overall water splitting. Chem. Sci. 2017, 8, 5261–5274. [Google Scholar] [CrossRef] [Green Version]
- Lu, L.; Lv, Z.; Si, Y.; Liu, M.; Zhang, S. Recent progress on band and surface engineering of graphitic carbon nitride for artificial photosynthesis. Appl. Surf. Sci. 2018, 462, 693–712. [Google Scholar] [CrossRef]
- Singla, S.; Sharma, S.; Basu, S.; Shetti, N.P.; Reddy, K.R. Graphene/graphitic carbon nitride-based ternary nanohybrids: Synthesis methods, properties, and applications for photocatalytic hydrogen production. FlatChem 2020, 24, 100200. [Google Scholar] [CrossRef]
- Gkini, K.; Martinaiou, I.; Falaras, P. A review on emerging efficient and stable perovskite solar cells based on g-C3N4 nanostructures. Materials 2021, 14, 1679. [Google Scholar] [CrossRef]
- Chen, L.; Song, J. Tailored Graphitic Carbon Nitride Nanostructures: Synthesis, Modification, and Sensing Applications. Adv. Funct. Mater. 2017, 27, 1702695. [Google Scholar] [CrossRef]
- Liao, G.; He, F.; Li, Q.; Zhong, L.; Zhao, R.; Che, H.; Gao, H.; Fang, B. Emerging graphitic carbon nitride-based materials for biomedical applications. Prog. Mater. Sci. 2020, 112, 100666. [Google Scholar] [CrossRef]
- Che, S.; Zhang, L.; Wang, T.; Su, D.; Wang, C. Graphitic Carbon Nitride-Based Photocatalysts for Biological Applications. Adv. Sustain. Syst. 2022, 6, 2100294. [Google Scholar] [CrossRef]
- Rono, N.; Kibet, J.K.; Martincigh, B.S.; Nyamori, V.O. A review of the current status of graphitic carbon nitride. Crit. Rev. Solid State Mater. Sci. 2021, 46, 189–217. [Google Scholar] [CrossRef]
- Zheng, Y.; Lin, L.; Wang, B.; Wang, X. Graphitic Carbon Nitride Polymers toward Sustainable Photoredox Catalysis. Angew. Chem.—Int. Ed. 2015, 54, 12868–12884. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Blechert, S.; Antonietti, M. Polymeric graphitic carbon nitride for heterogeneous photocatalysis. ACS Catal. 2012, 2, 1596–1606. [Google Scholar] [CrossRef]
- Wang, A.; Wang, C.; Fu, L.; Wong-Ng, W.; Lan, Y. Recent advances of graphitic carbon nitride-based structures and applications in catalyst, sensing, imaging, and leds. Nano-Micro Lett. 2017, 9, 47. [Google Scholar] [CrossRef] [PubMed]
- Gaddam, S.K.; Pothu, R.; Boddula, R. Graphitic carbon nitride (g-C3N4) reinforced polymer nanocomposite systems—A review. Polym. Compos. 2020, 41, 430–442. [Google Scholar] [CrossRef]
- Akhundi, A.; Badiei, A.; Ziarani, G.M.; Habibi-Yangjeh, A.; Muñoz-Batista, M.J.; Luque, R. Graphitic carbon nitride-based photocatalysts: Toward efficient organic transformation for value-added chemicals production. Mol. Catal. 2020, 488, 110902. [Google Scholar] [CrossRef]
- Vasiljević, J.; Jerman, I.; Simončič, B. Graphitic carbon nitride as a new sustainable photocatalyst for textile functionalization. Polymers 2021, 13, 2568. [Google Scholar] [CrossRef]
- Jourshabani, M.; Lee, B.K.; Shariatinia, Z. From Traditional Strategies to Z-scheme Configuration in Graphitic Carbon Nitride Photocatalysts: Recent Progress and Future Challenges. Appl. Catal. B Environ. 2020, 276, 119157. [Google Scholar] [CrossRef]
- Wang, F.; Li, W.; Zhang, W.; Ye, R.; Tan, X. Facile fabrication of the Ag nanoparticles decorated graphitic carbon nitride photocatalyst film for indoor air purification under visible light. Build. Environ. 2022, 222, 109402. [Google Scholar] [CrossRef]
- elbakry, S.; Ali, M.E.A.; Abouelfadl, M.; Badway, N.A.; Salam, K.M.M. Effective removal of organic compounds using a novel cellulose acetate coated by PA/g-CN/Ag nanocomposite membranes. Surf. Interfaces 2022, 29, 101748. [Google Scholar] [CrossRef]
- Zedan, M.; Zedan, A.F.; Amin, R.M.; Li, X. Visible-light active metal nanoparticles@carbon nitride for enhanced removal of water organic pollutants. J. Environ. Chem. Eng. 2022, 10, 107780. [Google Scholar] [CrossRef]
