Hybrid Nanoparticles of Citrate-Coated Manganese Ferrite and Gold Nanorods in Magneto-Optical Imaging and Thermal Therapy
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Methods
2.2.1. Preparation of Ci-MnFe2O4 NPs
2.2.2. Preparation of CTAB-GNRs
Gold Seed NPs
Preparation of Growth Solution
Purification of CTAB-GNRs
2.2.3. Preparation of Ci-MnFe2O4_CTAB-GNRs Hybrid NPs
2.3. Characterization of NPs
Magnetic Separation
2.4. Gelatin Tissue-Mimicking Phantom
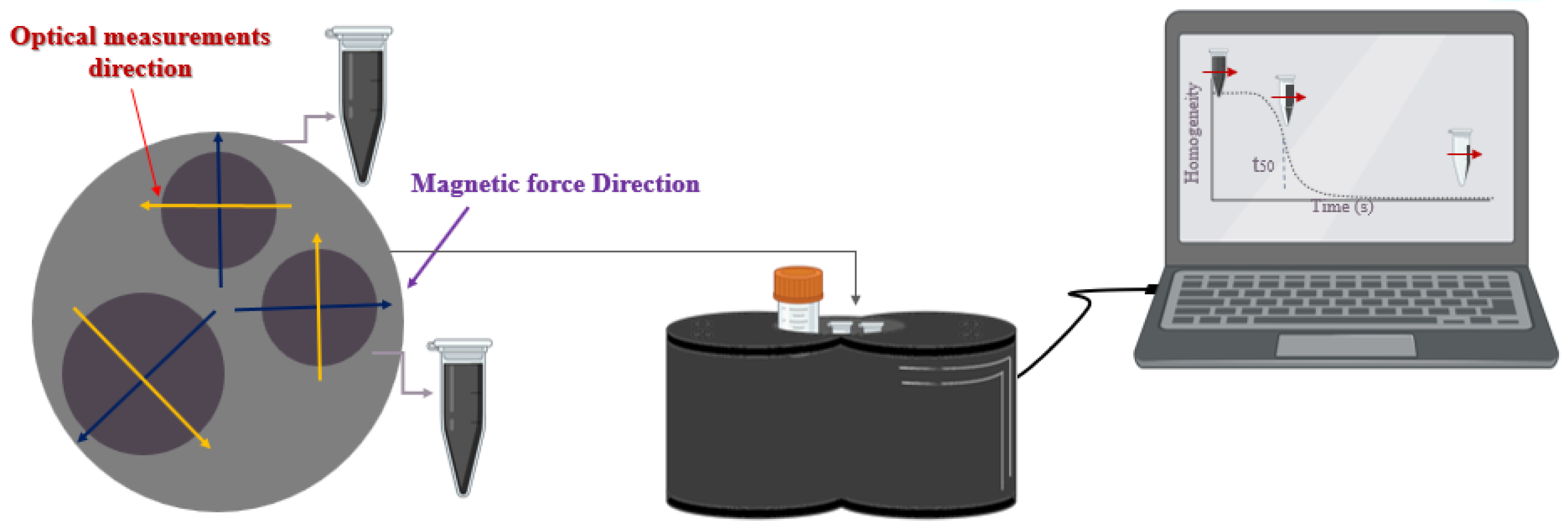
2.5. MMUS Experimental Setup
2.6. PAI Setup
2.7. MH Experiments
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, X.; Neretina, S.; El-Sayed, M.A. Gold nanorods: From synthesis and properties to biological and biomedical applications. Adv. Mater. 2009, 21, 4880–4910. [Google Scholar] [CrossRef] [PubMed]
- Al-Eryani, Y.; Dadashi, M.; Aftabi, S.; Sattarifard, H.; Ghavami, G.; Oldham, Z.W.; Ghoorchian, A.; Ghavami, S. Toxicity, therapeutic applicability, and safe handling of magnetic nanomaterials. In Magnetic Nanomaterials in Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2021; pp. 61–83. [Google Scholar]
- Arsalani, S.; Arsalani, S.; Hadadian, Y.; Sampaio, D.R.T.; Baffa, O.; Pavan, T.Z.; Carneiro, A.A.O. The effect of magnetization of natural rubber latex-coated magnetite nanoparticles on shear wave dispersion magneto-motive ultrasound. Phys. Med. Biol. 2019, 64, 215019. [Google Scholar] [CrossRef] [PubMed]
- Durr, N.J.; Larson, T.; Smith, D.K.; Korgel, B.A.; Sokolov, K.; Ben-Yakar, A. Two-photon luminescence imaging of cancer cells using molecularly targeted gold nanorods. Nano Lett. 2007, 7, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, K.; Bai, Y.Y.; Lu, Z.; Tang, Y.; Deng, Y.; Chen, J.; Qian, W.; Shen, H.; Zhang, Z.; et al. Synthesis of gold nanorods and their functionalization with bovine serum albumin for optical Hyperthermia. J. Biomed. Nanotechnol. 2014, 10, 1440–1449. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-S.; Zhao, Y.; Yoon, S.J.; Gambhir, S.S.; Emelianov, S. Miniature gold nanorods for photoacoustic molecular imaging in the second near-infrared optical window. Nat. Nanotechnol. 2019, 14, 465–472. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Nagaria, P.K.; Hexel, C.R.; Shaw, T.J.; Murphy, C.J.; Wyatt, M.D. Cellular uptake and cytotoxicity of gold nanorods: Molecular origin of cytotoxicity and surface effects. Small 2009, 5, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Arami, H.; Khandhar, A.; Liggitt, D.; Krishnan, K.M. In vivo delivery, pharmacokinetics, biodistribution and toxicity of iron oxide nanoparticles. Chem. Soc. Rev. 2015, 44, 8576–8607. [Google Scholar] [CrossRef] [PubMed]