- Li, M.; Zhang, J.; He, Y.; Zhang, X.; Cui, Z.; Fu, P.; Liu, M.; Shi, G.; Qiao, X.; Pang, X. Dual enhancement of carrier generation and migration on Au/g-C3N4 photocatalysts for highly-efficient broadband PET-RAFT polymerization. Polym. Chem. 2022, 13, 1022–1030. [Google Scholar] [CrossRef]
- Li, H.; Zhang, N.; Zhao, F.; Liu, T.; Wang, Y. Facile fabrication of a novel Au/phosphorus-doped g-C3N4 photocatalyst with excellent visible light photocatalytic activity. Catalysts 2020, 10, 701. [Google Scholar] [CrossRef]
- Olatunde, O.C.; Onwudiwe, D.C. A comparative study of the effect of graphene oxide, graphitic carbon nitride, and their composite on the photocatalytic activity of Cu3SnS4. Catalysts 2022, 12, 14. [Google Scholar] [CrossRef]
- Ahmad, K.; Kim, H. Design and preparation of g-C3N4/rGO modified screen printed electrode for hydrogen peroxide sensing application. Synth. Met. 2022, 286, 117047. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, D.; Chen, Q.; Chao, C.; Sun, J.; Dong, S.; Sun, Y. Synthesis of rGO/g-C3N4 for methyl orange degradation in activating peroxydisulfate under simulated solar light irradiation. J. Alloy. Compd. 2022, 907, 164500. [Google Scholar] [CrossRef]
- Hao, D.; Liu, J.; Sun, H.; Fu, B.; Liu, J.; Zhou, J. Integration of g-C3N4 into cellulose/graphene oxide foams for efficient photocatalytic Cr(VI) reduction. J. Phys. Chem. Solids 2022, 169, 110813. [Google Scholar] [CrossRef]
- Kobkeatthawin, T.; Chaveanghong, S.; Trakulmututa, J.; Amornsakchai, T.; Kajitvichyanukul, P.; Smith, S.M. Photocatalytic Activity of TiO2/g-C3N4 Nanocomposites for Removal of Monochlorophenols from Water. Nanomaterials 2022, 12, 2852. [Google Scholar] [CrossRef]
- Li, W.; Zhang, H.; Chen, W.; Yang, L.; Wu, H.; Mao, N. The effects of cotton cellulose on both energy band gap of g-C3N4–TiO2 nanoparticles and enhanced photocatalytic properties of cotton-g-C3N4–TiO2 composites. Cellulose 2022, 29, 193–212. [Google Scholar] [CrossRef]
- Girish, Y.R.; Udayabhanu; Alnaggar, G.; Hezam, A.; Nayan, M.B.; Nagaraju, G.; Byrappa, K. Facile and rapid synthesis of solar-driven TiO2/g-C3N4 heterostructure photocatalysts for enhanced photocatalytic activity. J. Sci. Adv. Mater. Devices 2022, 7, 100419. [Google Scholar] [CrossRef]
- Yu, B.; Miao, C.; Wang, D.; Li, H.; Sun, D.; Jiang, W.; Liu, C.; Che, G. Preparation of visible light responsive g-C3N4/H-TiO2 Z-scheme heterojunction with enhanced photocatalytic activity for RhB degradation. J. Mater. Sci. Mater. Electron. 2022, 33, 17587–17598. [Google Scholar] [CrossRef]
- Sundaram, I.M.; Kalimuthu, S.; P, G.P.; Sekar, K.; Rajendran, S. Hierarchical TiO2 spheroids decorated g-C3N4 nanocomposite for solar driven hydrogen production and water depollution. Int. J. Hydrog. Energy 2022, 47, 3709–3721. [Google Scholar] [CrossRef]
- Narkbuakaew, T.; Sattayaporn, S.; Saito, N.; Sujaridworakun, P. Investigation of the Ag species and synergy of Ag-TiO2 and g-C3N4 for the enhancement of photocatalytic activity under UV–Visible light irradiation. Appl. Surf. Sci. 2022, 573, 151617. [Google Scholar] [CrossRef]
- Ibrahim, I.; Belessiotis, G.V.; Antoniadou, M.; Kaltzoglou, A.; Sakellis, E.; Katsaros, F.; Sygellou, L.; Arfanis, M.K.; Salama, T.M.; Falaras, P. Silver decorated TiO2/g-C3N4 bifunctional nanocomposites for photocatalytic elimination of water pollutants under UV and artificial solar light. Results Eng. 2022, 14, 100470. [Google Scholar] [CrossRef]
- Liu, Y.; Yuan, Y.; Ni, S.; Liu, J.; Xie, S.; Liu, Y. Construction of g-C3N4/Ag/TiO2 Z-scheme photocatalyst and Its improved photocatalytic U(VI) reduction application in water. Water Sci. Technol. 2022, 85, 2639–2651. [Google Scholar] [CrossRef]