- Arsalani, S.; Hadadian, Y.; Mazon, E.E.; Guidelli, E.J.; Kava, E.; Ramos, A.P.; Gualdi, A.J.; Pavan, T.Z.; Baffa, O.; Carneiro, A.A.O. Uniform Size PEGylated Iron Oxide Nanoparticles as a Potential Theranostic Agent Synthesized by a Simple Optimized Coprecipitation Route. J. Magn. Magn. Mater 2022, 564, 170091. [Google Scholar] [CrossRef]
- Arsalani, S.; Guidelli, E.J.; Silveira, M.A.; Salmon, C.E.G.; Araujo, J.F.D.F.; Bruno, A.C.; Baffa, O. Magnetic Fe3O4 Nanoparticles Coated by Natural Rubber Latex as MRI Contrast Agent. J. Magn. Magn. Mater. 2019, 475, 458–464. [Google Scholar] [CrossRef]
- Arsalani, S.; Radon, P.; Schier, P.; Jaufenthaler, A.; Liebl, M.; Baumgarten, D.; Wiekhorst, F. Developing Magnetorelaxometry Imaging for Human Applications. Phys. Med. Biol. 2022, 67, 225007. [Google Scholar] [CrossRef] [PubMed]
- Arsalani, S.; Guidelli, E.J.; Araujo, J.F.D.F.; Bruno, A.C.; Baffa, O. Green Synthesis and Surface Modification of Iron Oxide Nanoparticles with Enhanced Magnetization Using Natural Rubber Latex. ACS Sustain. Chem. Eng. 2018, 6, 13756–13765. [Google Scholar] [CrossRef]
- Hadadian, Y.; Masoomi, H.; Dinari, A.; Ryu, C.; Hwang, S.; Kim, S.; Cho, B.K.; Lee, J.Y.; Yoon, J. From Low to High Saturation Magnetization in Magnetite Nanoparticles: The Crucial Role of the Molar Ratios between the Chemicals. ACS Omega 2022, 7, 15996–16012. [Google Scholar] [CrossRef]
- Hadadian, Y.; Sampaio, D.R.T.; Ramos, A.P.; Carneiro, A.A.O.; Mozaffari, M.; Cabrelli, L.C.; Pavan, T.Z. Synthesis and Characterization of Zinc Substituted Magnetite Nanoparticles and Their Application to Magneto-Motive Ultrasound Imaging. J. Magn. Magn. Mater 2018, 465, 33–43. [Google Scholar] [CrossRef]
- Arsalani, S.; Hadadian, Y.; Sampaio, D.R.T.; Arsalani, S.; Almedia, T.W.J.; Pavan, T.Z.; Carneiro, A.A.O. A Study on Magnetic Nanoparticles Concentration in Shear Wave Dispersion Magnetomotive Ultrasound. IFMBE Proc. 2019, 70, 727–730. [Google Scholar]
- Gutierrez, F.V.; de Falco, A.; Yokoyama, E.; Mendoza, L.A.F.; Luz-Lima, C.; Perez, G.; Loreto, R.P.; Pottker, W.E.; la Porta, F.A.; Solorzano, G.; et al. Magnetic Characterization by Scanning Microscopy of Functionalized Iron Oxide Nanoparticles. Nanomaterials 2021, 11, 2197. [Google Scholar] [CrossRef]
- Próspero, A.G.; Quini, C.C.; Bakuzis, A.F.; Fidelis-de-Oliveira, P.; Moretto, G.M.; Mello, F.P.F.; Calabresi, M.F.F.; Matos, R.V.R.; Zandoná, E.A.; Zufelato, N.; et al. Real-Time in Vivo Monitoring of Magnetic Nanoparticles in the Bloodstream by AC Biosuscep-tometry. J. Nanobiotechnology 2017, 15, 2121. [Google Scholar] [CrossRef] [PubMed]
- Soares, G.A.; Prospero, A.G.; Calabresi, M.F.; Rodrigues, D.S.; Simoes, L.G.; Quini, C.C.; Matos, R.R.; Pinto, L.A.; Sousa-Junior, A.A.; Bakuzis, A.F.; et al. Multichannel AC Biosusceptometry System to Map Biodistribution and Assess the Pharmacokinetic Profile of Magnetic Nanoparticles by Imaging. IEEE Trans. Nanobioscience 2019, 18, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Prospero, A.G.; Buranello, L.P.; Fernandes, C.A.H.; dos Santos, L.D.; Soares, G.; C Rossini, B.; Zufelato, N.; Bakuzis, A.F.; de Mattos Fontes, M.R.; de Arruda Miranda, J.R. Corona Protein Impacts on Alternating Current Biosusceptometry Signal and Circulation Times of Differently Coated MnFe2O4 Nanoparticles. Nanomedicine 2021, 16, 2189–2206. [Google Scholar] [CrossRef]
- Quini, C.C.; Matos, J.F.; Próspero, A.G.; Calabresi, M.F.F.; Zufelato, N.; Bakuzis, A.F.; Baffa, O.; Miranda, J.R.A. Renal Perfusion Evaluation by Alternating Current Biosusceptometry of Magnetic Nanoparticles. J. Magn. Magn. Mater 2015, 380, 2–6. [Google Scholar] [CrossRef]
- Nunes, A.D.C.; Ramalho, L.S.; Souza, Á.P.S.; Mendes, E.P.; Colugnati, D.B.; Zufelato, N.; Sousa, M.H.; Bakuzis, A.F.; Castro, C.H. Manganese Ferrite-Based Nanoparticles Induce Ex Vivo, but Not in Vivo, Cardiovascular Effects. Int. J. Nanomed. 2014, 9, 3299. [Google Scholar]
- Nunes, A.D.C.; Gomes-Silva, L.A.; Zufelato, N.; Prospero, A.G.; Quini, C.C.; Matos, R.V.R.; Miranda, J.R.A.; Bakuzis, A.F.; Castro, C.H. Albumin Coating Prevents Cardiac Effect of the Magnetic Nanoparticles. IEEE Trans. Nanobioscience 2019, 18, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shi, H.; Wang, Y.; Shi, B.; Guo, L.; Wu, D.; Yang, S.; Wu, H. Graphene Oxide/Manganese Ferrite Nanohybrids for Magnetic Resonance Imaging, Photothermal Therapy and Drug Delivery. J. Biomater. Appl. 2015, 30, 810–822. [Google Scholar] [CrossRef] [PubMed]