- Nekooie, R.; Ghasemi, J.B.; Badiei, A.; Shamspur, T.; Mostafavi, A.; Moradian, S. Design and synthesis of g-C3N4/(Cu/TiO2) nanocomposite for the visible light photocatalytic degradation of endosulfan in aqueous solutions. J. Mol. Struct. 2022, 1258, 132650. [Google Scholar] [CrossRef]
- Ding, P.; Ji, H.; Li, P.; Liu, Q.; Wu, Y.; Guo, M.; Zhou, Z.; Gao, S.; Xu, W.; Liu, W.; et al. Visible-light degradation of antibiotics catalyzed by titania/zirconia/graphitic carbon nitride ternary nanocomposites: A combined experimental and theoretical study. Appl. Catal. B Environ. 2022, 300, 120633. [Google Scholar] [CrossRef]
- Sattari, M.; Farhadian, M.; Reza Solaimany Nazar, A.; Moghadam, M. Enhancement of Phenol degradation, using of novel Z-scheme Bi2WO6/C3N4/TiO2 composite: Catalyst and operational parameters optimization. J. Photochem. Photobiol. A Chem. 2022, 431, 114065. [Google Scholar] [CrossRef]
- Rashid, M.M.; Simončič, B.; Tomšič, B. Recent advances in TiO2-functionalized textile surfaces. Surf. Interfaces 2021, 22, 100890. [Google Scholar] [CrossRef]
- Patnaik, S.; Sahoo, D.P.; Parida, K. An overview on Ag modified g-C3N4 based nanostructured materials for energy and environmental applications. Renew. Sustain. Energy Rev. 2018, 82, 1297–1312. [Google Scholar] [CrossRef]
- Kavitha, R.; Nithya, P.M.; Girish Kumar, S. Noble metal deposited graphitic carbon nitride based heterojunction photocatalysts. Appl. Surf. Sci. 2020, 508, 145142. [Google Scholar] [CrossRef]
- Simončič, B.; Klemenčič, D. Preparation and performance of silver as an antimicrobial agent for textiles: A review. Text. Res. J. 2016, 86, 210–223. [Google Scholar] [CrossRef]
- Yang, Y.; Guo, Y.; Liu, F.; Yuan, X.; Guo, Y.; Zhang, S.; Guo, W.; Huo, M. Preparation and enhanced visible-light photocatalytic activity of silver deposited graphitic carbon nitride plasmonic photocatalyst. Appl. Catal. B Environ. 2013, 142–143, 828–837. [Google Scholar] [CrossRef]
- Nagajyothi, P.C.; Pandurangan, M.; Vattikuti, S.V.P.; Tettey, C.O.; Sreekanth, T.V.M.; Shim, J. Enhanced photocatalytic activity of Ag/g-C3N4 composite. Sep. Purif. Technol. 2017, 188, 228–237. [Google Scholar] [CrossRef]
- Ye, M.; Wang, R.; Shao, Y.; Tian, C.; Zheng, Z.; Gu, X.; Wei, W.; Wei, A. Silver nanoparticles/graphitic carbon nitride nanosheets for improved visible-light-driven photocatalytic performance. J. Photochem. Photobiol. A Chem. 2018, 351, 145–153. [Google Scholar] [CrossRef]
- Paul, D.R.; Sharma, R.; Panchal, P.; Nehra, S.P.; Gupta, A.P.; Sharma, A. Synthesis, characterization and application of silver doped graphitic carbon nitride as photocatalyst towards visible light photocatalytic hydrogen evolution. Int. J. Hydrog. Energy 2020, 45, 23937–23946. [Google Scholar] [CrossRef]
- Luo, T.; Hu, X.; She, Z.; Wei, J.; Feng, X.; Chang, F. Synergistic effects of Ag-doped and morphology regulation of graphitic carbon nitride nanosheets for enhanced photocatalytic performance. J. Mol. Liq. 2021, 324, 114772. [Google Scholar] [CrossRef]
- Wen, K.; Wei, L.; Ren, Z.; Wang, B.; Lu, J. Enhanced photocatalytic degradation of cationic and anionic dyes by Ag-modified g-C3N4 composite: Insights on different mechanisms under visible light. J. Mater. Res. 2021, 36, 1549–1560. [Google Scholar] [CrossRef]
- Fontelles-Carceller, O.; Muñoz-Batista, M.J.; Fernández-García, M.; Kubacka, A. Interface Effects in Sunlight-Driven Ag/g-C3N4 Composite Catalysts: Study of the Toluene Photodegradation Quantum Efficiency. ACS Appl. Mater. Interfaces 2016, 8, 2617–2627. [Google Scholar] [CrossRef]