- Islam, K.; Haque, M.; Kumar, A.; Hoq, A.; Hyder, F.; Hoque, S.M. Manganese Ferrite Nanoparticles (MnFe2O4): Size Dependence for Hyperthermia and Negative/Positive Contrast Enhancement in MRI. Nanomaterials 2020, 10, 2297. [Google Scholar] [CrossRef] [PubMed]
- Branquinho, L.C.; Carrião, M.S.; Costa, A.S.; Zufelato, N.; Sousa, M.H.; Miotto, R.; Ivkov, R.; Bakuzis, A.F. Effect of magnetic dipolar interactions on nanoparticle heating efficiency: Implications for cancer hyperthermia. Sci. Rep. 2013, 3, 2887. [Google Scholar] [CrossRef] [PubMed]
- Zufelato, N.; Aquino, V.R.R.; Shrivastava, N.; Mendanha, S.; Miotto, R.; Bakuzis, A.F. Heat Generation in Magnetic Hyperthermia by Manganese Ferrite-Based Nanoparticles Arises from Néel Collective Magnetic Relaxation. ACS Appl. Nano Mater. 2022, 5, 7521–7539. [Google Scholar] [CrossRef]
- Verde, E.L.; Landi, G.T.; Carrião, M.S.; Drummond, A.L.; Gomes, J.A.; Vieira, E.D.; Sousa, M.H.; Bakuzis, A.F. Field dependent transition to the non-linear regime in magnetic hyperthermia experiments: Comparison between maghemite, copper, zinc, nickel and cobalt ferrite nanoparticles of similar sizes. AIP Adv. 2012, 2, 032120. [Google Scholar] [CrossRef]
- Mehrmohammadi, M.; Homan, K.; Joshi, P.; Emelianov, S.; Chen, Y.-S.; Sokolov, K.; Mallidi, S.; Truby, R.; Qu, M. Magne-to-Photo-Acoustic Imaging. Biomed. Opt. Express 2011, 2, 385–396. [Google Scholar]
- Qu, M.; Mallidi, S.; Mehrmohammadi, M.; Ma, L.L.; Johnston, K.P.; Sokolov, K.; Emelianov, S. Combined Photoacoustic and Magneto-Acoustic Imaging. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2009, 2009, 4763–4766. [Google Scholar]
- Qu, M.; Mehrmohammadi, M.; Truby, R.; Graf, I.; Homan, K.; Emelianov, S. Contrast-Enhanced Magneto-Photo-Acoustic Imaging in Vivo Using Dual-Contrast Nanoparticles. Photoacoustics 2014, 2, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Urries, I.; Muñoz, C.; Gomez, L.; Marquina, C.; Sebastian, V.; Arruebo, M.; Santamaria, J. Magneto-Plasmonic Nanoparticles as Theranostic Platforms for Magnetic Resonance Imaging, Drug Delivery and NIR Hyperthermia Applications. Nanoscale 2014, 6, 9230–9240. [Google Scholar] [CrossRef]
- Qu, M.; Mehrmohammadi, M.; Emelianov, S. Detection of nanoparticle endocytosis using magneto-photoacoustic imaging. Small 2011, 7, 2858–2862. [Google Scholar] [CrossRef] [PubMed]
- Mehrmohammadi, M.; Ma, L.L.; Chen, Y.-S.; Qu, M.; Joshi, P.; Chen, R.M.; Johnston, K.P.; Emelianov, S. Combined Photothermal Therapy and Magneto-Motive Ultrasound Imaging Using Multifunctional Nanoparticles. In Nanoscale Imaging Sensing and Actuation for Biomedical Applications VII; International Society for Optics and Photonics: Bellingham, WA, USA, 2010; Volume 7574, pp. 20–27. [Google Scholar]
- Morasso, C.; Picciolini, S.; Schiumarini, D.; Mehn, D.; Ojea-Jiménez, I.; Zanchetta, G.; Vanna, R.; Bedoni, M.; Prosperi, D.; Gramatica, F. Control of Size and Aspect Ratio in Hydroquinone-Based Synthesis of Gold Nanorods. J. Nanoparticle Res. 2015, 17, 1–7. [Google Scholar] [CrossRef]
- Arsalani, S.; Löwa, N.; Kosch, O.; Radon, P.; Baffa, O.; Wiekhorst, F. Magnetic Separation of Iron Oxide Nanoparticles to Improve Their Application for Magnetic Particle Imaging. Phys. Med. Biol. 2021, 66, 015002. [Google Scholar] [CrossRef] [PubMed]
- Leong, S.S.; Ahmad, Z.; Lim, J. Magnetophoresis of superparamagnetic nanoparticles at low field gradient: Hydrodynamic effect. Soft Matter 2015, 11, 6968–6980. [Google Scholar] [CrossRef] [PubMed]
- Andreu, J.; Camacho, J.; Faraudo, J.; Benelmekki, M.; Rebollo, C.; Martínez, L.M. Simple analytical model for the magnetophoretic separation of superparamagnetic dispersions in a uniform magnetic gradient. Phys. Rev. E 2011, 84, 021402. [Google Scholar] [CrossRef]
- Leong, S.S.; Yeap, S.P.; Lim, J.K. Working principle and application of magnetic separation for biomedical diagnostic at high-and low-field gradients. Interface Focus 2016, 6, 20160048. [Google Scholar] [CrossRef] [PubMed]
- De Las Cuevas., G.; Faraudo, J.; Camacho, J. Low-gradient magnetophoresis through field-induced reversible aggregation. J. Phys. Chem. C 2008, 112, 945–950. [Google Scholar] [CrossRef]
- Lim, J.; Lanni, C.; Evarts, E.R.; Lanni, F.; Tilton, R.D.; Majetich, S.A. Magnetophoresis of nanoparticles. ACS Nano 2011, 5, 217–226. [Google Scholar] [CrossRef]
- Leong, S.S.; Ahmad, Z.; Low, S.C.; Camacho, J.; Faraudo, J.; Lim, J.K. Unified view of magnetic nanoparticle separation under magnetophoresis. Langmuir 2020, 36, 8033–8055. [Google Scholar] [CrossRef]