- Hak, C.H.; Sim, L.C.; Leong, K.H.; Lim, P.F.; Chin, Y.H.; Saravanan, P. M/g-C3N4 (M=Ag, Au, and Pd) composite: Synthesis via sunlight photodeposition and application towards the degradation of bisphenol A. Environ. Sci. Pollut. Res. 2018, 25, 25401–25412. [Google Scholar] [CrossRef]
- Pandiyarajan, C.; Rameshkumar, P.; Murugesan, S.; Selvaraj, M. Silver nanoparticles-supported graphitic-like carbon nitride for the electrochemical sensing of nitrobenzene and its derivatives. J. Mater. Sci. Mater. Electron. 2021, 32, 19912–19924. [Google Scholar] [CrossRef]
- Tian, C.; Tao, X.; Luo, S.; Qing, Y.; Lu, X.; She, J.; Wu, Y. Cellulose nanofibrils anchored Ag on graphitic carbon nitride for efficient photocatalysis under visible light. Environ. Sci. Nano 2018, 5, 2129–2143. [Google Scholar] [CrossRef]
- Song, Y.; Qi, J.; Tian, J.; Gao, S.; Cui, F. Construction of Ag/g-C3N4 photocatalysts with visible-light photocatalytic activity for sulfamethoxazole degradation. Chem. Eng. J. 2018, 341, 547–555. [Google Scholar] [CrossRef]
- Minh Tri, N.L.; Kim, J.; Giang, B.L.; Al Tahtamouni, T.M.; Huong, P.T.; Lee, C.; Viet, N.M.; Quang Trung, D. Ag-doped graphitic carbon nitride photocatalyst with remarkably enhanced photocatalytic activity towards antibiotic in hospital wastewater under solar light. J. Ind. Eng. Chem. 2019, 80, 597–605. [Google Scholar] [CrossRef]
- Starukh, H.; Koštejn, M.; Matějka, V.; Praus, P. Graphitic Carbon Nitride as a Platform for the Synthesis of Silver Nanoclusters. Nanoscale Res. Lett. 2021, 16, 166. [Google Scholar] [CrossRef]
- Wang, J.; Cong, J.; Xu, H.; Wang, J.; Liu, H.; Liang, M.; Gao, J.; Ni, Q.; Yao, J. Facile Gel-Based Morphological Control of Ag/g-C3N4 Porous Nanofibers for Photocatalytic Hydrogen Generation. ACS Sustain. Chem. Eng. 2017, 5, 10633–10639. [Google Scholar] [CrossRef]
- Muñoz-Batista, M.J.; Fontelles-Carceller, O.; Ferrer, M.; Fernández-García, M.; Kubacka, A. Disinfection capability of Ag/g-C3N4 composite photocatalysts under UV and visible light illumination. Appl. Catal. B Environ. 2016, 183, 86–95. [Google Scholar] [CrossRef]
- Khan, M.E.; Han, T.H.; Khan, M.M.; Karim, M.R.; Cho, M.H. Environmentally sustainable fabrication of Ag@g-C3N4 nanostructures and their multifunctional efficacy as antibacterial agents and hotocatalysts. ACS Appl. Nano Mater. 2018, 1, 2912–2922. [Google Scholar] [CrossRef]
- Mohanraj, J.; Durgalakshmi, D.; Saravanan, R. Water-soluble graphitic carbon nitride for clean environmental applications. Environ. Pollut. 2021, 269, 116172. [Google Scholar] [CrossRef]
- Cheng, Q.; He, Y.; Ge, Y.; Zhou, J.; Song, G. Ultrasensitive detection of heparin by exploiting the silver nanoparticle-enhanced fluorescence of graphitic carbon nitride (g-C3N4) quantum dots. Microchim. Acta 2018, 185, 332. [Google Scholar] [CrossRef]
- Lu, Q.; Wang, H.; Liu, Y.; Hou, Y.; Li, H.; Zhang, Y. Graphitic carbon nitride nanodots: As reductant for the synthesis of silver nanoparticles and its biothiols biosensing application. Biosens. Bioelectron. 2017, 89, 411–416. [Google Scholar] [CrossRef]
- Veerakumar, P.; Rajkumar, C.; Chen, S.M.; Thirumalraj, B.; Lin, K.C. Ultrathin 2D graphitic carbon nitride nanosheets decorated with silver nanoparticles for electrochemical sensing of quercetin. J. Electroanal. Chem. 2018, 826, 207–216. [Google Scholar] [CrossRef]
- Meng, P.; Xu, J. Colorful Silver/Carbon Nitride Composites Obtained by Photoreduction. Chem. Res. Chin. Univ. 2020, 36, 1136–1140. [Google Scholar] [CrossRef]
- Xin, J.; Li, F.; Li, Z.; Zhao, J.; Wang, Y. Controlling the band structure and photocatalytic performance of single atom Ag/C3N4 catalysts by variation of silver concentration. Inorg. Chem. Front. 2022, 9, 302–309. [Google Scholar] [CrossRef]