- Pavan, T.Z.; Madsen, E.L.; Frank, G.R.; Adilton O Carneiro, A.; Hall, T.J. Nonlinear elastic behavior of phantom materials for elastography. Phys. Med. Biol 2010, 55, 2679–2692. [Google Scholar] [CrossRef]
- Mazon, E.E.; Arsalani, S.; Uliana, J.H.; Carneiro, A.A.O.; Gualdi, A.J.; Pavan, T.Z. A Pulsed Magnetomotive Ultrasound Imaging System for Magnetic Nanoparticle Detection. In Proceedings of the 2021 IEEE UFFC Latin America Ultrasonics Symposium (LAUS), Gainesville, FL, USA, 4–5 October 2021; pp. 1–4. [Google Scholar]
- Sampaio, D.R.T.; Grillo, F.W.; Bruno, A.C.; Pavan, T.Z.; Carneiro, A.A.O. A magneto-motive ultrasound platform designed for pre-clinical and clinical applications. Res. Biomed. Eng. 2017, 32, 337–346. [Google Scholar] [CrossRef]
- Uliana, J.H.; Sampaio, D.R.T.; Fernandes, G.S.P.; Brassesco, M.S.; Nogueira-Barbosa, M.H.; Carneiro, A.A.O.; Pavan, T.Z. Multiangle long-axis lateral illumination photoacoustic imaging using linear array transducer. Sensors 2020, 20, 4052. [Google Scholar] [CrossRef] [PubMed]
- Hadadian, Y.; Azimbagirad, M.; Navas, E.A.; Pavan, T.Z. A versatile induction heating system for magnetic hyperthermia studies under different experimental conditions. Rev. Sci. Instrum. 2019, 90, 074701. [Google Scholar] [CrossRef]
- Patil, R.; Thorat, N.D.; Shete, P.B.; Otari, S.V.; Tiwale, B.M.; Pawar, S.H. In vitro hyperthermia with improved colloidal stability and enhanced SAR of magnetic core/shell nanostructures. Mater. Sci. Eng. 2016, 59, 702–709. [Google Scholar] [CrossRef]
- Hadadian, Y.; Ramos, A.P.; Pavan, T.Z. Role of zinc substitution in magnetic hyperthermia properties of magnetite nanoparticles: Interplay between intrinsic properties and dipolar interactions. Sci. Rep. 2019, 9, 18048. [Google Scholar] [CrossRef] [PubMed]
- Hadadian, Y.; Uliana, J.H.; Carneiro, A.A.O.; Pavan, T.Z. A novel theranostic platform: Integration of magnetomotive and thermal ultrasound imaging with magnetic hyperthermia. IEEE Trans. Biomed. Eng. 2020, 68, 68–77. [Google Scholar] [CrossRef]
- Mazon, E.; Villa-Martínez, E.; Hernández-Sámano, A.; Córdova-Fraga, T.; Ibarra-Sánchez, J.J.; Calleja, H.A.; Leyva Cruz, J.A.; Barrera, A.; Estrada, J.C.; Paz, J.A.; et al. A high-resolution frequency variable experimental setup for studying ferrofluids used in magnetic hyperthermia. Rev. Sci. Instrum. 2017, 88, 084705. [Google Scholar] [CrossRef]
- Kumbhar, A.S.; Kinnan, M.K.; Chumanov, G. Multipole plasmon resonances of submicron silver particles. J. Am. Chem. Soc. 2005, 127, 12444–12445. [Google Scholar] [CrossRef]
- Guidelli, E.J.; Araujo, L.F.; Assunção, A.C.A.; Carvalho, I.C.S.; Clarke, D.R.; Baffa, O. Microwave-Assisted Growth of Silver Nanoparticle Films with Tunable Plasmon Properties and Asymmetrical Particle Geometry for Applications as Radiation Sensors. Plasmonics 2020, 15, 1551–1564. [Google Scholar] [CrossRef]
- Amendola, V.; Bakr, O.M.; Stellacci, F. A study of the surface plasmon resonance of silver nanoparticles by the discrete dipole approximation method: Effect of shape, size, structure, and assembly. Plasmonics 2010, 5, 85–97. [Google Scholar] [CrossRef]
- Truby, R.; Emelianov, S.; Homan, K.A. Ligand-mediated self-assembly of hybrid plasmonic and superparamagnetic nanostructures. Langmuir 2013, 29, 2465–2470. [Google Scholar] [CrossRef] [PubMed]
- Bakuzis, A.F.; Branquinho, L.C.; Luiz E Castro, L.; de Amaral E Eloi, M.T.; Miotto, R. Chain formation and aging process in biocompatible polydisperse ferrofluids: Experimental investigation and Monte Carlo simulations. Adv. Colloid Interface Sci. 2013, 191, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Shaw, D.J. Introduction to Colloid and Surface Chemistry; Butterworths: Oxford, UK; Boston, MA, USA, 1980. [Google Scholar]
- Chen, Z.P.; Zhang, Y.; Xu, K.; Xu, R.Z.; Uu, J.W.; Gu, N. Stability of hydrophilic magnetic nanoparticles under biologically relevant conditions. J. Nanosci. Nanotechnol. 2008, 8, 6260–6265. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Wang, J.H.; Liu, T.; Xie, Z.; Yu, X.F.; Li, W. Surface chemistry but not aspect ratio mediates the biological toxicity of gold nanorods in vitro and in vivo. Sci. Rep. 2015, 5, 11398. [Google Scholar] [CrossRef]
- Cao, Q.; Zhao, H.; He, Y.; Li, X.; Zeng, L.; Ding, N.; Wang, J.; Yang, J.; Wang, G. Hydrogen-bonding-induced colorimetric detection of melamine by nonaggregation-based Au-NPs as a probe. Biosens. Bioelectron. 2010, 25, 2680–2685. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, L.; Turrina, C.; Schwaminger, S. The Effect of pH and Viscosity on Magnetophoretic Separation of Iron Oxide Nanoparticles. Magnetochemistry 2021, 7, 80. [Google Scholar] [CrossRef]