- Fu, Y.; Huang, T.; Zhang, L.; Zhu, J.; Wang, X. Ag/g-C3N4 catalyst with superior catalytic performance for the degradation of dyes: A borohydride-generated superoxide radical approach. Nanoscale 2015, 7, 13723–13733. [Google Scholar] [CrossRef]
- Zhao, S.; Wu, J.; Xu, Y.; Wang, Z.; Han, Y.; Zhang, X. Ag2CO3-derived Ag/g-C3N4 composite with enhanced visible-light photocatalytic activity for hydrogen production from water splitting. Int. J. Hydrogen Energy 2020, 45, 20851–20858. [Google Scholar] [CrossRef]
- Khan, M.R.; Chuan, T.W.; Yousuf, A.; Chowdhury, M.N.K.; Cheng, C.K. Schottky barrier and surface plasmonic resonance phenomena towards the photocatalytic reaction: Study of their mechanisms to enhance photocatalytic activity. Catal. Sci. Technol. 2015, 5, 2522–2531. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.P.; Sharma, A.S.; Sharma, V.S.; Shimpi, N.G. Polyacrylonitrile Nanofibers Incorporating Silver-Decorated Graphitic Carbon Nitride for the Visible-Light-Activated Selective Oxidation of Styrene, Benzylic Methylene Groups, and Benzene. ACS Appl. Nano Mater. 2020, 3, 1922–1933. [Google Scholar] [CrossRef]
- Amedlous, A.; Majdoub, M.; Anfar, Z.; Amaterz, E. Self-supporting g-C3N4 nanosheets/Ag nanoparticles embedded onto polyester fabric as “dip-catalyst” for synergic 4-nitrophenol hydrogenation. Catalysts 2021, 11, 1533. [Google Scholar] [CrossRef]
- Bayan, S.; Pal, S.; Ray, S.K. Interface engineered silver nanoparticles decorated g-C3N4 nanosheets for textile based triboelectric nanogenerators as wearable power sources. Nano Energy 2022, 94, 106928. [Google Scholar] [CrossRef]
- Yan, H.; Yang, H. TiO2-g-C3N4 composite materials for photocatalytic H2 evolution under visible light irradiation. J. Alloy. Compd. 2011, 509, L26–L29. [Google Scholar] [CrossRef]
- Zhao, S.; Chen, S.; Yu, H.; Quan, X. g-C3N4/TiO2 hybrid photocatalyst with wide absorption wavelength range and effective photogenerated charge separation. Sep. Purif. Technol. 2012, 99, 50–54. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, T.; Li, B.; Jiang, S.; Zhang, X.; Hai, L.; Chen, X.; Wu, W. An ingenious strategy of preparing TiO2/g-C3N4 heterojunction photocatalyst: In situ growth of TiO2 nanocrystals on g-C3N4 nanosheets via impregnation-calcination method. Appl. Surf. Sci. 2018, 433, 963–974. [Google Scholar] [CrossRef]
- Jang, E.; Kim, W.J.; Kim, D.W.; Hong, S.H.; Ali, I.; Park, Y.M.; Park, T.J. Atomic layer deposition with rotary reactor for uniform hetero-junction photocatalyst, g-C3N4@TiO2 core-shell structures. RSC Adv. 2019, 9, 33180–33186. [Google Scholar] [CrossRef] [PubMed]
- Mei, P.; Wang, H.; Guo, H.; Zhang, N.; Ji, S.; Ma, Y.; Xu, J.; Li, Y.; Alsulami, H.; Alhodaly, M.S.; et al. The enhanced photodegradation of bisphenol A by TiO2/C3N4 composites. Environ. Res. 2020, 182, 109090. [Google Scholar] [CrossRef]
- Porcu, S.; Castellino, M.; Roppolo, I.; Carbonaro, C.M.; Palmas, S.; Mais, L.; Casula, M.F.; Neretina, S.; Hughes, R.A.; Secci, F.; et al. Highly efficient visible light phenyl modified carbon nitride/TiO2 photocatalyst for environmental applications. Appl. Surf. Sci. 2020, 531, 147394. [Google Scholar] [CrossRef]
- Ma, J.; Wang, X.; Zong, X.; Li, L.; Sun, H.; He, P.; Yang, Y.; Ding, Y.; Han, Y.; Fan, X. Photoelectrochemical properties of TiO2/g-C3N4 composited electrodes fabricated by a co-electrodeposited method. J. Phys. D. Appl. Phys. 2021, 54, 145104. [Google Scholar] [CrossRef]
- Ranjithkumar, R.; Lakshmanan, P.; Devendran, P.; Nallamuthu, N.; Sudhahar, S.; Kumar, M.K. Investigations on effect of graphitic carbon nitride loading on the properties and electrochemical performance of g-C3N4/TiO2 nanocomposites for energy storage device applications. Mater. Sci. Semicond. Process. 2021, 121, 105328. [Google Scholar] [CrossRef]
- Khan, I.; Khan, S.; Chen, J.; Shah, S.A.; Yuan, A. Biological Inspired Green Synthesis of TiO2 Coupled g-C3N4 Nanocomposites and Its Improved Activities for Sulfadiazine and Bisphenol A Degradation. J. Clust. Sci. 2022. [Google Scholar] [CrossRef]
- Liu, Y.; Ren, M.; Zhang, X.; Yang, G.; Qin, L.; Meng, J.; Guo, Y. Supramolecule self-assembly approach to direct Z-scheme TiO2/g-C3N4 heterojunctions for efficient photocatalytic degradation of emerging phenolic pollutants. Appl. Surf. Sci. 2022, 593, 153401. [Google Scholar] [CrossRef]
- Yu, J.; Wang, S.; Low, J.; Xiao, W. Enhanced photocatalytic performance of direct Z-scheme g-C3N4-TiO2 photocatalysts for the decomposition of formaldehyde in air. Phys. Chem. Chem. Phys. 2013, 15, 16883–16890. [Google Scholar] [CrossRef]
- Gu, L.; Wang, J.; Zou, Z.; Han, X. Graphitic-C3N4-hybridized TiO2 nanosheets with reactive {0 0 1} facets to enhance the UV- and visible-light photocatalytic activity. J. Hazard. Mater. 2014, 268, 216–223. [Google Scholar] [CrossRef]
- Li, H.; Zhou, L.; Wang, L.; Liu, Y.; Lei, J.; Zhang, J. In situ growth of TiO2 nanocrystals on g-C3N4 for enhanced photocatalytic performance. Phys. Chem. Chem. Phys. 2015, 17, 17406–17412. [Google Scholar] [CrossRef]
- Tong, Z.; Yang, D.; Xiao, T.; Tian, Y.; Jiang, Z. Biomimetic fabrication of g-C3N4/TiO2 nanosheets with enhanced photocatalytic activity toward organic pollutant degradation. Chem. Eng. J. 2015, 260, 117–125. [Google Scholar] [CrossRef]
- Huang, S.; Zhong, J.; Li, J.; Chen, J.; Xiang, Z.; Hu, W.; Li, M. Z-scheme TiO2/g-C3N4 composites with improved solar-driven photocatalytic performance deriving from remarkably efficient separation of photo-generated charge pairs. Mater. Res. Bull. 2016, 84, 65–70. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Li, H.; Chen, C. Fabrication of g-C3N4/TiO2 composite photocatalyst with extended absorption wavelength range and enhanced photocatalytic performance. J. Photochem. Photobiol. A Chem. 2016, 317, 151–160. [Google Scholar] [CrossRef]
- Kočí, K.; Reli, M.; Troppová, I.; Šihor, M.; Kupkova, J.; Kustrovski, P.; Praus, P. Photocatalytic decomposition of N2O over TiO2/g-C3N4 photocatalysts heterojunction. Appl. Surf. Sci. 2017, 396, 1685–1695. [Google Scholar] [CrossRef]
- Sun, M.; Shen, S.; Wu, Z.; Tang, Z.; Shen, J.; Yang, J. Rice spike-like g-C3N4/TiO2 heterojunctions with tight-binding interface by using sodium titanate ultralong nanotube as precursor and template. Ceram. Int. 2018, 44, 8125–8132. [Google Scholar] [CrossRef]
- Etacheri, V.; Di Valentin, C.; Schneider, J.; Bahnemann, D.; Pillai, S.C. Visible-light activation of TiO2 photocatalysts: Advances in theory and experiments. J. Photochem. Photobiol. C Photochem. Rev. 2015, 25, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Nam, Y.; Lim, J.H.; Ko, K.C.; Lee, J.Y. Photocatalytic activity of TiO2 nanoparticles: A theoretical aspect. J. Mater. Chem. A 2019, 7, 13833–13859. [Google Scholar] [CrossRef]
- Su, F.; Mathew, S.C.; Lipner, G.; Fu, X.; Antonietti, M.; Blechert, S.; Wang, X. mpg-C3N4-catalyzed selective oxidation of alcohols using O2 and visible light. J. Am. Chem. Soc. 2010, 132, 16299–16301. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Zhang, L.; Yu, J.; Wageh, S.; Al-Ghamdi, A.A.; Jaroniec, M. Direct Z-scheme photocatalysts: Principles, synthesis, and applications. Mater. Today 2018, 21, 1042–1063. [Google Scholar] [CrossRef]
- Low, J.; Yu, J.; Jaroniec, M.; Wageh, S.; Al-Ghamdi, A.A. Heterojunction Photocatalysts. Adv. Mater. 2017, 29, 1601694. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Bahadur, N.; Kong, L.; O’Dell, L.A.; Merenda, A.; Dumée, L.F. Engineering Schottky-like and heterojunction materials for enhanced photocatalysis performance—A review. Mater. Adv. 2022, 3, 2309–2323. [Google Scholar] [CrossRef]