- Mehrmohammadi, M.; Yoon, K.Y.; Qu, M.; Johnston, K.P.; Emelianov, S.Y. Enhanced pulsed magneto-motive ultrasound imaging using superparamagnetic nanoclusters. Nanotechnology 2010, 22, 045502. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.Y.; Mehrmohammadi, M.; Borwankar, A.; Emelianov, S.Y.; Johnston, K.P. Synthesis of Iron Oxide Nanoclusters with Enhanced Magnetization and Their Applications in Pulsed Magneto-Motive Ultrasound Imaging. Nano 2015, 10, 1550073. [Google Scholar] [CrossRef]
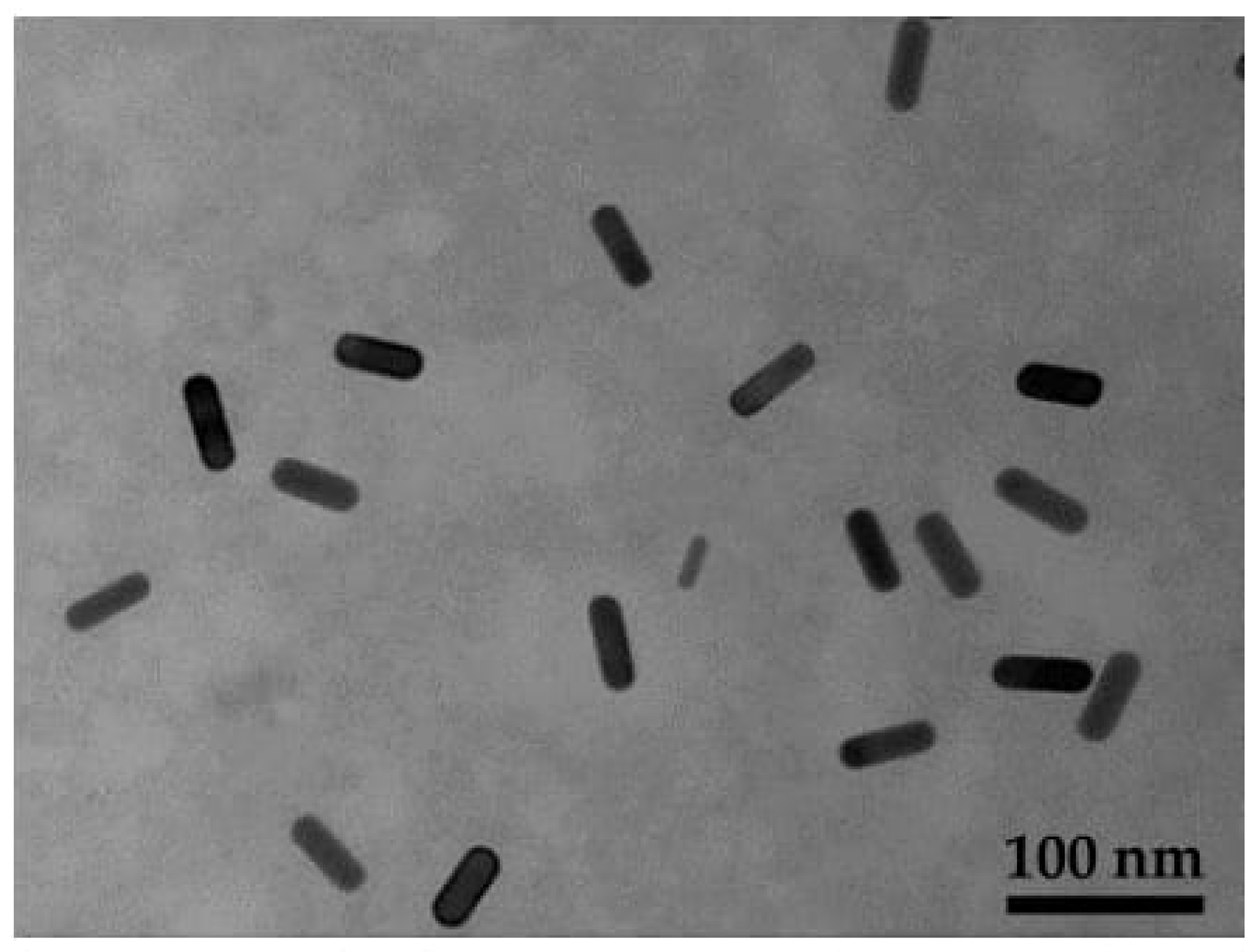
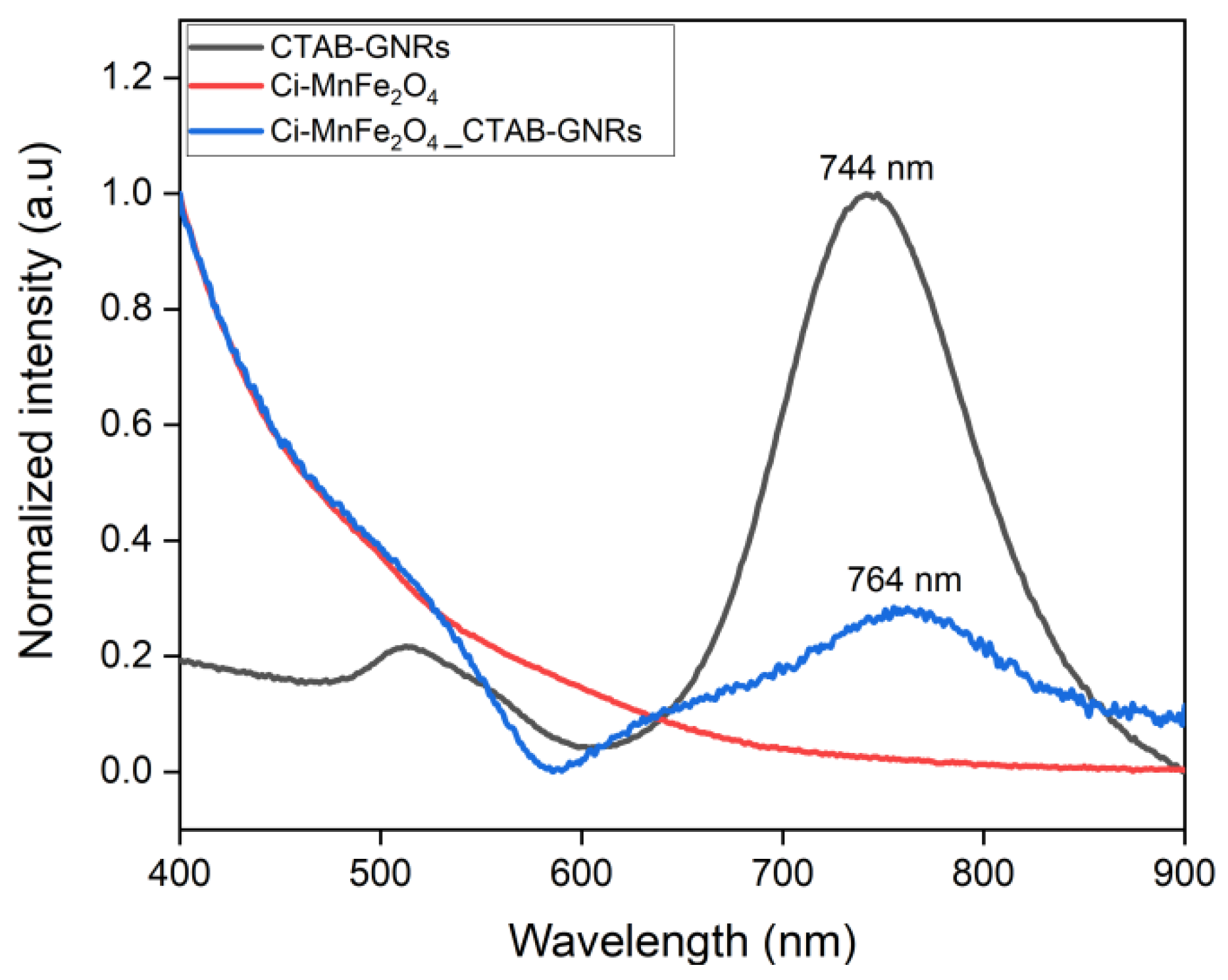
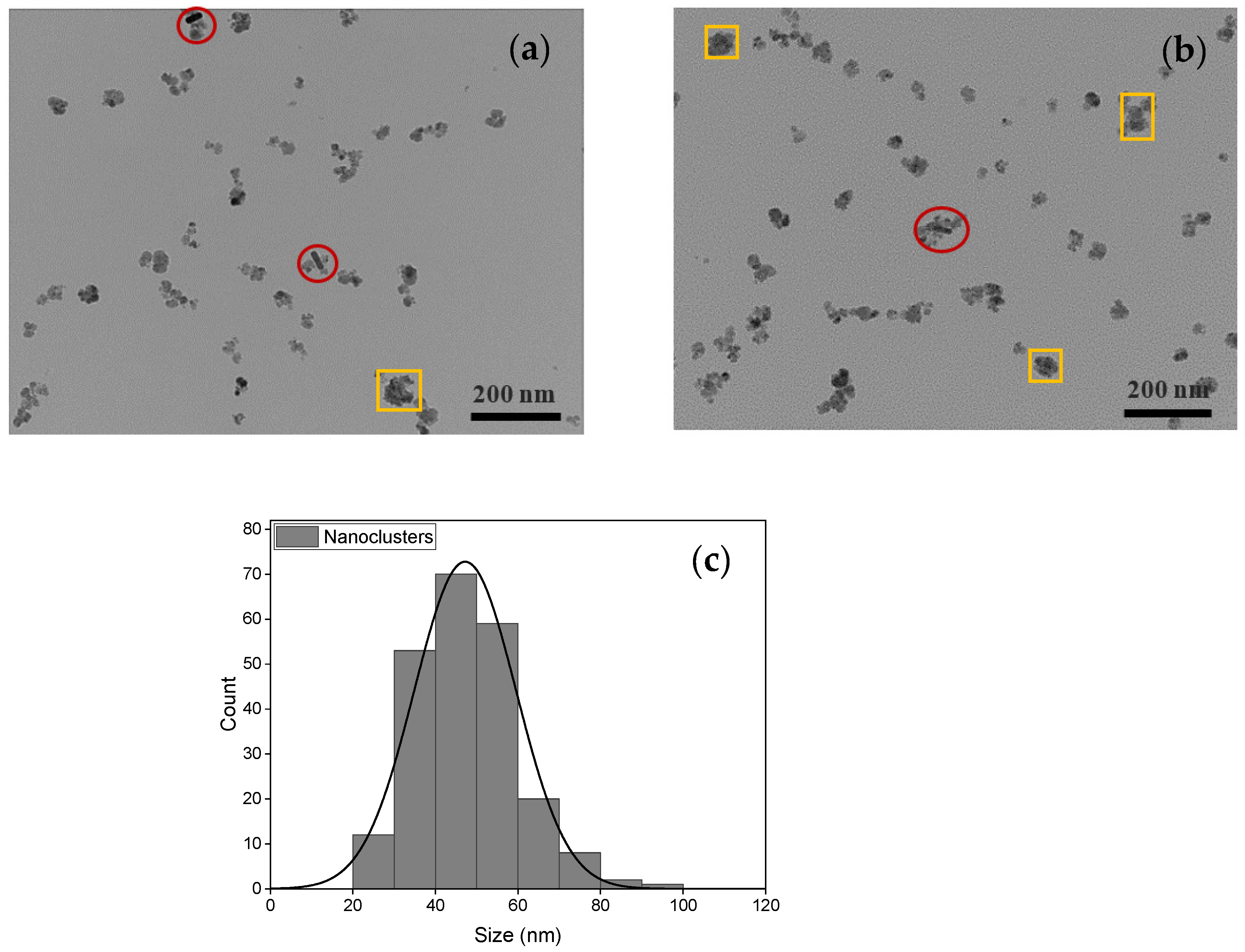
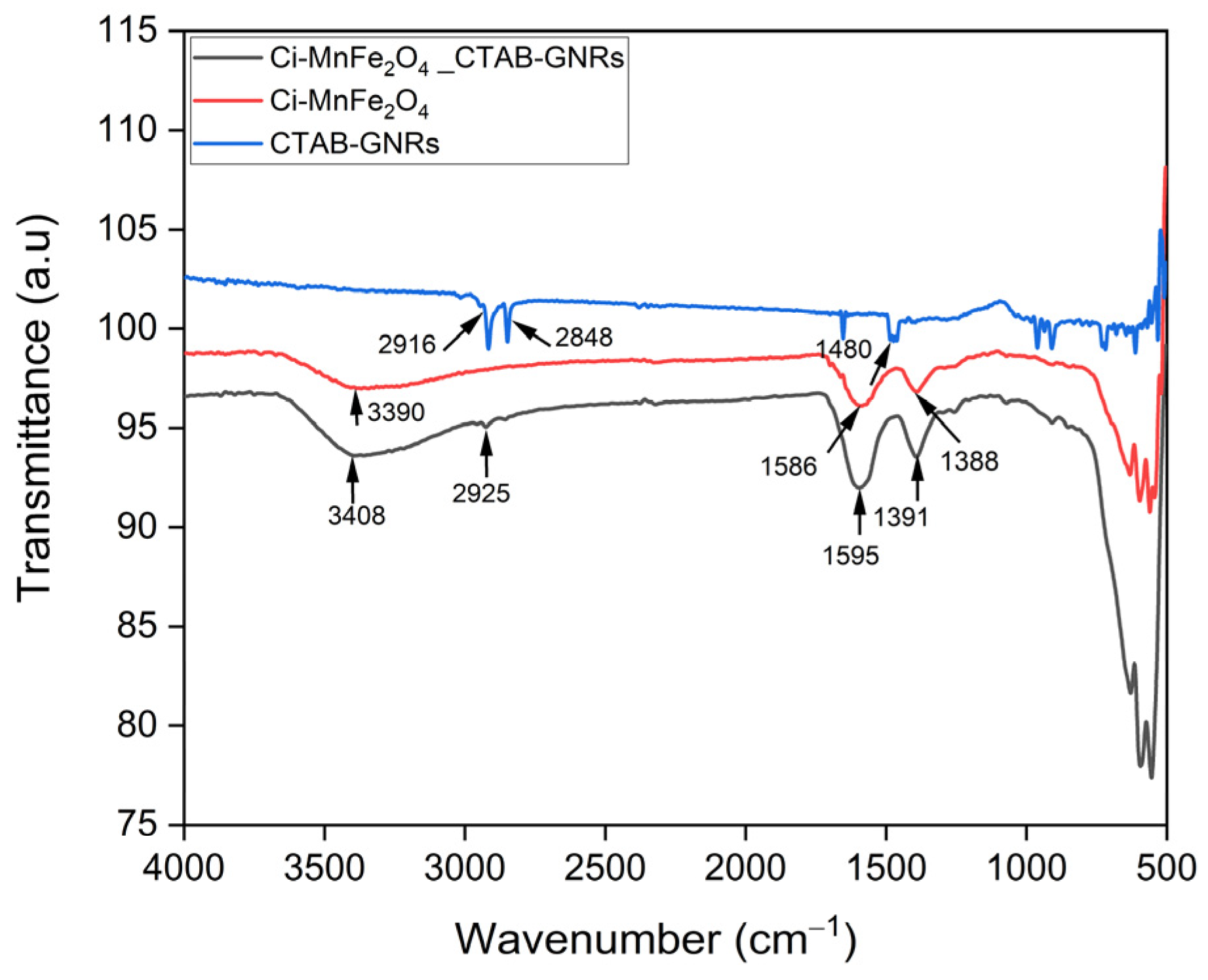


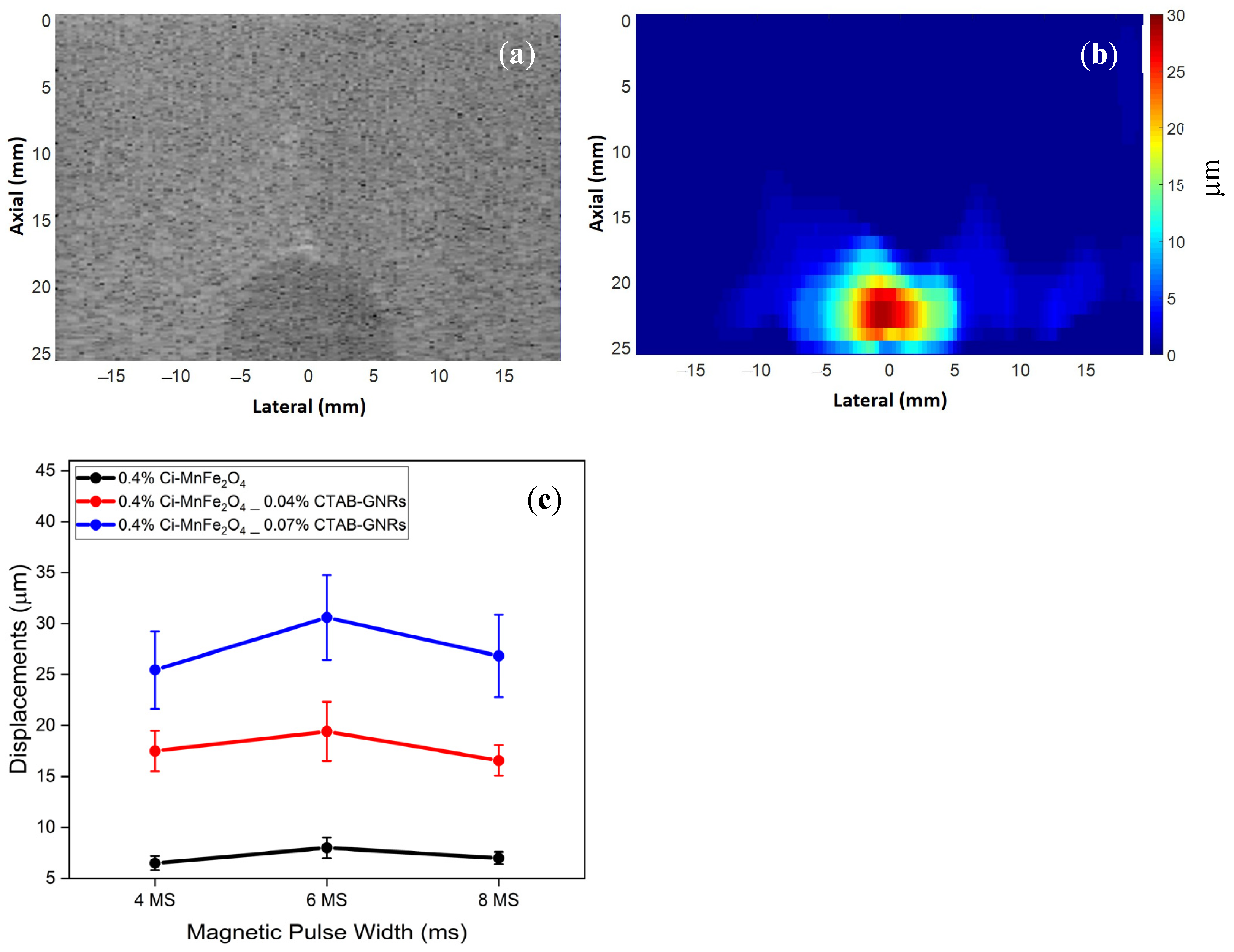
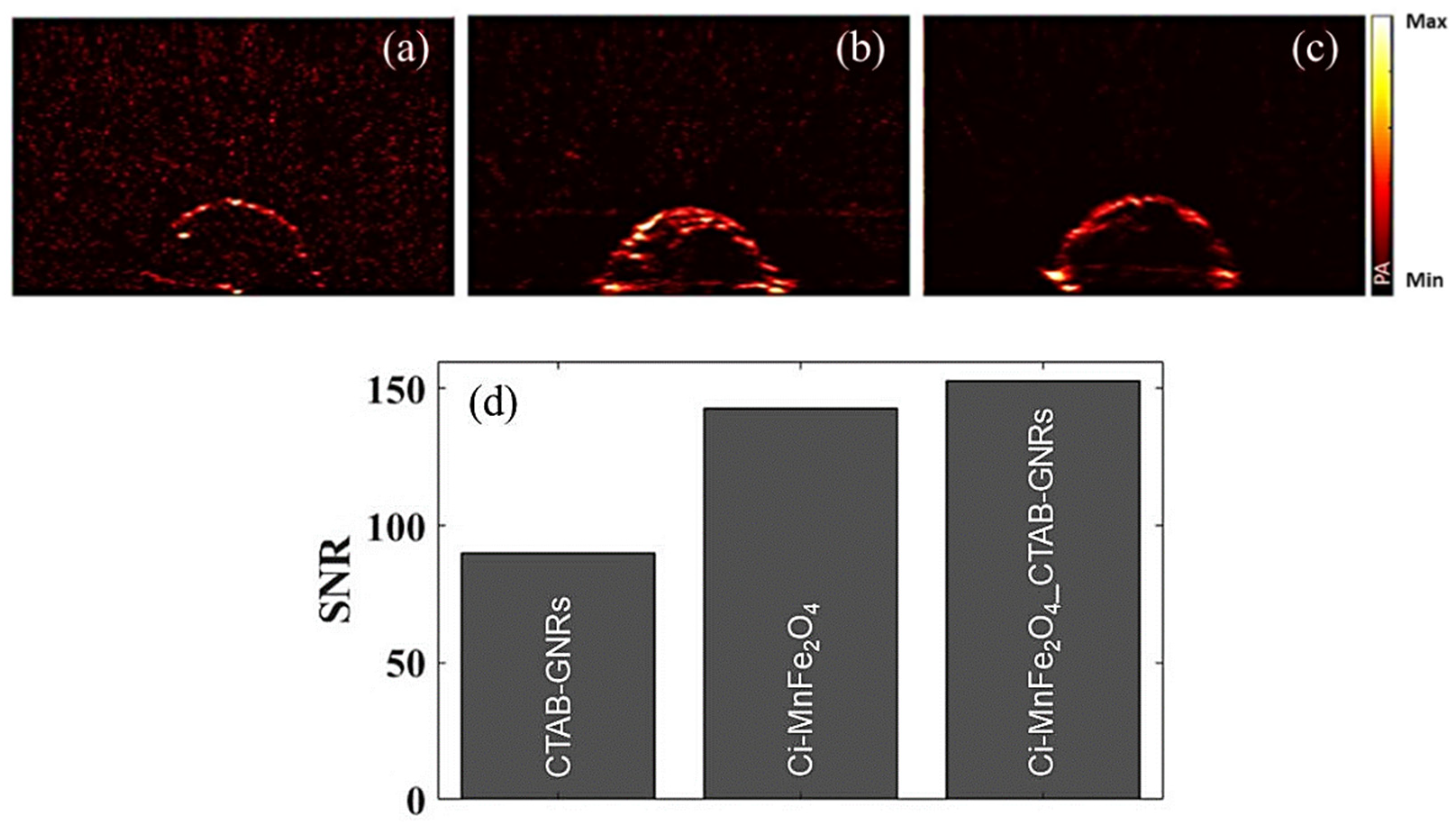
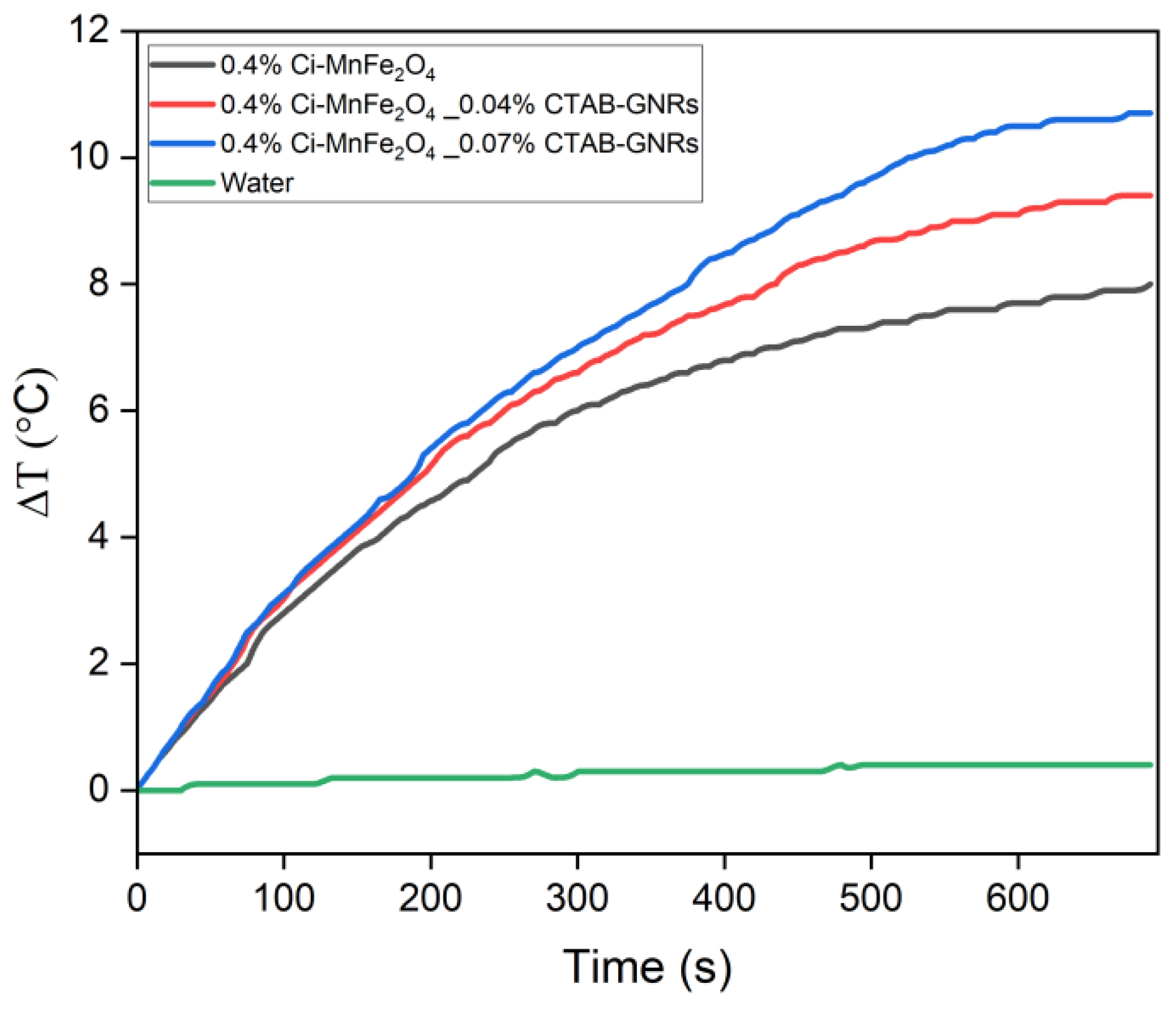
| Samples | Solution | Zeta Potential (mV) | Hydrodynamic Size (nm) | PDI |
|---|---|---|---|---|
| CTAB-GNRs | Water | 41 | - | - |
| Ci-MnFe2O4 | Water | −43.5 | 38 | 0.32 |
| 0.4 wt% Ci-MnFe2O4_0.04 wt% CTAB-GNRs | Water | −30.4 | 43 | 0.34 |
| 0.4 wt% Ci-MnFe2O4_0.07 wt% CTAB-GNRs | Water | −31.1 | 37.5 | 0.33 |
| 0.4 wt% Ci-MnFe2O4_0.04 wt% CTAB-GNRs | PBS | −37.3 | 38 | 0.4 |
| 0.4 wt% Ci-MnFe2O4_0.07 wt% CTAB-GNRs | PBS | −33.8 | 44 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arsalani, S.; Arsalani, S.; Isikawa, M.; Guidelli, E.J.; Mazon, E.E.; Ramos, A.P.; Bakuzis, A.; Pavan, T.Z.; Baffa, O.; Carneiro, A.A.O. Hybrid Nanoparticles of Citrate-Coated Manganese Ferrite and Gold Nanorods in Magneto-Optical Imaging and Thermal Therapy. Nanomaterials 2023, 13, 434. https://doi.org/10.3390/nano13030434
Arsalani S, Arsalani S, Isikawa M, Guidelli EJ, Mazon EE, Ramos AP, Bakuzis A, Pavan TZ, Baffa O, Carneiro AAO. Hybrid Nanoparticles of Citrate-Coated Manganese Ferrite and Gold Nanorods in Magneto-Optical Imaging and Thermal Therapy. Nanomaterials. 2023; 13(3):434. https://doi.org/10.3390/nano13030434
Chicago/Turabian StyleArsalani, Saeideh, Soudabeh Arsalani, Mileni Isikawa, Eder J. Guidelli, Ernesto E. Mazon, Ana Paula Ramos, Andris Bakuzis, Theo Z. Pavan, Oswaldo Baffa, and Antonio A. O. Carneiro. 2023. "Hybrid Nanoparticles of Citrate-Coated Manganese Ferrite and Gold Nanorods in Magneto-Optical Imaging and Thermal Therapy" Nanomaterials 13, no. 3: 434. https://doi.org/10.3390/nano13030434