- Wang, Y.; Ding, X.; Zhang, P.; Wang, Q.; Zheng, K.; Chen, L.; Ding, J.; Tian, X.; Zhang, X. Convenient and Recyclable TiO2/g-C3N4 Photocatalytic Coating: Layer-by-Layer Self-assembly Construction on Cotton Fabrics Leading to Improved Catalytic Activity under Visible Light. Ind. Eng. Chem. Res. 2019, 58, 3978–3987. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, W.; Shen, H.; Gu, Y.; Xu, T.; Zhu, Z.; Wang, G.; Chen, W. Solar-driven efficient degradation of emerging contaminants by g-C3N4-shielding polyester fiber/TiO2 composites. Appl. Catal. B Environ. 2019, 258, 117960. [Google Scholar] [CrossRef]
- Xiong, Q.; Chen, Y.; Xu, T.; Zhu, Z.; Chen, W.; Lu, W. Highly efficient purification of emerging pollutants and bacteria in natural water by g-C3N4-sheltered fibers containing TiO2. Appl. Surf. Sci. 2021, 559, 149839. [Google Scholar] [CrossRef]
- Ghafoor, S.; Inayat, A.; Aftab, F.; Duran, H.; Kirchhoff, K.; Waseem, S.; Arshad, S.N. TiO2 nanofibers embedded with g-C3N4 nanosheets and decorated with Ag nanoparticles as Z-scheme photocatalysts for environmental remediation. J. Environ. Chem. Eng. 2019, 7, 103452. [Google Scholar] [CrossRef]
- Kuang, J.; Xing, Z.; Yin, J.; Li, Z.; Zhu, Q.; Zhou, W. Surface plasma Ag-decorated single-crystalline TiO2−x(B) nanorod/defect-rich g-C3N4 nanosheet ternary superstructure 3D heterojunctions as enhanced visible-light-driven photocatalyst. J. Colloid Interface Sci. 2019, 542, 63–72. [Google Scholar] [CrossRef]
- Sobahi, T.R.; Amin, M.S. Upgrading the photocatalytic achievement of g-C3N4 nanosheets along decoration with Ag@TiO2 nanospheres for the preparation of vitamin B3. Appl. Nanosci. 2019, 9, 1621–1636. [Google Scholar] [CrossRef]
- Ahmed, A.; Niazi, M.B.K.; Jahan, Z.; Ahmad, T.; Hussain, A.; Pervaiz, E.; Janjua, H.A.; Hussain, Z. In-vitro and in-vivo study of superabsorbent PVA/Starch/g-C3N4/Ag@TiO2 NPs hydrogel membranes for wound dressing. Eur. Polym. J. 2020, 130, 109650. [Google Scholar] [CrossRef]
- Mahvelati-Shamsabadi, T.; Lee, B.K. Design of Ag/g-C3N4 on TiO2 nanotree arrays via ultrasonic-assisted spin coating as an efficient photoanode for solar water oxidation: Morphology modification and junction improvement. Catal. Today 2020, 358, 412–421. [Google Scholar] [CrossRef]
- Sui, G.; Li, J.; Du, L.; Zhuang, Y.; Zhang, Y.; Zou, Y.; Li, B. Preparation and characterization of g-C3N4/Ag–TiO2 ternary hollowsphere nanoheterojunction catalyst with high visible light photocatalytic performance. J. Alloy Compd. 2020, 823, 153851. [Google Scholar] [CrossRef]
- Soh, M.F.; Noh, M.F.M.; Mohamed, N.A.; Safaei, J.; Rosli, N.N.; Lim, E.L.; Yap, C.C.; Teridi, M.A.M. Incorporation of g-C3N4/Ag dopant in TiO2 as electron transport layer for organic solar cells. Mater. Lett. 2019, 253, 117–120. [Google Scholar] [CrossRef]
- Yang, Y.; Lu, C.; Ren, J.; Li, X.; Ma, Y.; Huang, W.; Zhao, X. Enhanced photocatalytic hydrogen evolution over TiO2/g-C3N4 2D heterojunction coupled with plasmon Ag nanoparticles. Ceram. Int. 2020, 46, 5725–5732. [Google Scholar] [CrossRef]
- Yu, B.; Meng, F.; Khan, M.W.; Qin, R.; Liu, X. Facile synthesis of AgNPs modified TiO2@g-C3N4 heterojunction composites with enhanced photocatalytic activity under simulated sunlight. Mater. Res. Bull. 2020, 121, 110641. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, D.; Sun, P.; Ji, Z.; Duan, J. High efficiency photocatalytic degradation of indoor formaldehyde by Ag/g-C3N4/TiO2 composite catalyst with ZSM-5 as the carrier. Microporous Mesoporous Mater. 2021, 322, 111134. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, W.; He, D.; Situ, Y.; Huang, H. Construction of heterostructured g-C3N4/Ag/TiO2 microspheres with enhanced photocatalysis performance under visible-light irradiation. ACS Appl. Mater. Interfaces 2014, 6, 14405–14414. [Google Scholar] [CrossRef]
- Wang, C.; Rao, Z.; Mahmood, A.; Wang, X.; Wang, Y.; Xie, X.; Sun, J. Improved photocatalytic oxidation performance of gaseous acetaldehyde by ternary g-C3N4/Ag-TiO2 composites under visible light. J. Colloid Interface Sci. 2021, 602, 699–711. [Google Scholar] [CrossRef]
- Leong, K.H.; Liu, S.L.; Sim, L.C.; Saravanan, P.; Jang, M.; Ibrahim, S. Surface reconstruction of titania with g-C3N4 and Ag for promoting efficient electrons migration and enhanced visible light photocatalysis. Appl. Surf. Sci. 2015, 358, 370–376. [Google Scholar] [CrossRef]
- Zang, M.; Shi, L.; Liang, L.; Li, D.; Sun, J. Heterostructured g-C3N4/Ag-TiO2 composites with efficient photocatalytic performance under visible-light irradiation. RSC Adv. 2015, 5, 56136–56144. [Google Scholar] [CrossRef]
- Li, H.; Gao, Y.; Wu, X.; Lee, P.H.; Shih, K. Fabrication of Heterostructured g-C3N4/Ag-TiO2 Hybrid Photocatalyst with Enhanced Performance in Photocatalytic Conversion of CO2 Under Simulated Sunlight Irradiation. Appl. Surf. Sci. 2017, 402, 198–207. [Google Scholar] [CrossRef]
- Zhou, B.; Hong, H.; Zhang, H.; Yu, S.; Tian, H. Heterostructured Ag/g-C3N4/TiO2 with enhanced visible light photocatalytic performances. J. Chem. Technol. Biotechnol. 2019, 94, 3806–3814. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhao, D.; Cao, F.; Luo, Q.; Xiang, Y.; Deng, Y. Highly photoactive Ag-based urchin-like E-g-C3N4 /TiO2 ternary composite photocatalyst. Micro Nano Lett. 2019, 14, 154–157. [Google Scholar] [CrossRef]
- Jo, W.K.; Yoo, H.J. Combination of ultrasound-treated 2D g-C3N4 with Ag/black TiO2 nanostructure for improved photocatalysis. Ultrason.—Sonochem. 2018, 42, 517–525. [Google Scholar] [CrossRef]
- Geng, R.; Yin, J.; Zhou, J.; Jiao, T.; Feng, Y.; Zhang, L.; Chen, Y.; Bai, Z.; Peng, Q. In Situ Construction of Ag/TiO2/g-C3N4 Heterojunction Nanocomposite Based on Hierarchical Co-Assembly with Sustainable Hydrogen Evolution. Nanomaterials 2020, 10, 1. [Google Scholar] [CrossRef] [Green Version]
- Chai, B.; Peng, T.; Mao, J.; Li, K.; Zan, L. Graphitic carbon nitride (g-C3N4)-Pt-TiO2 nanocomposite as an efficient photocatalyst for hydrogen production under visible light irradiation. Phys. Chem. Chem. Phys. 2012, 14, 16745–16752. [Google Scholar] [CrossRef]
- Tangwongputti, C.; Reubroycharoen, P.; Sujaridworakun, P. Facile synthesis of heterostructured g-C3N4/Ag -TiO2 photocatalysts with enhanced visible-light photocatalytic performance. J. Met. Mater. Miner. 2022, 32, 48–54. [Google Scholar] [CrossRef]











Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Glažar, D.; Jerman, I.; Tomšič, B.; Chouhan, R.S.; Simončič, B. Emerging and Promising Multifunctional Nanomaterial for Textile Application Based on Graphitic Carbon Nitride Heterostructure Nanocomposites. Nanomaterials 2023, 13, 408. https://doi.org/10.3390/nano13030408
Glažar D, Jerman I, Tomšič B, Chouhan RS, Simončič B. Emerging and Promising Multifunctional Nanomaterial for Textile Application Based on Graphitic Carbon Nitride Heterostructure Nanocomposites. Nanomaterials. 2023; 13(3):408. https://doi.org/10.3390/nano13030408
Chicago/Turabian StyleGlažar, Dominika, Ivan Jerman, Brigita Tomšič, Raghuraj Singh Chouhan, and Barbara Simončič. 2023. "Emerging and Promising Multifunctional Nanomaterial for Textile Application Based on Graphitic Carbon Nitride Heterostructure Nanocomposites" Nanomaterials 13, no. 3: 408. https://doi.org/10.3390/nano13030408






